Significance
Stacking of cisternae in the mammalian Golgi apparatus is known to be a highly complicated process that involves a large number of adhesive proteins, including Golgi reassembly and stacking proteins (GRASPs) and Golgin tethers, as well as coordinated disassembly/reassembly of Golgi stacks during mitotic cell division. Using extensive quantitative analysis of electron microscopic data, we show here that Golgi stacking with flattened cisternae is maintained in HeLa cells depleted of GRASP65/55 proteins when the GRASP-deficient cells are complemented by exogenous expression of either Golgi matrix protein of 130 kDa or Golgin45. These results strongly suggest that the Golgi stack assembly and cisternal morphology are governed by simple membrane adhesion at the core, explaining how Golgi stacking occurs in organisms, which do not express (or use) GRASP-type adhesion proteins such as plants and yeast.
Keywords: tethers, GRASPs
Abstract
Two classes of proteins that bind to each other and to Golgi membranes have been implicated in the adhesion of Golgi cisternae to each other to form their characteristic stacks: Golgi reassembly and stacking proteins 55 and 65 (GRASP55 and GRASP65) and Golgin of 45 kDa and Golgi matrix protein of 130 kDa. We report here that efficient stacking occurs in the absence of GRASP65/55 when either Golgin is overexpressed, as judged by quantitative electron microscopy. The Golgi stacks in these GRASP-deficient HeLa cells were normal both in morphology and in anterograde cargo transport. This suggests the simple hypothesis that the total amount of adhesive energy gluing cisternae dictates Golgi cisternal stacking, irrespective of which molecules mediate the adhesive process. In support of this hypothesis, we show that adding artificial adhesive energy between cisternae and mitochondria by dimerizing rapamycin-binding domain and FK506-binding protein domains that are attached to cisternal adhesive proteins allows mitochondria to invade the stack and even replace Golgi cisternae within a few hours. These results indicate that although Golgi stacking is a highly complicated process involving a large number of adhesive and regulatory proteins, the overriding principle of a Golgi stack assembly is likely to be quite simple. From this simplified perspective, we propose a model, based on cisternal adhesion and cisternal maturation as the two core principles, illustrating how the most ancient form of Golgi stacking might have occurred using only weak cisternal adhesive processes because of the differential between the rate of influx and outflux of membrane transport through the Golgi.
The Golgi apparatus plays a central role in the processing, sorting, and secretion of various cargo molecules destined for various intracellular and extracellular destinations (1). In animal and plant cells, its unique structure of four to six stacked, roughly planar cisternae serves, among other things, as a platform to organize Golgi resident glycosyltransferases into distinct membrane-bound subcompartments (the cis-, medial-, and trans-Golgi cisternae) for proper and sequential posttranslational maturation of the transiting cargo proteins (2, 3).
Although these characteristic features of Golgi morphology have drawn the attention of many researchers for many decades, the molecular mechanisms underlying them are still unclear (4). Pioneering functional reconstitution studies using a cell-free system in which Golgi stacks (but not ribbons) reassemble from mitotic extracts (5–8) yielded two classes of purified proteins, each clearly contributing to stacking: globular Golgi reassembly and stacking proteins (GRASPs; the homologous proteins GRASP65 and GRASP55) (7, 9) and the helical rod-like and partially homologous proteins Golgi matrix protein of 130 kDa (GM130) and Golgin of 45 kDa (Golgin45) (10, 11). One member of each family (GRASP65 and GM130) is located in the cis-most cisterna (7, 12), and another member of each family (GRASP55 and Golgin45) is located in this (and more so in later cisternae) (9, 10). The GRASP proteins bind to the Golgins (note that we use the term “Golgin” in a limited sense in this article to refer either to GM130 or Golgin45) (10, 11). An appealing mechanism for intercisternal adhesion has been proposed for the GRASP proteins based on X-ray crystallography and biochemistry that involves PSD-95, Dlg1, Zo-1 domain-dependent homo-oligomerization in trans (13–15). In recent years, with the advent of RNAi-based technologies, knock-down studies have broadly confirmed a role for GRASP proteins and Golgins in controlling Golgi morphology but have not agreed with each other on many notable details, leaving the field in a somewhat confused and conflictory state (10, 15–20). In the simpler case of Drosophila, dsRNA-mediated depletion of dGRASP results in ∼30% loss of Golgi stacks, whereas double depletion of dGRASP and dGM130 was shown to unstack the Golgi, as examined by EM (21). In Schizosaccharomyces pombe, depletion of yeast GRASP homolog Grh1 has no effect on Golgi stacking (22). This situation can be extended to plants, where no GRASP or even Golgin homolog has been identified thus far (23).
To address this discrepancy, we studied the relative contribution of these four “stacking factors” (GM130, Golgin45, GRASP65, and GRASP55) for Golgi stacking by extensive quantitative analysis of EM-based studies. The results of these experiments strongly indicate that the overriding principle of Golgi stack assembly is simple cisternal adhesion, regardless of which molecule mediates the cisternal adhesive process. We show that adhesive energy that binds cisternae to each other at physiological equilibrium can be generated by many different combinations of Golgins+GRASPs or even in the absence of GRASPs. On the basis of this new understanding, we propose a simple mechanistic model illustrating how the most ancient form of Golgi stacking might have been facilitated by the principles described in our adhesion model and cisternal maturation (i.e., Rab conversion) in organisms, such as yeast (S. pombe) and plants, for which these four adhesive proteins either are not found or are not important for stacking.
Results
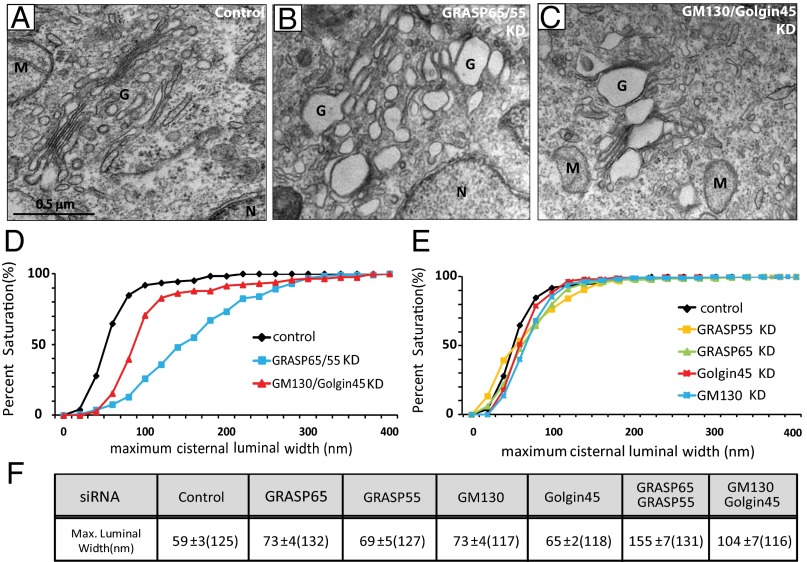
Simultaneous Depletion of GRASP65/55 or GM130/Golgin45 Disrupts Cisternal Flatness of Golgi Stacks but Does Not Result in Golgi Unstacking.
A recent study reported that double knockdown of GRASP65/55 results in complete unstacking of Golgi in HeLa cells (15). To better understand the relative contribution of Golgins and GRASPs in Golgi stack assembly and to find out whether GRASPs are both necessary and sufficient for Golgi stacking in vivo, we decided to simultaneously deplete both GRASPs or both Golgins and study the effects on Golgi stacking, using electron microscopy. In contrast to the earlier report, we observed that simultaneous depletion of GRASP65/55 or their Golgin binding partners, GM130/Golgin45, results in disruption of Golgi cisternal flatness (cf. EM photos in Fig. 1 A–C) without significant Golgi unstacking. As shown in Fig. S1, double depletion of GRASPs resulted in a significant reduction of Golgins, and vice versa. These phenomena suggest that Golgins and GRASPs are likely to affect the stability of each other to a certain extent (GM130–GRASP65 and Golgin45–GRASP55), mainly because of the role they play in the membrane association of their binding partners. Importantly, however, even with this additional reduction in the level of other adhesion proteins, we did not observe any significant Golgi unstacking in either GRASP or Golgin double-depleted HeLa cells. To exclude the possibility that this difference is a result of siRNA oligos used for GRASP65/55 depletion, we designed an additional set of siRNA oligos against GRASP65 and GRASP55, respectively. The efficiency of GRASP65/55 double knockdown routinely reached above 98% for both sets of siRNAs against GRASP65/55 (Figs. S1 and S2). Neither siRNA combinations led to significant unstacking of the Golgi when examined under EM. We have no explanation for this discrepancy, although we also observed a significant unstacking of the Golgi in a small percentage (<1%) of the knockdown cells we analyzed.
Fig. 1.
Double knockdown of GRASP65/55 or GM130/Golgin45 leads to significant disruption in Golgi cisternal flatness, but not Golgi disassembly. (A–C) Representative EM micrographs of HeLa cells treated with siRNAs against indicated adhesive proteins for 96 h. (Scale bar, 0.5 μm.) Control siRNA (A); GRASP65/55 siRNA (B); GM130/Golgin45 siRNA (C). Double knockdown of the two GRASPs or two Golgins resulted in significant disruption of Golgi cisternal flatness, but we did not observe significant Golgi unstacking under any double-knockdown conditions. For morphological criteria of Golgi unstacking, we looked for significant separation of cisternae from main body of the Golgi or significant vesiculation of the Golgi at the EM level. (D and E) K-S plots showing distribution of maximum cisternal luminal width in double-knockdown cells (D) or in single-knockdown cells (E), based on luminal width measurement using ImageJ software. (F) Table summarizing maximum luminal width measurement for both single- and double-knockdown cells. Results are expressed as the mean ± SEM. Numbers in parenthesis indicate the number of Golgi cisternae for which luminal width was measured. Note that all analysis passed the Student t test (P < 0.05), except for Golgin45-depleted cells. G, Golgi; n, nucleus; M, mitochondria; tER, transitional endoplasmic reticulum.
Morphologically, we noticed that Golgi membranes appeared to have lost the characteristic flattened cisternae. As a way to quantify the change in Golgi cisternal morphology, we measured the maximum luminal width of the Golgi cisternae (see Fig. S1 for an example). In control siRNA-transfected cells, the average maximum luminal width of Golgi cisternae was around 59 ± 3 nm (Fig. 1F). In single-knockdown cells, we observed a small but consistent increase (∼10–15 nm) in the average luminal width, whereas GRASP65/55 or GM130/Golgin45 double depletion led to a two- to threefold increase in the luminal width (Fig. 1 D and E for Kolmogorov–Smirnov (K-S) plots; see Fig. S1 for frequency distribution graphs).
We reasoned that increased cisternal width in these knockdown cells might be a result of a block in anterograde cargo transport through the Golgi. To test this, we used CD8 fused to conditional aggregation domain (24) to assess the efficiency of bulk cargo transport under these knockdown conditions. Surprisingly, we found that GRASP65/55 double knockdown consistently led to a ∼30–40% increase in CD8 transport to the plasma membrane compared with control cells and GM130/Golgin45 depleted cells (Fig. S2; see Fig. S3 for raw data), suggesting that the increased cisternal width in these cells is not likely to be caused by accumulation of anterograde cargo within the lumen of the Golgi cisterna.
Additional Knockdown of Golgin45 in GRASP65/55-Depleted Cells Results in Golgi Disassembly.
These findings naturally led us to the question of whether the changes in cisternal flatness in the GRASP- or Golgin-depleted cells are associated with Golgi stacking. In other words, the disrupted cisternal flatness may or may not be indicative of the progression toward Golgi disassembly in these cells. To find an answer to this seemingly elusive question, we tested whether additional depletion of Golgin45 or GM130 leads to Golgi disassembly in GRASP65/55-depleted HeLa cells. If so, the result would suggest that both cisternal flatness and cisternal stacking are determined by the collective cisternal adhesive process from these GRASPs and Golgins. In support of this idea, we found that triple knockdown of GRASP65/55 and Golgin45 (and, to a lesser extent, GM130) in HeLa cells results in significant Golgi unstacking (Fig. S4 A and B). Qualitatively, depletion of Golgin45/GRASP65/55 led to almost complete Golgi disassembly, but only partial Golgi unstacking was observed in GM130/GRASP65/55-depleted cells (compare EM photos in Fig. S4 A and B).
Is the Disruption in Golgi Cisternal Flatness Reversible?
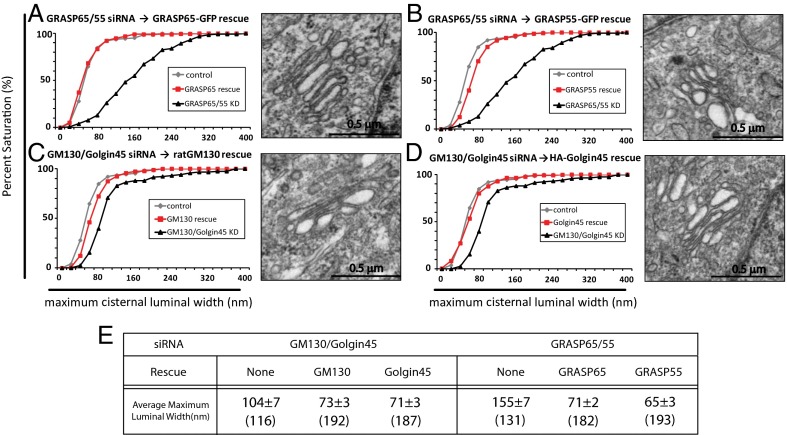
We then asked whether the disruption in cisternal flatness can be restored by rescue transfection. To do this, we expressed an RNAi-resistant form of each adhesion protein in double-knockdown cells that eliminate both GRASP proteins or both Golgins. As expected, Golgi stack morphology was restored by either exogenously expressing GRASP65 or GRASP55 in GRASPs double-depleted cells (Fig. 2 A and B) or by expressing either Golgin GM130 or Golgin45 in Golgin double-depleted cells (Fig. 2 C and D; see Fig. 2E for summary). The average maximum luminal width for rescued Golgi cisternae was very close to the values we saw in single-knockdown cells. The knockdown and rescue expressions were confirmed by Western blots, as shown in Fig. S5. Altogether, this is consistent with the idea that GRASP55 and GRASP65 play a complementary role in Golgi stacking and cisternal morphology, as can Golgin45 and GM130, despite their differing physiological cis-trans distributions.
Fig. 2.
Disrupted Golgi cisternal flatness in the double-depletion cells can be reversed by rescue transfection. (A–D) K-S plots showing rescue of Golgi cisternal flatness by exogenous expression of the RNAi-resistant form of GRASP65 (A) or GRASP55 (B) in cells depleted of both GRASPs and by exogenous expression of the RNAi-resistant form of GM130 (C) or Golgin45 (D) in cells depleted of both Golgins. Representative electron micrographs of Golgi stacks in cells rescued with indicated cDNAs are shown in the right panel of each graph. (Scale bar, 0.5 μm.) (E) Table summarizing the results from rescue experiments. Results are expressed as the mean ± SEM. Numbers in parenthesis indicate the number of Golgi cisternae that luminal width was measured.
Are GRASP Proteins Dispensable for Golgi Stacking?
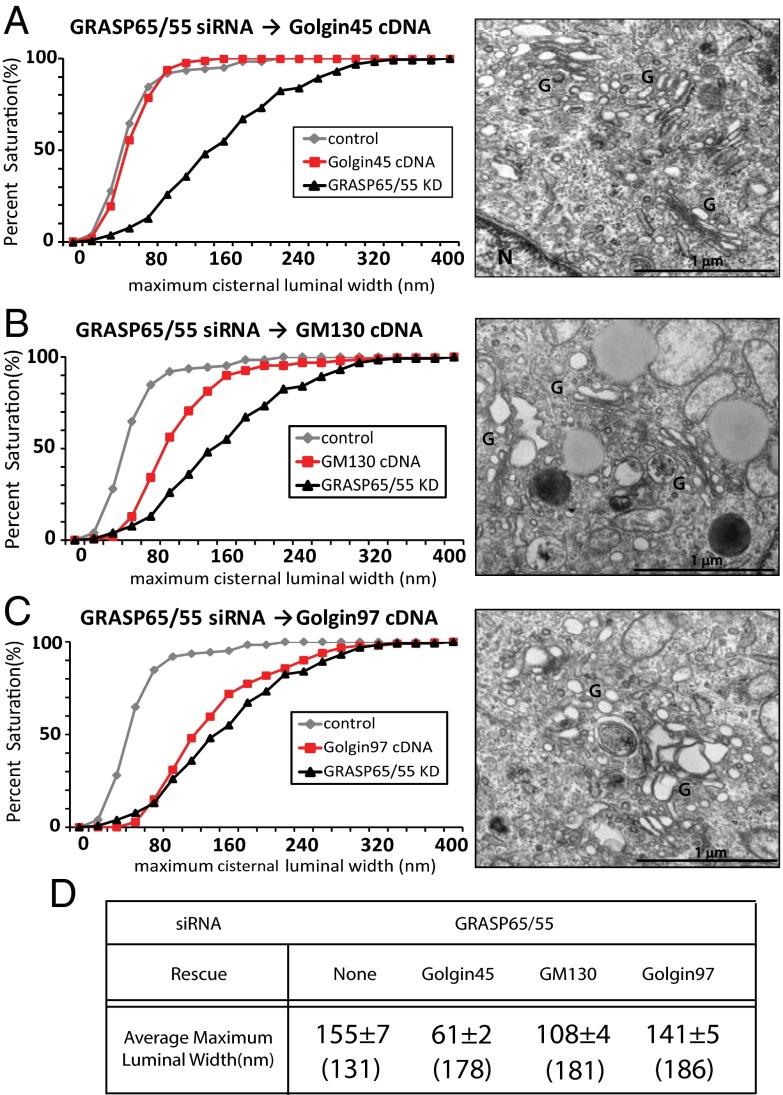
As shown in the triple-knockdown experiments (Fig. S4), there was a strong indication that GRASPs and Golgins may be playing functionally complementary roles in Golgi stacking and cisternal morphology. If this is true, is it possible that Golgins may be able to substitute for GRASPs either entirely or partially in maintaining Golgi stacking and cisternal morphology? To test this, we exogenously expressed Golgins in GRASP65/55-depleted HeLa cells to see whether Golgins can restore cisternal flatness in GRASP-depleted HeLa cells. Remarkably, crossing the functional barrier between GRASPs and Golgins was equally effective. Expressing either Golgin protein in GRASPs double-depleted cells restored the Golgi stack morphology, as judged qualitatively by EM (Fig. 3 A and B, Right). Golgi cisternal flatness was not restored by expressing trans-Golgi network localized Golgin97 (Fig. 3C). As shown in Fig. S6, we also performed reverse experiments, in which we depleted GM130/Golgin45, followed by exogenous expression of GRASP55-GFP. Although the degree of restoration is smaller compared with the effect of Golgin overexpression in GRASP-depleted cells, we still found a significant restoration in cisternal flatness in these cells (P < 0.05).
Fig. 3.
Morphologically and functionally normal Golgi stacks in GRASP65/55-deficient HeLa cells (A–C) K-S plots showing rescue of Golgi cisternal flatness by exogenous expression of Golgin45 (A) or GM130 (B) or Golgin97 as a control (C) in cells depleted of both GRASP65/55. Representative electron micrographs of Golgi stacks in cells rescued with indicated cDNAs are shown in the right panel of each graph. (Scale bar, 1 μm.) (D) Table summarizing the results from substitution experiments. Results are expressed as the mean ± SEM. Numbers in parenthesis indicate the number of Golgi cisternae that luminal width was measured.
We measured maximum cisternal luminal width to quantify the degree of restoration (see K-S plots in Fig. 3 A–D). Exogenous expression of Golgin45 in GRASP65/55-depleted cells resulted in Golgi with a 61 ± 2 nm average maximum cisternal luminal width (from 155 ± 7 nm in GRASP65/55-depleted cells). Compared with control siRNA-transfected cells (59 ± 3 nm), there was essentially no significant difference. Thus, our results strongly indicate that the GRASPs and Golgin45 (and, to a lesser extent, GM130) are likely to play a functionally redundant and complementary role for Golgi stacking under physiological conditions. We measured the anterograde cargo transport in these cells (Fig. S6D) and found that the amount of CD8 transported to the plasma membrane in these cells is slightly lower compared with GRASP65/55-depleted cells.
Golgin45-Dependent Restoration of Golgi Cisternal Flatness and Morphology Is Not Cell Type-Specific.
To see whether these observations are restricted to HeLa cells, we tested human fibrosarcoma HT1080 cells depleted of either GRASP65/55 or GM130/Golgin45 for 96 h and processed these knockdown cells for EM. We found that the morphological changes in HT1080 cells depleted of GRASPs or Golgins were essentially identical (Fig. S7A). No significant unstacking of the Golgi was observed in either knockdown cell. Exogenous expression of Golgin45 in GRASP65/55-depleted HT-1080 for 18 h resulted in similar restoration of Golgi cisternal flatness as in HeLa cells (Fig. S7 A–C). Because HT-1080 cells are known to secrete matrix metalloproteinase MMP-2 abundantly, the knockdown cells were tested for the secretion of endogenous MMP-2. Strikingly, we found that both GRASP and Golgin double-depleted HT1080 cells secreted significantly increased amounts of endogenous MMP-2 (∼1.5-fold increase; Fig. S7 E and F) during the 4-h secretion assays compared with the control transfected cells. These results suggest that flattening of Golgi cisternae might have a role in determining the rate of anterograde cargo transport through the Golgi.
Can an Ectopic Adhesion Process Introduce Another Organelle into the Golgi Stack?
Our results so far have suggested that cisternal adhesion, irrespective of its molecular source, is likely to be the overriding principle of Golgi stack assembly. If so, then adding adhesive bonds between Golgi cisternae and another organelle (mitochondria) should lead to the formation of hybrid stacks within a few hours, in which mitochondria can be incorporated into preexisting interphase Golgi stacks as if they were Golgi cisternae, provided the ectopic binding energy exceeds that normally stabilizing the Golgi stack.
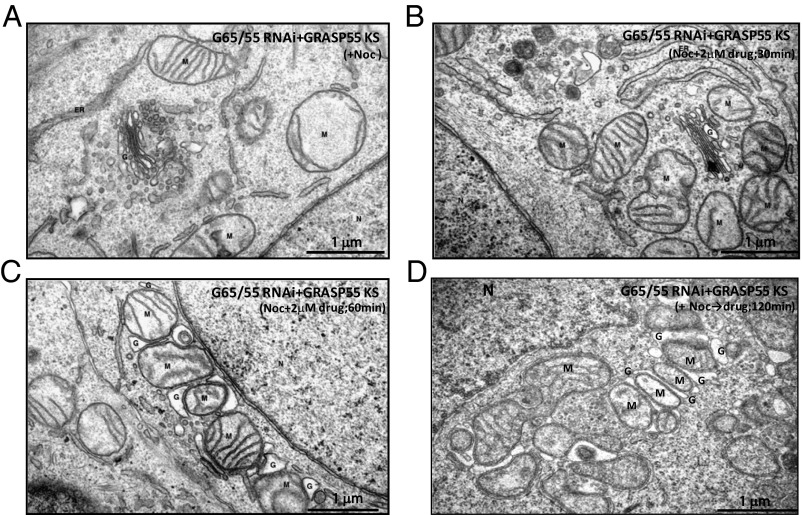
To engineer ectopic adhesion between Golgi cisternae and mitochondria that can be controlled pharmacologically, we used a “knock-sideways” approach (13, 25) involving the ligand-induced formation of heterodimers between the FK506-binding protein (FKBP) and a rapamycin-binding domain (FRB). These two proteins bind to each other when a rapamycin analog (AP21967, which simultaneously binds to the FRB and FKBP domains) is added to cells. The Golgi adhesion protein GRASP55 was expressed as a hybrid protein with FKBP (and a fluorescent protein [tag blue fluorescent protein (tagBFP)]) to enable visualization (Fig. S8A). FRB was expressed as a hybrid with a protein targeted to the mitochondrial outer membrane (OMP25) also tagged with the myc epitope. In this way, the expressed GRASP55 hybrid protein should relocalize from the Golgi (and cytosol if present in excess) to the mitochondria when the ligand AP21967 is added to the cells (Fig. S8B). More specifically, endogenous GRASPs were replaced by the GRASP55 hybrid protein by using GRASP double-depleted cells that also express an RNAi-resistant form of the GRASP55-FKBP-tagBFP hybrid. To simplify the analysis and focus on the effects of ectopic adhesion on the stack (compared with the ribbon), these cells were treated with nocodazole to produce ministacks before initiating knock-sideways with AP21967.
Electron microscopy was then used to follow the fate of preexisting Golgi stacks and mitochondria (Fig. 4 A–D). The result was a dramatic reorganization of the Golgi stacks within 30–60 min after addition of ligand (2 μM), in which mitochondria cluster around ministacks at earlier times (Fig. 4B) and then invade them at later times, often forming hybrid stacks (Fig. 4 C and D). In some cases, Golgi cisternae appeared to wrap around mitochondria similar to a hot dog bun wrapping around a sausage. The diversity of the resulting hybrid organelles makes it impossible to quantify the results. However, it is nonetheless clear that these results support the idea that established Golgi stacks can be dissected by ectopic adhesion to mitochondria within a few hours. This implies that the stacks had been stabilized by reversible adhesion that could then be overcome at equilibrium by an engineered alternative.
Fig. 4.
Golgi–mitochondria hybrid stack formation driven by engineered ectopic adhesive interaction Representative electron micrographs from knock-sideways experiments. HeLa cells treated with siRNAs against GRASP65/55 for 72 h were rescued with GRASP55-FKBP-tagBFP for 24 h, followed by 3 h of treatment with Nocodazole (A); GRASP55-FKBP-tagBFP-rescued cells treated with AP21967 for indicated time to rapidly target the exogenous GRASP55 fusion protein onto mitochondria, which leads to Golgi-mitochondria hybrid stacks (B–D). G, Golgi; n, nucleus; M, mitochondria.
Discussion
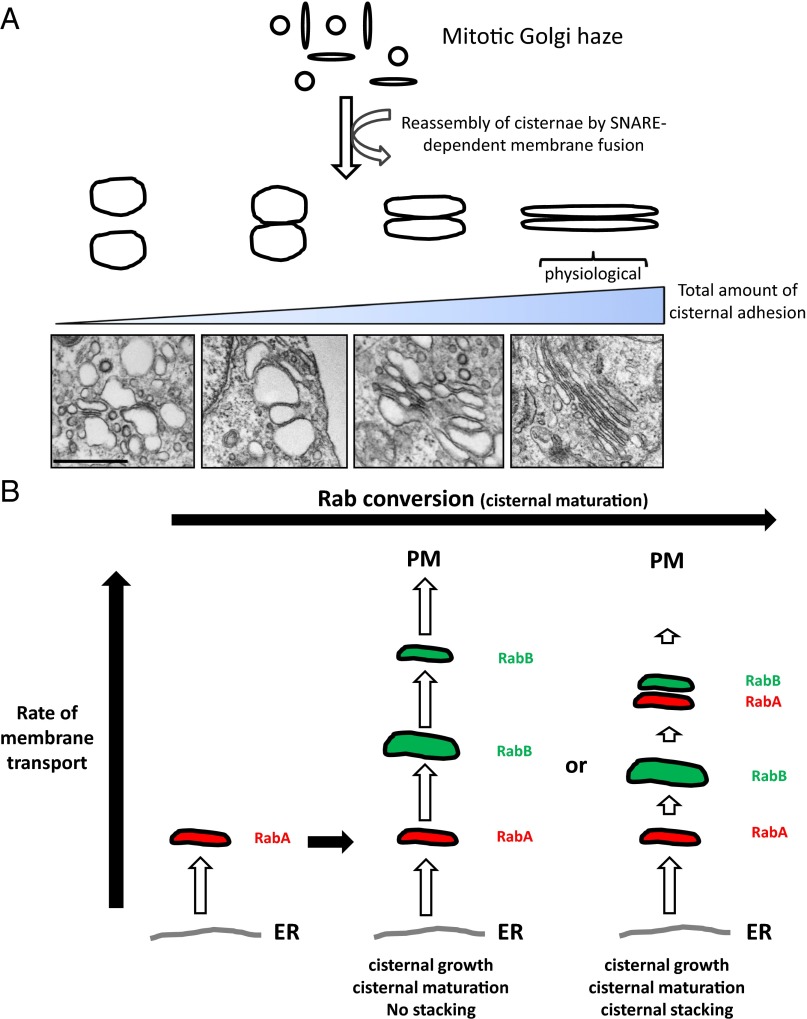
In this study, we report that cisternal adhesion is likely to be a key governing principle that dictates the two characteristic features of the Golgi apparatus (flattened cisternae and cisternal stacking) as illustrated in Fig. 5A along with EM photos, showing examples of Golgi stacks at different stages of cisternal adhesion, and although GRASPs seem to play an important role in Golgi stacking under physiological conditions, they are not absolutely essential even in mammalian cells in the sense that they could be replaced with Golgins, as shown in our experiments. This implies that Golgi stacking may be possible even in organisms lacking GRASP-type adhesive proteins.
Fig. 5.
Illustrations describing the concept of the adhesion model and the simplest form of cisternal stacking that could occur via cisternal adhesion and Rab conversion as two fundamental principles of stacking mechanism. (A) Illustration explaining diverse morphologies of Golgi stacks based on the adhesion model: Golgi cisternae are analogous to a water-filled balloon that is densely covered with Velcro-like molecules (tethering proteins, GRASPs, or other adhesive proteins). At the low adhesive condition, two of these water-filled balloons (cisternae) would be barely adhered together. As the adhesive energy is progressively increased broadly across the surface of cisternae, the cisternae gets flattened as the cisternal adhesive strength overcomes the osmotic pressure within the cisternae. Examples of Golgi EM photos for progressively adhesive conditions are shown here. (Scale bar, 500 nm.) (B) Illustration depicting the simplest form of cisternal stacking, based on the adhesion model and Rab conversion. According to this model, cisternal stacking occurs because of significantly faster ER-to-Golgi anterograde cargo transport compared with cargo export from the trans-Golgi to the plasma membrane. Only weak tethering or adhesive processes may suffice if the rate of the ER-Golgi transport far exceeds that of Golgi–plasma membrane transport. Cisternal maturation initiates the stacking of newly forming cis-cisternae to now more distal cisternae.
In the case of yeast (S. pombe) and plants, in which none of the homologs for these four proteins have been either identified or found to be necessary for Golgi stacking, similar adhesive proteins or new adhesive interactions of existing Golgi proteins could potentially mediate cisternal adhesion. It is possible that even weak tethering processes between neighboring cisterna could promote cisternal stacking theoretically (i.e., multiple tether–Rab binding, tether–SNARE binding, etc). In Fig. 5B, we propose a model illustrating how the most primitive form of cisternal stacking might have occurred by this kind of weak tethering (adhesion) processes and Rab conversion, a compartmental maturation process, in which one Rab-GTP recruits the Rab–GTP exchange factor for the subsequent Rab protein and is known to be well-conserved through the evolution from yeast to mammals (26–29). This model requires that the cargo export from the trans-Golgi be slower than cargo import into the cis-Golgi. In this case, the slower the older (distal) cisternae dissipates from the trans side of the Golgi via cargo export to the plasma membrane, the better the chance of cisternal stacking because newly formed cis-cisternae would accumulate at the cis face of now more distal cisternae. Naturally, the number of cisternae per stack would be determined by dynamic ratio of cargo influx/outflux through the Golgi in a given cell type.
One of the reasons that newly formed nascent cis-cisternae may stack with older cis-cisternae (rather than fuse with older cis-cisternae) could be cisternal maturation (i.e., Rab conversion) of older cis-cisternae. Thus, newly forming cis-cisternae continues to grow in volume as a result of rapid membrane influx from the endoplasmic reticulum (ER), until Rab conversion (i.e., RabA → RabB) is fully completed, at which point the matured cisternae begins to stack with more nascent cis-cisternae. In this way, the Golgi stack could be built from the trans side of the Golgi toward the cis side in terms of a purely mechanistic point of view.
If one assumes that Golgi stacking could occur as described here, why do these Golgins and GRASPs play crucial roles in mammalian and fly Golgi stacking under physiological conditions? The answer to this question may be found from the fact that yeast and plant Golgi stacks do not undergo mitotic disassembly/reassembly, whereas mammalian and fly Golgi stacks do. It is possible that the necessity for rapid mitotic disassembly/reassembly of Golgi stacks might have brought about the evolutionary adaptation of this particular group of adhesive proteins for Golgi stacking in mammals and fly. Thus, yeast and plants may be using the more ancient strategy (i.e., multiple weak adhesive processes) for building stable Golgi stacks, whereas mammals and flies have adapted a small group of proteins for Golgi stacks that can be dynamically disassembled and reassembled in sync with mitotic cell division.
Materials and Methods
Reagents and Antibodies.
All common reagents were purchased from Sigma-Aldrich unless otherwise mentioned. The following antibodies were used: mouse monoclonal GM130 (BD Transduction Laboratories), goat polyclonal GRASP65 (Santa Cruz Biotechnology), rabbit polyclonal GRASP55 (Proteintech Inc.), rabbit polyclonal p115 (Santa Cruz Biotechnology), HRP-conjugated mouse β-actin antibody (GenScript). Rabbit polyclonal Golgin97 (AbCAM). Rabbit polyclonal Golgin45 antibody was made by injecting synthetic Golgin45 peptide (AA40-53) conjugated to KLH (GenScript). pcDNA-hGRASP55-GFP was kindly provided by Adam Linstedt (Carnegie Mellon University). Rat GM130 cDNA was obtained from Nobuhiro Nakamura (Kyoto Sangyo University). Human Golgin45 cDNA was purchased from Addgene. Human GRASP65 cDNA was from Yanzhuang Wang (University of Michigan, Ann Arbor, MI). Human Golgin97 cDNA was obtained from Sean Munro (MRC Laboratories, Cambridge, United Kingdom).
See SI Materials and Methods for further information.
Supplementary Material
Acknowledgments
We thank Adam Linstedt, Gregory Lavieu, Nobuhiro Nakamura, Yanzhuang Wang, and Sean Munro for kindly sharing reagents and Stuart Kornfeld for critical reading of the manuscript. We are especially grateful to Vivek Malhotra for insightful inputs and discussion during the preparation of this manuscript.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1323895111/-/DCSupplemental.
References
- 1.Nakamura N, Wei JH, Seemann J. Modular organization of the mammalian Golgi apparatus. Curr Opin Cell Biol. 2012;24(4):467–474. doi: 10.1016/j.ceb.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Warren G, Malhotra V. The organisation of the Golgi apparatus. Curr Opin Cell Biol. 1998;10(4):493–498. doi: 10.1016/s0955-0674(98)80064-1. [DOI] [PubMed] [Google Scholar]
- 3.Kornfeld R, Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- 4.Wang Y, Seemann J. Golgi biogenesis. Cold Spring Harb Perspect Biol. 2011;3(10):a005330. doi: 10.1101/cshperspect.a005330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rabouille C, Misteli T, Watson R, Warren G. Reassembly of Golgi stacks from mitotic Golgi fragments in a cell-free system. J Cell Biol. 1995;129(3):605–618. doi: 10.1083/jcb.129.3.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rabouille C, Levine TP, Peters JM, Warren G. An NSF-like ATPase, p97, and NSF mediate cisternal regrowth from mitotic Golgi fragments. Cell. 1995;82(6):905–914. doi: 10.1016/0092-8674(95)90270-8. [DOI] [PubMed] [Google Scholar]
- 7.Barr FA, Puype M, Vandekerckhove J, Warren G. GRASP65, a protein involved in the stacking of Golgi cisternae. Cell. 1997;91(2):253–262. doi: 10.1016/s0092-8674(00)80407-9. [DOI] [PubMed] [Google Scholar]
- 8.Shorter J, Warren G. A role for the vesicle tethering protein, p115, in the post-mitotic stacking of reassembling Golgi cisternae in a cell-free system. J Cell Biol. 1999;146(1):57–70. doi: 10.1083/jcb.146.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shorter J, et al. GRASP55, a second mammalian GRASP protein involved in the stacking of Golgi cisternae in a cell-free system. EMBO J. 1999;18(18):4949–4960. doi: 10.1093/emboj/18.18.4949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Short B, et al. A GRASP55-rab2 effector complex linking Golgi structure to membrane traffic. J Cell Biol. 2001;155(6):877–883. doi: 10.1083/jcb.200108079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barr FA, Nakamura N, Warren G. Mapping the interaction between GRASP65 and GM130, components of a protein complex involved in the stacking of Golgi cisternae. EMBO J. 1998;17(12):3258–3268. doi: 10.1093/emboj/17.12.3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lowe M, et al. Cdc2 kinase directly phosphorylates the cis-Golgi matrix protein GM130 and is required for Golgi fragmentation in mitosis. Cell. 1998;94(6):783–793. doi: 10.1016/s0092-8674(00)81737-7. [DOI] [PubMed] [Google Scholar]
- 13.Sengupta D, Truschel S, Bachert C, Linstedt AD. Organelle tethering by a homotypic PDZ interaction underlies formation of the Golgi membrane network. J Cell Biol. 2009;186(1):41–55. doi: 10.1083/jcb.200902110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bachert C, Linstedt AD. Dual anchoring of the GRASP membrane tether promotes trans pairing. J Biol Chem. 2010;285(21):16294–16301. doi: 10.1074/jbc.M110.116129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xiang Y, Wang Y. GRASP55 and GRASP65 play complementary and essential roles in Golgi cisternal stacking. J Cell Biol. 2010;188(2):237–251. doi: 10.1083/jcb.200907132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feinstein TN, Linstedt AD. GRASP55 regulates Golgi ribbon formation. Mol Biol Cell. 2008;19(7):2696–2707. doi: 10.1091/mbc.E07-11-1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duran JM, et al. The role of GRASP55 in Golgi fragmentation and entry of cells into mitosis. Mol Biol Cell. 2008;19(6):2579–2587. doi: 10.1091/mbc.E07-10-0998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marra P, et al. The biogenesis of the Golgi ribbon: The roles of membrane input from the ER and of GM130. Mol Biol Cell. 2007;18(5):1595–1608. doi: 10.1091/mbc.E06-10-0886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Puthenveedu MA, Bachert C, Puri S, Lanni F, Linstedt AD. GM130 and GRASP65-dependent lateral cisternal fusion allows uniform Golgi-enzyme distribution. Nat Cell Biol. 2006;8(3):238–248. doi: 10.1038/ncb1366. [DOI] [PubMed] [Google Scholar]
- 20.Ramirez IB, Lowe M. Golgins and GRASPs: Holding the Golgi together. Semin Cell Dev Biol. 2009;20(7):770–779. doi: 10.1016/j.semcdb.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 21.Kondylis V, Spoorendonk KM, Rabouille C. dGRASP localization and function in the early exocytic pathway in Drosophila S2 cells. Mol Biol Cell. 2005;16(9):4061–4072. doi: 10.1091/mbc.E04-10-0938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levi SK, Bhattacharyya D, Strack RL, Austin JR, 2nd, Glick BS. The yeast GRASP Grh1 colocalizes with COPII and is dispensable for organizing the secretory pathway. Traffic. 2010;11(9):1168–1179. doi: 10.1111/j.1600-0854.2010.01089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hawes C, Schoberer J, Hummel E, Osterrieder A. Biogenesis of the plant Golgi apparatus. Biochem Soc Trans. 2010;38(3):761–767. doi: 10.1042/BST0380761. [DOI] [PubMed] [Google Scholar]
- 24.Volchuk A, et al. Megavesicles implicated in the rapid transport of intracisternal aggregates across the Golgi stack. Cell. 2000;102(3):335–348. doi: 10.1016/s0092-8674(00)00039-8. [DOI] [PubMed] [Google Scholar]
- 25.Robinson MS, Sahlender DA, Foster SD. Rapid inactivation of proteins by rapamycin-induced rerouting to mitochondria. Dev Cell. 2010;18(2):324–331. doi: 10.1016/j.devcel.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matsuura-Tokita K, Takeuchi M, Ichihara A, Mikuriya K, Nakano A. Live imaging of yeast Golgi cisternal maturation. Nature. 2006;441(7096):1007–1010. doi: 10.1038/nature04737. [DOI] [PubMed] [Google Scholar]
- 27.Losev E, et al. Golgi maturation visualized in living yeast. Nature. 2006;441(7096):1002–1006. doi: 10.1038/nature04717. [DOI] [PubMed] [Google Scholar]
- 28.Rink J, Ghigo E, Kalaidzidis Y, Zerial M. Rab conversion as a mechanism of progression from early to late endosomes. Cell. 2005;122(5):735–749. doi: 10.1016/j.cell.2005.06.043. [DOI] [PubMed] [Google Scholar]
- 29.Rivera-Molina FE, Novick PJ. A Rab GAP cascade defines the boundary between two Rab GTPases on the secretory pathway. Proc Natl Acad Sci USA. 2009;106(34):14408–14413. doi: 10.1073/pnas.0906536106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.