Abstract
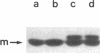
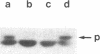
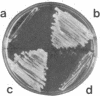
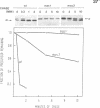
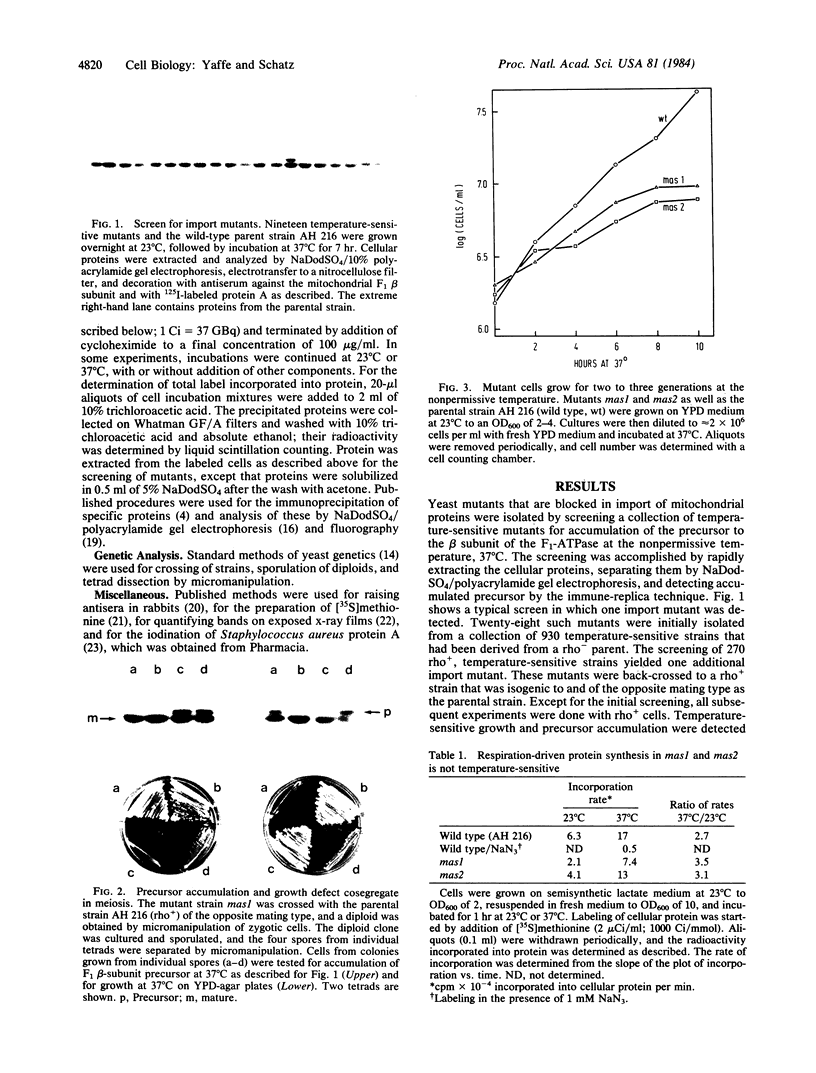
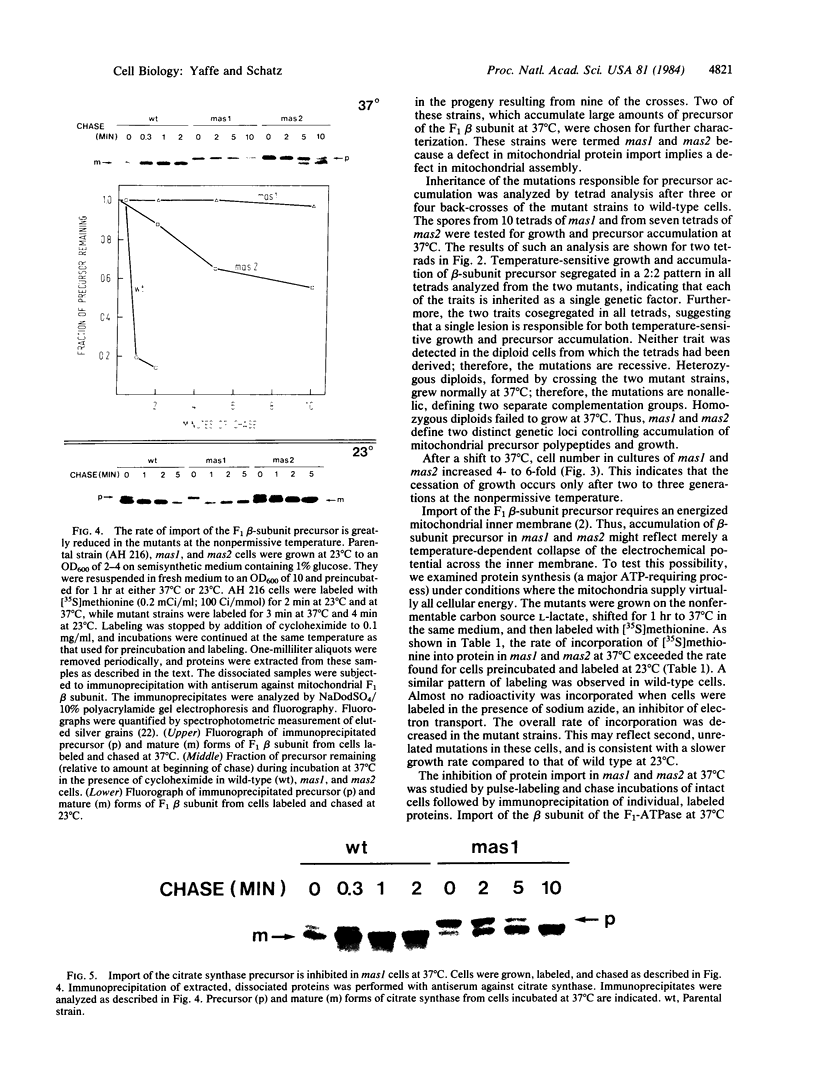
We isolated two yeast mutants that are temperature-sensitive for import of mitochondrial proteins. Each strain contains a single mutation that results in arrest of growth and accumulation of precursor to the beta subunit of the mitochondrial F1-ATPase after incubation at 37 degrees C. These lesions (mas1 and mas2) are nonallelic and recessive. Cells harboring either mutation stop growing only after 2-3 generations at 37 degrees C. Import of the F1 beta subunit at 37 degrees C is more than 250 times slower in mas1 and 15 times slower in mas2 than in wild-type cells. At 23 degrees C, import occurs with similar rates in mutant and wild-type cells. The two mutations also reduce the rate of import of other proteins; however, import of different precursors is affected to different degrees in the two strains. The temperature-sensitive step in import in both mas1 and mas2 occurs before arrival of precursors in the mitochondrial matrix.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Argan C., Lusty C. J., Shore G. C. Membrane and cytosolic components affecting transport of the precursor for ornithine carbamyltransferase into mitochondria. J Biol Chem. 1983 Jun 10;258(11):6667–6670. [PubMed] [Google Scholar]
- Chamberlain J. P. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal Biochem. 1979 Sep 15;98(1):132–135. doi: 10.1016/0003-2697(79)90716-4. [DOI] [PubMed] [Google Scholar]
- Crawford L. V., Gesteland R. F. Synthesis of polyoma proteins in vitro. J Mol Biol. 1973 Mar 15;74(4):627–634. doi: 10.1016/0022-2836(73)90053-3. [DOI] [PubMed] [Google Scholar]
- Daum G., Böhni P. C., Schatz G. Import of proteins into mitochondria. Cytochrome b2 and cytochrome c peroxidase are located in the intermembrane space of yeast mitochondria. J Biol Chem. 1982 Nov 10;257(21):13028–13033. [PubMed] [Google Scholar]
- Douglas M. G., Butow R. A. Variant forms of mitochondrial translation products in yeast: evidence for location of determinants on mitochondrial DNA. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1083–1086. doi: 10.1073/pnas.73.4.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasser S. M., Daum G., Schatz G. Import of proteins into mitochondria. Energy-dependent uptake of precursors by isolated mitochondria. J Biol Chem. 1982 Nov 10;257(21):13034–13041. [PubMed] [Google Scholar]
- Gasser S. M., Ohashi A., Daum G., Böhni P. C., Gibson J., Reid G. A., Yonetani T., Schatz G. Imported mitochondrial proteins cytochrome b2 and cytochrome c1 are processed in two steps. Proc Natl Acad Sci U S A. 1982 Jan;79(2):267–271. doi: 10.1073/pnas.79.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gbelská Y., Subík J., Svoboda A., Goffeau A., Kovác L. Intramitochondrial ATP and cell functions: yeast cells depleted of intramitochondrial ATP lose the ability to grow and multiply. Eur J Biochem. 1983 Feb 1;130(2):281–286. doi: 10.1111/j.1432-1033.1983.tb07148.x. [DOI] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Hartwell L. H., Culotti J., Pringle J. R., Reid B. J. Genetic control of the cell division cycle in yeast. Science. 1974 Jan 11;183(4120):46–51. doi: 10.1126/science.183.4120.46. [DOI] [PubMed] [Google Scholar]
- Hennig B., Neupert W. Assembly of cytochrome c. Apocytochrome c is bound to specific sites on mitochondria before its conversion to holocytochrome c. Eur J Biochem. 1981 Dec;121(1):203–212. doi: 10.1111/j.1432-1033.1981.tb06450.x. [DOI] [PubMed] [Google Scholar]
- Ito H., Fukuda Y., Murata K., Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983 Jan;153(1):163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen R., Sprague G. F., Jr, Herskowitz I. Regulation of yeast mating-type interconversion: feedback control of HO gene expression by the mating-type locus. Proc Natl Acad Sci U S A. 1983 May;80(10):3035–3039. doi: 10.1073/pnas.80.10.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccecchini M. L., Rudin Y., Blobel G., Schatz G. Import of proteins into mitochondria: precursor forms of the extramitochondrially made F1-ATPase subunits in yeast. Proc Natl Acad Sci U S A. 1979 Jan;76(1):343–347. doi: 10.1073/pnas.76.1.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura S., Mori M., Tatibana M. Transport of ornithine carbamoyltransferase precursor into mitochondria. Stimulation by potassium ion, magnesium ion, and a reticulocyte cytosolic protein(s). J Biol Chem. 1983 Jun 10;258(11):6671–6674. [PubMed] [Google Scholar]
- Nelson N., Schatz G. Energy-dependent processing of cytoplasmically made precursors to mitochondrial proteins. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4365–4369. doi: 10.1073/pnas.76.9.4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta S., Schatz G. A purified precursor polypeptide requires a cytosolic protein fraction for import into mitochondria. EMBO J. 1984 Mar;3(3):651–657. doi: 10.1002/j.1460-2075.1984.tb01862.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poyton R. O., Schatz G. Cytochrome c oxidase from bakers' yeast. IV. Immunological evidence for the participation of a mitochondrially synthesized subunit in enzymatic activity. J Biol Chem. 1975 Jan 25;250(2):762–766. [PubMed] [Google Scholar]
- Reid G. A., Schatz G. Import of proteins into mitochondria. Yeast cells grown in the presence of carbonyl cyanide m-chlorophenylhydrazone accumulate massive amounts of some mitochondrial precursor polypeptides. J Biol Chem. 1982 Nov 10;257(21):13056–13061. [PubMed] [Google Scholar]
- Riezman H., Hay R., Witte C., Nelson N., Schatz G. Yeast mitochondrial outer membrane specifically binds cytoplasmically-synthesized precursors of mitochondrial proteins. EMBO J. 1983;2(7):1113–1118. doi: 10.1002/j.1460-2075.1983.tb01554.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatz G., Butow R. A. How are proteins imported into mitochondria? Cell. 1983 Feb;32(2):316–318. doi: 10.1016/0092-8674(83)90450-6. [DOI] [PubMed] [Google Scholar]
- Schleyer M., Schmidt B., Neupert W. Requirement of a membrane potential for the posttranslational transfer of proteins into mitochondria. Eur J Biochem. 1982 Jun 15;125(1):109–116. doi: 10.1111/j.1432-1033.1982.tb06657.x. [DOI] [PubMed] [Google Scholar]
- Suissa M. Spectrophotometric quantitation of silver grains eluted from autoradiograms. Anal Biochem. 1983 Sep;133(2):511–514. doi: 10.1016/0003-2697(83)90117-3. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]