Abstract
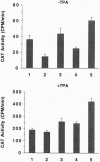
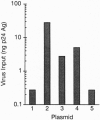
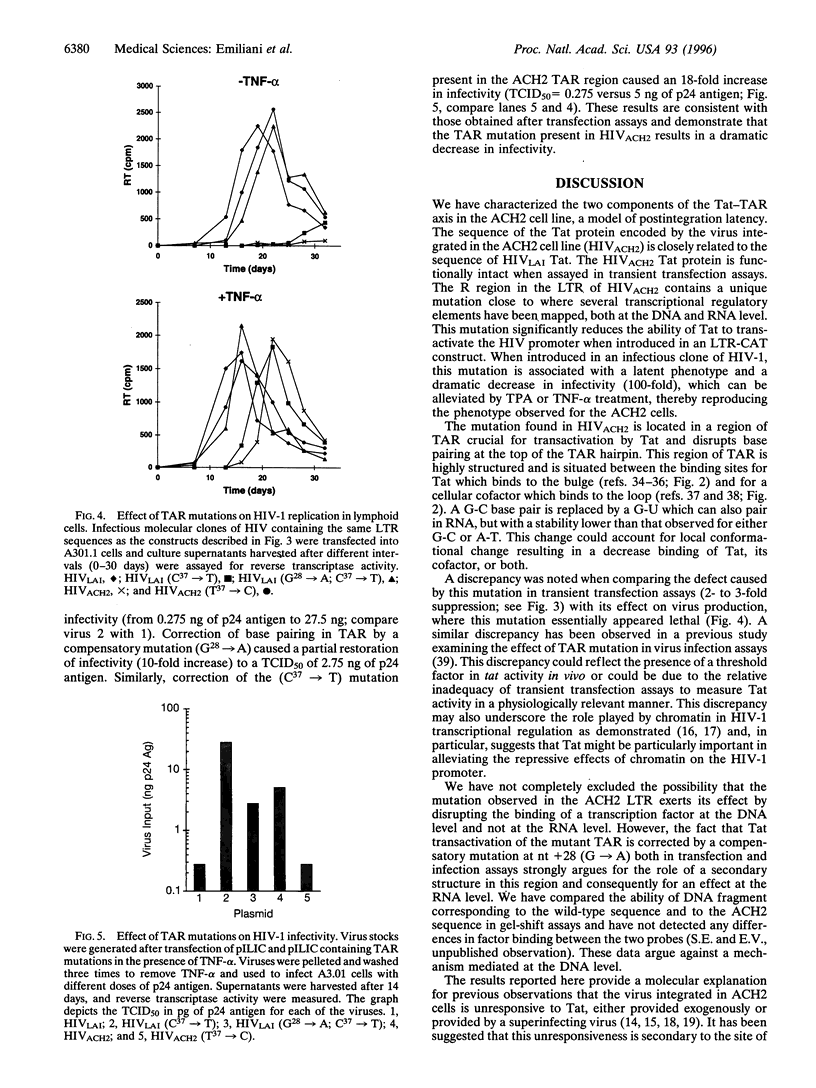
Study of the mechanism of HIV-1 postintegration latency in the ACH2 cell line demonstrates that these cells failed to increase HIV-1 production following treatment with exogenous Tat. Reasoning that the defect in ACH2 cells involves the Tat response, we analyzed the sequence of tat cDNA and Tat responsive element (TAR) from the virus integrated in ACH2. Tat cDNA sequence is closely related to that of HIV LAI, and the encoded protein is fully functional in terms of long terminal repeat (LTR) transactivation. Cloning of a region corresponding to the 5'-LTR from ACH2, however, identified a point mutation (C37 -> T) in TAR. This mutation impaired Tat responsiveness of the LTR in transient transfection assays, and the measured defect was complemented in cells that had been treated with tetradecanoyl phorbol acetate or tumor necrosis factor type alpha (TNF-alpha). A compensatory mutation in TAR (G28 -> A), designed to reestablish base pairing in the TAR hairpin, restored wild-type Tat responsiveness. When the (C37 -> T) mutation was introduced in an infectious clone of HIV-1, no viral production was measured in the absence of TNF-alpha, whereas full complementation was observed when the infection was conducted in the presence of TNF-alpha or when a compensatory mutation (G28 -> A) was introduced into TAR. These experiments identify a novel mutation associated with HIV-1 latency and suggest that alterations in the Tat-TAR axis can be a crucial determinant of the latent phenotype in infected individuals.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adachi A., Gendelman H. E., Koenig S., Folks T., Willey R., Rabson A., Martin M. A. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J Virol. 1986 Aug;59(2):284–291. doi: 10.1128/jvi.59.2.284-291.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams M., Sharmeen L., Kimpton J., Romeo J. M., Garcia J. V., Peterlin B. M., Groudine M., Emerman M. Cellular latency in human immunodeficiency virus-infected individuals with high CD4 levels can be detected by the presence of promoter-proximal transcripts. Proc Natl Acad Sci U S A. 1994 Apr 26;91(9):3862–3866. doi: 10.1073/pnas.91.9.3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arya S. K., Guo C., Josephs S. F., Wong-Staal F. Trans-activator gene of human T-lymphotropic virus type III (HTLV-III). Science. 1985 Jul 5;229(4708):69–73. doi: 10.1126/science.2990040. [DOI] [PubMed] [Google Scholar]
- Butera S. T., Roberts B. D., Lam L., Hodge T., Folks T. M. Human immunodeficiency virus type 1 RNA expression by four chronically infected cell lines indicates multiple mechanisms of latency. J Virol. 1994 Apr;68(4):2726–2730. doi: 10.1128/jvi.68.4.2726-2730.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calnan B. J., Biancalana S., Hudson D., Frankel A. D. Analysis of arginine-rich peptides from the HIV Tat protein reveals unusual features of RNA-protein recognition. Genes Dev. 1991 Feb;5(2):201–210. doi: 10.1101/gad.5.2.201. [DOI] [PubMed] [Google Scholar]
- Cannon P., Kim S. H., Ulich C., Kim S. Analysis of Tat function in human immunodeficiency virus type 1-infected low-level-expression cell lines U1 and ACH-2. J Virol. 1994 Mar;68(3):1993–1997. doi: 10.1128/jvi.68.3.1993-1997.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang K. Y., Tinoco I., Jr Characterization of a "kissing" hairpin complex derived from the human immunodeficiency virus genome. Proc Natl Acad Sci U S A. 1994 Aug 30;91(18):8705–8709. doi: 10.1073/pnas.91.18.8705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B. K., Saksela K., Andino R., Baltimore D. Distinct modes of human immunodeficiency virus type 1 proviral latency revealed by superinfection of nonproductively infected cell lines with recombinant luciferase-encoding viruses. J Virol. 1994 Feb;68(2):654–660. doi: 10.1128/jvi.68.2.654-660.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clouse K. A., Powell D., Washington I., Poli G., Strebel K., Farrar W., Barstad P., Kovacs J., Fauci A. S., Folks T. M. Monokine regulation of human immunodeficiency virus-1 expression in a chronically infected human T cell clone. J Immunol. 1989 Jan 15;142(2):431–438. [PubMed] [Google Scholar]
- Delassus S., Meyerhans A., Cheynier R., Wain-Hobson S. Absence of selection of HIV-1 variants in vivo based on transcription/transactivation during progression to AIDS. Virology. 1992 Jun;188(2):811–818. doi: 10.1016/0042-6822(92)90536-x. [DOI] [PubMed] [Google Scholar]
- Dingwall C., Ernberg I., Gait M. J., Green S. M., Heaphy S., Karn J., Lowe A. D., Singh M., Skinner M. A. HIV-1 tat protein stimulates transcription by binding to a U-rich bulge in the stem of the TAR RNA structure. EMBO J. 1990 Dec;9(12):4145–4153. doi: 10.1002/j.1460-2075.1990.tb07637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan L., Oakes J. W., Ferraro A., Bagasra O., Pomerantz R. J. Tat and rev differentially affect restricted replication of human immunodeficiency virus type 1 in various cells. Virology. 1994 Mar;199(2):474–478. doi: 10.1006/viro.1994.1148. [DOI] [PubMed] [Google Scholar]
- Embretson J., Zupancic M., Ribas J. L., Burke A., Racz P., Tenner-Racz K., Haase A. T. Massive covert infection of helper T lymphocytes and macrophages by HIV during the incubation period of AIDS. Nature. 1993 Mar 25;362(6418):359–362. doi: 10.1038/362359a0. [DOI] [PubMed] [Google Scholar]
- Folks T. M., Clouse K. A., Justement J., Rabson A., Duh E., Kehrl J. H., Fauci A. S. Tumor necrosis factor alpha induces expression of human immunodeficiency virus in a chronically infected T-cell clone. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2365–2368. doi: 10.1073/pnas.86.7.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel A. D., Pabo C. O. Cellular uptake of the tat protein from human immunodeficiency virus. Cell. 1988 Dec 23;55(6):1189–1193. doi: 10.1016/0092-8674(88)90263-2. [DOI] [PubMed] [Google Scholar]
- Green M., Ishino M., Loewenstein P. M. Mutational analysis of HIV-1 Tat minimal domain peptides: identification of trans-dominant mutants that suppress HIV-LTR-driven gene expression. Cell. 1989 Jul 14;58(1):215–223. doi: 10.1016/0092-8674(89)90417-0. [DOI] [PubMed] [Google Scholar]
- Harper M. E., Marselle L. M., Gallo R. C., Wong-Staal F. Detection of lymphocytes expressing human T-lymphotropic virus type III in lymph nodes and peripheral blood from infected individuals by in situ hybridization. Proc Natl Acad Sci U S A. 1986 Feb;83(3):772–776. doi: 10.1073/pnas.83.3.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrich D., Hsu C., Race E., Gaynor R. B. Differential growth kinetics are exhibited by human immunodeficiency virus type 1 TAR mutants. J Virol. 1994 Sep;68(9):5899–5910. doi: 10.1128/jvi.68.9.5899-5910.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashanchi F., Duvall J. F., Brady J. N. Electroporation of viral transactivator proteins into lymphocyte suspension cells. Nucleic Acids Res. 1992 Sep 11;20(17):4673–4674. doi: 10.1093/nar/20.17.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard J., Parrott C., Buckler-White A. J., Turner W., Ross E. K., Martin M. A., Rabson A. B. The NF-kappa B binding sites in the human immunodeficiency virus type 1 long terminal repeat are not required for virus infectivity. J Virol. 1989 Nov;63(11):4919–4924. doi: 10.1128/jvi.63.11.4919-4924.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann D. A., Frankel A. D. Endocytosis and targeting of exogenous HIV-1 Tat protein. EMBO J. 1991 Jul;10(7):1733–1739. doi: 10.1002/j.1460-2075.1991.tb07697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCune J. M. Viral latency in HIV disease. Cell. 1995 Jul 28;82(2):183–188. doi: 10.1016/0092-8674(95)90305-4. [DOI] [PubMed] [Google Scholar]
- McElrath M. J., Pruett J. E., Cohn Z. A. Mononuclear phagocytes of blood and bone marrow: comparative roles as viral reservoirs in human immunodeficiency virus type 1 infections. Proc Natl Acad Sci U S A. 1989 Jan;86(2):675–679. doi: 10.1073/pnas.86.2.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muesing M. A., Smith D. H., Capon D. J. Regulation of mRNA accumulation by a human immunodeficiency virus trans-activator protein. Cell. 1987 Feb 27;48(4):691–701. doi: 10.1016/0092-8674(87)90247-9. [DOI] [PubMed] [Google Scholar]
- Pantaleo G., Graziosi C., Demarest J. F., Butini L., Montroni M., Fox C. H., Orenstein J. M., Kotler D. P., Fauci A. S. HIV infection is active and progressive in lymphoid tissue during the clinically latent stage of disease. Nature. 1993 Mar 25;362(6418):355–358. doi: 10.1038/362355a0. [DOI] [PubMed] [Google Scholar]
- Patterson B. K., Till M., Otto P., Goolsby C., Furtado M. R., McBride L. J., Wolinsky S. M. Detection of HIV-1 DNA and messenger RNA in individual cells by PCR-driven in situ hybridization and flow cytometry. Science. 1993 May 14;260(5110):976–979. doi: 10.1126/science.8493534. [DOI] [PubMed] [Google Scholar]
- Pearson L., Garcia J., Wu F., Modesti N., Nelson J., Gaynor R. A transdominant tat mutant that inhibits tat-induced gene expression from the human immunodeficiency virus long terminal repeat. Proc Natl Acad Sci U S A. 1990 Jul;87(13):5079–5083. doi: 10.1073/pnas.87.13.5079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poli G., Kinter A., Justement J. S., Kehrl J. H., Bressler P., Stanley S., Fauci A. S. Tumor necrosis factor alpha functions in an autocrine manner in the induction of human immunodeficiency virus expression. Proc Natl Acad Sci U S A. 1990 Jan;87(2):782–785. doi: 10.1073/pnas.87.2.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomerantz R. J., Bagasra O., Baltimore D. Cellular latency of human immunodeficiency virus type 1. Curr Opin Immunol. 1992 Aug;4(4):475–480. doi: 10.1016/s0952-7915(06)80042-7. [DOI] [PubMed] [Google Scholar]
- Pomerantz R. J., Trono D., Feinberg M. B., Baltimore D. Cells nonproductively infected with HIV-1 exhibit an aberrant pattern of viral RNA expression: a molecular model for latency. Cell. 1990 Jun 29;61(7):1271–1276. doi: 10.1016/0092-8674(90)90691-7. [DOI] [PubMed] [Google Scholar]
- Psallidopoulos M. C., Schnittman S. M., Thompson L. M., 3rd, Baseler M., Fauci A. S., Lane H. C., Salzman N. P. Integrated proviral human immunodeficiency virus type 1 is present in CD4+ peripheral blood lymphocytes in healthy seropositive individuals. J Virol. 1989 Nov;63(11):4626–4631. doi: 10.1128/jvi.63.11.4626-4631.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross E. K., Buckler-White A. J., Rabson A. B., Englund G., Martin M. A. Contribution of NF-kappa B and Sp1 binding motifs to the replicative capacity of human immunodeficiency virus type 1: distinct patterns of viral growth are determined by T-cell types. J Virol. 1991 Aug;65(8):4350–4358. doi: 10.1128/jvi.65.8.4350-4358.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S., Delling U., Chen C. H., Rosen C. A., Sonenberg N. A bulge structure in HIV-1 TAR RNA is required for Tat binding and Tat-mediated trans-activation. Genes Dev. 1990 Aug;4(8):1365–1373. doi: 10.1101/gad.4.8.1365. [DOI] [PubMed] [Google Scholar]
- Schnittman S. M., Psallidopoulos M. C., Lane H. C., Thompson L., Baseler M., Massari F., Fox C. H., Salzman N. P., Fauci A. S. The reservoir for HIV-1 in human peripheral blood is a T cell that maintains expression of CD4. Science. 1989 Jul 21;245(4915):305–308. doi: 10.1126/science.2665081. [DOI] [PubMed] [Google Scholar]
- Sheline C. T., Milocco L. H., Jones K. A. Two distinct nuclear transcription factors recognize loop and bulge residues of the HIV-1 TAR RNA hairpin. Genes Dev. 1991 Dec;5(12B):2508–2520. doi: 10.1101/gad.5.12b.2508. [DOI] [PubMed] [Google Scholar]
- Van Lint C., Ghysdael J., Paras P., Jr, Burny A., Verdin E. A transcriptional regulatory element is associated with a nuclease-hypersensitive site in the pol gene of human immunodeficiency virus type 1. J Virol. 1994 Apr;68(4):2632–2648. doi: 10.1128/jvi.68.4.2632-2648.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdin E. DNase I-hypersensitive sites are associated with both long terminal repeats and with the intragenic enhancer of integrated human immunodeficiency virus type 1. J Virol. 1991 Dec;65(12):6790–6799. doi: 10.1128/jvi.65.12.6790-6799.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdin E., Paras P., Jr, Van Lint C. Chromatin disruption in the promoter of human immunodeficiency virus type 1 during transcriptional activation. EMBO J. 1993 Aug;12(8):3249–3259. doi: 10.1002/j.1460-2075.1993.tb05994.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winslow B. J., Pomerantz R. J., Bagasra O., Trono D. HIV-1 latency due to the site of proviral integration. Virology. 1993 Oct;196(2):849–854. doi: 10.1006/viro.1993.1545. [DOI] [PubMed] [Google Scholar]
- Wu F., Garcia J., Sigman D., Gaynor R. tat regulates binding of the human immunodeficiency virus trans-activating region RNA loop-binding protein TRP-185. Genes Dev. 1991 Nov;5(11):2128–2140. doi: 10.1101/gad.5.11.2128. [DOI] [PubMed] [Google Scholar]