Abstract
Dysregulation of glutamate handling ensuing downregulation of expression and activity levels of the astroglial glutamate transporter EAAT2 is implicated in excitotoxic degeneration of motor neurons in amyotrophic lateral sclerosis (ALS). We previously reported that EAAT2 (a.k.a. GLT-1) is cleaved by caspase-3 at its cytosolic carboxy-terminus domain. This cleavage results in impaired glutamate transport activity and generates a proteolytic fragment (CTE) that we found to be post-translationally conjugated by SUMO1. We show here that this sumoylated CTE fragment accumulates in the nucleus of spinal cord astrocytes of the SOD1-G93A mouse model of ALS at symptomatic stages of disease. Astrocytic expression of CTE, artificially tagged with SUMO1 (CTE-SUMO1) to mimic the native sumoylated fragment, recapitulates the nuclear accumulation pattern of the endogenous EAAT2-derived proteolytic fragment. Moreover, in a co-culture binary system, expression of CTE-SUMO1 in spinal cord astrocytes initiates extrinsic toxicity by inducing caspase-3 activation in motor neuron-derived NSC-34 cells or axonal growth impairment in primary motor neurons. Interestingly, prolonged nuclear accumulation of CTE-SUMO1 is intrinsically toxic to spinal cord astrocytes, although this gliotoxic effect of CTE-SUMO1 occurs later than the indirect, non-cell autonomous toxic effect on motor neurons. As more evidence on the implication of SUMO substrates in neurodegenerative diseases emerges, our observations strongly suggest that the nuclear accumulation in spinal cord astrocytes of a sumoylated proteolytic fragment of the astroglial glutamate transporter EAAT2 could participate to the pathogenesis of ALS and suggest a novel, unconventional role for EAAT2 in motor neuron degeneration.
Keywords: Amyotrophic lateral sclerosis, post-translational modification, SUMO, excitotoxicity
INTRODUCTION
In the CNS, astrocytes are essential partners of neurons, providing trophic support (Taylor et al. 2007a) and mediating rapid binding and clearance of synaptic glutamate mainly through the glutamate transporter EAAT2 (Tzingounis and Wadiche 2007). In amyotrophic lateral sclerosis (ALS), astrocytes have emerged as key determinants of the progressive degeneration of motor neurons (Yamanaka et al. 2008), either directly by releasing toxic factors (Gandelman et al. 2010; Nagai et al. 2007) and/or contributing indirectly through the loss of physiological function. Excitotoxicity ensuing impairment in EAAT2-mediated synaptic clearance of glutamate is amongst various proposed mechanisms implicated in the propagation of motor neuron death in ALS (Foran and Trotti 2009).
Studies in ALS mice and patients also revealed that activation of caspase-3 within motor neurons and astrocytes contribute to ALS pathogenesis (Pasinelli et al. 2000; Rossi et al. 2008). Linking excitotoxicity and caspase-3 activation as converging mechanisms in ALS, we showed that caspase-3 cleaves EAAT2 within a unique cleavage consensus site (-DTID-) in its cytosolic carboxy-terminus domain, impairing the activity (Boston-Howes et al. 2006). In addition, familial ALS-linked mutant SOD1 proteins (mutSOD1) inhibit EAAT2 via caspase-3 cleavage. Recently, we showed that a proteolytic fragment of apparent molecular mass of ~25 kDa derived from caspase-3 cleavage of the EAAT2 cytoplasmic C-terminus, is SUMO1 conjugated and significantly accumulates in the spinal cord of SOD1-G93A mice as early as disease onset. Interestingly, accumulation of this fragment is ALS disease and spinal cord-specific (Gibb et al. 2007).
Evidence indicate that sumoylation can affect stability of polypeptides and proteins, protein-protein interactions, sub-cellular re-localization and transcriptional regulation (Geiss-Friedlander and Melchior 2007). Using immunocytochemistry, we report here the evidence of nuclear localization of a sumoylated EAAT2 fragment in vivo, in spinal cord astrocytes of transgenic SOD1-G93A mice, and in spinal cord astrocytes cultured from these diseased mice. In addition, we found that astrocytic expression of CTE-SUMO1, an artificial peptide that models the sumoylated fragment of EAAT2, leads to activation of caspase-3 in co-cultured motor neuron-derived NSC-34 cells or axon impairment in co-cultured primary motor neurons. As more evidence on the implication of SUMO substrates in neurodegenerative diseases emerges (Lieberman 2004; Steffan et al. 2004), and considering the active role of astrocytes in the progressive degeneration of motor neurons in ALS, our observations strongly suggest that nuclear accumulation of CTE-SUMO1 in spinal cord astrocytes could play a key role in the non-cell autonomous mechanisms of ALS pathogenesis.
MATERIALS AND METHODS
Trangenic mice
Mutant SOD1-G93A mice model of ALS [B6.Cg-Tg(SOD1-G93A)1Gur/J] were purchase from Jackson Laboratories (stock #004435) and bred in our animal facility.
Antibodies
Affinity purified EAAT2 antibodies ABR518 (polyclonal, epitope 518-536 in mouse EAAT2 sequence) and ABR372 (polyclonal, epitope 372-386 in mouse EAAT2 sequence) were custom-made (Thermo Fisher). The EAAT2 antibody ABR556 (polyclonal, epitope 556-573 in mouse EAAT2 sequence) was purchased from Thermo Fisher. The EAAT1 antibody A522 (polyclonal, epitope 522-541 in the C-terminus of rat EAAT1 sequence) was obtained from Dr. Danbolt. Antibodies against Myc (MS Clontech), GFAP (MS Cell Signaling), p75NTR (MS Chemicon), SUMO1 (RB SantaCruz), ChAT (RB Millipore), PML (MS Chemicon), active caspase-3 (RB Cell Signaling), FUS/TLS (RB Proteintech), netrin-1 (RB Abcam) and nestin (MS Millipore) were also used.
Primary cell cultures
Primary astrocytes were prepared from spinal cord of newborn mice (P2-P4) as previously described (Gibb et al. 2007). Cell monolayers were >95% astrocyte-pure as determined by GFAP immunoreactivity. Mixed glia cultures from spinal cord of adult, diseased SOD1-G93A mice (~150 days old) were prepared according to the protocol and media used for neonatal astrocytes, with the exception of omitting the shaking step needed for astrocyte purification. Adult mixed glia cells were plated on T25 flasks, harvested at confluency and plated on coverslips for confocal microscopy analysis.
Motor neurons were prepared from E12.5 mouse spinal cords (Taylor et al. 2007b). Briefly, 10-12 spinal cords were dissected and incubated in 0.05% trypsin for 15 minutes at 37°C, followed by mechanic dissociation and purification by centrifugation on an Optiprep (Iodioxanol) gradient and BSA cushion. For co-culture experiments, spinal cord astrocytes were transfected with plasmid cDNA encoding Myc-CTE-SUMO1 or Myc-CTE using Nucleofector (Lonza) and then cultured in 4-chamber slides (Lab Tek). At 6 days post-transfection, astrocytes were washed twice with PBS and motor neurons were seeded onto the astrocyte monolayer at a constant density of 500 cells/cm2. Co-cultures were maintained for additional 72 hours in L15 medium supplemented with 0.63 mg/ml NaHCO3, 5 μg/ml insulin, 0.1 mg/ml conalbumin, 0.1 mM putrescine, 30 nM sodium selenite, 20 nM progesterone, 20 mM glucose, 0.1 mg/ml Primocin (invivogen), and 2% horse serum.
Assessment of axonal length
Seventytwo hours after motor neurons were seeded on a bed of treated spinal astrocytes axonal length was assessed by counting p75NTR positive cells displaying neurites shorter than 4 cells in diameter. Counts were performed using the Optical Fractionator Workflow tool of the Steroinvestigator Software (MBF bioscience) and performed by one investigator blind to the experimental groups.
Immunofluorescence in cultured cells and nuclei isolation
Cells grown on glass coverslips were rinsed with PBS, fixed with 4% PFA/PBS and treated with blocking buffer (0.4% BSA, 5% goat-serum and 0.2% Triton X-100 in PBS) for 20 minutes at room temperature. Selected antibodies were incubated overnight at 4°C in blocking buffer. Anti-mouse Alexa 488-conjugated antibody or anti-rabbit Alexa 555-conjugated antibody (1:1,000) was added for 90 min at room temperature.
Nuclei were isolated from adult spinal cords of SOD1-G93A mice. The cords were homogenized at 500 rpm in an isotonic saline buffer (NIM, pH 7.4) containing (in mM) 250 sucrose, 25 KCl, 5 MgCl2, 1 CaCl2, 10 Tris-HCl with 0.5% Egepal, 40 μM Cytochalasin B, and a proteinase inhibitor pellet (Roche). The homogenate was centrifuged at 10,000 × g through an Optiprep (Iodioxanol) gradient (25%, 30%, 35%) and rested upon a 35% layer. The nuclei were collected and then concentrated by centrifugation at 2,000 × g in NIM. The pellet was resuspended in PBS in the presence of 20 mM NEM and 10 mM Iodoacetamide, plated on cover slips coated with CellTak (BD Scientific) and stained as above.
Histology
Spinal cords of SOD1-G93A mice were fixed with a buffered 4% formaldehyde solution, lumbar tract removed, embedded in TissueTek, frozen and sectioned at 10 μm. Sections were stained for GFAP as astrocyte marker (Alexa Fluor 546 conjugated monoclonal antibody, 1:500), EAAT1 or EAAT2 (Alexa Fluor 488, 1:1,000) and treated with Invitrogen Prolong Gold Antifade with DAPI. Z-axis image stacks (z-step size: 0.4 μm) were collected to three-dimensional data sets of spinal cord sections on Zeiss 510 Confocal Microscope with a 63x oil-immersion objective in condition of optimal iris diameter as defined by Carl Zeiss AIM software.
Constructs
CTE and CTE-SUMO1 fragments were obtained as previously described (Gibb et al. 2007). The N-terminus of SUMO1 was fused in frame with the C-terminus of the CTE fragment (Suppl. Fig.1). The reactive C-terminus of the SUMO1 moiety was inactivated by removing one glycine residue of the conserved “GG” active region (Martin et al. 2007), hence preventing unspecific sumoylations. Both CTE and CTE-SUMO1 were inserted into the pCMV-Myc plasmid (Clontech) at Kpn1 and EcoR1-blunted sites. To allow further Gateway recombination in adenoviral expression plasmids, Bgl2-EcoR1 fragment consisting of the Gfa2 astrocyte-specific promoter sequence (Brenner et al. 1994) was inserted into pENTR plasmid (Invitrogen) opened at EcoR1 and BamH1 sites. The expression cassettes accommodating Gfa2 promoter element, Myc-CTE or Myc-CTE-SUMO1 coding sequences, and SV40 pA signal were released from pCMV-Myc-CTE and pCMV-CTE-SUMO1 plasmids, respectively at Xba1 flanking sites and ligated to pENTR-Gfa2 plasmid opened with EcoR1 by Xba1-EcoR1 adapters. Finally, both expression cassettes were transferred by Gateway recombinase into adenoviral expression constructs, which were produced and amplified in HEK 293A cells (ViraPower Adenoviral Expression System; Invitrogen). Adenoviral constructs were added on astrocyte monolayers in fresh OPTI-MEM medium with primacin and the medium was changed 18 h later. Multiplicity of infection (m.o.i.) ranged from 1 to 3. The constructs pENTR-Gfa2-Myc-CTE or CTE-SUMO1 were used for electroporation with nucleofector (Lonza).
Microarray experiments and data analysis
Total RNA was isolated from spinal cord astrocytes six days after transfection with CTE or CTE-SUMO1 constructs using TRIzol (Invitrogen) and purified using RNeasy Mini kit (Qiagen). RNA quality was assessed with Agilent 2100 Bioanalyzer (Agilent Technologies) and quantified with a NanoDrop 1000 spectrophotometer (Thermo Scientific). 2 μg each of total RNA sample was labeled according to standard one-cycle amplification and labeling protocol (Affymetrix). Labeled cRNA was hybridized on Affymetrix GeneChip Mouse Genome 430A 2.0 Arrays, containing 22,690 probe sets corresponding to approximately 14,000 well-characterized mouse genes. Hybridized arrays were stained and washed (GeneChip Fluidics Station 450) and scanned (GeneChip Scanner 3000 7G). Cell intensity values and probe detection calls were computed using the Affymetrix GeneChip Operating Software (GCOS). Further data processing was performed in the R computing environment (http://www.r-project.org/) version 2.8.0 with BioConductor packages (http://www.bioconductor.org/). Robust Multi-Array Average (RMA) normalization was applied (Irizarry et al. 2003). Data were then filtered based on Affymetrix detection call and probe set intensity, so that only probe sets that had a present call and intensity value >100 in at least one of the arrays were retained. Statistical analysis was performed with limma (Smyth 2004). P-values were adjusted for multiple testing using Benjamini and Hochberg's method to control the false discovery rate (Hochberg and Benjamini 1990). Genes with adjusted P values below 0.05 were considered differentially expressed. Data were analyzed by Ingenuity Pathways Analysis (www.ingenuity.com).
Quantitative real-time PCR
Total RNA was isolated from cultured mouse spinal cord astrocytes. First strand cDNA was synthesized by reverse transcription from 1μg of total RNA using High Capacity RNA-to-cDNA Master Mix (AppliedBiosystems). Real-time PCR was performed for Fus/TLS, Netrin-1, EAAT2, EAAT1, nestin, PTEN and TDP43 genes (primer set part # Mm00836363_g1, Mm00500896_m1, Mm00441457_m1, Mm00450205_m1, AppliedBiosystems). The thermocycler parameters were 50°C for 2 min, 95°C for 10 min, followed by 40 cycles of 95°C for 15 sec and 60°C for 1 min. The results were analyzed by the comparative Ct method and normalized with Mouse ACTB Endogenous Control Assay (Part # 4352341E).
Statistical analysis
Comparisons were done by t-test analysis. Data are expressed as average ± sem. P values less than 0.05 were considered statistically significant.
RESULTS
Nuclear localization of a sumoylated fragment generated by a caspase-3 cleavage of the cytosolic C-terminus of EAAT2 in spinal cord astrocytes of SOD1-G93A mice
Caspase-3 cleaves the astroglial glutamate transporter EAAT2 creating a truncated, yet functional transporter and a cytosolic C-terminus fragment (Boston-Howes et al. 2006). The cleavage is both EAAT2 and CNS area specific. It takes place once symptom progression begins in the spinal cord of SOD1-G93A mice resulting in a progressive accumulation of the C-terminus EAAT2 fragment (CTE), which is SUMO1 conjugated (Gibb et al. 2007).
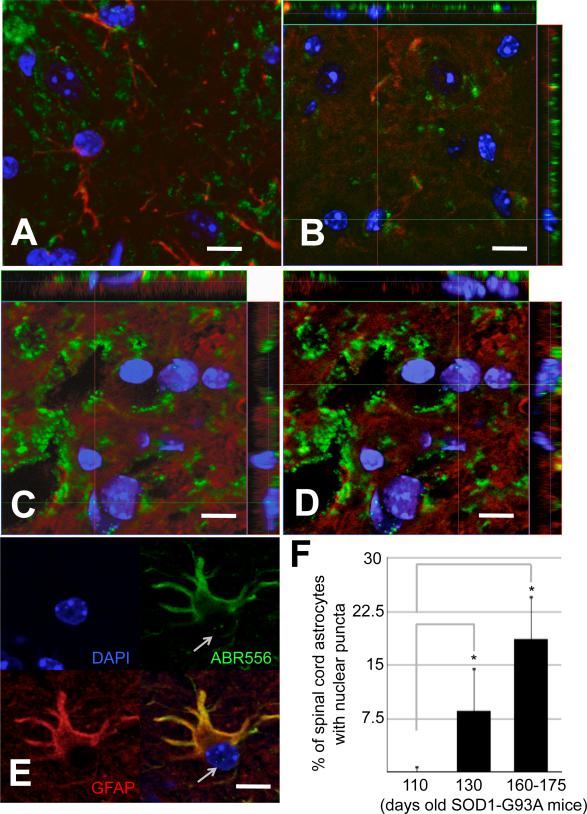
Spinal cord astrocytes transfected with CTE translationally fused to SUMO1 (CTE-SUMO1) exhibited, contrary to astrocytes transfected with the unmodified CTE fragment, accumulation of CTE-SUMO1 in PML nuclear bodies (PML-NBs) displaying a characteristic puncta staining that overlapped with PML staining in the nucleus (Suppl.Figs.1A-C,4B,4C) (Gibb et al. 2007). To determine whether a nuclear pattern for the endogenous sumoylated CTE fragment occurred in vivo, we immunostained lumbar spinal cord sections of SOD1-G93A mice at different stages of disease with anti-GFAP to mark astrocytes and ABR556 to stain for the C-terminus domain of EAAT2 (Suppl. Fig.1A). Along with EAAT2 full-length staining of astrocytes, we detected EAAT2-immunopositive puncta in the nucleus of GFAP-positive cells in the ventral horn of symptomatic ALS mice, but not in pre-symptomatic mice. This nuclear staining pattern was significant in symptomatic mice, involving ~10% of EAAT2 and GFAP-positive ventral horn cells, and it was more notable at advanced disease stages, reaching ~20% of total bona fide astrocytes in the ventral horn area of the spinal cord, suggesting a dependence on disease progression (Fig.1A-F). Conversely, dorsal horn astrocytes did not display EAAT2 C-terminus positive nuclear puncta (Suppl.Fig.2A), suggesting a possible relationship between the nuclear accumulation and motor neuron degeneration in ALS mice. Nuclear localization of the immunopositive puncta was confirmed with DAPI and orthogonal confocal imaging analyses (Fig.1C-E). We did not observe puncta-like immunoreactivity when an antibody raised against the extracellular domain of EAAT2 was used for staining (epitope 372-386, Suppl.Fig.1A,D and Suppl.Fig.2B,C), indicating that these puncta likely consisted of the cytosolic C-terminus domain of EAAT2. Puncta immunoreactivity was also observed with an antibody directed against the epitope 518-536 in the C-terminus domain of EAAT2. Isolated nuclei from spinal cord of diseased SOD1-G93A mice displayed puncta immunopositive for ABR518 (Fig.2B-E). As expected, puncta immunoreactivity was absent in isolated nuclei purified from pre-symptomatic mice (Fig.2A). ABR518 positive nuclear puncta in diseased mice co-stained for SUMO1 and overlapped with PML staining (Fig.2B-E), strongly indicating that the EAAT2 C-terminus fragment was sumoylated and accumulated in PML-NBs. This co-localization pattern is unique to EAAT2, as we failed to observe similar nuclear accumulation in vivo for EAAT1, the other major glial glutamate transporter (Suppl.Fig.2D). Interestingly, nuclear accumulation of EAAT2 C-terminus occurred exclusively in astrocytes of the grey matter in the motor neuron rich ventral horn of the spinal cord and not in astrocytes of either the dorsal horn or white matter (Suppl.Fig.2E). EAAT2 is a predominantly astroglial transporter in the spinal cord (Regan et al. 2007). Accordingly, we did not find any evidence of either expression of EAAT2 or nuclear localization of the EAAT2 fragment in both oligodendrocytes (Suppl.Fig.3), microglia cells and motor neurons (not shown) of the spinal cord grey matter. Occasionally, we observed that astrocytes displaying EAAT2 C-terminus nuclear punctate staining were directly apposing dystrophic motor neurons (Suppl.Fig.4).
Fig.1. EAAT2-immunopositive puncta in spinal cord astrocyte nuclei of SOD1-G93A mice at disease progression.
Ten μm spinal cord cross-sections from (A) presymptomatic (70 days old), (B) onset (110 days old) and (C-E) diseased (160-170 days old) G93A-SOD1 mice were immunostained with EAAT2 (ABR556; green; dil. 1:100) and GFAP (red; dil. 1:100) antibodies. The ventral portion of the lumbar spinal cord was imaged. (F) Quantification of GFAP-positive astrocytes displaying C-terminus EAAT2 immunoreactive nuclear punctate. For quantification, spinal cords of 3 animals from each disease stage were cut into 10um cross sections and probed with GFAP, EAAT2 and DAPI. From each mouse the ventral horn of 3 random sections were analyzed with LSM Image software. Asterisk indicates P<0.05 versus onset stage group. Nuclear puncta were absent at presymptomatic stage of disease and not plotted. Scale bar, 10 μm.
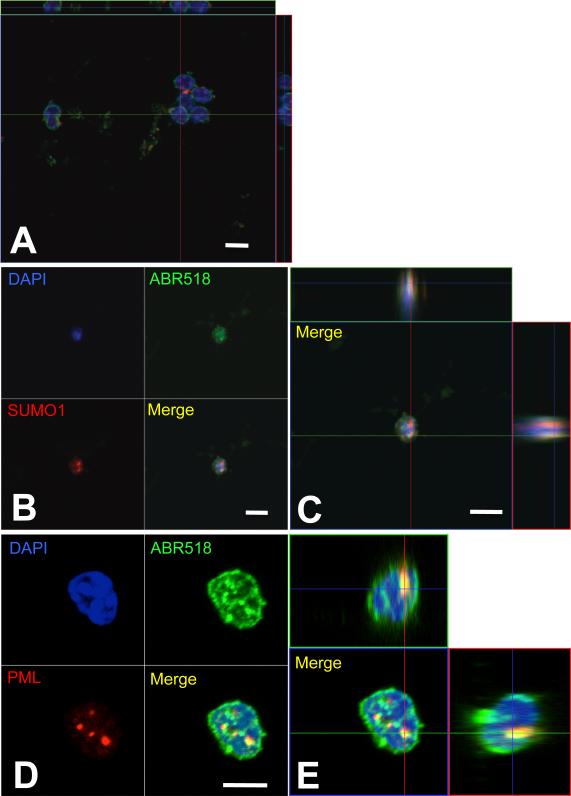
Fig.2. The C-terminus fragment of EAAT2 is sumoylated in the nucleus of spinal cord astrocytes of SOD1-G93A mice.
Nuclei were isolated from spinal cords of (A) pre-symptomatic (70 days old) and (B-E) diseased G93A-SOD1 mice (~160 days old) and stained for ABR518 (green, 1.5μg/ml), SUMO1 (red, dil. 1:40), and DAPI (blue) (B,C). Puncta immunopositive for the EAAT2 C-terminus antibody ABR518 were also immunoreactive for PML (dil. 1:100) (D,E). No ABR518 immunopositive puncta were detected in nuclei of pre-symptomatic mice (A). Images were taken with 63X oil-immersed objective. Scale bar, 10 μm.
To determine whether this nuclear accumulation pattern of the EAAT2 sumoylated fragment could be recapitulated in vitro, we cultured spinal cord astrocytes from adult, diseased SOD1-G93A mice and immunostained them with GFAP and ABR556 antibodies. Cultured astrocytes displayed nuclear localization pattern of the EAAT2 C-terminus. Punctate immunoreactivity positive for ABR556 was apparent in SOD1-G93A spinal cord astrocyte nuclei (Fig.2 D-G) and absent in spinal cord astrocytes derived from non-transgenic mice (Fig.2 A-C). We must compare adult astrocytes cultured from diseased mice to neonatal astrocytes. Despite numerous attempts, we failed to culture astrocytes from non-transgenic adult mice or pre-symptomatic adult ALS mice, suggesting that our standard culturing protocol was somehow selecting for reactive astrocytes, which are preponderant in diseased mice (Blackburn et al. 2009). In support of this hypothesis, ABR556 positive nuclear puncta were absent in SOD1-G93A spinal cord astrocytes cultured from neonatal mice (P2-P4; not shown), in which the reactive astrogliosis characteristic of ALS is not yet occurring. Nuclear puncta staining was not detected when spinal cord astrocytes cultured from diseased SOD1-G93A mice were probed with ABR372 antibody (epitope located upstream of the caspase-3 cleavage site), again indicating that the immunoreactivity of the puncta was likely directed against the cytosolic C-terminus domain of EAAT2 (Suppl.Fig.5A-C).
As in diseased SOD1-G93A spinal cord in vivo, nuclear puncta in cultured adult SOD1-G93A astrocytes were immunoreactive to ABR556 (Fig.4A,B) as well as SUMO1 (Fig.4C,D) and co-localized with PML (Fig.4E-H), indicating that sumoylation of the C-terminus fragment of EAAT2 and association to PML-NBs has occurred . Interestingly, not all the nuclear EAAT2 C-terminus puncta were positive for SUMO1 (Fig.4 D, 1 of 6, green arrow) suggesting that, perhaps, sumoylation of the EAAT2 C-terminus fragment could take place in situ in the nucleus after caspase-3 cleavage of EAAT2 and nuclear translocation of the fragment. Similarly, not all the nuclear EAAT2 C-terminus puncta overlapped with PML-NBs. In the nucleus of the astrocyte shown in Fig.4E-H, there are 5 ABR518 immunopositive puncta that displayed different degree of association with PML-NBs, ranging from full to lack of association (see also Suppl.Fig.6A). However, puncta association with PML-NBs was comprehensive in spinal cord astrocytes transfected with CTE-SUMO1 (Suppl.Fig.1A,B; Suppl.Fig.6B,C). Discrepancy between the artificial and endogenous CTE-SUMO1 fragment may suggest that the latter is in a dynamic equilibrium between its PML-associated and dissociated forms, most likely because the endogenous fragment retains the ability to be de-sumoylated and, hence, to dissociate from PML-NBs, contrary to the artificial CTE-SUMO1 fragment.
Fig.4. The C-terminus fragment of EAAT2 is immunoreactive for SUMO1 and partition to PML-nuclear bodies in spinal cord astrocytes cultured from SOD1-G93A diseased mice.
Nuclear EAAT2 puncta were also SUMO1 immunoreactive (A-C), although some of them are not (green arrow in D). Puncta either localized in close proximity or were associated with PML immunopositive nuclear bodies (E-H). EAAT2 puncta were immunopositive for both ABR518 and ABR556 (B,F). Scale bar, 10 μm. An higher magnification of panel H is presented in Suppl.Fig.6A.
Expression of CTE-SUMO1 in astrocytes triggers non-cell autonomous toxicity
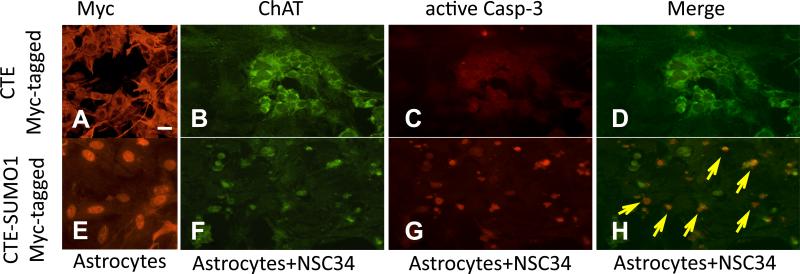
Accumulation of the sumoylated C-terminus fragment of EAAT2 in ALS mice occurs in a disease progression-dependent manner and is unique to the disease-affected spinal cord (Gibb et al. 2007) (Fig.1). Several lines of evidence indicate that astrocytes actively contribute to the degeneration of spinal motor neurons in ALS (Ilieva et al. 2009). We hypothesized that the caspase-3-derived, sumoylated EAAT2 fragment could be involved in motor neuron degeneration based on the evidence that its accumulation correlates with the progression of the disease. To look for evidence of a CTE-SUMO1-mediated, non-cell autonomous toxicity of astrocytes, we used a co-culture system consisting of undifferentiated motor neuron-derived NSC34 cells plated on a monolayer of spinal cord astrocytes, which were transduced three days before with AdV-Myc-CTE-SUMO1 or AdV-Myc-CTE as control. Spinal cord astrocytes were transduced with the two constructs at MOI 2 to achieve nearly 100% transfection efficiency (Fig.5A,E; Suppl.Fig.7); expression levels of Myc-CTE or Myc-CTE-SUMO1 were comparable and occurred only in astrocytes (not shown) because we used the astrocyte-specific promoter element gfa2 to drive expression (Lee et al. 2008). In this co-culture system, CTE-SUMO1-expressing astrocytes induced activation of caspase-3 in NSC34 cells after three day post-seeding (Fig.5B-D, 5F-H).
Fig.5. Nuclear accumulation in astrocytes of CTE-SUMO1 leads to activation of caspase-3 in co-cultured NSC-34 cells.
Nontransgenic spinal cord astrocytes were transduced with either AdV-myc-CTE or with AdV-myc-CTE-SUMO1 (MOI 2). Three days post transduction NSC34 cells were plated on the bed of either (A) Myc-CTE or (E) Myc-CTE-SUMO1 expressing astrocytes. Nuclear localization of CTE-SUMO1 is clearly noticeable (E) compared to the cytosolic distribution of CTE (A). NSC34 cells can be identified by ChAT (dil.1:100) staining (B,F). NSC34 cells plated on CTE-SUMO1 astrocytes were positive for active caspase 3 immunostaining (dil. 1:100; arrows in merged panel) (G,H) compared to NSC34 cells plated on CTE-expressing astrocytes (C,D). Representative fluorescence microscopy images are displayed. Scale bar, 10 μm.
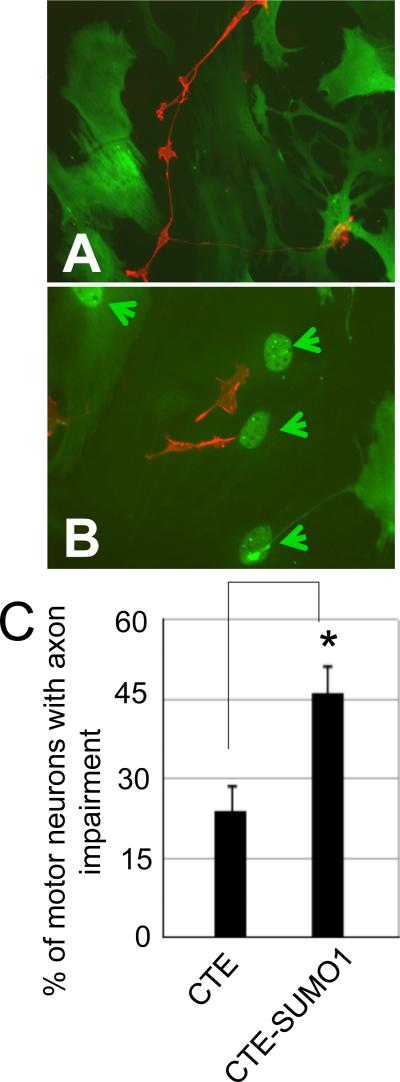
Nuclear accumulation of CTE-SUMO1 in spinal cord astrocytes promotes motor neuron impairment
To examine whether astrocyte expression of CTE-SUMO1 also affected primary spinal motor neurons, we plated purified motor neurons on a layer of spinal cord astrocytes expressing either Myc-CTE or Myc-CTE-SUMO1. Instead of adenovirus-based constructs we used plasmid cDNA constructs, which were electroporated in astrocytes (Nucleofector®, Lonza). With the Nucleofector protocol, we were able to achieve both high transfection efficiency (~60% as determined by staining with and antibody to the myc tag) and comparable expression levels between Myc-CTE and Myc-CTE-SUMO1 (Fig.7B). We did not use adenovirus constructs to transduce astrocytes as we found that, although the astrocyte monolayer was thoroughly rinsed after transduction, there was extensive motor neuron death (~100%) in both groups. This aspecific toxicity was most likely caused by residual traces of contaminants derived from the adenovirus isolation steps and to which motor neurons, contrary to NSC34 cells, could be sensitive. It was also unlikely that motor neuron toxicity could be caused by transduction of CTE or CTE-SUMO1 in motor neurons because we used the gfa2 promoter element to selectively drive astrocyte expression. Neurons were plated at the same density among experimental groups, resulting initially in equal numbers of primary motor neurons identified as both p75NTR and ChAT positive cells featuring long neurites (Suppl.Fig.8). As we did for NSC-34 cells, motor neurons were seeded on a monolayer of astrocytes and 72 hours later motor neuron toxicity was indexed by counting the number of motor neurons featuring neurites shorter than ~4 times the cell diameter. Motor neurons on CTE-SUMO1-expressing astrocytes displayed a significantly higher frequency of short neurites compared to CTE astrocytes (Fig.6A-C). These findings demonstrate that, under our in vitro experimental condition, nuclear expression of CTE-SUMO1 in astrocytes causes abnormal axon growth of spinal motor neurons.
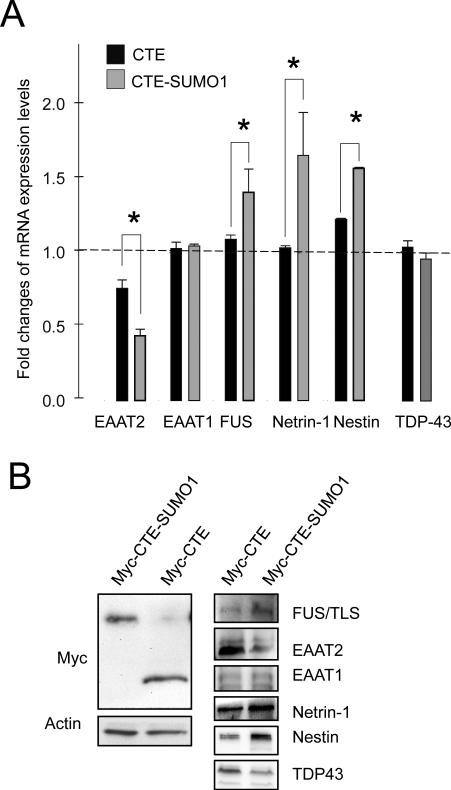
Fig.7. Validation of microarray data.
Nontransgenic spinal cord astrocytes were transfected with Myc-CTE or Myc-CTE-SUMO1. Analysis was performed either by qRT-PCR or western blot. (A) Six genes from the microarray experiment, which displayed statistically significant changes in the CTE-SUMO1 group, were tested with quantitative real time PCR. Data are normalized for β-actin mRNA and are the average of 6 independent experiments. Of these genes, EAAT1 and TDP-43 did not show significant differences in RNA expression levels. Two-tail t-test, *P<0.05. (B) Protein expression levels of the same six genes were also tested by western blot to confirm that the mRNA changes carried on to protein changes. TDP-43 protein expression levels decreased in contrast with the qPCR data set but in agreement with microarray analysis. Spinal cord astrocytes showed equal levels of CTE or CTE-SUMO1 assessed by western blot with anti-Myc antibody. A representative western blot analysis is shown. Astrocytes expressing CTE or CTE-SUMO1 were lysed in modified RIPA buffer and run on a 16% TrisTricine gel, transferred to PVDF membrane and probed as indicated.
Fig.6. Spinal cord astrocytes displaying nuclear localization of CTE-SUMO1 are toxic to motor neurons in culture.
Nontransgenic spinal cord astrocytes were transfected with either myc-CTE or with myc-CTE-SUMO1 plasmid cDNA. After six days purified non-transgenic motor neurons were plated on either Myc-CTE (A) or Myc-CTE-SUMO1 expressing (B) astrocytes. Expression of the constructs was assessed with anti-Myc antibody (dil. 1:100; green). Motor neurons plated on spinal cord astrocytes displaying nuclear accumulation of CTE-SUMO1 (arrowheads) had a larger percent of cells with axonal impairment (C). Motor neurons were immunostained with P75NTR antibody (red; dil. 1:100). Representative images of 4 different experiments are presented. *P<0.01, one-tail t-test.
To gain initial insights into possible extrinsic mechanisms of CTE-SUMO1 toxicity, we compared the genomic profiling of astrocytes expressing either CTE, as reference, or CTE-SUMO1. Data analysis showed that 4242 probes (18.7 % of all probes on the microarray) differed significantly in their intensity between CTE vs. CTE-SUMO1 expressing astrocytes. This corresponds to 3503 genes (Suppl.Table1), 477 of which changed more than 1.5 times between the two conditions (Suppl.Table2). Microarray data were deposited in the GEO database (accession number GSE26070). We investigated the differentially expressed genes’ function through the use of Ingenuity Pathways Analysis and the Database for Annotation, Visualization and Integrated Discovery (DAVID; (Huang da et al. 2009). These analyses highlighted that 827 genes are localized mainly to the nucleus (nucleome), 152 are secreted into the extracellular space (secretome), and 416 localized to the plasma membrane (plasma membranome). A representative list of these genes is shown in Suppl.Tables 3-5. Among the secreted differentially expressed genes, the top up-regulated one is netrin-1, known to be involved in axon migration. Similarly, we found 17 growth factor genes among the differentially expressed genes, but they are both up- and down-regulated. Among the 827 differentially expressed genes whose protein products are mainly located to the nucleus, 300 are transcription regulators, 25 of which increase at least 4 times (60 at least 2.8 times). Given that CTE-SUMO1 localizes in the nucleus and associates with PML-NBs, the number of significantly different genes whose protein product localizes mainly to the nucleus, seems to agree with the hypothesis that the sumoylated fragment may perturb nuclear function in astrocytes. Mutations in two RNA processing proteins (FUS/TLS and TARDBP/TDP43) have been recently linked to ALS (Kwiatkowski et al. 2009; Sreedharan et al. 2008). Interestingly, we found that in CTE-SUMO1 expressing astrocytes the levels of these two genes are also altered, along with various other factors involved in RNA processing and metabolism, suggesting an overall alteration in these processes. We also observed over-representation of genes involved in zinc ion binding among the down-regulated genes. While it is known that the mutations in SOD1 selectively destabilizes the remote metal binding region, thereby affecting the intermolecular protein-protein interactions which cause formation of protein aggregates (Museth et al. 2009) our data indicates that there is a general decrease in the expression level of genes whose protein product are normally able to interact with this important ion. Toxicity brought about by mutant SOD1 has long been hypothesized as a causal mechanism of ALS, causing damage to mitochondria, proteasomes, and protein folding chaperones. In agreement with this theory, our data show evident dysregulation in genes involved in oxidative phosphorylation, mitochondrial function, cellular respiration, protein modification/ubiquitination, and protein processing in general (metabolism, modification, localization, transport, phosphorylation, folding). Our genome profiling data also indicates a dramatic increase in nestin. During neuro- and gliogenesis, nestin is replaced by cell type-specific intermediate filaments, e.g. neurofilaments and glial fibrillary acidic protein (GFAP). Interestingly, nestin expression is reinduced in the adult during pathological situations, such as the formation of the glial scar after CNS injury and during regeneration of injured muscle tissue. We also measured a decrease in the glial glutamate transporter EAAT2 (GLT1). This may be in agreement with observations that downregulation of EAAT2 (GLT1) is occurring in spinal cord astrocytes during progression of the disease.
Validation of the microarray data was obtained by qRT-PCR for some of the genes of interest in the ALS field (Fig.7A). Changes in protein expression levels for EAAT2, FUS/TLS, TDP-43, netrin-1 and nestin, as determined by western blot analysis, were found in CTE-SUMO1 expressing astrocytes (Fig.7B), further confirming the microarray data. For some of these genes that were validated by qRT-PCR, such as PTEN (not shown), we did not observe differences in the protein levels among treatment groups, perhaps suggesting that further regulation at the translational levels could occur.
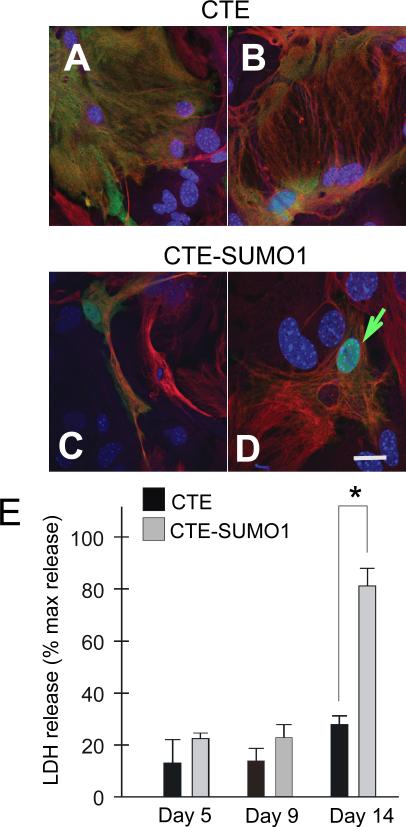
Persistent nuclear accumulation of CTE-SUMO1 is intrinsically toxic to astrocytes
Degeneration of a subpopulation of astrocytes has been previously reported in the SOD1-G93A mouse, in particular those astrocytes directly surrounding motor neurons (Rossi et al. 2008). To ascertain whether prolonged nuclear expression of CTE-SUMO1 is also directly toxic to astrocytes, we looked for morphological and biochemical changes typical of degenerating astrocytes in CTE-SUMO1 or CTE-expressing spinal cord astrocytes. Clear morphological changes with derangement of the intracellular GFAP filament network and appearance of large vacuoles were noticeable at day 14 post-transfection in CTE-SUMO1 (Fig.8C,D) compared to CTE expressing astrocytes (Fig.8A,B). These morphological changes were paralleled by a marked increase in extracellular accumulation of lactate dehydrogenase (LDH), a cytosolic enzyme used as marker of cellular integrity (Fig.8E), indicating that CTE-SUMO1 was inducing vast cellular degradation in astrocytes. It is interesting to note that the toxic effect of CTE-SUMO1 expressing astrocytes on motor neurons temporally preceded CTE-SUMO1 gliotoxicity. Motor neuron toxicity was, indeed, assessed at day 7 - 9 post-transfection of astrocytes with CTE-SUMO1 or CTE (3 days post-seeding of motor neurons) when there were no evident signs of morphological alterations in astrocytes and no significant LDH release differences among the experimental groups (Fig.8E).
Fig.8. Persistent nuclear accumulation of CTE-SUMO1 is intrinsically toxic to astrocytes.
Nontransgenic spinal cord astrocytes were transfected with either myc-CTE or myc-CTE-SUMO1. Lactate dehydrogenase (LDH) levels released in the medium was assessed at days 5, 9, and 14 post-transfection using the Clontech LDH cytotoxicity detection kit (cat.# 630117) following manufacturer's instructions and compared to LDH released by exposure of the cells to 30 min of 1% Triton X-100. At day 14 a subset of transfected astrocytes were fixed and stained with GFAP (red), Myc (green) and DAPI. Astrocytes expressing CTE retained their normal morphology after two weeks in culture (A), while nuclear accumulation of CTE-SUMO1 in led to dramatic altered morphology at 14 days in vitro in astrocytes including elongated processes (C) and large vacuoles (D). In A-D are shown representative confocal microscopy images. Results in E are the average ± s.e.m. of 3 independent experiments, each run in triplicate.
DISCUSSION
A role for non-neuronal cells in motor neuron degeneration in mutSOD1 ALS mice has been firmly established (Ilieva et al. 2009). Less established are the underlying molecular pathways, although mounting lines of evidence suggest a convergence of different mechanisms (Gandelman et al. 2010; Nagai et al. 2007; Vargas et al. 2006). This study identifies one unexpected pathway that may contribute to astrocyte-mediated motor neuron toxicity. We identified a C-terminus fragment of the astroglial glutamate transporter EAAT2 (a.k.a GLT-1), which originates from a caspase-3 cleavage and accumulates in the spinal cord of mutSOD1 mice as disease progresses (Gibb et al. 2007). EAAT2 impairment in ALS is regarded as a major mechanism responsible for initiating motor neuron excitotoxicity (Foran and Trotti 2009). In this study, we show that EAAT2 could play a role in motor neuron degeneration beyond excitoxicity. A gain-of-toxic-function is conferred to the EAAT2, caspase-3 generated fragment by SUMO1 conjugation (CTE-SUMO1), which results in its accumulation in astrocytes’ nucleus and partition to PML nuclear bodies. We showed an unique pattern of accumulation for CTE-SUMO1 in vivo in the SOD1-G93A mice, in spinal cord astrocytes cultured from these diseased mice and in astrocytes transfected with an artificial CTE construct tagged with SUMO1. Astrocytes that accumulate CTESUMO1 within PML nuclear bodies acquire toxic properties that affect motor neurons in a non-cell autonomous fashion. In co-culture experiments, CTE-SUMO1 expressing astrocytes induced caspase-3 activation and axonal growth impairment in NSC-34 cells and primary spinal motor neurons, respectively. Interestingly, we found that a prolonged accumulation of CTE-SUMO1 is also gliotoxic, although the time frame for mediating astrocytic degeneration is longer than that required for mediating motor neuron impairment. Gliotoxicity of CTE-SUMO1 would be consistent with the observation that the proportion of astrocytes displaying nuclear accumulation of CTE-SUMO1 in symptomatic ALS mice is restricted to 10-20% of all of the GFAP-positive cells at disease end-stage, suggesting that these astrocytes may die and be cleared at earlier phases of disease. Supporting this hypothesis, Rossi and colleagues reported degeneration of astrocytes in SOD1-G93A mice (Rossi et al. 2008). That activation of caspase-3 is required for generation of the C-terminus fragment of EAAT2 may suggest that astrocytes that display this fragment in vivo might have executed apoptotic processes, although not necessarily. Since there is no evidence of apoptotic astrocyte death in ALS, generation of CTE-SUMO1 would imply a controlled, non-apoptotic activation of caspase-3 could be occuring in these cells. Recent lines of evidence support the participation of this protease in non-apoptotic cellular events, including cell proliferation, cell cycle regulation, cellular differentiation (McLaughlin 2004; Noyan-Ashraf et al. 2005; Oomman et al. 2006; Oomman et al. 2005; Rohn et al. 2004). Interestingly, a recent study found that caspase-3 activation in astrocytes following excitotoxic damage correlated with cytoskeletal remodeling, but not with cell death (Acarin et al. 2007). Moreover, constitutively active caspase-3 not associated with apoptosis was detected in brain astrocytes of rats systemically injected with 3-nitropropionic acid to induce Huntington disease-like lesions, pointing to a non-apoptotic role for caspase-3 (Duran-Vilaregut et al. 2010). In this regard, it would be worth pursuing the study of molecular mechanisms and signaling molecule(s) that lead to caspase-3 activation and CTE-SUMO1 accumulation in astrocytes in ALS and what role mutant SOD1 could play in this process. Rossi and colleagues have suggested that excessive glutamate-mediated activation of mGluR5 on astrocytes could be responsible for the gliotoxicity seen in SOD1-G93A mice (Rossi et al. 2008). It is, therefore, tempting to speculate that glutamate released from excitatory presynapses by motor neurons during transmission can activate receptors in the perisynaptic astrocytic processes, which is turn can trigger a controlled activation of caspase-3 in astrocytes. During progression of the disease these astrocytes, altered by the chronic pathological presence of mutSOD1, may produce and accumulate CTE-SUMO1, which could cause exacerbation of non-cell autonomous mechanisms of motor neuron pathology and ultimately death of the astrocytes themself.
In vitro transient expression of CTE-SUMO1 is not initially associated with cell death, but rather triggers genotypic changes that affect astrocyte physiology initially compatible with their viability. Only chronic nuclear presence of CTE-SUMO1 (14 day-in-vitro) ultimately leads to astrocyte death. The time line of these effects, however, may be an underestimation when translated in vivo, because artificial SUMO1 tagging of CTE used above cannot be reversed by desumoylation enzymes that are normally located in the nucleus due to the translational fusion of SUMO1 with CTE (Suppl.Fig.1B), instead of an isopeptide bond as it occurs in vivo. In fact, endogenously sumoylated CTE is maintained at equilibrium with its corresponding desumoylated form by desumoylation enzymes (Geiss-Friedlander and Melchior 2007), attenuating the impact on nuclear function.
Research aimed at understanding the cellular functions of PML-NBs has led to the identification of many proteins that localize in these nuclear structures and, as a consequence, PML-NBs have been implicated in the regulation of many biological functions (Dellaire et al. 2003). The diverse nature of the proteins that accumulate in PML-NBs has made it difficult to attribute a specific and distinctive biochemical function to these structures. It is apparent that PML-NBs are unique nuclear domains that regulate functions like induction of apoptosis (Takahashi et al. 2004), DNA-damage response, regulation of cellular senescence, transcriptional regulation (Zhong et al. 2000), and that this is achieved by different biochemical means, including recruitment and post-translational modification of proteins (i.e. sumoylation), as well as modification or maintenance of chromatin domains (Bernardi and Pandolfi 2007). Experimental evidence has led to hypothesize that PML in PML-NBs could serve as an E3 ligase for sumoylation (Quimby et al. 2006). This might explain why we occasionally observed punctate immunoreactivity for the C-terminus of EAAT2 that did not react with anti-SUMO1 antibodies (Fig.4D) and did not overlap, but rather were in close proximity, with PML-NBs (Fig.4H), suggesting that sumoylation of CTE has not yet occurred, leaving open the possibility that the C-terminus fragment would get sumoylated once recruited into PML-NBs. Indeed, we currently have no evidence to indicate whether sumoylation of EAAT2 occurs before or after caspase-3 cleavage of the EAAT2 C-terminus. It has been proposed that PML-NBs operate as nuclear storage for the accumulation of proteins both in normal and pathological conditions (Bernardi and Pandolfi 2007). The sumoylated CTE fragment may be recruited or retained, in case sumoylation occurred in situ, in PML-NBs by interacting with SUMO-interaction motifs (SIMs) present in PML molecules that are co-assembled in these nuclear bodies (Shen et al. 2006). Once bound or associated with PML-NBs, CTE-SUMO1 could affect their normal functions such as regulation of gene transcription. Not surprisingly, the gene profile of cultured spinal cord astrocytes displaying nuclear accumulation of CTE-SUMO1 changed drastically. Of interest, one of the genes that are significantly reduced is the glutamate transporter EAAT2 from which the CTE-SUMO1 fragment is generated, implicating regulation of CTE-SUMO1 on the expression levels of the parent molecule. This effect of CTE-SUMO1 might contribute further to the excitotoxic damage of motor neurons and explain the downregulation in expression levels of EAAT2 that has been reported in ALS, perhaps an event that could be in addition to the recently described regulation by KBBP of EAAT2 protein levels in normal physiological condition and in CNS disease (Yang et al. 2009). However, whether ambient glutamate elevation caused by CTE-SUMO1-mediated down-regulation of EAAT2 could, in part or fully, account for motor axon growth impairment is still unknown and currently under investigation. It also remains unknown if other key proteins among the list of genes that are differentially regulated in astrocytes by CTE-SUMO1 are responsible for the indirect motor neuron toxicity and the late gliotoxicity. What is notable is that CTE-SUMO1 is dramatically changing the expression profile of spinal cord astrocytes, at least in culture, to the extent of causing fully differentiated cells to express markers of neural precursors. An example of this phenomenon, the intermediate filament protein nestin, which is expressed at high levels in neural precursor cells (Dahlstrand et al. 1995) and is downregulated when they differentiate into astrocytes, oligodendrocytes and neurons (Messam et al. 2000) is upregulated by CTE-SUMO1. Increase in nestin positive cells were reported in the brainstem of diseased SOD1-G93A mice (Juan et al. 2007). We also detected increased expression of nestin in the ventral spinal cord of diseased mice (Suppl.Fig.9). At this point of the investigation, however, it would be premature to hypothesize that CTE-SUMO1-expressing astrocytes have rolled back to the stage of progenitor cells or, once degenerated, have been replaced by nestin-expressing cells.
Netrin-1 is among the top up-regulated genes encoding secreted proteins in CTE-SUMO1 expressing astrocytes. This protein has been reported to inhibit in vitro axon growth of embryonic rat spinal cord motor neurons and axonal regeneration in the adult injured spinal cord (Low et al. 2008). Markers identifying glial cell types in the spinal cord indicated that netrin-1 is expressed by most, if not all, oligodendrocytes but not by astrocytes (Manitt et al. 2001). Therefore, aberrant expression and secretion of netrin-1 by CTE-SUMO1 expressing astrocytes may cause the inhibition of axon growth seen in vitro. An attractive hypothesis to test, which could explain motor neuron and axon impairments in vivo in the SOD1-G93A mice, would be that CTE-SUMO1-induced release of netrin-1 by astrocytes may trigger profound alterations of the normal motor neuron physiology and contribute to aggravate their degeneration in ALS.
Taken together, our findings indicate a novel role for the astroglial glutamate transporter EAAT2 in motor neuron degeneration, which goes beyond the dysregulation of glutamate homeostasis and excitotoxicity. Impairment in EAAT2 activity and expression levels is one of the few firm mechanistic events described in inherited and sporadic ALS, making the increase in EAAT2 an attractive target for a successful therapeutic approach to ALS. However, in light of our results, the strategy of inducing astrocytes to upregulate EAAT2, which potentially leads to more accumulation of pathogenic fragment, would seem inadequate. Instead, the effort should be focused not only on increasing synaptic glutamate clearance, but also on preventing the formation and post-translational processing of EAAT2.
Supplementary Material
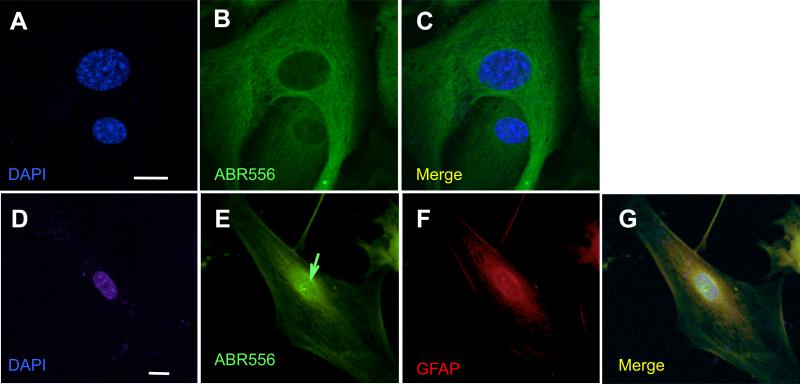
Fig.3. Spinal cord astrocytes cultured from SOD1-G93A diseased mice accumulate a C-terminus fragment of EAAT2 in the nucleus.
Astrocytes were isolated from spinal cords of neonatal non-transgenic mice (P2-P4) or from diseased SOD1-G93A mice (~150 days old), expanded in culture, and analyzed by immunofluorescence. Non-transgenic astrocytes did not display nuclear immunoreactivity for EAAT2 (A-C). Instead, there were discrete EAAT2 C-terminus immunopositive puncta (green arrow) in the nucleus of astrocytes isolated from spinal cord of diseased mutant SOD1 mice (D-G), which were not immunoreactive for ABR372 (Suppl.Fig.5A-C). Scale bars, 10 μm.
ACKNOWLEDGMENTS
This work was supported by the National Institute of Health grant RO1-NS44292 (to DT), RO1-NS051488 (to PP), and the Muscular Dystrophy Association (to DT). The Weinberg Unit for ALS research is also supported by the Farber Family Foundation.
REFERENCES
- Acarin L, Villapol S, Faiz M, Rohn TT, Castellano B, Gonzalez B. Caspase-3 activation in astrocytes following postnatal excitotoxic damage correlates with cytoskeletal remodeling but not with cell death or proliferation. Glia. 2007;55(9):954–65. doi: 10.1002/glia.20518. [DOI] [PubMed] [Google Scholar]
- Bernardi R, Pandolfi PP. Structure, dynamics and functions of promyelocytic leukaemia nuclear bodies. Nat Rev Mol Cell Biol. 2007;8(12):1006–16. doi: 10.1038/nrm2277. [DOI] [PubMed] [Google Scholar]
- Blackburn D, Sargsyan S, Monk PN, Shaw PJ. Astrocyte function and role in motor neuron disease: a future therapeutic target? Glia. 2009;57(12):1251–64. doi: 10.1002/glia.20848. [DOI] [PubMed] [Google Scholar]
- Boston-Howes W, Gibb SL, Williams EO, Pasinelli P, Brown RH, Jr., Trotti D. Caspase-3 cleaves and inactivates the glutamate transporter EAAT2. J Biol Chem. 2006;281(20):14076–84. doi: 10.1074/jbc.M600653200. [DOI] [PubMed] [Google Scholar]
- Brenner M, Kisseberth WC, Su Y, Besnard F, Messing A. GFAP promoter directs astrocyte-specific expression in transgenic mice. J Neurosci. 1994;14(3 Pt 1):1030–7. doi: 10.1523/JNEUROSCI.14-03-01030.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlstrand J, Lardelli M, Lendahl U. Nestin mRNA expression correlates with the central nervous system progenitor cell state in many, but not all, regions of developing central nervous system. Brain Res Dev Brain Res. 1995;84(1):109–29. doi: 10.1016/0165-3806(94)00162-s. [DOI] [PubMed] [Google Scholar]
- Dellaire G, Farrall R, Bickmore WA. The Nuclear Protein Database (NPD): sub-nuclear localisation and functional annotation of the nuclear proteome. Nucleic Acids Res. 2003;31(1):328–30. doi: 10.1093/nar/gkg018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duran-Vilaregut J, Del Valle J, Manich G, Junyent F, Camins A, Pallas M, Pelegri C, Vilaplana J. Systemic administration of 3-nitropropionic acid points out a different role for active caspase-3 in neurons and astrocytes. Neurochem Int. 2010;56(3):443–50. doi: 10.1016/j.neuint.2009.12.001. [DOI] [PubMed] [Google Scholar]
- Foran E, Trotti D. Glutamate transporters and the excitotoxic path to motor neuron degeneration in amyotrophic lateral sclerosis. Antioxid Redox Signal. 2009;11(7):1587–602. doi: 10.1089/ars.2009.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandelman M, Peluffo H, Beckman JS, Cassina P, Barbeito L. Extracellular ATP and the P2X(7) receptor in astrocyte-mediated motor neuron death: implications for amyotrophic lateral sclerosis. J Neuroinflammation. 2010;7:33. doi: 10.1186/1742-2094-7-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiss-Friedlander R, Melchior F. Concepts in sumoylation: a decade on. Nat Rev Mol Cell Biol. 2007;8(12):947–56. doi: 10.1038/nrm2293. [DOI] [PubMed] [Google Scholar]
- Gibb SL, Boston-Howes W, Lavina SZ, Gustincich S, Brown RH, Jr., Pasinelli P, Trotti D. A caspase-3 cleaved fragment of the glial glutamate transporter EAAT2 is sumoylated and targeted to promyelocytic leukemia nuclear bodies in mutant SOD1 linked ALS. J Biol Chem. 2007:M704314200. doi: 10.1074/jbc.M704314200. [DOI] [PubMed] [Google Scholar]
- Hochberg Y, Benjamini Y. More powerful procedures for multiple significance testing. Stat Med. 1990;9(7):811–8. doi: 10.1002/sim.4780090710. [DOI] [PubMed] [Google Scholar]
- Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4(1):44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- Ilieva H, Polymenidou M, Cleveland DW. Non-cell autonomous toxicity in neurodegenerative disorders: ALS and beyond. J Cell Biol. 2009;187(6):761–72. doi: 10.1083/jcb.200908164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irizarry RA, Hobbs B, Collin F, Beazer-Barclay YD, Antonellis KJ, Scherf U, Speed TP. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics. 2003;4(2):249–64. doi: 10.1093/biostatistics/4.2.249. [DOI] [PubMed] [Google Scholar]
- Juan L, Dawei Z, Julie AD. Increased number and differentiation of neural precursor cells in the brainstem of superoxide dismutase 1(G93A) (G1H) transgenic mouse model of amyotrophic lateral sclerosis. Neurol Res. 2007;29(2):204–9. doi: 10.1179/174313206X152519. [DOI] [PubMed] [Google Scholar]
- Kwiatkowski TJ, Jr., Bosco DA, Leclerc AL, Tamrazian E, Vanderburg CR, Russ C, Davis A, Gilchrist J, Kasarskis EJ, Munsat T. Mutations in the FUS/TLS gene on chromosome 16 cause familial amyotrophic lateral sclerosis. Science. 2009;323(5918):1205–8. doi: 10.1126/science.1166066. others. [DOI] [PubMed] [Google Scholar]
- Lee Y, Messing A, Su M, Brenner M. GFAP promoter elements required for region-specific and astrocyte-specific expression. Glia. 2008;56(5):481–93. doi: 10.1002/glia.20622. [DOI] [PubMed] [Google Scholar]
- Lieberman AP. SUMO, a ubiquitin-like modifier implicated in neurodegeneration. Exp Neurol. 2004;185(2):204–7. doi: 10.1016/j.expneurol.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Low K, Culbertson M, Bradke F, Tessier-Lavigne M, Tuszynski MH. Netrin-1 is a novel myelin-associated inhibitor to axon growth. J Neurosci. 2008;28(5):1099–108. doi: 10.1523/JNEUROSCI.4906-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manitt C, Colicos MA, Thompson KM, Rousselle E, Peterson AC, Kennedy TE. Widespread expression of netrin-1 by neurons and oligodendrocytes in the adult mammalian spinal cord. J Neurosci. 2001;21(11):3911–22. doi: 10.1523/JNEUROSCI.21-11-03911.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin S, Wilkinson KA, Nishimune A, Henley JM. Emerging extranuclear roles of protein SUMOylation in neuronal function and dysfunction. Nat Rev Neurosci. 2007;8(12):948–59. doi: 10.1038/nrn2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin B. The kinder side of killer proteases: caspase activation contributes to neuroprotection and CNS remodeling. Apoptosis. 2004;9(2):111–21. doi: 10.1023/B:APPT.0000018793.10779.dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messam CA, Hou J, Major EO. Coexpression of nestin in neural and glial cells in the developing human CNS defined by a human-specific anti-nestin antibody. Exp Neurol. 2000;161(2):585–96. doi: 10.1006/exnr.1999.7319. [DOI] [PubMed] [Google Scholar]
- Museth AK, Brorsson AC, Lundqvist M, Tibell LA, Jonsson BH. The ALS-associated mutation G93A in human copper-zinc superoxide dismutase selectively destabilizes the remote metal binding region. Biochemistry. 2009;48(37):8817–29. doi: 10.1021/bi900703v. [DOI] [PubMed] [Google Scholar]
- Nagai M, Re DB, Nagata T, Chalazonitis A, Jessell TM, Wichterle H, Przedborski S. Astrocytes expressing ALS-linked mutated SOD1 release factors selectively toxic to motor neurons. Nat Neurosci. 2007;10(5):615–622. doi: 10.1038/nn1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noyan-Ashraf MH, Brandizzi F, Juurlink BH. Constitutive nuclear localization of activated caspase 3 in subpopulations of the astroglial family of cells. Glia. 2005;49(4):588–93. doi: 10.1002/glia.20140. [DOI] [PubMed] [Google Scholar]
- Oomman S, Strahlendorf H, Dertien J, Strahlendorf J. Bergmann glia utilize active caspase-3 for differentiation. Brain Res. 2006;1078(1):19–34. doi: 10.1016/j.brainres.2006.01.041. [DOI] [PubMed] [Google Scholar]
- Oomman S, Strahlendorf H, Finckbone V, Strahlendorf J. Non-lethal active caspase-3 expression in Bergmann glia of postnatal rat cerebellum. Brain Res Dev Brain Res. 2005;160(2):130–45. doi: 10.1016/j.devbrainres.2005.07.010. [DOI] [PubMed] [Google Scholar]
- Pasinelli P, Houseweart MK, Brown RH, Jr., Cleveland DW. Caspase-1 and -3 are sequentially activated in motor neuron death in Cu,Zn superoxide dismutase-mediated familial amyotrophic lateral sclerosis. Proc Natl Acad Sci U S A. 2000;97(25):13901–6. doi: 10.1073/pnas.240305897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quimby BB, Yong-Gonzalez V, Anan T, Strunnikov AV, Dasso M. The promyelocytic leukemia protein stimulates SUMO conjugation in yeast. Oncogene. 2006;25(21):2999–3005. doi: 10.1038/sj.onc.1209335. [DOI] [PubMed] [Google Scholar]
- Regan MR, Huang YH, Kim YS, Dykes-Hoberg MI, Jin L, Watkins AM, Bergles DE, Rothstein JD. Variations in Promoter Activity Reveal a Differential Expression and Physiology of Glutamate Transporters by Glia in the Developing and Mature CNS. J Neurosci. 2007;27(25):6607–6619. doi: 10.1523/JNEUROSCI.0790-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohn TT, Cusack SM, Kessinger SR, Oxford JT. Caspase activation independent of cell death is required for proper cell dispersal and correct morphology in PC12 cells. Exp Cell Res. 2004;295(1):215–25. doi: 10.1016/j.yexcr.2003.12.029. [DOI] [PubMed] [Google Scholar]
- Rossi D, Brambilla L, Valori CF, Roncoroni C, Crugnola A, Yokota T, Bredesen DE, Volterra A. Focal degeneration of astrocytes in amyotrophic lateral sclerosis. Cell Death Differ. 2008;15(11):1691–700. doi: 10.1038/cdd.2008.99. [DOI] [PubMed] [Google Scholar]
- Shen TH, Lin HK, Scaglioni PP, Yung TM, Pandolfi PP. The mechanisms of PML-nuclear body formation. Mol Cell. 2006;24(3):331–9. doi: 10.1016/j.molcel.2006.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth GK. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol. 2004;3 doi: 10.2202/1544-6115.1027. Article3. [DOI] [PubMed] [Google Scholar]
- Sreedharan J, Blair IP, Tripathi VB, Hu X, Vance C, Rogelj B, Ackerley S, Durnall JC, Williams KL, Buratti E. TDP-43 mutations in familial and sporadic amyotrophic lateral sclerosis. Science. 2008;319(5870):1668–72. doi: 10.1126/science.1154584. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffan JS, Agrawal N, Pallos J, Rockabrand E, Trotman LC, Slepko N, Illes K, Lukacsovich T, Zhu YZ, Cattaneo E. SUMO modification of Huntingtin and Huntington's disease pathology. Science. 2004;304(5667):100–4. doi: 10.1126/science.1092194. others. [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Lallemand-Breitenbach V, Zhu J, de The H. PML nuclear bodies and apoptosis. Oncogene. 2004;23(16):2819–24. doi: 10.1038/sj.onc.1207533. [DOI] [PubMed] [Google Scholar]
- Taylor AR, Gifondorwa DJ, Newbern JM, Robinson MB, Strupe JL, Prevette D, Oppenheim RW, Milligan CE. Astrocyte and Muscle-Derived Secreted Factors Differentially Regulate Motoneuron Survival. J Neurosci. 2007a;27(3):634–644. doi: 10.1523/JNEUROSCI.4947-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor AR, Robinson MB, Milligan CE. In vitro methods to prepare astrocyte and motoneuron cultures for the investigation of potential in vivo interactions. Nat Protoc. 2007b;2(6):1499–507. doi: 10.1038/nprot.2007.208. [DOI] [PubMed] [Google Scholar]
- Tzingounis AV, Wadiche JI. Glutamate transporters: confining runaway excitation by shaping synaptic transmission. Nat Rev Neurosci. 2007;8(12):935–47. doi: 10.1038/nrn2274. [DOI] [PubMed] [Google Scholar]
- Vargas MR, Pehar M, Cassina P, Beckman JS, Barbeito L. Increased glutathione biosynthesis by Nrf2 activation in astrocytes prevents p75NTR-dependent motor neuron apoptosis. J Neurochem. 2006;97(3):687–96. doi: 10.1111/j.1471-4159.2006.03742.x. [DOI] [PubMed] [Google Scholar]
- Yamanaka K, Chun SJ, Boillee S, Fujimori-Tonou N, Yamashita H, Gutmann DH, Takahashi R, Misawa H, Cleveland DW. Astrocytes as determinants of disease progression in inherited amyotrophic lateral sclerosis. Nat Neurosci. 2008 doi: 10.1038/nn2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Gozen O, Watkins A, Lorenzini I, Lepore A, Gao Y, Vidensky S, Brennan J, Poulsen D, Won Park J. Presynaptic Regulation of Astroglial Excitatory Neurotransmitter Transporter GLT1. Neuron. 2009;61(6):880. doi: 10.1016/j.neuron.2009.02.010. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong S, Salomoni P, Pandolfi PP. The transcriptional role of PML and the nuclear body. Nat Cell Biol. 2000;2(5):E85–90. doi: 10.1038/35010583. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.