Significance
N-myc downstream regulated gene 1 (NDRG1) is a central and druggable molecular hub integrating diverse therapy-induced microenvironmental factors to promote resistance toward alkylating chemotherapy. We suggest that NDRG1-mediated chemoprotection is achieved via binding and stabilizing methyltransferases, such as O6-methylguanine-DNA methyltransferase.
Abstract
A hypoxic microenvironment induces resistance to alkylating agents by activating targets in the mammalian target of rapamycin (mTOR) pathway. The molecular mechanisms involved in this mTOR-mediated hypoxia-induced chemoresistance, however, are unclear. Here we identify the mTOR target N-myc downstream regulated gene 1 (NDRG1) as a key determinant of resistance toward alkylating chemotherapy, driven by hypoxia but also by therapeutic measures such as irradiation, corticosteroids, and chronic exposure to alkylating agents via distinct molecular routes involving hypoxia-inducible factor (HIF)-1alpha, p53, and the mTOR complex 2 (mTORC2)/serum glucocorticoid-induced protein kinase 1 (SGK1) pathway. Resistance toward alkylating chemotherapy but not radiotherapy was dependent on NDRG1 expression and activity. In posttreatment tumor tissue of patients with malignant gliomas, NDRG1 was induced and predictive of poor response to alkylating chemotherapy. On a molecular level, NDRG1 bound and stabilized methyltransferases, chiefly O6-methylguanine-DNA methyltransferase (MGMT), a key enzyme for resistance to alkylating agents in glioblastoma patients. In patients with glioblastoma, MGMT promoter methylation in tumor tissue was not more predictive for response to alkylating chemotherapy in patients who received concomitant corticosteroids.
Primary or acquired antitumor therapy resistance is one of the major obstacles in oncology. For glioma, to date, this is pivotal for the standard of care, radiotherapy, and temozolomide (TMZ) alkylating chemotherapy. The DNA repair protein O6-methylguanine-DNA methyltransferase (MGMT) plays a critical role in primary resistance to alkylating agents (1, 2). Serving as a central signaling hub integrating multiple intracellular and extracellular cues, the 289-kDa serine/threonine kinase mammalian target of rapamycin (mTOR) is an attractive anticancer target. Activation of the signaling network engaged by the protein inositol-3 kinase/AKT/mTOR axis frequently occurs by activation of receptor tyrosine kinases (RTK), chiefly the epidermal growth factor receptor (EGFR) being the most commonly altered RTK in glioblastomas. However, mere inhibition of EGFR or mTOR has been ineffective in glioblastomas.
The hypoxic microenvironment has been proposed to serve as germ center for more aggressive and therapy-resistant tumor cell phenotypes (3) especially preventing the efficacy of radiotherapy (4, 5). Hypoxia induces resistance to several anticancer agents in neurons (6) but also in glioma cells (7). In general, hypoxia causes the accumulation of the transcription factor hypoxia-inducible factor (HIF)-1 leading to the expression of hypoxia-inducible genes such as those for vascular endothelial growth factor (VEGF) and N-myc downstream regulated gene 1 (NDRG1) (8). The NDRG family of proteins consists of four evolutionary conserved members, NDRG1–4. The first member to be discovered and responsible for the family name was NDRG1 because its expression is repressed by the protooncogenes MYCN and MYC (9). It has been hypothesized that NDRG1 expression is inversely correlated with survival in glioblastomas (10), but the molecular and functional mechanisms involved in this association remain unclear.
To identify critical pathways involved in the chemoresistance of gliomas evoked by microenvironmental factors, particularly hypoxia, we initiated an unbiased proteomics approach.
Materials and Methods
Cell Culture, Reagents, Transfections, and Treatment Regimens.
Details are provided in SI Appendix, Methods.
Plasmid-Based Knockdown of NDRG1.
To silence NDRG1 gene expression, two short-hairpin RNA (shRNA) sequences targeting different sites were cloned into the pSUPER-puro vector (11, 12). The sequences are provided in SI Appendix.
Lentiviral Preparations.
Lentiviral particles for the knockdown experiments were produced by cotransfecting psPAX2, pMD2.G (both Addgene plasmid 12259), and pLKO.1 constructs (TRC1; Sigma-Aldrich) in HEK293T cells using TransIT LT1 (Mirus Bio). Details are given in SI Appendix.
Cloning of NDRG1 Variants.
NDRG1 dephospho-variants were generated by using site-directed mutagenesis PCR and changing the codons of the phospho-sites Thr and Ser to Val and Ala, respectively. Two different phosphorylation-deficient variants, NDRG1-T346V-T356V and NDRG1-T328V-S330A-T346V-T356V, were generated.
Immunoblot.
Preparation of cell lysates and immunoblots were performed as described before (12). Antibodies are given in SI Appendix, Table S8.
Proximity Ligation Assay.
T98G and LN-229 cells (n = 2 × 104) were confluently grown at O2 of 1% (vol/vol) on coverslips for 72 h. Fixation was done using 30 min Cytofixx Pump Spray cell path and 30 min 4% (vol/vol) paraformaldehyde. For detection the Red Duolink In Situ Proximity Ligation Assay (PLA) Kit was performed according to manufacturer´s instructions with anti-NDRG1 polyclonal rabbit (Sigma-Aldrich) and mouse anti-MGMT (Life Technologies) applied for 12 h. Mounting was done with VECTASHIELD HardSet Mounting Medium with DAPI.
Animal Experiments, Image Processing, and Histology.
All animal work was approved by the governmental authorities (Regierungspräsidium Karlsruhe) and supervised by institutional animal protection officials in accordance with the National Institutes of Health guidelines given in Guide for the Care and Use of Laboratory Animals. Details are provided in SI Appendix.
Clinical Data.
All clinically related research in this manuscript is covered by the Ethical Vote for the UKT-05 (13), NOA-04 (14), and NOA-08 (15) trials.
Statistical Analysis.
Quantitative in vitro data are expressed as mean ± SD (SD), as indicated. All in vitro experiments reported here represent at least three independent replications performed in triplicate if not otherwise stated. Statistical significance was assessed by two-sided Student’s t test or ANOVA (Microsoft Excel). Values of P < 0.05 were considered significant and asterisked without correction for multiple statistical tests. Mouse glioma volumes were corrected for outliers using Grubbs’ test. Survival data were plotted by the Kaplan–Meier method and analyzed by the log-rank test. SigmaPlot Software was used for all analyses.
Results
Hypoxia-Induced Alkylator but Not Radiotherapy Resistance in Malignant Gliomas Depends on NDRG1.
To identify factors that mediate hypoxia-induced alkylator resistance (SI Appendix, Fig. S1A), we screened human glioma cell lines for their response to alkylating chemotherapy in hypoxic conditions and subjected LN-229 glioma cells to a proteome screen (SI Appendix, Fig. S1B). This screen revealed hypoxia-specific up-regulation of seven and down-regulation of two proteins (SI Appendix, Table S1), of which up-regulated NDRG1 was further analyzed because (i) its up-regulation in hypoxia was unequivocally confirmed in all conducted assays (SI Appendix, Table S1), (ii) it had been implicated as a target of hypoxia (16) and a prognostic factor in other types of tumors (17), and (iii) it was found down-regulated in a transcriptome analysis following pharmaceutical mTOR inhibition with RAD001 (at www.ebi.ac.uk/arrayexpress under the accession number E-MEXP-3802). Hypoxia induced NDRG1 in all tested glioma cell lines (Fig. 1A). This was specific for NDRG1 because NDRG2–4 were not differentially regulated (Fig. 1B). In human glioma specimens, NDRG1 was associated with the degree of malignancy, and in glioblastomas it was prominently expressed in putatively hypoxic, perinecrotic areas (Fig. 1C and SI Appendix, Fig. S2A).
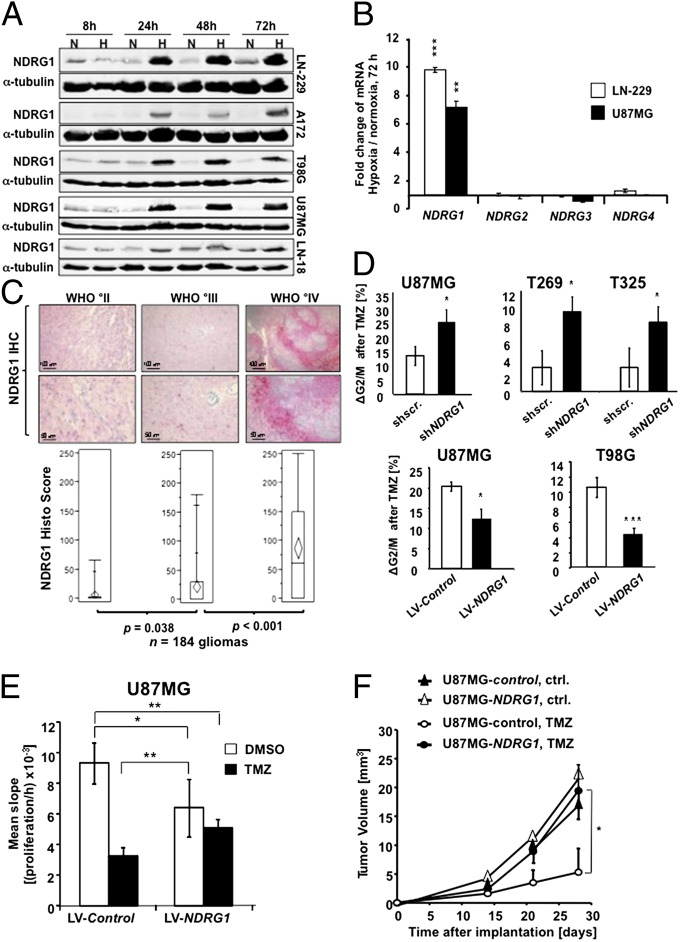
Fig. 1.
NDRG1 is a hypoxia-associated chemoresistance marker in glioma. (A) Immunoblot analyses for NDRG1 of lysates prepared from glioma cells exposed to 1% O2 (H) or 21% O2 (N) for the indicated intervals. α-tubulin served as a loading control. (B) qRT-PCR analysis of NDRG isoforms exposed to 1% O2 (mean ± SD, n = 3, **P < 0.01, ***P < 0.005). (C) NDRG1 staining in WHO °II (n = 46), WHO °III (n = 57), and WHO °IV (n = 81) gliomas presented as number of NDRG1+ cells per field (mean ± SD). Representative images of scattered NDRG1+ cells (Left), increased numbers of NDRG1+ cells (Center), and perinecrotic NDRG1+ cells (Right) are depicted by the specific red staining. (D) Cell cycle distributions and mean G2/M-arrest of TMZ-treated glioma cells relative to DMSO- (vehicle-) treated cells dependent on the NDRG1 status. TMZ concentrations used were 10 µM for U87MG, 40 µM for T269, 300 µM for T325, and 300 µM for T98G, and the medium was changed every 24 h with addition of fresh TMZ. (Upper) Lentiviral knockdown in U87MG, T269, and T325 GIC. (Lower) NDRG1 overexpression in U87MG and T98G cells. (E) Proliferation of TMZ/vehicle-treated U87MG cells overexpressing NDRG1 or control in RTCA. (F) MRI-determined tumor volumes of intracranially implanted U87MG gliomas overexpressing NDRG1 or control vector. TMZ was given on days 10–15 as described in Materials and Methods (*P < 0.05 versus control, t test, n = 6).
Knockdown of NDRG1 resulted in sensitization of established glioma cells and naturally highly NDRG1-expressing T269 and T325 primary glioma cells to TMZ (Fig. 1D and SI Appendix, Fig. S3A), indicating that NDRG1 mediates hypoxia-induced resistance to alkylating agents. Conversely, NDRG1-overexpressing cells showed a reduction in the TMZ-induced G2/M arrest (Fig. 1D and SI Appendix, Fig. S3B), which corresponded to a reduction in proliferation in vitro (Fig. 1E) and tumor growth in vivo (Fig. 1F), whereas proliferation or clonogenicity of glioma cells exposed to radiotherapy at 2 or 4 Gy remained unaffected (SI Appendix, Fig. S3D). It is notable that NDRG1-overexpressing cells not exposed to TMZ proliferated slower than the controls (Fig. 1E and SI Appendix, Fig. S4 D and F).
NDRG1 Is Transcriptionally Activated by Radiotherapy and Phosphorylated in the Course of TMZ Treatment.
Next, we analyzed the influence of therapeutic measures altering the tumor microenvironment on NDRG1 expression and activity. In vitro, irradiation (SI Appendix, Fig. S5A) but not TMZ induced NDRG1 mRNA and protein expression. In contrast to hypoxia, irradiation-induced NDRG1 expression was dependent on p53 expression (SI Appendix, Figs. S5B and S4 A and B) but was not impaired by HIF-1α or HIF-2α gene silencing (SI Appendix, Fig. S5C), indicating that hypoxia and irradiation use diverse signaling pathways to induce chemoresistance via NDRG1. Long-term exposure to TMZ led to an increased phosphorylation of NDRG1 at position T346 in surviving cells (SI Appendix, Fig. S5D). NDRG1 phosphorylation at T346 is associated with increased activity (18). Collectively, these data indicate that hypoxia and irradiation but not alkylating chemotherapy activate NDRG1 via distinct pathways resulting in resistance toward alkylating chemotherapy (SI Appendix, Fig. S6).
NDRG1 Is a Predictive Marker for Response to Alkylating Chemotherapy.
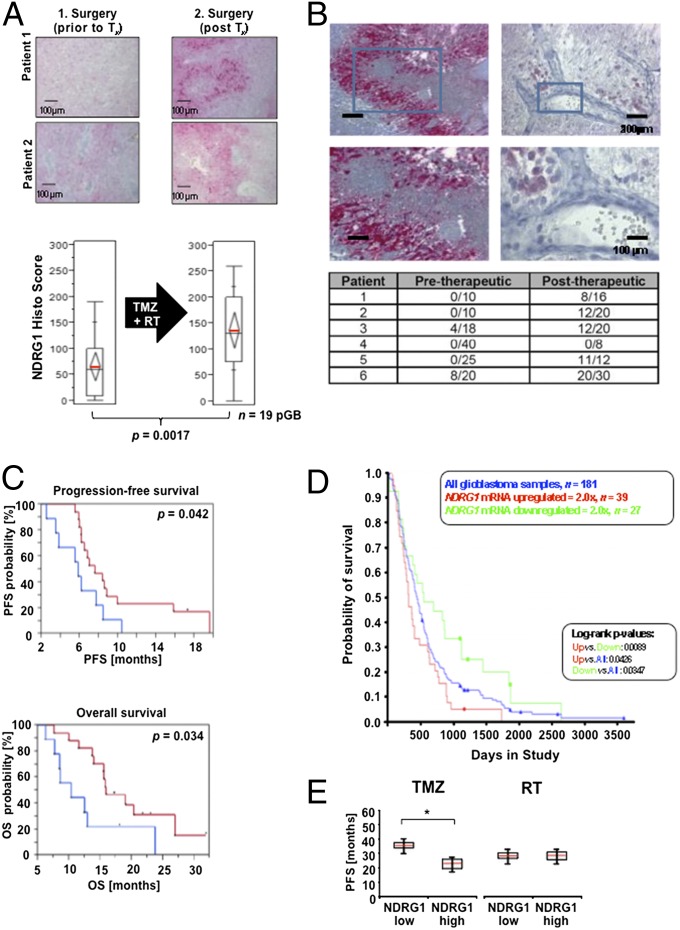
Next, we interrogated patient tumor tissue to recapitulate the relevance of inducible NDRG1 for therapy resistance. NDRG1 is induced at tumor recurrence (Fig. 2 and SI Appendix, Fig. S2B). As opposed to tumor tissue at diagnosis, NDRG1 expression in the treated tissues was not predominantly seen in perinecrotic areas anymore, but NDRG1 was widely expressed in glioblastoma cells even perivascularly opposed to the situation in the untreated tumors (Fig. 2B). NDRG1 expression in patients with low-grade gliomas progressing without interim genotoxic treatment also increased (SI Appendix, Fig. S2B). High NDRG1 levels at recurrence suggest a poor response to alkylating chemotherapy, but not to the antiangiogenic agent bevacizumab, in a small group of patients (SI Appendix, Table S2). A predictive role of NDRG1 for poor response to radiochemotherapy was suggested by post hoc NDRG1 expression analyses, which revealed that progression-free survival (PFS) and overall survival (OS) of glioblastoma patients from the UKT-05 trial (13) with moderate or high expression of NDRG1 was reduced compared with patients with low NDRG1-expressing tumors (Fig. 2C). This was supported by an analysis of the Repository for Molecular Brain Neoplasia Data (REMBRANDT) database, which revealed that the OS of glioblastoma patients with intratumoral up-regulation of NDRG1 was reduced compared with patients with intermediate or down-regulated expression of the NDRG1 transcript (Fig. 2D). To determine whether the prognostic impact of NDRG1 is specifically related to alkylating chemotherapy, tissue samples of the NOA-04 trial comparing primary radiotherapy with primary alkylating chemotherapy (14) were analyzed. NDRG1 expression was associated with reduced PFS in TMZ-treated patients but not with radiotherapy in this not preplanned subgroup analysis (Fig. 2E). Collectively, these data from several study patient populations suggest that NDRG1 expression in glioma tissue is associated with a poor response specifically to alkylating chemotherapy.
Fig. 2.
NDRG1 is induced by glioblastoma therapy and serves as a negative prognostic factor. (A) Representative tissues of 19 patients before and at recurrence after radiochemotherapy with TMZ were scored for the number of NDRG1-positive cells (mean ± SD). (B) Representative perivascular tumor region from 20 relapsed (after radiochemotherapy) glioblastoma tissue samples. (C) Correlation of NDRG1 levels and PFS (Upper) or OS (Lower) of glioblastoma patients of the UKT-05 trial. (D) NDRG1 expression relative to patient survival in glioblastoma (REMBRANDT). (E) Correlation of NDRG1 levels and PFS of patients with anaplastic gliomas of the NOA-04 trial separated for treatment.
NDRG1 Is an Effector of the mTORC2/SGK1 Pathway.
As opposed to transcriptional regulation of NDRG1 by hypoxia and radiation, the signaling cascade that mediates NDRG1 phosphorylation at the T346 residue in TMZ-resistant glioma cells is unclear but most likely downstream of mTOR. Knockdown of the mTORC2 subunit rapamycin-insensitive companion of mTOR (RICTOR) but not the mTORC1 subunit regulatory associated protein of mTOR (RAPTOR) resulted in a reduction of NDRG1 phosphorylation and expression. Control of NDRG1 phosphorylation by RICTOR is independent of its transcriptional regulation of NDRG1 as knockdown of RICTOR in cells with exogenous NDRG1 expression, resulting in a reduction of NDRG1 phosphorylation and subsequent sensitization toward TMZ (Fig. 3A).
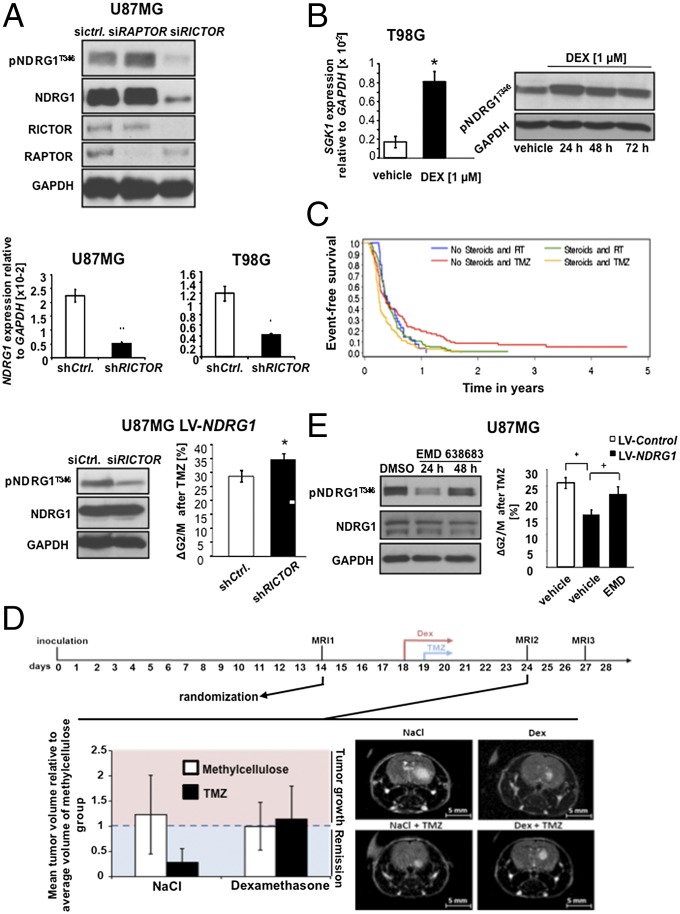
Fig. 3.
mTORC2 is a master regulator of NDRG1. (A) Immunoblot of siRNA-treated U87MG cells targeting RAPTOR or RICTOR (Top). Assessment of NDRG1 phosphorylation at T346 in siRICTOR transfected U87MG_LV-NDRG1 cells (Bottom Left) and ΔG2/M after treatment with TMZ (Bottom Right). NDRG1 mRNA expression 48 h after siRNA-mediated knockdown of RICTOR in U87MG and T98G cells (Middle). (B) SGK1 mRNA expression relative to GAPDH in T98G 72 h after treatment with dexamethasone (DEX; Left) and phosphorylation status of NDRG1 24–72 h after DEX treatment (Right). (C) Progression-free survival of the NOA-08 cohort patients differentiated according to treatment [radiotherapy (RT) versus TMZ] and steroid use. (D) (Upper) Timeline depicting course of animal experiment including dates of MRI measurements and treatment period. (Lower) Tumor volumes on postoperative day 24. The left segment shows a comparison of mean tumor volumes relative to average tumor volumes of methylcellulose group. The right segment shows representative MRI pictures of respective treatment groups. (E) Phosphorylation status of NDRG1 at T346 24 and 48 h after treatment with the SGK1 inhibitor EMD638683 (Left) and TMZ-mediated shift of G2/M-phase in U87MG cells treated with EMD638683 relative to DMSO (vehicle) (Right; *P < 0.05 for the effect of LV-NDRG1, +P < 0.05 for the effect of EMD).
Studies in pancreatic cancer indicated that phosphorylation of NDRG1 at this specific site is mediated by the putative mTOR downstream effector, serum glucocorticoid-induced protein kinase 1 (SGK1) (19). Dexamethasone (DEX), which is an integral part in the treatment of malignant gliomas as a means to control edema, induced SGK1 transcription and increased the phosphorylation of NDRG1 at T346 (Fig. 3B). To test the hypothesis that treatment with DEX blunts the efficacy of alkylating chemotherapy in patients with glioblastoma, we performed a subgroup analysis of the NOA-08 trial. In this trial, elderly patients with malignant astrocytoma received radiotherapy or TMZ until progression and were treated with steroids at clinical discretion to treat or prevent vasogenic cerebral edema (15). Subgroup analyses allowed us to generate the hypothesis that steroid administration was associated with reduced PFS of patients treated with TMZ but not radiotherapy (Fig. 3C and SI Appendix, Table S3). An animal experiment with U87MG cells supports a negative impact of DEX on the efficacy of TMZ (Fig. 3D and SI Appendix, Fig. S6B). Pharmacological inhibition of SGK1 by EMD638683 resulted in decreased phosphorylation of T346 and overcame the NDRG1-mediated protection from TMZ (Fig. 3E). This is specific neither for TMZ nor for glioma cells as EMD638683 treatment also decreased constitutive NDRG1 phosphorylation in pancreatic, breast, colon, and ovarian cancer cells (SI Appendix, Fig. S7A). EMD638683-mediated sensitization toward chemotherapy was specific for alkylating agents as a sensitization was seen for lomustine in breast and ovarian cell lines (SI Appendix, Fig. S7B), but resistance neither to 5-fluorouracil nor to cisplatin was decreased (SI Appendix, Fig. S7 C and D).
NDRG1 Interacts with Three DNA Repair Enzymes and Promotes Protein Stability/Activity of MGMT.
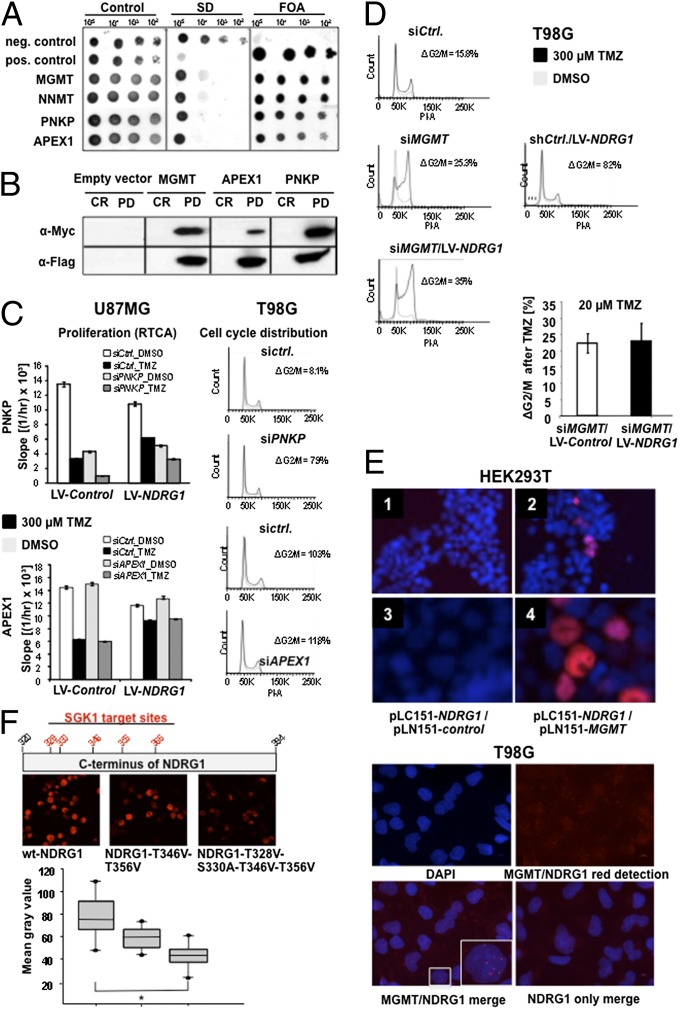
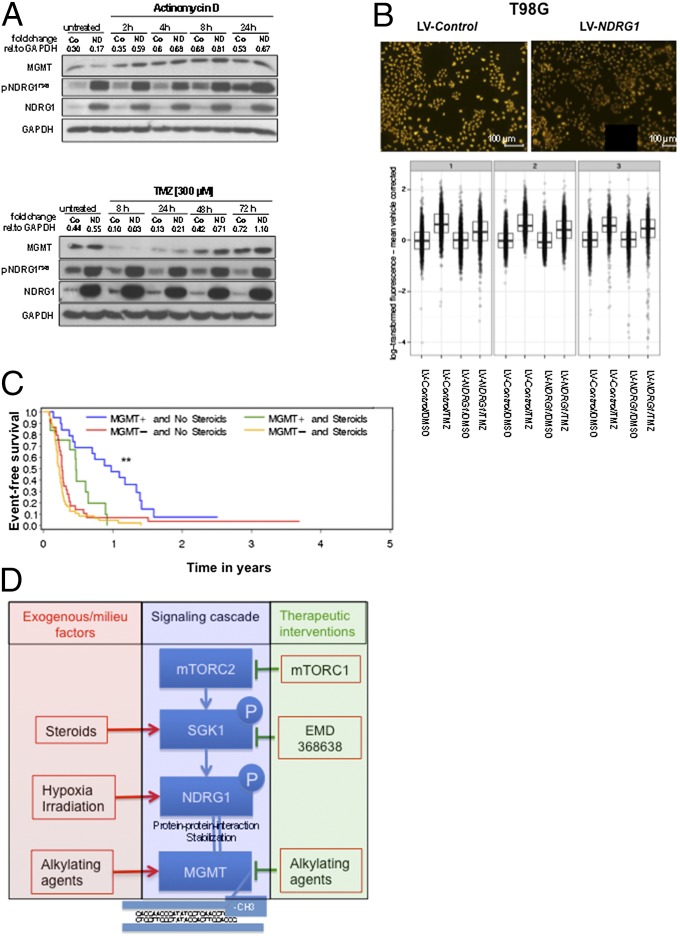
Although described for the mediation of cisplatin resistance in glioma via activation of the mTORC2-mediated cascade, in the present paradigm, nuclear factor (NF)κB is not influenced by the NDRG status (SI Appendix, Fig. S8C). To unravel the molecular mechanisms by which NDRG1 prevents TMZ-induced cytotoxicity, a yeast two-hybrid screen for potential interaction partners was performed. Of 119 possible interaction partners (SI Appendix, Table S4), three proteins were involved in DNA repair: polynucleotide 3′-phosphatase, polynucleotide 5′-hydroxyl-kinase (PNKP), DNA-(apurinic or apyrimidinic site) lyase (APEX1), and MGMT (Fig. 4A). Interaction of these three proteins with NDRG1 was verified in a pull-down assay using HEK293 cells (Fig. 4B). The interaction of MGMT and NDRG1 was studied further because (i) in particular, MGMT has been implicated in mediating the resistance of gliomas to alkylating agents (15, 20); (ii) only MGMT expression correlated with TMZ resistance in gliomas cells; and (iii) knockdown of MGMT but not PNKP or APEX1 rendered glioma cells more susceptible toward the antiproliferative effects of TMZ (Fig. 4 C and D). Bimolecular fluorescence complementation (BiFC) assays confirmed a direct interaction of MGMT and NDRG1 in both HEK293T (Fig. 4E, Upper) and T98G glioma cells (SI Appendix, Fig. S10A) exogenously overexpressing the two proteins. PLAs also revealed this interaction for native T98G cells exposed to hypoxia in the nucleus (Fig. 4E, Lower). This interaction critically depended on SGK1 activity (SI Appendix, Fig. S11) and phospho-threonine or -serine SGK1 target sites in the C terminus of NDRG1 because mutation of T328, 330, 346, and 356 significantly reduced the NDRG1/MGMT interaction in BiFC assays (Fig. 4F). Forced expression of NDRG1 did not render cells resistant to TMZ when MGMT was knocked down, indicating that TMZ resistance mediated by NDRG1 is dependent on MGMT (Fig. 4D). Next, the hypothesis was tested that NDRG1 increases MGMT activity resulting in enhanced repair of DNA damage mediated by alkylating agents. Exogenous expression of NDRG1 resulted in augmented MGMT levels at 8 h (Fig. 5A, Upper) when RNA synthesis was blocked. Exposure of these cells to TMZ resulted in an expected decrease via depletion of MGMT expression at 4 h independent from NDRG1 but a faster recovery of MGMT in NDRG1-overexpressing cells (Fig. 5A, Lower). Indeed, exogenous expression of NDRG1 resulted in enhanced demethylation activity of MGMT in glioma cells exposed to TMZ [−0.25, 95% CI (−0.41 to −0.09), P = 0.0219] (Fig. 5B). To test whether this is relevant for treatment outcome in patients with glioblastoma we again performed post hoc subgroup analyses of the NOA-08 trial. Stratification of the TMZ-treated group of NOA-08 despite all limitations of this approach revealed that steroid administration resulted in a reduced PFS only in patients with a methylated MGMT promoter and hence inactive MGMT (Fig. 5C and SI Appendix, Table S5), suggesting that treatment of patients with DEX by inducing NDRG1 activity compromises the efficacy of alkylating chemotherapy independent of DNA repair activity, although there may be confounding factors triggering a decision for or against DEX treatment. In addition, in patients who failed radiotherapy in this trial and who received salvage chemotherapy with TMZ (SI Appendix, Table S5), MGMT activity was no longer predictive of response to chemotherapy, as opposed to the primary situation (Fig. 5C). Collectively, these data indicate that multiple treatment measures including treatment with steroids and radiotherapy by inducing NDRG1 can impair inherent susceptibility of MGMT-methylated glioma cells to the therapeutic effects of alkylating chemotherapy (Fig. 5D).
Fig. 4.
NDRG1 interacts with the DNA repair proteins MGMT, APEX1, and PNKP and promotes MGMT-mediated protection from TMZ. (A) Split Ubiquitin Screen for interaction partners of NDRG1 (NNMT, Nicotinamide N-methyl transferase). (B) Validation of the interaction of NDRG1 with MGMT, PNKP, and APEX1 via coimmunoprecipitation using HEK293T lysates and Flag-/Myc-tagged constructs of NDRG1, MGMT, PNKP, and APEX1, respectively. (C) Cell cycle and proliferation analysis of siRNA-treated U87MG and T98G cells targeting APEX1 and PNKP. Experiments were performed three times with one representative example shown. (D) Cell cycle analysis after siRNA-mediated knockdown of MGMT in T98G cells in response to 300 µM (regular dose) or 20 µM TMZ. (E) (Upper) BiFC assay with NDRG1. HEK293T cells cotransfected with NDRG1 in pGW-myc-LC151 and bJun in pGW-HA-LN-151 as negative control (1, 3) or with MGMT in pGW-HA-LN-151 (2, 4). The overlay with the DAPI stain shows a clear nuclear localization of the NDRG1 interaction with MGMT (2, 4). (Lower) PLA with NDRG1 and MGMT. Parental T98G cells exposed to 1% O2 for 72 h are analyzed for interaction of NDRG1 and MGMT (n = 3). Relevant controls are depicted in SI Appendix, Fig. S9B. (F) (Top) Schematic overview on the location of SGK1 target residues within the C terminus of the NDRG1 protein. (Middle) BiFC assay with genetically modified versions of NDRG1 resulting in amino acid substitutions. HEK293 cells cotransfected with wt-NDRG1 (Left), T346V-T356V-NDRG1 (Center), or T328V-S330A-T346V-T356V-NDRG1 (Right). (Bottom) Quantification of interaction between MGMT and the three NDRG1 versions depicted as mean fluorescence intensity.
Fig. 5.
NDRG1 stabilizes MGMT. (A) Time-dependent abundance of MGMT, pNDRG1T346, and NDRG1 proteins in T98G cells (Co, control transfected cells; ND, cells lentivirally overexpressing NDRG1) after treatment with actinomycin D (Upper) or TMZ (Lower). (B) Immunofluorescent staining of O6-methylguanine of TMZ-treated T98G LV-Co and LV-NDRG1 cells (Upper) and quantification of relative O6-methylguanine content depicted as log-transformed fluorescence for three independent replications (Lower). (C) Progression-free survival of the NOA-08 cohort patients differentiated according to MGMT promoter methylation status (+, methylated; −, unmethylated) and steroid use in the temozolomide treatment group. (D) Schematic overview of the signaling cascade with iatrogenic and microenvironmental activating factors (left side) and options for therapeutic intervention (right side).
Discussion
Hypoxia-induced resistance (SI Appendix, Fig. S1A) is implicated in treatment resistance not only to radiotherapy but also to chemotherapy (5). We identified NDRG1 as a previously undescribed clinically relevant resistance factor induced by both hypoxia and iatrogenic stimuli such as irradiation and corticosteroids. In line with this, we found NDRG1 expression in gliomas, which increased with malignancy (Fig. 1C), in untreated tumors mostly restricted to the perinecrotic, hypoxic areas (Fig. 1C), whereas in the recurrent tumors, subjected to treatment with irradiation, chemotherapy, and steroids, NDRG1 was not only further up-regulated but also expressed in the tumor bulk and in perivascular regions (Fig. 2 A and B and SI Appendix, Fig. S2A), although some induction of NDRG1 with progression is also observed without genotoxic treatment (SI Appendix, Fig. S2B). These data do not support the previously proposed proapoptotic function of NDRG1 in gliomas (16) but rather hint that NDRG1 can play a pivotal role in therapy resistance.
Several analyses of tumor tissue specimens from clinical studies of malignant gliomas support the notion that NDRG1 is induced by radiochemotherapy with TMZ (Fig. 2A) and, more importantly, renders gliomas insensitive to chemotherapy with alkylating chemotherapy (Fig. 2 C–E and SI Appendix, Table S2). In vitro studies did not suggest a propensity of alkylating chemotherapy to induce NDRG1 expression (SI Appendix, Fig. S5D), whereas irradiation appeared to be a strong inducer of NDRG1 (SI Appendix, Fig. S5A). Another explanation for the increased expression of NDRG1 posttherapy might be the cytoprotective effect of NDRG1 providing an advantage for NDRG1-expressing cells during treatment with TMZ (Fig. 1 D–F) but not radiotherapy (SI Appendix, Fig. S3C) or induction with the tumor progression (SI Appendix, Fig. S2B).
An alternative mechanism of NDRG1 activation is phosphorylation at T346, which is increased in TMZ-resistant cell lines (SI Appendix, Fig. S8C). Phosphorylation of NDRG1 at T346 is triggered by SGK1 (19), an mTORC2 target. Tanaka et al. recently demonstrated that EGFRvIII-activated mTORC2 is relevant in the mediation of resistance toward cisplatin in glioblastoma. They used pNDRG1 as a marker for pathway activity (21). We demonstrate that mTORC2 regulates NDRG1 not only on a posttranslational level through SGK1 but also transcriptionally (Fig. 3A, Middle). In addition to mTORC1, which increases HIF-1α levels in normoxic conditions by stimulating the cap-dependent translation from the 5′-untranslated region of the HIF-1α mRNA, mTORC2 has been implicated in the regulation of HIF (22).
Pharmacological inhibition of the mTORC2 target SGK1 by EMD638683 overcame the NDRG1-mediated protection from TMZ (Fig. 3D). SGK1 has been shown to promote cell survival and cell cycle progression in a multitude of human tumors. Because the SGK1 promoter contains a glucocorticoid response element and SGK1 is well-known to be inducible by dexamethasone treatment (23), we were not surprised to find SGK1 induced by dexamethasone in glioblastoma cells as well. This transcriptional activation of SGK1 was accompanied by an increased phosphorylation of NDRG1 at T346 (Fig. 3B). In TMZ-treated patients with intratumoral MGMT promoter methylation from the NOA-08 cohort, cotreatment with steroids halved the PFS compared with TMZ treatment without steroid administration (Fig. 5C and SI Appendix, Table S5). One alternative contributing factor is that larger tumors, which may require higher steroid doses, are more difficult to control. Further, patients with inactive MGMT would also suffer most from blood–brain barrier normalizing effects of corticosteroids.
Our interpretation of the data led us to propose an interaction of NDRG1 with factors involved in the execution or prevention of DNA damage. In our yeast two-hybrid screen, we see a protein interaction of NDRG1 with the DNA repair enzymes APEX1, PNKP, and MGMT (2) (Fig. 4 A and B). In line with the observation that only the expression of MGMT correlated with TMZ resistance (SI Appendix, Fig. S8A), the interaction of NDRG1 with MGMT, but not with PNKP or APEX1, proved to be of functional relevance for the resistance phenotype in malignant glioma (Fig. 4 C and D). Considering the observed augmentation of MGMT levels under stress conditions in the presence of high NDRG1 levels (Fig. 5A) and the colocalization at subcellular levels (Fig. 4E), it is conceivable that NDRG1 stabilizes MGMT via a direct protein–protein interaction, thus fulfilling a chaperone-like function. Nevertheless, MGMT alone cannot account for the observed NDRG1-dependent resistance phenotype because MGMT-negative U87MG cells also become more resistant upon an elevated NDRG1 expression level (Fig. 1D). Patients with intratumoral methylation of the MGMT promoter and thus putatively no MGMT expression become more resistant in response to steroid treatment (SI Appendix, Table S4, and Fig. 5C). These data suggest that there may be additional mechanisms involved in the NDRG1-provoked resistance to alkylating chemotherapy in gliomas.
In conclusion, we identified NDRG1 as a unique hypoxia-, steroid-, and mTORC2/SGK1-regulated molecule in glioma that may be developed as a predictive biomarker for response to treatment with TMZ in high-grade gliomas. Its TMZ-protective effect makes NDRG1 an attractive candidate for targeted therapy not only in gliomas but also in a variety of other cancer types, potentially via inhibition of SGK1. The preclinical data suggest multiple levels of cell-intrinsic (mTORC1), microenvironmental (hypoxia), and iatrogenic (radiotherapy, dexamethasone) influences on this critical signaling pathway downstream of several growth factor receptors. The mTORC2/SGK1/NDRG1 pathway may serve as a target for future preclinical and clinical research on therapy resistance (Fig. 5D).
Supplementary Material
Acknowledgments
We thank Torsten Schmenger, Mona Friedrich, BSc, and Hans-Werner Pledl for assistance with some of the experiments. The HIF-knockdown cell lines were kindly provided by Prof. Dr. Till Acker (Justus Liebig University Giessen), and EMD638683 was provided by Dr. Thomas Fuchs (Merck KGaA, Merck Serono). Support by the German Cancer Research Center (DKFZ) Light Microscopy Facility is gratefully acknowledged. This work was supported within the Brain Tumor Network (BTN)plus, Subproject 6 by the Bundesminesterium für Forschung und Technologie, the Förderverein für Gehirntumorforschung Karlsruhe e.V., the Charitable Hertie Foundation, and the Deutsche Forschungsgemeinschaft [Sonderforschungsbereich (SFB) 773 and SFB 938 TP K]. J.B. is a doctoral student in the PhD Program of the DKFZ.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1314469111/-/DCSupplemental.
Change History
September 7, 2021: The SI Appendix has been updated to coincide with a formal Correction.
References
- 1.Sarkaria JN, et al. Mechanisms of chemoresistance to alkylating agents in malignant glioma. Clin Cancer Res. 2008;14(10):2900–2908. doi: 10.1158/1078-0432.CCR-07-1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dunn GP, et al. Emerging insights into the molecular and cellular basis of glioblastoma. Genes Dev. 2012;26(8):756–784. doi: 10.1101/gad.187922.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amberger-Murphy V. Hypoxia helps glioma to fight therapy. Curr Cancer Drug Targets. 2009;9(3):381–390. doi: 10.2174/156800909788166637. [DOI] [PubMed] [Google Scholar]
- 4.Harris AL. Hypoxia—A key regulatory factor in tumour growth. Nat Rev Cancer. 2002;2(1):38–47. doi: 10.1038/nrc704. [DOI] [PubMed] [Google Scholar]
- 5.Winkler F, et al. Kinetics of vascular normalization by VEGFR2 blockade governs brain tumor response to radiation: Role of oxygenation, angiopoietin-1, and matrix metalloproteinases. Cancer Cell. 2004;6(6):553–563. doi: 10.1016/j.ccr.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 6.Wick A, et al. Hypoxic neuroprotection requires sequential activation of vascular endothelial growth factor receptor and Akt. J Neurosci. 2002;22:6401–6407. doi: 10.1523/JNEUROSCI.22-15-06401.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Henze AT, et al. Prolyl hydroxylases 2 and 3 act in gliomas as protective negative feedback regulators of hypoxia-inducible factors. Cancer Res. 2010;70(1):357–366. doi: 10.1158/0008-5472.CAN-09-1876. [DOI] [PubMed] [Google Scholar]
- 8.Salnikow K, Blagosklonny MV, Ryan H, Johnson R, Costa M. Carcinogenic nickel induces genes involved with hypoxic stress. Cancer Res. 2000;60(1):38–41. [PubMed] [Google Scholar]
- 9.Melotte V, et al. The N-myc downstream regulated gene (NDRG) family: diverse functions, multiple applications. FASEB J. 2010;24(11):4153–4166. doi: 10.1096/fj.09-151464. [DOI] [PubMed] [Google Scholar]
- 10.Sun B, et al. Decreased expression of NDRG1 in glioma is related to tumor progression and survival of patients. J Neurooncol. 2009;94(2):213–219. doi: 10.1007/s11060-009-9859-7. [DOI] [PubMed] [Google Scholar]
- 11.Brummelkamp TR, Bernards R, Agami R. Stable suppression of tumorigenicity by virus-mediated RNA interference. Cancer Cell. 2002;2(3):243–247. doi: 10.1016/s1535-6108(02)00122-8. [DOI] [PubMed] [Google Scholar]
- 12.Weiler M, et al. Suppression of proinvasive RGS4 by mTOR inhibition optimizes glioma treatment. Oncogene. 2013;32(9):1099–1109. doi: 10.1038/onc.2012.137. [DOI] [PubMed] [Google Scholar]
- 13.Weiler M, et al. Phase II trial of radiochemotherapy with daily concomitant and adjuvant intensified (one week on / one week off) TMZ plus indomethacin in newly diagnosed glioblastoma: UKT-05. Int J Rad Biol Phys. 2010;77(3):670–676. doi: 10.1016/j.ijrobp.2009.05.031. [DOI] [PubMed] [Google Scholar]
- 14.Wick W, et al. NOA-04 randomized phase III trial of sequential radiochemotherapy of anaplastic glioma with procarbazine, lomustine, and vincristine or temozolomide. J Clin Oncol. 2009;27(35):5874–5880. doi: 10.1200/JCO.2009.23.6497. [DOI] [PubMed] [Google Scholar]
- 15.Wick W, et al. NOA-08 Study Group of Neuro-oncology Working Group (NOA) of German Cancer Society Temozolomide chemotherapy alone versus radiotherapy alone for malignant astrocytoma in the elderly: The NOA-08 randomised, phase 3 trial. Lancet Oncol. 2012;13(7):707–715. doi: 10.1016/S1470-2045(12)70164-X. [DOI] [PubMed] [Google Scholar]
- 16.Zhang P, Tchou-Wong KM, Costa M. Egr-1 mediates hypoxia-inducible transcription of the NDRG1 gene through an overlapping Egr-1/Sp1 binding site in the promoter. Cancer Res. 2007;67(19):9125–9133. doi: 10.1158/0008-5472.CAN-07-1525. [DOI] [PubMed] [Google Scholar]
- 17.Azuma K, et al. NDRG1/Cap43/Drg-1 may predict tumor angiogenesis and poor outcome in patients with lung cancer. J Thorac Oncol. 2012;7(5):779–789. doi: 10.1097/JTO.0b013e31824c92b4. [DOI] [PubMed] [Google Scholar]
- 18.García-Martínez JM, Alessi DR. mTOR complex 2 (mTORC2) controls hydrophobic motif phosphorylation and activation of serum- and glucocorticoid-induced protein kinase 1 (SGK1) Biochem J. 2008;416(3):375–385. doi: 10.1042/BJ20081668. [DOI] [PubMed] [Google Scholar]
- 19.Murakami Y, et al. Identification of sites subjected to serine/threonine phosphorylation by SGK1 affecting N-myc downstream-regulated gene 1 (NDRG1)/Cap43-dependent suppression of angiogenic CXC chemokine expression in human pancreatic cancer cells. Biochem Biophys Res Commun. 2010;396(2):376–381. doi: 10.1016/j.bbrc.2010.04.100. [DOI] [PubMed] [Google Scholar]
- 20.Hegi ME, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352(10):997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- 21.Tanaka K, et al. Oncogenic EGFR signaling activates an mTORC2-NF-κB pathway that promotes chemotherapy resistance. Cancer Discov. 2011;1(6):524–538. doi: 10.1158/2159-8290.CD-11-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Toschi A, Lee E, Gadir N, Ohh M, Foster DA. Differential dependence of hypoxia-inducible factors 1 alpha and 2 alpha on mTORC1 and mTORC2. J Biol Chem. 2008;283(50):34495–34499. doi: 10.1074/jbc.C800170200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mikosz CA, Brickley DR, Sharkey MS, Moran TW, Conzen SD. Glucocorticoid receptor-mediated protection from apoptosis is associated with induction of the serine/threonine survival kinase gene, sgk-1. J Biol Chem. 2001;276(20):16649–16654. doi: 10.1074/jbc.M010842200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.