Abstract
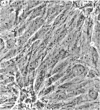
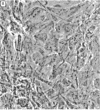
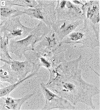
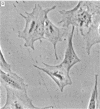
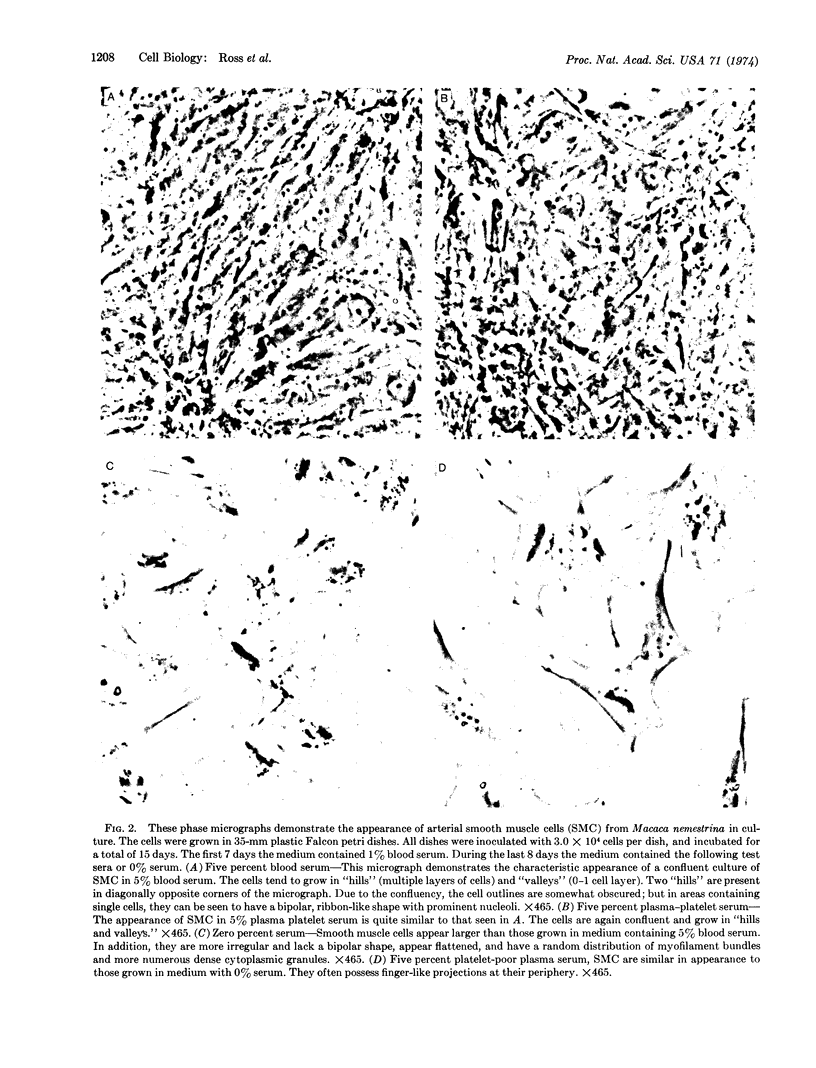
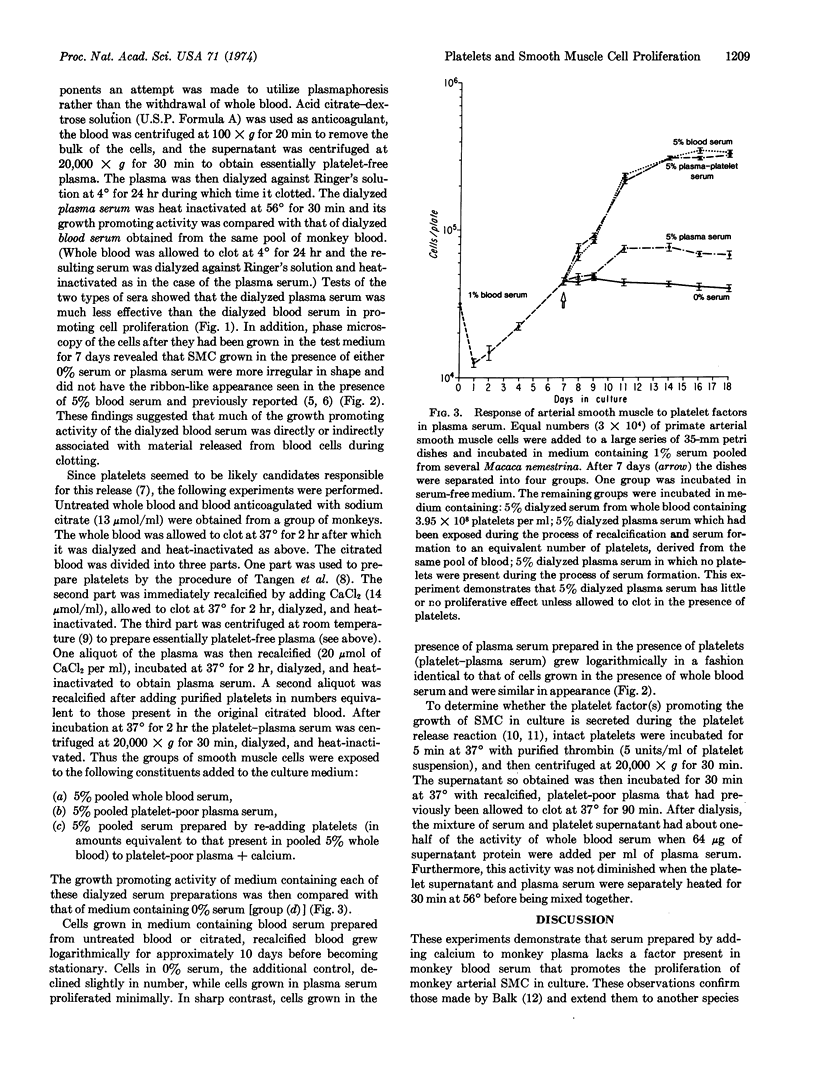
Dialyzed serum from clotted monkey blood (“blood serum”) promotes the proliferation of monkey arterial smooth muscle cells in culture, but dialyzed serum prepared from recalcified platelet-poor plasma (“plasma serum”) is much less effective. Addition of platelets and calcium to platelet-poor plasma increases the activity of plasma serum to the same level achieved with blood serum. Furthermore, addition to plasma serum of a platelet-free supernatant prepared by exposing purified platelets to thrombin also stimulates the proliferation of smooth muscle cells. Thus, much of the growth-promoting activity of dialyzed serum is directly or indirectly derived from platelets. This finding has important implications for the response of arteries to localized injury and provides a key to further understanding of the role of factors derived from blood serum in promoting cell proliferation in vitro.
Keywords: primate, cell culture, atherosclerosis
Full text
PDF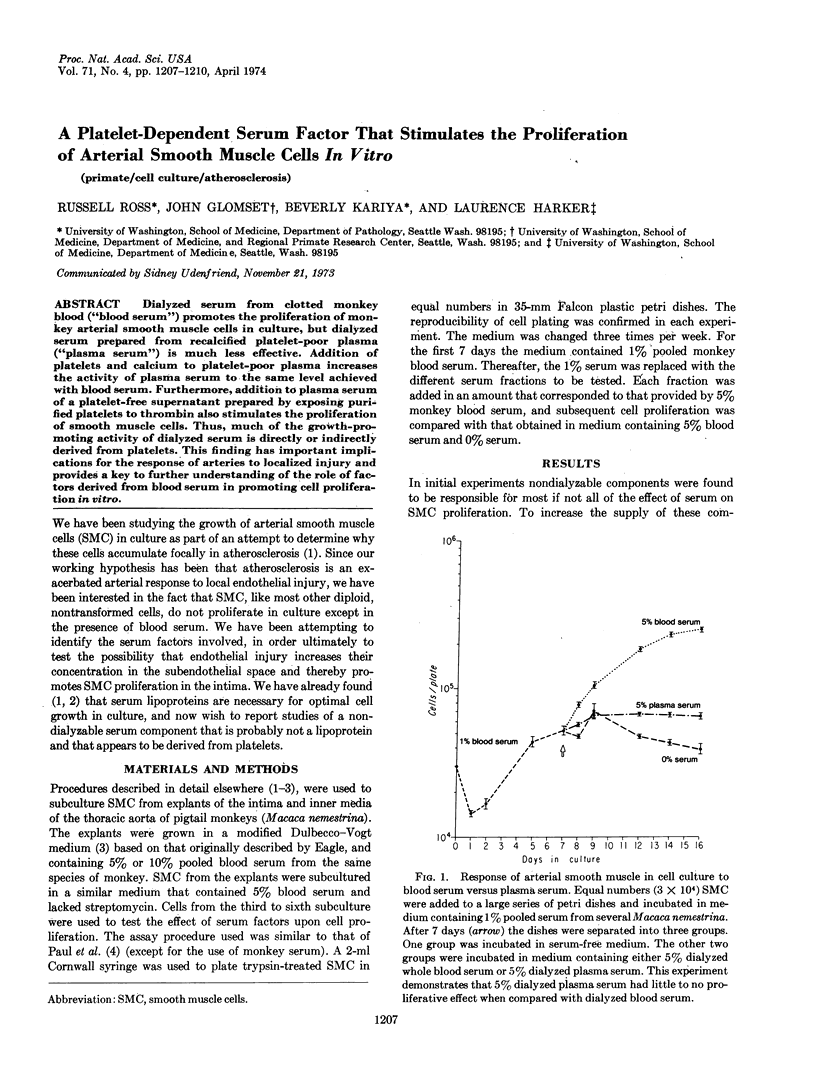

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balk S. D. Calcium as a regulator of the proliferation of normal, but not of transformed, chicken fibroblasts in a plasma-containing medium. Proc Natl Acad Sci U S A. 1971 Feb;68(2):271–275. doi: 10.1073/pnas.68.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamley J. H., Campbell G. R., Burnstock G. An analysis of the interactions between sympathetic nerve fibers and smooth muscle cells in tissue culture. Dev Biol. 1973 Aug;33(2):344–361. doi: 10.1016/0012-1606(73)90142-5. [DOI] [PubMed] [Google Scholar]
- Day H. J., Ang G. A., Holmsen H. Platelet release reaction during clotting of native human platelet-rich plasma. Proc Soc Exp Biol Med. 1972 Mar;139(3):717–721. doi: 10.3181/00379727-139-36223. [DOI] [PubMed] [Google Scholar]
- Gerschenson L. E., Okigaki T., Andersson M., Molson J., Davidson M. B. Fine structural and growth characteristics of cultured rat liver cells. Insulin effects. Exp Cell Res. 1972 Mar;71(1):49–58. doi: 10.1016/0014-4827(72)90262-5. [DOI] [PubMed] [Google Scholar]
- Holmsen H., Day H. J. The selectivity of the thrombin-induced platelet release reaction: subcellular localization of released and retained constituents. J Lab Clin Med. 1970 May;75(5):840–855. [PubMed] [Google Scholar]
- Jorgensen L., Packham M. A., Rowsell H. C., Mustard J. F. Deposition of formed elements of blood on the intima and signs of intimal injury in the aorta of rabbit, pig, and man. Lab Invest. 1972 Sep;27(3):341–350. [PubMed] [Google Scholar]
- LIEBERMAN I., OVE P. Growth factors for mammalian cells in culture. J Biol Chem. 1959 Oct;234:2754–2758. [PubMed] [Google Scholar]
- Mustard J. F., Packham M. A. Factors influencing platelet function: adhesion, release, and aggregation. Pharmacol Rev. 1970 Jun;22(2):97–187. [PubMed] [Google Scholar]
- Mustard J. F., Perry D. W., Ardlie N. G., Packham M. A. Preparation of suspensions of washed platelets from humans. Br J Haematol. 1972 Feb;22(2):193–204. doi: 10.1111/j.1365-2141.1972.tb08800.x. [DOI] [PubMed] [Google Scholar]
- Paul D., Lipton A., Klinger I. Serum factor requirements of normal and simian virus 40-transformed 3T3 mouse fibroplasts. Proc Natl Acad Sci U S A. 1971 Mar;68(3):645–652. doi: 10.1073/pnas.68.3.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierson R. W., Jr, Temin H. M. The partial purification from calf serum of a fraction with multiplication-stimulating activity for chicken fibroblasts in cell culture and with non-suppressible insulin-like activity. J Cell Physiol. 1972 Jun;79(3):319–330. doi: 10.1002/jcp.1040790302. [DOI] [PubMed] [Google Scholar]
- Ross R., Glomset J. A. Atherosclerosis and the arterial smooth muscle cell: Proliferation of smooth muscle is a key event in the genesis of the lesions of atherosclerosis. Science. 1973 Jun 29;180(4093):1332–1339. doi: 10.1126/science.180.4093.1332. [DOI] [PubMed] [Google Scholar]
- Ross R. The smooth muscle cell. II. Growth of smooth muscle in culture and formation of elastic fibers. J Cell Biol. 1971 Jul;50(1):172–186. doi: 10.1083/jcb.50.1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stemerman M. B., Ross R. Experimental arteriosclerosis. I. Fibrous plaque formation in primates, an electron microscope study. J Exp Med. 1972 Oct 1;136(4):769–789. doi: 10.1084/jem.136.4.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tangen O., Berman H. J., Marfey P. Gel filtration. A new technique for separation of blood platelets from plasma. Thromb Diath Haemorrh. 1971 Jun 30;25(2):268–278. [PubMed] [Google Scholar]