Summary
Nuclear sensing of viral DNA has emerged as an essential step in innate immune responses against herpesviruses. Here, we provide mechanistic insight into host recognition of human cytomegalovirus (HCMV) and subsequent immune evasion by this prominent DNA virus. We establish that the interferon inducible protein IFI16 acts as a nuclear DNA sensor following HCMV infection, binding viral DNA and triggering expression of antiviral cytokines via the STING-TBK1-IRF3 signaling pathway. The HCMV tegument protein pUL83 inhibits this response by interacting with the IFI16 pyrin domain, blocking its oligomerization upon DNA sensing and subsequent immune signals. pUL83 disrupts IFI16 by concerted action of its N- and C-terminal domains, in which an evolutionarily conserved N-terminal Pyrin Association Domain (PAD) binds IFI16. Additionally, phosphorylation of the N-terminal domain modulates pUL83-mediated inhibition of pyrin aggregation. Collectively, our data elucidate the interplay between host DNA sensing and HCMV immune evasion, providing targets for restoring antiviral immunity.
Introduction
An essential step to trigger host innate immune responses against viral infections is sensing of intracellular viral nucleic acids. It is widely accepted that sensing of viral DNAs by cellular sensor proteins occurs in the cytoplasm. However, recent findings from several laboratories, including ours, have established the concept of nuclear sensing, as nuclear viral DNAs can be recognized by a DNA sensor—the interferon inducible protein IFI16 (Kerur et al., 2011; Li et al., 2012; Orzalli et al., 2012). Nuclear sensing was shown to be critical for eliciting interferon response (Li et al., 2012; Orzalli et al., 2012) and inflammatory response (Ansari et al., 2013; Kerur et al., 2011; Singh et al., 2013) following herpesvirus infection.
During co-evolution with their hosts, viruses have acquired effective mechanisms for blocking host immune signaling. Defining these mechanisms is essential for understanding virus pathogenesis and developing new therapeutics. The human cytomegalovirus (HCMV), a β herpesvirus, causes global epidemics, complications in AIDS patients and organ transplant recipients, and is a major cause of birth defects (Cheeran et al., 2009). While nucleoside-analogues are used to treat HCMV infections, the emergence of drug-resistant strains underscores the demand for novel antiviral strategies (Jabs et al., 1998).
The HCMV major tegument protein pUL83 (a.k.a. phosphoprotein pp65) is thought to play critical roles in immune evasion. Present at >2,000 copies per mature virion, pUL83 is the most abundant virion component (Varnum et al., 2004). It is deposited into infected cells immediately after entry, thereby quickly subverting both adaptive and innate immunity. pUL83 was shown to block antigen presentation (Gilbert et al., 1996), activation of natural killer cells (Arnon et al., 2005), and suppress the induction of antiviral cytokines (Abate et al., 2004; Browne and Shenk, 2003). Consistently, guinea pig CMV lacking the UL83 homologue was attenuated in viral dissemination and pathology (McGregor et al., 2004). However, the mechanisms underlying pUL83-mediated innate immune evasion remain elusive. pUL83 may target antiviral host proteins, yet their identities or downstream signaling pathways remain unknown. Using a proteomic approach during HCMV infection, we reported that nuclear pUL83 interacts with two interferon-inducible proteins from the PYHIN family—IFI16 and IFIX (Cristea et al., 2010). The pUL83-IFI16 interaction is required for activating viral gene transcription at the major immediate early promoter (MIEP). However, it is unclear if this interaction is also connected to the pUL83-dependent immune evasion.
Recent reports showed that IFI16 can detect viral DNA in both the cytoplasm and nucleus (Horan et al., 2013; Johnson et al., 2013; Kerur et al., 2011; Li et al., 2012; Orzalli et al., 2012; Singh et al., 2013; Unterholzner et al., 2010) and act as a virus-restricting factor (Gariano et al., 2012). The sensing ability of IFI16, which is influenced by its subcellular distribution, is modulated by acetylations within its nuclear localization signal (Li et al., 2012). As herpesviruses DNA is protected by viral capsids in the cytoplasm prior to nuclear exposure, nuclear sensing is a critical aspect of defense against these nuclear replicating viruses. In endothelial cells, nuclear IFI16 can assemble inflammasomes during infection with Kaposi sarcoma-associated herpes virus (KSHV), leading to secretion of pro-inflammatory interleukins (Kerur et al., 2011). In U2OS cells and fibroblasts, nuclear IFI16 senses herpes simplex virus-1 (HSV-1) DNA, triggering Type I interferon (IFN) response (Li et al., 2012; Orzalli et al., 2012) and inflammasome formation (Johnson et al., 2013). In macrophages, where capsids may be vulnerable to degradation, leaked HSV-1 DNA can be detected by cytosolic IFI16 (Horan et al., 2013). Cytosolic IFI16 also detects transfected DNA, inducing Type I IFN in a STING-dependent manner (Unterholzner et al., 2010).
While these results highlight IFI16 as a critical antiviral factor during herpesvirus infection, our finding that pUL83 targets IFI16 raises the question whether IFI16 also acts as a DNA sensor during HCMV infection. Furthermore, the mechanisms involved in HCMV immune evasion remain to be further elucidated. Here, we aim to define the roles of IFI16 during HCMV infection and the mechanisms utilized by pUL83 for suppressing innate immunity. First, we establish that IFI16 and pUL83 play opposing roles in regulating HCMV-induced cytokines. Next, we identify conserved domains that directly mediate the pUL83-IFI16 interaction. We demonstrate that pUL83 sequesters the IFI16 pyrin domain by concerted action of its N- and C-termini, blocking nuclear IFI16 oligomerization. We determine that pUL83 has similar inhibitory effects on all nuclear PYHIN proteins (i.e., IFI16, IFIX and MNDA). Finally, we identify phosphorylation sites on pUL83, and show that site-specific phosphorylation by host kinases compromises pUL83-mediated pyrin interference. Altogether, this work defines a previously unrecognized mechanism underlying HCMV immune evasion, identifying a critical molecular hub that determines the outcome of host defense and viral pathogenesis.
Results
The host sensor IFI16 and viral protein pUL83 act in opposition to regulate host innate immune responses during HCMV infection
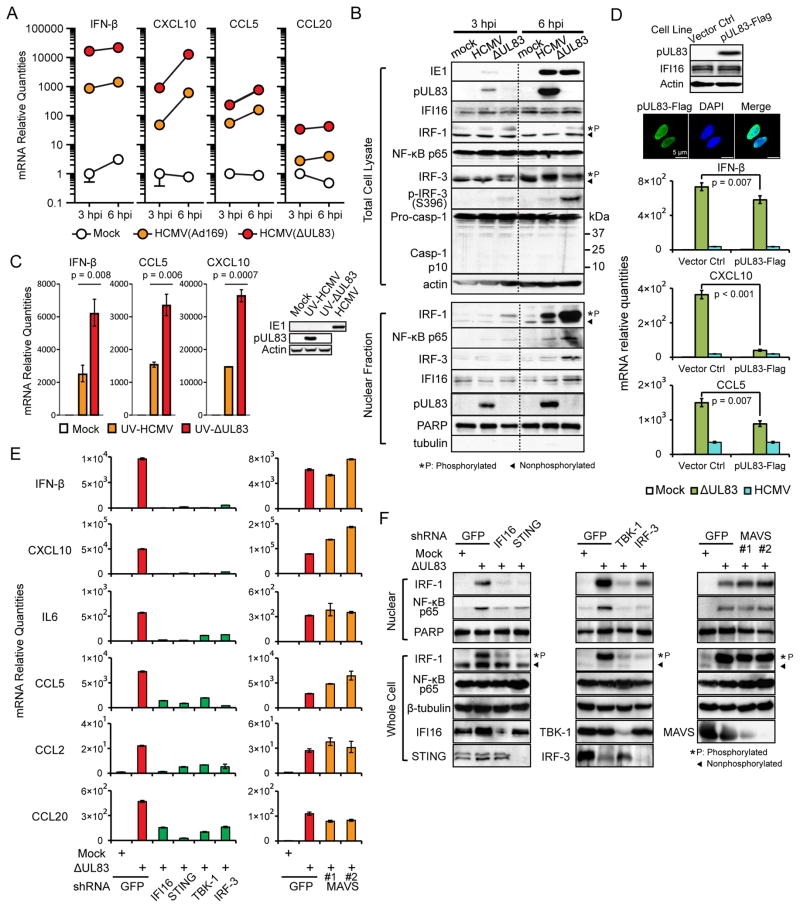
To confirm the immunosuppressive function of pUL83, we compared innate immune responses in human foreskin fibroblasts (HFF) infected with either wild type (WT, Ad169) or ΔUL83 mutant HCMV at a multiplicity of infection (moi) of 5 (hereinafter), which was selected to facilitate induction of immune response and comparison with other studies on pUL83 (Abate et al., 2004; Browne and Shenk, 2003). Antiviral responses were monitored at early stages of infection, 3 and 6 hours post infection (hpi), by measuring: 1) the expressions of antiviral cytokines, IFN-β, CXCL10, CCL5 and CCL20 by RT-qPCR, and 2) the nuclear levels of transcription factors (NF-κB, IRF-1 and IRF-3) by Western blot. The ΔUL83 strain induced antiviral cytokines ~10-fold higher than the WT strain (Figure 1A). This correlated with increased nuclear translocation of IRF-1 and NF-κB, and with phosphorylations of IRF-1 and IRF-3—markers of active immune signaling (Figures 1B and S1A). Notably, the ΔUL83 strain did not induce a higher inflammasome activity compared to the WT strain, reflected by caspase-1 cleavage (Figures 1B and S1B). As pUL83 is also required for optimal expression of immediate early (IE) proteins (Cristea et al., 2010), of which IE2 can block cytokine expression (Taylor and Bresnahan, 2005, 2006), we next assessed the independent role of pUL83 in immune evasion. WT and ΔUL83 HCMV were UV-irradiated to inactivate viral gene expressions (Figure 1C). Following infections of HFFs, the UV-treated ΔUL83 strain induced two-three-fold higher levels of antiviral cytokines (IFN-β, CXCL10 and CCL5) at 6 hpi than UV-treated WT HCMV (Figure 1C). Therefore, pUL83 has cytokine-blocking function that is independent of IE proteins. To test if pUL83 alone can counteract antiviral response, we stably expressed pUL83-Flag or vector control in HFFs, and infected the cell lines with the ΔUL83 strain (Figure 1D). Overexpressed pUL83 reduced the expressions of antiviral cytokines (IFN-β, CXCL10, CCL5, Figure 1D and IL-6, Figure S1C) at 6 hpi, confirming a role for pUL83 that may be complemented by other viral factors during infection. Together, these results support that pUL83 is critical for dampening innate antiviral response during HCMV infection.
Figure 1. pUL83 antagonizes IFI16-mediated antiviral response.
(A) mRNA levels of cytokines in HFFs infected with WT or ΔUL83 HCMV or mock-infected, at 3 or 6 hpi (moi = 5). Data shown as mean +/− SEM, n = 3.
(B) Protein levels of IRF-1, IRF-3, NF-κB p65, and other markers were measured by Western blot in total cell lysates and nuclear fractions of HFFs infected as in (A).
(C) mRNA levels of cytokines in HFFs infected with UV-treated WT or ΔUL83 HCMV, at 6 hpi (moi = 5). Western blots show IE-1, pUL83 and loading control (actin) protein levels.
(D) HFFs stably expressing pUL83-Flag or control were infected with mock or ΔUL83 strain. Western blots show protein levels for pUL83 and IFI16. IF images show nuclear localization of pUL83-Flag. mRNA levels of cytokines measured by qPCR as in (A).
(E) Endogenous IFI16, STING, TBK-1, IRF-3 and MAVS were stably knocked down in HFFs by shRNA. mRNA levels of cytokines in response to mock or ΔUL83 strain were measured as in (A).
(F) Protein levels for IRF-1, NF-κB p65, and other markers in nuclear fraction or total lysates were measured by Western blot.
See also Figure S1.
As we previously observed that pUL83 associates with IFI16 during infection (Cristea et al., 2010), we hypothesized that this interaction underlies its immune evasive functions, and that IFI16 acts as a DNA sensor during HCMV infection. This would predict that IFI16 is required for cytokine induction in the absence of pUL83. Indeed, shRNA knockdown of endogenous IFI16 and infection with the ΔUL83 strain significantly reduced cytokine expression (IFN-β, CXCL10, IL-6, CCL5, CCL2 and CCL20) and impeded nuclear translocations of IRF-1 and NF-κB when compared to control knockdown (Figure 1E–F). We next studied the downstream signaling pathway through which IFI16 may regulate this immune response. Components of the DNA sensing pathway, STING, TBK-1 and IRF3, shown to be important during HSV-1 infection (Orzalli et al., 2012; Unterholzner et al., 2010), were individually silenced by shRNA, and the cell lines were infected with the ΔUL83 strain. As a result, we observed significantly compromised induction of antiviral cytokines and reduced nuclear translocation of IRF-1 and NF-κB (Figure 1E and F). In contrast, silencing MAVS, a component of RNA sensing pathways (Seth et al., 2005), did not adversely affect antiviral response, supporting the specific engagement of DNA sensing. These results demonstrate that IFI16 and the STING-TBK1-IRF3 DNA sensing pathway are critical for eliciting antiviral response against HCMV infection in the absence of pUL83.
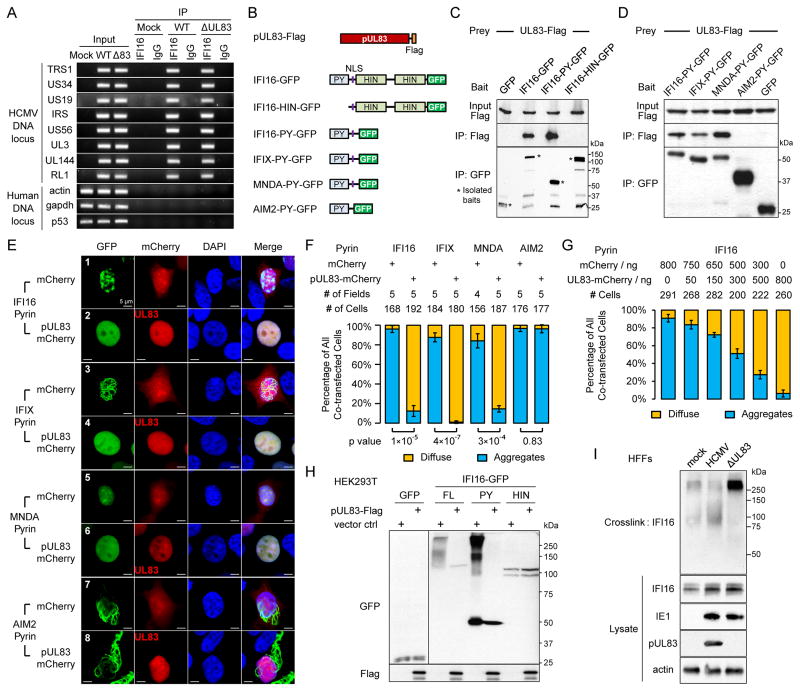
Next, we tested if IFI16 can recognize HCMV DNA during infection. ChIP assays at 6 hpi showed that endogenous IFI16 specifically recognized HCMV DNA at numerous loci, but not host chromosomal DNA (Figure 2A). This is consistent with our report that nuclear IFI16 binds HCMV MIEP in infected HFFs (Cristea et al., 2010). Altogether, our results establish IFI16 as a DNA sensor during HCMV infection, binding viral DNA and triggering expression of antiviral cytokines. The opposing roles of pUL83 and IFI16, in conjunction with their physical interaction, indicate that pUL83 may act as a critical viral factor to inhibit IFI16-mediated DNA sensing and antiviral response. In contrast to HSV-1 (Orzalli et al., 2012), HCMV infection did not reduce the level of IFI16 (Figure 1B), suggesting a distinct mode of inhibition.
Figure 2. pUL83 blocks nuclear pyrin aggregation.
(A) ChIP assays test the association of endogenous IFI16 with HCMV DNA and host DNA in infected HFFs.
(B) Constructs of pUL83, IFI16 and other PYHIN proteins.
(C) Co-IP assays of pUL83-Flag interaction with IFI16 constructs.
(D) Co-IP assays of pUL83 interaction with pyrin domains of PYHIN proteins.
(E) 293T cells were transfected with plasmids (1:5) of Pyrin-GFP and mCherry or pUL83-mCherry. Direct fluorescent images illustrate PY-GFPs.
(F) As in (E), cells from random microscopy fields were analyzed for PY-GFP distributions. Bars: percentages of diffuse (yellow) or aggregation (blue) patterns as mean +/− SEM.
(G) As in (E), bars show IFI16-PY-GFP distributions as a function of the pUL83-mCherry/mCherry ratio.
(H) HEK293T cells transiently transfected with GFP alone or GFP fusions with full-length (FL), PY or HIN domains of IFI16, in the presence or absence of exogenous pUL83. Cell lysates were cross-linked and the oligomerization status was assessed by Western blotting with an anti-GFP antibody.
(I) Western blot shows migration of endogenous IFI16 in infected HFFs cross-linked with glutaraldehyde. Protein levels in total lysates prior to crosslinking are shown.
pUL83 targets the pyrin domain of IFI16 and other nuclear PYHIN proteins
To explore how pUL83 inhibits IFI16 function, we mapped its binding site within IFI16. We constructed GFP fusions with full-length (FL) IFI16, the pyrin (PY) or the HIN domains, and performed co-IP experiments with pUL83-Flag in HEK 293T cells (Figure 2B). pUL83 only interacted with the FL or PY domain, but not with the HIN domains or GFP alone (Figure 2C). Therefore, the PY domain is both necessary and sufficient for pUL83 binding, and the DNA-binding HIN domains are not targeted. Indeed, ChIP assays showed that endogenous IFI16 bound HCMV DNA independent of pUL83 (Figure 2A). In conclusion, pUL83 specifically targets the PY domain of IFI16.
As pyrin domains are also present within other PYHIN proteins—IFIX, MNDA and AIM2, similar PY-GFP constructs were tested for pUL83 interaction. Interestingly, pUL83 bound the PY of all nuclear PYHIN proteins (IFI16, IFIX and MNDA), but not AIM2-PY (Figure 2D). Sequence alignment showed that nuclear PY domains are distantly related to AIM2-PY (Figure S2A). Co-IP assays with inflammasome adaptor ASC showed a similar division among PY domains, as only AIM2-PY binds ASC (Figure S2B), consistent with a previous report (Hornung et al., 2009). Therefore, the nuclear PY domains form a distinct subgroup from AIM2-PY. Together, these data indicate that the pUL83-PY interaction is a general mean for HCMV to target nuclear PYHIN proteins.
pUL83 blocks the ability of pyrin domains to form nuclear oligomers
Strikingly, the pyrin domains assembled into distinct aggregation patterns in transfected cells (Figure 2E, Rows 1, 3, 5 & 7; Movies S1A–D). The PY of IFI16, IFIX and AIM2 formed filaments with variable lengths. IFI16-PY filaments were shorter and clustered within discrete puncta. IFIX and AIM2 PY filaments formed extensive networks in the nucleus or cytoplasm, respectively; their filaments could also loop or branch (Figure S2C). In contrast, the MNDA-PY aggregates were not filamentous and less discrete. Previously, we observed that full-length IFI16-GFP did not form filamentous aggregates under unstimulated conditions (Li et al., 2012), consistent with the auto-inhibition of PY by HIN domains in resting state (Jin et al., 2012). The PY aggregation may reflect an active state, serving as a structural platform for signal amplification during DNA sensing.
To test the impact of pUL83 on PY aggregations, we co-expressed pUL83-mCherry with the pyrin proteins. Strikingly, pUL83 effectively dissipated PY aggregations for IFI16, IFIX and MNDA, but not for AIM2 (Figure 2E, Row 2, 4, 6, and 8 and Movies S1E–H). Furthermore, filamentous networks of AIM2-PY were predominantly cytoplasmic, being rarely observed in the nucleus, and were not disrupted by pUL83 (Figure S2D). Forced nuclear localization of AIM2-PY by an exogenous NLS still resulted in prominent oligomerization even in the presence of pUL83 (Figure S2E). These data support that pUL83 does not target AIM2-PY regardless of localization. Co-expression of mCherry alone did not affect aggregations, demonstrating that the inhibition is specific to pUL83. These results were validated by quantification in over a hundred cells (Figure 2F). Compared to mCherry alone, pUL83 drastically reduced the percentages of cells displaying PY aggregations for IFI16, IFIX and MNDA, but not for AIM2. Importantly, the pUL83-mediated inhibition was dose dependent (Figures 2G and S2F), supporting the specific engagement of pUL83 binding in PY interference.
Finally, we tested the effect of pUL83 on full-length endogenous IFI16 during HCMV infection. Endogenous IFI16 remained nuclear and did not display filamentous patterns during HCMV infection (Figure S2G), consistent with our report (Cristea et al., 2010). Similar nuclear localization was observed during HSV-1 infection (Orzalli et al., 2012), while another study reported partial cytoplasmic IFI16 (Johnson et al., 2013). The HIN domains may modulate PY behavior, reducing the degree of multimerization. Therefore, we adopted a crosslinking assay, similar to those previously used to show that AIM2 (Fernandes-Alnemri et al., 2009) or RNase L (Han et al., 2012) oligomerize upon activation. First, we tested the oligomerization of FL IFI16 in an overexpression model. 293T cells were transfected with GFP-tagged FL IFI16 or the PY or HIN domains and subjected to crosslinking in the presence or absence of co-transfected pUL83-Flag (Figure 2H). Only the FL and the PY displayed oligomerization, but not the HIN domains or GFP alone, confirming that PY is the oligomerization domain in IFI16. Consistent with the interactions of FL and PY with pUL83, their oligomerization was effectively dissipated by pUL83. We next assessed the oligomerization of endogenous IFI16 following either WT or ΔUL83 HCMV infection in HFF cells. Using a similar approach, we observed that endogenous IFI16 indeed oligomerized, shifting to higher mass following infection with ΔUL83, but not with WT HCMV (Figure 2I). Therefore, upon DNA sensing IFI16 forms oligomers via PY domain, which are effectively blocked by pUL83.
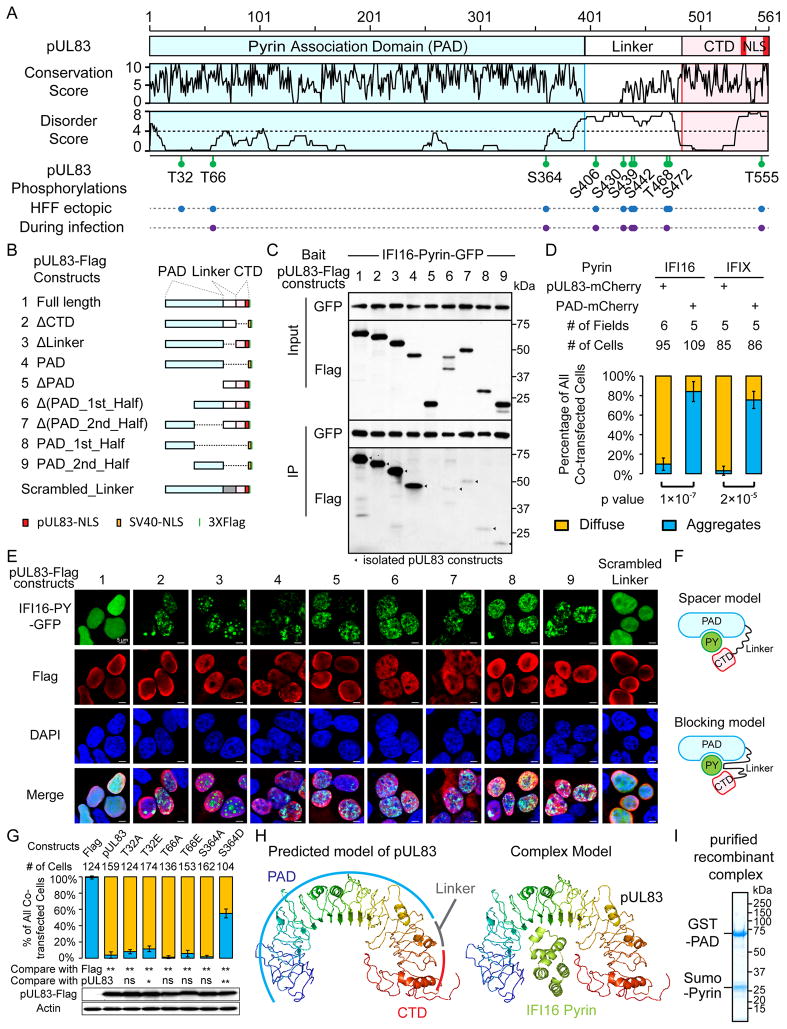
pUL83 contains conserved N- and C-terminus domains joined by a highly-phosphorylated linker
To explore how pUL83 blocks PY oligomerization, we characterized its sequence and posttranslational modifications (PTM). Sequence alignment of pUL83 homologs from primate CMVs (Figure S3A) revealed a conserved N-terminal domain (~386 residues), a divergent linker region, and a conserved C-terminus (CTD, ~90 residues) (Figure 3A), indicating a bipartite structure. The conservation correlated inversely with predicted disordered scores (Figures 3A and S3B).
Figure 3. Interference of pyrin aggregation requires both the Pyrin Association Domain (PAD) and the C-terminal domain of pUL83.
(A) Schematic of pUL83 shows its bipartite structure, conservation scores, predicted disorder degrees, and phosphorylation sites.
(B) Schematics of pUL83 constructs; those lacking NLS (red) were supplemented with SV40-NLS (orange) to ensure nuclear localization.
(C) Interactions of pUL83-Flag constructs in (B) (preys) with IFI16-PY-GFP (bait) were tested by co-IP.
(D) PAD-mCherry and pUL83-mCherry were tested for PY-interference as in Figure 2F; diffuse (yellow) or aggregation (blue) patterns shown as mean +/− SEM.
(E) IF shows the ability of pUL83 constructs in (B) to interfere with IFI16-PY-GFP aggregation.
(F) Two models for the role of the pUL83 linker in PY interference.
(G) Mutants of PAD phosphosites were tested for pyrin-interference as in (D). Statistics: ns, not significant; *, p< 0.05; **, p< 0.01. Western blots show levels of pUL83 constructs.
(H) Predicted structure models for pUL83 (rainbow) and IFI16 Pyrin (green). Docking shows arbitrary size fitting.
(I) SDS-PAGE of recombinant complex of GST-PAD and sumo-IFI16-Pyrin.
See also Figure S3
pUL83 is known to be phosphorylated (Roby and Gibson, 1986); however, to our knowledge, none of the phosphorylation sites are defined or have known functions. To identify these sites and test their roles in immune evasion, we isolated virally or ectopically expressed pUL83 from HFFs. Mass spectrometry analyses led to 95% pUL83 sequence coverage, and identified eight phosphorylated serine (S) or threonine (T) residues present in both virally and ectopically expressed pUL83, and two sites (T32 and S472) detected only in the latter (Figures 3A and S3C–D). These results suggest that most phosphorylations are regulated by host kinases. Notably, most sites are within the linker, with S406, T468, and S472 present at casein kinase II-like motifs (S/TXXE/D) (Figure S3A). Collectively, our results revealed a bipartite structure for pUL83 and numerous phosphorylation sites.
Phosphorylation modulates the pyrin interfering activity of pUL83
To test if phosphorylation modulates IFI16-PY interference by pUL83, we performed mutagenesis to alanine (A), aspartate (D) or glutamate (E), focusing on the three sites within the conserved N-terminal domain. While mutations at T32 or T66 or the S364A mutation had little impact on PY-interference, the phospho-mimic S364D reduced this activity (Figure 3G). As these mutations did not affect pUL83 stability or nuclear localization (Figures 3G and S3E), we conclude that S364 phosphorylation can partially compromise IFI16-PY interference. This phosphorylation was detected only at low levels (<1%, Figure S3F) during infection, suggesting a minor event, not affecting the majority of pUL83. However, our results show that host enzymes can modulate pUL83 activity at the posttranslational level.
The mechanism of pUL83-mediated IFI16 inhibition involves its N-terminus for pyrin binding and C-terminus for dissipation of nuclear aggregation
To map the PY-binding activity within pUL83, we generated deletion constructs to represent combinations of the above-defined pUL83 regions (Figure 3B). Co-IP assays showed that constructs including the entire N-terminus domain could efficiently bind IFI16-PY (Figure 3C, #1–4), while loss of this domain (#5) abolished PY-binding. Therefore, the N-terminal domain is necessary and sufficient for interaction with IFI16 PY, and we named this conserved region as Pyrin Association Domain (PAD). Moreover, both the first (1–200) and second (201–391) halves of PAD are necessary (#6–7), but not sufficient (#8–9) for strong association with IFI16-PY. These results establish PAD as the minimal unit required for optimal recruitment of IFI16-PY.
To see if PAD is sufficient for PY interference, we co-expressed PAD-mCherry with PY in 293T cells. Surprisingly, PAD was not sufficient for interfering with either IFI16- or tIFIX-PY (Figure 3D), suggesting another pUL83 region is also required. Therefore, we tested the aforementioned pUL83 constructs (Figure 3E). Neither PAD alone (#4) nor any PAD-deficient constructs (#5–9) disrupted IFI16-PY aggregation. Notably, the CTD-deficient pUL83 (#2) did not disrupt aggregation, indicating that CTD is also needed for PY interference. Strikingly, removal of the linker (#3) led to a distinct PY distribution, displaying nuclear spheres and nucleolar enrichment. This result implied two possible models for the role of the pUL83 linker as: (1) a spacer that correctly positions PAD and CTD, or (2) a direct contributor to PY interference (Figure 3F). To test these models, we replaced the native linker with a scrambled sequence (Figures 3B and 3E). Interestingly, this new linker restored the PY-interfering activity, supporting the spacer model. Collectively, our data show concerted action of the pUL83 domains, where the PAD initially recruits PY, and the CTD, appropriately separated by the linker, assists in dissipating PY oligomerization.
Lastly, to understand the molecular basis for this action, we predicted a structural model for pUL83. The model depicts an arched shape structured by solenoid folding (Figure 3H). The PAD contributes to most of the arch, followed by the solenoid linker and the CTD. The arch could accommodate the size of a PY domain, implying a one-to-one PAD-PY complex. Indeed, we co-purified a recombinant complex of GST-PAD and sumo-tagged IFI16-PY from E. coli that appeared at equivalent ratio (Figure 3I). The model suggests extensive contact between PY and pUL83, consistent with both halves of PAD being required for binding PY. The contact at CTD likely masks critical PY surfaces needed for oligomerization. Altogether, the predicted structure of pUL83 lends further mechanistic insights into the pUL83-IFI16 interaction.
Discussion
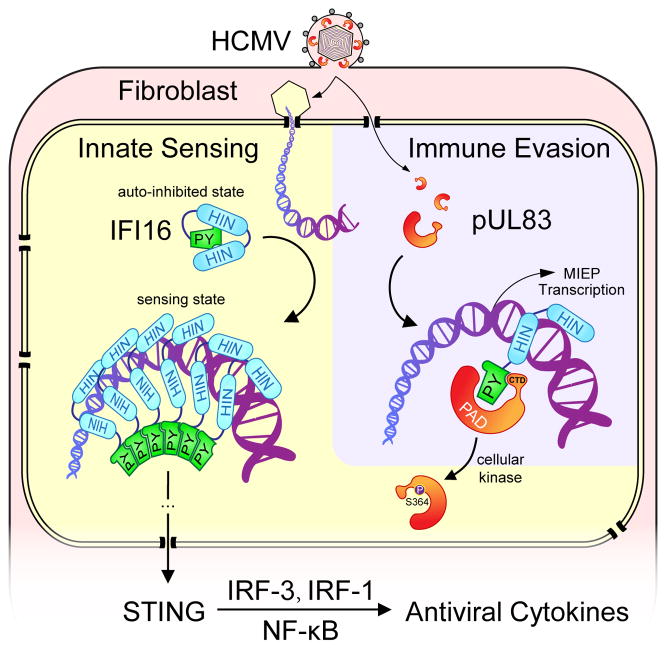
Here, we provide a mechanistic view of the conflict between host innate antiviral response and viral immune evasion during HCMV infection (Figure 4). First, we establish that IFI16 is a nuclear DNA sensor that is critical for triggering antiviral response during HCMV infection. Early during infection, IFI16 detects nuclear HCMV DNA, oligomerizes, and activates the STING-TBK1-IRF3 pathway to induce antiviral cytokines. Our findings also raise questions as to how nuclear IFI16 signals to activate cytoplasmic STING, and what other signaling pathways may be involved in antiviral responses. Second, we define a previously unknown mechanism in which the viral protein pUL83 blocks host IFI16-mediated sensing. Following cell entry, the abundant pUL83 enters the nucleus and binds IFI16 via its pyrin domain. Although IFI16 HIN domains can still bind viral DNA and release the auto-inhibited pyrin, pUL83 can effectively block pyrin oligomerization and subsequent immune signaling. Third, cellular kinase(s) can phosphorylate pUL83 at S364, compromising its pyrin interfering activity. Moreover, pUL83 recruits IFI16 to the MIEP to help activate viral gene expression (Cristea et al., 2010). Therefore, the pUL83-IFI16 interaction is a critical hub that modulates the outcome of HCMV infection, influencing the balance between innate immune response that initiates viral clearance and viral immune evasion that facilitates viral spread. Conceivably, a pharmaceutical agent that disrupts this interaction would shift the balance towards DNA sensing, boosting host immune responses to thwart HCMV infections.
Figure 4. Working model for IFI16-mediated nuclear DNA sensing and pUL83-mediated immune evasion.
IFI16 detects nuclear HCMV DNA via HIN domains, oligomerizes via the pyrin domain, and activates STING-mediated cytokine expression. pUL83 blocks IFI16 pyrin aggregation and hijacks IFI16 to help activate HCMV MIEP. pUL83 is partly inhibited by phosphorylation at S364. The balance between the DNA sensing and immune evasion determines the immunological outcome of HCMV infections.
Innate host sensors frequently utilize aggregation-dependent amplification mechanisms for signaling, e.g. the DNA sensor AIM2 (Fernandes-Alnemri et al., 2009) and RNA sensors RIG-I and MDA-5 (Jiang et al., 2012). Here, we showed that this principle also applies to nuclear DNA sensing, as IFI16 oligomerizes via its pyrin domain. To block pyrin oligomerization, the pUL83 PAD domain first recruits pyrin, and then locks pyrin in a monomeric state with the help of its CTD. This action also predicts that one pUL83 molecule is needed to inhibit one IFI16 molecule. This may explain why HCMV evolved to carry pUL83 as the most abundant virion component, delivered instantly after cell entry to aid immune evasion. This is distinct from HSV-1 infection, which uses the viral E3 ubiquitin ligase ICP0 to help target IFI16 for degradation, thereby dampening Type I IFN response (Orzalli et al., 2012) and inflammatory response (Johnson et al., 2013). In contrast, HCMV adopts a non-destructive strategy that may suit better its longer lytic cycle. As no homologs of pUL83 are present in other herpesvirus subfamilies, the pUL83-dependent immune evasion is likely a specific feature of CMVs. Overall, the distinct viral mechanisms used to target IFI16 underscore the essential role of this nuclear DNA sensor in innate antiviral response. Interestingly, the pUL83-mediated immune evasion is similar to subversion of RNA sensing by paramyxoviruses, e.g. measles, parainfluenza and sendai viruses. Their V proteins bind the SF2 domain of the RNA sensor MDA5, blocking its aggregation required for immune signaling (Motz et al., 2013). Therefore, our study points to both divergent and convergent evolutions of viruses to breach host nucleic acids sensing activities.
Altogether, we demonstrate that pUL83 inhibits IFI16-mediated antiviral cytokine expression. Additionally, previous reports have shown that pUL83 can indirectly contribute to immune evasion by modulating the expression of viral immediate early proteins IE1 and IE2 (Cristea et al., 2010; Taylor and Bresnahan, 2006). IE1 binds STAT1/2, thereby antagonizing the antiviral effect of IFN-β (Paulus et al., 2006). IE2 was shown to block the HCMV-induced expression of IFN-β and antiviral chemokines (Taylor and Bresnahan, 2005, 2006). The additive impact of different viral strategies for immune evasion may contribute to the partial suppression of ΔUL83 HCMV-induced cytokines by pUL83-Flag, while this could also result from insufficient ectopic expression of pUL83. Nevertheless, our observation that the ΔUL83 strain triggered substantially higher levels of antiviral cytokines than WT HCMV, even after UV treatment blocked viral gene expression, demonstrated an independent role for pUL83 in suppressing cytokine expression.
In summary, here we defined a molecular mechanism for viral immune evasion, an important aspect of HCMV pathogenesis. This knowledge may guide the future design of therapeutics to restore host immune response as a possible antiviral strategy.
Experimental Procedures
Full descriptions of materials and methods are provided in Supplemental Experimental procedures.
shRNA-mediated silencing, viral infections, nuclear fractionation, and RNA quantification
Lentiviral particles were produced in HEK293T cells co-transfected with pLKO.1 shRNA vectors (Open Biosystems) and packaging vectors. HFFs were transduced for 24 hrs with resulting particles and 8 μg/mL polybrene, and selected with 2 μg/mL puromycin. 3×105 HFFs were infected with HCMV strains at moi = 5 for 3 or 6 hpi. Nuclear fractions were collected by hypotonic lysis of cells, followed by washes and centrifugation. Protein levels were measured by Western blot. RNA levels were quantified by reverse transcription and qPCR.
IFI16 oligomerization by microscopy and cross-linking
PYHIN and pUL83 plasmids were constructed as detailed in Supplemental Experimental Procedures. 293T cells were transfected with PYHIN and pUL83 plasmids at indicated ratios using lipofectamine 2000 (Invitrogen) and visualized after 16 hr by confocal direct fluorescence microscopy. To test IFI16 oligomerization, HFFs lysates were cross-linked with 5 mM glutaraldehyde for 10 min, quenched with 50 mM Glycine, boiled in SDS buffer, and analyzed by Western blot.
IFI16-DNA interactions by chromatin IP
Infected HFF cells were cross-linked with 1% paraformaldehyde. Endogenous IFI16 was immunoisolated from lysates on magnetic beads (Invitrogen) conjugated with anti-IFI16 antibodies (Abcam). After a denaturing elution, cross-linking was reversed at 65°C overnight, and DNA was recovered by column purification and analyzed by PCR.
pUL83 isolation and mass spectrometry
HFFs stably expressing pUL83-Flag or infected with HCMV UL83-TAP (moi = 3) for 6 hr were used to immunoaffinity purify pUL83 via anti-FLAG antibody or IgG (TAP) conjugated magnetic beads, as in (Cristea et al., 2005). Isolated proteins were digested with trypsin, and analyzed by LC-MS/MS using an LTQ Orbitrap Velos (Thermo Scientific). Raw data were searched in Proteome Discoverer (V1.3) against a database containing human and herpesvirus sequences (Swiss-Prot), with peptide false discovery rate of 1%, and further refined in Scaffold 3.0 (Proteome Software).
Supplementary Material
Highlights.
IFI16 senses HCMV DNA to trigger antiviral cytokines via the STING/TBK1/IRF3 pathway
HCMV blocks IFI16 nuclear DNA sensing via the tegument protein pUL83
pUL83 clamps the IFI16 pyrin domain to block nuclear aggregation and immune signals
pUL83 site-specific phosphorylation inactivates its control over pyrin aggregation
Acknowledgments
We thank Y. Han and T.M. Greco for support with structure prediction or mass spectrometry, respectively, and B.A. Diner and T.M. Greco for critical reading of the manuscript. Reagents were kindly shared by T.E. Shenk (HCMV strains), Y. Kang (pCMV5 vectors) and Y.C. Tsai (mCherry vectors). This work was supported by NIH grants DP1DA026192 and R21AI102187, and HFSPO award RGY0079/2009-C to IMC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abate DA, Watanabe S, Mocarski ES. Major human cytomegalovirus structural protein pp65 (ppUL83) prevents interferon response factor 3 activation in the interferon response. Journal of virology. 2004;78:10995–11006. doi: 10.1128/JVI.78.20.10995-11006.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansari MA, Singh VV, Dutta S, Veettil MV, Dutta D, Chikoti L, Lu J, Everly D, Chandran B. Constitutive Interferon-Inducible Protein 16-Inflammasome Activation during Epstein-Barr Virus Latency I, II, and III in B and Epithelial Cells. Journal of virology. 2013;87:8606–8623. doi: 10.1128/JVI.00805-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnon TI, Achdout H, Levi O, Markel G, Saleh N, Katz G, Gazit R, Gonen-Gross T, Hanna J, Nahari E, et al. Inhibition of the NKp30 activating receptor by pp65 of human cytomegalovirus. Nat Immunol. 2005;6:515–523. doi: 10.1038/ni1190. [DOI] [PubMed] [Google Scholar]
- Browne EP, Shenk T. Human cytomegalovirus UL83-coded pp65 virion protein inhibits antiviral gene expression in infected cells. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:11439–11444. doi: 10.1073/pnas.1534570100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheeran MC, Lokensgard JR, Schleiss MR. Neuropathogenesis of congenital cytomegalovirus infection: disease mechanisms and prospects for intervention. Clin Microbiol Rev. 2009;22:99–126. doi: 10.1128/CMR.00023-08. Table of Contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cristea IM, Moorman NJ, Terhune SS, Cuevas CD, O’Keefe ES, Rout MP, Chait BT, Shenk T. Human cytomegalovirus pUL83 stimulates activity of the viral immediate-early promoter through its interaction with the cellular IFI16 protein. J Virol. 2010;84:7803–7814. doi: 10.1128/JVI.00139-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cristea IM, Williams R, Chait BT, Rout MP. Fluorescent proteins as proteomic probes. Molecular & cellular proteomics: MCP. 2005;4:1933–1941. doi: 10.1074/mcp.M500227-MCP200. [DOI] [PubMed] [Google Scholar]
- Fernandes-Alnemri T, Yu JW, Datta P, Wu J, Alnemri ES. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature. 2009;458:509–513. doi: 10.1038/nature07710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gariano GR, Dell’Oste V, Bronzini M, Gatti D, Luganini A, De Andrea M, Gribaudo G, Gariglio M, Landolfo S. The intracellular DNA sensor IFI16 gene acts as restriction factor for human cytomegalovirus replication. PLoS pathogens. 2012;8:e1002498. doi: 10.1371/journal.ppat.1002498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert MJ, Riddell SR, Plachter B, Greenberg PD. Cytomegalovirus selectively blocks antigen processing and presentation of its immediate-early gene product. Nature. 1996;383:720–722. doi: 10.1038/383720a0. [DOI] [PubMed] [Google Scholar]
- Han Y, Whitney G, Donovan J, Korennykh A. Innate immune messenger 2-5A tethers human RNase L into active high-order complexes. Cell reports. 2012;2:902–913. doi: 10.1016/j.celrep.2012.09.004. [DOI] [PubMed] [Google Scholar]
- Horan KA, Hansen K, Jakobsen MR, Holm CK, Soby S, Unterholzner L, Thompson M, West JA, Iversen MB, Rasmussen SB, et al. Proteasomal degradation of herpes simplex virus capsids in macrophages releases DNA to the cytosol for recognition by DNA sensors. J Immunol. 2013;190:2311–2319. doi: 10.4049/jimmunol.1202749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornung V, Ablasser A, Charrel-Dennis M, Bauernfeind F, Horvath G, Caffrey DR, Latz E, Fitzgerald KA. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature. 2009;458:514–518. doi: 10.1038/nature07725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabs DA, Enger C, Forman M, Dunn JP. Incidence of foscarnet resistance and cidofovir resistance in patients treated for cytomegalovirus retinitis. The Cytomegalovirus Retinitis and Viral Resistance Study Group. Antimicrob Agents Chemother. 1998;42:2240–2244. doi: 10.1128/aac.42.9.2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Kinch LN, Brautigam CA, Chen X, Du F, Grishin NV, Chen ZJ. Ubiquitin-induced oligomerization of the RNA sensors RIG-I and MDA5 activates antiviral innate immune response. Immunity. 2012;36:959–973. doi: 10.1016/j.immuni.2012.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin T, Perry A, Jiang J, Smith P, Curry JA, Unterholzner L, Jiang Z, Horvath G, Rathinam VA, Johnstone RW, et al. Structures of the HIN domain: DNA complexes reveal ligand binding and activation mechanisms of the AIM2 inflammasome and IFI16 receptor. Immunity. 2012;36:561–571. doi: 10.1016/j.immuni.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KE, Chikoti L, Chandran B. Herpes Simplex Virus 1 Infection Induces Activation and Subsequent Inhibition of the IFI16 and NLRP3 Inflammasomes. Journal of virology. 2013;87:5005–5018. doi: 10.1128/JVI.00082-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerur N, Veettil MV, Sharma-Walia N, Bottero V, Sadagopan S, Otageri P, Chandran B. IFI16 acts as a nuclear pathogen sensor to induce the inflammasome in response to Kaposi Sarcoma-associated herpesvirus infection. Cell host & microbe. 2011;9:363–375. doi: 10.1016/j.chom.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Diner BA, Chen J, Cristea IM. Acetylation modulates cellular distribution and DNA sensing ability of interferon-inducible protein IFI16. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:10558–10563. doi: 10.1073/pnas.1203447109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor A, Liu F, Schleiss MR. Molecular, biological, and in vivo characterization of the guinea pig cytomegalovirus (CMV) homologs of the human CMV matrix proteins pp71 (UL82) and pp65 (UL83) Journal of virology. 2004;78:9872–9889. doi: 10.1128/JVI.78.18.9872-9889.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motz C, Schuhmann KM, Kirchhofer A, Moldt M, Witte G, Conzelmann KK, Hopfner KP. Paramyxovirus V Proteins Disrupt the Fold of the RNA Sensor MDA5 to Inhibit Antiviral Signaling. Science. 2013;339:690–693. doi: 10.1126/science.1230949. [DOI] [PubMed] [Google Scholar]
- Orzalli MH, DeLuca NA, Knipe DM. Nuclear IFI16 induction of IRF-3 signaling during herpesviral infection and degradation of IFI16 by the viral ICP0 protein. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:E3008–3017. doi: 10.1073/pnas.1211302109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus C, Krauss S, Nevels M. A human cytomegalovirus antagonist of type I IFN-dependent signal transducer and activator of transcription signaling. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:3840–3845. doi: 10.1073/pnas.0600007103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roby C, Gibson W. Characterization of phosphoproteins and protein kinase activity of virions, noninfectious enveloped particles, and dense bodies of human cytomegalovirus. Journal of virology. 1986;59:714–727. doi: 10.1128/jvi.59.3.714-727.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth RB, Sun L, Ea CK, Chen ZJ. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-kappaB and IRF 3. Cell. 2005;122:669–682. doi: 10.1016/j.cell.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Singh VV, Kerur N, Bottero V, Dutta S, Chakraborty S, Ansari MA, Paudel N, Chikoti L, Chandran B. Kaposi’s sarcoma-associated herpesvirus latency in endothelial and B cells activates gamma interferon-inducible protein 16-mediated inflammasomes. Journal of virology. 2013;87:4417–4431. doi: 10.1128/JVI.03282-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor RT, Bresnahan WA. Human cytomegalovirus immediate-early 2 gene expression blocks virus-induced beta interferon production. Journal of virology. 2005;79:3873–3877. doi: 10.1128/JVI.79.6.3873-3877.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor RT, Bresnahan WA. Human cytomegalovirus immediate-early 2 protein IE86 blocks virus-induced chemokine expression. Journal of virology. 2006;80:920–928. doi: 10.1128/JVI.80.2.920-928.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unterholzner L, Keating SE, Baran M, Horan KA, Jensen SB, Sharma S, Sirois CM, Jin T, Latz E, Xiao TS, et al. IFI16 is an innate immune sensor for intracellular DNA. Nat Immunol. 2010;11:997–1004. doi: 10.1038/ni.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varnum SM, Streblow DN, Monroe ME, Smith P, Auberry KJ, Pasa-Tolic L, Wang D, Camp DG, 2nd, Rodland K, Wiley S, et al. Identification of proteins in human cytomegalovirus (HCMV) particles: the HCMV proteome. Journal of virology. 2004;78:10960–10966. doi: 10.1128/JVI.78.20.10960-10966.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.