Abstract
We recently discovered elevated β-secretase 1 (BACE1) activity in brains with sporadic Alzheimer disease (AD). Moreover, we also found high levels of BACE1 enzymatic activity in the cerebrospinal fluid from patients with both mild cognitive impairment (MCI) and AD. These results suggest that elevation of BACE1 enzymatic activity may occur early or may contribute to AD. We therefore examined whether BACE1 enzymatic activity was changed in MCI brains. BACE1 activity and tumor necrosis factor (TNF)-α levels were measured by enzymatic assay and ELISA in the temporal cortex from 18 patients with clinically well-characterized AD, 18 patients with MCI, and 18 healthy controls. We found a significant increase in BACE1 activity and protein level in brains of MCI and AD patients. Moreover, increased BACE1 activity correlated with plaque numbers and cognition status. We also found an increase in TNF-α in MCI brains. In vitro study revealed that TNF-α rather than other cytokines can up-regulate BACE1 protein expression. These findings suggest that BACE1 increase occurs early in MCI and is possibly induced by TNF-α and that BACE1 enzymatic activity may be important for conversion of MCI to AD.
Searching the early events of Alzheimer disease (AD) is becoming critical for effective diagnosis and treatment. Neuritic plaques and neurofibrillary tangles are two major pathologic characteristics of AD and major targets for AD diagnosis. Amyloid β (Aβ) peptide is a major component of neuritic plaques. β-Secretase 1 (BACE1), also known as β-site amyloid precursor protein (APP) cleaving enzyme, is an aspartic protease, a critical enzyme to promote Aβ generation.1–4 We originally discovered that BACE1 activity and protein expression are significantly increased in AD brains.5,6 Because the signals from cerebrospinal fluid (CSF) reflect most of neuropathologic changes in AD, we have studied BACE1 activity in the CSF from patients with mild cognitive impairment (MCI) and AD and found elevated BACE1 activity in the CSF of both MCI and AD patients.7,8 These results indicate that activation of BACE1 may play an important role in the early stage of AD when cognitive deficits are first observed and in progressive dementia associated with AD. However, whether BACE1 enzymatic activity and protein expression were altered in the brain as early as at the MCI stage was not known, possibly because of limits of sources of autopsied brain tissues. To examine whether BACE1 was activated in the brain during the early stages of AD (ie, MCI), we took advantage of the brain bank9 of rapidly autopsied MCI and AD brain tissues and biochemically measured BACE1 activity in brains of nondemented (ND) controls, MCI patients, and AD patients. We found that BACE1 enzymatic activity was significantly increased in both MCI and AD brains in the present study. Moreover, we also found that BACE1 activity was positively correlated with numbers of plaques and negatively correlated with Mini-Mental State Examination (MMSE) scores in MCI patients. Interestingly however, we also observed that there was no significant difference in BACE1 activity between MCI and AD patients. Because recent studies demonstrated that BACE1 may affect neuronal activity and brain metabolism,10–13 we conclude that BACE1 activity precedes the clinical diagnosis of AD and could be an early indicator of neuronal dysfunction or disease in AD.
Materials and Methods
Brain Samples for Research
Human tissue used in this study was collected with informed consent of participants or next of kin and with ethical approval from the institutional review boards of Sun Health Research Institute (Sun City, AZ). Details of the pathologic evaluation, including brain autopsy, and Braak staging, as well as the clinical evaluation, have been previously reported.9 All procedures that involved experiments on humans were performed in accordance with the ethical standards of the Committee on Human Experimentation of Sun Health Research Institute. Temporal cortex samples from AD, MCI, and ND patients with clinically diagnosed, neuropathologically confirmed conditions were frozen at autopsy and stored in vacuum-sealed plastic bags at −80°C until assayed. AD was diagnosed by National Institute of Neurological and Communicative Disorders and Stroke–Alzheimer’s Disease and Related Disorders Association14 criteria and confirmed by autopsy using National Institute on Aging–Reagan Institute Working Group on Diagnostic Criteria for the Neurological Assessment of Alzheimer’s Disease.15 MCI was diagnosed according to the Petersen criteria16 (ie, MCI patients performed 1.5 SDs below the age-adjusted reference mean in memory scales, as assessed using the Consortium to Establish a Registry for Alzheimer’s Disease cognitive battery,17 including verbal learning, recognition, and recall tests). Global cognitive function and activities of daily living were unimpaired in the MCI subjects. A board-certified neuropathologist using Consortium to Establish a Registry for Alzheimer’s Disease criteria18 performed the neuropathologic evaluation. Postmortem intervals averaged <3 hours. We randomly selected 54 cases, consisting of 18 AD cases, 18 MCI cases, and 18 ND control cases. All of the patients had died from lung or kidney failure, and they did not have any other central nervous system (CNS) diseases, sudden death, immunologic disorders, or severe infectious diseases (based on clinical records and pathologic examination). These 3 groups were well matched with regard to sex, age, postmortem interval, and sample preparations (Table 1).
Table 1.
Demographic Characteristics of the Study Participants
| Group | Total study participants (No.) | Men/women (No.) | Age (years) | PMI (h) | History (years) | APOE ε4 carrier (%) | Last MMSE (No.)∗ | Plaques (No.) |
|---|---|---|---|---|---|---|---|---|
| Controls | 18 | 11/7 | 82.3 ± 6.9 | 2.6 ± 0.8 | 27.8 | 28.1 ± 1.3 | 3.1 ± 3.5 | |
| MCI patients | 18 | 11/7 | 87.1 ± 4.9 | 2.7 ± 0.7 | 2 ± 1.7 | 44.4 | 27.2 ± 1.8 | 6.2 ± 4.5 |
| AD patients | 18 | 8/10 | 84.6 ± 6.7 | 2.6 ± 0.8 | 8.7 ± 5.2 | 66.7 | 10.1 ± 9.4 | 12.5 ± 1.0 |
Data are expressed as means ± SD unless otherwise indicated.
PMI, postmortem interval.
Control group, available in nine individuals; MCI group, available in 11 patients; AD group, available in 10 patients.
BACE1 Enzymatic Activity Assay
Activity assays of BACE1 were performed as described previously.19 In brief, samples of human temporal cortex were lyzed with a lysis buffer (10 mmol/L Tris-HCl, pH 7.4, 150 mmol/L NaCl, 1 mmol/L EDTA, 1 mmol/L EGTA, 1 mmol/L Na3VO4, 10% glycerol, and 0.5% Triton X-100). BACE1 enzymatic activity assays were performed by using synthetic peptide substrates containing Swedish mutant BACE1 cleavage site (Calbiochem, San Diego, CA). BACE1 substrate was dissolved in dimethyl sulfoxide and mixed with a 100 mmol/L Tris-HCl and 100 mmol/L NaCl, pH 4.5 (pH adjusted with acetic acid), reaction buffer. An equal amount of protein was mixed with 100 μL of substrate, and fluorescence intensity was measured with a microplate reader (BioTek, Winooski, VT) at an excitation wavelength of 430 nm and an emission wavelength of 520 nm. For the inhibitor test experiment, human brain lysate or BACE1 stable cell lysate was preincubated with 10 nmol/L BACE1-specific inhibitor (BACE1 inhibitor IV; Millipore, Billerica, MA) or 1 μmol/L cathepsin D inhibitor pepstatin-A (Millipore) on ice for 30 minutes and then mixed with substrate for fluorescence intensity measurement.
Western Blot
The samples of human temporal cortex were individually homogenized in 1× radioimmunoprecipitation assay buffer, including a protease inhibitor cocktail (Boehringer Mannheim, Indianapolis, IN). Protein concentration was measured by protein assay (Bio-Rad Laboratories, Hercules, CA), and 50 to 100 μg of total protein was subjected to SDS-PAGE (6% to 12% acrylamide). Separated proteins were then transferred onto polyvinylidene fluoride membranes. The blots were probed with the following antibodies: anti-BACE1 polyclonal antibody (Calbiochem), anti-BACE1 monoclonal antibody (R&D Systems, Minneapolis, MN), and anti–β-actin monoclonal antibody (1:5000; Sigma-Aldrich, St. Louis, MO). Western blot measurements were repeated three times independently.
ELISA
BACE1 protein levels were measured by enzyme-linked immunosorbent assay (ELISA) as described previously,5,19 with some modifications. The capture antibody was anti-BACE1 monoclonal antibody (R&D Systems), and the detection antibody was biotinylated anti-BACE1 polyclonal antibody B280. TMB substrate (Thermo Scientific Pierce, Rockford, IL) was used to visualize the reaction product, which was read at OD 450 with a microplate reader (Sigma-Aldrich). BACE1 protein (Calbiochem) was used as a standard. Data are presented as means ± SD of three experiments.
Tumor necrosis factor (TNF)-α levels in the lyzed extracts were measured by a commercially available ELISA kit for human TNF-α (R&D Systems). The manufacturer instructions were used throughout. Dilution curve homogenate samples of the brain were parallel to the standard dilution curve. The absorbance was measured at 450 nm with an automatic wavelength correction of 540 nm, and each experiment was repeated three times.
Neurotypical Cell Line Culture
Human SH-SY5Y cells, which can be terminally differentiated into cells that bear numerous specific characteristics of neurons as described previously,20 were cultured using 50% minimal essential medium plus 50% F12 medium, 10% heat-inactivated fetal calf serum, and 10 μmol/L retinoic acid. The culture medium was replaced every 3 days, and cells were differentiated for 6 days. Differentiated cells were seeded on 6-well plates that were precoated with 100 μg/mL of poly-l-lysine hydrobromide (Sigma-Aldrich) at a density of 1 × 105 cells/cm2. Differentiated neurotypical SH-SY5Y cells were grown with the medium based on Neurobasal A supplemented with 2% of B27, 2 mmol/L of l-glutamine, and 1% of N2 supplement (Invitrogen, Carlsbad, CA) at 37°C with 5% CO2. Half volume of the medium was changed every 3 days. Differentiated SH-SY5Y cells were treated at 6 days with different concentration of TNF-α and IL-1β (R&D Systems). After 24 hours, cells were collected and lyzed for Western blot. Experiments were repeated three times.
Cell Transfection and Luciferase Assay
HEK293 cells were transfected with pB1P-A vector containing a BACE1 promoter (−1941 to +292) upstream from a luciferase reporter gene using lipofectamine (Invitrogen). After transfection, cells were treated with different concentrations of TNF-α, IL-8, IL-12, and granulocyte-macrophage colony-stimulating factor (GM-CSF) (R&D Systems) or NF-κB inhibitor 6-amino-4(4-phenoxyphenylethylamino) quinazoline (Calbiochem). Cells were collected at different time points after treatment, and luciferase assay (Promega, Madison, WI) was performed and luminescence intensity measured as previously described.19
Statistical Analysis
Data are presented as means ± SD. Differences in means were determined by analysis of variance. P < 0.05 was considered statistically significant.
Results
Elevated BACE1 Protein Levels in Brains with MCI
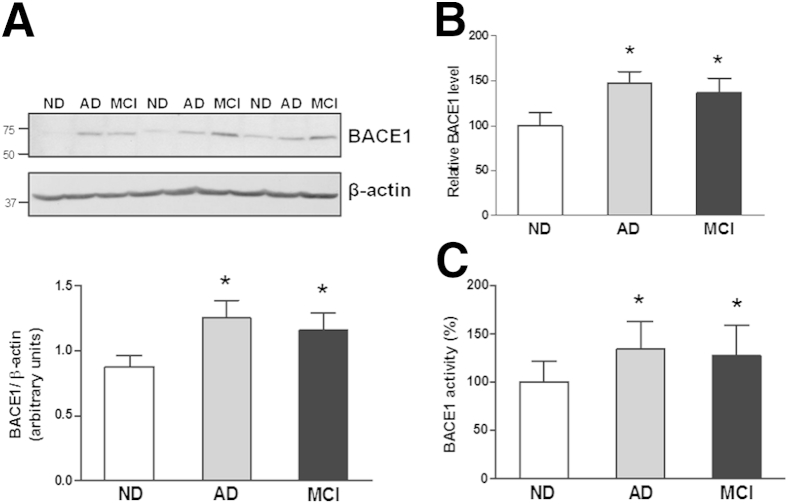
We and others have previously demonstrated that significantly increased protein levels of BACE1 are observed in the AD brain.5,6,21,22 By using the same BACE1 antibodies as we previously described,5,6 Western blots were used to measure BACE1 protein expression levels. As previously mentioned,6 the yield of total protein did not correlate with age at the time of death or with the postmortem interval for ND, MCI, or AD patients (data not shown). It was not surprising that similar and significant elevation of BACE1 protein levels were observed in AD temporal cortex samples compared with that of ND samples (Figure 1, A and B). Interestingly, the Western blot analysis in the samples also revealed a higher level of BACE1 protein expression in the temporal cortex of MCI patients than that in the same brain regions of ND controls. The statistical significance (P < 0.05) was reached after all cases were examined (n = 18) in each group (Figure 1, A and B). However, the difference between AD and MCI was not significant (Figure 1), which is consistent with our previous findings.5,6 Similar significant differences were found in Western blots detected with additional antibodies as published (data not shown).
Figure 1.
A: Western blot shows BACE1 protein expression in the temporal cortex from the brains of non-demented (ND) controls, Alzheimer disease (AD) patients, and mild cognitive impairment (MCI) patients. Quantitative analysis reveals a significant increase in BACE1 protein levels in the brains of AD and MCI patients compared with ND controls. B: BACE1 ELISA confirms the results of Western blot. C: BACE1 activity assay reveals elevated BACE1 activity in brains of AD and MCI patients compared with ND controls. The activity was normalized to the input protein amount and indicated as an arbitrary unit. n = 18 for each group. ∗P < 0.05 versus ND brains.
Increased BACE1 Enzymatic Activity Levels in MCI Brains
An increased BACE1 protein level is observed in MCI brains, suggesting similar BACE1 enzymatic activity in MCI brains as we observed in AD brains.5,6 As for AD brains, fluorescent-labeled peptide bearing the APP containing the Swedish mutation (APPsw) sequence was used as a BACE1 enzymatic substrate to test whether the enzyme from the AD brain crude extracts could cleave the substrate. As expected, there was an approximately 37% increased fluorescent intensity in AD temporal cortex extracts compared with that in ND controls. Importantly, we found that the fluorescent intensity in MCI temporal cortex extracts was significantly increased by 27% (P < 0.05) compared with that of ND controls (Figure 1C). In a parallel test model for another positive control as previously reported,5,6 we transiently transfected BACE1 cDNA into HEK293 cells stably expressing APPsw. We observed a high-fluorescent intensity in BACE1-transfected cells, suggesting that BACE1-overexpressing cells have higher BACE1 activity. In summary, a mixture of APPsw peptide and MCI and AD crude extracts generated higher fluorescent fragment products (127% and 137%, P < 0.05) than that of the mixture of APPsw peptide and ND extracts (Figure 1C). Our results suggest that elevated BACE1 enzymatic activity was observed not only in AD brains but also in MCI brains.
Association of BACE1 Activity with Amyloid Plaque Burden in the Temporal Cortex Not Only in AD but Also in MCI Patients
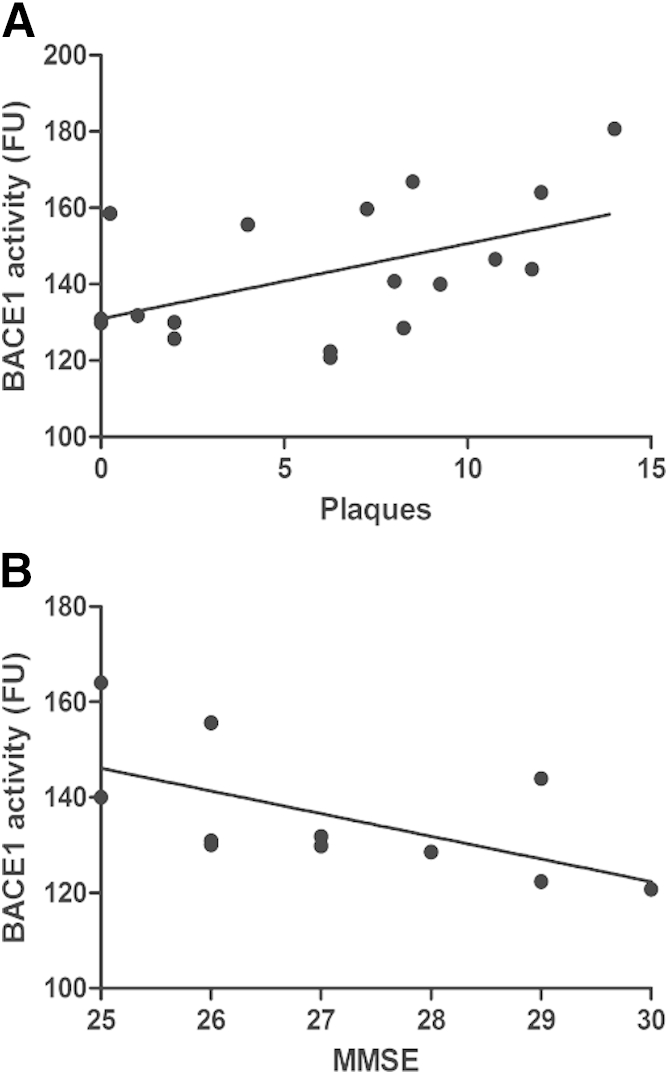
We previously reported that there is a correlation between BACE1 enzymatic activity and plaque numbers.6 Because plaques that contain Aβ are one of the established pathologic hallmarks in the AD brain, BACE1 is one of the major enzymes in Aβ production, and a high level of BACE1 was found in not only AD but also MCI brains (Figure 1), we examined a possibility of whether BACE1 activity was correlated with plaque burden in MCI brains. By using linear regression analysis, we observed a significant correlation between levels of BACE1 enzymatic activity and plaque numbers in MCI brains (Figure 2A). The data suggest that there is an active and dynamic AD-related pathologic process during the MCI status, an early stage of AD.
Figure 2.
A: BACE1 enzymatic activity significantly correlates with plaque numbers in mild cognitive impairment (MCI) brains by linear regression analysis (rs = 0.48, P < 0.05). B: Among the 11 MCI patients who had their last MMSE before death, BACE1 enzymatic activity negatively correlates with their cognitive function (MMSE scores) by linear regression analysis (rs = −0.67, P < 0.05).
Selective Changes in Cortical BACE1 Activity as Cognitive Deficits Merge
We and others have reported the abnormal increase of BACE1 enzymatic activity in sporadic AD.5,6,21 Moreover, we also discovered that CSF BACE1 activity was an indicator of the impaired cognitive function in MCI patients.7 We found increased BACE1 activity in MCI brains (Figure 1). We therefore performed within-subject correlational analyses to determine whether changes in the MCI brain BACE1 activity (Figure 1C) correspond to cognitive decline as assessed by the MMSE (Figure 2B). Among 18 MCI patients, 11 had last MMSE before death. Closer inspection of enzymatic activity levels across study participants sorted by cognitive status (Figure 2B) confirmed that MCI brain cortical BACE1 activity increases during the earliest stages of dementia. This increase was then followed by a precipitous decrease as cognitive decline progressed, which likely accounts for the slightly positive correlation between MCI brain BACE1 activity and MMSE scores (Figure 2B).
Increased Proinflammatory Cytokine TNF-α Protein Levels in MCI Brains
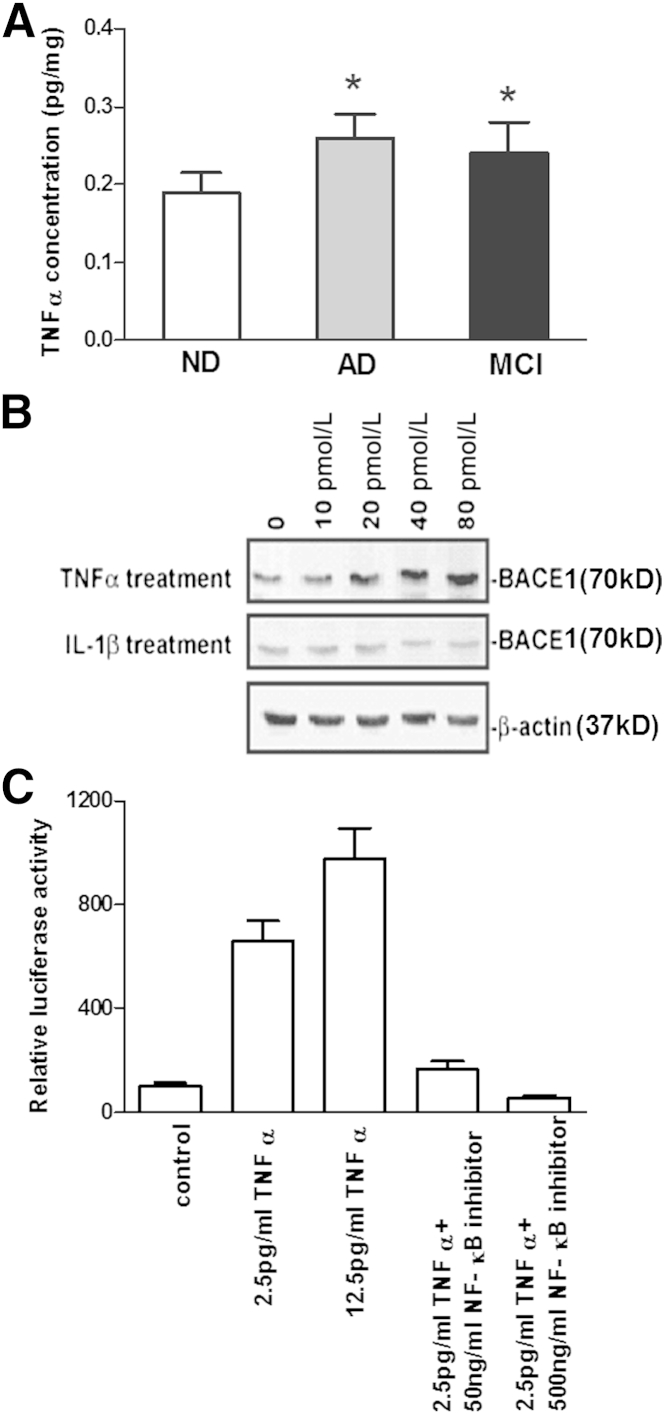
Because TNF-α is one of the major inflammatory cytokines in AD brains19,23 and is required for amyloid protein–induced neuron death,19 it is essential to understand whether protein levels of TNF-α are changed in the MCI brain. TNF-α ELISA from identical brain regions of the same patients revealed a 26% and 36% increase in TNF-α in MCI and AD temporal tissues, respectively, when compared with that found in ND controls (Figure 3A). In addition, the TNF-α increase may not be entirely due to neuronal loss. The elevation of TNF-α levels may be due to the activation of glial cells that normally express TNF-α protein in affected regions of MCI and AD brains. However, no significant difference was observed in TNF-α protein levels between AD and MCI brains (Figure 3A).
Figure 3.
A: TNF-α concentration elevates in mild cognitive impairment (MCI) or Alzheimer disease (AD) brains compared with non-demented (ND) brains. n = 18 each group. ∗P < 0.05 versus ND brains. B: With exogenous TNF-α treatment on human neurons, BACE1 protein expression levels elevate in a dose-dependent manner, whereas IL-1β appears to have little effect on BACE1 expression. C: HEK293 cells transfected with a BACE1 promoter luciferase reporter vector were treated with different concentrations of TNF-α with or without NF-κB inhibitor. TNF-α induces BACE1 promoter activity in a dose-dependent manner, whereas NF-κB inhibits TNF-α–induced BACE1 promoter activity.
TNF-α Up-Regulates BACE1 Protein Expression
Because both BACE1 and TNF-α protein levels were significantly increased in the brains of AD and MCI patients, the next question was whether there was any relationship between these two molecules. With exogenous TNF-α treatment on primary human neurons, BACE1 protein levels were significantly increased in a dose-dependent manner, whereas IL-1β appeared to have little effect on BACE1 protein expression (Figure 3B).
TNF-α Up-Regulates BACE1 Transcription through the NF-κB Pathway
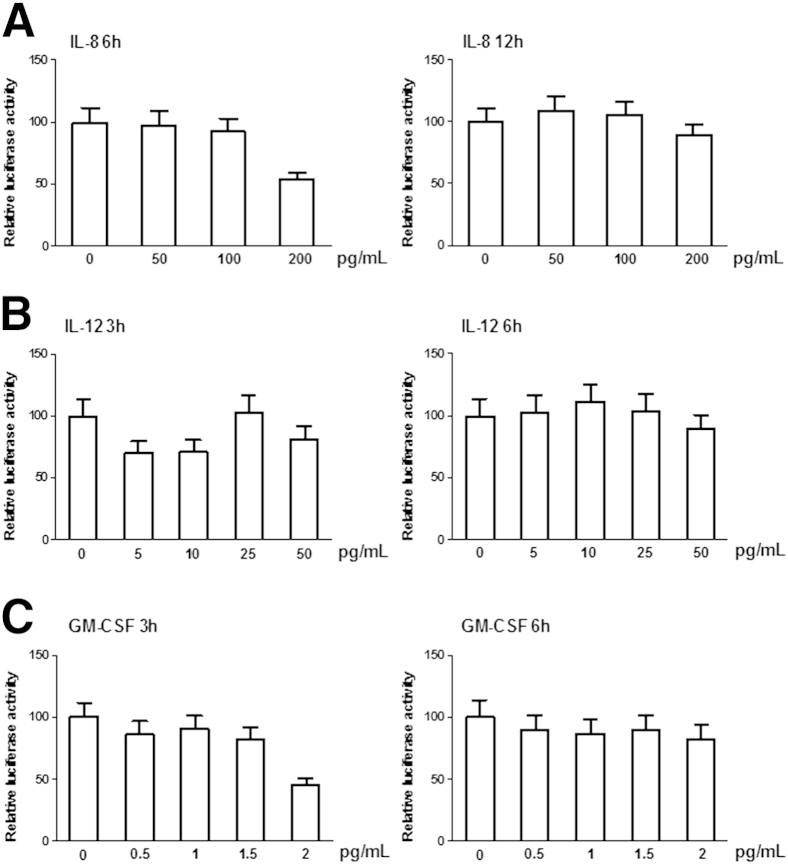
To clarify how TNF-α up-regulates BACE1 protein expression, HEK293 cells were transfected with a BACE1 promoter luciferase reporter vector and then treated with different concentrations of TNFα, IL-8, Il-12, and GM-CSF, respectively. We found significantly increased BACE1 promoter activity in a concentration-dependent manner with exogenous TNF-α treatment (Figure 3C). However, there are little effects of IL-8, IL-12, and GM-CSF treatments on BACE1 promoter activity (Figure 4). Because NF-κB is one of the major mediators of TNF-α–activated signaling24 and multiple NF-κB binding sites are located in the vicinity of the BACE1 promoter,25 an NF-κB activation inhibitor was used to block NF-κB signaling in TNF-α–treated HEK293 cells transfected with the BACE1 promoter. Treating the cells with an NF-κB inhibitor significantly reduced TNF-α–induced BACE1 promoter activity (Figure 3C), suggesting that the up-regulation of BACE1 transcription may be through TNF-α–mediated activation of NF-κB.
Figure 4.
HEK293 cells transfected with a BACE1 promoter luciferase reporter vector were treated with different concentrations of IL-8 (A), IL-12 (B), and GM-CSF (C). There are few effects of IL-8, IL-12, and GM-CSF treatments on BACE1 promoter activity.
Discussion
In the present study, we have examined whether BACE1 is activated in older individuals with ND, MCI, and AD. This is the first report to demonstrate that active BACE1 protein is intimately associated with MCI patients. The most significant finding is that specific BACE1 activity exhibits an obvious elevation in the cortical region, which may be considered a functional marker of neuropathologic disease and dementia. Some observed changes were associated with the mildest stages of cognitive decline, suggesting that BACE1 activity may be an antecedent to other pathophysiologic changes that arise during the disease.
In regard to the specificity of BACE1 enzymatic assay, because the BACE1 activity assay chosen may also detect the activity of cathepsin D, we treated some representative samples with pepstatin-A, an inhibitor of a number of aspartyl proteases, such as cathepsin D, that is known to not affect BACE1 activity. We also have a BACE1 stable cell line with treatment of BACE1 inhibitor IV C3 and pepstatin-A. As shown in Supplemental Figure S1, we found that BACE1 activity significantly decreased with BACE1 inhibitor C3 treatment as expected but not with pepstatin-A exposure (Supplemental Figure S1), suggesting pepstatin-A indeed does not affect BACE1. Thus, our assay of BACE1 activity used in the present study is specific.
Interestingly, we found elevated BACE1 enzymatic activity in MCI patients despite the virtual absence or small presence of Aβ plaques. In general, MCI is considered to be variable because we know that MCI patients are under conversion; some of MCI patients convert to AD, whereas some do not. However, the patients with MCI we studied had died already of different causes. We assume that these MCI brain tissues were at the same or similar mental status, which might be one reason why we have less variability among MCI brains.
Furthermore, in MCI individuals, the levels of BACE1 activity and protein expression in the temporal cortex are correlated with the level of cognition. Moreover, the proinflammatory cytokine TNF-α is significantly associated with BACE1 activity in both MCI and AD brains. Our in vitro studies have found that TNF-α may up-regulate BACE1 promoter activity (Figure 3C). Although there is no direct evidence to indicate that Aβ and inflammation can cause AD, we found that BACE1, which is regulated by TNF-α, is increased in MCI brains. Therefore, the present results indicate that the elevated BACE1 activity occurs before clinical dementia. Such changes may be regulated by inflammation in the brain, such as TNF-α, a nonspecific but potent factor in the development of brain disorders, including AD and depression.26 We have previously found that TNF-α and its receptors exhibit differential expression in AD brains.27 TNF-α is produced by activated microglia, mainly in response to Aβ40 and Aβ42 peptides, as well as to oxidative stress.5,6,19,28 Thus, an increased TNF-α level in MCI brains is a novel pathologic observation and is considered only one of the early changed molecules during MCI. The results suggest that certain nonspecific stimulus or environmental changes may trigger expression or release of inflammatory factors (ie, TNF-α), which then further chronically stimulates BACE1 transcription and translation. Our results are consistent with previous reports that indicate that oxidative stress is involved in the prodromal stage of AD.29,30 As we discussed above, oxidative stress and cytokines are active, dynamic, and critical early events of MCI. However, a series of rigorous studies would be needed before we can draw any conclusions that these are the causes of AD. Our results provide possible explanations for previous pathologic observations: elevation of CSF BACE1 activity in MCI patients or the presence of an active and dynamic AD-related pathologic process during the MCI status, an early stage of AD.
The increased association of BACE1 activity with Aβ plaque burdens from MCI and AD cortex tissues suggests that BACE1 activity is elevated as a function of disease state. Increasing evidence suggests that BACE1 is intricately involved in the pathogenesis of AD.31 The present study investigated BACE1 activity in MCI brains using our established assays, which allows more powerful statistical analyses. We confirmed and extended our earlier discoveries that BACE1 expression is elevated in AD5,6 compared with age-matched control cases.
Footnotes
Supported by grant NIA025888 (Y.S.) and NIAR01AG032441 (R.L.) from the National Institutes of Health, grants IIRG-09-61521 (Y.S.) and IIRG-07-59510 (R.L.) from the Alzheimer Association, grants G2006-118 (R.L.) and A2008-642 (P.H.) from the American Health Assistance Foundation, grant 81100861 from the National Natural Science Foundation of China (X.C.), and grant 20100071120081 from the Specialized Research Fund for the Doctoral Program of Higher Education (X.C.).
Human tissue samples were received from the Brain and Tissue Bank, Sun Health Research Institute (Sun City, AZ). The Brain and Body Donation Program is supported by the National Institute of Neurological Disorders and Stroke (grant U24NS072026 from the National Brain and Tissue Resource), the National Institute on Aging (grant P30AG19610 from the Arizona Alzheimer's Disease Core Center), and the Arizona Department of Health Services (contract 211002 from the Arizona Alzheimer's Research Center).
Supplemental Data
Ten-nanomolar β-secretase inhibitor C3 (BACE1 inhibitor IV; Millipore) inhibits BACE1 activity, unlike 1 μmol/L cathepsin D inhibitor pepstatin-A (Millipore), in human brain sample and BACE1 cell lysate. All data are means ± SD of three independent measurements. ∗P < 0.05 versus control sample.
References
- 1.Vassar R., Bennett B.D., Babu-Khan S., Kahn S., Mendiaz E.A., Denis P., Teplow D.B., Ross S., Amarante P., Loeloff R., Luo Y., Fisher S., Fuller J., Edenson S., Lile J., Jarosinski M.A., Biere A.L., Curran E., Burgess T., Louis J.C., Collins F., Treanor J., Rogers G., Citron M. Beta-secretase cleavage of Alzheimer’s amyloid precursor protein by the transmembrane aspartic protease BACE. Science. 1999;286:735–741. doi: 10.1126/science.286.5440.735. [DOI] [PubMed] [Google Scholar]
- 2.Yan R., Bienkowski M.J., Shuck M.E., Miao H., Tory M.C., Pauley A.M., Brashier J.R., Stratman N.C., Mathews W.R., Buhl A.E., Carter D.B., Tomasselli A.G., Parodi L.A., Heinrikson R.L., Gurney M.E. Membrane-anchored aspartyl protease with Alzheimer’s disease beta-secretase activity. Nature. 1999;402:533–537. doi: 10.1038/990107. [DOI] [PubMed] [Google Scholar]
- 3.Sinha S., Anderson J.P., Barbour R., Basi G.S., Caccavello R., Davis D., Doan M., Dovey H.F., Frigon N., Hong J., Jacobson-Croak K., Jewett N., Keim P., Knops J., Lieberburg I., Power M., Tan H., Tatsuno G., Tung J., Schenk D., Seubert P., Suomensaari S.M., Wang S., Walker D., Zhao J., McConlogue L., John V. Purification and cloning of amyloid precursor protein beta-secretase from human brain. Nature. 1999;402:537–540. doi: 10.1038/990114. [DOI] [PubMed] [Google Scholar]
- 4.Lin X., Koelsch G., Wu S., Downs D., Dashti A., Tang J. Human aspartic protease memapsin 2 cleaves the beta-secretase site of beta-amyloid precursor protein. Proc Natl Acad Sci U S A. 2000;97:1456–1460. doi: 10.1073/pnas.97.4.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang L.B., Lindholm K., Yan R., Citron M., Xia W., Yang X.L., Beach T., Sue L., Wong P., Price D., Li R., Shen Y. Elevated beta-secretase expression and enzymatic activity detected in sporadic Alzheimer disease. Nat Med. 2003;9:3–4. doi: 10.1038/nm0103-3. [DOI] [PubMed] [Google Scholar]
- 6.Li R., Lindholm K., Yang L.B., Yue X., Citron M., Yan R., Beach T., Sue L., Sabbagh M., Cai H., Wong P., Price D., Shen Y. Amyloid beta peptide load is correlated with increased beta-secretase activity in sporadic Alzheimer’s disease patients. Proc Natl Acad Sci U S A. 2004;101:3632–3637. doi: 10.1073/pnas.0205689101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhong Z., Ewers M., Teipel S., Burger K., Wallin A., Blennow K., He P., McAllister C., Hampel H., Shen Y. Levels of beta-secretase (BACE1) in cerebrospinal fluid as a predictor of risk in mild cognitive impairment. Arch Gen Psychiatry. 2007;64:718–726. doi: 10.1001/archpsyc.64.6.718. [DOI] [PubMed] [Google Scholar]
- 8.Ewers M., Zhong Z., Burger K., Wallin A., Blennow K., Teipel S.J., Shen Y., Hampel H. Increased CSF-BACE 1 activity is associated with ApoE-epsilon 4 genotype in subjects with mild cognitive impairment and Alzheimer’s disease. Brain. 2008;131:1252–1258. doi: 10.1093/brain/awn034. [DOI] [PubMed] [Google Scholar]
- 9.Beach T.G., Sue L.I., Walker D.G., Roher A.E., Lue L., Vedders L., Connor D.J., Sabbagh M.N., Rogers J. The Sun Health Research Institute Brain Donation Program: description and experience, 1987-2007. Cell Tissue Bank. 2008;9:229–245. doi: 10.1007/s10561-008-9067-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim D.Y., Carey B.W., Wang H., Ingano L.A., Binshtok A.M., Wertz M.H., Pettingell W.H., He P., Lee V.M., Woolf C.J., Kovacs D.M. BACE1 regulates voltage-gated sodium channels and neuronal activity. Nat Cell Biol. 2007;9:755–764. doi: 10.1038/ncb1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’Connor T., Sadleir K.R., Maus E., Velliquette R.A., Zhao J., Cole S.L., Eimer W.A., Hitt B., Bembinster L.A., Lammich S., Lichtenthaler S.F., Hebert S.S., De Strooper B., Haass C., Bennett D.A., Vassar R. Phosphorylation of the translation initiation factor eIF2alpha increases BACE1 levels and promotes amyloidogenesis. Neuron. 2008;60:988–1009. doi: 10.1016/j.neuron.2008.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tesco G., Koh Y.H., Kang E.L., Cameron A.N., Das S., Sena-Esteves M., Hiltunen M., Yang S.H., Zhong Z., Shen Y., Simpkins J.W., Tanzi R.E. Depletion of GGA3 stabilizes BACE and enhances beta-secretase activity. Neuron. 2007;54:721–737. doi: 10.1016/j.neuron.2007.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu X., Zhou X., He W., Yang J., Xiong W., Wong P., Wilson C.G., Yan R. BACE1 deficiency causes altered neuronal activity and neurodegeneration. J Neurosci. 2010;30:8819–8829. doi: 10.1523/JNEUROSCI.1334-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McKhann G., Drachman D., Folstein M., Katzman R., Price D., Stadlan E.M. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 15.Hyman B.T., Trojanowski J.Q. Consensus recommendations for the postmortem diagnosis of Alzheimer disease from the National Institute on Aging and the Reagan Institute Working Group on diagnostic criteria for the neuropathological assessment of Alzheimer disease. J Neuropathol Exp Neurol. 1997;56:1095–1097. doi: 10.1097/00005072-199710000-00002. [DOI] [PubMed] [Google Scholar]
- 16.Petersen R.C. Clinical practice: mild cognitive impairment. N Engl J Med. 2011;364:2227–2234. doi: 10.1056/NEJMcp0910237. [DOI] [PubMed] [Google Scholar]
- 17.Morris J.C., Heyman A., Mohs R.C., Hughes J.P., van Belle G., Fillenbaum G., Mellits E.D., Clark C. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD), part I: clinical and neuropsychological assessment of Alzheimer’s disease. Neurology. 1989;39:1159–1165. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- 18.Mirra S.S., Heyman A., McKeel D., Sumi S.M., Crain B.J., Brownlee L.M., Vogel F.S., Hughes J.P., van Belle G., Berg L. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD), part II: standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology. 1991;41:479–486. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- 19.He P., Zhong Z., Lindholm K., Berning L., Lee W., Lemere C., Staufenbiel M., Li R., Shen Y. Deletion of tumor necrosis factor death receptor inhibits amyloid beta generation and prevents learning and memory deficits in Alzheimer’s mice. J Cell Biol. 2007;178:829–841. doi: 10.1083/jcb.200705042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He P., Liu Q., Wu J., Shen Y. Genetic deletion of TNF receptor suppresses excitatory synaptic transmission via reducing AMPA receptor synaptic localization in cortical neurons. FASEB J. 2012;26:334–345. doi: 10.1096/fj.11-192716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fukumoto H., Cheung B.S., Hyman B.T., Irizarry M.C. Beta-secretase protein and activity are increased in the neocortex in Alzheimer disease. Arch Neurol. 2002;59:1381–1389. doi: 10.1001/archneur.59.9.1381. [DOI] [PubMed] [Google Scholar]
- 22.Holsinger R.M., McLean C.A., Beyreuther K., Masters C.L., Evin G. Increased expression of the amyloid precursor beta-secretase in Alzheimer’s disease. Ann Neurol. 2002;51:783–786. doi: 10.1002/ana.10208. [DOI] [PubMed] [Google Scholar]
- 23.Shen Y., He P., Zhong Z., McAllister C., Lindholm K. Distinct destructive signal pathways of neuronal death in Alzheimer’s disease. Trends Mol Med. 2006;12:574–579. doi: 10.1016/j.molmed.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 24.Li R., Lindholm K., Yang L.B., Konishi Y., Hampel H., Zhang D., Shen Y. TNF death receptor signaling cascade is required for amyloid-β-protein induced neuron death. J Neurosci. 2004;28:1760–1771. doi: 10.1523/JNEUROSCI.4580-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sambamurti K., Kinsey R., Maloney B., Ge Y.W., Lahiri D.K. Gene structure and organization of the human beta-secretase (BACE) promoter. FASEB J. 2004;18:1034–1036. doi: 10.1096/fj.03-1378fje. [DOI] [PubMed] [Google Scholar]
- 26.Simen B.B., Duman C.H., Simen A.A., Duman R.S. TNFalpha signaling in depression and anxiety: behavioral consequences of individual receptor targeting. Biol Psychiatry. 2006;59:775–785. doi: 10.1016/j.biopsych.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 27.Cheng X., Yang L., He P., Li R., Shen Y. Differential activation of tumor necrosis factor receptors distinguishes between brains from Alzheimer’s disease and non-demented patients. J Alzheimers Dis. 2010;19:621–630. doi: 10.3233/JAD-2010-1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee M., Schwab C., Yu S., McGeer E., McGeer P.L. Astrocytes produce the anti-inflammatory and neuroprotective agent hydrogen sulfide. Neurobiol Aging. 2009;30:1523–1534. doi: 10.1016/j.neurobiolaging.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 29.Bonda D.J., Wang X., Perry G., Nunomura A., Tabaton M., Zhu X., Smith M.A. Oxidative stress in Alzheimer disease: a possibility for prevention. Neuropharmacology. 2010;59:290–294. doi: 10.1016/j.neuropharm.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 30.Nunomura A., Tamaoki T., Motohashi N., Nakamura M., McKeel D.W., Jr., Tabaton M., Lee H.G., Smith M.A., Perry G., Zhu X. The earliest stage of cognitive impairment in transition from normal aging to Alzheimer disease is marked by prominent RNA oxidation in vulnerable neurons. J Neuropathol Exp Neurol. 2012;71:233–241. doi: 10.1097/NEN.0b013e318248e614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang H., Li R., Shen Y. beta-Secretase: its biology as a therapeutic target in diseases. Trends Pharmacol Sci. 2013;34:215–225. doi: 10.1016/j.tips.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Ten-nanomolar β-secretase inhibitor C3 (BACE1 inhibitor IV; Millipore) inhibits BACE1 activity, unlike 1 μmol/L cathepsin D inhibitor pepstatin-A (Millipore), in human brain sample and BACE1 cell lysate. All data are means ± SD of three independent measurements. ∗P < 0.05 versus control sample.