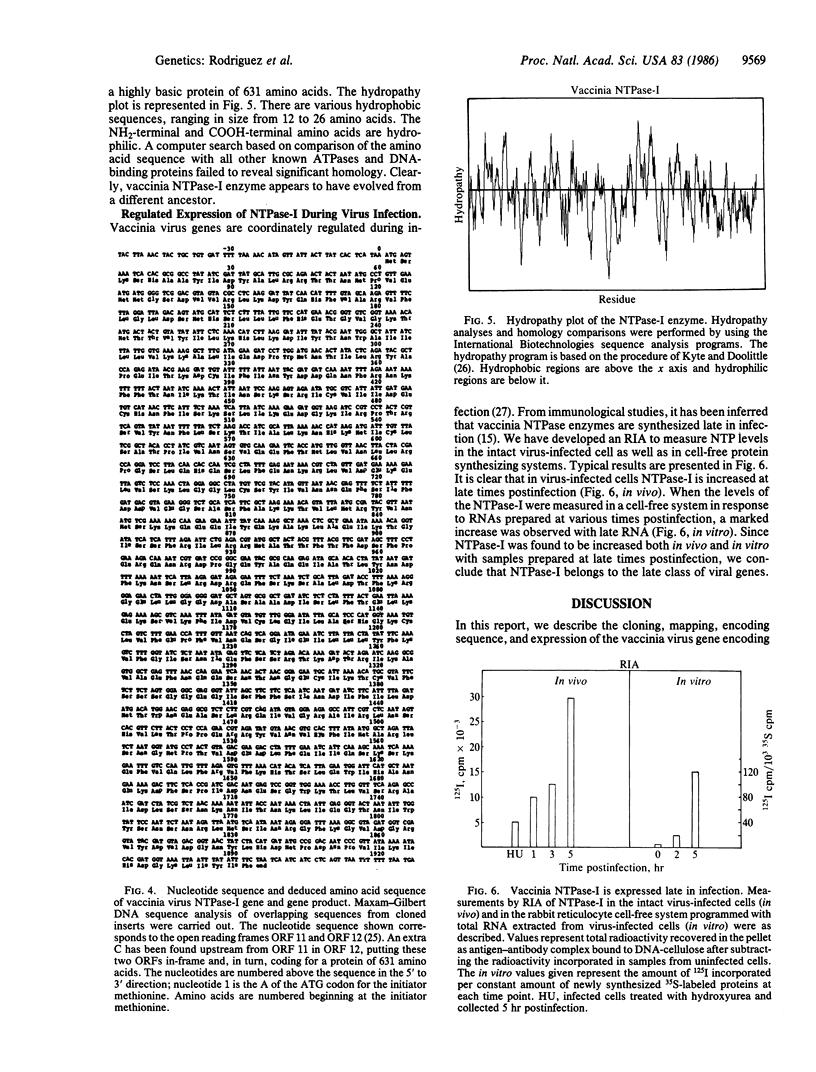
Abstract
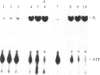
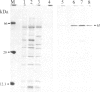
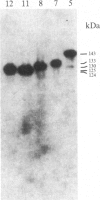
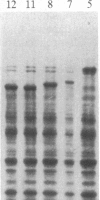
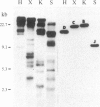
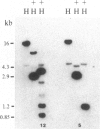
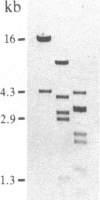
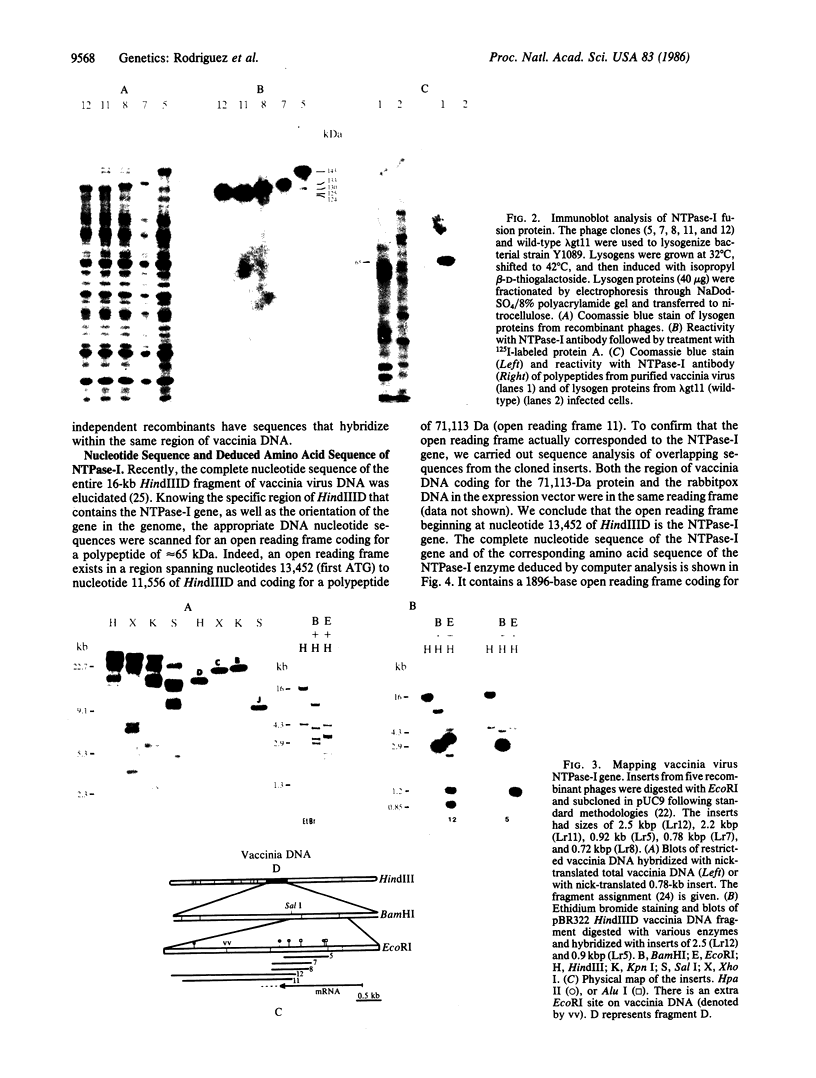
A rabbit poxvirus genomic library contained within the expression vector lambda gt11 was screened with polyclonal antiserum prepared against vaccinia virus nucleic acid-dependent nucleoside triphosphatase (NTPase)-I enzyme. Five positive phage clones containing from 0.72- to 2.5-kilobase-pair (kbp) inserts expressed a beta-galactosidase fusion protein that was reactive by immunoblotting with the NTPase-I antibody. Hybridization analysis allowed the location of this gene within the vaccinia HindIIID restriction fragment. From the known nucleotide sequence of the 16-kbp vaccinia HindIIID fragment, we identified a region that contains a 1896-base open reading frame coding for a 631-amino acid protein. Analysis of the complete sequence revealed a highly basic protein, with hydrophilic COOH and NH2 termini, various hydrophobic domains, and no significant homology to other known proteins. Translational studies demonstrate that NTPase-I belongs to a late class of viral genes. This protein is highly conserved among Orthopoxviruses.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bajszár G., Wittek R., Weir J. P., Moss B. Vaccinia virus thymidine kinase and neighboring genes: mRNAs and polypeptides of wild-type virus and putative nonsense mutants. J Virol. 1983 Jan;45(1):62–72. doi: 10.1128/jvi.45.1.62-72.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertholet C., Drillien R., Wittek R. One hundred base pairs of 5' flanking sequence of a vaccinia virus late gene are sufficient to temporally regulate late transcription. Proc Natl Acad Sci U S A. 1985 Apr;82(7):2096–2100. doi: 10.1073/pnas.82.7.2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomquist M. C., Hunt L. T., Barker W. C. Vaccinia virus 19-kilodalton protein: relationship to several mammalian proteins, including two growth factors. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7363–7367. doi: 10.1073/pnas.81.23.7363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandl C. J., Green N. M., Korczak B., MacLennan D. H. Two Ca2+ ATPase genes: homologies and mechanistic implications of deduced amino acid sequences. Cell. 1986 Feb 28;44(4):597–607. doi: 10.1016/0092-8674(86)90269-2. [DOI] [PubMed] [Google Scholar]
- Brown J. P., Twardzik D. R., Marquardt H., Todaro G. J. Vaccinia virus encodes a polypeptide homologous to epidermal growth factor and transforming growth factor. Nature. 1985 Feb 7;313(6002):491–492. doi: 10.1038/313491a0. [DOI] [PubMed] [Google Scholar]
- Esteban M., Benavente J., Paez E. Effect of interferon on integrity of vaccinia virus and ribosomal RNA in infected cells. Virology. 1984 Apr 15;134(1):40–51. doi: 10.1016/0042-6822(84)90270-8. [DOI] [PubMed] [Google Scholar]
- Gold P. H., Dales S. Localization of nucleotide phosphohydrolase activity within vaccinia. Proc Natl Acad Sci U S A. 1968 Jul;60(3):845–852. doi: 10.1073/pnas.60.3.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt P., Hiller G., Wittek R. Localization and fine structure of a vaccinia virus gene encoding an envelope antigen. J Virol. 1986 Jun;58(3):757–764. doi: 10.1128/jvi.58.3.757-764.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hruby D. E., Ball L. A. Mapping and identification of the vaccinia virus thymidine kinase gene. J Virol. 1982 Aug;43(2):403–409. doi: 10.1128/jvi.43.2.403-409.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones E. V., Moss B. Mapping of the vaccinia virus DNA polymerase gene by marker rescue and cell-free translation of selected RNA. J Virol. 1984 Jan;49(1):72–77. doi: 10.1128/jvi.49.1.72-77.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanazawa H., Futai M. Structure and function of H+-ATPase: what we have learned from Escherichia coli H+-ATPase. Ann N Y Acad Sci. 1982;402:45–64. doi: 10.1111/j.1749-6632.1982.tb25731.x. [DOI] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Lengyel P. Biochemistry of interferons and their actions. Annu Rev Biochem. 1982;51:251–282. doi: 10.1146/annurev.bi.51.070182.001343. [DOI] [PubMed] [Google Scholar]
- Mackett M., Archard L. C. Conservation and variation in Orthopoxvirus genome structure. J Gen Virol. 1979 Dec;45(3):683–701. doi: 10.1099/0022-1317-45-3-683. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Morgan J. R., Cohen L. K., Roberts B. E. Identification of the DNA sequences encoding the large subunit of the mRNA-capping enzyme of vaccinia virus. J Virol. 1984 Oct;52(1):206–214. doi: 10.1128/jvi.52.1.206-214.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D. K., Carter J. K., Moyer R. W. Isolation and characterization of monoclonal antibodies directed against two subunits of rabbit poxvirus-associated, DNA-directed RNA polymerase. J Virol. 1985 Sep;55(3):670–680. doi: 10.1128/jvi.55.3.670-680.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munyon W., Paoletti E., Ospina J., Grace J. T., Jr Nucleotide phosphohydrolase in purified vaccinia virus. J Virol. 1968 Mar;2(3):167–172. doi: 10.1128/jvi.2.3.167-172.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niles E. G., Condit R. C., Caro P., Davidson K., Matusick L., Seto J. Nucleotide sequence and genetic map of the 16-kb vaccinia virus HindIII D fragment. Virology. 1986 Aug;153(1):96–112. doi: 10.1016/0042-6822(86)90011-5. [DOI] [PubMed] [Google Scholar]
- Paez E., Esteban M. Nature and mode of action of vaccinia virus products that block activation of the interferon-mediated ppp(A2'p)nA-synthetase. Virology. 1984 Apr 15;134(1):29–39. doi: 10.1016/0042-6822(84)90269-1. [DOI] [PubMed] [Google Scholar]
- Paez E., Esteban M. Resistance of vaccinia virus to interferon is related to an interference phenomenon between the virus and the interferon system. Virology. 1984 Apr 15;134(1):12–28. doi: 10.1016/0042-6822(84)90268-x. [DOI] [PubMed] [Google Scholar]
- Paolette E., Rosemond-Hornbeak H., Moss B. Two nucleid acid-dependent nucleoside triphosphate phosphohydrolases from vaccinia virus. Purification and characterization. J Biol Chem. 1974 May 25;249(10):3273–3280. [PubMed] [Google Scholar]
- Paoletti E., Cooper N., Moss B. Regulation of synthesis of two immunologically distinct nucleic acid-dependent nucleoside triphosphate phosphohydrolases in vaccinia virus-infected HeLa cells. J Virol. 1974 Sep;14(3):578–586. doi: 10.1128/jvi.14.3.578-586.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoletti E., Moss B. Two nucleic acid-dependent nucleoside triphosphate phosphohydrolases from vaccinia virus. Nucleotide substrate and polynucleotide cofactor specificities. J Biol Chem. 1974 May 25;249(10):3281–3286. [PubMed] [Google Scholar]
- Plucienniczak A., Schroeder E., Zettlmeissl G., Streeck R. E. Nucleotide sequence of a cluster of early and late genes in a conserved segment of the vaccinia virus genome. Nucleic Acids Res. 1985 Feb 11;13(3):985–998. doi: 10.1093/nar/13.3.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisner A. H. Similarity between the vaccinia virus 19K early protein and epidermal growth factor. 1985 Feb 28-Mar 6Nature. 313(6005):801–803. doi: 10.1038/313801a0. [DOI] [PubMed] [Google Scholar]
- Rodriguez J. F., Janeczko R., Esteban M. Isolation and characterization of neutralizing monoclonal antibodies to vaccinia virus. J Virol. 1985 Nov;56(2):482–488. doi: 10.1128/jvi.56.2.482-488.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosel J., Moss B. Transcriptional and translational mapping and nucleotide sequence analysis of a vaccinia virus gene encoding the precursor of the major core polypeptide 4b. J Virol. 1985 Dec;56(3):830–838. doi: 10.1128/jvi.56.3.830-838.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shull G. E., Schwartz A., Lingrel J. B. Amino-acid sequence of the catalytic subunit of the (Na+ + K+)ATPase deduced from a complementary DNA. Nature. 1985 Aug 22;316(6030):691–695. doi: 10.1038/316691a0. [DOI] [PubMed] [Google Scholar]
- Tartaglia J., Paoletti E. Physical mapping and DNA sequence analysis of the rifampicin resistance locus in vaccinia virus. Virology. 1985 Dec;147(2):394–404. doi: 10.1016/0042-6822(85)90141-2. [DOI] [PubMed] [Google Scholar]
- Tartaglia J., Piccini A., Paoletti E. Vaccinia virus rifampicin-resistance locus specifies a late 63,000 Da gene product. Virology. 1986 Apr 15;150(1):45–54. doi: 10.1016/0042-6822(86)90264-3. [DOI] [PubMed] [Google Scholar]
- Traktman P., Sridhar P., Condit R. C., Roberts B. E. Transcriptional mapping of the DNA polymerase gene of vaccinia virus. J Virol. 1984 Jan;49(1):125–131. doi: 10.1128/jvi.49.1.125-131.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesan S., Baroudy B. M., Moss B. Distinctive nucleotide sequences adjacent to multiple initiation and termination sites of an early vaccinia virus gene. Cell. 1981 Sep;25(3):805–813. doi: 10.1016/0092-8674(81)90188-4. [DOI] [PubMed] [Google Scholar]
- Venkatesan S., Gershowitz A., Moss B. Complete nucleotide sequences of two adjacent early vaccinia virus genes located within the inverted terminal repetition. J Virol. 1982 Nov;44(2):637–646. doi: 10.1128/jvi.44.2.637-646.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinrich S. L., Niles E. G., Hruby D. E. Transcriptional and translational analysis of the vaccinia virus late gene L65. J Virol. 1985 Aug;55(2):450–457. doi: 10.1128/jvi.55.2.450-457.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir J. P., Bajszár G., Moss B. Mapping of the vaccinia virus thymidine kinase gene by marker rescue and by cell-free translation of selected mRNA. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1210–1214. doi: 10.1073/pnas.79.4.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir J. P., Moss B. Regulation of expression and nucleotide sequence of a late vaccinia virus gene. J Virol. 1984 Sep;51(3):662–669. doi: 10.1128/jvi.51.3.662-669.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittek R., Richner B., Hiller G. Mapping of the genes coding for the two major vaccinia virus core polypeptides. Nucleic Acids Res. 1984 Jun 25;12(12):4835–4848. doi: 10.1093/nar/12.12.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]