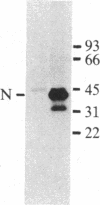
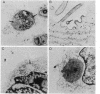
Abstract
The T-lymphocyte response to respiratory syncytial (RS) virus has been invoked to explain the bronchiolitis and pneumonia caused by RS virus in human infants. However, T cells also appear to play a role in protection against RS virus infection. Although RS virus-specific human lymphocytes have been demonstrated, neither the phenotype nor the function of the lymphocytes was characterized. We describe here the induction of anti-RS virus cytotoxic T lymphocytes, in both bulk culture and restimulated cell lines, from human peripheral blood. Infection of Epstein-Barr virus-transformed human B-cell lines with RS virus in vitro readily caused a persistent infection; these cells continued to synthesize RS viral proteins and secrete infectious RS virus 4 months after infection. The persistently infected cells were used both to restimulate cytotoxic-T-cell precursors and as targets for RS virus-specific cytotoxic T cells.
Full text
PDF
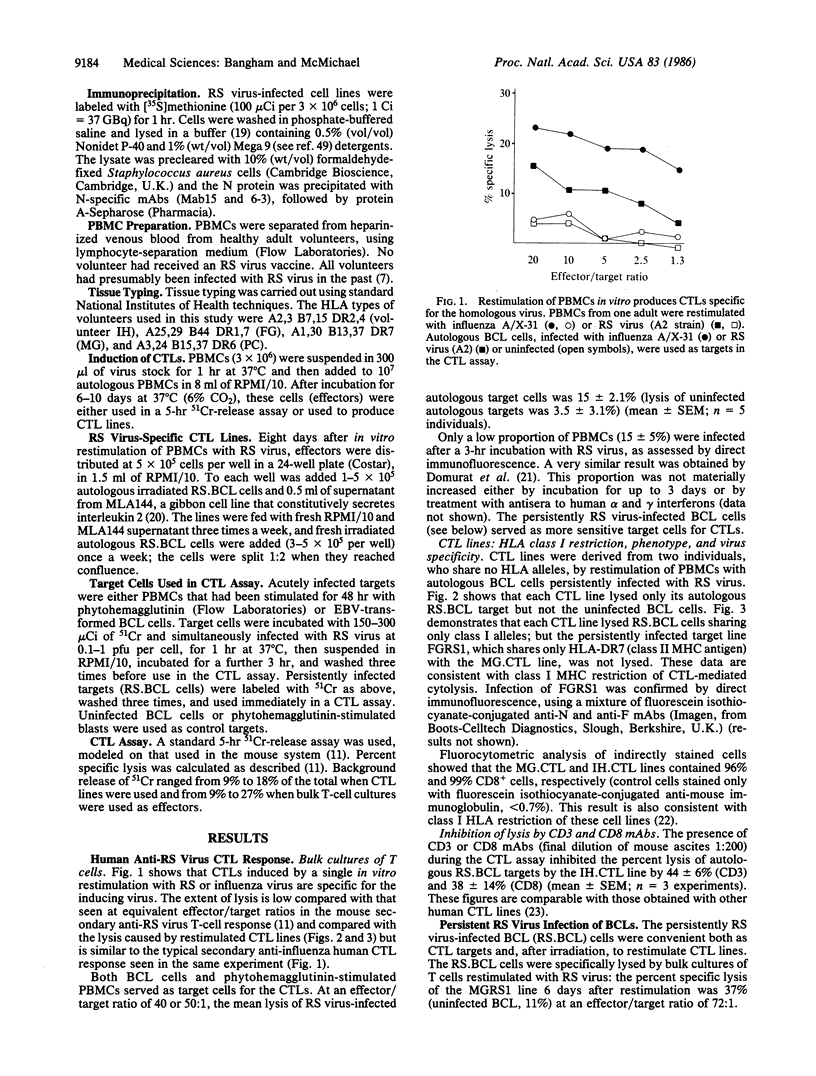
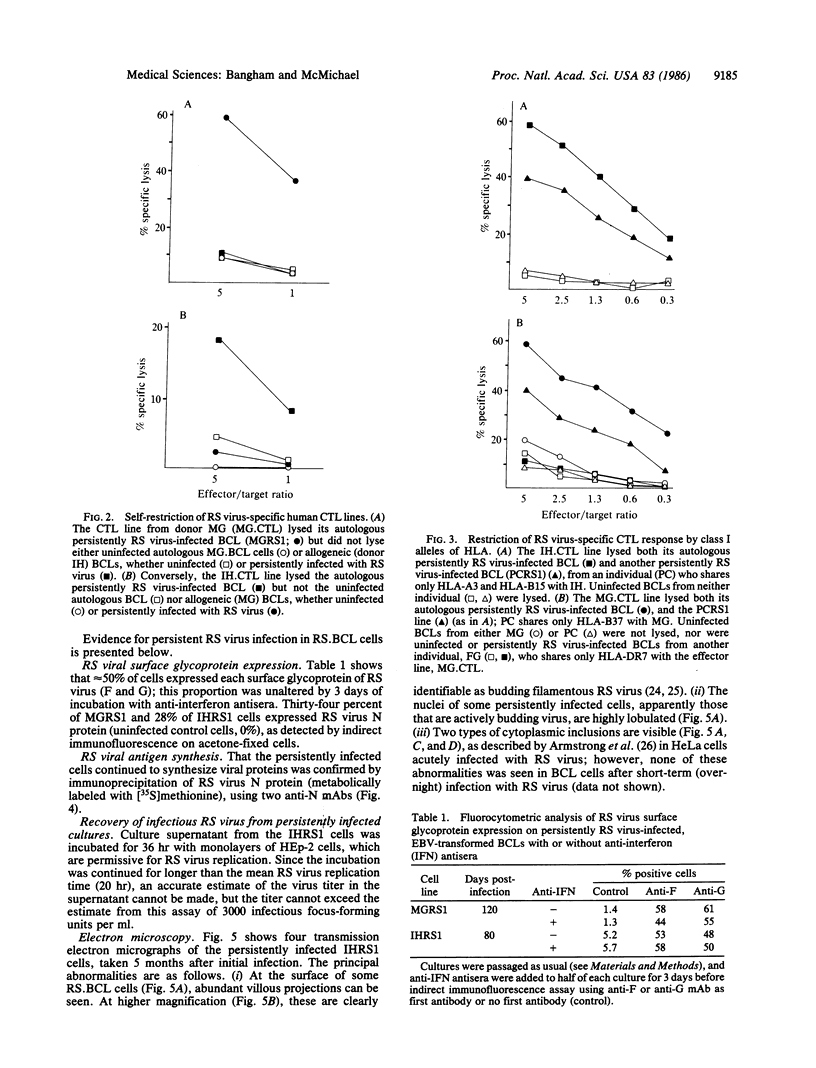


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldridge P., Senterfit L. B. Persistent infection of cells in culture by respiratory syncytial virus. Proc Soc Exp Biol Med. 1976 Apr;151(4):684–688. doi: 10.3181/00379727-151-39286. [DOI] [PubMed] [Google Scholar]
- Bangham C. R., Askonas B. A. Murine cytotoxic T cells specific to respiratory syncytial virus recognize different antigenic subtypes of the virus. J Gen Virol. 1986 Apr;67(Pt 4):623–629. doi: 10.1099/0022-1317-67-4-623. [DOI] [PubMed] [Google Scholar]
- Bangham C. R., Cannon M. J., Karzon D. T., Askonas B. A. Cytotoxic T-cell response to respiratory syncytial virus in mice. J Virol. 1985 Oct;56(1):55–59. doi: 10.1128/jvi.56.1.55-59.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beverley P. C., Callard R. E. Distinctive functional characteristics of human "T" lymphocytes defined by E rosetting or a monoclonal anti-T cell antibody. Eur J Immunol. 1981 Apr;11(4):329–334. doi: 10.1002/eji.1830110412. [DOI] [PubMed] [Google Scholar]
- Bächi T., Howe C. Morphogenesis and ultrastructure of respiratory syncytial virus. J Virol. 1973 Nov;12(5):1173–1180. doi: 10.1128/jvi.12.5.1173-1180.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cote P. J., Jr, Fernie B. F., Ford E. C., Shih J. W., Gerin J. L. Monoclonal antibodies to respiratory syncytial virus: detection of virus neutralization and other antigen-antibody systems using infected human and murine cells. J Virol Methods. 1981 Oct;3(3):137–147. doi: 10.1016/0166-0934(81)90048-3. [DOI] [PubMed] [Google Scholar]
- Creager R. S., Whitaker-Dowling P., Frey T. K., Youngner J. S. Varied responses of human B-lymphoblastoid cell lines to infection with vesicular stomatitis virus. Virology. 1982 Sep;121(2):414–419. doi: 10.1016/0042-6822(82)90179-9. [DOI] [PubMed] [Google Scholar]
- Domurat F., Roberts N. J., Jr, Walsh E. E., Dagan R. Respiratory syncytial virus infection of human mononuclear leukocytes in vitro and in vivo. J Infect Dis. 1985 Nov;152(5):895–902. doi: 10.1093/infdis/152.5.895. [DOI] [PubMed] [Google Scholar]
- Dongworth D. W., McMichael A. J. Inhibition of human T lymphocyte function with monoclonal antibodies. Br Med Bull. 1984 Jul;40(3):254–261. doi: 10.1093/oxfordjournals.bmb.a071986. [DOI] [PubMed] [Google Scholar]
- Evans R. L., Wall D. W., Platsoucas C. D., Siegal F. P., Fikrig S. M., Testa C. M., Good R. A. Thymus-dependent membrane antigens in man: inhibition of cell-mediated lympholysis by monoclonal antibodies to TH2 antigen. Proc Natl Acad Sci U S A. 1981 Jan;78(1):544–548. doi: 10.1073/pnas.78.1.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernie B. F., Ford E. C., Gerin J. L. The development of Balb/c cells persistently infected with respiratory syncytial virus: presence of ribonucleoprotein on the cell surface. Proc Soc Exp Biol Med. 1981 May;167(1):83–86. doi: 10.3181/00379727-167-41129. [DOI] [PubMed] [Google Scholar]
- Fishaut M., Tubergen D., McIntosh K. Cellular response to respiratory viruses with particular reference to children with disorders of cell-mediated immunity. J Pediatr. 1980 Feb;96(2):179–186. doi: 10.1016/s0022-3476(80)80799-2. [DOI] [PubMed] [Google Scholar]
- Goswami K. K., Cameron K. R., Russell W. C., Lange L. S., Mitchell D. N. Evidence for the persistence of paramyxoviruses in human bone marrows. J Gen Virol. 1984 Nov;65(Pt 11):1881–1888. doi: 10.1099/0022-1317-65-11-1881. [DOI] [PubMed] [Google Scholar]
- Hildreth J. E. N-D-Gluco-N-methylalkanamide compounds, a new class of non-ionic detergents for membrane biochemistry. Biochem J. 1982 Nov 1;207(2):363–366. doi: 10.1042/bj2070363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson S., McFarland H. F. Measles virus persistence in human lymphocytes: a role for virus-induced interferon. J Gen Virol. 1982 Dec;63(2):351–357. doi: 10.1099/0022-1317-63-2-351. [DOI] [PubMed] [Google Scholar]
- Jacobson S., Richert J. R., Biddison W. E., Satinsky A., Hartzman R. J., McFarland H. F. Measles virus-specific T4+ human cytotoxic T cell clones are restricted by class II HLA antigens. J Immunol. 1984 Aug;133(2):754–757. [PubMed] [Google Scholar]
- Joseph B. S., Lampert P. W., Oldstone M. B. Replication and persistence of measles virus in defined subpopulations of human leukocytes. J Virol. 1975 Dec;16(6):1638–1649. doi: 10.1128/jvi.16.6.1638-1649.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannagi M., Sugamura K., Kinoshita K., Uchino H., Hinuma Y. Specific cytolysis of fresh tumor cells by an autologous killer T cell line derived from an adult T cell leukemia/lymphoma patient. J Immunol. 1984 Aug;133(2):1037–1041. [PubMed] [Google Scholar]
- Kannagi M., Sugamura K., Sato H., Okochi K., Uchino H., Hinuma Y. Establishment of human cytotoxic T cell lines specific for human adult T cell leukemia virus-bearing cells. J Immunol. 1983 Jun;130(6):2942–2946. [PubMed] [Google Scholar]
- Kim H. W., Arrobio J. O., Brandt C. D., Jeffries B. C., Pyles G., Reid J. L., Chanock R. M., Parrott R. H. Epidemiology of respiratory syncytial virus infection in Washington, D.C. I. Importance of the virus in different respiratory tract disease syndromes and temporal distribution of infection. Am J Epidemiol. 1973 Sep;98(3):216–225. doi: 10.1093/oxfordjournals.aje.a121550. [DOI] [PubMed] [Google Scholar]
- Kim H. W., Canchola J. G., Brandt C. D., Pyles G., Chanock R. M., Jensen K., Parrott R. H. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol. 1969 Apr;89(4):422–434. doi: 10.1093/oxfordjournals.aje.a120955. [DOI] [PubMed] [Google Scholar]
- Kim H. W., Leikin S. L., Arrobio J., Brandt C. D., Chanock R. M., Parrott R. H. Cell-mediated immunity to respiratory syncytial virus induced by inactivated vaccine or by infection. Pediatr Res. 1976 Jan;10(1):75–78. doi: 10.1203/00006450-197601000-00015. [DOI] [PubMed] [Google Scholar]
- Kreth H. W., Kress L., Kress H. G., Ott H. F., Eckert G. Demonstration of primary cytotoxic T cells in venous blood and cerebrospinal fluid of children with mumps meningitis. J Immunol. 1982 Jun;128(6):2411–2415. [PubMed] [Google Scholar]
- Mahy B. W. Strategies of virus persistence. Br Med Bull. 1985 Jan;41(1):50–55. doi: 10.1093/oxfordjournals.bmb.a072024. [DOI] [PubMed] [Google Scholar]
- Malissen B., Rebai N., Liabeuf A., Mawas C. Human cytotoxic T cell structures associated with expression of cytolysis. I. Analysis at the clonal cell level of the cytolysis-inhibiting effect of 7 monoclonal antibodies. Eur J Immunol. 1982 Sep;12(9):739–747. doi: 10.1002/eji.1830120908. [DOI] [PubMed] [Google Scholar]
- McChesney M. B., Fujinami R. S., Lampert P. W., Oldstone M. B. Viruses disrupt functions of human lymphocytes. II. Measles virus suppresses antibody production by acting on B lymphocytes. J Exp Med. 1986 May 1;163(5):1331–1336. [PMC free article] [PubMed] [Google Scholar]
- McIntosh K., Fishaut J. M. Immunopathologic mechanisms in lower respiratory tract disease of infants due to respiratory syncytial virus. Prog Med Virol. 1980;26:94–118. [PubMed] [Google Scholar]
- McMichael A. J., Askonas B. A. Influenza virus-specific cytotoxic T cells in man; induction and properties of the cytotoxic cell. Eur J Immunol. 1978 Oct;8(10):705–711. doi: 10.1002/eji.1830081007. [DOI] [PubMed] [Google Scholar]
- McMichael A. J., Gotch F. M., Noble G. R., Beare P. A. Cytotoxic T-cell immunity to influenza. N Engl J Med. 1983 Jul 7;309(1):13–17. doi: 10.1056/NEJM198307073090103. [DOI] [PubMed] [Google Scholar]
- Meuer S. C., Hodgdon J. C., Cooper D. A., Hussey R. E., Fitzgerald K. A., Schlossman S. F., Reinherz E. L. Human cytotoxic T cell clones directed at autologous virus-transformed targets: further evidence for linkage of genetic restriction to T4 and T8 surface glycoproteins. J Immunol. 1983 Jul;131(1):186–190. [PubMed] [Google Scholar]
- Mills B. G., Singer F. R., Weiner L. P., Suffin S. C., Stabile E., Holst P. Evidence for both respiratory syncytial virus and measles virus antigens in the osteoclasts of patients with Paget's disease of bone. Clin Orthop Relat Res. 1984 Mar;(183):303–311. [PubMed] [Google Scholar]
- Moss D. J., Wallace L. E., Rickinson A. B., Epstein M. A. Cytotoxic T cell recognition of Epstein-Barr virus-infected B cells. I. Specificity and HLA restriction of effector cells reactivated in vitro. Eur J Immunol. 1981 Sep;11(9):686–693. doi: 10.1002/eji.1830110904. [DOI] [PubMed] [Google Scholar]
- P'ringle C. R., Shirodaria P. V., Cash P., Chiswell D. J., Malloy P. Initiation and maintenance of persistent infection by respiratory syncytial virus. J Virol. 1978 Oct;28(1):199–211. doi: 10.1128/jvi.28.1.199-211.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrott R. H., Kim H. W., Arrobio J. O., Hodes D. S., Murphy B. R., Brandt C. D., Camargo E., Chanock R. M. Epidemiology of respiratory syncytial virus infection in Washington, D.C. II. Infection and disease with respect to age, immunologic status, race and sex. Am J Epidemiol. 1973 Oct;98(4):289–300. doi: 10.1093/oxfordjournals.aje.a121558. [DOI] [PubMed] [Google Scholar]
- Parry J. E., Shirodaria P. V., Pringle C. R. Pneumoviruses: the cell surface of lytically and persistently infected cells. J Gen Virol. 1979 Aug;44(2):479–491. doi: 10.1099/0022-1317-44-2-479. [DOI] [PubMed] [Google Scholar]
- Prince G. A., Horswood R. L., Camargo E., Koenig D., Chanock R. M. Mechanisms of immunity to respiratory syncytial virus in cotton rats. Infect Immun. 1983 Oct;42(1):81–87. doi: 10.1128/iai.42.1.81-87.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince G. A., Horswood R. L., Chanock R. M. Quantitative aspects of passive immunity to respiratory syncytial virus infection in infant cotton rats. J Virol. 1985 Sep;55(3):517–520. doi: 10.1128/jvi.55.3.517-520.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinnan G. V., Jr, Kirmani N., Esber E., Saral R., Manischewitz J. F., Rogers J. L., Rook A. H., Santos G. W., Burns W. H. HLA-restricted cytotoxic T lymphocyte and nonthymic cytotoxic lymphocyte responses to cytomegalovirus infection of bone marrow transplant recipients. J Immunol. 1981 May;126(5):2036–2041. [PubMed] [Google Scholar]
- Rabin H., Hopkins R. F., 3rd, Ruscetti F. W., Neubauer R. H., Brown R. L., Kawakami T. G. Spontaneous release of a factor with properties of T cell growth factor from a continuous line of primate tumor T cells. J Immunol. 1981 Nov;127(5):1852–1856. [PubMed] [Google Scholar]
- Ron D., Tal J. Coevolution of cells and virus as a mechanism for the persistence of lymphotropic minute virus of mice in L-cells. J Virol. 1985 Aug;55(2):424–430. doi: 10.1128/jvi.55.2.424-430.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Routledge E. G., McQuillin J., Samson A. C., Toms G. L. The development of monoclonal antibodies to respiratory syncytial virus and their use in diagnosis by indirect immunofluorescence. J Med Virol. 1985 Mar;15(3):305–320. doi: 10.1002/jmv.1890150311. [DOI] [PubMed] [Google Scholar]
- Scott R., Pullan C. R., Scott M., McQuillin J. Cell-mediated immunity in respiratory syncytial virus disease. J Med Virol. 1984;13(1):105–114. doi: 10.1002/jmv.1890130112. [DOI] [PubMed] [Google Scholar]
- Taylor G., Stott E. J., Bew M., Fernie B. F., Cote P. J., Collins A. P., Hughes M., Jebbett J. Monoclonal antibodies protect against respiratory syncytial virus infection in mice. Immunology. 1984 May;52(1):137–142. [PMC free article] [PubMed] [Google Scholar]
- Townsend A. R., Gotch F. M., Davey J. Cytotoxic T cells recognize fragments of the influenza nucleoprotein. Cell. 1985 Sep;42(2):457–467. doi: 10.1016/0092-8674(85)90103-5. [DOI] [PubMed] [Google Scholar]
- Walsh E. E., Schlesinger J. J., Brandriss M. W. Purification and characterization of GP90, one of the envelope glycoproteins of respiratory syncytial virus. J Gen Virol. 1984 Apr;65(Pt 4):761–767. doi: 10.1099/0022-1317-65-4-761. [DOI] [PubMed] [Google Scholar]
- Zinkernagel R. M., Doherty P. C. MHC-restricted cytotoxic T cells: studies on the biological role of polymorphic major transplantation antigens determining T-cell restriction-specificity, function, and responsiveness. Adv Immunol. 1979;27:51–177. doi: 10.1016/s0065-2776(08)60262-x. [DOI] [PubMed] [Google Scholar]