Abstract
Claudins are pivotal building blocks of tight junctions that form the paracellular barrier in epithelia and endothelia. In mammals, claudins are a 27-gene family that encodes tetraspan membrane proteins, playing a crucial role in the formation and integrity of tight junctions and regulate the barrier function. Claudin isoforms are expressed in a tissue- and/or developmental stage-dependent manner. A growing body of evidence indicates that pathological states characterized by neuroinflammation, such as Alzheimer disease, multiple sclerosis, diabetic retinopathy and retinopathy of prematurity share a common feature: the barrier breakdown. This review aims integrating and summarizing the most relevant and recent work developed in the field of claudins, with particular attention to their role in blood-brain and blood-retinal barriers, as well as describing their regulation in the aforementioned human diseases.
Keywords: claudins, blood-retinal barrier, blood-brain barrier, tight junctions, paracellular transport
Tight Junctions and Paracellular Transport in the Brain and Retina
The blood-brain barrier (BBB) concept was first derived from experimental observations made by Ehrlich in 18851 and later by Goldman in 1913,2 after injecting colored dyes into the blood stream. The experiments conducted by Goldman showed that the trypan blue dye stained almost all tissues except the brain. When the dye was injected into the cerebrospinal fluid (CSF) that surrounds the brain, this organ was stained, but the rest of the tissues in the body were not, providing the first demonstration that there is a kind of compartmentalization between the bloodstream and the CSF. Subsequent studies performed by Stern confirmed the presence of a special filter at the blood-brain interface that protects the brain, which was called blood-brain barrier (until then called hematoencephalic barrier).
Several decades later, morphological studies using transmission electron microscopy, showed the presence of “zonula occludens” between retinal endothelial cells. Moreover, permeability measurements, after systemic or intravitreal injection of fluorescein, were the basis of the concept of a blood-retinal barrier (BRB).3,4 Based on those and other findings, it was proposed that the BRB consists of two anatomical components, an inner BRB (iBRB) composed by tight junctions (TJ) between retinal capillary endothelial cells and an outer BRB (oBRB) formed by TJ between retinal pigment epithelial (RPE) cells.5,6
In the brain and retina, the presence of a barrier between the vascular lumen and neural layers and parenchyma, respectively, allows the maintenance of a regulated microenvironment and the proper neuronal function. BBB is formed by a continuous monolayer of endothelial cells, separating the nervous system from circulating blood.
The BBB is comprised of brain microvascular endothelial cells, astrocytes and smooth muscle cells, the pericytes. It has been claimed that astrocytes secrete soluble factors that are important to strengthen the barrier function in BBB.7,8 Pericytes have also an important role in maintaining and strengthen the barrier function, as demonstrated by in vitro BBB models.9
Cerebral homeostasis also results from the ability of the blood-cerebrospinal fluid barrier (BCSFB) at the choroid plexus to control the composition of the CSF and cerebral extracellular fluid. Unlike the capillaries that form the BBB, choroid plexus capillaries do not have TJ and are fenestrated and therefore do not form a barrier to the passage of small molecules. Instead, the BCSFB at the choroid plexus is formed by TJ between the epithelial cells and TJ linked to the arachnoid membrane that envelops the brain.
The permeability barriers, as a result of the compartmentalization created by epithelia and endothelia with TJ, regulate the paracellular movement of ions and small molecules between adjacent cells. TJ strands are complex structures, composed of transmembrane and cytosolic proteins that function as a gate, which is sensitive to rapid changes on the microenvironment. TJ provide at least dual functions to the tissues, as barrier and fence, which are essential for the tissue development and homeostasis, as well as for the maintenance of cell polarity as a boundary between the apical and basolateral plasma membrane domains.10-12 Recent advances have improved our understanding about the molecular components, regulation and function of the TJ permeability. TJ are dynamic complexes in which the extracellular domains of TJ proteins associate with extracellular domains of proteins on adjacent cells. The branching network of sealing strands of proteins found in endothelial and epithelial TJ includes a series of transmembrane proteins embedded in the plasma membrane, such as junctional adhesion molecules (JAMS), claudins, occludin and tricellulin,13,14 which in turn are attached to several cytoskeleton and cytoplasmic scaffold proteins, including zonula occludens (ZO)-1/2/3, MAGI-1, MAGI-3, CASK/LIN-2, MUPP1, AF6, ASIP, PALS1, PATJ and cingulin.15
It is now clear that size-, charge-selectivity and permeability of TJ are tissue specific and depend on their essential components, the claudins.16-20 In this review, we will summarize recent progress with respect to claudins, giving particular attention to their function and regulation in the brain and retinal barriers in health and pathological conditions.
Claudins Family: Structure, Function and Distribution
TJ constitute the most apical intercellular junctional complex between adjacent endothelial and epithelial cells. The TJ, a multiprotein complex consisting of transmembrane and cytosolic proteins, control and restrict the paracellular diffusion of macromolecules, ions and polar solutes.21
Based on transmission electron microscopy studies, TJ were first described as structures in which the outer leaflets of the membranes of two adjacent cells are merged into a single line or into a series of apparent fusions (kissing points).22 Afterward, freeze-fracture electron microscopy showed that these fusions were composed by strands of intramembranous particles, corresponding to TJ.23
One of the major components of the TJ strands is the claudin family, which comprises 27 members in mice and humans.16 Claudins were first purified and identified by Furuse and colleagues in 1998.17 The name claudin derives from the latin claudere which means close. Based on sequence analysis of the mouse claudin proteins, Krause and colleagues proposed a subdivision of the claudin family into classic (claudin-1–10, 14, 15, 17, 19) and non-classic (claudin-11–13, 16, 18, 20–24) groups for mouse proteins.24 Later on, it was proposed a similar division for the human proteins, with slight differences, considering that claudin-1–9, 14, 17, 19, 20 are classic claudins and claudin-10–12, 15, 16, 18, 21–24 are non-classic claudins.25 Recently, it was proposed another classification of the claudin family, based on their permeability attributes, dividing each member into different categories: sealing, channel forming (anion- or cation-selective and water-permeable), inconsistent functionality and limited functional characterization.26
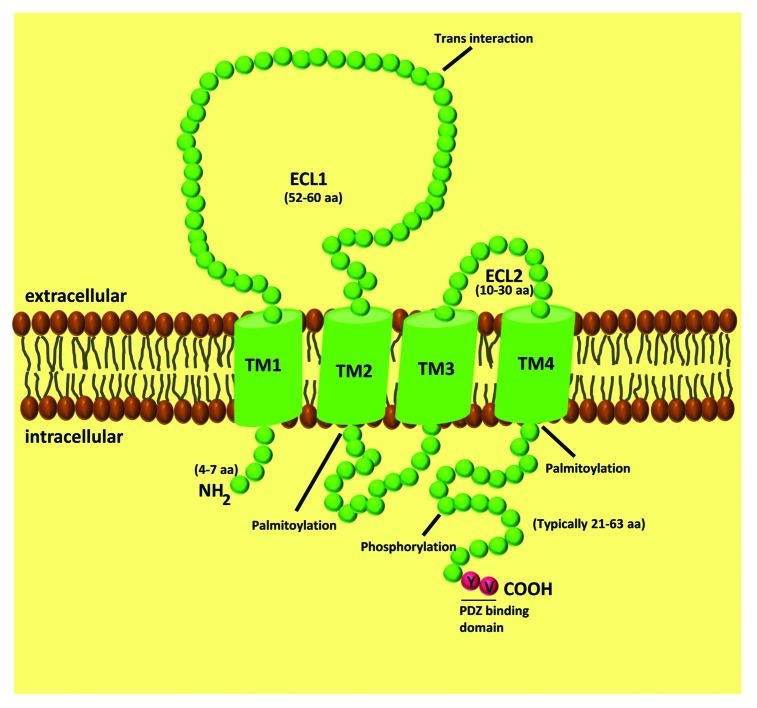
Claudins are 20–34 kDa proteins, containing four transmembrane domais, N- and C-terminal cytoplasmic domains and two extracellular loops (ECL) (Fig. 1). The C-terminal tail of claudins is essential for their stability and intracellular transport to the TJ.27-29 For some claudins, this domain can be phosphorylated to regulate barrier function, and its phosphorylation has been linked to either increases or decreases in TJ assembly and function. For example, it has been shown that cyclic AMP induces phosphorylation of Thr-207 in claudin-5 by protein kinase A (PKA), increasing the barrier function in brain endothelium.30 However, when the same residue of claudin-5 is phosphorylated by PKA in lung endothelial cells, a size selective loosening of claudin-5-based barrier against small molecules was found.31 In brain endothelial cells, Rho/ROCK signaling leads to the phosphorylation of claudin-5 at Ser and Tyr residues, increasing BBB permeability.32 In lung endothelial cells, phosphorylation of claudin-1 at Thr-203 by MAPK promotes barrier functions.33 In colon carcinoma cells, phosphorylation of claudin-4 at Tyr-208 by ephrin type-A receptor 2 (EphA2) attenuates its interaction with ZO-1 and reduces the integration of claudin-4 into TJ, enhancing paracellular permeability.34 Similarly, PKA-dependent phosphorylation of claudin-3 at Thr-192 leads to its cytoplasmic localization and barrier dysfunction in ovarian cancer cells.35 In summary, claudins can be phosphorylated by different kinases, which controls claudin localization and/or function. However, since the phosphorylation of claudins triggers different outcomes, it is not possible to make a general functional conclusion since the results differ enormously.
Figure 1. Schematic representation of claudin proteins. Claudins have four transmembrane spanning regions, two extracellular loops, one intracellular domain, with the amino and carboxyl terminus oriented toward the cytoplasm.
Other posttranslational modifications of claudins, including palmitoylation, have been described. Palmitoylation occurs at the conserved di-cysteine motifs, located right after the second and fourth transmembrane domains.36 Palmitoylation of these motifs is thought to be required for incorporation of claudin-2, 4 and 14 into the TJ, enabling their translocation to detergent-resistant plasma membranes (lipid rafts).36
Additionally, almost every claudin has a PDZ binding motif at the C-terminus that allows binding to the PDZ domain of cytoplasmic scaffolding proteins: ZO-1/2/3,37 PATJ38 and MUPP1.39 Association with the scaffold proteins indirectly links claudin strands to the actin cytoskeleton and regulates claudins function.
The first ECL (ECL1) of the claudin family is composed by ~50–60 amino acids being much longer than the second one, which is composed only by ~10–30 amino acids. ECL1 appears to be involved in the ionic properties of claudin strands. The role of ECL1 in determining the selectivity for ions was demonstrated using mutagenesis assays. For example, the replacement of acidic by basic amino acid residues in claudin-15 led to a reversal in paracellular charge selectivity, from a preference for cations to anions.40 This has also been demonstrated in other claudin isoforms.40-42 ECL1 also contains a GLWCC motif, which acts as a receptor for Hepatitis C virus (HCV) entry. This has been demonstrated for claudin-1, 6 and 9, which are widely expressed in the liver and in peripheral blood mononuclear cells, precisely the major HCV replication sites.43,44
The second ECL (ECL2), due to its predicted helix-turn-helix motif, appears to be more important for the transcellular binding that narrows the paracellular space.45 Moreover, it has been demonstrated that the ECL2 of claudin-3 and 4 is a receptor for Clostridium perfringens enterotoxin.46,47
The ECL from different claudins can interact with each other between opposing cells (trans-interaction) or in the same cell (cis-interaction).24 The cis-interaction can involve the same claudin subtype (homomeric interaction) or different claudin subtypes (heteromeric interaction). The trans-interaction can be homotypic or heterotypic.24,45,48
Transfection of L-fibroblasts with different claudin isoforms, and their co-cultivation, show that both claudin-1 and claudin-2 can trans-interact with claudin-3 but not to each other.49 More recently, Piontek and colleagues have demonstrated homotypic trans-interactions between claudin-1, 2, 3 and 5 (but not between claudin-12) and heterotypic trans-interactions between claudin-1 and 5, claudin-1 and 3 and claudin-3 and 5.48 Despite nearly identical ECL domains and cis-type interaction, claudin-3 and 4 are heterotypically incompatible,50 pointing out that the amino acid sequence of the ECL is not the only determinant for the trans-interaction between claudins.
Claudins form ladders of stable homomultimers in native gel electrophoresis with increased molecular mass, corresponding to hexamers, which suggests a similar strand conformation to connexins in gap junctions.51,52 The formation of claudin-4 monomers and claudin-2 homodimers occurs via the second transmembrane domain (helix–helix interaction).53 Cis homo-dimerization was detected by fluorescence resonance energy transfer (FRET) analysis in claudin-5.54 Cis-homomeric interactions have also been described for claudin-1, 2 and 3, along with heteromeric interactions between claudin-5 and 1, claudin-3 and 1 and claudin-3 and 5.45,48 Interactions between claudin-4 and 8 and claudin-16 and 19, have also been studied. Claudin-4 and claudin-8 are co-transported from the Golgi apparatus to the TJ, cis-interacting with each other.55 Claudin-16 and claudin-19, like claudin-3 and 4 share high similarity in their ECL. They show cis, but not trans-interaction.56 The claudin-claudin interactions, in the same cell and between two adjacent cells, are responsible for the establishment of a continuous barrier within the intercellular clefts of the monolayer. This should mean that the network of strands formed by various claudin isoforms in each tissue leads to a selective permeability of the solutes or compounds with different molecular weights or differently charged ions.
The diverse claudin subtypes are expressed in a tissue- and cell type-specific manner varying with the stage of development and differing in their barrier properties.24 They can functionally be divided in barrier-forming or sealing claudins (for example claudin-1, 3 and 5) that are found in several epithelia and endothelia with a moderate to high transepithelial or endothelial resistance (TER), respectively.57 Additionally, they can be pore-forming claudins; claudin-2 and a complex between claudin-16 and claudin-19 form pores for cations,56,58 while claudin-17 forms an anion-selective pore.59 Claudin-2 also forms a water channel, mediating paracellular water transport in leaky epithelia.60 Interestingly, alternative splicing of claudin-10 gene originates claudin-10a and b, a cation and anion-selective claudin pore-forming, respectively.61
The function of several claudins, like for example claudin-4, 7 or 8, are not yet fully understood, mainly because very different and sometimes contradictory results have been obtained, namely in studies where claudins are overexpressed or knockdown. However, this strongly depends on the cell type used, and one cannot discard the possibility of interactions, or lack of them, with other claudins or other TJ proteins endogenously expressed in a specific cell type.
Some physiological roles of claudins have been clarified from studies with transgenic and knockout mice or human diseases. Claudin-1 knockout mice die shortly after birth due to dermal water loss indicating the essential role of claudin-1 in contributing to the tightness of skin epithelia.20 Mutations in claudin-1 gene cause neonatal ichthyosis and sclerosing cholangitis in humans.62 Deficiency in claudin-5 also causes neonatal death, due to size-selective loosening of the BBB.63 Mice deficient in claudin-11 show myelin defects and the male animals are sterile due to the breakdown of the blood-testis barrier.64 Mutations in claudin-14 gene lead to autosomal recessive deafness in mice and humans.65 Several mutations in claudin-16 gene are seen in patients of familial hypomagnesemia with hypercalciuria and nephrocalcinosis, an autosomal recessive disorder that leads to renal calcification processes and renal failure.66,67 Mutations in claudin-19 cause renal hypomagnesemia with ocular involvement.68
Claudins in Blood-Brain and Blood-Retinal Barriers
The BBB is a selective interface between the blood and the brain that maintains ionic homeostasis within the brain microenvironment.69 The lack of fenestrations, decreased pinocytotic activity and presence of TJ, contribute to a high TER (1500–2000 Ω/cm2) and to the restrictive nature of the BBB. On the contrary, systemic capillaries present a TER of only 5–10 Ω/cm2.70,71
At the BBB, claudins-1, 3, 5 and 12 participate in the formation of TJ between brain microvascular endothelial cells (see Table 1).18,63,72,73 Claudin-5 is the most abundant claudin at the BBB and is a critical regulator of brain endothelial cells permeability. In claudin-5 knockout mice, Nitta and colleagues demonstrated that the size-selectivity of the BBB was affected, allowing the diffusion of molecules smaller than 800 Da, but not of larger molecules. These mice present morphologically normal TJ but died within 10 h of birth. It remains unclear whether this was due to BBB defects. Furthermore, overexpression of claudin-5 in cultured brain microvascular endothelial cells increases barrier properties.74 Moreover, the expression of the TJ protein claudin-1 is lost in brain tumor microvessels, while claudin-5 is only downregulated, suggesting a relationship between claudin-1 suppression and the alteration of TJ morphology, which is likely to be correlated with the increase in endothelial cell permeability.72 Similarly, the selective loss of claudin-3 from the TJ in pathological conditions demonstrates that it may be important for determining permeability and BBB integrity.73 Claudin-12 at the cell-cell boundaries of brain capillary endothelial cells was detected in the brain of mice embryo.63 In adult tissue, the levels of claudin-12 mRNA were relatively lower than those of claudin-5 at rat brain capillaries, and when the whole brain was analyzed, claudin-12 was shown not to be restricted only to capillaries.57 Possibly, claudin-12 expression in BBB changes during development.
Table 1. Claudin expression changes in blood-brain and blood-retinal barriers in several neuroinflammatory diseases.
| Barrier | Claudin | Expression | Alterations |
|---|---|---|---|
| Brain |
|
|
|
| BBB |
Claudin-1 |
mRNA and protein detected in mouse57 and human72 |
Expression suppressed in brain tumor vessels72 |
| |
Claudin-3 |
mRNA and protein detected in mouse57 and human73 |
Loss of the TJ strands in a MS animal model and in brain tumor vessels73 |
| |
Claudin-5 |
mRNA and protein detected in mouse18,57,63 and human72 |
Knockout mice have selective blood–brain barrier dysfunction for molecules < 800 Da;63 Decreased expression124 and subcellular redistribution114 in a MS animal model |
| |
Claudin-12 |
High mRNA levels in mice embryos, relative low expression in adult tissue63 |
No known alteration |
| BCSFB |
Claudin-1 |
mRNA and protein detected in mouse,75 rat152 and human76 |
No known alteration |
| |
Claudin-2 |
mRNA and protein detected in mouse,75 rat152 and human76 |
No known alteration |
| |
Claudin-3 |
mRNA and protein detected in rat and human76 |
No known alteration |
| |
Claudin-11 |
Protein detected in mouse75 |
No known alteration |
| Retina |
|
|
|
| iBRB |
Claudin-1 |
mRNA and protein detected in mouse,77 rat79 and rabbit78 |
No known alteration |
| |
Claudin-2 |
mRNA and protein detected in mouse77 |
Overexpression in OIR animal model77 |
| |
Claudin-5 |
mRNA and protein detected in mouse77 and rat79,130 |
Overexpression in OIR animal model;77 Reduced expression and subcellular redistribution in diabetes animal models79,132,136,137 |
| oBRB |
Claudin-1 |
mRNA and protein detected in chick embryo80,81 |
Increased expression after ER stress induction;141 Subcellular redistribution after high glucose and IL-1β exposure95 |
| |
Claudin-2 |
mRNA and protein detected in chick embryo80,82 |
No known alteration |
| |
Claudin-3 |
mRNA and protein detected in human83,84 |
No known alteration |
| |
Claudin-4L2 |
mRNA detected in chick embryo81 |
No known alteration |
| |
Claudin-5 |
mRNA and protein detected in chick embryo80,81 |
No known alteration |
| |
Claudin-10b |
mRNA and protein detected in human84 |
No known alteration |
| |
Claudin-11 |
mRNA detected in chick embryo81 |
No known alteration |
| |
Claudin-12 |
mRNA and protein detected in chick embryo80 |
No known alteration |
| |
Claudin-19 |
mRNA and protein detected in human83,84 |
siRNA against claudin-19 eliminates TER in vitro;83 Mutations in claudin-19 gene cause renal hypomagnesemia with severe visual impairment68 |
| Claudin-20 | mRNA detected in chick embryo81,82 | No known alteration |
The barriers that surround the central nervous system are critical for its protection and homeostasis. While the BBB has been investigated intensively, only recently the choroid plexus-blood barrier, also known as the BCSFB, has received attention. BCSFB is characterized by having lower TER values (150 Ω/cm2) and being less restrictive than the BBB. The molecular organization underlying that difference is probably related to the expression of different claudins, since those proteins play an important role in barrier size-selectivity and in the control of paracellular movement of ions. In fact, claudins-1, 2, 3 and 11 are expressed in the choroid plexus epithelium (see Table 1).75,76 The expression of claudin-2 greatly increases the permeability of this barrier to both cations and water, and one cannot forget that interactions between different claudins can also influence paracelullar tightening. Despite low evidences showing the contribution of claudin-11 in the BCSFB, an important role of this isoform was pointed out in other cell types, namely in oligodendrocytes and Sertoli cells.64 Immunohistochemical analysis of human/rat fetal and postnatal brains for claudin-1, 2 and 3 demonstrated their early presence and localization at the apico-lateral border of the choroid plexus epithelial cells.76 Increased mRNA expression of claudin-6, 9, 19 and 22 also displayed a previously undescribed choroidal selectivity, although the authors could not confirm the presence of claudin-6 and 22 at the TJ due to lack of appropriate antibodies.76 It was also detected a developmental upregulation of claudin-2, 9 and 22 and downregulation of claudin-6, reflecting changes in selective blood to CSF transport functions during development, which may be crucial for brain protection.
In the eye, the blood-tissue barrier is divided into two regions. The iBRB is formed by two beds of capillary endothelia. The inner capillary bed lies in the ganglion cell layer and its barrier function is modulated by astrocytes. The outer capillary bed lies in the inner and outer plexiform layers, where the function of astrocytes is replaced by Muller cells. The oBRB is formed by the RPE and lies on the outer surface of the photoreceptor layer.
At the iBRB, claudin-1, 2 and 5 are the most abundant claudins (see Table 1).77-79 In comparison with BBB, the iBRB might be more permeable to ions in general, due to the lack of claudin-3 and more permeable still to the cation sodium, due to the presence of claudin-2.
The expression of claudins during the formation of the iBRB TJ also seems to vary. The mRNA levels of claudin-1, 2, 3, 4, 5, 12, 22 and 23 were shown to be developmentally altered in the retinas of mice pups, from postnatal day 8 (P8) until P21. Claudin-22 mRNA increased throughout this period, but the others exhibited transient peaks. The protein levels of claudin-1 and 5 remained high, even though the amount of their mRNA decreased at P21. On the contrary, the protein levels of claudin-2 paralleled the decrease in mRNA expression. Among all the claudins expressed in neural retina, only claudin-1, 2 and 5 were found in the blood vessels, which are present in the inner, outer and ganglion cell vascular layers. All three claudins co-localized with occludin in the lateral membranes of endothelial cells.77 By contrast, claudin-3, 4, 12 and 23 were localized in extravascular cells.
The oBRB regulates the movement of solutes between the fenestrated capillaries of the choroid and the photoreceptor layer of the retina. The formation of TJ and TJ protein expression in RPE seem to vary considerably between species and developmental stage. The analysis of RPE from chick embryos demonstrates that claudins continue to be tightly regulated even after the barrier is fully functional. Claudin-1, 2, 4L2, 5, 11, 12 and 20 mRNA have been detected (see Table 1). For example, claudin-5 is transiently expressed, while claudin-1 appears in an intermediate phase and others, like claudin-20, appear later.80-82 These results suggest that from the time that functional TJ form to the time they mature, the selectivity and permeability of the oBRB are likely to change. Human RPE expresses predominantly claudin-19 mRNA and protein, also with significant amounts of claudin-3 (see Table 1).83-85 In a monolayer of a human fetal RPE, claudin isoforms have a diverse localization. For instance, although claudin-19 and claudin-3 were uniformly expressed across the monolayer, claudin-10 and claudin-1 were only detected in a subset of cells.83,84 Moreover, knockdown of claudin-19 by siRNA in the same in vitro model eliminated the TER, while siRNAs for other claudins had minimal effects,83 supporting the assumption that this claudin has an important role in ocular complications.68
Role of Claudins in Human Diseases
Changes in the integrity of the brain and retinal barriers may affect the neurovascular unit, a functional association of neurons, astrocytes and microvasculature. Findings have shown that blood stream derived-factors and signals from astrocytes and pericytes are involved in the regulation of claudins expression in endothelial cells.86-88 In several barrier dysfunction-related diseases, the levels of claudins and occludin present in microvessels are altered, contributing to the barrier breakdown. In several pathologies of the nervous system characterized by a prominent neuroinflammatory component, such as Alzheimer disease, multiple sclerosis, diabetic retinopathy and retinopathy of prematurity, it has been claimed that brain and retinal barriers dysfunction contributes to the pathogenesis of those diseases, even in the early stages.77,89-95 The increase in passive diffusion of blood-borne substances through TJ detected in several pathological conditions will be discussed below. The redistribution, protein levels and mRNA expression changes of claudin isoforms observed in those pathologies are outlined in Table 1.
Alzheimer disease
Alzheimer disease (AD), the most common dementia in elderly, is characterized by learning and memory impairments.96 AD patients present cerebral amyloid angiopathy and profound changes in cerebral microvessels.97 It has been shown that β-amyloid peptide (1–42) might alter BBB integrity by affecting the TJ complexes.98,99 The disruption of BBB is well documented in AD and may contribute to the progression of disease. Indeed, several reports have shown a positive correlation between increased BBB leakage in aged brains and the degree of AD,100-102 suggesting that BBB disruption may be an early event in AD progression or even an independent factor involved in brain aging. Increased levels of oxidative stress markers associated with downregulation of TJ proteins and increased BBB permeability103 have been found in the early stages of AD.104-107
Moreover, a detailed immunohistochemistry analysis for claudins in AD brains revealed higher levels of claudin-2, 5 and 11 (in neurons), claudin-2 and 11 (in astrocytes) and claudin-11 (in oligodendrocytes), as compared with aged controls.108 The upregulation of these claudin isoforms in AD might be an endogenous protective response of the brain tissue. Neurons expressing claudins were identified as being mainly of pyramidal type, which are thought to support cognition, and are known to be affected in the early stage of AD,109 bearing neurofibrillary tangles. In addition, changes in BBB may result from a deregulation of the interplay between different claudin isoforms, such as claudin-2, which induces a leaky strand type,110 while claudin-5 and 11 are known to be responsible for increasing the TER.111 Although the role of claudins in AD is currently poorly understood, the regulation of claudin expression in different cell types, others than endothelial cells, also suggests a role of claudins in cellular responses to neurodegeneration.
Multiple Sclerosis
Multiple sclerosis (MS) is characterized by microvascular inflammation and demyelination of the nerves of the central nervous system (brain and spinal cord),112 with a relapsing-remitting profile. In MS, increased leukocyte migration leads to a reorganization of the actin cytoskeleton and loss or subcellular redistribution of the brain endothelial TJ proteins claudin-5, occludin and ZO-1,113-115 with a consequent disruption of both BBB and BCSFB. The BBB breakdown allows the infiltration of activated immune effector cells, including T lymphocytes, which in turn activate a complex cascade leading to tissue damage.116,117 Several pro-inflammatory cytokines (tumor necrosis factor (TNF)-α, interferon (IFN)-γ, interleukin (IL)-6 and IL-12)118 and activated matrix metalloproteinases (MMPs) target TJ proteins in brain endothelial cells, compromising the BBB integrity.115,119 In experimental autoimmune encephalomyelitis (EAE), a mouse model used for brain inflammation and MS, it has been suggested that the inflammatory cytokines TNF-α and IL-1 are key mediators that induce alterations in BBB permeability.120 Moreover, it has been consistently shown that TNF-α is detected upon post-mortem examination of MS brain lesions, being abnormally elevated in the CSF of patients. Moreover, the levels of this cytokine have been correlated with the progression and severity of this disease.121-123 Although the molecular mechanisms underlying the regulation of BBB and BCSFB in MS remain poorly understood, recent evidences have shown that Irgm-1, an immune-related GTPase, is involved in the regulation of those barriers. During the initiation and progression of EAE, Irgm-1 is upregulated in epithelial cells of the choroid plexus, ependymal layers and ventricular system, as well as in reactive astrocytes, promoting the disruption of BBB and BCSFB via downregulation of claudin-5 expression on brain microvascular endothelial cells and upregulation of CCL-20 expression in choroid plexus and ependymal cells, respectively.124 It has been also described a compromised endothelial barrier function (decreased TER) that is associated with a protein and mRNA downregulation of claudin-5 and occludin and an upregulation of the matrix metalloproteinase MMP-9 when brain endothelial cells are incubated with sera from patients in the exacerbation or remission phase of MS.125 The downregulation of claudin-5 and occludin, accompanied with an upregulation of vascular endothelial growth factor (VEGF)-A, correlated with the BBB breakdown in an animal model of EAE.88 In the same animal model, a specific loss of claudin-3 immunostaining from the brain microvessels that were surrounded by inflammatory infiltrates was observed,73 suggesting a direct role for inflammatory cells in disrupting BBB TJ.
Diabetic Retinopathy and Retinopathy of Prematurity
The breakdown of the outer and inner BRB in patients with diabetic retinopathy, due to disorganization of TJ proteins, is one of the main factors accounting for macular edema and major vision complications that frequently lead to severe vision loss in patients with diabetes.5,126 Therefore, the BRB is a relevant target for the treatment of retinal diseases, as we have previously discussed.127
The increase in BRB permeability has been described to be associated with changes in the expression, protein levels, phosphorylation, ubiquitination and subcellular distribution of TJ proteins in retinal endothelial cells.128-131 Several studies have shown that the levels of pro-inflammatory cytokines, namely IL-1β and TNF-α, and adhesion molecules, are increased in the retina, vitreous and serum of diabetic patients and rats, being key mediators of TJ proteins disorganization and consequently BRB breakdown.94,132-135 Moreover, alterations in claudin-5 in retinal vessels have been associated with increased vessel leakage in the early stages of diabetes.79,132,136,137 In addition to pro-inflammatory cytokines, growth factors like VEGF, also mediate the increase in BRB permeability and contribute to the pathophysiology of diabetic retinopathy. In vitro studies demonstrate that VEGF treatment disrupts cell border staining of claudin-1138 and 5 and contributes to clathrin-mediated endocytosis of claudin-5.139 Recent reports have also shown that claudin-5 may be downregulated in retinal endothelial cells due to endoplasmic reticulum (ER) stress, which has been involved in vascular impairment in diabetic retinopathy.140 In contrast, in a RPE cell line, ER stress promotes the increase in both protein and mRNA claudin-1 expression, which is accompanied by an increase in TER.141
Retinopathy of prematurity (ROP), the major cause of vision loss in children, is associated with younger gestational age and lower birth weight as risk factors.142 The key pathological change, namely retinal neovascularization, is associated with local ischemia followed by subsequent neovascularization. In more severe forms of the disease, the abnormal vascular changes may progress to retinal detachment, and once retinal detachment occurs the prognosis for recovery of good visual acuity is very low.143 The oxygen-induced retinopathy (OIR) model is widely used for studies of retinal neovascular diseases such as ROP and proliferative diabetic retinopathy.144 Normally, in the OIR model, there is neovascularization in the retina and increased vascular permeability,145 being also detected an overexpression of claudin-2 and 5 (mRNA and protein), while the levels of occludin and claudin-1 were unaffected.77 Moreover, each claudin was also mislocalized to the cytosolic compartment or distributed to nonjunctional regions of the plasma membrane, suggesting a break in tight junctional strands of each cell thus contributing to the formation of new leaky vessels.77
While the breakdown of the iBRB has been investigated extensively, the involvement of the breakdown of the oBRB (RPE barrier) in the progression of certain retinal diseases has not been widely addressed. The leakage through the RPE barrier causes excessive water influx to the retina, and so the breakdown of this barrier is likely to play a causative role in the development of some forms of diabetic macular edema, a major cause of vision loss in diabetic retinopathy, being also involved in the development of age-related macular degeneration.
As mentioned above, inflammation underlies many alterations detected in these retinal pathologies. In ARPE-19 cells, a spontaneously transformed cell line of human adult RPE, the pro-inflammatory cytokine IL-1β promotes a decrease in the TER, while stimulating the expression of claudin-1, although the expression of claudin-11 and 12 remains constant.146 In the same cell line, exposure to high glucose and IL-1β leads to the disruption of claudin-1 staining, despite an increase in its protein levels, which is associated with an increase in the monolayer permeability.95 Upregulation of claudin-1 can induce changes in TJ function by different arrangements, either by altering side-to-side oligomerization that is essential for the formation of TJ within a cell or head-to-head interactions (homophilic or heterophilic) between opposing cells.
Although ARPE-19 cells are widely used and studied, one should be careful when interpreting data from studies regarding the RPE tight junctions using this cell line. Cultured RPE can manifest a greater heterogeneity than the one observed in vivo. In fact, Luo and colleagues reported that the heterogeneity of the tight junctions in ARPE-19 cells was manifested by a nonuniform distribution of claudin-1 and 2 and that the expression of the claudins was very dependent on culture conditions.147 Also, transcriptome analysis revealed that this cell line does not express claudin-19,85 which is, as mentioned before, one of the most important claudin isoform in maintaining the monolayer resistance.
One of the most suitable culture models appears to be derived from a primary human fetal RPE cell culture, as it mimics the normal physiology, function and structure of native fetal and adult RPE, preserving the function of tight junctions and retaining barrier function.148 In human fetal RPE, a mixture of inflammatory cytokines including IL-1β, TNF-α and IFN-γ, or IFN-γ alone, decreased TER after 24 h with an increase in net epithelial fluid absorption.149,150 With longer incubation periods (two days) only TNF-α alone significantly decreased TER, although this decrease was not correlated with changes in claudin-2, claudin-3 or claudin-19 expression.151 Moreover, the authors did not detect any alterations in TER when exposing the human fetal RPE cells to IL-1β,151 contrarily to what was observed in the ARPE-19 cells.146
Conclusion and Perspectives
Claudins are key components of TJ that regulate the paracellular permeability. Although it is well established that claudins can polymerize into TJ strands in heteromeric and heterotypic claudin-claudin interactions, the mechanisms underlying claudin assembly are not yet well understood, as well as whether and how this heterogeneity contributes to barrier properties and tissue homeostasis. Disturbances in the content, distribution and postranslational modifications of claudins have been detected in several brain and retinal diseases characterized by barrier breakdown. Although the elucidation of the molecular mechanisms involved in TJ deregulation are pivotal to find a potential common denominator in many disease states, a better understanding of claudin biology may facilitate the development of novel claudin-targeted therapies.
Acknowledgments
This manuscript was prepared under the scope of Grants funded by the European Foundation for the Study of Diabetes (EFSD)/ Glaxo Smith Kline (GSK) Programme and the Foundation for Science and Technology (PEst-C/SAU/UI3282/2011 and COMPETE-FEDER).
Glossary
Abbreviations:
- AD
Alzheimer disease
- BBB
blood-brain barrier
- BCSFB
blood-cerebrospinal fluid barrier
- BRB
blood-retinal barrier
- CSF
cerebrospinal fluid
- EAE
experimental autoimmune encephalomyelitis
- ECL
extracellular loops
- ECL1
first extracellular loop
- ECL2
second extracellular loop
- EphA2
ephrin type-A receptor 2
- ER
endoplasmic reticulum
- FRET
Fluorescence resonance energy transfer
- HCV
hepatitis C virus
- iBRB
inner blood-retinal barrier
- IFN
interferon
- IL
interleukin
- JAMS
junctional adhesion molecules
- MMPs
matrix metalloproteinases
- MS
multiple sclerosis
- oBRB
outer blood-retinal barrier
- OIR
oxygen-induced retinopathy
- PKA
protein kinase A
- ROP
retinopathy of prematurity
- RPE
retinal pigment epithelium
- TER
transepithelial or endothelial resistance
- TJ
tght junctions
- TNF
tumor necrosis factor
- VEGF
vascular endothelial growth factor
- ZO
Zonula occludens
Disclosure of Potential Conflicts of Interest
No potential conflict of interest was disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/tissuebarriers/article/24782
References
- 1.Ehrlich P. Das sauerstufbudurfnis des organismus. Eine Farbenanalytische Studie. Berlin: Hirschwald 1885. [Google Scholar]
- 2.Goldman EE. Vitalbarfung am Zentralnervensystem.: Preuss Akad Wiss, 1913. [Google Scholar]
- 3.Cunha-Vaz JG, Maurice DM. The active transport of fluorescein by the retinal vessels and the retina. J Physiol. 1967;191:467–86. doi: 10.1113/jphysiol.1967.sp008262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cunha Vaz JGFD. Permeability of the retinal vessels in health and disease. University of London (Institute of Ophthalmology), 1965. [Google Scholar]
- 5.Cunha-Vaz J, Faria de Abreu JR, Campos AJ. Early breakdown of the blood-retinal barrier in diabetes. Br J Ophthalmol. 1975;59:649–56. doi: 10.1136/bjo.59.11.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cunha-Vaz JG. The blood-retinal barriers. Doc Ophthalmol. 1976;41:287–327. doi: 10.1007/BF00146764. [DOI] [PubMed] [Google Scholar]
- 7.Hori S, Ohtsuki S, Hosoya K, Nakashima E, Terasaki T. A pericyte-derived angiopoietin-1 multimeric complex induces occludin gene expression in brain capillary endothelial cells through Tie-2 activation in vitro. J Neurochem. 2004;89:503–13. doi: 10.1111/j.1471-4159.2004.02343.x. [DOI] [PubMed] [Google Scholar]
- 8.Kim JH, Kim JH, Park JA, Lee SW, Kim WJ, Yu YS, et al. Blood-neural barrier: intercellular communication at glio-vascular interface. J Biochem Mol Biol. 2006;39:339–45. doi: 10.5483/BMBRep.2006.39.4.339. [DOI] [PubMed] [Google Scholar]
- 9.Sariola H, Saarma M. Novel functions and signalling pathways for GDNF. J Cell Sci. 2003;116:3855–62. doi: 10.1242/jcs.00786. [DOI] [PubMed] [Google Scholar]
- 10.Anderson JM, Van Itallie CM, Fanning AS. Setting up a selective barrier at the apical junction complex. Curr Opin Cell Biol. 2004;16:140–5. doi: 10.1016/j.ceb.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 11.Balda MS, Matter K. Tight junctions. J Cell Sci. 1998;111:541–7. doi: 10.1242/jcs.111.5.541. [DOI] [PubMed] [Google Scholar]
- 12.Tsukita S, Furuse M, Itoh M. Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol. 2001;2:285–93. doi: 10.1038/35067088. [DOI] [PubMed] [Google Scholar]
- 13.Ikenouchi J, Furuse M, Furuse K, Sasaki H, Tsukita S, Tsukita S. Tricellulin constitutes a novel barrier at tricellular contacts of epithelial cells. J Cell Biol. 2005;171:939–45. doi: 10.1083/jcb.200510043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chiba H, Osanai M, Murata M, Kojima T, Sawada N. Transmembrane proteins of tight junctions. Biochim Biophys Acta. 2008;1778:588–600. doi: 10.1016/j.bbamem.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 15.Bauer HC, Traweger A, Zweimueller-Mayer J, Lehner C, Tempfer H, Krizbai I, et al. New aspects of the molecular constituents of tissue barriers. J Neural Transm. 2011;118:7–21. doi: 10.1007/s00702-010-0484-6. [DOI] [PubMed] [Google Scholar]
- 16.Mineta K, Yamamoto Y, Yamazaki Y, Tanaka H, Tada Y, Saito K, et al. Predicted expansion of the claudin multigene family. FEBS Lett. 2011;585:606–12. doi: 10.1016/j.febslet.2011.01.028. [DOI] [PubMed] [Google Scholar]
- 17.Furuse M, Fujita K, Hiiragi T, Fujimoto K, Tsukita S. Claudin-1 and -2: novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin. J Cell Biol. 1998;141:1539–50. doi: 10.1083/jcb.141.7.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morita K, Sasaki H, Furuse M, Tsukita S. Endothelial claudin: claudin-5/TMVCF constitutes tight junction strands in endothelial cells. J Cell Biol. 1999;147:185–94. doi: 10.1083/jcb.147.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsukita S, Furuse M. Occludin and claudins in tight-junction strands: leading or supporting players? Trends Cell Biol. 1999;9:268–73. doi: 10.1016/S0962-8924(99)01578-0. [DOI] [PubMed] [Google Scholar]
- 20.Furuse M, Hata M, Furuse K, Yoshida Y, Haratake A, Sugitani Y, et al. Claudin-based tight junctions are crucial for the mammalian epidermal barrier: a lesson from claudin-1-deficient mice. J Cell Biol. 2002;156:1099–111. doi: 10.1083/jcb.200110122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Furuse M. Molecular basis of the core structure of tight junctions. Cold Spring Harb Perspect Biol. 2010;2:a002907. doi: 10.1101/cshperspect.a002907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Farquhar MG, Palade GE. Junctional complexes in various epithelia. J Cell Biol. 1963;17:375–412. doi: 10.1083/jcb.17.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Staehelin LA, Mukherjee TM, Williams AW. Freeze-etch appearance of the tight junctions in the epithelium of small and large intestine of mice. Protoplasma. 1969;67:165–84. doi: 10.1007/BF01248737. [DOI] [PubMed] [Google Scholar]
- 24.Krause G, Winkler L, Mueller SL, Haseloff RF, Piontek J, Blasig IE. Structure and function of claudins. Biochim Biophys Acta. 2008;1778:631–45. doi: 10.1016/j.bbamem.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 25.Lal-Nag M, Morin PJ. The claudins. Genome Biol. 2009;10:235. doi: 10.1186/gb-2009-10-8-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schulzke JD, Günzel D, John LJ, Fromm M. Perspectives on tight junction research. Ann N Y Acad Sci. 2012;1257:1–19. doi: 10.1111/j.1749-6632.2012.06485.x. [DOI] [PubMed] [Google Scholar]
- 27.Rüffer C, Gerke V. The C-terminal cytoplasmic tail of claudins 1 and 5 but not its PDZ-binding motif is required for apical localization at epithelial and endothelial tight junctions. Eur J Cell Biol. 2004;83:135–44. doi: 10.1078/0171-9335-00366. [DOI] [PubMed] [Google Scholar]
- 28.Arabzadeh A, Troy TC, Turksen K. Role of the Cldn6 cytoplasmic tail domain in membrane targeting and epidermal differentiation in vivo. Mol Cell Biol. 2006;26:5876–87. doi: 10.1128/MCB.02342-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Müller D, Kausalya PJ, Meij IC, Hunziker W. Familial hypomagnesemia with hypercalciuria and nephrocalcinosis: blocking endocytosis restores surface expression of a novel Claudin-16 mutant that lacks the entire C-terminal cytosolic tail. Hum Mol Genet. 2006;15:1049–58. doi: 10.1093/hmg/ddl020. [DOI] [PubMed] [Google Scholar]
- 30.Ishizaki T, Chiba H, Kojima T, Fujibe M, Soma T, Miyajima H, et al. Cyclic AMP induces phosphorylation of claudin-5 immunoprecipitates and expression of claudin-5 gene in blood-brain-barrier endothelial cells via protein kinase A-dependent and -independent pathways. Exp Cell Res. 2003;290:275–88. doi: 10.1016/S0014-4827(03)00354-9. [DOI] [PubMed] [Google Scholar]
- 31.Soma T, Chiba H, Kato-Mori Y, Wada T, Yamashita T, Kojima T, et al. Thr(207) of claudin-5 is involved in size-selective loosening of the endothelial barrier by cyclic AMP. Exp Cell Res. 2004;300:202–12. doi: 10.1016/j.yexcr.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 32.Persidsky Y, Heilman D, Haorah J, Zelivyanskaya M, Persidsky R, Weber GA, et al. Rho-mediated regulation of tight junctions during monocyte migration across the blood-brain barrier in HIV-1 encephalitis (HIVE) Blood. 2006;107:4770–80. doi: 10.1182/blood-2005-11-4721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fujibe M, Chiba H, Kojima T, Soma T, Wada T, Yamashita T, et al. Thr203 of claudin-1, a putative phosphorylation site for MAP kinase, is required to promote the barrier function of tight junctions. Exp Cell Res. 2004;295:36–47. doi: 10.1016/j.yexcr.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 34.Tanaka M, Kamata R, Sakai R. EphA2 phosphorylates the cytoplasmic tail of Claudin-4 and mediates paracellular permeability. J Biol Chem. 2005;280:42375–82. doi: 10.1074/jbc.M503786200. [DOI] [PubMed] [Google Scholar]
- 35.D’Souza T, Agarwal R, Morin PJ. Phosphorylation of claudin-3 at threonine 192 by cAMP-dependent protein kinase regulates tight junction barrier function in ovarian cancer cells. J Biol Chem. 2005;280:26233–40. doi: 10.1074/jbc.M502003200. [DOI] [PubMed] [Google Scholar]
- 36.Van Itallie CM, Gambling TM, Carson JL, Anderson JM. Palmitoylation of claudins is required for efficient tight-junction localization. J Cell Sci. 2005;118:1427–36. doi: 10.1242/jcs.01735. [DOI] [PubMed] [Google Scholar]
- 37.Itoh M, Furuse M, Morita K, Kubota K, Saitou M, Tsukita S. Direct binding of three tight junction-associated MAGUKs, ZO-1, ZO-2, and ZO-3, with the COOH termini of claudins. J Cell Biol. 1999;147:1351–63. doi: 10.1083/jcb.147.6.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roh MH, Liu CJ, Laurinec S, Margolis B. The carboxyl terminus of zona occludens-3 binds and recruits a mammalian homologue of discs lost to tight junctions. J Biol Chem. 2002;277:27501–9. doi: 10.1074/jbc.M201177200. [DOI] [PubMed] [Google Scholar]
- 39.Hamazaki Y, Itoh M, Sasaki H, Furuse M, Tsukita S. Multi-PDZ domain protein 1 (MUPP1) is concentrated at tight junctions through its possible interaction with claudin-1 and junctional adhesion molecule. J Biol Chem. 2002;277:455–61. doi: 10.1074/jbc.M109005200. [DOI] [PubMed] [Google Scholar]
- 40.Colegio OR, Van Itallie CM, McCrea HJ, Rahner C, Anderson JM. Claudins create charge-selective channels in the paracellular pathway between epithelial cells. Am J Physiol Cell Physiol. 2002;283:C142–7. doi: 10.1152/ajpcell.00038.2002. [DOI] [PubMed] [Google Scholar]
- 41.Hou J, Paul DL, Goodenough DA. Paracellin-1 and the modulation of ion selectivity of tight junctions. J Cell Sci. 2005;118:5109–18. doi: 10.1242/jcs.02631. [DOI] [PubMed] [Google Scholar]
- 42.Alexandre MD, Jeansonne BG, Renegar RH, Tatum R, Chen YH. The first extracellular domain of claudin-7 affects paracellular Cl- permeability. Biochem Biophys Res Commun. 2007;357:87–91. doi: 10.1016/j.bbrc.2007.03.078. [DOI] [PubMed] [Google Scholar]
- 43.Evans MJ, von Hahn T, Tscherne DM, Syder AJ, Panis M, Wölk B, et al. Claudin-1 is a hepatitis C virus co-receptor required for a late step in entry. Nature. 2007;446:801–5. doi: 10.1038/nature05654. [DOI] [PubMed] [Google Scholar]
- 44.Zheng A, Yuan F, Li Y, Zhu F, Hou P, Li J, et al. Claudin-6 and claudin-9 function as additional coreceptors for hepatitis C virus. J Virol. 2007;81:12465–71. doi: 10.1128/JVI.01457-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Piontek J, Winkler L, Wolburg H, Müller SL, Zuleger N, Piehl C, et al. Formation of tight junction: determinants of homophilic interaction between classic claudins. FASEB J. 2008;22:146–58. doi: 10.1096/fj.07-8319com. [DOI] [PubMed] [Google Scholar]
- 46.Fujita K, Katahira J, Horiguchi Y, Sonoda N, Furuse M, Tsukita S. Clostridium perfringens enterotoxin binds to the second extracellular loop of claudin-3, a tight junction integral membrane protein. FEBS Lett. 2000;476:258–61. doi: 10.1016/S0014-5793(00)01744-0. [DOI] [PubMed] [Google Scholar]
- 47.Katahira J, Sugiyama H, Inoue N, Horiguchi Y, Matsuda M, Sugimoto N. Clostridium perfringens enterotoxin utilizes two structurally related membrane proteins as functional receptors in vivo. J Biol Chem. 1997;272:26652–8. doi: 10.1074/jbc.272.42.26652. [DOI] [PubMed] [Google Scholar]
- 48.Piontek J, Fritzsche S, Cording J, Richter S, Hartwig J, Walter M, et al. Elucidating the principles of the molecular organization of heteropolymeric tight junction strands. Cell Mol Life Sci. 2011;68:3903–18. doi: 10.1007/s00018-011-0680-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Furuse M, Sasaki H, Tsukita S. Manner of interaction of heterogeneous claudin species within and between tight junction strands. J Cell Biol. 1999;147:891–903. doi: 10.1083/jcb.147.4.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Daugherty BL, Ward C, Smith T, Ritzenthaler JD, Koval M. Regulation of heterotypic claudin compatibility. J Biol Chem. 2007;282:30005–13. doi: 10.1074/jbc.M703547200. [DOI] [PubMed] [Google Scholar]
- 51.Mitic LL, Unger VM, Anderson JM. Expression, solubilization, and biochemical characterization of the tight junction transmembrane protein claudin-4. Protein Sci. 2003;12:218–27. doi: 10.1110/ps.0233903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Coyne CB, Gambling TM, Boucher RC, Carson JL, Johnson LG. Role of claudin interactions in airway tight junctional permeability. Am J Physiol Lung Cell Mol Physiol. 2003;285:L1166–78. doi: 10.1152/ajplung.00182.2003. [DOI] [PubMed] [Google Scholar]
- 53.Van Itallie CM, Mitic LL, Anderson JM. Claudin-2 forms homodimers and is a component of a high molecular weight protein complex. J Biol Chem. 2011;286:3442–50. doi: 10.1074/jbc.M110.195578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Blasig IE, Winkler L, Lassowski B, Mueller SL, Zuleger N, Krause E, et al. On the self-association potential of transmembrane tight junction proteins. Cell Mol Life Sci. 2006;63:505–14. doi: 10.1007/s00018-005-5472-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hou J, Renigunta A, Yang J, Waldegger S. Claudin-4 forms paracellular chloride channel in the kidney and requires claudin-8 for tight junction localization. Proc Natl Acad Sci U S A. 2010;107:18010–5. doi: 10.1073/pnas.1009399107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hou J, Renigunta A, Gomes AS, Hou M, Paul DL, Waldegger S, et al. Claudin-16 and claudin-19 interaction is required for their assembly into tight junctions and for renal reabsorption of magnesium. Proc Natl Acad Sci U S A. 2009;106:15350–5. doi: 10.1073/pnas.0907724106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ohtsuki S, Yamaguchi H, Katsukura Y, Asashima T, Terasaki T. mRNA expression levels of tight junction protein genes in mouse brain capillary endothelial cells highly purified by magnetic cell sorting. J Neurochem. 2008;104:147–54. doi: 10.1111/j.1471-4159.2007.05008.x. [DOI] [PubMed] [Google Scholar]
- 58.Amasheh S, Meiri N, Gitter AH, Schöneberg T, Mankertz J, Schulzke JD, et al. Claudin-2 expression induces cation-selective channels in tight junctions of epithelial cells. J Cell Sci. 2002;115:4969–76. doi: 10.1242/jcs.00165. [DOI] [PubMed] [Google Scholar]
- 59.Krug SM, Günzel D, Conrad MP, Rosenthal R, Fromm A, Amasheh S, et al. Claudin-17 forms tight junction channels with distinct anion selectivity. Cell Mol Life Sci. 2012;69:2765–78. doi: 10.1007/s00018-012-0949-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rosenthal R, Milatz S, Krug SM, Oelrich B, Schulzke JD, Amasheh S, et al. Claudin-2, a component of the tight junction, forms a paracellular water channel. J Cell Sci. 2010;123:1913–21. doi: 10.1242/jcs.060665. [DOI] [PubMed] [Google Scholar]
- 61.Günzel D, Stuiver M, Kausalya PJ, Haisch L, Krug SM, Rosenthal R, et al. Claudin-10 exists in six alternatively spliced isoforms that exhibit distinct localization and function. J Cell Sci. 2009;122:1507–17. doi: 10.1242/jcs.040113. [DOI] [PubMed] [Google Scholar]
- 62.Hadj-Rabia S, Baala L, Vabres P, Hamel-Teillac D, Jacquemin E, Fabre M, et al. Claudin-1 gene mutations in neonatal sclerosing cholangitis associated with ichthyosis: a tight junction disease. Gastroenterology. 2004;127:1386–90. doi: 10.1053/j.gastro.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 63.Nitta T, Hata M, Gotoh S, Seo Y, Sasaki H, Hashimoto N, et al. Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. J Cell Biol. 2003;161:653–60. doi: 10.1083/jcb.200302070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gow A, Southwood CM, Li JS, Pariali M, Riordan GP, Brodie SE, et al. CNS myelin and sertoli cell tight junction strands are absent in Osp/claudin-11 null mice. Cell. 1999;99:649–59. doi: 10.1016/S0092-8674(00)81553-6. [DOI] [PubMed] [Google Scholar]
- 65.Ben-Yosef T, Belyantseva IA, Saunders TL, Hughes ED, Kawamoto K, Van Itallie CM, et al. Claudin 14 knockout mice, a model for autosomal recessive deafness DFNB29, are deaf due to cochlear hair cell degeneration. Hum Mol Genet. 2003;12:2049–61. doi: 10.1093/hmg/ddg210. [DOI] [PubMed] [Google Scholar]
- 66.Weber S, Schneider L, Peters M, Misselwitz J, Rönnefarth G, Böswald M, et al. Novel paracellin-1 mutations in 25 families with familial hypomagnesemia with hypercalciuria and nephrocalcinosis. J Am Soc Nephrol. 2001;12:1872–81. doi: 10.1681/ASN.V1291872. [DOI] [PubMed] [Google Scholar]
- 67.Müller D, Kausalya PJ, Claverie-Martin F, Meij IC, Eggert P, Garcia-Nieto V, et al. A novel claudin 16 mutation associated with childhood hypercalciuria abolishes binding to ZO-1 and results in lysosomal mistargeting. Am J Hum Genet. 2003;73:1293–301. doi: 10.1086/380418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Konrad M, Schaller A, Seelow D, Pandey AV, Waldegger S, Lesslauer A, et al. Mutations in the tight-junction gene claudin 19 (CLDN19) are associated with renal magnesium wasting, renal failure, and severe ocular involvement. Am J Hum Genet. 2006;79:949–57. doi: 10.1086/508617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37:13–25. doi: 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 70.Butt AM, Jones HC, Abbott NJ. Electrical resistance across the blood-brain barrier in anaesthetized rats: a developmental study. J Physiol. 1990;429:47–62. doi: 10.1113/jphysiol.1990.sp018243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Crone C, Christensen O. Electrical resistance of a capillary endothelium. J Gen Physiol. 1981;77:349–71. doi: 10.1085/jgp.77.4.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liebner S, Fischmann A, Rascher G, Duffner F, Grote EH, Kalbacher H, et al. Claudin-1 and claudin-5 expression and tight junction morphology are altered in blood vessels of human glioblastoma multiforme. Acta Neuropathol. 2000;100:323–31. doi: 10.1007/s004010000180. [DOI] [PubMed] [Google Scholar]
- 73.Wolburg H, Wolburg-Buchholz K, Kraus J, Rascher-Eggstein G, Liebner S, Hamm S, et al. Localization of claudin-3 in tight junctions of the blood-brain barrier is selectively lost during experimental autoimmune encephalomyelitis and human glioblastoma multiforme. Acta Neuropathol. 2003;105:586–92. doi: 10.1007/s00401-003-0688-z. [DOI] [PubMed] [Google Scholar]
- 74.Ohtsuki S, Sato S, Yamaguchi H, Kamoi M, Asashima T, Terasaki T. Exogenous expression of claudin-5 induces barrier properties in cultured rat brain capillary endothelial cells. J Cell Physiol. 2007;210:81–6. doi: 10.1002/jcp.20823. [DOI] [PubMed] [Google Scholar]
- 75.Wolburg H, Wolburg-Buchholz K, Liebner S, Engelhardt B. Claudin-1, claudin-2 and claudin-11 are present in tight junctions of choroid plexus epithelium of the mouse. Neurosci Lett. 2001;307:77–80. doi: 10.1016/S0304-3940(01)01927-9. [DOI] [PubMed] [Google Scholar]
- 76.Kratzer I, Vasiljevic A, Rey C, Fevre-Montange M, Saunders N, Strazielle N, et al. Complexity and developmental changes in the expression pattern of claudins at the blood-CSF barrier. Histochem Cell Biol. 2012;138:861–79. doi: 10.1007/s00418-012-1001-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Luo Y, Xiao W, Zhu X, Mao Y, Liu X, Chen X, et al. Differential expression of claudins in retinas during normal development and the angiogenesis of oxygen-induced retinopathy. Invest Ophthalmol Vis Sci. 2011;52:7556–64. doi: 10.1167/iovs.11-7185. [DOI] [PubMed] [Google Scholar]
- 78.Morcos Y, Hosie MJ, Bauer HC, Chan-Ling T. Immunolocalization of occludin and claudin-1 to tight junctions in intact CNS vessels of mammalian retina. J Neurocytol. 2001;30:107–23. doi: 10.1023/A:1011982906125. [DOI] [PubMed] [Google Scholar]
- 79.Klaassen I, Hughes JM, Vogels IM, Schalkwijk CG, Van Noorden CJ, Schlingemann RO. Altered expression of genes related to blood-retina barrier disruption in streptozotocin-induced diabetes. Exp Eye Res. 2009;89:4–15. doi: 10.1016/j.exer.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 80.Rahner C, Fukuhara M, Peng S, Kojima S, Rizzolo LJ. The apical and basal environments of the retinal pigment epithelium regulate the maturation of tight junctions during development. J Cell Sci. 2004;117:3307–18. doi: 10.1242/jcs.01181. [DOI] [PubMed] [Google Scholar]
- 81.Rizzolo LJ, Chen X, Weitzman M, Sun R, Zhang H. Analysis of the RPE transcriptome reveals dynamic changes during the development of the outer blood-retinal barrier. Mol Vis. 2007;13:1259–73. [PubMed] [Google Scholar]
- 82.Sun R, Peng S, Chen X, Zhang H, Rizzolo LJ. Diffusible retinal secretions regulate the expression of tight junctions and other diverse functions of the retinal pigment epithelium. Mol Vis. 2008;14:2237–62. [PMC free article] [PubMed] [Google Scholar]
- 83.Peng S, Rao VS, Adelman RA, Rizzolo LJ. Claudin-19 and the barrier properties of the human retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2011;52:1392–403. doi: 10.1167/iovs.10-5984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Peng S, Adelman RA, Rizzolo LJ. Minimal effects of VEGF and anti-VEGF drugs on the permeability or selectivity of RPE tight junctions. Invest Ophthalmol Vis Sci. 2010;51:3216–25. doi: 10.1167/iovs.09-4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Strunnikova NV, Maminishkis A, Barb JJ, Wang F, Zhi C, Sergeev Y, et al. Transcriptome analysis and molecular signature of human retinal pigment epithelium. Hum Mol Genet. 2010;19:2468–86. doi: 10.1093/hmg/ddq129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kröll S, El-Gindi J, Thanabalasundaram G, Panpumthong P, Schrot S, Hartmann C, et al. Control of the blood-brain barrier by glucocorticoids and the cells of the neurovascular unit. Ann N Y Acad Sci. 2009;1165:228–39. doi: 10.1111/j.1749-6632.2009.04040.x. [DOI] [PubMed] [Google Scholar]
- 87.Ronaldson PT, Demarco KM, Sanchez-Covarrubias L, Solinsky CM, Davis TP. Transforming growth factor-beta signaling alters substrate permeability and tight junction protein expression at the blood-brain barrier during inflammatory pain. J Cereb Blood Flow Metab. 2009;29:1084–98. doi: 10.1038/jcbfm.2009.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Argaw AT, Gurfein BT, Zhang Y, Zameer A, John GR. VEGF-mediated disruption of endothelial CLN-5 promotes blood-brain barrier breakdown. Proc Natl Acad Sci U S A. 2009;106:1977–82. doi: 10.1073/pnas.0808698106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Carvey PM, Hendey B, Monahan AJ. The blood-brain barrier in neurodegenerative disease: a rhetorical perspective. J Neurochem. 2009;111:291–314. doi: 10.1111/j.1471-4159.2009.06319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Coisne C, Engelhardt B. Tight junctions in brain barriers during central nervous system inflammation. Antioxid Redox Signal. 2011;15:1285–303. doi: 10.1089/ars.2011.3929. [DOI] [PubMed] [Google Scholar]
- 91.Grammas P, Martinez J, Miller B. Cerebral microvascular endothelium and the pathogenesis of neurodegenerative diseases. Expert Rev Mol Med. 2011;13:e19. doi: 10.1017/S1462399411001918. [DOI] [PubMed] [Google Scholar]
- 92.Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev. 2005;57:173–85. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- 93.Petty MA, Lo EH. Junctional complexes of the blood-brain barrier: permeability changes in neuroinflammation. Prog Neurobiol. 2002;68:311–23. doi: 10.1016/S0301-0082(02)00128-4. [DOI] [PubMed] [Google Scholar]
- 94.Leal EC, Manivannan A, Hosoya K, Terasaki T, Cunha-Vaz J, Ambrósio AF, et al. Inducible nitric oxide synthase isoform is a key mediator of leukostasis and blood-retinal barrier breakdown in diabetic retinopathy. Invest Ophthalmol Vis Sci. 2007;48:5257–65. doi: 10.1167/iovs.07-0112. [DOI] [PubMed] [Google Scholar]
- 95.Trudeau K, Roy S, Guo W, Hernández C, Villarroel M, Simó R, et al. Fenofibric acid reduces fibronectin and collagen type IV overexpression in human retinal pigment epithelial cells grown in conditions mimicking the diabetic milieu: functional implications in retinal permeability. Invest Ophthalmol Vis Sci. 2011;52:6348–54. doi: 10.1167/iovs.11-7282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nabeshima T, Nitta A. Memory impairment and neuronal dysfunction induced by beta-amyloid protein in rats. Tohoku J Exp Med. 1994;174:241–9. doi: 10.1620/tjem.174.241. [DOI] [PubMed] [Google Scholar]
- 97.Thal DR, Griffin WS, Braak H. Parenchymal and vascular Abeta-deposition and its effects on the degeneration of neurons and cognition in Alzheimer’s disease. J Cell Mol Med. 2008;12(5B):1848–62. doi: 10.1111/j.1582-4934.2008.00411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Marco S, Skaper SD. Amyloid beta-peptide1-42 alters tight junction protein distribution and expression in brain microvessel endothelial cells. Neurosci Lett. 2006;401:219–24. doi: 10.1016/j.neulet.2006.03.047. [DOI] [PubMed] [Google Scholar]
- 99.Kook SY, Hong HS, Moon M, Ha CM, Chang S, Mook-Jung I. Aβ₁₋₄₂-RAGE interaction disrupts tight junctions of the blood-brain barrier via Ca²⁺-calcineurin signaling. J Neurosci. 2012;32:8845–54. doi: 10.1523/JNEUROSCI.6102-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Starr JM, Farrall AJ, Armitage P, McGurn B, Wardlaw J. Blood-brain barrier permeability in Alzheimer’s disease: a case-control MRI study. Psychiatry Res. 2009;171:232–41. doi: 10.1016/j.pscychresns.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 101.Ujiie M, Dickstein DL, Carlow DA, Jefferies WA. Blood-brain barrier permeability precedes senile plaque formation in an Alzheimer disease model. Microcirculation. 2003;10:463–70. doi: 10.1038/sj.mn.7800212. [DOI] [PubMed] [Google Scholar]
- 102.Viggars AP, Wharton SB, Simpson JE, Matthews FE, Brayne C, Savva GM, et al. Alterations in the blood brain barrier in ageing cerebral cortex in relationship to Alzheimer-type pathology: a study in the MRC-CFAS population neuropathology cohort. Neurosci Lett. 2011;505:25–30. doi: 10.1016/j.neulet.2011.09.049. [DOI] [PubMed] [Google Scholar]
- 103.Krizbai IA, Bauer H, Bresgen N, Eckl PM, Farkas A, Szatmári E, et al. Effect of oxidative stress on the junctional proteins of cultured cerebral endothelial cells. Cell Mol Neurobiol. 2005;25:129–39. doi: 10.1007/s10571-004-1378-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nunomura A, Castellani RJ, Zhu X, Moreira PI, Perry G, Smith MA. Involvement of oxidative stress in Alzheimer disease. J Neuropathol Exp Neurol. 2006;65:631–41. doi: 10.1097/01.jnen.0000228136.58062.bf. [DOI] [PubMed] [Google Scholar]
- 105.Nunomura A, Hofer T, Moreira PI, Castellani RJ, Smith MA, Perry G. RNA oxidation in Alzheimer disease and related neurodegenerative disorders. Acta Neuropathol. 2009;118:151–66. doi: 10.1007/s00401-009-0508-1. [DOI] [PubMed] [Google Scholar]
- 106.Nunomura A, Perry G, Aliev G, Hirai K, Takeda A, Balraj EK, et al. Oxidative damage is the earliest event in Alzheimer disease. J Neuropathol Exp Neurol. 2001;60:759–67. doi: 10.1093/jnen/60.8.759. [DOI] [PubMed] [Google Scholar]
- 107.Simpson JE, Ince PG, Lace G, Forster G, Shaw PJ, Matthews F, et al. MRC Cognitive Function and Ageing Neuropathology Study Group Astrocyte phenotype in relation to Alzheimer-type pathology in the ageing brain. Neurobiol Aging. 2010;31:578–90. doi: 10.1016/j.neurobiolaging.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 108.Romanitan MO, Popescu BO, Spulber S, Băjenaru O, Popescu LM, Winblad B, et al. Altered expression of claudin family proteins in Alzheimer’s disease and vascular dementia brains. J Cell Mol Med. 2010;14:1088–100. doi: 10.1111/j.1582-4934.2009.00999.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Romanitan MO, Popescu BO, Winblad B, Bajenaru OA, Bogdanovic N. Occludin is overexpressed in Alzheimer’s disease and vascular dementia. J Cell Mol Med. 2007;11:569–79. doi: 10.1111/j.1582-4934.2007.00047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Furuse M, Furuse K, Sasaki H, Tsukita S. Conversion of zonulae occludentes from tight to leaky strand type by introducing claudin-2 into Madin-Darby canine kidney I cells. J Cell Biol. 2001;153:263–72. doi: 10.1083/jcb.153.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Furuse M, Tsukita S. Claudins in occluding junctions of humans and flies. Trends Cell Biol. 2006;16:181–8. doi: 10.1016/j.tcb.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 112.Lassmann H, Brück W, Lucchinetti C. Heterogeneity of multiple sclerosis pathogenesis: implications for diagnosis and therapy. Trends Mol Med. 2001;7:115–21. doi: 10.1016/S1471-4914(00)01909-2. [DOI] [PubMed] [Google Scholar]
- 113.Couraud PO. Infiltration of inflammatory cells through brain endothelium. Pathol Biol (Paris) 1998;46:176–80. [PubMed] [Google Scholar]
- 114.Errede M, Girolamo F, Ferrara G, Strippoli M, Morando S, Boldrin V, et al. Blood-brain barrier alterations in the cerebral cortex in experimental autoimmune encephalomyelitis. J Neuropathol Exp Neurol. 2012;71:840–54. doi: 10.1097/NEN.0b013e31826ac110. [DOI] [PubMed] [Google Scholar]
- 115.Förster C, Kahles T, Kietz S, Drenckhahn D. Dexamethasone induces the expression of metalloproteinase inhibitor TIMP-1 in the murine cerebral vascular endothelial cell line cEND. J Physiol. 2007;580:937–49. doi: 10.1113/jphysiol.2007.129007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Engelhardt B. Molecular mechanisms involved in T cell migration across the blood-brain barrier. J Neural Transm. 2006;113:477–85. doi: 10.1007/s00702-005-0409-y. [DOI] [PubMed] [Google Scholar]
- 117.Yednock TA, Cannon C, Fritz LC, Sanchez-Madrid F, Steinman L, Karin N. Prevention of experimental autoimmune encephalomyelitis by antibodies against alpha 4 beta 1 integrin. Nature. 1992;356:63–6. doi: 10.1038/356063a0. [DOI] [PubMed] [Google Scholar]
- 118.Minagar A, Alexander JS. Blood-brain barrier disruption in multiple sclerosis. Mult Scler. 2003;9:540–9. doi: 10.1191/1352458503ms965oa. [DOI] [PubMed] [Google Scholar]
- 119.Szczuciński A, Losy J. Chemokines and chemokine receptors in multiple sclerosis. Potential targets for new therapies. Acta Neurol Scand. 2007;115:137–46. doi: 10.1111/j.1600-0404.2006.00749.x. [DOI] [PubMed] [Google Scholar]
- 120.Körner H, Sedgwick JD. Tumour necrosis factor and lymphotoxin: molecular aspects and role in tissue-specific autoimmunity. Immunol Cell Biol. 1996;74:465–72. doi: 10.1038/icb.1996.77. [DOI] [PubMed] [Google Scholar]
- 121.Beck J, Rondot P, Catinot L, Falcoff E, Kirchner H, Wietzerbin J. Increased production of interferon gamma and tumor necrosis factor precedes clinical manifestation in multiple sclerosis: do cytokines trigger off exacerbations? Acta Neurol Scand. 1988;78:318–23. doi: 10.1111/j.1600-0404.1988.tb03663.x. [DOI] [PubMed] [Google Scholar]
- 122.Sharief MK, Hentges R. Association between tumor necrosis factor-alpha and disease progression in patients with multiple sclerosis. N Engl J Med. 1991;325:467–72. doi: 10.1056/NEJM199108153250704. [DOI] [PubMed] [Google Scholar]
- 123.Hofman FM, Hinton DR, Johnson K, Merrill JE. Tumor necrosis factor identified in multiple sclerosis brain. J Exp Med. 1989;170:607–12. doi: 10.1084/jem.170.2.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wang C, Wang C, Dong H, Wu XM, Wang C, Xia F, et al. Immune-related GTPase Irgm1 exacerbates experimental auto-immune encephalomyelitis by promoting the disruption of blood-brain barrier and blood-cerebrospinal fluid barrier. Mol Immunol. 2013;53:43–51. doi: 10.1016/j.molimm.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 125.Blecharz KG, Haghikia A, Stasiolek M, Kruse N, Drenckhahn D, Gold R, et al. Glucocorticoid effects on endothelial barrier function in the murine brain endothelial cell line cEND incubated with sera from patients with multiple sclerosis. Mult Scler. 2010;16:293–302. doi: 10.1177/1352458509358189. [DOI] [PubMed] [Google Scholar]
- 126.The Diabetes Control and Complications Trial Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–86. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 127.Fernandes R, Gonçalves A, Cunha-Vaz J. Blood–Retinal Barrier: The Fundamentals. In: Thassu D, Chader G, eds. Ocular Drug Delivery Systems: Barriers and Application of Nanoparticulate Systems: CRC Press, 2012:111-32. [Google Scholar]
- 128.Antonetti DA, Barber AJ, Hollinger LA, Wolpert EB, Gardner TW. Vascular endothelial growth factor induces rapid phosphorylation of tight junction proteins occludin and zonula occluden 1. A potential mechanism for vascular permeability in diabetic retinopathy and tumors. J Biol Chem. 1999;274:23463–7. doi: 10.1074/jbc.274.33.23463. [DOI] [PubMed] [Google Scholar]
- 129.Antonetti DA, Barber AJ, Khin S, Lieth E, Tarbell JM, Gardner TW, Penn State Retina Research Group Vascular permeability in experimental diabetes is associated with reduced endothelial occludin content: vascular endothelial growth factor decreases occludin in retinal endothelial cells. Diabetes. 1998;47:1953–9. doi: 10.2337/diabetes.47.12.1953. [DOI] [PubMed] [Google Scholar]
- 130.Barber AJ, Antonetti DA. Mapping the blood vessels with paracellular permeability in the retinas of diabetic rats. Invest Ophthalmol Vis Sci. 2003;44:5410–6. doi: 10.1167/iovs.03-0244. [DOI] [PubMed] [Google Scholar]
- 131.Barber AJ, Antonetti DA, Gardner TW, The Penn State Retina Research Group Altered expression of retinal occludin and glial fibrillary acidic protein in experimental diabetes. Invest Ophthalmol Vis Sci. 2000;41:3561–8. [PubMed] [Google Scholar]
- 132.Gonçalves A, Leal E, Paiva A, Teixeira Lemos E, Teixeira F, Ribeiro CF, et al. Protective effects of the dipeptidyl peptidase IV inhibitor sitagliptin in the blood-retinal barrier in a type 2 diabetes animal model. Diabetes Obes Metab. 2012;14:454–63. doi: 10.1111/j.1463-1326.2011.01548.x. [DOI] [PubMed] [Google Scholar]
- 133.Kowluru RA, Odenbach S. Role of interleukin-1beta in the pathogenesis of diabetic retinopathy. Br J Ophthalmol. 2004;88:1343–7. doi: 10.1136/bjo.2003.038133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Joussen AM, Murata T, Tsujikawa A, Kirchhof B, Bursell SE, Adamis AP. Leukocyte-mediated endothelial cell injury and death in the diabetic retina. Am J Pathol. 2001;158:147–52. doi: 10.1016/S0002-9440(10)63952-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Joussen AM, Poulaki V, Mitsiades N, Kirchhof B, Koizumi K, Döhmen S, et al. Nonsteroidal anti-inflammatory drugs prevent early diabetic retinopathy via TNF-alpha suppression. FASEB J. 2002;16:438–40. doi: 10.1096/fj.01-0707fje. [DOI] [PubMed] [Google Scholar]
- 136.Leal EC, Martins J, Voabil P, Liberal J, Chiavaroli C, Bauer J, et al. Calcium dobesilate inhibits the alterations in tight junction proteins and leukocyte adhesion to retinal endothelial cells induced by diabetes. Diabetes. 2010;59:2637–45. doi: 10.2337/db09-1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bucolo C, Ward KW, Mazzon E, Cuzzocrea S, Drago F. Protective effects of a coumarin derivative in diabetic rats. Invest Ophthalmol Vis Sci. 2009;50:3846–52. doi: 10.1167/iovs.08-3328. [DOI] [PubMed] [Google Scholar]
- 138.Deissler H, Deissler H, Lang S, Lang GE. VEGF-induced effects on proliferation, migration and tight junctions are restored by ranibizumab (Lucentis) in microvascular retinal endothelial cells. Br J Ophthalmol. 2008;92:839–43. doi: 10.1136/bjo.2007.135640. [DOI] [PubMed] [Google Scholar]
- 139.Murakami T, Felinski EA, Antonetti DA. Occludin phosphorylation and ubiquitination regulate tight junction trafficking and vascular endothelial growth factor-induced permeability. J Biol Chem. 2009;284:21036–46. doi: 10.1074/jbc.M109.016766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Adachi T, Yasuda H, Nakamura S, Kamiya T, Hara H, Hara H, et al. Endoplasmic reticulum stress induces retinal endothelial permeability of extracellular-superoxide dismutase. Free Radic Res. 2011;45:1083–92. doi: 10.3109/10715762.2011.595408. [DOI] [PubMed] [Google Scholar]
- 141.Yoshikawa T, Ogata N, Izuta H, Shimazawa M, Hara H, Takahashi K. Increased expression of tight junctions in ARPE-19 cells under endoplasmic reticulum stress. Curr Eye Res. 2011;36:1153–63. doi: 10.3109/02713683.2011.606592. [DOI] [PubMed] [Google Scholar]
- 142.Darlow BA, Hutchinson JL, Henderson-Smart DJ, Donoghue DA, Simpson JM, Evans NJ, Australian and New Zealand Neonatal Network Prenatal risk factors for severe retinopathy of prematurity among very preterm infants of the Australian and New Zealand Neonatal Network. Pediatrics. 2005;115:990–6. doi: 10.1542/peds.2004-1309. [DOI] [PubMed] [Google Scholar]
- 143.Hartnett ME, Penn JS. Mechanisms and management of retinopathy of prematurity. N Engl J Med. 2012;367:2515–26. doi: 10.1056/NEJMra1208129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Smith LE, Wesolowski E, McLellan A, Kostyk SK, D’Amato R, Sullivan R, et al. Oxygen-induced retinopathy in the mouse. Invest Ophthalmol Vis Sci. 1994;35:101–11. [PubMed] [Google Scholar]
- 145.Zhang S, Leske DA, Holmes JM. Neovascularization grading methods in a rat model of retinopathy of prematurity. Invest Ophthalmol Vis Sci. 2000;41:887–91. [PubMed] [Google Scholar]
- 146.Abe T, Sugano E, Saigo Y, Tamai M. Interleukin-1beta and barrier function of retinal pigment epithelial cells (ARPE-19): aberrant expression of junctional complex molecules. Invest Ophthalmol Vis Sci. 2003;44:4097–104. doi: 10.1167/iovs.02-0867. [DOI] [PubMed] [Google Scholar]
- 147.Luo Y, Zhuo Y, Fukuhara M, Rizzolo LJ. Effects of culture conditions on heterogeneity and the apical junctional complex of the ARPE-19 cell line. Invest Ophthalmol Vis Sci. 2006;47:3644–55. doi: 10.1167/iovs.06-0166. [DOI] [PubMed] [Google Scholar]
- 148.Maminishkis A, Chen S, Jalickee S, Banzon T, Shi G, Wang FE, et al. Confluent monolayers of cultured human fetal retinal pigment epithelium exhibit morphology and physiology of native tissue. Invest Ophthalmol Vis Sci. 2006;47:3612–24. doi: 10.1167/iovs.05-1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Shi G, Maminishkis A, Banzon T, Jalickee S, Li R, Hammer J, et al. Control of chemokine gradients by the retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2008;49:4620–30. doi: 10.1167/iovs.08-1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Li R, Maminishkis A, Banzon T, Wan Q, Jalickee S, Chen S, et al. IFNgamma regulates retinal pigment epithelial fluid transport. Am J Physiol Cell Physiol. 2009;297:C1452–65. doi: 10.1152/ajpcell.00255.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Peng S, Gan G, Rao VS, Adelman RA, Rizzolo LJ. Effects of proinflammatory cytokines on the claudin-19 rich tight junctions of human retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2012;53:5016–28. doi: 10.1167/iovs.11-8311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lippoldt A, Liebner S, Andbjer B, Kalbacher H, Wolburg H, Haller H, et al. Organization of choroid plexus epithelial and endothelial cell tight junctions and regulation of claudin-1, -2 and -5 expression by protein kinase C. Neuroreport. 2000;11:1427–31. doi: 10.1097/00001756-200005150-00015. [DOI] [PubMed] [Google Scholar]