Abstract
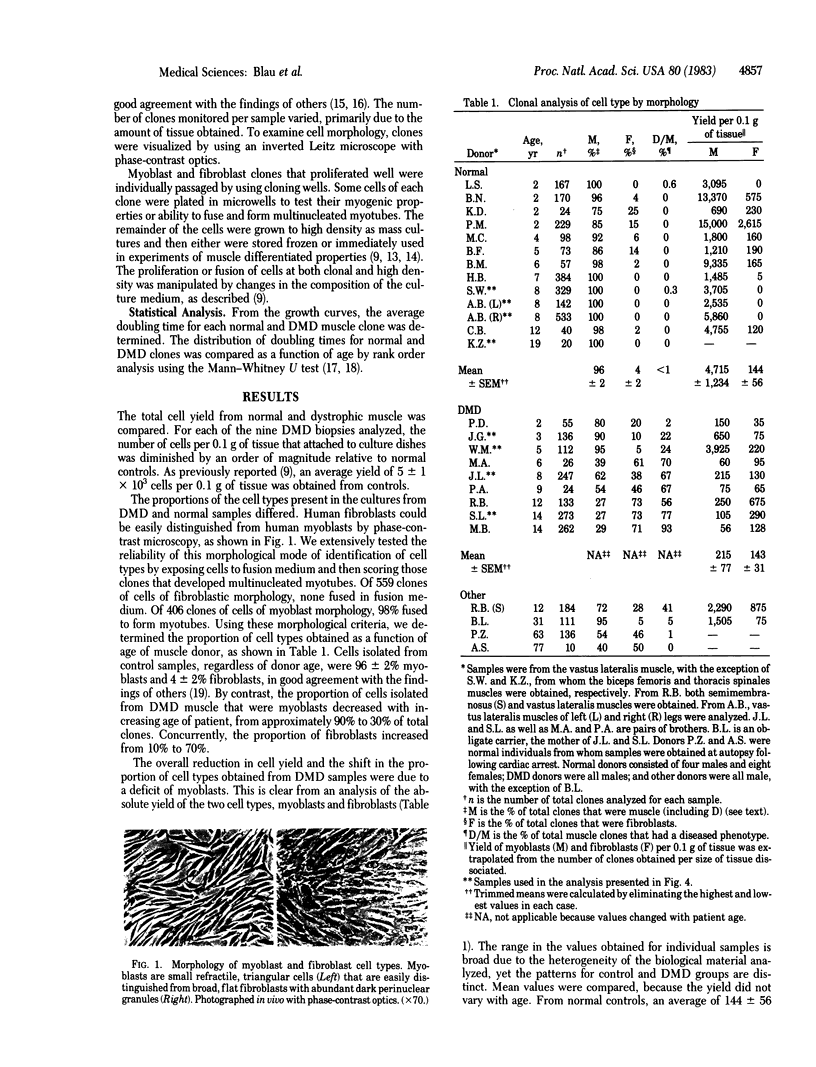
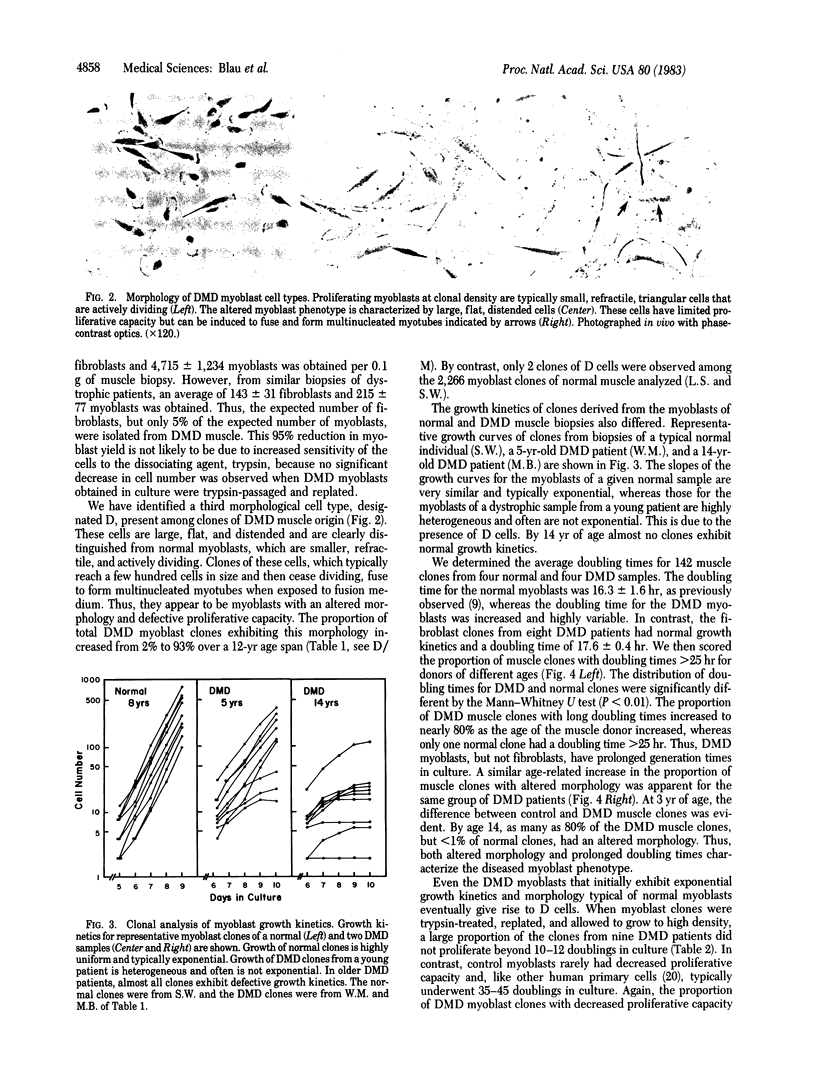
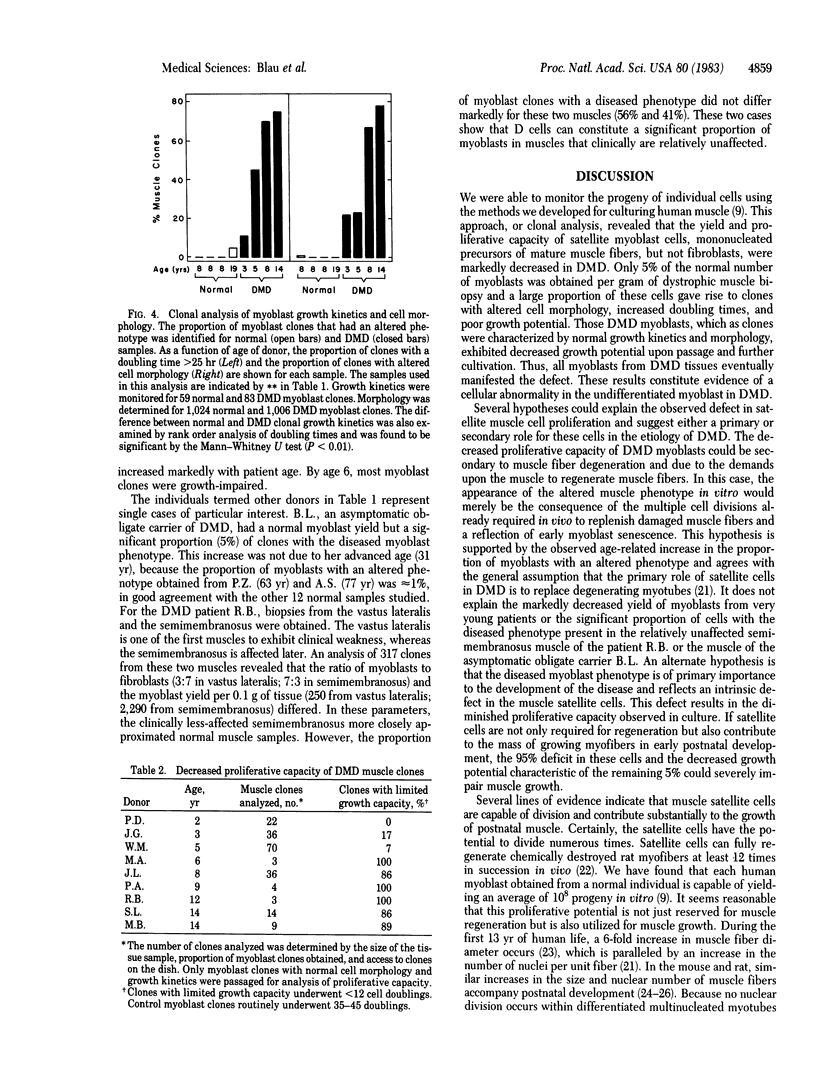
A defect in the proliferative capacity of satellite cells, mononucleated precursors of mature muscle fibers, was found in clonal analyses of cells cultured from Duchenne muscular dystrophy (DMD) patients. The total yield of myoblasts per gram of muscle biopsy was decreased to 5% of normal. Of the DMD myoblast clones obtained, a large proportion contained a morphological class of flat distended cells that had an increased generation time and ceased to proliferate beyond 100-1,000 cells but could be induced to fuse and form myotubes. The altered muscle phenotype was detected in all cultures from DMD patients but was rarely found among myoblasts of controls. By age 14 yr, it comprised as man as 90% of DMD myoblasts. The remaining DMD myoblast clones, which initially grew well, had severely impaired proliferative capacity upon passage and further cultivation. Eventually all myoblasts from DMD muscle tissue exhibited defective growth potential. In contrast, the fibroblast yield and proliferative capacity from DMD samples did not differ from normal. Based on these findings, we propose a hypothesis for the etiology of DMD: Dividing myoblasts are required for muscle growth and maintenance, and the limited capacity of DMD myoblasts to grow is directly related to the progressive muscle degeneration characteristic of the disease.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bischoff R. Enzymatic liberation of myogenic cells from adult rat muscle. Anat Rec. 1974 Dec;180(4):645–661. doi: 10.1002/ar.1091800410. [DOI] [PubMed] [Google Scholar]
- Blau H. M., Webster C., Chiu C. P., Guttman S., Chandler F. Differentiation properties of pure populations of human dystrophic muscle cells. Exp Cell Res. 1983 Apr 1;144(2):495–503. doi: 10.1016/0014-4827(83)90431-7. [DOI] [PubMed] [Google Scholar]
- Blau H. M., Webster C. Isolation and characterization of human muscle cells. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5623–5627. doi: 10.1073/pnas.78.9.5623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooke M. H., Engel W. K. The histographic analysis of human muscle biopsies with regard to fiber types. 4. Children's biopsies. Neurology. 1969 Jun;19(6):591–605. doi: 10.1212/wnl.19.6.591. [DOI] [PubMed] [Google Scholar]
- Cardasis C. A., Cooper G. W. An analysis of nuclear numbers in individual muscle fibers during differentiation and growth: a satellite cell-muscle fiber growth unit. J Exp Zool. 1975 Mar;191(3):347–358. doi: 10.1002/jez.1401910305. [DOI] [PubMed] [Google Scholar]
- Cavanagh N. P., Franklin G. I., Hughes B. P., Yasin R., Phillips E., van Beers G., Thompson E. J. Creatine kinase isoenzymes in cultured human muscle cells. II. A study of carrier females for Duchenne muscular dystrophy by needle and open biopsy. Clin Chim Acta. 1981 Sep 10;115(2):191–198. doi: 10.1016/0009-8981(81)90075-9. [DOI] [PubMed] [Google Scholar]
- Franklin G. I., Cavanagh N. P., Hughes B. P., Yasin R., Thompson E. J. Creatine kinase isoenzymes in cultured human muscle cells. I. Comparison of Duchenne muscular dystrophy with other myopathic and neurogenic disease. Clin Chim Acta. 1981 Sep 10;115(2):179–189. doi: 10.1016/0009-8981(81)90074-7. [DOI] [PubMed] [Google Scholar]
- Golbus M. S., Stephens J. D., Mahoney M. J., Hobbins J. C., Haseltine F. P., Caskey C. T., Banker B. Q. Failure of fetal creatine phosphokinase as a diagnostic indicator of Duchenne muscular dystrophy. N Engl J Med. 1979 Apr 12;300(15):860–861. doi: 10.1056/NEJM197904123001515. [DOI] [PubMed] [Google Scholar]
- Hauschka S. D. Clonal analysis of vertebrate myogenesis. 3. Developmental changes in the muscle-colony-forming cells of the human fetal limb. Dev Biol. 1974 Apr;37(2):345–368. doi: 10.1016/0012-1606(74)90154-7. [DOI] [PubMed] [Google Scholar]
- Ionasescu V., Ionasescu R., Feld R., Witte D., Cancilla P., Kaeding L., Stern L. Z. Alterations in creatine kinase in fresh muscle and cell cultures in Duchenne dystrophy. Ann Neurol. 1981 Apr;9(4):394–399. doi: 10.1002/ana.410090413. [DOI] [PubMed] [Google Scholar]
- Ionasescu V., Monaco L., Sandra A., Ionasescu R., Burmeister L., Deprosse C., Stern L. Z. Alterations in lipid incorporation in Duchenne muscular dystrophy. Studies of fresh and cultured muscle. J Neurol Sci. 1981 May;50(2):249–251. doi: 10.1016/0022-510x(81)90171-4. [DOI] [PubMed] [Google Scholar]
- Ionasescu V., Zellweger H., Burmeister L. Detection of carriers and genetic counseling in duchenne muscular dystrophy by ribosomal protein synthesis. Acta Neurol Scand. 1976 Nov;54(5):442–452. doi: 10.1111/j.1600-0404.1976.tb04376.x. [DOI] [PubMed] [Google Scholar]
- Konigsberg U. R., Lipton B. H., Konigsberg I. R. The regenerative response of single mature muscle fibers isolated in vitro. Dev Biol. 1975 Aug;45(2):260–275. doi: 10.1016/0012-1606(75)90065-2. [DOI] [PubMed] [Google Scholar]
- MAURO A. Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol. 1961 Feb;9:493–495. doi: 10.1083/jcb.9.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin G. M., Sprague C. A., Epstein C. J. Replicative life-span of cultivated human cells. Effects of donor's age, tissue, and genotype. Lab Invest. 1970 Jul;23(1):86–92. [PubMed] [Google Scholar]
- O'STEEN W. K. THE GROWTH OF HUMAN DYSTROPHIC SKELETAL MUSCLE IN DIFFUSION CHAMBERS. Tex Rep Biol Med. 1963;21:369–379. [PubMed] [Google Scholar]
- Okazaki K., Holtzer H. Myogenesis: fusion, myosin synthesis, and the mitotic cycle. Proc Natl Acad Sci U S A. 1966 Nov;56(5):1484–1490. doi: 10.1073/pnas.56.5.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papayannopoulou T., Brice M., Stamatoyannopoulos G. Hemoglobin F synthesis in vitro: evidence for control at the level of primitive erythroid stem cells. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2923–2927. doi: 10.1073/pnas.74.7.2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papayannopoulou T., Kalmantis T., Stamatoyannopoulos G. Cellular regulation of hemoglobin switching: evidence for inverse relationship between fetal hemoglobin synthesis and degree of maturity of human erythroid cells. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6420–6424. doi: 10.1073/pnas.76.12.6420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STOCKDALE F. E., HOLTZER H. DNA synthesis and myogenesis. Exp Cell Res. 1961 Sep;24:508–520. doi: 10.1016/0014-4827(61)90450-5. [DOI] [PubMed] [Google Scholar]
- Thompson E. J. Tissue culture of dystrophic muscle cells. Br Med Bull. 1980 May;36(2):181–185. doi: 10.1093/oxfordjournals.bmb.a071635. [DOI] [PubMed] [Google Scholar]
- Thompson E. J., Yasin R., Van Beers G., Nurse K., Al-Ani S. Myogenic defect in human muscular dystrophy. Nature. 1977 Jul 21;268(5617):241–243. doi: 10.1038/268241a0. [DOI] [PubMed] [Google Scholar]




