Abstract
Background
Healing chronic wounds is an ongoing challenge for clinicians and poses a serious public health burden. Electrical stimulation (ES), broadly defined as the application of electrical current via electrodes placed on the skin adjacent to or directly within the wound, has been proposed as a therapeutic modality over a century ago, and recent advances in understanding the biology of electrical phenomena in the skin have rekindled an interest in this modality.
The Problem
Despite evidence that has shown ES to be effective for wound healing, it has been slow to gain acceptance in the United States. Also, there has been no consensus in terms of standardization of parameters to devise a systematic protocol for implementation of this technology.
Basic/Clinical Science Advances
The epidermis maintains a “skin battery” that generates an endogenous electric field and current flow when wounded. Experimental models have demonstrated that most of the cell types within the wound can sense an electric field in the range of that endogenously generated in the wound, and respond with a variety of biological and functional responses that can contribute to healing. Multiple animal wound models have demonstrated enhancement of a number of parameters of healing when ES is exogenously supplied.
Clinical Care Relevance
Clinical trials have investigated the efficacy of multiple forms of ES for improving healing in a wide variety of human chronic wounds. In 2002 the Centers for Medicare and Medicaid Services approved reimbursement for use of ES in a clinical setting for certain chronic wounds.
Conclusion
There remain many voids in our knowledge base: clinical evidence is limited by deficiencies in the design of many of the trials, a multiplicity of ES application modes and waveforms used in trials prevent selection of an optimal modality, and lack of uniformity in reporting ES dosages leave us not much advanced from our clinical knowledge base a decade ago.

Sara Dahle
Background
Despite recent advances in the understanding of the biology of healing, the clinical problem of the chronic skin wound remains an unmet need. It has been proposed that in the absence of vascular or infection issues, a 50% reduction in wound size by 4 weeks of treatment is a good predictor that the wound will completely heal.1 If this reduction in size has not been achieved, “advanced wound care therapies” have been advocated to speed up the healing process. These include bioengineered skin substitutes, negative pressure wound devices, oxygen, ultrasound, and electrical stimulation (ES).2
ES technology broadly defined is the application of electrical current through electrodes placed on the skin near or directly within the wound. Although proposed as a therapeutic hundreds of years ago, recent advances in understanding the biology of electrical phenomena in the skin have rekindled an interest in this modality. Delivery of current has been shown to promote cellular activity in nearly all phases of experimental wound healing, and a comprehensive review of the physiology underpinning ES has been recently published.3 Here, we update the reader with the latest clinical studies in the field and critically assess the evidence for ES.
Target Articles.
1. Lee BY, Al-Waili N, Stubbs D, Wendell K, Butler G, Al-Waili T, and Al-Waili A: Ultra-low microcurrent in the management of diabetes mellitus, hypertension and chronic wounds: report of twelve cases and discussion of mechanism of action. Int J Med Sci 2009; 7: 29.
2. Petrofsky JS, Lawson D, Berk L, and Suh H: Enhanced healing of diabetic foot ulcers using local heat and electrical stimulation of 30 min three times per week. J Diabetes 2010; 2: 41.
3. Houghton PE, Campbell KE, Fraser CH, Harris C, Keast DH, Potter PJ, Hayes KC, and Woodbury MG: Electrical stimulation therapy increases rate of healing of pressure ulcers in community-dwelling people with spinal injury. Arch Phys Med Rehabil 2010; 91: 669.
Clinical Problem
Healing chronic wounds is an ongoing challenge for clinicians and poses a serious public health burden, with skyrocketing costs and a spiral of morbidity issues associated with nonhealing wounds. On average, the cost of care is $8,000 for one ulcer and $17,000 for an infected ulcer. Of those with diabetes and limb amputations, 85% were preceded by ulceration. Globally, advanced therapies have been estimated to cost 5 billion.4,5
An important initial step when approaching a wound is to identify its etiology (e.g., pressure, ischemic, venous stasis, neuropathic, or mixed) and implement the necessary standard of care (e.g., offloading, revascularization, compression, and/or debridement). Once a wound stagnates into a chronic phase, the next decision clinicians face is determining which of the advanced therapies to use given the differences of modalities available as well as cost and time invested. Among the options of advanced therapies available, ES offers an alternative that has been shown to have a positive effect at different stages of wound healing in many preclinical studies. However, as ES is not a new technology and numerous animal and clinical studies have been published to support its use, it is perhaps surprising that it is less widely adopted in the United States than might be expected.6 This may in part derive from late (2002) approval by Centers for Medicare and Medicaid Services for reimbursement for ES treatment of chronic ulcers (>30 days) that had failed standard wound therapies for diabetic, pressure (stage III or IV), stasis, and arterial ulcers—this approval came only after a lawsuit brought by the American Physical Therapy Association.7 Although the Food and Drug Administration (FDA) has granted premarket application for ES devices for bone, deep brain, and muscle stimulation, it has not currently approved ES for the treatment of skin wounds.8 In addition to the “off-label” use of ES devices for wound healing, the clinical problem faced is that there is no clearly defined method of use. ES devices vary in voltage, current settings, mode and length of time of application, mono- or bipolar, bi or tri-electrodes, as well as types of wounds indicated.
Relevant Basic Science Context
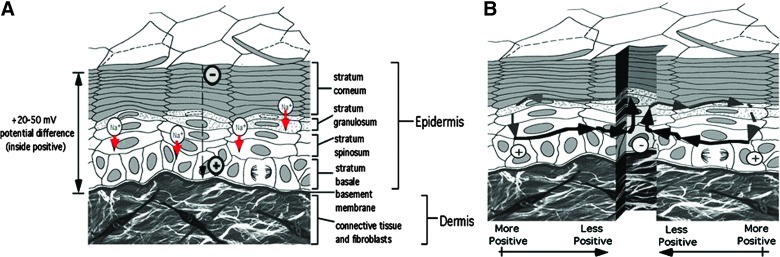
It has been established that human epidermis maintains an endogenous battery and, when its integrity is breached, generates an electric field in the immediate wound vicinity (Fig. 1).9,10 Application of external electrical current to wounds has been shown to facilitate some aspects of the repair process. Preclinical studies have demonstrated that cellular activities, such as collagen and DNA synthesis, ATP concentration, and generation of chemotaxic factors, are enhanced by ES.3 ES has also been demonstrated to increase tissue perfusion, decrease edema, and promote angiogenesis and galvanotaxis, directing cell migration in the wound tissue to promote wound healing.11,12
Figure 1.
Generation of skin wound electric fields. Unbroken skin maintains a “skin battery,” derived by apical-basal transport of Na+ and generation of a transepithelial potential (A). When wounded, the potential drives current flow through the newly formed low-resistance pathway (B), generating an electric field whose negative vector points toward the wound center at the lower portion of the epidermis.10 Color images available online at www.liebertpub.com/wound
Experimental Model or Material: Advantages and Limitations
A number of in vivo, in vitro, and animal model studies have revealed the effects of ES on healing processes, demonstrating increased collagen deposition, enhanced angiogenesis, greater wound tensile strength, and a faster wound contraction rate.3 ES has also been shown to play a role in bacteriostatic and bactericidal effects demonstrated both in vitro and in vivo.3 Animal models have advantages in the study of ES, as they allow the investigator to vary the parameters to determine optimal healing potential, studies that are difficult to carry out in humans. Overall, animal studies have demonstrated positive effects of ES in experimental wound healing, providing an experimental venue to fine-tune the parameters necessary for development of clinical devices. The shortcomings are that there are few animal model wounds for human chronic wounds, particularly for venous and diabetic wounds. Even in diabetic animal models, the neuropathic component thought to impact on human diabetic ulceration is lacking. Most animal studies have been conducted on rodents, which significantly differ from human skin in epidermal thickness and in mechanism of healing by contraction. Comparing animals' dermis, which differs in thickness, presence or absence of hair, and location of injury, may affect electrical current flow.10 The randomized human clinical trial will remain the gold standard for assessing clinical efficacy of proposed ES modalities.
Discussion of Findings and Relevant Literature
Some recent reviews have listed a number of clinical trials to support the use of ES for wound healing.3 However, the reported studies demonstrate great variability in the parameters of ES application, leading to an inability to generate sufficient evidence to support any one standard therapeutic approach. Indeed, the U.S. FDA has not yet approved any ES devices for the indication of healing skin wounds (a potential exception may be Procellera™, a woven metallic bandage with embedded microbatteries, which has received premarket approval in 200813 as a dressing, and whose inventor proposes the mechanism of action is delivery of ES to the wound). Although some advisory bodies, such as the National Pressure Ulcer Advisory Panel and the European Pressure Ulcer Advisory Panel, have deemed that “direct contact (capacitative) ES” is supported by level A evidence, it is neither clear what parameters for this ES should be used, nor what device(s) can be used to deliver it. Indeed, they note that “all of these biophysical energies should be delivered using government-agency-approved medical devices,” yet there are no devices so approved for this indication. It is also important to note that although many U.S. major health insurance carriers provide coverage for ES, this is only when used in a clinical setting with direct professional supervision.14,15 Thus, confusion persists as to what kind of ES has strong clinical evidence for improving wound healing. The Cochrane Wound Group has an ongoing protocol to review ES for chronic wounds.16 Here, we review three target articles that provide recent clinical evidence for the various devices used to deliver ES to different chronic wounds, which will highlight the problems associated with evaluating published work supporting ES in wound healing.
Houghton et al. conducted a randomized, single-blinded, parallel-group study evaluating high-voltage pulsed current on patients with pressure ulcers and spinal cord injuries, in a community setting.17 This study is well controlled, matching cases and controls, stratifying for wound duration, with additional secondary outcome measurement of wound quality assessment, and with 3 months of follow-up period. The results showed that wound area decreased significantly in the ES group compared with the standard of care group.17 Limited drawbacks of this study are that (1) the study size was relatively small (34 subjects) and (2) ES test protocols were varied to accommodate to the patient and caregiver skills, making it difficult to analyze what treatment was actually delivered. Yet, a major advance of this study is the demonstration that ES could be effectively delivered in the community, or at home, and did not need to be relegated to the clinic with direct oversight by healthcare providers.
An ongoing debate of whether heat generated by ES contributes to its potential to heal wounds was addressed by Petrofsky et al. This study compared healing in diabetic foot ulcers treated either with heat alone or ES plus heat and found a significant decrease in wound area after a month treatment with the ES plus heat group, compared with heat alone.18 This study used biphasic symmetrical pulsed current with low-current flow, 20 mA) delivered using three electrodes (one active and two reference). However, this study has significant shortcomings, in the small sample size (n = 10 in each group), the short follow-up period (1 month), and the lack of an ES alone control. It is also unclear whether the addition of heat to treatment with other ES devices that have different ES characteristics would have the same effects. So it is not entirely clear that application of additional heating to ES devices globally improves healing.
Another touted form of ES is “microcurrent” application. Lee et al.19 used direct-current (DC) ES at 3 mA to treat 12 patients with a wide variety of morbidities that included diabetes and hypertension, including two patients with ulcers. Although the authors conclude that this case series demonstrates that “that ultra-low microcurrent has apparent therapeutic effects on diabetes, hypertension, and wound healing,” the very small number of patients with wounds2 limits the nature of the evidence that can be gleaned from this study.
Overall, authors of these studies reported successful positive outcomes using ES to accelerate wound healing. Yet, the wide differences in types of ulcerations, ES parameter settings, and limited power of study design make it difficult to draw conclusions as to an optimal mode of ES usage or ES device.
Innovation
Despite the new products being introduced to the market for wound ES (Fig. 2), there remains much to be accomplished to demonstrate clinical utility and optimize and standardize its application to the wound. The study by Houghton et al. advances the field by demonstrating that ES may be effectively applied in a home or nonclinical setting. Nevertheless, there were issues with compliance, as the authors noted the patients were less likely to wear ES for full 8 h recommended while sleeping, but those who had complied had better outcomes.17 This study may translate into expanded reimbursements by third-party insurers to include home ES use; at this time, reimbursement is only available for ES applied under direct supervision of a professional healthcare provider.
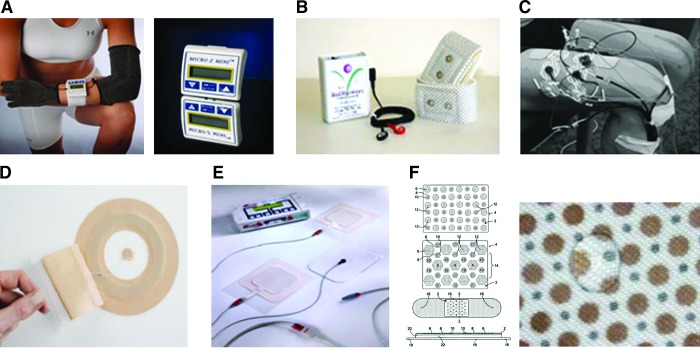
Figure 2.
Electrical stimulation devices used for healing chronic wounds. Devices used in the target studies: (A) Houghton et al.17—the Micro Z©, a portable monophasic twin peak wave form with high-voltage pulsed current and electroconductive sock (monopolar or bipolar method used). Voltage ranges from 50 to 150 V (www.advancedtherapyconcepts.com); (B) Lee et al.19—the Electro Pressure Regeneration Therapy™ (EPRT) device, an ultra-low microcurrent that delivers square wave bipolar, direct current. Voltage ranges from 5 to 40 V (www.bodiharmoni.com); (C) Petrofsky et al.18—customized three-channel stimulator, biphasic wave with 20 mA, and two pathways of current created rotated every second in sequence.20 Internationally available marketed devices: (D) Posifect® by BIOFiSica (UK) Ltd., used in the United Kingdom, is a bandage that delivers direct current microamps with embedded metallic anode in outer and inner bandages with embedded metallic anode in a hydrogel placed over the wound; external power source required (www.sumed.co.uk); (E) Wound EL® by GerroMed Dressing electrode and dispersing electrode, with patient-programmable external power source, delivers pulsed direct current stimulation; (F) Procellera® by Vomaris, Inc., is the only U.S. FDA 510 K, over-the-counter, or by prescription approved medical device for partial and full-thickness wounds. A small voltage (2–10 mV) is produced by microbatteries of Ag and Zn metals printed onto woven bandage activated by moisture of the wound (http://procellera.com). Color images available online at www.liebertpub.com/wound
The other target studies attempt to use other ES modalities, such as microcurrent (in target article 3)19 or novel application procedures such as multiple electrodes (in target article 2 and other studies by this group that compared two, three, and four-electrode system, finding the four-electrode with the rationale that increasing the number of electrodes to deliver the ES will result in more even and deeper dispersion of delivered ES).20 These studies are insufficiently powered to provide more than preliminary trends that require further investigation. It has been suggested that to allow for comparisons of modalities and ES dosing, all studies should report ES delivery as current density.3 This would certainly enable comparisons and modality optimization.
Future Development of Interest
Although intrinsic electric fields have been demonstrated in acute skin wounds, there is no evidence yet that they are deficient in chronic wounds to provide support for the current clinical practice of application of externally generated ES. It will be important to determine whether this is the case to provide a scientific rationale for exogenous application of ES to chronic wounds. Work by several investigators is ongoing to address this important unresolved issue.
Take-Home Message.
Basic science advances
ES enhances aspects of wound healing. This has been established in vitro and in animal models with
• understanding that skin wounds themselves create a current and generate an electric field;
• demonstration of the cellular pathways that signal electrical stimuli and induce biological and functional responses; and
• documentation of changes in multiple wound-related phenomena in experimental wound in response to ES, including increased ATP and DNA synthesis, galvanotaxic directional migration of epithelial cells, decreasing edema, increased angiogenesis, and increased nerve sprouting.
Clinical science advances
• ES (DC, pulsed, microcurrent) has been applied in various settings and types of ulcers such as pressure, neuropathic, diabetic, and ischemic wounds, each demonstrating some potential for accelerating wound healing.
• Possible synergistic effect of wound healing was found in combination with heat in promoting wound healing.
• Microcurrent ES may be an effective alternative to high-voltage pulsed ES.
• New devices are being tested, to improve ease of use and to more uniformly deliver current with electrodes.
• Home application of ES may be as efficacious as ES applied in a clinical setting under the direct supervision of a healthcare provider.
Relevance to clinical care
• Nonhealing wounds have tremendous impact on the healthcare system, costing billions of dollars every year. Design of devices that can be used for delivery of community-based ES as adjunctive therapy may prove to be more cost effective than other current standard therapies available.
• New technologies offering novel electrode design and dressings with embedded batteries may make the ES delivery devices more tractable for clinical uses.
• Ultimate adoption of devices will depend on reimbursement by third-party payers, and this in turn is dependent on the generation of substantive clinical evidence.
• Widespread adoption will also be dependent on the availability of FDA-approved devices. These are currently under investigation, and none is approved for the indication of healing chronic skin wounds.
There has been a new interest in the role of stochastic electrical noise and wound healing, with a recent preliminary study showing promising results in promoting wound healing.21 Using an ES of stochastic electrical noise has been found to activate sensory nerves in humans, which may have positive effects at stimulating the wound healing process. This finding may help provide a unifying explanation as to why disparate and widely differing ES modalities used to date have all been interpreted as improving healing outcomes.
Caution, Critical Remarks, and Recommendations
Thus far, no significant adverse reactions have been demonstrated in clinical trials using ES for healing chronic wounds. Studies have reported only findings of minor rash and skin irritations. Proponents of ES therapy suggest that this can be used on all types of wounds, except in the setting of bone infection, in malignancy, and in patients with implanted electronic devices.3 In the future, well-designed clinical studies specifically investigating safety issues of the various devices are needed. This will undoubtedly be part of the FDA approval process for proposed ES devices.
It is unclear as to why certain ES devices and waveform modalities have been selected for clinical investigation, compared with others. The epidermal “skin battery” generates constant, monophasic DC at the wound site, and thus, the rationale for the application of pulsed modalities and biphasic waveforms is not clear. ES device choices by investigators may be governed by accessibility, familiarity with a particular modality, and intellectual property or commercial development potential. In these latter cases, conflict of interest should be disclosed and experimental rigor enhanced to limit the potential for bias.
Abbreviations and Acronyms
- DC
direct current
- ES
electrical stimulation
- FDA
Food and Drug Administration
Acknowledgments and Funding Sources
The authors gratefully acknowledge Dr. Luther Kloth's thoughtful discussion. Partial support for R.R.I. and S.E.D. was provided via VA Merit Award 8044900.
Author Disclosure and Ghostwriting
The authors have no disclosures and no ghostwriters were used.
References
- 1.Sheehan P. Jones P. Caselli A. Giurini JM. Veves A. Percent change in wound area of diabetic foot ulcers over a 4-week period is a robust predictor of complete healing in a 12-week prospective trial. Diabetes Care. 2003;26:1879. doi: 10.2337/diacare.26.6.1879. [DOI] [PubMed] [Google Scholar]
- 2.Snyder RJ. Kirsner RS. Warriner RA., 3rd Lavery LA. Hanft JR. Sheehan P. Consensus recommendations on advancing the standard of care for treating neuropathic foot ulcers in patients with diabetes. Ostomy Wound Manage. 2010;56(4 Suppl):S1. [PubMed] [Google Scholar]
- 3.Kloth L. Zhao M. Endogenous, exogenous electrical fields for wound healing. In: McCulloch J, editor; Kloth L, editor. Wound Healing: Evidence-Based Management. 4th. Brick, NJ: F.A. Davis; 2009. pp. 450–513. [Google Scholar]
- 4.Reiber GE. Ledoux WR. Epidemiology of diabetic foot ulcers, amputations: evidence for prevention. In: Williams R, editor; Herman W, editor; Kinmonth AL, editor; Wareham NJ, editor. The Evidence Base for Diabetes Care. Hoboken, NJ: John Wiley & Sons; 2002. pp. 641–665. [Google Scholar]
- 5.Boulton AJ. Vileikyte L. Ragnarson-Tennvall G. Apelqvist J. The global burden of diabetic foot disease. Lancet. 2005;366:1719. doi: 10.1016/S0140-6736(05)67698-2. [DOI] [PubMed] [Google Scholar]
- 6.Hinchliffe RJ. Valk GD. Apelqvist J, et al. A systematic review of the effectiveness of interventions to enhance the healing of chronic ulcers of the foot in diabetes. Diabetes Metab Res Rev. 2008;24(Suppl 1):S119. doi: 10.1002/dmrr.825. [DOI] [PubMed] [Google Scholar]
- 7.Tunis S. Shuren J. Ballantine L. Chin J. Decision memo for electrostimulation for wounds (CAG-00068N) 2002. www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?NCAId=27&ver=11&NcaName=Electrostimulation+for+Wounds&NCDId=190&ncdver=2&IsPopup=y&bc=AAAAAAAAIAAA& www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?NCAId=27&ver=11&NcaName=Electrostimulation+for+Wounds&NCDId=190&ncdver=2&IsPopup=y&bc=AAAAAAAAIAAA& ;
- 8.U.S. Food and Drug Administration: Center for Devices and Radiological Health medical device database. 2011. www.accessdata.fda.gov/scripts/cdrh/devicesatfda/index.cfm www.accessdata.fda.gov/scripts/cdrh/devicesatfda/index.cfm
- 9.Jaffe LF. Vanable JW., Jr Electric fields and wound healing. Clin Dermatol. 1984;2:34. doi: 10.1016/0738-081x(84)90025-7. [DOI] [PubMed] [Google Scholar]
- 10.Ojingwa JC. Isseroff RR. Electrical stimulation of wound healing. J Invest Dermatol. 2003;121:1. doi: 10.1046/j.1523-1747.2003.12454.x. [DOI] [PubMed] [Google Scholar]
- 11.Nishimura KY. Isseroff RR. Nuccitelli R. Human keratinocytes migrate to the negative pole in direct current electric fields comparable to those measured in mammalian wounds. J Cell Sci. 1996;109(Pt 1):199. doi: 10.1242/jcs.109.1.199. [DOI] [PubMed] [Google Scholar]
- 12.Ennis WJ. Lee C. Plummer M. Meneses P. Current status of the use of modalities in wound care: electrical stimulation and ultrasound therapy. Plast Reconstr Surg. 2011;127(Suppl 1):93S. doi: 10.1097/PRS.0b013e3181fbe2fd. [DOI] [PubMed] [Google Scholar]
- 13.Melkerson MN. Memo to Vomaris Innovations, Inc. Center for Devices and Radiological Health. 2008. www.accessdata.fda.gov/cdrh_docs/pdf8/K081977.pdf www.accessdata.fda.gov/cdrh_docs/pdf8/K081977.pdf
- 14.Aetna. Clinical policy bulletin: electrical stimulation for chronic ulcers. 2011. www.aetna.com/cpb/medical/data/600_699/0680.html www.aetna.com/cpb/medical/data/600_699/0680.html
- 15.CIGNA. CIGNA medical coverage policy: electrical stimulation for wound healing. 2011. www.cigna.com/customer_care/healthcare_professional/coverage_positions/medical/mm_0351_coveragepositioncriteria_electrical_stimulation_for_wound_healing.pdf www.cigna.com/customer_care/healthcare_professional/coverage_positions/medical/mm_0351_coveragepositioncriteria_electrical_stimulation_for_wound_healing.pdf
- 16.Fernandez-Chimeno M. Houghton PE. Holey L. Electrical stimulation for chronic wounds (protocol) Cochrane Libr. 2008.
- 17.Houghton PE. Campbell KE. Fraser CH, et al. Electrical stimulation therapy increases rate of healing of pressure ulcers in community-dwelling people with spinal cord injury. Arch Phys Med Rehabil. 2010;91:669. doi: 10.1016/j.apmr.2009.12.026. [DOI] [PubMed] [Google Scholar]
- 18.Petrofsky JS. Lawson D. Berk L. Suh H. Enhanced healing of diabetic foot ulcers using local heat and electrical stimulation for 30 min three times per week. J Diabetes. 2010;2:41. doi: 10.1111/j.1753-0407.2009.00058.x. [DOI] [PubMed] [Google Scholar]
- 19.Lee BY. Al-Waili N. Stubbs D, et al. Ultra-low microcurrent in the management of diabetes mellitus, hypertension and chronic wounds: report of twelve cases and discussion of mechanism of action. Int J Med Sci. 2009;7:29. doi: 10.7150/ijms.7.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petrofsky J. Lawson D. Prowse M. Suh HJ. Effects of a 2-, 3- and 4-electrode stimulator design on current dispersion on the surface and into the limb during electrical stimulation in controls and patients with wounds. J Med Eng Technol. 2008;32:485. doi: 10.1080/03091900701574407. [DOI] [PubMed] [Google Scholar]
- 21.Ricci E. Afaragan M. The effect of stochastic electrical noise on hard-to-heal wounds. J Wound Care. 2010;19:96. doi: 10.12968/jowc.2010.19.3.47278. [DOI] [PubMed] [Google Scholar]