Abstract
A long-held tenet of molecular pharmacology is that canonical signal transduction mediated by G-protein-coupled receptor (GPCR) coupling to heterotrimeric G proteins is confined to the plasma membrane. Evidence supporting this traditional view is based on analytical methods that provide limited or non-subcellular resolution1. It has been subsequently proposed that signalling by internalized GPCR is restricted to G-protein-independent mechanisms such as scaffolding by arrestins2,3, or GPCR activation elicits a discrete form of persistent G protein activation4–9, or that internalized GPCR can indeed contribute to the acute G protein-mediated response10. Evidence supporting these various latter hypotheses is indirect or subject to alternate interpretation, and it remains unknown if endosome-localized GPCR are even present in an active form. Here we describe the application of conformation-specific single domain antibodies (nanobodies) to directly probe activation of the β2-adrenoceptor, a prototypical GPCR11, and its cognate G protein, Gs (ref. 12) in living mammalian cells. We show that the adrenergic agonist isoprenaline promotes receptor and G protein activation in the plasma membrane as expected, but also in the early endosome membrane; and that internalized receptors contribute to the overall cellular cyclic AMP response within several minutes after agonist application. These findings provide direct support for the hypothesis that canonical GPCR signalling occurs from endosomes as well as the plasma membrane, and suggest a versatile strategy for probing dynamic conformational change in vivo.
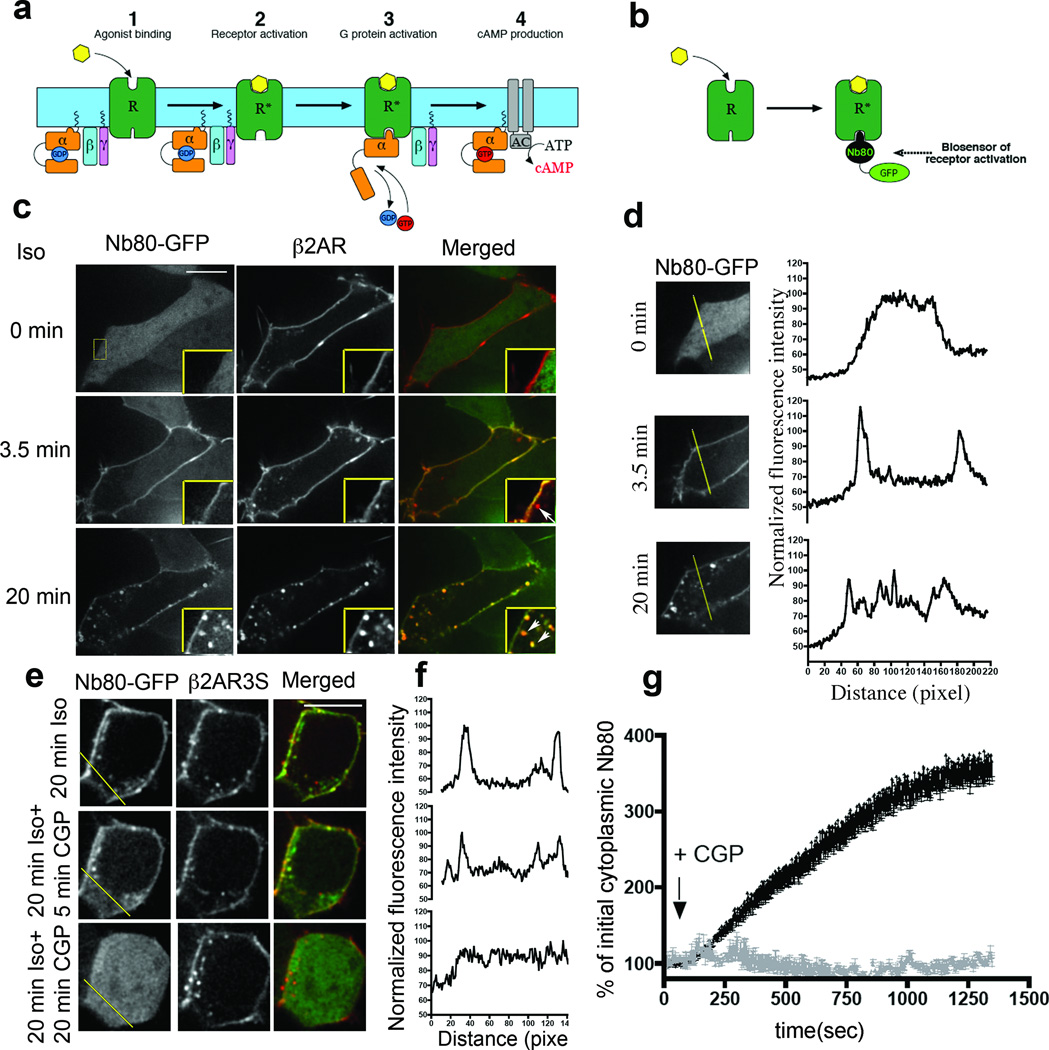
Ligand binding to the extracellular surface of the β2-adrenoceptor (β2-AR) produces an activating conformational change in the receptor that promotes guanine nucleotide dissociation from the cytoplasmic GTP-binding protein Gαs; this represents the critical biochemical event initiating classical GPCR signal transduction (Fig. 1a)13. Activated β2-ARs are substrates for phosphorylation and binding of β-arrestins, events which inhibit interaction with G proteins and promote endocytosis of receptors via clathrin-coated pits (CCPs)14,15. Acute β2-AR Gαs signalling is thus traditionally thought to be restricted to the plasma membrane14,16,17. However, to our knowledge, this assumption has not been directly tested. To do so, we generated a biosensor of activated β2-AR based on a conformation-specific single-domain camelid antibody (Nb80) used in recent structural studies18,19. We reasoned that this nanobody, which selectively binds the agonist-occupied β2-AR and is able to stabilize an activated receptor conformation when present in vitro at high concentration, might act as a sensor of receptor activation when expressed at relatively low concentration in intact cells (Fig. 1b). This proved to be the case; in cells maintained in the absence of agonist, Nb80 fused to enhanced GFP (Nb80–GFP) localized to the cytoplasm and not with β2-ARs present in the plasma membrane(Fig. 1c, 0 min, top row; Pearson’s coefficient = 0.135). Line scan analysis verified the cytoplasmic distribution of Nb80–GFP before β2-AR activation (Fig. 1d, top row) was as expected because the cytoplasmic concentration of Nb80–GFP achieved in our experiments (approximately equal to 20 nM) was considerably lower than the equilibrium dissociation constant estimated in vitro for Nb80 binding to purified β2-ARs in the absence of agonist (0.76 ± 0.14 µM; Supplementary Fig. 1a–d). After application of the adrenergic agonist isoprenaline (10 µM), Nb80–GFP was rapidly recruited to the plasma membrane and co-localized there with β2-ARs (Fig. 1c, middle row; Pearson’s coefficient = 0.625). Line scan analysis verified robust Nb80–GFP recruitment to the plasma membrane and concomitant depletion from the cytoplasm (Fig. 1d, middle row), consistent with the much higher affinity of Nb80 for isoprenaline-activated β2-ARs (2.9 ± 0.5 nM; Supplementary Fig. 1d). Agonist-induced membrane recruitment of Nb80–GFP was specific because the D1 Dopamine receptor (DRD1), which is also Gs-coupled but does not bind Nb80 in vitro (data not shown), failed to recruit Nb80–GFP to the plasma membrane in response to dopamine (10 µM) application (Supplementary Fig. 2). Furthermore, β2-AR–CFP and Nb80–YFP generated a pronounced fluorescence (Förster) resonance energy transfer (FRET) signal after isoprenaline application whereas DRD1–CFP did not (Supplementary Fig. 3a, b).
Figure 1. Nb80–GFP detects activated β2-ARs in the plasma membrane and endosomes.
a, The main events in β2-AR cAMP signalling include agonist binding (step 1), conformational activation of the receptor (step 2) that is coupled to conformational activation of Gs (step 3) that produces guanine nucleotide exchange on Gs and subsequent activation of adenylyl cyclase (AC) (step 4). b, Scheme for detecting conformational activation of β2-AR with Nb80–GFP. c, Representative Nb80-GFP (green) and β2-AR (red) localization at the indicated time (left) after 10 µM isoprenaline addition (>30 Nb80–GFP positive endosomes per cell observed at 20 min; n = 29 cells, 10 experiments). d, Representative individual Nb80–GFP line scans (shown at the same magnification as panel c). e, Representative Nb80–GFP (green) and β2-AR-3S (red) localization after 20 min of isoprenaline treatment (top) followed by reversal with 50 µM CGP-12177 for the indicated times (6.4 Nb80–GFP positive endosomes per cell; n = 40 cells, 3 experiments). f, Representative individual Nb80–GFP line scans. g, Recovery of cytoplasmic Nb80–GFP fluorescence (mean ± s.e.m., n = 5 experiments). Scale bars, 10 µm.
β2-AR internalization began 1 to 2 min after Nb80–GFP recruitment to the plasma membrane, indicated by the emergence of surface-labelled β2-AR in peripheral cytoplasmic vesicles. Nb80–GFP did not co-localize with β2-AR-containing endocytic vesicles upon first appearance (Fig. 1c, middle row, arrow in merged image points to an example) but was recruited at later time points (Fig. 1c, bottom row, Pearson’s coefficient = 0.702; examples are indicated by arrowheads). Endosome recruitment of Nb80–GFP was evident by line scan analysis (Fig. 1d, bottom row; line scans are from the representative individual examples with further quantification in legend) and localized to EEA1-marked early endosomes (Pearson’s coefficient = 0.846; Supplementary Fig. 4) through which β2-ARs iteratively cycle in the presence of agonist20. β2-AR-containing endosomes were initially devoid of Nb80–GFP and later acquired Nb80–GFP during their movement (Supplementary Videos 1 and 2). Interaction at endosomes was verified by β2-AR–CFP and Nb80-YFP normalized FRET (nFRET) (Supplementary Fig. 3c). These results suggest that β2-AR activation initiates a precisely choreographed series of events: Nb80–GFP is first recruited from the cytoplasm to the plasma membrane, then β2-ARs internalize devoid of Nb80–GFP, followed by a second phase of Nb80–GFP recruitment to the internalized β2-ARs.
Nb80–GFP recruitment to endosomes required β2-ARs because a phosphorylation-deficient mutant version of the β2-AR (β2AR-3S) that couples to Gαs but is impaired in agonist-induced endocytosis21 recruited Nb80–GFP to the plasma membrane but produced much less recruitment to endosomes (Fig. 1e, top row). Nb80–GFP co-localized with β2-AR-3S after agonist-induced activation (Pearson’s coefficient = 0.674) but this was largely restricted to the plasma membrane (line scan analysis in Fig. 1f is representative; further quantification in the figure legend). Nb80–GFP membrane recruitment was also reversible because the biosensor returned to a cytoplasmic distribution after addition of the competitive antagonist CGP-12177 (Fig. 1e, f, middle and bottom rows; Pearson’s coefficient = 0.106), verified by recovery of Nb80–GFP fluorescence intensity in the cytoplasm (Fig. 1g; see also Supplementary Video 3). Thus, Nb80–GFP detected activated β2-ARs both in the plasma membrane and endosomes after acute agonist application.
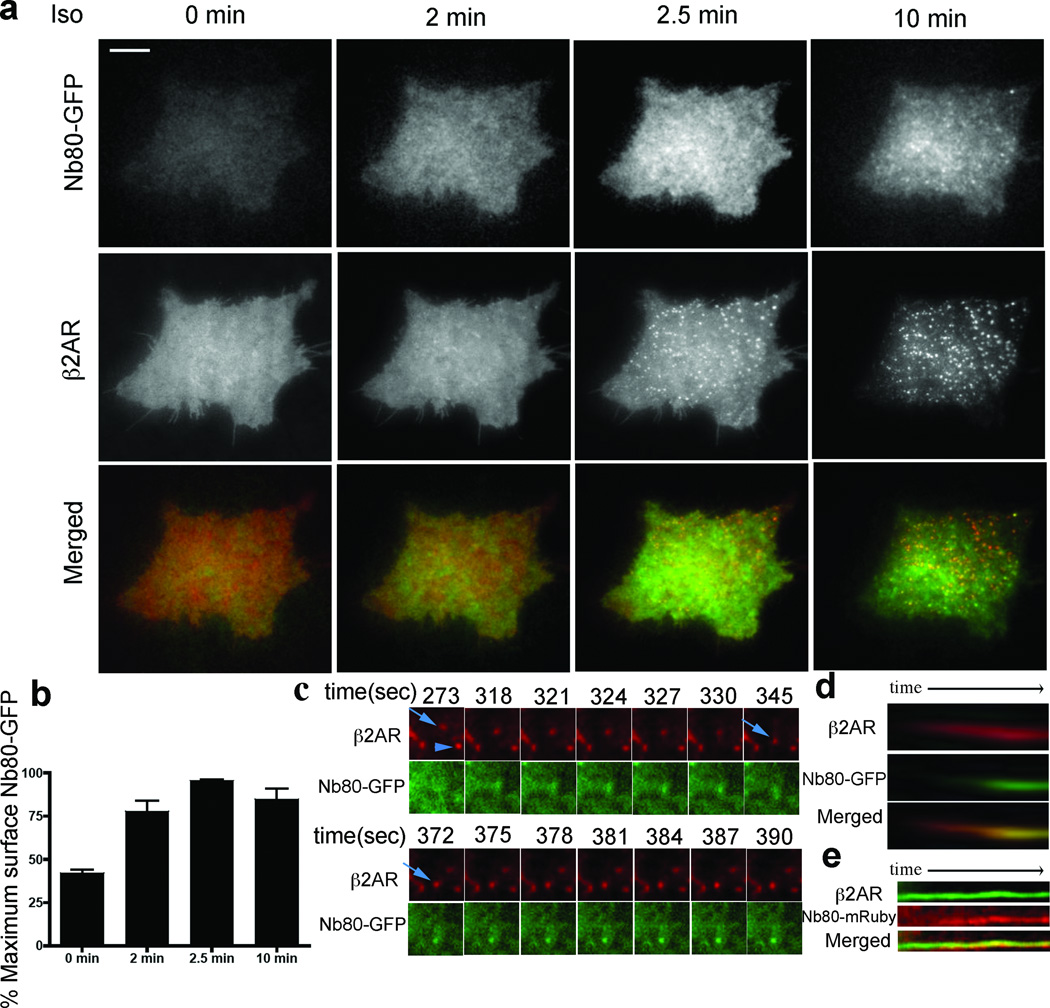
Discrete Nb80–GFP recruitment phases were clearly resolved by total internal reflection fluorescence (TIRF) microscopy that selectively detects events occurring in the plasma membrane and extending approximately equal to 100 nm into the peripheral cytoplasm22. First, within 2 min after agonist application, Nb80–GFP was progressively recruited to the plasma membrane in a diffuse distribution (Fig. 2a, compare first and second columns from left; quantification in Fig. 2b). Nb80–GFP did not co-localize with β2-ARs when they clustered in relatively static punctae characteristic of receptor-containing CCPs23 (Fig. 2a, third column). Second, over a period of several additional minutes, Nb80–GFP was recruited to a discrete population of highly mobile punctae (Fig. 2a, fourth column) representing peripheral β2-AR-containing endocytic vesicles23.
Figure 2. Nb80–GFP accumulates on β2-AR-containing endosomes after their formation.
a, β2-AR (red) and Nb80–GFP (green) at the indicated times after isoprenaline addition. Scale bar, 10 µm. b, Average Nb80–GFP fluorescence measured in the TIRF illumination field at the indicated times (mean ± s.e.m., n = 7 cells). c, TIRF image series showing β2-AR (red) and Nb80–GFP (green) in sequential frames. d, Kymograph of an individual β2-AR-containing endosome (red, Alexa555) showing Nb80–GFP (green) acquisition over 4 min. e, Kymograph of β2-AR (green, Alexa488) and Nb80–mRuby (red) over 6 min.
Sequential TIRF imaging emphasized the distinction between relatively static β2-AR puncta not co-localizing with Nb80–GFP (Fig. 2c, arrowhead indicates an example) and mobile endosomes that did (Fig. 2c, arrow indicates a representative example; many are visible in Supplementary Video 4). Later recruitment of Nb80–GFP occurred rather suddenly, typically within approximately 5 s (Supplementary Video 5), as also evident in kymographs of individual endosome trajectories (Fig. 2d). Ruling out potential artefacts of wavelength-dependent differences in TIRF microscopy illumination depth, later recruitment of the biosensor to endosomes was similarly observed when the excitation wavelengths used to detect receptor and biosensor were reversed (Fig. 2e).
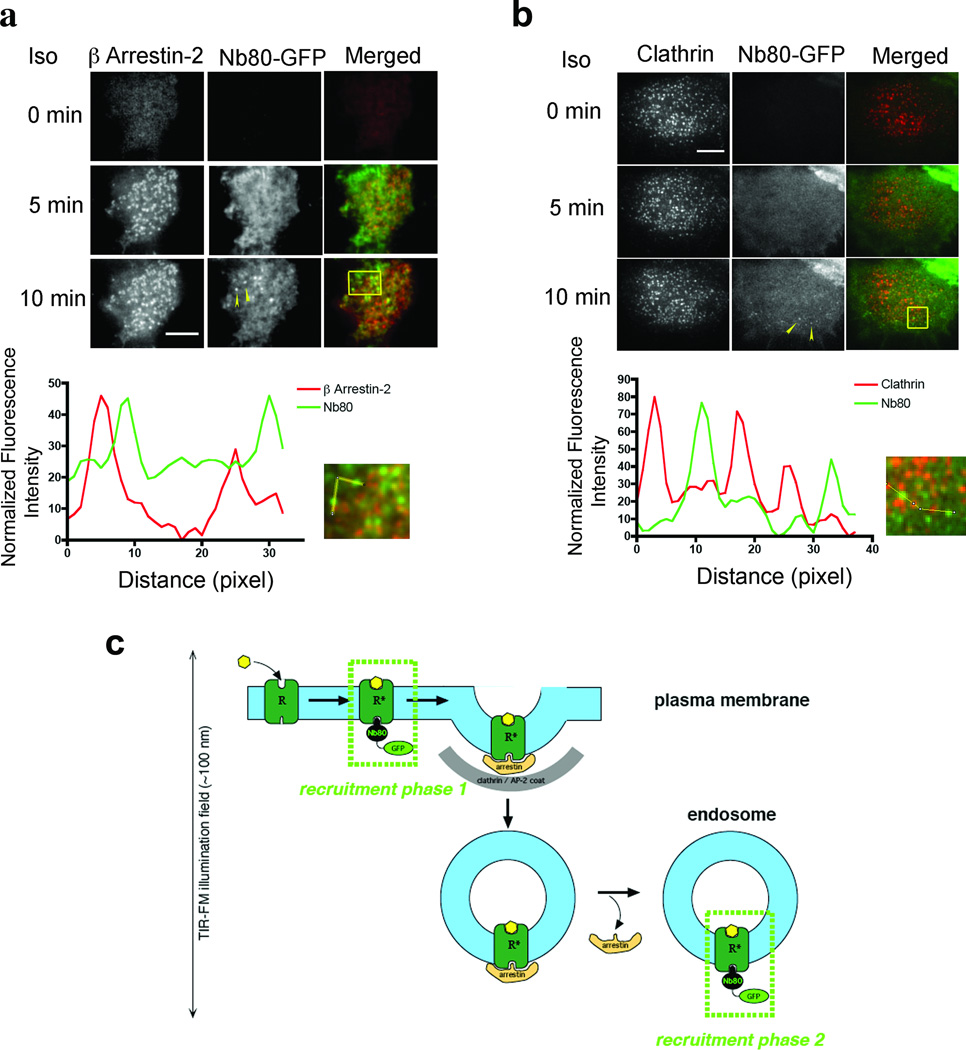
Nb80–GFP did not detectably concentrate in CCPs labelled with either of two independent markers, the adaptor protein β-arrestin (Fig. 3a and Supplementary Video 6) or the coat protein component clathrin light chain (Fig. 3b and Supplementary Video 7). Representative examples are shown and this was verified across multiple cells and experiments (Pearson’s coefficient = 0.365 and 0.319, respectively, with numbers of replicates specified in the legend). In contrast, and as expected based on previous work, extensive co-localization of β2-AR with β-arrestin was observed under the same conditions (Pearson's coefficient = 0.677). Separation of Nb80–GFP localization from that of either β-arrestin or clathrin was clear in line scans (bottom panels of Fig. 3a, b were derived from the images shown and are representative; further quantification is in the legend).
Figure 3. Nb80-GFP does not accumulate in clathrin-coated pits.
a, Representative TIRF microscopy frames showing Nb80–GFP (green) and β-arrestin-2–mCherry (red) before and after agonist addition. Fluorescence intensity profiles are shown below for the indicated region and path; representative of n = 39 cells, 5 experiments and 4,849 punctae. b, Equivalent analysis comparing Nb80–GFP (green) to clathrin light chain-dsRed (red); representative of n = 26 cells, 3 experiments and 3,965 punctae. Arrowheads indicate examples of Nb80-GFP labeled endosomes. Scale bars, 5 µm. c, Model for two phases of Nb80–GFP recruitment by the activated β2-AR, first at the plasma membrane and then at endosomes.
A simple interpretation is that Nb80–GFP associates with activated β2-ARs in the plasma membrane but then dissociates before receptors cluster in CCPs and internalize. This was surprising because β2-AR clustering in CCPs occurs much more rapidly23 than the overall reversal rate of Nb80–GFP recruitment observed after agonist washout in intact cells (Fig. 1g), or the kinetics of Nb80 dissociation from purified β2-ARs in vitro (Supplementary Fig. 1b, e). One possibility is that Nb80-GFP dissociation is accelerated during the clustering process, by mechanisms such as by receptor phosphorylation or steric exclusion mediated by β-arrestins or other CCP-associated components. An alternative possibility is that the fraction of β2-ARs that have bound Nb80–GFP in the plasma membrane by the time of the clustering reaction are unable to enter CCPs, and only those β2-ARs not initially bound to Nb80–GFP in the plasma membrane are able to cluster in CCPs and subsequently internalize. In either case, the data clearly indicate that Nb80–GFP associates with β2-ARs after endocytosis, and after uncoating of the endocytic vesicle has occurred (Fig. 3c). Accordingly, Nb80–GFP recruitment to β2-AR-containing endosomes cannot represent an artefact of persistent nanobody binding from the plasma membrane; instead, this observation reveals that β2-ARs present in early endosomes are in an activated conformation.
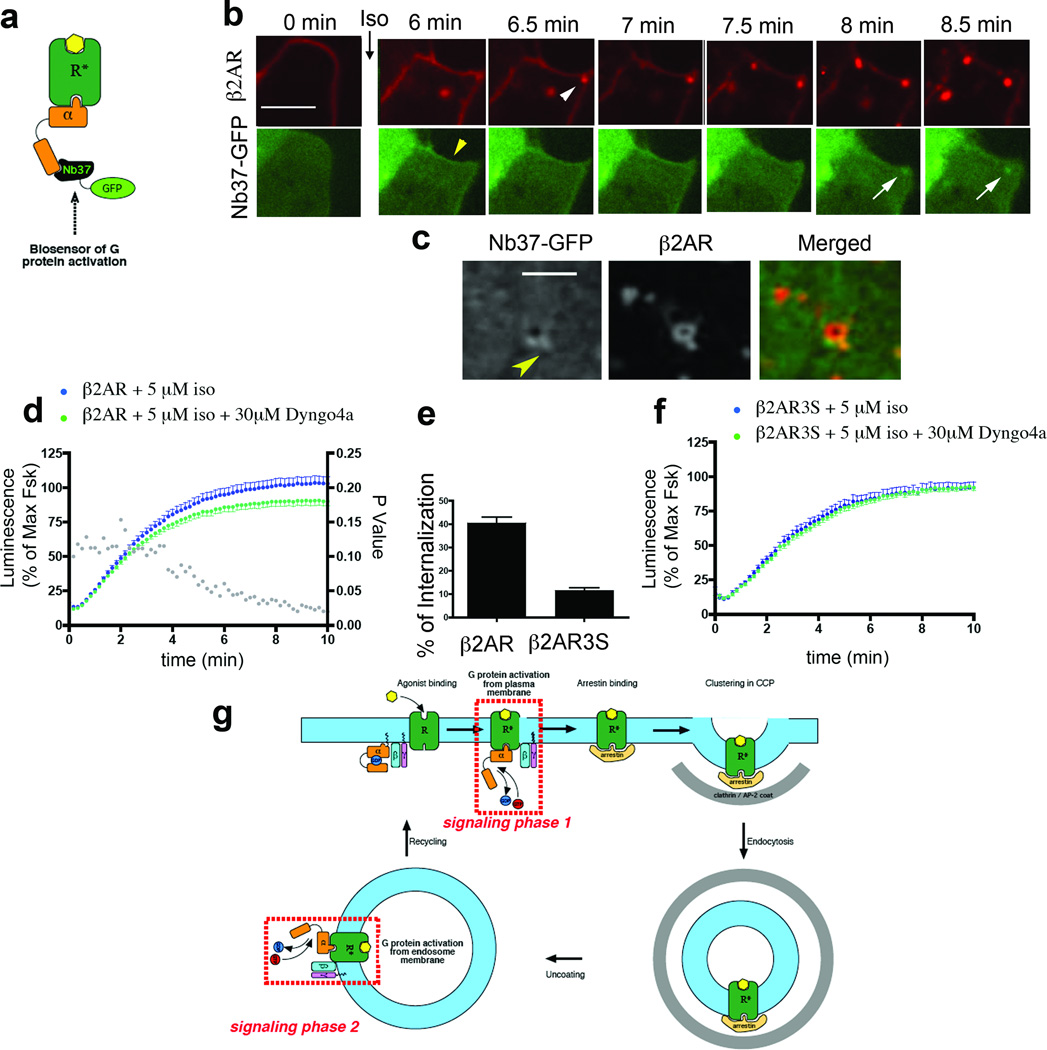
Because endosomes contain activated β2-ARs, we next asked if receptors engage their cognate G protein from this compartment. Heterotrimeric G proteins and adenylyl cyclase can be observed in endosomes as well as at the plasma membrane, supporting the concept of endosome-based G protein signalling4,6,10,24. To directly investigate the subcellular location of G protein activation, we developed a distinct biosensor based on another nanobody, Nb37, which specifically recognizes the guanine-nucleotide-free form of Gαs representing the catalytic intermediate of G protein activation (Fig. 4a)25. We hoped that because Nb37 binds a surface of the alpha-helical domain that is accessible only in the nucleotide-free form, we would be able to detect production of this critical but fleeting activation intermediate in living cells. This was indeed the case because Nb37–GFP localized in the cytoplasm of untreated cells and was rapidly recruited from the cytoplasm to the plasma membrane in response to isoprenaline application (Fig. 4b, yellow arrowhead, Pearson coefficient = 0.627). Membrane recruitment of Nb37–GFP was agonist-dependent even in cells overexpressing Gαs and, importantly, the cytoplasmic concentration of Nb37–GFP achieved in our experiments (also approximately 20 nM) was substantially lower than that producing detectable inhibition of G protein activation in vitro (Supplementary Fig. 5). Together, these observations suggest that Nb37–GFP indeed functions as a specific biosensor of Gs activation under the experimental conditions used.
Figure 4. Internalized β2-ARs contribute to the acute cAMP response.
a, Scheme for detecting conformational activation of Gs with Nb37–GFP. b, Confocal image frames showing Nb37–GFP (green) and β2-AR (red) at the indicated time points after isoprenaline addition (representative of n = 14 cells; estimated Nb37–GFP recruitment delay ranged from 0.7 to 2.65 min). Scale bar, 5µm. Yellow arrowhead indicates Nb37-GFP recruitment to the PM. White arrowhead indicates an endosome containing recently internalized β2-AR and not associated with Nb37-GFP, white arrow indicates Nb37–GFP recruitment. c, Representative confocal frames showing a discrete endosomal structure labelled with Nb37–GFP (green) and β2-AR (red) at higher magnification. Scale bar, 2 µm. Arrowhead indicates non-uniform localization of Nb37-GFP to the endosome. d, Forskolin-normalized β2-AR-mediated cAMP response in the absence or presence of 30 µM Dyngo-4a (mean ± s.e.m., n = 10 experiments, P values in grey). e, Isoprenaline (20 min)-induced β2-AR and β2-AR-3S internalization measured by flow cytometry (n = 4 experiments). f, Forskolin-normalized β2-AR-3S-mediated cAMP response in the absence or presence of 30 µM Dyngo-4a (n = 8 experiments; P = 0.1192). g, Model for two phases of β2-AR Gs activation, first at the plasma membrane and then at endosomes, separated by the endocytic event.
Nb37–GFP was recruited not only to the plasma membrane (Fig. 4b, yellow arrowhead) but also to β2-AR-containing endosomes. Notably, endosome recruitment of Nb37–GFP occurred after the appearance of β2-ARs in the endosome. Such later recruitment was evident in dual label confocal image series (Fig. 4b shows a representative example: white arrowhead indicates recently internalized β2-AR, white arrow indicates Nb37–GFP recruitment, Pearson coefficient = 0.710; see also Supplementary Video 8). Nb37–GFP recruitment was specifically dependent on receptor activation because a mutant β2-AR that is defective in G-protein coupling (β2-AR-Cys 341 Gly)26 did not produce detectable recruitment (Supplementary Fig. 6), whereas the distinct Gs-coupled DRD1 recruited Nb37–GFP to both the plasma membrane and endosomes (data not shown). Nb37–GFP localized uniformly on β2-AR-containing endosomes in cells overexpressing Gαs (Fig. 4b and Supplementary Video 8) but was recruited non-uniformly in cells expressing endogenous levels (Fig. 4c, arrowhead indicates an example). Moreover, Nb37–GFP recruitment to endosomes had a scintillating appearance, fluctuating rapidly when viewed in live image series (Supplementary Video 9), suggesting that G protein activation detected by Nb37–GFP occurs dynamically from limited regions of the endosome membrane.
We then asked if active β2-AR Gs coupling from endosomes contributes to the cellular response, focusing on cAMP as a classical second messenger carrying the downstream signal, using a previously described real-time assay of cAMP accumulation in living cells and normalizing to the signal produced by receptor-independent activation of adenylyl cyclase with forskolin (5 µM)27. Isoprenaline produced a rapid increase in cytoplasmic cAMP accumulation, reaching a maximum within approximately 10 min (Fig. 4d, blue line). Dyngo-4a, a dynamin inhibitor that blocks β2-AR endocytosis by inhibiting CCP function28,29, did not detectably affect the earliest portion of the forskolin-normalized cAMP accumulation curve but significantly reduced its later rise (Fig. 4d, green line, grey dots indicate P values), consistent with the two phases of endosome-localized activation detected by the nanobody-derived biosensors. A similar inhibition was observed in cells expressing a mutant β2-AR with a C-terminal alanine residue added that prevents efficient recycling (β2-AR-Ala)30 (Supplementary Fig. 7), suggesting that the Dyngo-sensitive component of the cAMP response did not represent a secondary consequence of resensitization by receptor recycling17. Dyngo-4a did not produce the same effect on cAMP accumulation elicited by the internalization-defective β2-AR-3S mutant receptor21 (Fig. 4e, f), further supporting the conclusion that the second signalling phase indeed requires receptor localization in endosomes.
The present findings revise a long-held tenet of molecular pharmacology, that acute signal transduction mediated by the canonical β2-AR Gs signal transduction mechanism is plasma membrane delimited. The results provide direct evidence supporting the hypothesis that β2-AR endocytosis contributes to a second phase of the acute cellular cAMP response, which represents a significant component of the overall biochemical signal developed within several minutes after the initial agonist application. Thus, while it remains clear that β2-ARs can elicit Gs-mediated signal transduction from the plasma membrane, the present data reveal a discrete component of the acute signalling response that is initiated from endosomes (Fig. 4g). It remains unknown if β2-ARs are continuously bound by agonist in endosomes, as depicted in the figure for simplicity, but conformational activation of Gs in endosomes is both receptor and agonist-dependent. Unambiguous detection of endosome-based activation of acute G-protein-linked signalling is presently limited to the β2-AR Gs system for which the critical nanobodies are available. However, we believe that endosome-based contribution to the acute signalling response is likely widespread in the GPCR superfamily because the β2-AR belongs to the largest group (family A) of GPCR and is often considered a prototype. We also suggest, more generally, that nanobody-based biosensors represent a versatile strategy for probing other types of dynamic conformational change with high spatiotemporal resolution in living cells.
METHODS
Cell Culture, cDNA constructs and transfection
HEK293 cells were grown in Dulbecco’s minimal essential medium supplemented with 10% Fetal Bovine Serum (FBS)(UCSF Cell Culture Facility) without antibiotics. Stably transfected HEK293 cell clones expressing Flag-tagged β2-AR-3S were created using previously described Flag-tagged β2-AR30. A plasmid encoding a cyclic permuted luciferase reporter construct, based on a mutated RIIβB cAMP-binding domain from PKA (pGloSensor-20F, Promega). Nb80–eGFP and Nb37–eGFP were created by amplifying Nb80 and Nb37 nanobody complementary DNAs using 5′-CTTGAAAAGCTTGCCGCCACCATGGGACAGGTGCAGCTGCA-3′; 5′-TTCAAGGGATCCATGTGATGGTGATGGTGGTGTGAGGAGACGGT-3′ and 5′-CTTGAAAAGCTTGCCGCCACCATGGGACAGGTGCAGCTGCA-3′; 5′-TTCAAGGGATCCATGTGATGGGCTTCAGGTTCGTGATGGTGATG-3′ primers, respectively, and cloning into the peGFP-N1 vector using HindIII and BamH1. β-arrestin-2–GFP, Clathrin–DsRed, EEA1–DsRed and Gαs–HA were gifts from M. Caron, W. Almers, K. Mostov and P. Wedegaertner, respectively. β-arrestin-2–mCherry was generated by subcloning β-arrestin-2 to pmCherry (Clontech) and β2-AR–CFP was generated from the Flag-tagged β2-AR construct. Transfections were performed using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. Flag tagged human β2-AR and β2-AR-3S constructs (Ser 355 Gly, Ser 356 Gly, Ser 364 Gly were mutated simultaneously) were labelled with Alexa555 or Alexa488-conjugated M1 anti-FLAG monoclonal antibody (Sigma) as described previously31.
Live-cell confocal imaging
Live cell imaging was carried out using Yokagawa CSU22 spinning disk confocal microscope with a 100 ×, 1.4 numerical aperture, oil objective and a CO2 and 37 °C temperature-controlled incubator. A 488 nm argon laser and a 568 nm argon/krypton laser (Melles Griot) were used as light sources for imaging GFP and Flag signals, respectively. Cells expressing both Flag-tagged receptor and the indicated nanobody–GFP were plated onto glass coverslips. Receptors were surface labelled by addition of M1 anti-Flag antibody (1:1000, Sigma) conjugated to Alexa 555 (A10470, Invitrogen) to the media for 30 min, as described previously32. Indicated agonist (isoprenaline, Sigma) or antagonist (CGP-12177, TOCRIS) were added and cells were imaged every 3 s for 20 min in DMEM without phenol red supplemented with 30 mM HEPES, pH 7.4 (UCSF cell culture facility). Time-lapse images were acquired with a Cascade II EM-charge-coupled device (CCD) camera (Photometrics) driven by Micro-Manager 1.4 (http://www.micro-manager.org).
Live cell total internal reflection fluorescence (TIRF) microscopy
TIRF imaging was carried out as described previously33. Briefly, HEK293 cells co-expressing either Nb80–eGFP and β-arrestin-2–mCherry or Clathrin light chain–DsRed, were imaged in DMEM without phenol red supplemented with 30 mM HEPES, pH 7.4 (UCSF cell culture facility). Imaging was carried out using a Nikon TE-2000E inverted microscope with a 100 ×, 1.49 numerical aperture TIRF objective, equipped for through-the-objective TIRF illumination, a 37 °C temperature-controlled stage (Bioscience Tools) and an objective warmer (Bioptechs). A 488 nm argon laser (Melles Griot) and a 543 nm helium-neon laser (Spectra Physics) were used as light sources. Time-lapse sequences were acquired with a C9100-12 camera (Hamamatsu Photonics) driven by iQ software (Andor). Cells were imaged every 3 s for 20 min.
Image analysis and statistical analysis
Images were saved as 16 bit TIFF files. Quantitative image analysis was carried out on unprocessed images using ImageJ software (http://rsb.info.nih.gov/ij). Co-localization analysis was estimated by calculating the Pearson’s correlation coefficient between the indicated image channels using the co-localization plug-in for ImageJ. Analysis of Nb80–GFP intensity profile along the straight line and Nb80–GFP/β-arrestin or Nb80–GFP/clathrin along the segmented line were carried out using the ImageJ plot profile function. For estimating changes in Nb80–GFP surface fluorescence over time in TIRF images, individual cells were selected manually and fluorescence values measured over the entire stack. A blank area of the image lacking cells was used to estimate background fluorescence. Average fluorescence intensity was measured in each frame, background-subtracted and normalized to the maximum value. P values are from one-tailed unpaired Student’s t-tests. For visual presentation (but not quantitative analysis) image series were processed using Kalman stack filter in ImageJ.
Luminescence-based rapid cAMP assay
HEK293 cells were transfected with a plasmid encoding a cyclic-permuted luciferase reporter construct, based on a mutated RIIβB cAMP-binding domain from PKA (pGloSensor-20F, Promega), which produces rapid and reversible cAMP-dependent activation of luciferase activity in intact cells. Cells were plated in 24-well dishes containing approximately 200,000 cells per well in 500 µl DMEM without phenol red and no serum and equilibrated to 37 °C in a light-proof cabinet. An image of the plate was focused on a 512 × 512 pixel electron multiplying CCD sensor (Hamamatsu C9100-13), cells were equilibrated for 1 h in the presence of 250 µg ml−1 luciferin (Biogold), and sequential luminescence images were collected every 10 s to obtain basal luminescence values. The camera shutter was closed, the cabinet opened and the indicated concentration of isoprenaline was bath applied, with gentle manual rocking before replacing in the dark cabinet and resuming luminescence image acquisition. In endocytic manipulation experiments, cells were pre-incubated with 30 µM Dyngo-4a (abcam Biochemicals) for 15 min. Every 10 s, sequential images were acquired using Micro-Manager (http://www.micro-manager.org) and integrated luminescence intensity detected from each well was calculated after background subtraction and correction for vignetting using scripts written in MATLAB (MathWorks). In each multi-well plate, and for each experimental condition, a reference value of luminescence was measured in the presence of 5 µM forskolin, a manipulation that stimulates a moderate amount of receptor-independent activation of adenylyl cyclase. The average luminescence value—measured across duplicate wells—was normalized to the maximum luminescence value measured in the presence of 5 µM forskolin.
FRET imaging
FRET imaging was carried out as described previously10. Briefly, HEK293 cells co-expressing β2-AR–CFP or DRD1–CFP and Nb80–YFP were imaged in wide field at 37 °C using a shuttered mercury arc lamp and standard CFP excitation (ET430/24 ×) and YFP emission (ET500/20 ×) band pass filters (Chroma). YFP emission was collected using a 535/30 m filter, and CFP emission was collected through a 470/24 m filter. Corrected FRET ratios were obtained using the following equation: NFRET = [(IFRET − BGFRET) − (ICFP − BGCFP) X BTDONOR − (IYFP−BGYFP) X DEACCEPTOR]/ICFP. BTDONOR, donor bleed through; DEACCEPTOR, direct excitation of the acceptor; BGX, background fluorescence; and IX, integrated fluorescence intensity measured in a given channel.
Flow cytometric assay of receptor endocytosis
Surface fluorescence of FLAG– β2-AR or FLAG– β2-AR-3S expressing HEK293 cells was used to measure receptor endocytosis. Cells were incubated with 10 µM isoprenaline for 20 min at 37 °C to drive receptor internalization to steady state and were subsequently rinsed 3 times with ice-cold PBS, then mechanically lifted and incubated with 1µg ml−1 Alexa647 (Invitrogen)-conjugated M1 anti-FLAG monoclonal antibody (Sigma-Aldrich) at 4 °C for 1 h. Mean fluorescence intensity of 10,000 cells was measured using FACSCalibur instrument (Becton Dickinson). Each condition was performed in triplicate.
Enhanced GFP (eGFP ) calibration
Recombinant eGFP (BioVision) was used for calibrating average fluorescence intensity of the biosensors, imaged in confocal optical sections through the cytoplasm of cells not exposed to agonist (to achieve diffuse cytoplasmic distribution of the biosensors). eGFP was diluted in Hank's balanced salt solution and confocal sections were imaged through droplets of each using the same illumination and acquisition parameters as for imaging the biosensors in cells. For each cell, a background fluorescence value was determined by average fluorescence intensity of a blank region in the same image. The cytoplasmic concentration of biosensors was estimated by interpolation of the background-subtracted value using a linear least-squares fit to the standard plot.
Generation of β2-AR-rHDL nanoparticles
Apolipoprotein-AI (Apo-AI) was biotinylated using NHS-PEG4-biotin (Pierce Biotechnology) at a 1:1 molar ratio. Following a 30-min biotinylation reaction at room temperature, the sample was dialysed to remove free biotin. FLAG-tagged β2-AR was incorporated into recombinant high density lipoprotein (rHDL) particles as previously described34,35 using biotinylated Apo-AI. Receptor-containing particles were then purified by M1 anti-FLAG immunoaffinity chromatography36. Particles containing purified monobromobimane-labelled β2-AR were generated similarly except not using biotinylated HDL, with receptor labelling and fluorescence analysis carried out as described previously18.
Assessing Nb80 binding to immobilized β2AR-rHDL
Nb80 binding to unliganded and agonist-occupied β2-AR was measured using the OctetRED biolayer interferometry system (Pall FortéBio). In this assay, a target protein is immobilized on the functionalized tip of a fibre optic probe that is dipped into an analyte solution to observe analyte association to the target protein. A dissociation step is then performed by transferring the biosensor into buffer lacking analyte. Analyte association/dissociation is measured by monitoring changes in the interference pattern of a light beam reflected from the biosensor tip as the total mass bound at the tip surface changes37. Streptavidin-coated biosensors (Pall FortéBio) were loaded with biotinylated β2-AR-rHDL particles for 15 min at room temperature and the biosensors were transferred to the OctetRED instrument. Sensors were placed into assay buffer (20 mM HEPES, pH 7.7, 100 mM NaCl, 1 mM EDTA, 0.02% w/v ascorbic acid, 0.05% w/v BSA) with or without 100 µM isoprenaline for 30 min. To measure Nb80 association, the sensor was transferred to assay buffer with Nb80 (at indicated concentrations) for 5 min, followed by a 30 min dissociation step in assay buffer. Isoprenaline (100 µM) was included in the association and dissociation steps when measuring Nb80 binding to agonist-occupied receptor. All experiments were carried out at 25 °C with the assay plate shaking at 1,000 r.p.m. Buffer-only controls were included in each experiment to monitor for baseline drift, and nonspecific Nb80 binding was measured in a parallel assay using sensors loaded with empty rHDL particles. Raw data were processed to remove baseline and nonspecific binding using Octet Data Analysis 7.0 software (Pall FortéBio) and exported to Prism 5 (GraphPad) for curve fitting. All association and dissociation curves were fit using a single-phase exponential association or decay curves, respectively. Equilibrium binding affinity of Nb80 for β2-AR in the presence or absence of the agonist isoprenaline was assessed by monitoring the maximal interference shift generated by Nb80 binding (at varying Nb80 concentrations) to the probe containing β2-AR reconstituted in rHDL. The maximal shift was plotted against the Nb80 concentration and fitted by nonlinear regression in Prism 5 (GraphPad) to generate the apparent affinity.
Inhibition of bodipy-GTPγS-FL binding by Nb37
The effect of Nb37 on GTP loading of purified G proteins was measured using 100 nM bodipy-GTPγs-FL (Invitrogen) essentially as described25. In this assay we used the fluorescence emission of bodipy-GTPγs-FL (λex~470 nm, λem~515nm) that accompanies binding of the labelled nucleotide to G protein38. Briefly, the fluorescence of 100 nM bodipy-GTPγs-FL was measured in the presence of 1 mM of the indicated G protein using a 96-well microtitre plate format on a M5 fluorescence plate reader (Molecular Devices). Nb37 was added together with bodipy-GTPγs-FL and the binding reaction was initiated by the addition of G protein (1 mM) in 20 mM Tris-HCl, pH 8.0, 3 mM MgCl2, 1 mM dithiothreitol in a final volume of 200 ml. Bodipy-GTPγs-FL binding to heterotrimeric G protein included 0.1% dodecylmaltoside (final). Gαs was purified as described39. Gαsβ γ was purified as described13. Myristoylated Gαi was purified as described40. The time scans were limited to 240 s to minimize the accumulation of hydrolysis of the product of bodipy-GTPγs- FL, bodipy-phosphate41.
Supplementary Material
Acknowledgements
We thank B. Kobilka, P. Robinson, A. Kruse, E. Pardon, P. Temkin, M. Puthenveedu, A. Henry, A. Marley and K. Thorn for assistance, advice and valuable discussion. These studies were supported by the National Institute on Drug Abuse of the U.S. National Institutes of Health (DA010711 and DA012864 to M.v.Z. and F32 DA029993 to J.C.T.). R.I. is supported by the American Heart Association. R.K.S. and J.P.M are supported by the National Institute of General Medical Sciences (GM083118 to R.K.S. and T32 GM007767 to J.P.M). S.G.F.R. is supported by the Lundbeck Foundation. J.S. is supported by FWO-Vlaanderen grants (FWO551 and FWO646) and Innoviris-Brussels (BRGEOZ132). B.H. is supported by a Packard Fellowship for Science and Engineering.
Footnotes
Supplementary Information is available in the online version of the paper.
Author Contributions R.I. constructed and validated the nanobody biosensors, carried out most of the cell biological experiments and analysis, contributed to overall experimental strategy and took a lead role in writing the manuscript. J.C.T. carried out early experiments identifying endocytic inhibitor effects on cellular cAMP signaling, and contributed to initial project planning. J.R.T. built the luminometer system, developed software for analysis of luminometry data, and contributed to early experiments on cellular cAMP signaling. M.C. contributed to experimental design and data analysis, and modelled effects of endocytic inhibitors on the cellular cAMP response. J.P.M. contributed to the production of receptor-containing rHDL particles and carried out in vitro studies of Nb80 binding and dissociation. J.S. developed the nanobody reagents used as the basis for the biosensors described in this study and advised on biosensor design and expression. S.G.F.R. contributed to developing and screening the initial nanobody reagents, and carried out in vitro studies of Nb80 binding and dissociation in rHDL particles reconstituted with bimane-labeled receptors. R.K.S. contributed to overall experimental interpretation, supervised J.P.M. in carrying out in vitro studies of Nb80 binding to receptors, and performed in vitro experiments evaluating Nb37 effects on G protein activation. H.E.-S. contributed to experimental design and data interpretation, and supervised efforts to model endocytic effects on the cellular cAMP response. B.H. contributed to overall experimental design and interpretation, implementation of biosensors and advised on image analysis. M.v.Z. was responsible for overall project strategy, carried out some of the imaging experiments, and drafted the manuscript together with R.I.
The authors declare no competing financial interests.
References
- 1.Lohse MJ, Nuber S, Hoffmann C. Fluorescence/bioluminescence resonance energy transfer techniques to study G-protein-coupled receptor activation and signaling. Pharmacol. Rev. 2012;64:299–336. doi: 10.1124/pr.110.004309. [DOI] [PubMed] [Google Scholar]
- 2.Shenoy SK, Lefkowitz RJ. Seven-transmembrane receptor signaling through β-arrestin. Sci. STKE 2005. 2005:cm10. doi: 10.1126/stke.2005/308/cm10. [DOI] [PubMed] [Google Scholar]
- 3.Murphy JE, Padilla BE, Hasdemir B, Cottrell GS, Bunnett NW. Endosomes: a legitimate platform for the signaling train. Proc. Natl Acad. Sci. USA. 2009;106:17615–17622. doi: 10.1073/pnas.0906541106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferrandon S, et al. Sustained cyclic AMP production by parathyroid hormone receptor endocytosis. Nature Chem. Biol. 2009;5:734–742. doi: 10.1038/nchembio.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feinstein TN, et al. Retromer terminates the generation of cAMP by internalized PTH receptors. Nature Chem. Biol. 2011;7:278–284. doi: 10.1038/nchembio.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calebiro D, et al. Persistent cAMP-signals triggered by internalized G-protein-coupled receptors. PLoS Biol. 2009;7:e1000172. doi: 10.1371/journal.pbio.1000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Werthmann RC, Volpe S, Lohse MJ, Calebiro D. Persistent cAMP signaling by internalized TSH receptors occurs in thyroid but not in HEK293 cells. FASEB J. 2012;26:2043–2048. doi: 10.1096/fj.11-195248. [DOI] [PubMed] [Google Scholar]
- 8.Mullershausen F, et al. Persistent signaling induced by FTY720-phosphate is mediated by internalized S1P1 receptors. Nature Chem. Biol. 2009;5:428–434. doi: 10.1038/nchembio.173. [DOI] [PubMed] [Google Scholar]
- 9.Calebiro D, Nikolaev VO, Lohse MJ. Imaging of persistent cAMP signaling by internalized G protein-coupled receptors. J. Mol. Endocrinol. 2010;45:1–8. doi: 10.1677/JME-10-0014. [DOI] [PubMed] [Google Scholar]
- 10.Kotowski SJ, Hopf FW, Seif T, Bonci A, von Zastrow M. Endocytosis promotes rapid dopaminergic signaling. Neuron. 2011;71:278–290. doi: 10.1016/j.neuron.2011.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lefkowitz RJ. Seven transmembrane receptors: something old, something new. Acta Physiol. (Oxf.) 2007;190:9–19. doi: 10.1111/j.1365-201X.2007.01693.x. [DOI] [PubMed] [Google Scholar]
- 12.Gilman AG. Transmembrane signaling, G proteins, and adenylyl cyclase. Harvey Lect. 1989;85:153–172. [PubMed] [Google Scholar]
- 13.Rasmussen SG, et al. Crystal structure of the β2 adrenergic receptor-Gs protein complex. Nature. 2011;477:549–555. doi: 10.1038/nature10361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moore CA, Milano SK, Benovic JL. Regulation of receptor trafficking by GRKs and arrestins. Annu. Rev. Physiol. 2007;69:451–482. doi: 10.1146/annurev.physiol.69.022405.154712. [DOI] [PubMed] [Google Scholar]
- 15.Sorkin A, von Zastrow M. Endocytosis and signalling: intertwining molecular networks. Nature Rev. Mol. Cell Biol. 2009;10:609–622. doi: 10.1038/nrm2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luttrell LM, Lefkowitz RJ. The role of β-arrestins in the termination and transduction of G-protein-coupled receptor signals. J. Cell Sci. 2002;115:455–465. doi: 10.1242/jcs.115.3.455. [DOI] [PubMed] [Google Scholar]
- 17.Gainetdinov RR, Premont RT, Bohn LM, Lefkowitz RJ, Caron MG. Desensitization of G protein-coupled receptors and neuronal functions. Annu. Rev. Neurosci. 2004;27:107–144. doi: 10.1146/annurev.neuro.27.070203.144206. [DOI] [PubMed] [Google Scholar]
- 18.Rasmussen SG, et al. Structure of a nanobody-stabilized active state of the β2 adrenoceptor. Nature. 2011;469:175–180. doi: 10.1038/nature09648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steyaert J, Kobilka BK. Nanobody stabilization of G protein-coupled receptor conformational states. Curr. Opin. Struct. Biol. 2011;21:567–572. doi: 10.1016/j.sbi.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lauffer BE, et al. SNX27 mediates PDZ-directed sorting from endosomes to the plasma membrane. J. Cell Biol. 2010;190:565–574. doi: 10.1083/jcb.201004060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hausdorff WP, et al. A small region of the β-adrenergic receptor is selectively involved in its rapid regulation. Proc. Natl Acad. Sci. USA. 1991;88:2979–2983. doi: 10.1073/pnas.88.8.2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steyer JA, Almers W. A real-time view of life within 100 nm of the plasma membrane. Nature Rev. Mol. Cell Biol. 2001;2:268–275. doi: 10.1038/35067069. [DOI] [PubMed] [Google Scholar]
- 23.Puthenveedu MA, von Zastrow M. Cargo regulates clathrin-coated pit dynamics. Cell. 2006;127:113–124. doi: 10.1016/j.cell.2006.08.035. [DOI] [PubMed] [Google Scholar]
- 24.Slessareva JE, Routt SM, Temple B, Bankaitis VA, Dohlman HG. Activation of the phosphatidylinositol 3-kinase Vps34 by a G protein α subunit at the endosome. Cell. 2006;126:191–203. doi: 10.1016/j.cell.2006.04.045. [DOI] [PubMed] [Google Scholar]
- 25.Westfield GH, et al. Structural flexibility of the Gαs α-helical domain in the β2-adrenoceptor Gs complex. Proc. Natl Acad. Sci. USA. 2011;108:16086–16091. doi: 10.1073/pnas.1113645108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Campbell PT, et al. Mutations of the human β2-adrenergic receptor that impair coupling to Gs interfere with receptor down-regulation but not sequestration. Mol. Pharmacol. 1991;39:192–198. [PubMed] [Google Scholar]
- 27.Violin JD, et al. β2-adrenergic receptor signaling and desensitization elucidated by quantitative modeling of real time cAMP dynamics. J. Biol. Chem. 2008;283:2949–2961. doi: 10.1074/jbc.M707009200. [DOI] [PubMed] [Google Scholar]
- 28.Harper CB, et al. Dynamin inhibition blocks botulinum neurotoxin type A endocytosis in neurons and delays botulism. J. Biol. Chem. 2011;286:35966–35976. doi: 10.1074/jbc.M111.283879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Howes MT, et al. Clathrin-independent carriers form a high capacity endocytic sorting system at the leading edge of migrating cells. J. Cell Biol. 2010;190:675–691. doi: 10.1083/jcb.201002119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cao TT, Deacon HW, Reczek D, Bretscher A, von Zastrow M. A kinase-regulated PDZ-domain interaction controls endocytic sorting of the β2-adrenergic receptor. Nature. 1999;401:286–290. doi: 10.1038/45816. [DOI] [PubMed] [Google Scholar]
- 31.Gage RM, Matveeva EA, Whiteheart SW, von Zastrow M. Type I PDZ ligands are sufficient to promote rapid recycling of G protein-coupled receptors independent of binding to N-ethylmaleimide-sensitive factor. J. Biol. Chem. 2005;280:3305–3313. doi: 10.1074/jbc.M406934200. [DOI] [PubMed] [Google Scholar]
- 32.Puthenveedu MA, et al. Sequence-dependent sorting of recycling proteins by actin-stabilized endosomal microdomains. Cell. 2010;143:761–773. doi: 10.1016/j.cell.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yudowski GA, Puthenveedu MA, Henry AG, von Zastrow M. Cargo-mediated regulation of a rapid Rab4-dependent recycling pathway. Mol. Biol. Cell. 2009;20:2774–2784. doi: 10.1091/mbc.E08-08-0892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Whorton MR, et al. A monomeric G protein-coupled receptor isolated in a high-density lipoprotein particle efficiently activates its G protein. Proc. Natl Acad. Sci. USA. 2007;104:7682–7687. doi: 10.1073/pnas.0611448104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuszak AJ, et al. Purification and functional reconstitution of monomeric µ-opioid receptors: allosteric modulation of agonist binding by Gi2. J. Biol. Chem. 2009;284:26732–26741. doi: 10.1074/jbc.M109.026922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yao X, et al. Coupling ligand structure to specific conformational switches in the beta2-adrenoceptor. Nature Chem. Biol. 2006;2:417–422. doi: 10.1038/nchembio801. [DOI] [PubMed] [Google Scholar]
- 37.Abdiche Y, Malashock D, Pinkerton A, Pons J. Determining kinetics and affinities of protein interactions using a parallel real-time label-free biosensor, the Octet. Anal. Biochem. 2008;377:209–217. doi: 10.1016/j.ab.2008.03.035. [DOI] [PubMed] [Google Scholar]
- 38.McEwen DP, Gee KR, Kang HC, Neubig RR. Fluorescent BODIPY-GTP analogs: real-time measurement of nucleotide binding to G proteins. Anal. Biochem. 2001;291:109–117. doi: 10.1006/abio.2001.5011. [DOI] [PubMed] [Google Scholar]
- 39.Sunahara RK, Tesmer JJ, Gilman AG, Sprang SR. Crystal structure of the adenylyl cyclase activator Gsα. Science. 1997;278:1943–1947. doi: 10.1126/science.278.5345.1943. [DOI] [PubMed] [Google Scholar]
- 40.Lee E, Linder ME, Gilman AG. Expression of G-protein α subunits in Escherichia coli. Methods Enzymol. 1994;237:146–164. doi: 10.1016/s0076-6879(94)37059-1. [DOI] [PubMed] [Google Scholar]
- 41.Jameson EE, et al. Real-time detection of basal and stimulated G protein GTPase activity using fluorescent GTP analogues. J. Biol. Chem. 2005;280:7712–7719. doi: 10.1074/jbc.M413810200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.