Abstract
Movement of material between intracellular compartments takes place through the production of transport vesicles derived from donor membranes. Vesicle budding that results from the interaction of cytoplasmic coat proteins (coatomer and clathrin) with intracellular organelles requires a type of GTP-binding protein termed ADP-ribosylation factor (ARF). The GTPase cycle of ARF proteins that allows the uncoating and fusion of a transport vesicle with a target membrane is mediated by ARF-dependent GTPase-activating proteins (GAPs). A previously identified yeast protein, Gcs1, exhibits structural similarity to a mammalian protein with ARF-GAP activity in vitro. We show herein that the Gcs1 protein also has ARF-GAP activity in vitro using two yeast Arf proteins as substrates. Furthermore, Gcs1 function is needed for the efficient secretion of invertase, as expected for a component of vesicle transport. The in vivo role of Gcs1 as an ARF GAP is substantiated by genetic interactions between mutations in the ARF1/ARF2 redundant pair of yeast ARF genes and a gcs1-null mutation; cells lacking both Gcs1 and Arf1 proteins are markedly impaired for growth compared with cells missing either protein. Moreover, cells with decreased levels of Arf1 or Arf2 protein, and thus with decreased levels of GTP-Arf, are markedly inhibited for growth by increased GCS1 gene dosage, presumably because increased levels of Gcs1 GAP activity further decrease GTP-Arf levels. Thus by both in vitro and in vivo criteria, Gcs1 is a yeast ARF GAP.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Archambault J., Drebot M. A., Stone J. C., Friesen J. D. Isolation and phenotypic analysis of conditional-lethal, linker-insertion mutations in the gene encoding the largest subunit of RNA polymerase II in Saccharomyces cerevisiae. Mol Gen Genet. 1992 Apr;232(3):408–414. doi: 10.1007/BF00266244. [DOI] [PubMed] [Google Scholar]
- Bednarek S. Y., Ravazzola M., Hosobuchi M., Amherdt M., Perrelet A., Schekman R., Orci L. COPI- and COPII-coated vesicles bud directly from the endoplasmic reticulum in yeast. Cell. 1995 Dec 29;83(7):1183–1196. doi: 10.1016/0092-8674(95)90144-2. [DOI] [PubMed] [Google Scholar]
- Boman A. L., Kahn R. A. Arf proteins: the membrane traffic police? Trends Biochem Sci. 1995 Apr;20(4):147–150. doi: 10.1016/s0968-0004(00)88991-4. [DOI] [PubMed] [Google Scholar]
- Carlson M., Botstein D. Two differentially regulated mRNAs with different 5' ends encode secreted with intracellular forms of yeast invertase. Cell. 1982 Jan;28(1):145–154. doi: 10.1016/0092-8674(82)90384-1. [DOI] [PubMed] [Google Scholar]
- Cukierman E., Huber I., Rotman M., Cassel D. The ARF1 GTPase-activating protein: zinc finger motif and Golgi complex localization. Science. 1995 Dec 22;270(5244):1999–2002. doi: 10.1126/science.270.5244.1999. [DOI] [PubMed] [Google Scholar]
- Donaldson J. G., Cassel D., Kahn R. A., Klausner R. D. ADP-ribosylation factor, a small GTP-binding protein, is required for binding of the coatomer protein beta-COP to Golgi membranes. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6408–6412. doi: 10.1073/pnas.89.14.6408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drebot M. A., Johnston G. C., Singer R. A. A yeast mutant conditionally defective only for reentry into the mitotic cell cycle from stationary phase. Proc Natl Acad Sci U S A. 1987 Nov;84(22):7948–7952. doi: 10.1073/pnas.84.22.7948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finazzi D., Cassel D., Donaldson J. G., Klausner R. D. Aluminum fluoride acts on the reversibility of ARF1-dependent coat protein binding to Golgi membranes. J Biol Chem. 1994 May 6;269(18):13325–13330. [PubMed] [Google Scholar]
- Franco M., Chardin P., Chabre M., Paris S. Myristoylation of ADP-ribosylation factor 1 facilitates nucleotide exchange at physiological Mg2+ levels. J Biol Chem. 1995 Jan 20;270(3):1337–1341. doi: 10.1074/jbc.270.3.1337. [DOI] [PubMed] [Google Scholar]
- Hartwell L. H. Macromolecule synthesis in temperature-sensitive mutants of yeast. J Bacteriol. 1967 May;93(5):1662–1670. doi: 10.1128/jb.93.5.1662-1670.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ireland L. S., Johnston G. C., Drebot M. A., Dhillon N., DeMaggio A. J., Hoekstra M. F., Singer R. A. A member of a novel family of yeast 'zn-finger' proteins mediates the transition from stationary phase to cell proliferation. EMBO J. 1994 Aug 15;13(16):3812–3821. doi: 10.1002/j.1460-2075.1994.tb06692.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn R. A., Gilman A. G. The protein cofactor necessary for ADP-ribosylation of Gs by cholera toxin is itself a GTP binding protein. J Biol Chem. 1986 Jun 15;261(17):7906–7911. [PubMed] [Google Scholar]
- Kahn R. A., Kern F. G., Clark J., Gelmann E. P., Rulka C. Human ADP-ribosylation factors. A functionally conserved family of GTP-binding proteins. J Biol Chem. 1991 Feb 5;266(4):2606–2614. [PubMed] [Google Scholar]
- Makler V., Cukierman E., Rotman M., Admon A., Cassel D. ADP-ribosylation factor-directed GTPase-activating protein. Purification and partial characterization. J Biol Chem. 1995 Mar 10;270(10):5232–5237. doi: 10.1074/jbc.270.10.5232. [DOI] [PubMed] [Google Scholar]
- Nair J., Müller H., Peterson M., Novick P. Sec2 protein contains a coiled-coil domain essential for vesicular transport and a dispensable carboxy terminal domain. J Cell Biol. 1990 Jun;110(6):1897–1909. doi: 10.1083/jcb.110.6.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orcl L., Palmer D. J., Amherdt M., Rothman J. E. Coated vesicle assembly in the Golgi requires only coatomer and ARF proteins from the cytosol. Nature. 1993 Aug 19;364(6439):732–734. doi: 10.1038/364732a0. [DOI] [PubMed] [Google Scholar]
- Schekman R., Orci L. Coat proteins and vesicle budding. Science. 1996 Mar 15;271(5255):1526–1533. doi: 10.1126/science.271.5255.1526. [DOI] [PubMed] [Google Scholar]
- Sikorski R. S., Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989 May;122(1):19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
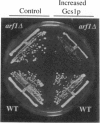
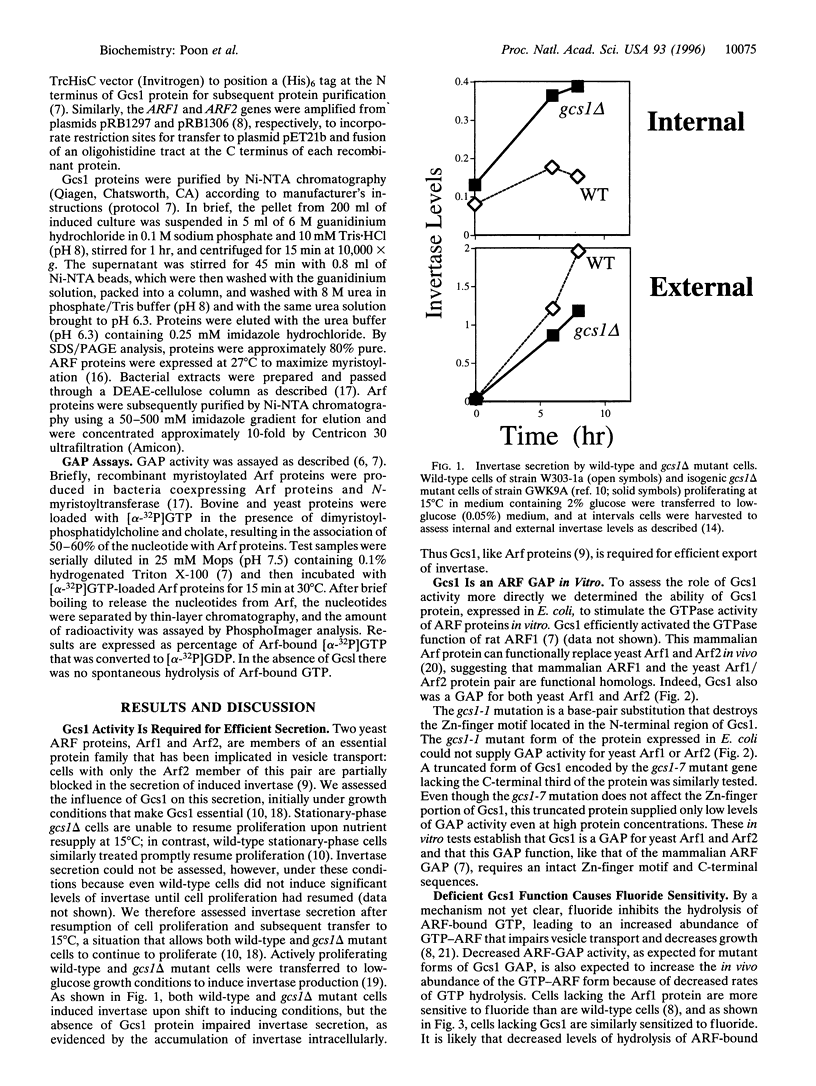
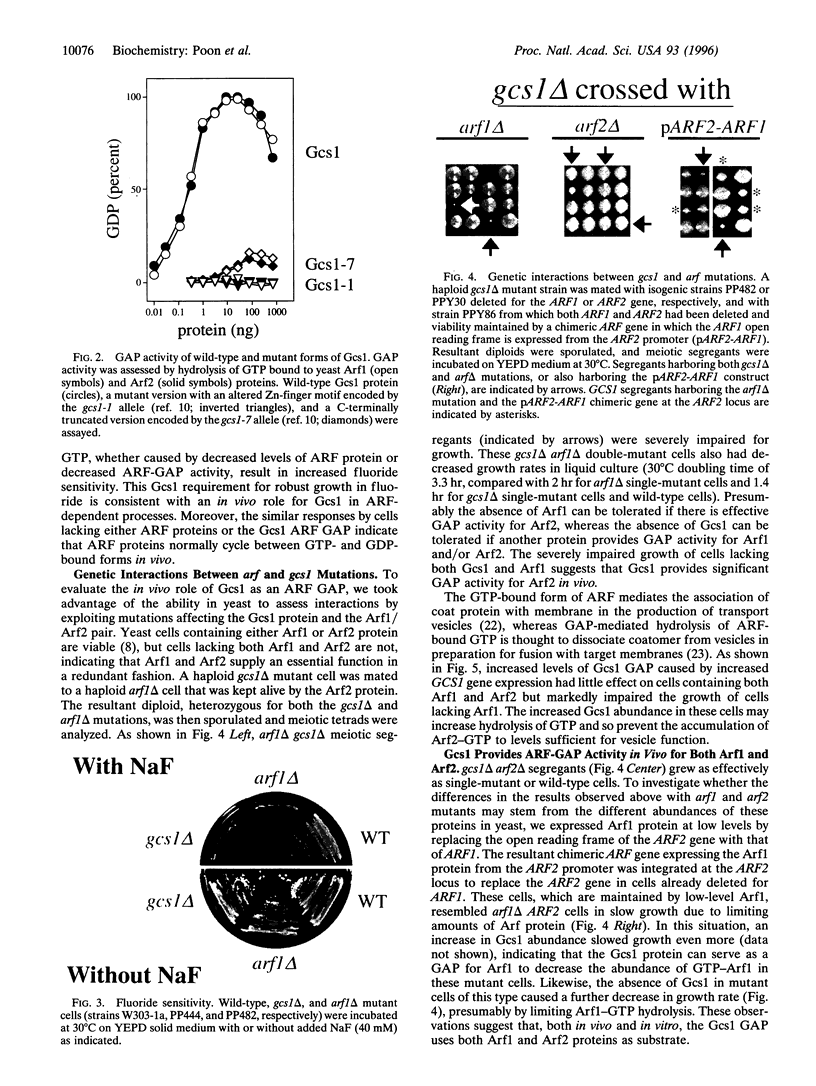
- Stearns T., Kahn R. A., Botstein D., Hoyt M. A. ADP ribosylation factor is an essential protein in Saccharomyces cerevisiae and is encoded by two genes. Mol Cell Biol. 1990 Dec;10(12):6690–6699. doi: 10.1128/mcb.10.12.6690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stearns T., Willingham M. C., Botstein D., Kahn R. A. ADP-ribosylation factor is functionally and physically associated with the Golgi complex. Proc Natl Acad Sci U S A. 1990 Feb;87(3):1238–1242. doi: 10.1073/pnas.87.3.1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanigawa G., Orci L., Amherdt M., Ravazzola M., Helms J. B., Rothman J. E. Hydrolysis of bound GTP by ARF protein triggers uncoating of Golgi-derived COP-coated vesicles. J Cell Biol. 1993 Dec;123(6 Pt 1):1365–1371. doi: 10.1083/jcb.123.6.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss O., Holden J., Rulka C., Kahn R. A. Nucleotide binding and cofactor activities of purified bovine brain and bacterially expressed ADP-ribosylation factor. J Biol Chem. 1989 Dec 15;264(35):21066–21072. [PubMed] [Google Scholar]
- Werner-Washburne M., Braun E., Johnston G. C., Singer R. A. Stationary phase in the yeast Saccharomyces cerevisiae. Microbiol Rev. 1993 Jun;57(2):383–401. doi: 10.1128/mr.57.2.383-401.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney J. A., Gomez M., Sheff D., Kreis T. E., Mellman I. Cytoplasmic coat proteins involved in endosome function. Cell. 1995 Dec 1;83(5):703–713. doi: 10.1016/0092-8674(95)90183-3. [DOI] [PubMed] [Google Scholar]