Abstract
Introduction
Maternal obesity (MO) remains a serious obstetric problem with acute and chronic morbidities for both mothers and offspring. The mechanisms underlying these adverse consequences of MOremain unknown. Endocannabinoids (ECB) are neuromodulatory lipids derived from adipocytes. Metabolic crosstalk between placenta and adipocytes may mediate sequelae of MO. The goal of this study was to elucidate placental and systemic ECBs in MO.
Material and methods
Placentas, serum, and subcutaneous fat were collected at Cesarean sections performed near term (0.9 G) in four non-obese (nOB) and four obese (OB) baboons (Papio spp.). Concentrations of anandamide (AEA) and 2-arachidonoylglycerol (2-AG) were measured by liquid chromatography coupled to tandem mass spectrometry. AEA and 2-AG pathways were characterized in placentas by Q-RT-PCR, Western blot, and immunohistochemistry.
Results
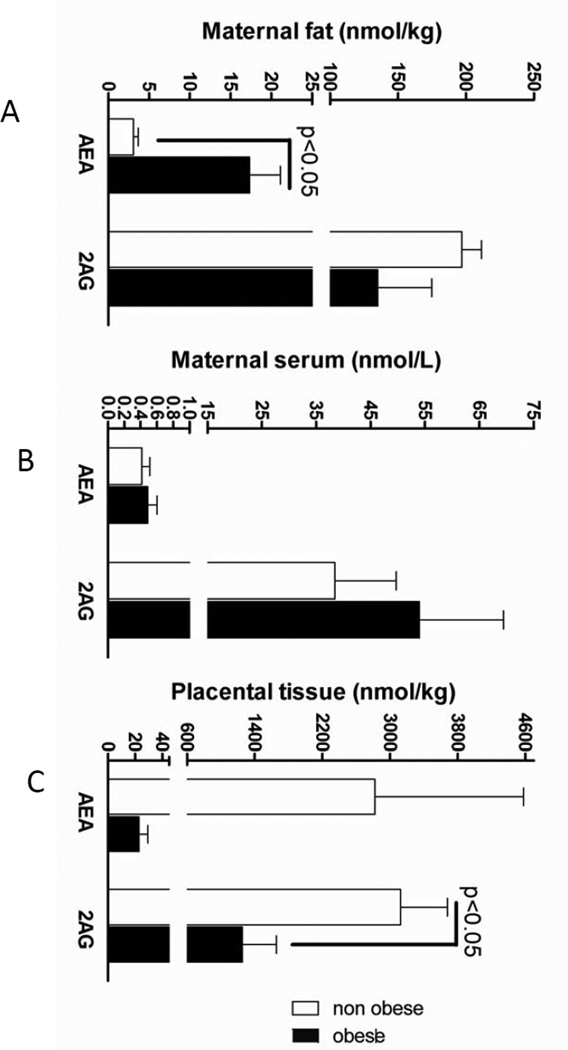
Placental 2-AG levels were lower and maternal fat AEA levels were higher in OB (1254.1 ± 401.3 nmol/kg and 17.3 ± 4 nmol/kg ) vs. nOB (3124.2 ± 557.3 nmol/kg and 3.1 ± 0.6 nmol/kg) animals. Concentrations of 2-AG correlated positively between maternal fat and placenta (r=0.82, p=0.013), but correlated negatively with maternal leptin concentrations (r=−0.72, p=0.04 and r=−0.83, p=0.01, respectively).
Conclusion
This is the first study to demonstrate differential ECB pathway regulation in maternal fat and placenta in MO. Differential regulation and function exist for AEA and 2-AG as the major ECB pathways in placenta.
INTRODUCTION
Maternal obesity (MO) remains at epidemic dimensions in developed countries [1, 2]] and exerts adverse consequences across the lifespan upon the mother, her fetus, the newborn, and later adult life [3]. Mechanisms linking MO and negative pregnancy outcomes remain unknown. Activation of the endocannabinoid system (ECBs) has been reported as a consequence of obesity [4, 5] and been associated with higher endocannabinoid (ECB) levels in visceral fat and serum from obese subjects [6, 7].
The ECB system includes N-arachidonoylethanolamide (anandamide, AEA), 2-arachidonoylglycerol (2-AG), cannabinoid receptors 1 and 2 (CB1 and CB2), the recently added orphan receptor, GPR55, and specified enzymes for their synthesis and degradation [8, 9]. AEA biosynthesis occurs via cleavage of N-arachidonoylphosphatidylethanolamine (NAPE) by the ECB-specific enzyme NAPE-phospholipase D (NAPE-PLD). Combined actions of phospholipase C and diacylglycerol lipase (DAGL) produce 2-AG. [10]. Degradation of AEA is mediated by fatty acid amide hydrolase (FAAH), which hydrolyzes the amide bond to yield arachidonic acid (AA) and ethanolamine. The enzyme monoacylglycerol lipase (MAGL) is the main catabolic enzyme producing AA and glycerol, however FAAH, α-β-hydrolase 6 (ABHD6) and 12 (ABHD12) can also catabolize 2-AG [9–12].
CB1 receptors are abundant in the central nervous system but also detected in peripheral organs, including adrenal gland, heart, uterus, gonads, and liver [13, 14]. CB2 receptors have been identified mainly in the central nervous system on microglia besides peripheral occurrence [15, 16]. ECBs and ECB signaling are emerging as key regulators of reproduction, as evidenced by tight regulation of uterine ECB signaling and their critical roles in preimplantation embryo development, oviductal embryo transport, implantation, and placentation[17, 18]. Moreover, the human fetus may depend upon placental and maternal sources of ECBs [19]. We previously described that placental, fetal, and maternal responses to MO in baboons (Papio spp.) strikingly resemble the changes described in human pregnancies [20, 21]. The present work aimed to characterize ECB responses to MO in a baboon model, based on hypothesis that local (placental and maternal fat) and circulating ECB tone would increase in obese mothers.
MATERIAL AND METHODS
Animals housing and handling
Animals’ characteristics and procedures were previously described in details[20]. Briefly, animals housed in harem cages at the AALAC-approved facilities of the Southwest National Primate Center at the Texas Biomedical Research Institute were divided into two groups [Obese (Ob) n=4 (two male and two female fetuses), and non-Obese (nOb) n=4 (all fetuses are males)] based on the Rh index. Rh index is an obesity index defined as body weight divided by the square of the crown-rump length in another non-human primate species (Rhesus monkeys). Rh indices were 48.7 ± 1.0 kg/m2 for Ob and 39.1 ± 3.2 kg/m2 for nOb animals [20]. All procedures were approved by the Animal Care and Use Committee of the Texas Biomedical Research Institute.
Tissue collection and processing
Farley et al., 2009 detailed the Cesarean procedures performed at the end of gestation [165 days of gestation (dGA, term = 180 dGA)], maternal and fetal tissue collections, and organ weighing [20]. Maternal subcutaneous fat tissue was collected under the umbilicus during necropsy. Maternal and fetal organ weights are presented on Table 1. Subcutaneous fat and placental tissues were flash-frozen in liquid nitrogen and stored at −80°C until further use. Additionally, a sample from the central part of the placenta was embedded in paraffin, cut at 5microns and processed as described below.
Table 1.
Weight of maternal and fetal organs of obese and non-obese baboons (Papiospp) (Data presented as mean±SE).
| Non-Obese n=4 |
Obese n=4 |
|
|---|---|---|
| Weight of maternal organs | ||
| Heart (g) | 61.7 ± 2.09 | 57.3 ± 7.45 |
| Lungs (g) | 98.48 ± 7.55 | 88.6 ± 3.43 |
| Thymus (g) | 3.26 ± 0.39 | 3.06 ± 0.89 |
| Thyroids (Combined) (g) | 1.49 ± 0.33 | 1.45 ± 0.39 |
| Liver (g) | 292.00 ± 19.61 | 319.55 ± 23.14 |
| Spleen (g) | 19.38 ± 1.76 | 21.17 ± 3.00 |
| Pancreas (g) | 17.42 ± 3.00 | 16.7 ± 1.87 |
| Kidney (combined) (g) * | 72.00± 2.00 | 65.7 ± 2.00A |
| Brain (g) | 134.69 ± 3.09 | 132.34 ± 11.00 |
| Weight of fetal organs * | ||
| Heart (g) | 4.4 ± 0.6 | 4.5 ± 0.5 |
| Lungs (g) | 23.01 ± 2.94 | 22.28 ± 3.63 |
| Thymus (g) | 3.42 ± 0.34 | 3.78 ± 0.44 |
| Thyroids (Combined) (g) | 0.29 ± 0.09 | 0.31 ± 0.04 |
| Liver (g) | 24.93 ± 1.57 | 25.36 ± 4.85 |
| Spleen (g) | 1.61 ± 0.10 | 1.54 ± 0.15 |
| Pancreas (g) | 0.66 ± 0.10 | 0.56 ± 0.22 |
| Kidney (Combined) | 2.58 ± 0.17 | 2.02 ± 0.23 |
| Brain (g) | 80.22 ± 1.54 | 76.1 ± 6.88 |
p<0.05 compared to non-obese group.
Data obtained from [20].
Measurements of circulating and peripheral hormones
Leptin
Circulating leptin was measured by radioimmunoassay in a single assay according to the manufacturer’s instructions (LINCO Research, Inc., St. Charles, MO) as described previously Intra-assay coefficient of variation (%CV) was 3.0% at leptin concentration of 4.9 ng/mL[21].
AEA and 2AG
AEA and 2-AG concentrations were determined by liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS) as previously described [22]. Briefly, 1 mL serum was spiked with the internal standards (IS) d4-AEA and d5-2-AG to a final concentration of 1.2 nM and 1.8 nM, respectively, immediately after thawing on ice. Liquid-liquid extraction was performed with 1 mL toluene. For placental tissue, the IS (final concentration of 12.5 nmol/kg d4-AEA and 3.8 nM/kg d5-2-AG) was added to ≈100 mg deep-frozen tissue. One-step homogenization and liquid-liquid extraction with toluene was performed by means of a cooled Precellys 24 homogenizer (Bertin Technologies, France). Maternal fat tissue (≈100 mg) was spiked with a fixed volume of IS solution resulting in varying concentrations (≈30–40 nmol/kg d4-AEA and 9–14 nmol/kg d5-2-AG). Solid phase extraction of ECBs was performed after homogenization of fat tissue, IS, and 1 mL chloroform with a Precellys 24 homogenizer.
RT-PCR
Total RNA was extracted from 70 mg of homogenized placental tissues using TRIzol reagent (Invitrogen Life Technologies, Carlsbad, CA, USA), cDNA was synthesized with first strand cDNA synthesis kit (Roche Diagnostics GmbH, Mannheim, Germany) from 5 µg of total RNA following the manufacturer’s instructions as previously described. Quantitative PCR was performed in triplicates with custom-synthesized (Thermo Fisher Scientific Inc, Waltham, MA, USA) and commercially available (Roche, Applied sciences Indianapolis, IN, USA) sequence-specific oligonucleotides (Table 2A), using TaqMan Mix (Roche, Applied sciences Indianapolis, IN, USA) or SYBR green mix (Kapa Bio systems Inc. Woburn, MA, USA) (Table 2A) [23]. Data were collected on a LightCycler® 480(Roche, Applied Sciences, Indianapolis, IN, USA). Relative mRNA expression data were calculated using the standard ΔCt method [23].
Table 2.
Primers, probes and conditions (A), antibodies and blocking peptides (B) used.
| A. Q-RT-PCR conditions | |||
|---|---|---|---|
| Transcripts | Left(l) and Right(R)Primers | Probes* | Reactionconditions** |
| CB1 | L 5’-AAAATTCTGTAATGTTCACCTGGTC-3’ R 5’-AAAATTCTGTAATGTTCACCTGGTC-3’ |
n/a | 95°C-20sec 58°C- 30sec 72°C- 20sec |
| CB2 | L 5’-GGAGAGGACAGAAAACAACTG-3’ R 5’-GAGCTTGTCTAGAAGGCTTTGG-3’ |
n/a | 95°C-20sec 58°C- 30sec 72°C- 20sec |
| NAPE PLD | L 5’-AGATGGCTGTAATGGGAA-3’ R 5’-TTCTCCTCCCACCAGTC-3’ |
n/a | 95°C-15sec 58°C- 30sec 72°C- 10sec |
| MAGL | L 5’-AGCTGACTGTGCCCTTCCT-3’ R 5’-GGTAGGCACCTTCATAAATCTTG-3’ |
#1,cat.no.04684974001 | 95°C-15sec 55°C- 30sec 72°C- 10sec |
| DAGL- A | L 5’-AAGCACCAAGCCCAAATG—3’ R 5’-CTCAGGCAGCTCCGACTT—3’ |
#49,cat.no.04688104001 | 95°C-15sec 55°C- 30sec 72°C- 10sec |
| DAGL B | L 5’-GCCTTGCTCAGCAGTTACTTG-3’ R 5’-CCAGGGTTACAAATCGTTCC-3’ |
n/a | 95°C-15sec 55°C- 30sec 72°C- 10sec |
| FAAH | L 5’-CTCTGCTGCCAAGGCTGT-3’ R 5’- TGCAGTTCCCAGAGTTTTCC-3’ |
#73,cat.no.04688961001 | 95°C-15sec 55°C- 30sec 72°C- 10sec |
| β-ACTIN | L 5’-CCAACCGCGAGAAGATGA-3’ R 5’-CCAGAGGCGTACAGGGATAG-3’ |
#64,cat.no.04688635001 | 95°C-15sec 55°C- 30sec 72°C- 10sec |
| B. Antibodies and blockingpeptides (IH) | ||||
|---|---|---|---|---|
| Catalognumber | Company | Host | Dilution | |
| CB1 | SP5262P | AcrisAntibodiesGmbH, Herford, Germany | Rabbit | 1:1000(IH, WB) |
| CB2 | H00001269-MO1 | Abnova, Taipei City, Taiwan | Mouse | 1:500(IH),1:2000 (WB) |
| NAPE PLD | 10305 Blockingpeptide: 10303, dilution 1:5 | CaymanChemical, Ann Arbor, MI, USA | Rabbit | 1:50 1:50 |
| MAGL | 100035 Blockingpeptide 300014, dilution 1:1 | CaymanChemical, Ann Arbor, MI, USA | Rabbit | 1:50 |
| DAGL α | sc-133307, Blockingpeptide sc-133307, dilution 1:5 | Santa Cruz Biotechnology, Inc., Dallas, TX, USA | Rabbit | 1:50 |
| FAAH1 | ab54615 | Abcam, Cambridge, MA USA | Mouse | 1:500 |
| β-ACTIN | A3854 | Sigma, St. Louis, MS, USA | Mouse | 1:20000 (WB) |
Abbreviations: IH- Immunohistochemistry; WB-Western Blot
Immunohistochemistry
Deparafinized (5 µm thick) baboon’s placenta slides were placed in antigen retrieval solution (Citrate Buffer, pH=6) and pressure cooked for 30 minutes (FAAH antibodies), or microwaved twice for 2 min (for CB1 and CB2) or 4 min (other antibodies). The avidin-biotin complex (ABC) method was employed according to the manufacturer’s guidelines (Vectastain ABC kit, Vector Laboratories, Inc. Burlingame, CA). Table 2 B lists primary antibodies and dilutions used. Immunological complexes were visualized by the use of H2O2/DAB (3,3′-diaminobenzidine-tetrahydrochloride) as substrate/chromogen for CB1, FAAH, and NAPE-PLD; MAGL and H2O2/AEC (3-Amino-9-Ethylcarbazole) were used as substrate/chromogen for CB2 and DAGL-α. Slides with FAAH immunostaining were counterstained with hematoxylin. Slides were mounted with Dako Mounting Medium (Dako, Carpinteria, CA).
Histomorphometry
Slides were scanned using an AperioScanScope® instrument at 40X. Images were analyzed using the ImageScope™ v11.1.2.752 by Aperio® available positive pixel count algorithm as described elsewhere [24]. One randomly selected slide from the central part of the placenta was used. Immunostaining levels in the villi (trophoblast, mesenchymal core, fetal capillaries) and the basal plates were obtained separately using histoscore method [25, 26].
Western Blot
To evaluate expression changes observed in CB1 and CB2 receptors, Western blot (WB) was performed with suitable antibodies (Table 1B), as described elsewhere [23]. Protein expression was quantified using arbitrary unites (optical density of the bands relative to the beta-actin expression) with Image J (NIH) program[23].
Statistical methods
Comparisons between obese and non-obese groups were performed by two-tailed Student’s t-tests. Correlation of variables was performed with linear regression analysis to calculate Pearson’s correlation coefficient. Data are presented as mean ± SE. Significance was set at p< 0.05.
RESULTS
Maternal and fetal leptin concentrations
Maternal serum leptin concentration was lower in nOB(48.2 ±11.4 ng/ml) animals compared to OB (116.4 ± 12.4 ng/ml) (p<0.05). There were no differences in fetal leptin concentrations between two groups [20].
Serum, placental and maternal adipose tissue content of AEA and 2-AG
Placental AEA as well as serum and maternal fat 2-AG concentrations were similar in both groups (Fig. 1). AEA concentration in maternal fat was higher in the obese animals (Fig. 1A). Concentrations of 2-AG in placenta were lower in obese baboons (1254.1 ± 401.3 nmol/kg) vs. non-obese (3124.2 ± 557.3 nmol/kg) (Fig. 1C). There were no correlations between placental/maternal fat and serum ECB concentrations. AEA contents were lower than 2-AG contents in maternal serum and fat (Fig. 1). Maternal serum AEA concentrations correlated negatively with the weight of maternal liver (r=−0.72, p=0.04) and also showed slight, but significant correlations with the weights of fetal brain (r=0.74, p=0.04) and fetal pancreas (r=0.75, p=0.03). Maternal fat and placental 2-AG concentrations positively correlated with each other (r=0.82, p=0.013) and with maternal leptin concentrations (r=−0.72, p=0.04 and r=−0.83, p=0.01 respectively).
Figure 1.
Expression of ECB mRNA and proteins in placenta
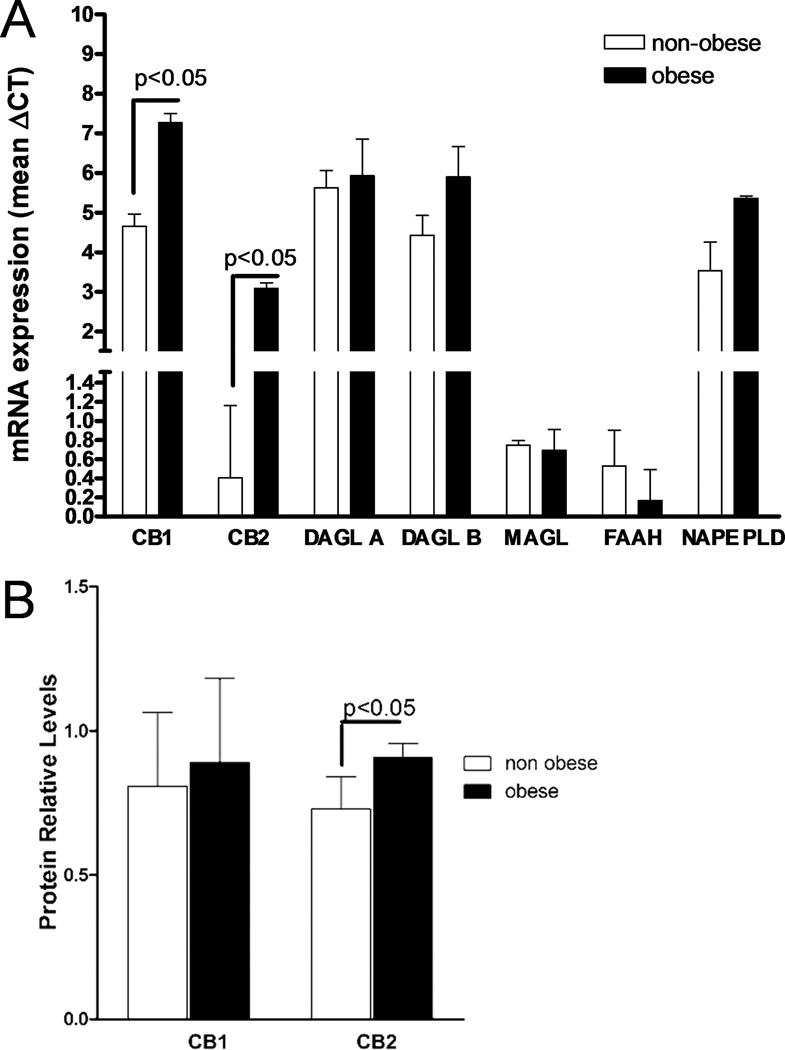
Expressions of CB1 and CB2 receptor mRNA were lower in obese placentas, compared to the non-obese (Fig. 2A). Expressions of DAGL-A, DAGL-B, NAPE-PLD, MAGLand FAAH did not differ between obese and non-obese animals. Relative expression of CB2 receptor protein was higher in obese compared to non-obese placentas (Fig 2B).
Figure 2.
Expression of ECB proteins in the placental villous tree
Localization
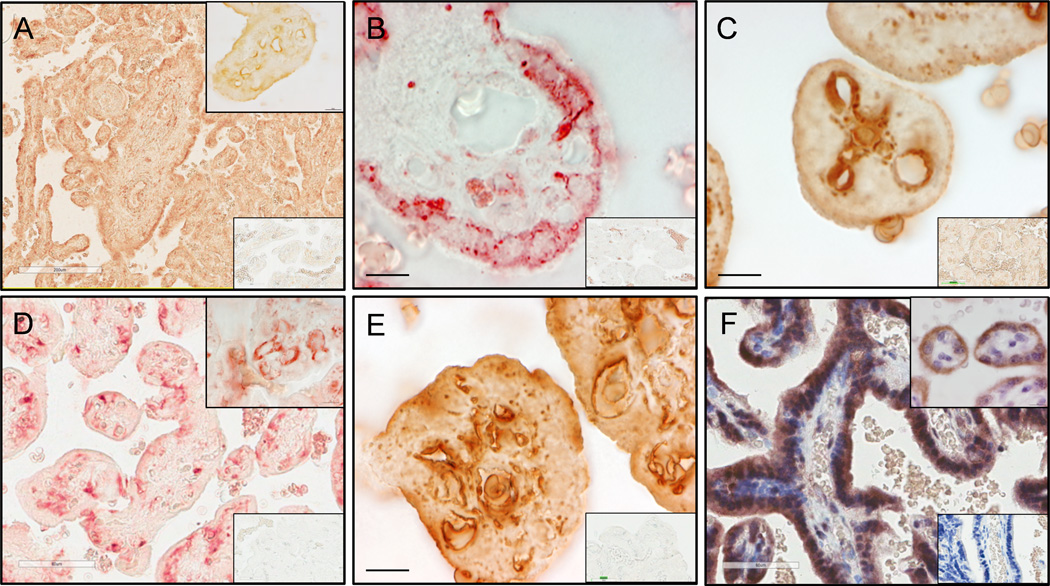
Villous trophoblastic cells demonstrated relatively weak expression of CB1 and CB2 receptors (Fig. 3A, 3B) and intense expression of MAGL and FAAH (Fig. 3E and 3F). Villous capillary endothelium expressed CB1 receptors (Fig. 3A), NAPE-PLD (Fig. 3C), DAGL α (Fig. 3D), and MAGL (Fig. 3E).
Figure 3.
Histomorphometry
There were no observed differences in the villous and basal plateprotein expression of ECB ligands, their receptors, and metabolizing enzymes between obese and non-obese animals (Table 3).
Table 3.
Endocannabinoid system staining intensities (calculated as histoscore) in placentas from obese and non-obese baboons (Papiospp) (Data presented as Mean ± SE)
| Antigen | Obese (n=4) | Non-Obese (n=4) | p values | |
|---|---|---|---|---|
| CB1 | Villi | 53.75 ± 19.2 | 47.01 ± 25.2 | 0.84 |
| Basal plate | 63.78 ± 24.5 | 47.2 ± 25.2 | 0.654 | |
| CB2 | Villi | 95.50± 21.7 | 96.18 ± 9.5 | 0.98 |
| Basal plate | 76.87 ± 5.2 | 53.5 ± 11.6 | 0.17 | |
| FAAH | Villi | 7.48 ± 1.9 | 3.64 ± 0.8 | 0.12 |
| Basal plate | 0.3 ± 0.1 | 1.19 ± 0.5 | 0.14 | |
| MAGL | Villi | 232.6 ± 9.0 | 250.30 ± 5.3 | 0.14 |
| Basal plate | 251.53 ± 9.0 | 253.20 ± 20.0 | 0.94 | |
| DAGL | Villi | 150.97 ± 14.7 | 157.15 ± 23.2 | 0.83 |
| Basal plate | 53.10 ± 13.11 | 93.30 ± 17.3 | 0.12 | |
| NAPE-PLD | Villi | 163.19 ± 8.7 | 181.16 ± 18.6 | 0.42 |
| Basal plate | 193.75 ± 30 | 168.74 ± 19.64 | 0.51 | |
DISCUSSION
Maternal obesity exerts significant morbidities during pregnancy and birth (e.g., stillbirth, pre-eclampsia) and throughout the offspring’s lifespan [27]. To our knowledge, this study is the first to report the responses of placental and maternal ECBs to maternal obesity in any model. Subcutaneous fat has been shown to act as an endocrine organ [28] and accounts for almost 90% of total fat deposition in women [29].ECBs and their receptors are essential regulators of metabolism for multiple cardiovascular, neurologic, reproductive, and immune processes [30, 31]. Dysregulation of ECBs within subcutaneous fat is a hallmark of obesity [32]. Thus, it is reasonable to hypothesize that metabolic cross-talk between placenta and fat could mediate negative consequences of maternal obesity.
Components of 2AG enzymatic pathway
Despite the confirmed role of the ECB system to maintain pregnancy [33], the role of 2-AG in placenta remains unclear [9]. In our current study, 2-AG concentration was 10-fold higher in placenta than maternal serum, suggesting placenta as a source or depot for this key ECB ligand in pregnancy. This observation agrees with data published for rat placenta; however, the placental concentration of 2-AG in the baboons was higher than that reported for rodents [9]. Subcutaneous fat concentration of 2-AG was lower in baboons (vs. humans [32]); but serum values resembled those reported for humans and rodents [18]. In addition to biologic reasons, these differences in absolute concentrations could be attributed, to different analytical methodologies used.
The decrease in placental 2-AG content observed in obese baboons is intriguing. Similar decreases have been described in subcutaneous fat from obese humans [32]. In jejunum, 2-AG accumulation was associated with the dietary consumption of 18:1 oleic acid [34]. Keeping in mind that Syncytiotrophoblast (ST) shares striking structural and functional similarities with the small intestine [35], and also that maternal obesity significantly influences placental oleic acid uptake [36], it is logical to posit that the regulation of placental 2-AG pathways could be coupled to placental fatty acid transport. Recent evidence of association between maternal fat intake and offspring ECBs function in rats supports this idea [37].
Another mechanism for ECB regulation could involve leptin, and maternal concentration of leptin is increased in MO [20]. In brain and subcutaneous fat, leptin regulates energy homeostasis via down-regulation of 2-AG and CB1 receptor expression [38, 39]. In our study, maternal fat and placental 2-AG concentrations correlated directly; 2-AG concentrations in both tissues showed negative correlations with the maternal serum leptin concentration. Similar effect of leptin on 2-AG metabolism has been reported in the pregnant rodent uterus [40], yet the exact mechanism remains unclear. In neural tissues, 2-AG content is regulated by its enzyme-dependentsynthesis (via DAGL-A and DAGL-B), as well as degradation (via MAGL) [41]. The enzyme activity has not been measured in our study, and our data are limited to these proteins per se.MAGL expression was detectable in ST, which is also the site of leptin expression in humans and non-human primates [42], Notably, proteomic analyses of the ST apical brush border by Vandre et al. [43] revealed presence of another 2-AG hydrolyzing enzyme-ABHD12. ABHD12, plays an important role in brain microglial 2-AG - CB2 interaction and is responsible for 9% of 2AG hydrolysis. Thus ST might be an important site of ECBs hydrolyses. ECB hydrolysis could lead to a described in maternal obesity placental inflammatory response (macrophages and neutrophils accumulation)[20, 44] through the formation of AA and COX-2 [45].However, in rodents, decreased 2-AG production was protective against inflammatory liver injury [46]. The higher amount of 2-AG—compared to AEA in serum and fat in our study— agrees with data published for other tissues, e.g. brain [47] and decidua [48].
Components of AEA enzymatic pathway
The increase of the AEA in the subcutaneous fat of obese mothers in our study agrees with data from non-pregnant women [6]. In contrast, the serum AEA concentrations did not differ between obese and non-obese animals in our study and were similar to those reported for human pregnancies [18, 49]. Associations between the maternal circulating AEA, weight of maternal liver, and weight of insulin-sensitive fetal tissues (brain and pancreas) are intriguing The role of AEA as a central player in the regulation of the insulin-sensitivity, including development of Type 2 diabetes has been recently emerged [50].
CB1 and CB2 receptors
The CB1 receptor is closely involved in pregnancy maintenance, as shown in human miscarriages [18] and in spontaneous preterm labor in CB1 receptor knock-out mice [51]. Down-regulation of placental CB1 and CB2 receptors mRNA in MO here agrees with data published for mRNA expression in brown fat of obese Zuckerrats [52] and might provide a mechanism for the epidemiological link between maternal obesity and preterm labor [53]. The protein levels of CB1 receptors as evaluated by WB and IHC did not differ between two groups in our study. Theup-regulation in protein expressions of CB2 receptor in WB is in agreement with published data regarding the role of CB2 in inflammation[54].However, the mechanisms of CB2 regulation of inflammation and energy homeostasis are still remain obscure[54, 55]. The controversies in different published studies as well as our observed discrepancies between mRNA, WB and histomorphometry could result from the complexity of ECB molecular biology[55] and the limitations, associated with each detection. Distinct mRNA transcripts for example, could arise from different isoforms and splicing variants of both receptors [56]. The protein content in WB mirrors the diverse populations of placental cells from maternal and fetal origins, each of which could have distinct regulatory mechanisms and functions. Mitochondria express bothCB1 and CB2 receptors [57]. The control of mitochondrial function by ECBs may influence placental metabolic rate and therefore fetal development. For example, changes in placental metabolism related to higher altitude have been described as a cause of fetal growth retardation [58]. The localization of CB1 and CB2 receptors in trophoblast makes these receptors ideal candidates for regulation of placental metabolism [59].
In summary, the present study demonstrated for the first time the differential regulation of the ECBs in maternal fat and placental tissue related to maternal obesity. Differential regulation and function exists for the AEA and 2-AG pathways in both tissues, with decreased 2-AG concentration in placenta and increased AEA content in the subcutaneous fat from obese mothers. Our data suggest these pathways might exert different roles during fetal development.
Acknowledgements
The ideas, encouragement, and support of Dr. K.U. Malik (UTHSC) were highly appreciated. Authors thank personnel of the Texas Biomedical Institute and Center for Pregnancy and Newborn Research (UTHSC—San Antonio) for their help. Authors are grateful to Dr. Anand Kulkarni and Ms. Ashley Ezekiel for their expertise with an AperioScanScope. This study was partially supported by Texas Biomedical Research Institute Grant C06 RR013556 and NIH grant HD21350 to Dr. Peter Nathanielsz (UTHSC—San Antonio), UTHSCSA ERC New Investigator Award to N.S.-L., NIH NCRR grant P51 RR013986 to the Southwest National Primate Research Center. R.F. gratefully acknowledges research support of Le Bonheur Foundation (Memphis TN) and discloses unrelated research support from NIH R21 HD059292, NIH U01 DK085465, JDRF 1-2011-597, Gabrielle’s Angel Foundation, MacroGenics, Eli Lilly & Co., Bristol-Myers Squibb, Novo Nordisk A/S, Ipsen, Diamyd Therapeutics AB, Pfizer, Tolerx, GlaxoSmithKline, and Takeda.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010 Jan 20;303(3):235–241. doi: 10.1001/jama.2009.2014. PubMed PMID: 20071471. Epub 2010/01/15. eng. [DOI] [PubMed] [Google Scholar]
- 2.Guelinckx I, Devlieger R, Beckers K, Vansant G. Maternal obesity: pregnancy complications, gestational weight gain and nutrition. Obes Rev. 2008 Mar;9(2):140–150. doi: 10.1111/j.1467-789X.2007.00464.x. PubMed PMID, 18221480. Epub 2008/01/29. eng. [DOI] [PubMed] [Google Scholar]
- 3.Rkhzay-Jaf J, O'Dowd JF, Stocker CJ. Maternal Obesity and the Fetal Origins of the Metabolic Syndrome. Curr Cardiovasc Risk Rep. 2012 Oct;6(5):487–495. doi: 10.1007/s12170-012-0257-x. PubMed PMID, 23002417. Pubmed Central PMCID, 3433666. Epub 2012/09/25. Eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bellocchio L, Cervino C, Pasquali R, Pagotto U. The endocannabinoid system and energy metabolism. J Neuroendocrinol. 2008 Jun;20(6):850–857. doi: 10.1111/j.1365-2826.2008.01728.x. PubMed PMID, 18601709. Epub 2008/07/08. eng. [DOI] [PubMed] [Google Scholar]
- 5.Bermudez-Silva FJ, Cardinal P, Cota D. The role of the endocannabinoid system in the neuroendocrine regulation of energy balance. J Psychopharmacol. 2012 Jan;26(1):114–124. doi: 10.1177/0269881111408458. PubMed PMID, 21824982. Epub 2011/08/10. eng. [DOI] [PubMed] [Google Scholar]
- 6.Engeli S, Bohnke J, Feldpausch M, Gorzelniak K, Janke J, Batkai S, et al. Activation of the peripheral endocannabinoid system in human obesity. Diabetes. 2005 Oct;54(10):2838–2843. doi: 10.2337/diabetes.54.10.2838. PubMed PMID, 16186383. Pubmed Central PMCID, 2228268. Epub 2005/09/28. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bluher M, Engeli S, Kloting N, Berndt J, Fasshauer M, Batkai S, et al. Dysregulation of the peripheral and adipose tissue endocannabinoid system in human abdominal obesity. Diabetes. 2006 Nov;55(11):3053–3060. doi: 10.2337/db06-0812. PubMed PMID, 17065342. Pubmed Central PMCID, 2228260. Epub 2006/10/27. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maccarrone M, Gasperi V, Catani MV, Diep TA, Dainese E, Hansen HS, et al. The endocannabinoid system and its relevance for nutrition. Annu Rev Nutr. 2010 Aug 21;30:423–440. doi: 10.1146/annurev.nutr.012809.104701. PubMed PMID, 20645854. Epub 2010/07/22. eng. [DOI] [PubMed] [Google Scholar]
- 9.Fonseca BM, Correia-da-Silva G, Taylor AH, Lam PM, Marczylo TH, Konje JC, et al. Characterisation of the endocannabinoid system in rat haemochorial placenta. Reprod Toxicol. 2012 Nov;34(3):347–356. doi: 10.1016/j.reprotox.2012.05.036. PubMed PMID, 22613199. Epub 2012/05/23. eng. [DOI] [PubMed] [Google Scholar]
- 10.Di Marzo V. Endocannabinoids: synthesis and degradation. Rev Physiol Biochem Pharmacol. 2008;160:1–24. doi: 10.1007/112_0505. PubMed PMID, 18481028. Epub 2008/05/16. eng. [DOI] [PubMed] [Google Scholar]
- 11.Blankman JL, Simon GM, Cravatt BF. A comprehensive profile of brain enzymes that hydrolyze the endocannabinoid 2-arachidonoylglycerol. Chem Biol. 2007 Dec;14(12):1347–1356. doi: 10.1016/j.chembiol.2007.11.006. PubMed PMID, 18096503. Pubmed Central PMCID, 2692834. Epub 2007/12/22. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Petrocellis L, Di Marzo V. An introduction to the endocannabinoid system: from the early to the latest concepts. Best Pract Res Clin Endocrinol Metab. 2009 Feb;23(1):1–15. doi: 10.1016/j.beem.2008.10.013. PubMed PMID, 19285257. Epub 2009/03/17. eng. [DOI] [PubMed] [Google Scholar]
- 13.Felder CC, Nielsen A, Briley EM, Palkovits M, Priller J, Axelrod J, et al. Isolation and measurement of the endogenous cannabinoid receptor agonist, anandamide, in brain and peripheral tissues of human and rat. FEBS Lett. 1996 Sep 16;393(2–3):231–235. doi: 10.1016/0014-5793(96)00891-5. PubMed PMID, 8814296. Epub 1996/09/16. eng. [DOI] [PubMed] [Google Scholar]
- 14.Das SK, Paria BC, Chakraborty I, Dey SK. Cannabinoid ligand-receptor signaling in the mouse uterus. Proc Natl Acad Sci U S A. 1995 May 9;92(10):4332–4336. doi: 10.1073/pnas.92.10.4332. PubMed PMID, 7753807. Pubmed Central PMCID, 41938. Epub 1995/05/09. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Sickle MD, Duncan M, Kingsley PJ, Mouihate A, Urbani P, Mackie K, et al. Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science. 2005 Oct 14;310(5746):329–332. doi: 10.1126/science.1115740. PubMed PMID, 16224028. Epub 2005/10/15. eng. [DOI] [PubMed] [Google Scholar]
- 16.Galiegue S, Mary S, Marchand J, Dussossoy D, Carriere D, Carayon P, et al. Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. Eur J Biochem. 1995 Aug 15;232(1):54–61. doi: 10.1111/j.1432-1033.1995.tb20780.x. PubMed PMID, 7556170. Epub 1995/08/15. eng. [DOI] [PubMed] [Google Scholar]
- 17.Sun X, Xie H, Yang J, Wang H, Bradshaw HB, Dey SK. Endocannabinoid signaling directs differentiation of trophoblast cell lineages and placentation. Proc Natl Acad Sci U S A. 2010 Sep 28 39;107:16887–16892. doi: 10.1073/pnas.1010892107. PubMed PMID, 20837524. Pubmed Central PMCID, 2947871. Epub 2010/09/15. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taylor AH, Amoako AA, Bambang K, Karasu T, Gebeh A, Lam PM, et al. Endocannabinoids and pregnancy. Clin Chim Acta. 2010 Jul 4;411(13–14):921–930. doi: 10.1016/j.cca.2010.03.012. PubMed PMID, 20302856. Epub 2010/03/23. eng. [DOI] [PubMed] [Google Scholar]
- 19.Marczylo TH, Lam PM, Amoako AA, Konje JC. Anandamide levels in human female reproductive tissues: solid-phase extraction and measurement by ultraperformance liquid chromatography tandem mass spectrometry. Anal Biochem. 2010 May 15;400(2):155–162. doi: 10.1016/j.ab.2009.12.025. PubMed PMID, 20026294. Epub 2009/12/23. eng. [DOI] [PubMed] [Google Scholar]
- 20.Farley D, Tejero ME, Comuzzie AG, Higgins PB, Cox L, Werner SL, et al. Feto304 placental adaptations to maternal obesity in the baboon. Placenta. 2009 Sep;30(9):752–760. doi: 10.1016/j.placenta.2009.06.007. PubMed PMID, 19632719. Pubmed Central PMCID, 3011231. Epub 2009/07/28. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Samson JE, Mari G, Dick EJ, Jr, Hubbard GB, Ferry RJ, Jr, Schlabritz-Loutsevitch NE. The morphometry of materno-fetal oxygen exchange barrier in a baboon model of obesity. Placenta. 2011 Nov;32(11):845–851. doi: 10.1016/j.placenta.2011.07.083. PubMed PMID, 21872927. Pubmed Central PMCID, 3304583. Epub 2011/08/30. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zoerner AA, Batkai S, Suchy MT, Gutzki FM, Engeli S, Jordan J, et al. Simultaneous UPLC-MS/MS quantification of the endocannabinoids 2-arachidonoyl glycerol (2AG), 1-arachidonoyl glycerol (1AG), and anandamide in human plasma: minimization of matrix-effects, 2AG/1AG isomerization and degradation by toluene solvent extraction. J Chromatogr B Analyt Technol Biomed Life Sci. 2012 Feb 1;883–884:161–171. doi: 10.1016/j.jchromb.2011.06.025. PubMed PMID, 21752730. Epub 2011/07/15. eng. [DOI] [PubMed] [Google Scholar]
- 23.Janjetovic Z, Zmijewski MA, Tuckey RC, DeLeon DA, Nguyen MN, Pfeffer LM, et al. 20-Hydroxycholecalciferol, product of vitamin D3 hydroxylation by P450scc, decreases NF318 kappaB activity by increasing IkappaB alpha levels in human keratinocytes. PLoS One. 2009;4(6):e5988. doi: 10.1371/journal.pone.0005988. PubMed PMID, 19543524. Pubmed Central PMCID, 2694402. Epub 2009/06/23. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nassar A, Cohen C, Agersborg SS, Zhou W, Lynch KA, Heyman ER, et al. A new immunohistochemical ER/PR image analysis system: a multisite performance study. Appl Immunohistochem Mol Morphol. 2011 May;19(3):195–202. doi: 10.1097/PAI.0b013e3181fe53cb. PubMed PMID, 21217524. Epub 2011/01/11. eng. [DOI] [PubMed] [Google Scholar]
- 25.Taylor AH, Finney M, Lam PM, Konje JC. Modulation of the endocannabinoid system in viable and non-viable first trimester pregnancies by pregnancy-related hormones. Reprod Biol Endocrinol. 2011;9:152. doi: 10.1186/1477-7827-9-152. PubMed PMID, 22126420. Pubmed Central PMCID: PMC3266649. Epub 2011/12/01. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen Z, Wang T, Cai L, Su C, Zhong B, Lei Y, et al. Clinicopathological significance of non-small cell lung cancer with high prevalence of Oct-4 tumor cells. J Exp Clin Cancer Res. 2012;31:10. doi: 10.1186/1756-9966-31-10. PubMed PMID, 22300949. Pubmed Central PMCID: PMC3287152. Epub 2012/02/04. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taylor PD, Poston L. Developmental programming of obesity in mammals. Exp Physiol. 2007 Mar;92(2):287–298. doi: 10.1113/expphysiol.2005.032854. PubMed PMID, 17170060. Epub 2006/12/16. eng. [DOI] [PubMed] [Google Scholar]
- 28.Slominski AT, Zmijewski MA, Skobowiat C, Zbytek B, Slominski RM, Steketee JD. Sensing the environment: regulation of local and global homeostasis by the skin's neuroendocrine system. Adv Anat Embryol Cell Biol. 2012;212:v, vii, 1–115. doi: 10.1007/978-3-642-19683-6_1. PubMed PMID, 22894052. Pubmed Central PMCID, 3422784. Epub 2012/08/17. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wajchenberg BL. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev. 2000 Dec;21(6):697–738. doi: 10.1210/edrv.21.6.0415. PubMed PMID, 11133069. Epub 2001/01/02. eng. [DOI] [PubMed] [Google Scholar]
- 30.Bambang KN, Lambert DG, Lam PM, Quenby S, Maccarrone M, Konje JC. Immunity and early pregnancy events: are endocannabinoids the missing link? J Reprod Immunol. 2012 Dec;96(1–2):8–18. doi: 10.1016/j.jri.2012.10.003. PubMed PMID, 23177537. Epub 2012/11/28. eng. [DOI] [PubMed] [Google Scholar]
- 31.Pacher P, Steffens S. The emerging role of the endocannabinoid system in cardiovascular disease. Semin Immunopathol. 2009 Jun;31(1):63–77. doi: 10.1007/s00281-009-0145-8. PubMed PMID, 19357846. Pubmed Central PMCID, 2791499. Epub 2009/04/10. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bennetzen MF, Wellner N, Ahmed SS, Ahmed SM, Diep TA, Hansen HS, et al. Investigations of the human endocannabinoid system in two subcutaneous adipose tissue depots in lean subjects and in obese subjects before and after weight loss. Int J Obes (Lond) 2011 Nov;35(11):1377–1384. doi: 10.1038/ijo.2011.8. PubMed PMID, 21326208. Epub 2011/02/18. eng. [DOI] [PubMed] [Google Scholar]
- 33.Battista N, Meccariello R, Cobellis G, Fasano S, Di Tommaso M, Pirazzi V, et al. The role of endocannabinoids in gonadal function and fertility along the evolutionary axis. Mol Cell Endocrinol. 2012 May 15;355(1):1–14. doi: 10.1016/j.mce.2012.01.014. PubMed PMID, 22305972. Epub 2012/02/07. eng. [DOI] [PubMed] [Google Scholar]
- 34.Dipatrizio NV, Joslin A, Jung KM, Piomelli D. Endocannabinoid signaling in the gut mediates preference for dietary unsaturated fats. FASEB, J. 2013 Mar 5; doi: 10.1096/fj.13-227587. PubMed PMID, 23463697. Epub 2013/03/07. Eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boyd CAR. Review: Epithelial aspects of human placental trophoblast. Placenta. 2013;34:S24–S26. doi: 10.1016/j.placenta.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 36.Brass E, Hanson E, O'Tierney-Ginn PF. Placental oleic acid uptake is lower in male offspring of obese women. Placenta. 2013 Apr 17; doi: 10.1016/j.placenta.2013.03.009. PubMed PMID, 23602336. Epub 2013/04/23. Eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.D'Asti E, Long H, Tremblay-Mercier J, Grajzer M, Cunnane SC, Di Marzo V, et al. Maternal dietary fat determines metabolic profile and the magnitude of endocannabinoid inhibition of the stress response in neonatal rat offspring. Endocrinology. 2010 Apr;151(4):1685–1694. doi: 10.1210/en.2009-1092. PubMed PMID, 20160134. Epub 2010/02/18. eng. [DOI] [PubMed] [Google Scholar]
- 38.Di Marzo V, Goparaju SK, Wang L, Liu J, Batkai S, Jarai Z, et al. Leptin-regulated endocannabinoids are involved in maintaining food intake. Nature. 2001 Apr 12;410(6830):822–825. doi: 10.1038/35071088. PubMed PMID, 11298451. Epub 2001/04/12. eng. [DOI] [PubMed] [Google Scholar]
- 39.Bennetzen MF. Investigations of the endocannabinoid system in adipose tissue: effects of obesity/ weight loss and treatment options. Dan Med Bull. 2011 Apr;58(4):B4269. PubMed PMID, 21466769. Epub 2011/04/07. eng. [PubMed] [Google Scholar]
- 40.Maccarrone M, Fride E, Bisogno T, Bari M, Cascio MG, Battista N, et al. Up-regulation of the endocannabinoid system in the uterus of leptin knockout (ob/ob) mice and implications for fertility. Mol Hum Reprod. 2005 Jan;11(1):21–28. doi: 10.1093/molehr/gah130. PubMed PMID, 15563449. Epub 2004/11/26. eng. [DOI] [PubMed] [Google Scholar]
- 41.Alger BE, Kim J. Supply and demand for endocannabinoids. Trends Neurosci. 2011 Jun;34(6):304–315. doi: 10.1016/j.tins.2011.03.003. PubMed PMID, 21507493. Pubmed Central PMCID, 3106144. Epub 2011/04/22. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Henson MC, Swan KF, Edwards DE, Hoyle GW, Purcell J, Castracane VD. Leptin receptor expression in fetal lung increases in late gestation in the baboon: a model for human pregnancy. Reproduction. 2004 Jan;127(1):87–94. doi: 10.1530/rep.1.00037. PubMed PMID, 15056773. Epub 2004/04/02. eng. [DOI] [PubMed] [Google Scholar]
- 43.Vandre DD, Ackerman WEt, Tewari A, Kniss DA, Robinson JM. A placental sub385 proteome: the apical plasma membrane of the syncytiotrophoblast. Placenta. 2012 Mar;33(3):207–213. doi: 10.1016/j.placenta.2011.12.010. PubMed PMID, 22222045. Pubmed Central PMCID, 3277652. Epub 2012/01/10. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Challier JC, Basu S, Bintein T, Minium J, Hotmire K, Catalano PM, et al. Obesity in pregnancy stimulates macrophage accumulation and inflammation in the placenta. Placenta. 2008 Mar;29(3):274–281. doi: 10.1016/j.placenta.2007.12.010. PubMed PMID, 18262644. Epub 2008/02/12. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nomura DK, Morrison BE, Blankman JL, Long JZ, Kinsey SG, Marcondes MC, et al. Endocannabinoid hydrolysis generates brain prostaglandins that promote neuroinflammation. Science. 2011 Nov 11;334(6057):809–813. doi: 10.1126/science.1209200. PubMed PMID, 22021672. Pubmed Central PMCID, 3249428. Epub 2011/10/25. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cao Z, Mulvihill MM, Mukhopadhyay P, Xu H, Erdelyi K, Hao E, et al. Monoacylglycerol Lipase Controls Endocannabinoid and Eicosanoid Signaling and Hepatic Injury in Mice. Gastroenterology. 2013 Jan 4; doi: 10.1053/j.gastro.2012.12.028. PubMed PMID, 23295443. Epub 2013/01/09. Eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stella N, Schweitzer P, Piomelli D. A second endogenous cannabinoid that modulates long-term potentiation. Nature. 1997 Aug 21;388(6644):773–778. doi: 10.1038/42015. PubMed PMID, 9285589. Epub 1997/08/21. eng. [DOI] [PubMed] [Google Scholar]
- 48.Fonseca BM, Correia-da-Silva G, Taylor AH, Lam PM, Marczylo TH, Bell SC, et al. The endocannabinoid 2-arachidonoylglycerol (2-AG) and metabolizing enzymes during rat fetoplacental development: a role in uterine remodelling. Int J Biochem Cell Biol. 2010 Nov;42(11):1884–1892. doi: 10.1016/j.biocel.2010.08.006. PubMed PMID, 20727980. Epub 2010/08/24. eng. [DOI] [PubMed] [Google Scholar]
- 49.Gebeh AK, Willets JM, Bari M, Hirst RA, Marczylo TH, Taylor AH, et al. Elevated anandamide and related N-acylethanolamine levels occur in the peripheral blood of women with ectopic pregnancy and are mirrored by changes in peripheral fatty acid amide hydrolase activity. J Clin Endocrinol Metab. 2013 Mar;98(3):1226–1234. doi: 10.1210/jc.2012-3390. PubMed PMID, 23372171. Epub 2013/02/02. eng. [DOI] [PubMed] [Google Scholar]
- 50.Di Marzo V, Piscitelli F, Mechoulam R. Cannabinoids and endocannabinoids in metabolic disorders with focus on diabetes. Handb Exp Pharmacol. 2011;(203):75–104. doi: 10.1007/978-3-642-17214-4_4. PubMed PMID, 21484568. Epub 2011/04/13. eng. [DOI] [PubMed] [Google Scholar]
- 51.Wang H, Xie H, Dey SK. Loss of cannabinoid receptor CB1 induces preterm birth. PLoS One. 2008;3(10):e3320. doi: 10.1371/journal.pone.0003320. PubMed PMID, 18833324. Pubmed Central PMCID, 2553193. Epub 2008/10/04. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Iannotti FA, Piscitelli F, Martella A, Mazzarella E, Allara M, Palmieri V, et al. Analysis of the"endocannabinoidome" in peripheral tissues of obese Zucker rats. Prostaglandins Leukot Essent Fatty Acids. 2013 Aug;89(2–3):127–135. doi: 10.1016/j.plefa.2013.06.002. PubMed PMID, 23830028. Epub 2013/07/09. eng. [DOI] [PubMed] [Google Scholar]
- 53.Smith GC, Shah I, Pell JP, Crossley JA, Dobbie R. Maternal obesity in early pregnancy and risk of spontaneous and elective preterm deliveries: a retrospective cohort study. Am J Public Health. 2007 Jan;97(1):157–162. doi: 10.2105/AJPH.2005.074294. PubMed PMID, 17138924. Pubmed Central PMCID, 1716235. Epub 2006/12/02. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pacher P, Mechoulam R. Is lipid signaling through cannabinoid 2 receptors part of a protective system? Prog Lipid Res. 2011 Apr;50(2):193–211. doi: 10.1016/j.plipres.2011.01.001. PubMed PMID, 21295074. Pubmed Central PMCID: PMC3062638. Epub 2011/02/08. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Di Marzo V. Targeting the endocannabinoid system: to enhance or reduce? Nat Rev Drug Discov. 2008 May;7(5):438–455. doi: 10.1038/nrd2553. PubMed PMID, 18446159. Epub 2008/05/01. eng. [DOI] [PubMed] [Google Scholar]
- 56.Rossi F, Bellini G, Alisi A, Alterio A, Maione S, Perrone L, et al. Cannabinoid receptor type 2 functional variant influences liver damage in children with non-alcoholic fatty liver disease. PLoS One. 2012;7(8):e42259. doi: 10.1371/journal.pone.0042259. PubMed PMID, 22927922. Pubmed Central PMCID: PMC3426511. Epub 2012/08/29. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nunn A, Guy G, Bell JD. Endocannabinoids in neuroendopsychology: multiphasic control of mitochondrial function. Philos Trans R Soc Lond B Biol Sci. 2012 Dec 5;367(1607):3342–3352. doi: 10.1098/rstb.2011.0393. PubMed PMID, 23108551. Pubmed Central PMCID, 3481535. Epub 2012/10/31. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Illsley NP, Caniggia I, Zamudio S. Placental metabolic reprogramming: do changes in the mix of energy-generating substrates modulate fetal growth? Int J Dev Biol. 2010;54(2–3):409–419. doi: 10.1387/ijdb.082798ni. PubMed PMID, 19924633. Epub 2009/11/20. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Drewlo S, Baczyk D, Dunk C, Kingdom J. Fusion assays and models for the trophoblast. Methods Mol Biol. 2008;475:363–482. doi: 10.1007/978-1-59745-250-2_21. PubMed PMID, 18979255. Epub 2008/11/04. eng. [DOI] [PubMed] [Google Scholar]