Abstract
Despite increasing evidence that anti-tumor immune control exists in the pediatric brain, these findings have yet to be exploited successfully in the clinic. A barrier to development of immunotherapeutic strategies in pediatric brain tumors is that the immunophenotype of these tumors’ microenvironment has not been defined. To address this the present study used multicolor FACS of disaggregated tumor to systematically characterize the frequency and phenotype of infiltrating immune cells in the most common pediatric brain tumor types. The initial study cohort consisted of 7 pilocytic astrocytoma (PA), 19 ependymoma (EPN), 5 glioblastoma (GBM), 6 medulloblastoma (MED) and 5 non-tumor brain (NT) control samples obtained from epilepsy surgery. Immune cell types analyzed included both myeloid and T-cell lineages and respective markers of activated or suppressed functional phenotypes. Immune parameters that distinguished each of the tumor types were identified. PA and EPN demonstrated significantly higher infiltrating myeloid and lymphoid cells compared to GBM, MED or NT. Additionally, PA and EPN conveyed a comparatively activated/M1-skewed myeloid functional phenotype denoted in particular by HLA-DR and CD64 expression. In contrast, GBM and MED contained progressively fewer infiltrating leukocytes and more muted functional phenotype similar to that of NT. These findings were recapitulated using whole tumor expression of corresponding immune marker genes in a large gene expression microarray cohort of pediatric brain tumors. The results of this cross-tumor comparative analysis demonstrate that different pediatric brain tumor types exhibit distinct immunophenotypes, implying that specific immunotherapeutic approaches may be most effective for each tumor type.
Keywords: pediatric brain tumor, immune, infiltrate, FACS, microarray
INTRODUCTION
Childhood brain tumors are now the leading cause of death from childhood cancers. In general there has been no improvement in treatment options in the last 2 to 3 decades. Existing treatments often use radiation and chemotherapy, which carry with them damaging side effects. Immunotherapy is an appealing treatment modality because of the potential for tumor-specific cytotoxicity. Recent studies by our laboratory and others have identified an association between host immunity and improved survival in children with brain tumors, specifically EPN, GBM and MED (1-3). The finding that host immunity may already impact survival in these tumor types suggests that they may be better candidates for immunotherapy.
Effective application of immunotherapy in brain tumors is thought to be hindered by the dampened immunity inherent to the central nervous system and also to brain tumor-mediated immunosuppression. These assumptions are largely based upon extensive brain tumor immunology research that has been conducted in the context of adult GBM. These numerous studies have demonstrated that GBM evades host immunity by a variety of mechanisms that include tumor-secreted and cell surface immunosuppressive factors and tumor-induced immunosuppressive leukocyte populations (4-10). Whether this immunosuppressive phenotype extends to the types of brain tumor seen in childhood has not been clearly determined.
Polarization of the immune system into activated or suppressed phenotypes is thought to determine its anti-tumor activity. Classically activated Th-cells (Th1) and myeloid cells (M1) can deliver a cytotoxic respiratory burst and secrete pro-inflammatory cytokines that play an important role in fighting intracellular pathogens and neoplastic cells (11-14). In contrast, alternatively activated Th-cells (Th2) and myeloid cells (M2), characterized by low pro-inflammatory cytokine production, are involved in tissue repair and remodeling, angiogenesis, anti-parasitic and allergic reactions. Th2 T-cells and M2 myeloid cells have been ascribed pro-tumorigenic roles (15, 16).
A better understanding of the immunophenotype of pediatric brain tumors would give support to the development of effective immunotherapeutic approaches. To address this, the present study systematically measured the frequency and phenotype of tumor-infiltrating leukocytes in PA, EPN, MED and GBM – the four most common pediatric brain tumor types.
MATERIALS AND METHODS
Patient cohort
Surgical tumor samples were obtained from 42 patients who presented between 2009 and 2013 for treatment at Children’s Hospital Colorado (Aurora, CO) who were diagnosed with PA (n=7; median age 8 years), EPN (n=19; 3 years), GBM (n=5; 15 years), or MED (n=6; 5 years) according to World Health Organization guidelines. All tumor samples used in this study were obtained at the time of initial resection and prior to therapy, apart from a single EPN sample that was obtained at second look surgery of the initial presentation thus having received chemotherapy. Control NT tissue was obtained from pediatric epilepsy patients (median age 13 years) at the time of surgical intervention. Five epilepsy samples of temporal lobe were selected that had no neoplasm or frank immune cell infiltrate. Patient collection was conducted in compliance with Institutional Review Board regulations (COMIRB 95-500 and 09-0906).
Disaggregation of tissue samples
Samples were collected in Neurobasal A media (Gibco, Grand Island, NY) and immediately disaggregated as described previously (17). Briefly, resected tumor was disaggregated by finely mincing with a razor and further triturated by vigorous pipetting. A single cell suspension was obtained by passing the sample through a 70μm cell strainer that is of sufficiently large pore size to permit passage of all immune and tumor cells but not clumped tumor cells (Becton Dickinson, Franklin Lakes, NJ). Due to differences in the relative cohesiveness of the tumor types in the study, there may be different levels of tumor cell retention in the 70 um filters, resulting in a semi-quantitative measurement of tumor cells by FACS. Heterogenous distribution of infiltrating immune cells may be exist within the tumor margins, but this cannot be addressed by FACS once tissue samples have been dissagregated. Rather, FACS of disaggregated samples provides a net proportion of cellular subpopulations within the margins of each particular specimen. The majority of tumor samples processed measured at least one cm3 in total volume, providing a significant portion of the total tumor mass. Disaggregated cells were viably frozen in standard freezing media containing 10% DMSO and stored in liquid nitrogen for subsequent analysis by FACS. Different immune cell populations may be more or less succeptible to cell death after freeze/thaw, but comparative analysis of data generated from identically processed samples minimizes the effect of this potential deficiency.
FACS Ab panel design
Myeloid cells, predominantly macrophage/microglial cells in the context of the CNS, were distinguished in disaggregated tumor specimens by co-expression of CD45 and CD11b. HLA-DR and Fcγ-R CD64 were used as markers of an activated myeloid cells, CD64 also being a marker of the activated M1 polarized phenotype (18). These markers have been associated with improved outcome in microarray-based studies of EPN and GBM, providing further rationale for inclusion in this study (1, 2). Scavenger receptor CD163 and mannose receptor CD206 were used in the present study as markers of this suppressed inflammatory or alternatively activated M2 myeloid phenotype (19). CD50 (ICAM-3) is expressed by undifferentiated or immature myeloid cells (20, 21).
CD45 and CD3 coexpression was used to distinguish tumor-infiltrating lymphocytes. To reveal the phenotype of T-cells infiltrating pediatric brain tumors, expression of CD4 and CD8 was used to distinguish Th-cells and CTL respectively. Effector memory T-cells were identified by expression of CD45RO, a tumor infiltrating T-cell population that has been associated with a favorable outcome in a number of cancer types (22). Programmed cell death protein 1 (PD-1, CD279) expression was also measured, being a clinically targetable marker of inhibitory T-cell activity that has previously identified as a mediator of immunosuppression in GBM (23-25). Details of Abs used are described in Supplemental Table I.
FACS analysis
Viably frozen disaggregated cells were gently thawed and suspended in PBS supplemented with 10% human serum (Jackson Laboratories, Bar Harbor, ME) as a blocking agent and DAPI (Fisher, Pittsburgh, PA) for live/dead cell identification. After blocking for 15 minutes at 4°C samples were resuspended in FACS buffer (PBS/10% FBS) and multicolor Ab panels or corresponding isotype controls. Samples were stained for 30 minutes at 4°C and then washed with FACS buffer, fixed in 2.5% paraformaldehyde and immediately analyzed by FACS. PD-1 staining was performed on cells that had been incubated for 4 hours with 50ng/ml of PMA and 0.5μg/ml of ionomycin as previously described (26).
FACS was performed using a Beckman Coulter Gallios (Beckman Coulter, Brea, CA). All Abs were titered for optimal Ab fluorescence intensity and voltages were set using optimized volumes. Compensation was calculated using the autosetup-preprogrammed compensation on Gallios and settings were saved and used as starting settings for subsequent experiments.
FACS files were analyzed using FloJo version 9.5.2. (Tree Star Inc., Ashland, OR). All samples were first gated on size and singularity, followed by dead cell exclusion. Gating strategies for myeloid and T-cell populations and phenotype markers are shown in Supplemental Figure 1. All gates were drawn using IgG controls for each sample (Supplemental Figure 2). Population percentages were compiled for each sample to create a data matrix. Comparative and statistical analyses were performed on this matrix using Microsoft Excel two-tailed student’s t-test and GraphPad Prism (version 5) Pearson’s correlation analyses. Principal components analysis (PCA) and unsupervised clustering of flow data were performed using R (http://cran.r-project.org) using publicly available software packages from Bioconductor (http://www.bioconductor.org).
Gene expression analysis
Microarray processing was performed for pediatric brain tumor and NT samples as described previously (27). The gene expression study sample dataset included 15 PA, 46 EPN, 20 GBM, 22 MED and 13 NT brain samples. Tumors were obtained from pediatric patients at initial presentation and with no prior treatment. NT brain was collected from autopsy or epilepsy surgery. Sample collection was conducted in compliance with Institutional Review Board regulations (COMIRB 95-500 and 09-0906). Briefly, RNA was extracted from snap frozen surgical samples, processed and applied to HG-U133 Plus 2 GeneChip microarrays (Affymetrix). Scanned microarray data were background corrected and normalized using the guanine cytosine Robust Multiarray Average (gcRMA) algorithm resulting in log 2 gene expression values (28). Gene expression data corresponding to myeloid and T-cell markers were then extracted for further analysis. The probeset with the highest expression was selected in cases where multiple probesets for the same gene existed. Expression levels of PD-1 were below the threshold for accurate detection (log2 normalized expression <3) in all samples, and were thus excluded from this analysis. CD45R0 is a splice form of CD45, and could therefore not be distinguished in this analysis. Gene expression levels associated with the more prevalent myeloid cells were accordingly higher, allowing all myeloid markers to be assessed by this gene expression analysis. PCA and unsupervised clustering of gene expression data was performed as described above. The microarray data discussed in this publication have been deposited in the National Center for Biotechnology Information Gene Expression Omnibus (GEO) database and are publicly accessible through GEO Series accession number GSE50161 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE50161).
Statistical analyses
Statistical analyses including those described above were performed using Microsoft Excel, Graphpad Prism and Bioconductor.
RESULTS
Extent of myeloid cell infiltration varies widely dependent on pediatric brain tumor type
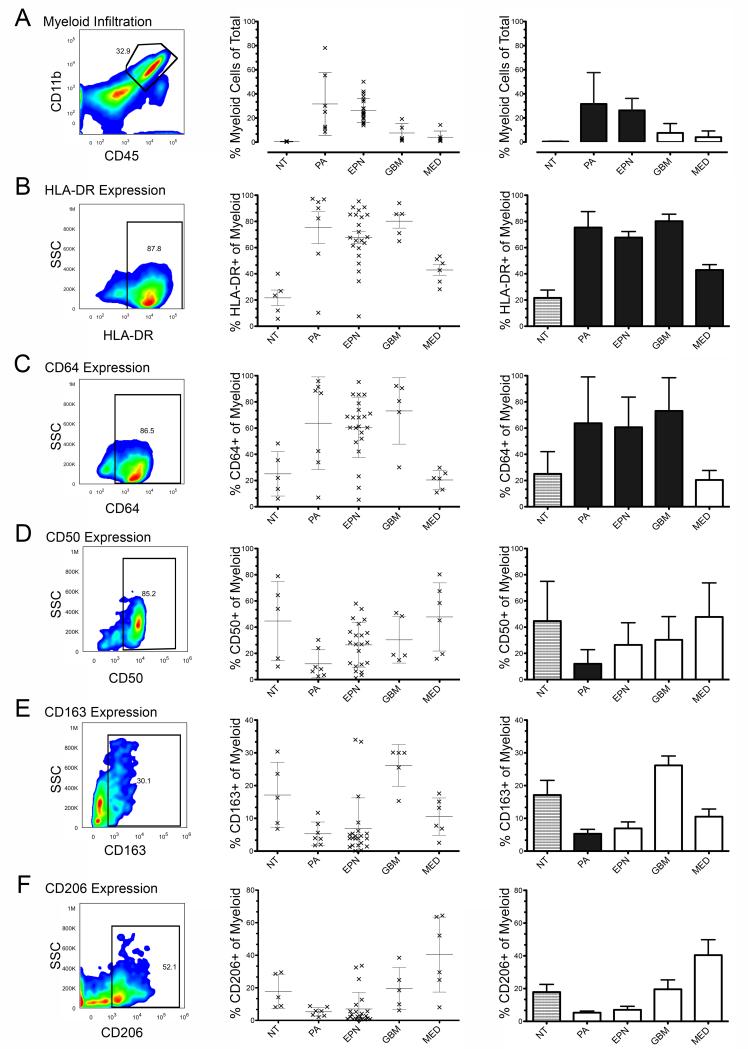
The average percentage of endogenous myeloid cells in the different tumor tissues varied widely, the most myeloid cell-rich being PA (31.6%) followed by EPN (27.1%), GBM (7.6%), MED (4.1%) and then NT (0.4%)(Figure 1A). The proportion of immune cells in patient tumor specimens was compared to that seen in control NT specimens to estimate the influence of malignancy on extent of myeloid cell infiltration. A large and significantly increased number of infiltrating myeloid cells was observed in both PA (72-fold, p=0.025) and EPN (61-fold, p=9.12×10−6) compared to NT. Although modest compared to PA and EPN, more infiltrating myeloid cells were also seen in GBM compared to NT (17.2-fold), showing a trend toward significance (p=0.074). MED exhibited the lowest levels in myeloid infiltration compared to NT (4.1-fold, p=0.154).
Figure 1. Characterization of infiltrating myeloid cells in pediatric brain tumors.
Multicolor FACS was used to measure myeloid cell infiltration and functional phenotype in disaggregated samples from PA, EPN, GBM, MED and NT. Representative FACS data with gating are shown for each marker (left panel). Histograms of individual patient sample data (middle) and mean average values (right) are shown (error bars = SD). Mean average values represented by black bars are those tumor types with values that were significantly different compared NT (p<0.05). (A) Percentage of infiltrating myeloid cells identified by co-expression of CD45 and CD11b (black arrow). Within the myeloid population, the percentage of (B) HLA-DR, (C) CD64, (D) CD50, (E) CD163 and (F) CD206 positive cells are shown. Abbreviations: PA, pilocytic astrocytoma; EPN, ependymoma; GBM, glioblastoma; MED, medulloblastoma; NT, non-tumor.
The functional phenotype of tumor-infiltrating myeloid cells is skewed differently between different pediatric brain tumor types
PA exhibited the most activated myeloid phenotype of pediatric tumor types in the present study. Myeloid activation markers HLA-DR and CD64 were both significantly higher (P<0.05) in PA compared to NT (3.5- and 2.5-fold respectively). Markers for undifferentiated and immune suppressed myeloid cells, CD50 and CD163 respectively, were significantly lower (3.7- and 3.2-fold respectively) in PA than NT. Myeloid suppression marker CD206 was also lower than NT (2.3-fold) showing a trend toward significance (p=0.053).
As observed in PA, EPN significantly over-expressed both myeloid HLA-DR (3.0-fold) and CD64 (2.3-fold) and under-expressed all three non-activated myeloid markers CD50, CD163 and 206 compared to NT (1.72-fold, 2.4-fold and 2.3-fold less respectively; all trending toward significance (p<0.1)).
GBM showed evidence of a suppressive M2 phenotype. Unlike PA and EPN, non-activated myeloid phenotype markers CD50, CD163 and CD206 in GBM were not significantly different from NT. M2 markers CD163 and CD206 were respectively 5.0- and 2.5-fold higher in GBMs than PA, and similarly overexpressed when compared to EPN. Despite the relatively elevated levels of non-activated myeloid markers, the level of activation markers in GBM was comparable to PA and EPN (HLA-DR 3.7-fold, CD64 2.9-fold) when compared to NT.
Compared to other tumor types, including GBM, MED contained a myeloid population that was more clearly skewed toward a suppressive M2 phenotype. MED were distinct from other brain tumors studied in that CD64, indicative of a classically activated M1 phenotype, showed no increase compared to NT. In addition, alternatively activated M2 marker CD206 was significantly higher in MED than both PA and EPN and showed a trend to significance when compared to NT. CD50 and CD163 were not different from NT.
T-cell infiltration is correlated with, but significantly lower, than myeloid cell infiltration across all tumor types
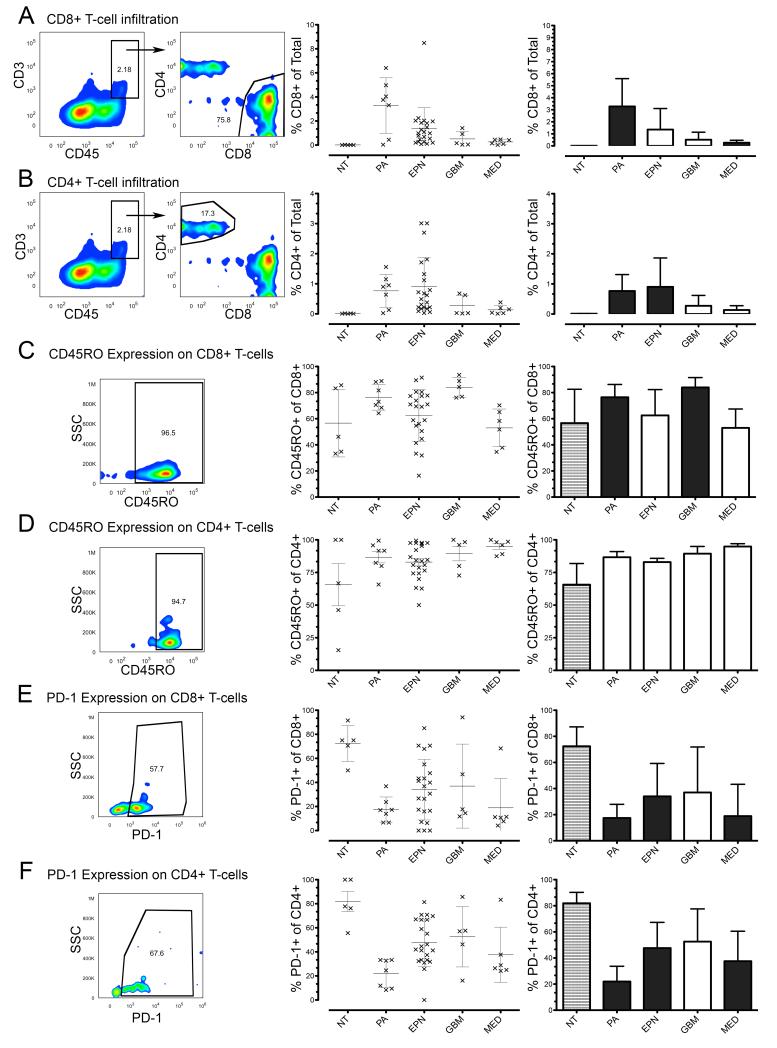
Total T-cell infiltration was determined using combined expression of CD45, CD3 and CD8 or CD4. The average number of infiltrating T-cells correlated strongly with myeloid cell infiltration in corresponding tumor and NT (Pearson R=0.96, p=0.0088). However, T-cells were consistently less common (~10%) than myeloid cells in each tumor type and NT. The average percentage of endogenous T-cells in the different tumor types varied widely but was in all cases higher than NT (0.02%), the most T-cell rich being PA (4.05%) followed by EPN (2.27%), GBM (0.79%) and MED (0.40%).
CD8+ and CD4+ T-cell infiltration varies significantly between tumor types and non-tumor brain
Compared to NT and other tumor types, PA contained on average the highest number of infiltrating CD8 T-cells (3.28%), approximately 300-fold more frequent than NT (Figure 2A). EPN, GBM and MED exhibited progressively lower CD8 T-cell infiltrations than PA, but all were higher than NT, 125-, 46- and 23-fold higher respectively. CD4 T-cell infiltration in EPN (1.40%) was higher than NT (83-fold) and all other tumor types (Figure 2B). PA, GBM and MED exhibited progressively lower CD4 T-cell infiltration than EPN but that was again higher than NT, 73-, 26- and 13-fold respectively, although GBM and MED were not statistically different from NT.
Figure 2. Characterization of infiltrating CD8 and CD4 T-cells in pediatric brain tumors.
Multicolor FACS was used to measure T-cell infiltration and functional phenotype in disaggregated samples from PA, EPN, GBM, MED and NT. Representative FACS data with gating are shown for each marker (left panel). Histograms of individual patient sample data (middle) and mean average values (right) are shown (error bars = SD). Mean average values represented by black bars are those tumor types with values that were significantly different compared NT (p<0.05). (A) Percentage of infiltrating T- cells was identified by co-expression of CD45 and CD3. Percentage of CD8 T-cell infiltration was then determined by expression of CD8 on T-cells. (B) Percentage of CD4 T-cell infiltration was determined similarly by expression of CD4 on CD45+CD3+ T-cells. Activated/memory T-cells were identified by expression of CD45RO in (C) CD8 and (D) CD4 T-cells. T-cell inactivation was measured by expression of PD-1 in (E) CD8 and (F) CD4 T-cells after PMA/ionomycin stimulation. Abbreviations: PA, pilocytic astrocytoma; EPN, ependymoma; GBM, glioblastoma; MED, medulloblastoma; NT, non-tumor.
The ratio of CD8 to CD4 T-cells has been identified as a prognostic factor in FACS studies of certain non-CNS tumors (29). In the present study CD8:CD4 T-cell ratios were higher in all tumor types than NT. The average ratio of CD8 to CD4 cells in PA was 3.91, significantly higher (p=0.0011) than the ratio in NT, where CD8 and CD4 T-cells were present in roughly equal numbers (average ratio CD8:CD4 = 0.83). The average ratio of CD8:CD4 ratio in EPN, GBM and MED was progressively lower (3.59, 2.83 and 2.78 respectively). The higher CD8:CD4 ratios in tumor compared to NT brain suggests a preferential recruitment of CD8 T-cells to the tumor microenvironment rather than a non-specific migration of CD8 and CD4 T-cells from adjacent normal brain. Activated/memory T-cell marker CD45RO was significantly higher (p<0.05) in PA and GBM than NT (1.9 and 2.1 fold respectively) (Figure 2C). EPN also demonstrated CD45RO expression on CD8 T-cells that was elevated above NT and this difference showed a trend toward significance (p=0.077). CD8 T-cells CD45RO was not significantly elevated in MED compared to NT. CD4 T-cells in all tumor types exhibited modestly elevated CD45RO compared to NT (Figure 2D).
Evidence for an active phenotype in the majority of pediatric brain tumor-infiltrating T-cells was presented by analysis of PD-1 expression, a marker of T-cell inactivation (Figure 2E,F). CD8 and CD4 T-cells in NT were predominantly expressed PD-1 (72.4% and 81.9% respectively). Compared to NT, all tumor types exhibited significantly (p<0.05) lower PD-1 expression on both CD8 and CD4 T-cells, apart from GBM, which trended toward significance with average expression of PD-1 on CD8 and CD4 T-cells 1.9- and 1.5-fold lower than NT (p<0.1) (Figure 2 E,F). Again PA exhibited the most activated immune cell phenotype exhibiting the lowest PD-1 expression in both CD8 and CD4 T-cells of any tumor analyzed.
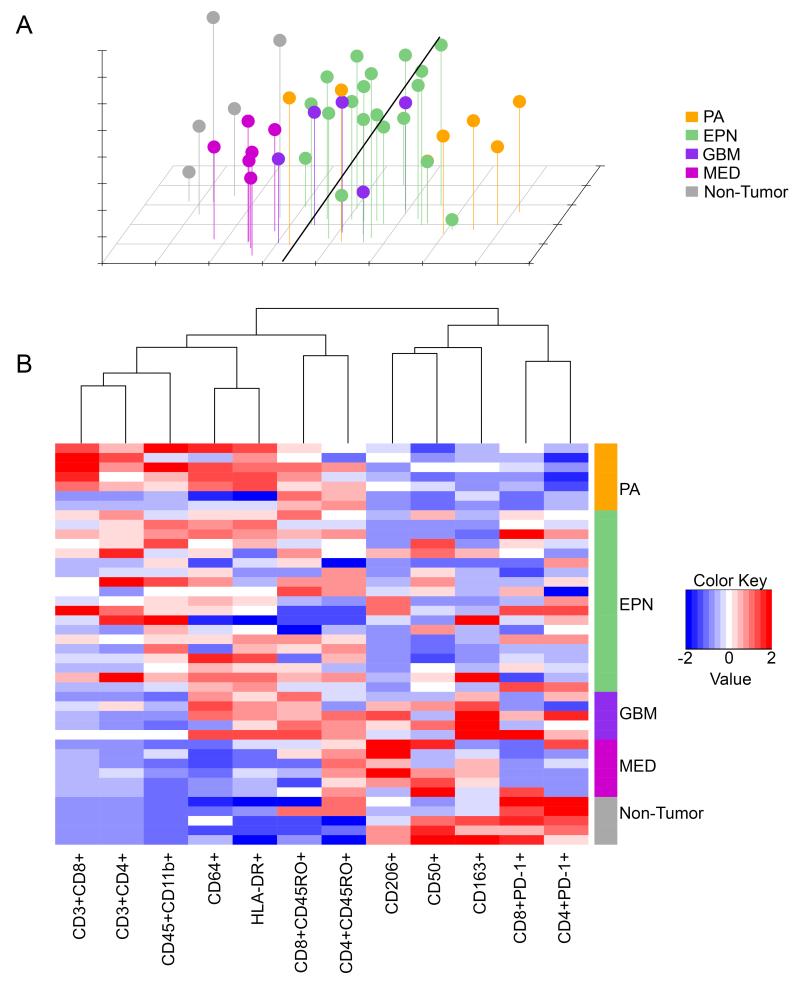
Unbiased clustering analyses of immune markers emphasizes distinct tumor type-specific immunophenotypes
The multiple immune markers measured in this study were combined to create a data matrix that was subjected to unbiased clustering analyses. Principle components analysis (PCA) demonstrated that based on these immune markers, partial grouping of samples occurs depending on tissue type (Figure 3A). The MED group overlapped with the NT group, with GBM, EPN and PA groups moving progressively farther away from the NT group. GBM, EPN and PA clusters showed some overlap with each other, demonstrating varying levels of heterogeneity within groups.
Figure 3. Clustering analyses of tumor-infiltrating leukocyte flow cytometry data in pediatric brain tumors.
(A) principal components analysis and (B) unsupervised hierarchical clustering of infiltrating leukocyte flow cytometry data in PA, EPN, GBM, MED and NT. Abbreviations: PA, pilocytic astrocytoma; EPN, ependymoma; GBM, glioblastoma; MED, medulloblastoma; NT, non-tumor.
Unbiased hierarchical clustering of the 12 myeloid and T-cell immune markers was performed to identify relationships between expression patterns of immune markers across all samples (Figure 3B). This analysis identified two distinct groups which corresponded to markers of activated and suppressed phenotypes respectively and not according to whether markers were myeloid or T-cell specific. This analysis also identified strong correlation between some markers, most notably HLA-DR and CD64, which showed the closest grouping of any marker used in the present study. The data matrix was further examined to directly measure correlations between myeloid markers HLA-DR, CD64, CD50, CD163 and CD206 across all samples. This analysis showed that HLA-DR and CD64 had the strongest correlation of any myeloid marker (Pearson’s R=0.79, p<0.0001), in accord with the clustering analysis. This analysis also demonstrated an inverse correlation of HLA-DR with CD50 (R= −0.33, p=0.032) and a trend to inverse correlation of HLA-DR with CD206 (R= −0.28, p=0.078). CD50 positively correlated with CD163 (R=0.31, p=0.045) and CD206 (R=0.31, p=0.044). The positive and inverse correlations observed between myeloid markers prompted re-analysis of FACS data to identify whether those markers identified distinct sub-populations of cells within the myeloid cell population. This analysis demonstrated that none of the studied markers was expressed exclusively. HLA-DR and CD64 showed the strongest co-expression, consistent with clustering analysis, however CD50, CD163 and CD206 expressing populations each showed expression of HLA-DR and CD64 (data not shown). This is consistent with previous studies that demonstrated overlapping marker expression across polarized myeloid phenotypes (18).
As observed by PCA, clustering analysis of FACS data (Figure 3B) illustrated the varying activation phenotype between sample types, the distinction between PA (high activation) and non-tumor brain (low activation) being particularly strong. Again, EPN, GBM and MED exhibit an immune phenotype that is progressively less distinct from NT, with GBM expressing markers of both activated and non-activated phenotypes.
Gene expression analysis distinguishes pediatric brain tumor-specific immunophenotypes
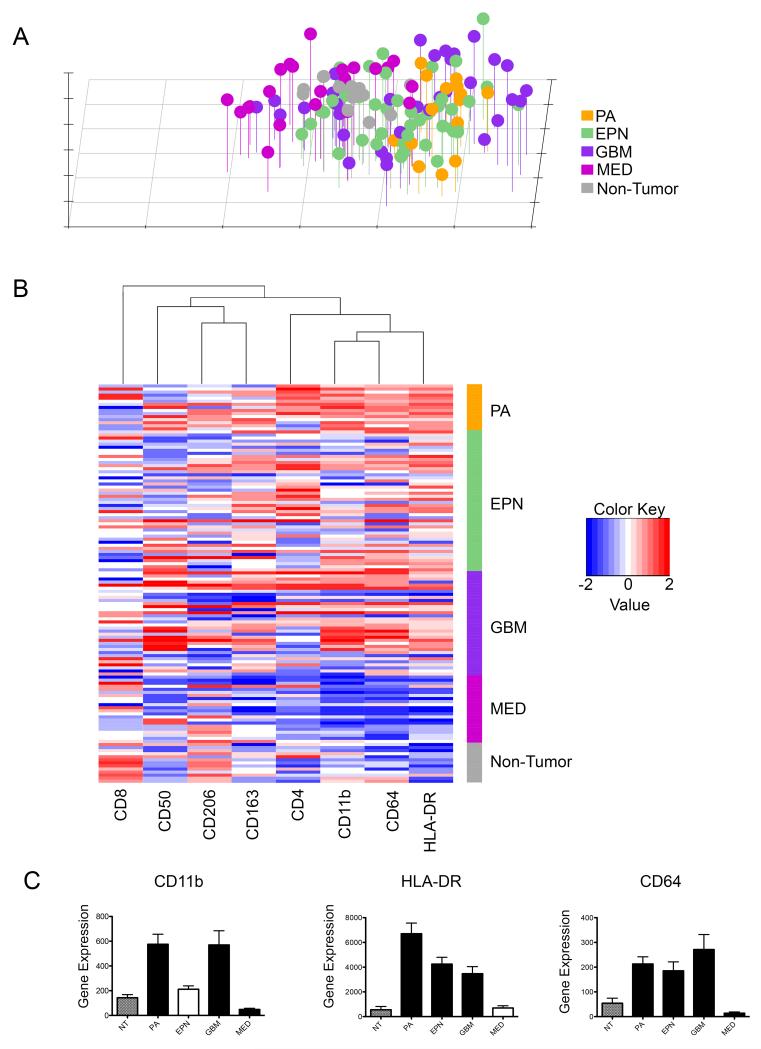
Gene expression microarray data was mined to determine whether immunophenotypes identified by FACS were reflected by corresponding immune marker gene expression in microarray data expression profiles of a larger cohort of pediatric brain tumor samples.
PCA analysis of immune marker gene expression correlated with results of the same analysis using FACS data, the biggest difference being that NT samples were less distinct from other brain tumor types (Figure 4A). The strongest separation was between MED and PA, as was seen with FACS immune markers. EPN and GBM clusters exhibited a more spread out pattern that overlapped with each other and to some extent with PA, but was centered between PA and MED. Hierarchical clustering demonstrated that activation (HLA-DR and CD64) and suppression markers (CD50, CD163 and CD206) clustered separately into two groups, as was seen with FACS data (Figure 4B). As was seen with clustering analysis of FACS data, gene expression of activation markers between PA and NT was distinct, with EPN, GBM and MED expressing progressively lower levels of activation marker genes. Thus the tumor type specific immunophenotypes observed by FACS analysis were recapitulated by gene expression analysis of a larger cohort of tumor samples.
Figure 4. Clustering analyses of immune marker gene expression data corresponding to flow cytometry markers in pediatric brain tumors.
(A) principal components analysis and (B) unsupervised hierarchical clustering of immune marker gene expression data in PA, EPN, GBM, MED and NT. (C) Average normalized gene expression analysis for immune markers CD11b, HLA-DR and CD64 in PA, EPN, GBM, MED and NT. Black bars represent tumor subset with significant differential expression (p<0.05) compared NT (error bars = SD). Abbreviations: PA, pilocytic astrocytoma; EPN, ependymoma; GBM, glioblastoma; MED, medulloblastoma; NT, non-tumor.
To determine if gene expression of specific immune markers can predict immune environment within a tumor, myeloid gene expression from the microarray database was correlated with corresponding population percentages obtained by FACS. Linearized gene expression and FACS data was averaged for each tissue type and correlated using Pearson’s correlation. CD11b gene expression (ITGAM; Affymetrix probeset ID 205786_s_at), was positively correlated with FACS data (R=0.83, p=0.044). With respect to the functional markers, significant correlation was observed with immune activation markers but not those associated with immune suppression. CD64 (FCGR1A/FCGR1C; 216950_s_at) had the best correlation (R= 0.91, p=0.057) for gene expression by microarray and expression level by FACS. Although not exclusively expressed by immune cells HLA-DR (210982_s_at) also had a positive statistically significant correlation with FACS data (R=0.84, p=0.037). In summary, myeloid activation marker gene expression microarray data reiterated FACS results, demonstrating that different brain tumor types confer distinct infiltrating myeloid cell phenotypes (Figure 4C). Based on these results, extent of myeloid cell infiltration and activation phenotype can be estimated by expression of these key markers in independent gene expression microarray datasets. No significant correlation between gene expression and FACS data for T-cell markers CD8or CD4 was observed, likely due to the relative rarity of infiltrating T-cells.
The use of control tissue from lateral temporal and frontal lobes the FACS study described above means that there is no location matched control NT for those tumors arising in the cerebellum, namely MED and approximately 50% of PA, due to the rare surgical availability of such tissue. This potential pitfall was partly addressed by estimation of myeloid infiltration and activation between different compartments of normal brain, specifically cerebrum, cerebellum and brainstem/thalamus using samples available in the gene expression microarray database. No significant difference in expression of immune markers CD11b, HLA-DR or CD64 was observed between cerebrum (n=8), cerebellum (n=2) or brainstem/thalamus (n=3)(Supplemental figure 3).
DISCUSSION
In the present study, comparative analysis of tumor-infiltrating leukocytes identified distinct immunophenotypes for PA, EPN, GBM and MED, the four most common pediatric brain tumor types. Although the nature of this study is descriptive, the use of well established functional immune markers allows for inference of the functional status of tumor-infiltrating immune cells. The vast majority of existing research in the immunobiology of brain tumors has been conducted in adult GBM, from which there is a consensus that GBM exhibit an immunosuppressed or actively immunosuppressive phenotype. The present study provides context to this body of knowledge, finding that the GBM-specific immunosuppressed phenotype does not necessarily extend to all other brain tumor types. In brief, it identified that PA and EPN, by comparison with GBM, MED and NT, have more leukocyte infiltration, and that these cells express markers of a more activated phenotype. Conversely, MED are less infiltrated with leukocytes and express markers that suggest a more immunosuppressed phenotype than GBM.
PA and EPN exhibited high myeloid and lymphocyte infiltration levels that were accompanied by high activation marker expression. Few published studies of tumor-infiltrating leukocytes in EPN exist. In a previous gene expression microarray study by our laboratory the predominant factor associated with improved survival in EPN was overexpression of immune function genes (1). Expression of the majority of those genes was found to be restricted to tumor-infiltrating leukocytes, and that the abundance of tumor-infiltrating leukocytes, specifically myeloid and CD4 T-cells, was associated with improved survival, corresponded to gene expression data. Based on these data it was inferred that the host immune system plays an anti-tumor role in EPN patients. In the present study, the identification of an activated EPN tumor-infiltrating leukocyte phenotype supports this hypothesis. As in EPN, PA is similarly little studied with respect to immune characteristics. In a recent histology-based tumor-infiltrating leukocytes study PA were used as a control against which to compare GBM (30). This semi-quantitative analysis concluded that significant differences existed in immunophenotype between diagnoses, consistent with the findings of the present study. An earlier gene expression microarray study of PA identified immune gene expression as a distinguishing factor in comparison with higher grade astrocytomas and normal brain, again consistent with the present study (31). As in EPN, it can be inferred that infiltrating immune cells in PA may also be exerting significant control of tumor growth, consistent with regression of minimal residual tumor in PA that are cured by surgical debulking alone.
Although fewer in number than PA or EPN, the majority of GBM-infiltrating myeloid cells were activated, expressing key mediators of cellular and humoral adaptive immune responses, HLA-DR and CD64 respectively. However, activity of these cells may be attenuated by co-expression of CD163 and CD206. Consistent with previously documented T-cell immunosuppression in GBM, the present study found that CD8 and CD4 T-cell infiltration was relatively infrequent. Additionally PD-1 expression, a marker of T-cell inactivation, was higher in GBM than all other tumor types studied.
Infiltrating myeloid cells in MED were rarer and potentially more immunosuppressed phenotype than GBM in the present analysis. MED exhibited a trend to an alternatively activated M2 phenotype, having lower levels of M1 marker CD64 and higher M2 marker CD206 than any other tumor or NT. As with PA and EPN, minimal published data exists with respect to the immune status of MED. A single immunohistochemistry (IHC) study identified tumor-infiltrating leukocytes in MED, but did not identify any difference in frequency of infiltration between MED and other brain tumor types including astrocytoma (32). This lack of distinction between diagnoses is likely a function of the less quantitative nature of histology versus FACS.
Tumor-infiltrating leukocytes represent a source of endogenous effector cells that can potentially be exploited therapeutically. The present study has implications for each of the 3 main categories of immunotherapeutic approach – passive, adoptive and active. Passive immunotherapy approaches are currently dominated by the use of therapeutic Abs. Ab-dependent cell-mediated cytotoxicity is considered to be the dominant mechanism of the in-vivo activity of Abs against tumors, and is particularly dependent on engagement of Ab with Fc-γ Rs expressed by immune effector cells (33). The results of the present study are particularly pertinent in this respect, identifying abundant expression of Fc-γ R CD64 on the majority of tumor-infiltrating myeloid cells in PA, EPN and GBM. Furthermore, CD64 has previously been implicated in brain tumor anti-tumor activity by its association with improved outcome in a gene expression microarray study of EPN (1). The extent of CD64 expression in brain tumor-infiltrating immune cells had not previously been appreciated partly because of technical limitations; specifically that CD64 cannot be measured by IHC in formalin-fixed paraffin-embedded material. The finding that PA-, EPN- and GBM-infiltrating myeloid cells are potentially primed for Ab-mediated tumor killing is therefore novel, and suggests that these tumor types might respond favorably to Ab-mediated immunotherapy.
Although the majority of adoptive cell and vaccine clinical trials in brain tumors are directed at adult GBM, a growing number of pediatric trials are being performed that include tumors other than GBM (34-36). Based on the findings of the present study, the less immunosuppressed PA and EPN would in theory provide a more permissive tumor micro-environment for such immunotherapeutic approaches. Conversely, MED would present a less favorable immunophenotype, an inference which is supported by preliminary results of a pediatric dendritic cell vaccine trial in which glial tumors responded more favorably than MED and primitive neuro-ectodermal tumors (34). The use of ex-vivo-expanded tumor-infiltrating autologous T-cells as adoptive T-cell therapy has been most effectively applied to malignant melanoma and treatment of advanced stage melanoma has demonstrated a 50% objective response rate (37). The more abundant T-cell infiltrations found in PA and EPN would facilitate adoptive T-cell immunotherapy for these tumor types, more so than for GBM or MED.
The distinct immunophenotypes across pediatric brain tumor types should be considered when developing immunotherapy approaches. Recently, a number of strategies that improve the immunogenicity of Ab-, cell- and vaccine-based immunotherapies have resulted in improved survival in refractory tumor clinical trials. Recent successes include the use of Ab-mediated depletion of immunosuppressive CTLA-4 cells in melanoma (38), chimeric antigen receptor-modified T-cell therapy in lymphoid leukemia (39) and tumor antigen/GM-CSF fusion protein-primed dendritic cell vaccine in prostate cancer (40). The novel data generated by this study will aid in adapting new immunotherapeutic approaches to the treatment of pediatric brain tumors and advancing these to clinical trials.
Supplementary Material
ACKNOWLEDGEMENTS
Jena French and the UC Denver FACS Core provided expertise in multicolor FACS. This study was presented at the 2013 Pediatric Neuro-Oncology Basic and Translational Research Conference.
Source of support
This work was supported by the National Institutes of Health (grant no. R01 CA140614) and the Morgan Adams Foundation.
Abbreviations
- EPN
ependymoma
- GBM
glioblastoma
- gcRMA
guanine cytosine Robust Multiarray Average
- MED
medulloblastoma
- NT
non-tumor brain
- PA
pilocytic astrocytoma
- PCA
principal components analysis
- PD-1
programmed cell death protein 1
REFERENCES
- 1.Donson AM, Birks DK, Barton VN, Wei Q, Kleinschmidt-Demasters BK, Handler MH, Waziri AE, Wang M, Foreman NK. Immune gene and cell enrichment is associated with a good prognosis in ependymoma. J Immunol. 2009;183:7428–7440. doi: 10.4049/jimmunol.0902811. [DOI] [PubMed] [Google Scholar]
- 2.Donson AM, Birks DK, Schittone SA, Kleinschmidt-Demasters BK, Sun DY, Hemenway MF, Handler MH, Waziri AE, Wang M, Foreman NK. Increased Immune Gene Expression and Immune Cell Infiltration in High-Grade Astrocytoma Distinguish Long-Term from Short-Term Survivors. J Immunol. 2012;189:1920–1927. doi: 10.4049/jimmunol.1103373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wiegering V, Eyrich M, Rutkowski S, Wolfl M, Schlegel PG, Winkler B. TH1 predominance is associated with improved survival in pediatric medulloblastoma patients. Cancer Immunol Immunother. 2011;60:693–703. doi: 10.1007/s00262-011-0981-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Martin R, Haendler B, Hofer-Warbinek R, Gaugitsch H, Wrann M, Schlusener H, Seifert JM, Bodmer S, Fontana A, Hofer E. Complementary DNA for human glioblastoma-derived T cell suppressor factor, a novel member of the transforming growth factor-beta gene family. Embo J. 1987;6:3673–3677. doi: 10.1002/j.1460-2075.1987.tb02700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rodrigues JC, Gonzalez GC, Zhang L, Ibrahim G, Kelly JJ, Gustafson MP, Lin Y, Dietz AB, Forsyth PA, Yong VW, Parney IF. Normal human monocytes exposed to glioma cells acquire myeloid-derived suppressor cell-like properties. Neuro Oncol. 2010;12:351–365. doi: 10.1093/neuonc/nop023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hishii M, Nitta T, Ishida H, Ebato M, Kurosu A, Yagita H, Sato K, Okumura K. Human glioma-derived interleukin-10 inhibits antitumor immune responses in vitro. Neurosurgery. 1995;37:1160–1166. doi: 10.1227/00006123-199512000-00016. discussion 1166-1167. [DOI] [PubMed] [Google Scholar]
- 7.Komohara Y, Ohnishi K, Kuratsu J, Takeya M. Possible involvement of the M2 anti-inflammatory macrophage phenotype in growth of human gliomas. J Pathol. 2008;216:15–24. doi: 10.1002/path.2370. [DOI] [PubMed] [Google Scholar]
- 8.Parsa AT, Waldron JS, Panner A, Crane CA, Parney IF, Barry JJ, Cachola KE, Murray JC, Tihan T, Jensen MC, Mischel PS, Stokoe D, Pieper RO. Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nat Med. 2007;13:84–88. doi: 10.1038/nm1517. [DOI] [PubMed] [Google Scholar]
- 9.Choi C, Xu X, Oh JW, Lee SJ, Gillespie GY, Park H, Jo H, Benveniste EN. Fas-induced expression of chemokines in human glioma cells: involvement of extracellular signal-regulated kinase 1/2 and p38 mitogen-activated protein kinase. Cancer Res. 2001;61:3084–3091. [PubMed] [Google Scholar]
- 10.Sippel TR, White J, Nag K, Tsvankin V, Klaassen M, Kleinschmidt-DeMasters BK, Waziri A. Neutrophil degranulation and immunosuppression in patients with GBM: restoration of cellular immune function by targeting arginase I. Clin Cancer Res. 2011;17:6992–7002. doi: 10.1158/1078-0432.CCR-11-1107. [DOI] [PubMed] [Google Scholar]
- 11.Mosser DM. The many faces of macrophage activation. J Leukoc Biol. 2003;73:209–212. doi: 10.1189/jlb.0602325. [DOI] [PubMed] [Google Scholar]
- 12.Edin S, Wikberg ML, Dahlin AM, Rutegard J, Oberg A, Oldenborg PA, Palmqvist R. The distribution of macrophages with a M1 or M2 phenotype in relation to prognosis and the molecular characteristics of colorectal cancer. PLoS One. 2012;7:e47045. doi: 10.1371/journal.pone.0047045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Braumuller H, Wieder T, Brenner E, Assmann S, Hahn M, Alkhaled M, Schilbach K, Essmann F, Kneilling M, Griessinger C, Ranta F, Ullrich S, Mocikat R, Braungart K, Mehra T, Fehrenbacher B, Berdel J, Niessner H, Meier F, van den Broek M, Haring HU, Handgretinger R, Quintanilla-Martinez L, Fend F, Pesic M, Bauer J, Zender L, Schaller M, Schulze-Osthoff K, Rocken M. T-helper-1-cell cytokines drive cancer into senescence. Nature. 2013;494:361–365. doi: 10.1038/nature11824. [DOI] [PubMed] [Google Scholar]
- 14.Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348–2357. [PubMed] [Google Scholar]
- 15.Heusinkveld M, van der Burg SH. Identification and manipulation of tumor associated macrophages in human cancers. J Transl Med. 2011;9:216. doi: 10.1186/1479-5876-9-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Monte L, Reni M, Tassi E, Clavenna D, Papa I, Recalde H, Braga M, Di Carlo V, Doglioni C, Protti MP. Intratumor T helper type 2 cell infiltrate correlates with cancer-associated fibroblast thymic stromal lymphopoietin production and reduced survival in pancreatic cancer. J Exp Med. 2011;208:469–478. doi: 10.1084/jem.20101876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Waziri A, Killory B, Ogden AT, 3rd, Canoll P, Anderson RC, Kent SC, Anderson DE, Bruce JN. Preferential in situ CD4+CD56+ T cell activation and expansion within human glioblastoma. J Immunol. 2008;180:7673–7680. doi: 10.4049/jimmunol.180.11.7673. [DOI] [PubMed] [Google Scholar]
- 18.Ambarus CA, Krausz S, van Eijk M, Hamann J, Radstake TR, Reedquist KA, Tak PP, Baeten DL. Systematic validation of specific phenotypic markers for in vitro polarized human macrophages. J Immunol Methods. 2012;375:196–206. doi: 10.1016/j.jim.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 19.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 20.Estecha A, Aguilera-Montilla N, Sanchez-Mateos P, Puig-Kroger A. RUNX3 regulates intercellular adhesion molecule 3 (ICAM-3) expression during macrophage differentiation and monocyte extravasation. PLoS One. 2012;7:e33313. doi: 10.1371/journal.pone.0033313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zahuczky G, Kristof E, Majai G, Fesus L. Differentiation and glucocorticoid regulated apopto-phagocytic gene expression patterns in human macrophages. Role of Mertk in enhanced phagocytosis. PLoS One. 2011;6:e21349. doi: 10.1371/journal.pone.0021349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fridman WH, Pages F, Sautes-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. 2012;12:298–306. doi: 10.1038/nrc3245. [DOI] [PubMed] [Google Scholar]
- 23.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parsa AT, Waldron JS, Panner A, Crane CA, Parney IF, Barry JJ, Cachola KE, Murray JC, Tihan T, Jensen MC, Mischel PS, Stokoe D, Pieper RO. Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nat Med. 2007;13:84–88. doi: 10.1038/nm1517. [DOI] [PubMed] [Google Scholar]
- 25.Zeng J, See AP, Phallen J, Jackson CM, Belcaid Z, Ruzevick J, Durham N, Meyer C, Harris TJ, Albesiano E, Pradilla G, Ford E, Wong J, Hammers HJ, Mathios D, Tyler B, Brem H, Tran PT, Pardoll D, Drake CG, Lim M. Anti-PD-1 blockade and stereotactic radiation produce long-term survival in mice with intracranial gliomas. Int J Radiat Oncol Biol Phys. 2013;86:343–349. doi: 10.1016/j.ijrobp.2012.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Agata Y, Kawasaki A, Nishimura H, Ishida Y, Tsubata T, Yagita H, Honjo T. Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int Immunol. 1996;8:765–772. doi: 10.1093/intimm/8.5.765. [DOI] [PubMed] [Google Scholar]
- 27.Birks DK, Donson AM, Patel PR, Dunham C, Muscat A, Algar EM, Ashley DM, Kleinschmidt-Demasters BK, Vibhakar R, Handler MH, Foreman NK. High expression of BMP pathway genes distinguishes a subset of atypical teratoid/rhabdoid tumors associated with shorter survival. Neuro Oncol. 2011;13:1296–1307. doi: 10.1093/neuonc/nor140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu Z, Irizarry RA, Gentleman R, Martinez-Murillo F, Spencer F. A Model-Based Background Adjustment for Oligonucleotide Expression Arrays. Journal of the American Statistical Association. 2004;99:909–917. [Google Scholar]
- 29.Diederichsen AC, Hjelmborg J, Christensen PB, Zeuthen J, Fenger C. Prognostic value of the CD4+/CD8+ ratio of tumour infiltrating lymphocytes in colorectal cancer and HLA-DR expression on tumour cells. Cancer Immunol Immunother. 2003;52:423–428. doi: 10.1007/s00262-003-0388-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang I, Han SJ, Sughrue ME, Tihan T, Parsa AT. Immune cell infiltrate differences in pilocytic astrocytoma and glioblastoma: evidence of distinct immunological microenvironments that reflect tumor biology. J Neurosurg. 2011;115:505–511. doi: 10.3171/2011.4.JNS101172. [DOI] [PubMed] [Google Scholar]
- 31.Huang H, Hara A, Homma T, Yonekawa Y, Ohgaki H. Altered expression of immune defense genes in pilocytic astrocytomas. J Neuropathol Exp Neurol. 2005;64:891–901. doi: 10.1097/01.jnen.0000183345.19447.8e. [DOI] [PubMed] [Google Scholar]
- 32.Bodey B, Bodey B, Jr., Siegel SE, Kaiser HE. Immunocytochemical detection of leukocyte-associated and apoptosis-related antigen expression in childhood brain tumors. Crit Rev Oncol Hematol. 2001;39:3–16. doi: 10.1016/s1040-8428(01)00119-6. [DOI] [PubMed] [Google Scholar]
- 33.Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytotoxicity against tumor targets. Nat Med. 2000;6:443–446. doi: 10.1038/74704. [DOI] [PubMed] [Google Scholar]
- 34.Ardon H, De Vleeschouwer S, Van Calenbergh F, Claes L, Kramm CM, Rutkowski S, Wolff JE, Van Gool SW. Adjuvant dendritic cell-based tumour vaccination for children with malignant brain tumours. Pediatr Blood Cancer. 2010;54:519–525. doi: 10.1002/pbc.22319. [DOI] [PubMed] [Google Scholar]
- 35.Peres E, Wood GW, Poulik J, Baynes R, Sood S, Abidi MH, Klein J, Bhambhani K, Dansey R, Abella E. High-dose chemotherapy and adoptive immunotherapy in the treatment of recurrent pediatric brain tumors. Neuropediatrics. 2008;39:151–156. doi: 10.1055/s-0028-1093333. [DOI] [PubMed] [Google Scholar]
- 36.Caruso DA, Orme LM, Neale AM, Radcliff FJ, Amor GM, Maixner W, Downie P, Hassall TE, Tang ML, Ashley DM. Results of a phase 1 study utilizing monocyte-derived dendritic cells pulsed with tumor RNA in children and young adults with brain cancer. Neuro Oncol. 2004;6:236–246. doi: 10.1215/S1152851703000668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dudley ME, Wunderlich JR, Yang JC, Sherry RM, Topalian SL, Restifo NP, Royal RE, Kammula U, White DE, Mavroukakis SA, Rogers LJ, Gracia GJ, Jones SA, Mangiameli DP, Pelletier MM, Gea-Banacloche J, Robinson MR, Berman DM, Filie AC, Abati A, Rosenberg SA. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol. 2005;23:2346–2357. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbe C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365:725–733. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims RB, Xu Y, Frohlich MW, Schellhammer PF. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.