Abstract
Here, for the first time, we test a novel hypothesis that systemic treatment of stroke with exosomes derived from multipotent mesenchymal stromal cells (MSCs) promote neurovascular remodeling and functional recovery after stroke in rats. Adult male Wistar rats were subjected to 2 hours of middle cerebral artery occlusion (MCAo) followed by tail vein injection of 100 μg protein from MSC exosome precipitates or an equal volume of vehicle phosphate-buffered saline (PBS) (n=6/group) 24 hours later. Animals were killed at 28 days after stroke and histopathology and immunohistochemistry were employed to identify neurite remodeling, neurogenesis, and angiogenesis. Systemic administration of MSC-generated exosomes significantly improved functional recovery in stroke rats compared with PBS-treated controls. Axonal density and synaptophysin-positive areas were significantly increased along the ischemic boundary zone of the cortex and striatum in MCAo rats treated with exosomes compared with PBS control. Exosome treatment significantly increased the number of newly formed doublecortin (a marker of neuroblasts) and von Willebrand factor (a marker of endothelial cells) cells. Our results suggest that intravenous administration of cell-free MSC-generated exosomes post stroke improves functional recovery and enhances neurite remodeling, neurogenesis, and angiogenesis and represents a novel treatment for stroke.
Keywords: exosome, functional recovery, mesenchymal stromal cell (MSC), microRNA, neurovascular plasticity, stroke
Introduction
Exosomes are endosomal origin small-membrane vesicles with a size of 40 to 100 nm in diameter.1 They are generated by many cell types and contain functional messenger RNAs and micro RNAs (miRNAs), as well as proteins.2 Exosomes are well suited for small functional molecule delivery3 and increasing evidence indicates that they have a pivotal role in cell-to-cell communication.4 Recent studies indicate that exosomes and microvesicles derived from multipotent mesenchymal stromal cells (MSCs) have therapeutic promise in cardiovascular, liver, and kidney diseases.5, 6, 7 Mesenchymal stromal cells decrease neurologic deficits in rodents after stroke by increasing neurite remodeling, neurogenesis, and angiogenesis.8 We have previously demonstrated that functional miRNAs are transferred between MSCs and neural cells via exosomes, and that exosome-encapsulated transfer of miRNAs promotes neurite remodeling and functional recovery of stroke in rat.9, 10 These data suggest that MSC-generated exosomes enhance the stroke recovery process. Thus, it is reasonable to test the hypothesis that exosomes alone when systemically administered to an animal with stroke improve functional outcome, with therapeutic benefit reflecting that observed with systemically administered MSCs. As a proof-of-principle study, we administer cell-free exosomes generated by MSCs to rats subjected to middle cerebral artery occlusion (MCAo) and investigate functional recovery as well as the mechanisms that underlie it.
Materials and Methods
All experimental procedures were carried out in accordance with the NIH Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of Henry Ford Hospital.
Mesenchymal Stromal Cells Exosome Generation and Collection
Bone marrow from adult male Wistar rats was mechanically dissociated, and the cells were washed and suspended in culture medium. Three days later, cells that tightly adhered to the plastic flasks were considered as P0 MSCs.11 Mesenchymal stromal cells were conventionally cultured with α-modified MEM medium (Hyclone, Logan, UT, USA) containing 20% fetal bovine serum (Gibco Laboratory, Grand Island, NY, USA) and penicillin–streptomycin on 75 cm2 tissue culture flasks (Corning, St Louis, MO, USA). For the exosome isolation, conventional culture medium was replaced with an exosome-depleted fetal bovine serum-contained (EXO-FBS-250 A-1, System Biosciences, Mountain View, CA, USA) medium when the cells reached 60% to 80% confluence, and the MSCs were cultured for an additional 24 hours. The media were then collected and exosomes were isolated via multistep centrifuging, as previously described.10 We quantitated the exosomes by measuring the total protein concentration, assessed by the micro Bicinchoninic Acid protocol (Pierce, Rockford, IL, USA).12
Middle Cerebral Artery Occlusion Model and Treatment
Adult male Wistar rats (weighing 270 to 300 g, n=12) purchased from Charles River (Wilmington, MA, USA) were subjected to 2 hours of right MCAo using suture intraluminal vascular occlusion, as modified in our laboratory.13 Twenty-four hours after induction of stroke, 100 μg total protein of MSC-generated exosomes in 0.5 mL phosphate-buffered saline (PBS, GIBCO) or 0.5 mL PBS were injected into the tail vein (n=6/group). To label cell proliferation, rats received intraperitoneal injections of the 5-bromodeoxyuridine (BrdU, 50 mg/kg) daily starting 24 hours after MCAo for 14 days.8
Behavioral Tests
For functional recovery evaluation, a Foot-fault test14 and a modified neurologic severity score (mNSS)15 were carried out before MCAo, and at 1, 3, 7, 14, 21, and 28 days after MCAo by an investigator masked to the treatments.
Lesion Volume Measurement
Rats were killed 28 days after MCAo by transcardial perfusion with saline, followed by perfusion and immersion in 4% paraformaldehyde before being embedded in paraffin. Seven coronal sections of forebrain tissues were processed and stained with hematoxylin and eosin for calculation of the lesion volume that was traced by using the Global Laboratory image analysis system (Data Translation). To measure the lesion volume, the MicroComputer Imaging Device (MCID, Imaging Research, St Catharines, Ontario, Canada) was employed to analyze the stained coronal sections, and the indirect lesion area was calculated, in which the intact area of the ipsilateral hemisphere was subtracted from the area of the contralateral hemisphere. Lesion volume was presented as a volume percentage of the lesion compared with the contralateral hemisphere.16
Histopathology and Immunohistochemistry
For histopathological and immunohistochemical staining, a standard paraffin block was obtained from the center of the lesion (bregma −1 to +1 mm), and a series of 8-μm-thick sections were prepared. To determine neurite remodeling in the ischemic boundary zone (IBZ), Bielschowsky silver (stains neuronal processes17) combined with Luxol fast blue (stains myelin sheath18) histochemistry staining as well as immunostaining with antibodies against the phosphorylated epitope of neurofilament heavy polypeptide, Clone SMI 31 (SMI 31, reacts broadly with thick and thin axons and some dendrites19), and synaptophysin (a marker for synapses, as synaptophysin is ubiquitously present at the synapses20) were employed, respectively. The newly generated cells in the IBZ were detected by immunostaining with the antibody against BrdU. Briefly, for immunostaining, adjacent brain sections were incubated with the primary antibodies against SMI 31 (dilution 1:500, Abcam, Cambridge, MA, USA; ab82259), synaptophysin (dilution 1:100, Chemicon, Billerica, MA, USA; MAB5258), and BrdU (1:100; Boehringer Mannheim, Indianapolis, IN, USA), followed by corresponding horseradish peroxidase conjugated to secondary antibodies and 3,3′-diaminobenzidine developing, respectively. To detect neurogenesis and angiogenesis in the IBZ, double immunofluorescent staining for BrdU with doublecortin (DCX, a marker of neuroblasts, 1:200; Santa Cruz Biotechnology, Santa Cruz, CA, USA), and von Willebrand factor (vWF, a marker of endothelial cells, 1:200, Santa Cruz Biotechnology), followed by their corresponding second antibody staining (fluorescein isothiocyanate labeled for BrdU and Cy3 labeled for DCX and vWF) were employed.
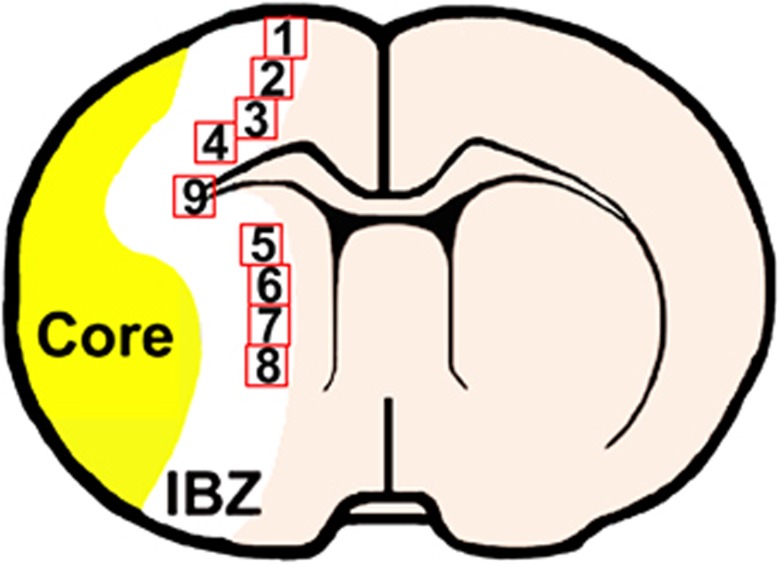
Positive staining within nine areas (see the schematic diagram at Figures 1 and 4 from the cortex, four from the striatum, and one from the corpus callosum) selected along the IBZ in these groups was digitized under a × 40 objective (BX40; Olympus Optical, Center Valley, PA, USA) using a 3-CCD color video camera (DXC-970MD, Sony, Teaneck, NJ, USA) interfaced with the MCID software.21 For the analysis of neurite remodeling, the area percentage of positive staining signals within the IBZ based on evaluation of an average of three histology slides (8-μm thick, every 10-slide interval) from the standard block of each animal was analyzed using the MCID software. For the quantification of new generated cells and double staining for neurogenesis and angiogenesis, the BrdU-labeled cells in each field and the percentage of double-stained cells were counted and calculated to present indices of neurogenesis and angiogenesis.
Figure 1.
Schematic diagram indicates the nine areas selected.
Statistical Analysis
Data were summarized and presented using mean±standard deviation (s.d.). The global test using generalized estimating equation was employed to test the group difference on functional recovery measured from multiple behavior tests.22 The differences between mean values were evaluated with the two-tailed Student's t-test (for 2 groups). All statistical analyses were conducted using SAS software (version 9.2; SAS Institute, Cary, NC, USA).
Results
Neurologic Outcome and Lesion Volume
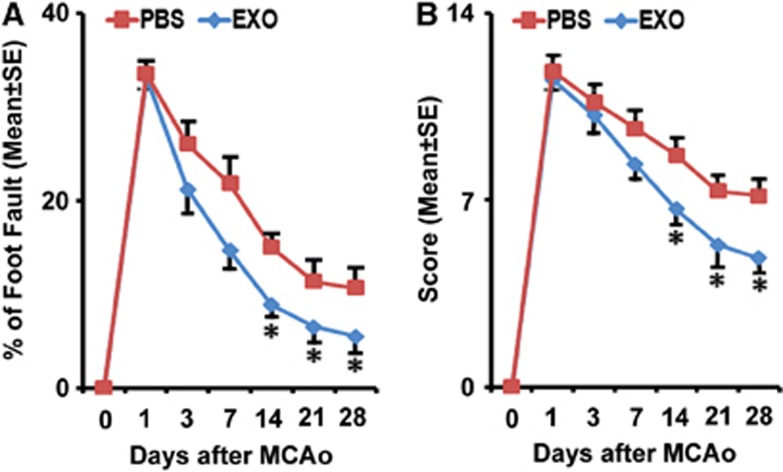
The sensorimotor performance of the stroke severity was assessed by measuring foot-fault and mNSS. All rats exhibited severe functional deficit at postoperative day 1, followed by gradual improvement within the 4-week experimental course. Compared with the PBS-treated group, animals that received exosomes exhibited significant functional enhancement in the foot-fault test (Figure 2A) and mNSS (Figure 2B) starting 2 weeks after treatment (P<0.05).
Figure 2.
Exosome treatment improves neurologic outcome. Foot-fault test (A) and modified neurologic severity score (B) data show that rats that received exosomes have significant functional enhancement starting 2 weeks after treatment, respectively. *P<0.05, mean±s.d., n=6/group. MCAo, middle cerebral artery occlusion; PBS, phosphate-buffered saline.
The ischemic infarct included the forelimb area of the sensorimotor cortex, striatum, and the supraoptic area in this MCAo model. At 28 days after the onset of stroke, the ischemic lesion volumes of the two groups were 32.9%±3.34% (PBS treatment) and 31.1%±3.79% (exosome treatment). There was no significant difference on lesion volume between the control and exosome treatment groups.
Exosomes Increase Neurite Remodeling in the Ischemic Boundary Zone
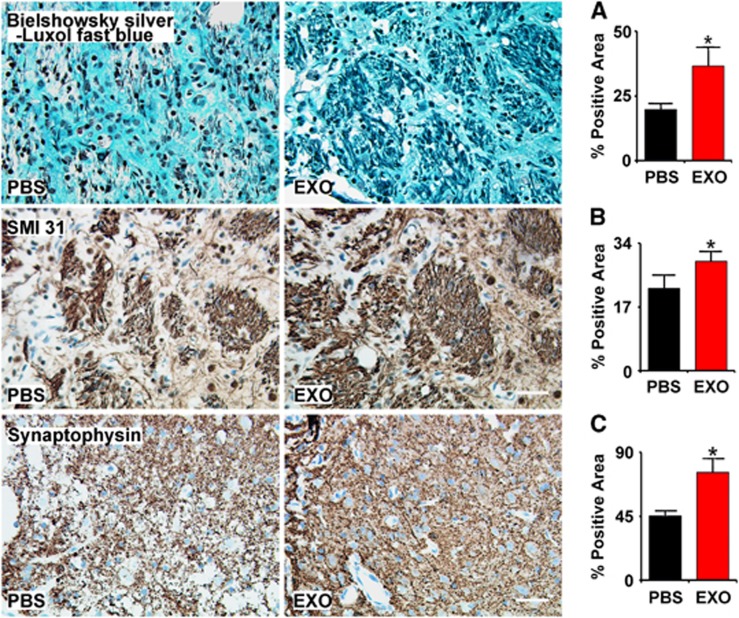
Double staining for Bielschowsky silver and Luxol fast blue was used to identify axons and myelin in the white matter in the brain, respectively. The white matter in the core lesion area was destroyed and axon–myelin bundles in the IBZ were partially damaged and disorganized after stroke. Axonal density along the IBZ was significantly increased by exosome treatment compared with PBS control at 28 days after MCAo (Figure 3, row A, P<0.05).
Figure 3.
Exosomes increase neurite remodeling in the ischemic boundary zone (IBZ). Bielschowsky silver and Luxol fast blue double staining (A row), SMI-31 immunostaining (B row), and synaptophysin immunostaining (C row) show that exosome treatment increased neurite remodeling and synaptic plasticity in the IBZ of ischemic rats compared with phosphate-buffered saline (PBS) treatment. *P<0.05, respectively. Mean±s.d., n=6/group. Scale bar, 50 μm.
Neurofilaments are dynamic structures during axonal growth, which involve the addition of neurofilament subunits along the filament length, as well as the addition of subunits at the filament ends.23 SMI-31 immunostaining revealed accumulation of phosphorylated neurofilament heavy polypeptide in axons and dendrites after stroke.24 Our data show that exosome treatment significantly increased the SMI-31 immunoreactive area in the IBZ compared with PBS control at 28 days after MCAo (Figure 3, row B, P<0.05).
As a presynaptic vesicle protein, synaptophysin is an indicator of synaptic plasticity and synaptogenesis.25 Exosome treatment significantly increased the synaptophysin immunoreactive area in the IBZ compared with PBS control at 28 days after MCAo (Figure 3, row C, P<0.05). These data suggest that exosome treatment increases neurite remodeling and synaptic plasticity in the IBZ of ischemic rats.
Mesenchymal Stromal Cell-Generated Exosomes Increase Neurogenesis And Angiogenesis in the Ischemic Boundary Zone
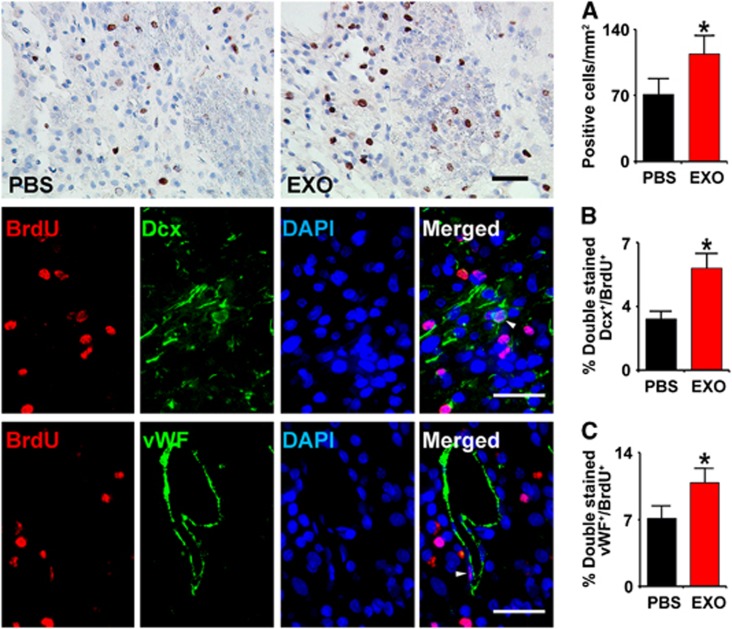
Middle cerebral artery occlusion increases cell proliferation within the subventricular zone of the adult rat.26 Newly formed neuroblasts migrate from the subventricular zone into the damaged striatum and cortex.27 Compared with PBS treatment, the BrdU-labeled cells in the IBZ were significantly increased after exosome treatment (Figure 4, row A, P<0.05). Doublecortin is a microtubule-associated protein expressed by migrating neuroblasts in the adult brain.28 To examine the effect of exosome treatment on neuroblasts, we measured the percentage of DCX- and BrdU-positive cells. Compared with PBS treatment, exosome treatment significantly increased the percentage of BrdU–DCX-positive cells in the IBZ (Figure 4, row B, P<0.05), suggesting that exosomes promote neurogenesis post stroke.
Figure 4.
Exosomes increase neurogenesis and angiogenesis in the ischemic boundary zone (IBZ). Compared with phosphate-buffered saline (PBS) treatment, the 5-bromodeoxyuridine (BrdU)-labeled cells in the IBZ were significantly increased after exosome treatment (A row). Representative micrographs show the double-stained cells with doublecortin (DCX) and BrdU (B row) or von Willebrand factor (vWF) and BrdU (C row). Compared with PBS treatment, exosome treatment significantly increased the percentage of BrdU–DCX stained cells (B) and BrdU–endothelial cells stained cells in the IBZ (C) in rats after stroke, respectively. *P<0.05, mean±s.d., n=6/group. Scale bar, 50 μm. DAPI, 4′,6-diamidino-2-phenylindole.
Angiogenesis involves endothelial cell proliferation and sprouting of new capillaries from pre-existing vessels, leading to formation of new capillary networks.29 To test whether exosome treatment affects cerebral endothelial cell proliferation, we measured BrdU- and vWF-positive cells. Rats that received exosomes exhibited a significant increase in the percentage of BrdU–vWF-positive cells (Figure 3, row C, P<0.05) in the IBZ compared with PBS treatment, suggesting that exosomes promote angiogenesis post stroke.
Discussion
Here, we demonstrate for the first time that systemic treatment of stroke with cell-free exosomes derived from MSCs significantly improve neurologic outcome and contribute to neurovascular remodeling. Although cell-based therapies are in clinical trials for stroke and other neurologic diseases30 and there is a robust literature on the efficacy of cell-based therapies for stroke,31 development of a treatment of stroke using exosomes generated by MSCs represents a novel and possibly a safer therapeutic approach. Exogenously administered cells accumulate in many organs in the body, possibly generate emboli and may replicate.32 If the benefits of cell-based therapies are mediated by exosomes produced and released by cells,9, 10 then direct treatment with exosomes may minimize potential adverse effects of administering replicating cells. In addition, we have the ability to alter the miRNAs within MSC-generated exosomes,9, 10 which may amplify the therapeutic benefit of the exosomes.9 Importantly, in the current study, we provide a novel treatment that cell-free exosomes derived from MSCs exert therapeutic restorative effects on rat's functional recovery after stroke. This therapeutic effect is consistent with the therapeutic effects of direct MSC treatment8, 33 and supports our hypothesis that treatment of stroke with MSCs is mediated by the in vivo release of exosomes and the actions of their miRNA content. In addition, the mechanisms underlying the beneficial effects of in vivo MSC and cell-free exosome treatments appear similar. They both increase neurite remodeling, neurogenesis, and angiogenesis. Thus, MSC-generated exosomes are novel candidates as a cell-free therapy.
Mesenchymal stromal cell exosomes contain miRNAs, messenger RNAs, and proteins, which can be transferred to recipient cells and thereby modify their characteristics.2, 9, 10 As miRNAs have a pivotal role in gene regulation, the miRNAs encapsulated into MSC exosomes have a primary effect on the stroke recovery. Exosomes are released by most cell types under physiologic conditions, and cellular activation or neoplastic transformation often increases their release.34 Increased release of microvesicles is associated with the acute and active phases of several neurologic disorders.35 In the current study, the 100 μg total protein of exosomes injected into each rat was collected from 3–5 × 107 MSCs, which exceeded the effective amount that we previously used in the MSC-based treatment (3 × 106 per rat).33 As an initial approach and proof-of-principle, a higher equivalent dose of exosomes was used for the cell-free exosome treatment. The underlying logic for the protein exosome dose is that the one-time injected cell-free exosomes were collected from MSCs cultured under normal conditions, whereas the cell-based injected MSCs are sustained under an ischemic condition in the brain of rats subjected to MCAo. As cellular stress increases the exosomes release from cell lines,36 MSCs within the ischemic tissue may release more exosomes to the brain, hence a higher equivalent dose of protein was used. Further studies to identify a dose response for this novel mode of exosome treatment are warranted.
Cellular condition affects the composition of the exosomes.37 In our previous study, we found that exosomes from MSCs exposed to the ischemic tissue contain increased miRNA (miR-133b), which enhances neurite remodeling.10 Further studies are warranted to identify the molecular constituents of the exosomes, including specific miRNAs that promote neurite remodeling, neurogenesis, and angiogenesis.
A caveat of the present study is that although we demonstrate a significant therapeutic and neuroplasticity effect of systemic exosome administration, we did not explicitly demonstrate the presence of the exosomes within the brain. Considering the nano size of exosomes, however, they likely enter into the brain.38 By engineering dendritic cells to express an exosomal membrane protein, Lamp2b, and fused it to the neuron-specific RVG peptide3, Alvarez-Erviti et al39 demonstrated effective delivery of functional siRNA into mouse brain by systemic injection of exosomes. Adhesive molecules are expressed on the exosome membrane,40 which may facilitate entry into the brain. Thus, systemic exosome administration may be a means by which to deliver the active components of cell-based therapy to the central nervous system. The aim of the present pilot study was to determine, for the first time, whether the administration of exosomes derived from MSCs enhance functional recovery after stroke. It is important to develop methods to quantify the amount of exosomes in the ischemic brain. Doing so would permit correlational analyses between levels of the brain-localized exosomes and functional recovery and may provide additional insight into the mechanism of action.
In the current study, we, for the first time, demonstrate that MSC-generated exosomes can be effectively employed for stroke treatment. This discovery provides a novel platform, which can treat stroke and possibly other neurologic diseases. Engineering exosomes to produce specific miRNAs that concurrently regulate multiple molecular pathways may further enhance restorative processes.9
Acknowledgments
We thank Cindi Roberts, Xia Shang, and Qing-e Lu for technical assistance.
The authors declare no conflict of interest.
Footnotes
This work was supported by NIH grants R01AG037506 (MC), R01 NS066041 (YL), and R01NS081189 (HX). This work was supported by National Institute of Aging (NIA), National Institute of Neurological Disorders and Stroke (NINDS) of the National Institutes of Health under award number R01AG037506 (MC), R01NS66041 (YL), and R01NS081189 (HX). The content is solely the responsibility of the authors and does not the necessarily represent the official views of the National Institutes of Health.
References
- Stoorvogel W, Kleijmeer MJ, Geuze HJ, Raposo G. The biogenesis and functions of exosomes. Traffic. 2002;3:321–330. doi: 10.1034/j.1600-0854.2002.30502.x. [DOI] [PubMed] [Google Scholar]
- Record M, Subra C, Silvente-Poirot S, Poirot M. Exosomes as intercellular signalosomes and pharmacological effectors. Biochem Pharmacol. 2011;81:1171–1182. doi: 10.1016/j.bcp.2011.02.011. [DOI] [PubMed] [Google Scholar]
- Zomer A, Vendrig T, Hopmans ES, van Eijndhoven M, Middeldorp JM, Pegtel DM. Exosomes: fit to deliver small rna. Commun Integr Biol. 2010;3:447–450. doi: 10.4161/cib.3.5.12339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathivanan S, Ji H, Simpson RJ. Exosomes: extracellular organelles important in intercellular communication. J Proteomics. 2010;73:1907–1920. doi: 10.1016/j.jprot.2010.06.006. [DOI] [PubMed] [Google Scholar]
- Li T, Yan Y, Wang B, Qian H, Zhang X, Shen L, et al. Exosomes derived from human umbilical cord mesenchymal stem cells alleviate liver fibrosis. Stem Cells Dev. 2013;22:845–854. doi: 10.1089/scd.2012.0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai RC, Chen TS, Lim SK. Mesenchymal stem cell exosome: a novel stem cell-based therapy for cardiovascular disease. Regen Med. 2011;6:481–492. doi: 10.2217/rme.11.35. [DOI] [PubMed] [Google Scholar]
- Gatti S, Bruno S, Deregibus MC, Sordi A, Cantaluppi V, Tetta C, et al. Microvesicles derived from human adult mesenchymal stem cells protect against ischaemia-reperfusion-induced acute and chronic kidney injury. Nephrol Dial Transplant. 2011;26:1474–1483. doi: 10.1093/ndt/gfr015. [DOI] [PubMed] [Google Scholar]
- Chen J, Li Y, Katakowski M, Chen X, Wang L, Lu D, et al. Intravenous bone marrow stromal cell therapy reduces apoptosis and promotes endogenous cell proliferation after stroke in female rat. J Neurosci Res. 2003;73:778–786. doi: 10.1002/jnr.10691. [DOI] [PubMed] [Google Scholar]
- Xin H, Li Y, Liu Z, Wang X, Shang X, Cui Y, et al. Mir-133b promotes neural plasticity and functional recovery after treatment of stroke with multipotent mesenchymal stromal cells in rats via transfer of exosome-enriched extracellular particles Stem Cells 2013(e-pub ahead of print). [DOI] [PMC free article] [PubMed]
- Xin H, Li Y, Buller B, Katakowski M, Zhang Y, Wang XL, et al. Exosome mediated transfer of mir-133b from multipotent mesenchymal stromal cells to neural cells contributes to neurite outgrowth. Stem Cells. 2012;30:1556–1564. doi: 10.1002/stem.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tropel P, Noel D, Platet N, Legrand P, Benabid AL, Berger F. Isolation and characterisation of mesenchymal stem cells from adult mouse bone marrow. Exp Cell Res. 2004;295:395–406. doi: 10.1016/j.yexcr.2003.12.030. [DOI] [PubMed] [Google Scholar]
- Bhatnagar S, Schorey JS. Exosomes released from infected macrophages contain mycobacterium avium glycopeptidolipids and are proinflammatory. J Biol Chem. 2007;282:25779–25789. doi: 10.1074/jbc.M702277200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Powers C, Jiang N, Chopp M. Intact, injured, necrotic and apoptotic cells after focal cerebral ischemia in the rat. J Neurol Sci. 1998;156:119–132. doi: 10.1016/s0022-510x(98)00036-7. [DOI] [PubMed] [Google Scholar]
- Stroemer RP, Kent TA, Hulsebosch CE. Neocortical neural sprouting, synaptogenesis, and behavioral recovery after neocortical infarction in rats. Stroke. 1995;26:2135–2144. doi: 10.1161/01.str.26.11.2135. [DOI] [PubMed] [Google Scholar]
- Li Y, Chen J, Chopp M. Adult bone marrow transplantation after stroke in adult rats. Cell Transplant. 2001;10:31–40. [PubMed] [Google Scholar]
- Zhang RL, Chopp M, Zhang ZG, Jiang Q, Ewing JR. A rat model of focal embolic cerebral ischemia. Brain Res. 1997;766:83–92. doi: 10.1016/s0006-8993(97)00580-5. [DOI] [PubMed] [Google Scholar]
- Stanely CBM. A method for intra-vital staining with silver ammonium oxide solution. J für Psychologie und Neurologie. 1925;31:4. [Google Scholar]
- Kluver H, Barrera E. A method for the combined staining of cells and fibers in the nervous system. J Neuropathol Exp Neurol. 1953;12:400–403. doi: 10.1097/00005072-195312040-00008. [DOI] [PubMed] [Google Scholar]
- Grady MS, McLaughlin MR, Christman CW, Valadka AB, Fligner CL, Povlishock JT. The use of antibodies targeted against the neurofilament subunits for the detection of diffuse axonal injury in humans. J Neuropathol Exp Neurol. 1993;52:143–152. doi: 10.1097/00005072-199303000-00007. [DOI] [PubMed] [Google Scholar]
- Calhoun ME, Jucker M, Martin LJ, Thinakaran G, Price DL, Mouton PR. Comparative evaluation of synaptophysin-based methods for quantification of synapses. J Neurocytol. 1996;25:821–828. doi: 10.1007/BF02284844. [DOI] [PubMed] [Google Scholar]
- Li Y, Sharov VG, Jiang N, Zaloga C, Sabbah HN, Chopp M. Ultrastructural and light microscopic evidence of apoptosis after middle cerebral artery occlusion in the rat. Am J Pathol. 1995;146:1045–1051. [PMC free article] [PubMed] [Google Scholar]
- Lu M, Chen J, Lu D, Yi L, Mahmood A, Chopp M. Global test statistics for treatment effect of stroke and traumatic brain injury in rats with administration of bone marrow stromal cells. J Neurosci Methods. 2003;128:183–190. doi: 10.1016/s0165-0270(03)00188-2. [DOI] [PubMed] [Google Scholar]
- Hulsmans M, Holvoet P.Microrna-containing microvesicles regulating inflammation in association with atherosclerotic disease Cardiovasc Resadvance online publication, 30 July 2013 (e-pub ahead of print). [DOI] [PubMed]
- Sceneay J, Smyth MJ, Moller A.The pre-metastatic niche: finding common ground Cancer Metastasis Rev 2013(e-pub ahead of print). [DOI] [PubMed]
- Ujike H, Takaki M, Kodama M, Kuroda S. Gene expression related to synaptogenesis, neuritogenesis, and map kinase in behavioral sensitization to psychostimulants. Ann N Y Acad Sci. 2002;965:55–67. doi: 10.1111/j.1749-6632.2002.tb04151.x. [DOI] [PubMed] [Google Scholar]
- Zhang RL, Zhang ZG, Zhang L, Chopp M. Proliferation and differentiation of progenitor cells in the cortex and the subventricular zone in the adult rat after focal cerebral ischemia. Neuroscience. 2001;105:33–41. doi: 10.1016/s0306-4522(01)00117-8. [DOI] [PubMed] [Google Scholar]
- Zhang R, Zhang Z, Wang L, Wang Y, Gousev A, Zhang L, et al. Activated neural stem cells contribute to stroke-induced neurogenesis and neuroblast migration toward the infarct boundary in adult rats. J Cereb Blood Flow Metab. 2004;24:441–448. doi: 10.1097/00004647-200404000-00009. [DOI] [PubMed] [Google Scholar]
- Couillard-Despres S, Winner B, Schaubeck S, Aigner R, Vroemen M, Weidner N, et al. Doublecortin expression levels in adult brain reflect neurogenesis. Eur J Neurosci. 2005;21:1–14. doi: 10.1111/j.1460-9568.2004.03813.x. [DOI] [PubMed] [Google Scholar]
- Chung AS, Ferrara N. Developmental and pathological angiogenesis. Ann Rev Cell Dev Biol. 2011;27:563–584. doi: 10.1146/annurev-cellbio-092910-154002. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Xu H, Xu W, Wang B, Wu H, Tao Y, et al. Exosomes released by human umbilical cord mesenchymal stem cells protect against cisplatin-induced renal oxidative stress and apoptosis in vivo and in vitro. Stem Cell Res Ther. 2013;4:34. doi: 10.1186/scrt194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess DC, Borlongan CV. Cell-based therapy in ischemic stroke. Expert Rev Neurother. 2008;8:1193–1201. doi: 10.1586/14737175.8.8.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Han ZB, Song YP, Han ZC. Safety of mesenchymal stem cells for clinical application. Stem Cells Int. 2012;2012:652034. doi: 10.1155/2012/652034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Li Y, Wang L, Zhang Z, Lu D, Lu M, et al. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke. 2001;32:1005–1011. doi: 10.1161/01.str.32.4.1005. [DOI] [PubMed] [Google Scholar]
- Hugel B, Martinez MC, Kunzelmann C, Freyssinet JM. Membrane microparticles: two sides of the coin. Physiology (Bethesda) 2005;20:22–27. doi: 10.1152/physiol.00029.2004. [DOI] [PubMed] [Google Scholar]
- Horstman LL, Jy W, Minagar A, Bidot CJ, Jimenez JJ, Alexander JS, et al. Cell-derived microparticles and exosomes in neuroinflammatory disorders. Int Rev Neurobiol. 2007;79:227–268. doi: 10.1016/S0074-7742(07)79010-4. [DOI] [PubMed] [Google Scholar]
- King HW, Michael MZ, Gleadle JM. Hypoxic enhancement of exosome release by breast cancer cells. BMC Cancer. 2012;12:421. doi: 10.1186/1471-2407-12-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldh M, Ekstrom K, Valadi H, Sjostrand M, Olsson B, Jernas M, et al. Exosomes communicate protective messages during oxidative stress; possible role of exosomal shuttle rna. PLoS One. 2010;5:e15353. doi: 10.1371/journal.pone.0015353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakhal S, Wood MJ. Exosome nanotechnology: an emerging paradigm shift in drug delivery: exploitation of exosome nanovesicles for systemic in vivo delivery of rnai heralds new horizons for drug delivery across biological barriers. Bioessays. 2011;33:737–741. doi: 10.1002/bies.201100076. [DOI] [PubMed] [Google Scholar]
- Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. 2011;29:341–345. doi: 10.1038/nbt.1807. [DOI] [PubMed] [Google Scholar]
- Clayton A, Turkes A, Dewitt S, Steadman R, Mason MD, Hallett MB. Adhesion and signaling by b cell-derived exosomes: the role of integrins. FASEB J. 2004;18:977–979. doi: 10.1096/fj.03-1094fje. [DOI] [PubMed] [Google Scholar]