Abstract
The multivesicular body (MVB) is a specialized Rab7+ late endosome (LE) containing multiple intralumenal vesicles that function in targeting ubiquitinylated cell surface proteins to the lysosome for degradation. African trypanosomes lack a morphologically well-defined MVB, but contain orthologues of the ESCRT machinery that mediates MVB formation. We investigate the role of TbVps23, an early ESCRT component, and TbVps4, the terminal ESCRT ATPase, in lysosomal trafficking in bloodstream form trypanosomes. Both localize to the TbRab7+ LE and RNAi silencing of each rapidly blocks growth. TbVps4 silencing results in ∼3-fold accumulation of TbVps23 at the LE, consistent with blocking terminal ESCRT disassembly. Trafficking of endocytic and biosynthetic cargo, but not default lysosomal reporters, is also negatively affected. Others reported that TbVps23 mediates ubiquitin-dependent lysosomal degradation of invariant surface glycoproteins (ISG65) (Traffic 2008, 9:1698). In contrast, we find that TbVps23 ablation does not affect ISG65 turnover, while TbVps4 silencing markedly enhances lysosomal degradation. We propose several models to accommodate these results, including that the ESCRT machinery actually retrieves ISG65 from the LE to earlier endocytic compartments, and in its absence ISG65 traffics more efficiently to the lysosome. Overall, these results confirm that the ESCRT machinery is essential in T. brucei and plays important and novel role(s) in LE function in trypanosomes.
Keywords: Trypanosome, Lysosome, Late Endosome, ESCRT, Invariant Surface Glycoprotein, MVB
Trypanosoma brucei ssp. are the causative agents of human and veterinary trypanosomiases in sub-Saharan Africa. The trypanosome life cycle alternates between the mammalian bloodstream form (BSF) and multiple stages in the insect vector, the tsetse fly, including the midgut procyclic form. BSF trypanosomes live solely within the bloodstream and extracellular fluids, and sustain infection by a complex process of antigenic variation (1, 2). Also crucial to pathogenesis is the lysosomal/endosomal pathway, which is greatly up-regulated relative to procyclic trypanosomes (3-5). Host serum proteins are aggressively taken up and delivered to a degradative lysosome for nutritional purposes (3), and this pathway may also eliminate potentially lytic host immune complexes bound to the surface of the parasite (6-8). Consequently, lysosomal enzymes have been investigated for many years as potential therapeutic targets (9).
As an anciently derived eukaryotic parasite, T. brucei has most of the core secretory and endocytic organelles present in higher eukaryotes, including the ER, the Golgi, endosomes (early, late, and recycling), and lysosome, although these are generally reduced in copy number (10-13). For instance, there is a single terminal lysosome that is reminiscent of the vacuole in yeast. There are two normal routes to the lysosome in trypanosomes, the endocytic pathway from the flagellar pocket and the biosynthetic pathway from the Golgi. Studies of endocytosis have focused mostly on glycosylphosphatidylinositol (GPI)-anchored variant surface glycoprotein (VSG) and the related transferrin receptor (TfR) (14). These proteins are taken up by clathrin-mediated endocytosis into the early endosome. From here they are recycled to the cell surface either directly via the recycling endosome, or by passing first through the late endosome and then the recycling endosome. Host serum proteins taken up for degradation, such as transferrin (Tf) or immunoglobulins, are sorted through the late endosome for subsequent delivery to the lysosome. These routes are broadly similar to those in higher eukaryotes (15). Less well understood is the biosynthetic route to the lysosome. Endogenous proteins, including the soluble thiol protease TbCatL and the membrane glycoprotein p67, are separated from secretory cargo in the Golgi, from which they are exported in a clathrin-dependent manner (16). By analogy to mammalian cells these proteins should sort through the late endosome (17), but this has not been formally demonstrated in T. brucei. There is a third cryptic pathway to the lysosome in BSF trypanosomes, the default route, which becomes apparent when normal trafficking signals of endogenous proteins are disrupted. One example is VSG in which GPI addition is blocked (18). Another is p67 in which innate targeting signals in the cytoplasmic domain are disrupted (19, 20). In the first case GPI-minus VSG is mis-targeted to the lysosome, and in the second mutated p67 reporters traffic to the lysosome with unaltered kinetics, despite the absence of normal targeting motifs. It is unclear the extent to which default trafficking utilizes the normal secretory/endocytic pathways.
As in other eukaryotes, trafficking through the various endosomal compartments is regulated by a core set of small GTPases called Rabs (21). Thus, TbRab5 and TbRab11 regulate trafficking through the early and recycling endosomes respectively (22-24). The situation with the late endosomal marker TbRab7 is unique however. TbRab7 regulates endocytic trafficking to the lysosome in BSF trypanosomes, but has no apparent role in biosynthetic trafficking of either native lysosomal proteins or default reporters (25). This contrasts markedly with mammalian cells where Rab7 regulates both biosynthetic and endocytic flux through the late endosome. It is alternatively possible that: 1) these cargos do not sort through the late endosome at all; 2) they do transit the late endosome but are not regulated by TbRab7; and/or 3) some other late endosomal Rab is in play.
One aspect of T. brucei endomembrane trafficking that is currently uncertain is the existence of a multivesicular body (MVB). In mammalian cells the MVB is defined as a specialized Rab7+ late endosome that contains intralumenal vesicles (ILVs) (26-28). As early endosomes mature into late endosomes, vesicles bud inwardly forming the MVB. Internalized cell surface proteins that have been tagged with ubiquitin are selected into these ILVs, which can then be sorted to lysosomes for degradation. MVB formation is mediated by the ESCRT (Endosomal Sorting Complex Required for Transport) machinery, consisting of four multi-subunit complexes: ESCRT 0 and ESCRT I cluster ubiquitinylated cargo, ESCRT I and ESCRT II initiate vesicle invagination, and ESCRT III causes internal vesicle membrane scission. The final step of the MVB pathway is mediated by Vps4, an AAA ATPase that disassembles terminal ESCRT III complexes. Vps4 also provides the only known energy input for ESCRT-driven vesicle budding and scission. Thus, blocking Vps4 activity is a powerful tool for studying ESCRT function since other ESCRT components can be functionally redundant and not all subunits and complexes participate in all ESCRT-driven processes (29).
The term ‘MVB’ has been used inconsistently for decades to label miscellaneous cytological features of trypanosomes (3, 30), and MVB-like structures have been observed in stressed parasites following RNAi silencing of components of the secretory and endosomal systems (31, 32). However, contrary to published claims (33-35), it is unclear whether a bona fide ILV-containing MVB normally exists in T. brucei. The late endosome has been characterized as a collection of complex membranous structures in close proximity to the lysosome, but these do not resemble a ‘classic’ MVB (14). This lack of morphological evidence may reflect the true situation, or may be due to the fragile nature of ILVs in standard EM fixation procedures (36). Nevertheless, evidence suggests that MVB functionalities do exist in trypanosomes. First, the T. brucei genome encodes orthologues for many components of the ESCRT machinery, including TbVps23 (ESCRT I) and TbVps4 (33). Second, BSF trypanosomes have a family of type-I transmembrane Invariant Surface Glycoproteins (ISGs) (37, 38). ISGs can be ubiquitinylated on their cytoplasmic domains, and this modification influences internalization, recycling and turnover (39-41). Finally, RNAi knockdown of TbVps23 reportedly has a negative effect on both BSF cell growth and degradation of ISG65 (33). These observations have been used to argue that the ESCRT machinery mediates lysosomal turnover of ubiquitinylated cargo in trypanosomes, much as it does in other systems (33).
Focusing primarily on TbVps4, which has not heretofore been studied, but also on TbVps23, we now define the localization of the ESCRT machinery in relationship to the well characterized TbRab7+ late endosome (25). We also present novel investigations of ESCRT function in all three modes of lysosomal trafficking: biosynthetic, endocytic and default. We next re-investigate the role of the ESCRT machinery in ISG65 trafficking and turnover, with results that contrast markedly with the published data on this process. Based on these findings we propose a new model of ISG65 trafficking to reconcile the currently available data.
Results
Location of ESCRT machinery in trypanosomes
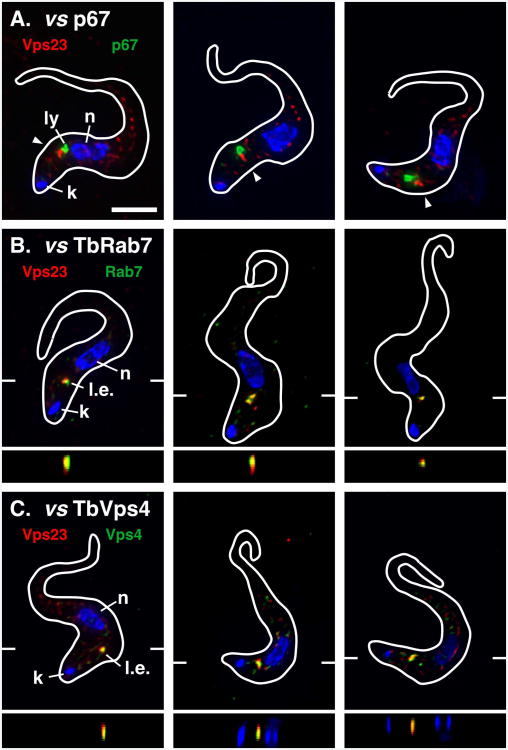
The localizations of TbVps23 and TbVps4 were investigated using BSF cell lines in which the endogenous proteins were epitope tagged by homologous recombination into the native chromosomal alleles. Three cell lines were generated: i) single tagged TbVps23-HA; ii) double tagged TbVps23-HA/Ty-TbRab7; and iii) double tagged TbVps23-HA/TbVps4-Ty [note that the order of epitope-to-orf indicates tagging at N- or C- termini]. We first examined TbVps23 because it had been previously shown to localize within the endosomal system (33). TbVps23-HA typically presented as a prominent structure in the post-nuclear region closely aligned with, but distinct from, the endogenous lysosomal marker p67 (Fig. 1A). Careful examination of raw non-deconvolved images (Fig. S1) also revealed considerable diffuse cytoplasmic staining for TbVps23, which has both membrane-bound and free cytoplasmic pools (33). The dominant signal proximal to the lysosome is reminiscent of the late endosomal marker TbRab7 (25), and double staining for TbVps23-HA and Ty-TbRab7 confirmed prominent colocalization of these proteins (Fig. 1B). TbVps23-HA also strongly colocalized with TbVps4-Ty (Fig. 1C). As with TbVps23-HA, there was also diffuse TbVps4-Ty staining throughout cells (Fig. S1) consistent with recruitment to membranes from a cytoplasmic pool. These results indicate the mammalian ESCRT machinery homologues TbVps23 and TbVps4 are predominantly associated with the TbRab7+ late endosome in BSF trypanosomes. Given the lack of evidence for a morphologically defined MVB in trypanosomes, and conversely the experimental precedent for the TbRab7+ late endosome (14, 25), we will refer to this compartment hereafter as the late endosome.
Figure 1. TbVps23 localization.
Immunofluorescence microscopy was performed on fixed/permeabilized BSF cells expressing in situ epitope tagged TbVps23-HA (A), TbVps23-HA and Ty-TbRab7 (B), or TbVps23-HA and TbVps4-Ty (C). In each case staining for TbVps23-HA is with rabbit anti-HA (red). Staining for p67 is with monoclonal anti-p67 (A, green) and staining for Ty-TbRab7 (B) and TbVps4-Ty (C) is with monoclonal anti-Ty (green). The target reporter proteins are color-coded, and the locations of the nucleus (n), kinetoplast (k), lysosome (l), and late endosome (l.e.) are indicated (left panels only). Arrowheads indicate regions of prominent TbVps23-HA localization (A only). In each case three representative deconvolved images are shown as merged three-channel summed stack projections with corresponding z transects through the late endosome (B & C only, indicated by marginal hatch marks in the x-y images). Cells were stained with DAPI to detect the nucleus and kinetoplast and cell outlines were traced from matching differential interference contrast (DIC) images. All images are of interphase cells (1k1n). Bar indicates 5 microns (top left only). See Fig. S1 for corresponding non-deconvolved red channel images for anti-HA (A, TbVps23-HA) and anti-Ty (C, TbVps4) stained cells.
TbVps4 RNAi silencing
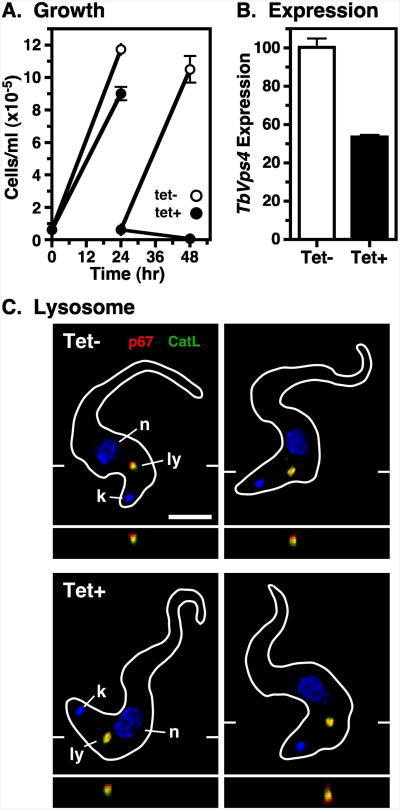
To investigate the function of trypanosomal ESCRT homologues, we silenced TbVps4 using an inducible stem-loop dsRNA construct. We first chose TbVps4 because of its crucial role in the terminal steps of the MVB pathway in mammalian cells. BSF growth was affected by 24 hrs of TbVps4 silencing and cell death occurred by 48 hrs (Fig. 2A). Subsequent analyses were done at 20 hrs because there were no defects in gross cell morphology as observed by light microscopy (data not shown). At that time qRT-PCR revealed that TbVps4 message levels were reduced 46.5±1.1% (Fig. 2B). However, the steady-state localizations of the lysosomal lumenal thiol protease TbCatL and membrane glycoprotein p67 were not altered as determined by fluorescence microscopy (Fig. 2C). These results indicate that while TbVps4 is essential in BSF trypanosomes, short-term TbVps4 silencing does not affect steady state lysosomal morphology.
Figure 2. TbVps4 RNAi: growth and morphology.
TbVps4 BSF RNAi cell line was cultured without (tet-) or with (tet+) tetracycline resulting in control or TbVps4 silenced cells, respectively. (A) Cell density was measured over time by hemocytometer and adjusted daily to starting density. Data are means ± SEM (n=3). All subsequent analyses using these cells were performed at 20 hr of induction, unless otherwise indicated. (B) TbVps4 message levels in control and silenced cells were determined by qRT-PCR. Normalized data are means ± SEM (n=3). (C) Immunofluorescence microscopy was performed as in Fig. 1 with anti-p67 (red) and anti-TbCatL (green). The target reporter proteins are color-coded, and the locations of the nucleus (n), kinetoplast (k), and lysosome (l) are indicated. Representative deconvolved summed stack images are presented with corresponding z transects through the lysosome. Bar indicates 5 microns (left only).
TbVps4 silencing affects TbVps23 localization
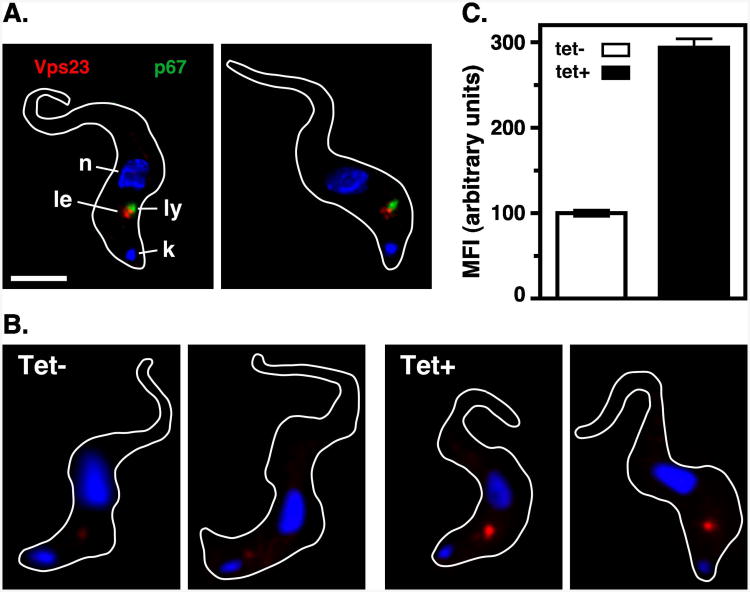
In mammalian cells, Vps4 disassembles the terminal ESCRT machinery from sites where ILVs have invaginated, consequently disruption of Vps4 function results in localized build up of ESCRT machinery (26, 27). To determine if TbVps4 functions in a similar manner, TbVps23 localization was investigated after specific silencing. As seen in control cells (Fig. 1A), following knockdown of TbVps4 prominent TbVps23-HA localization was apparent in close proximity to the lysosome (Fig. 3A). However, there was a dramatic increase in the localized TbVps23-HA signal in the TbVps4 silenced cells, which is best seen in non-deconvolved images (Fig. 3B). Quantification of raw unprocessed images revealed that localized TbVps23-HA fluorescence intensity was increased 2.9±0.1 fold after TbVps4 silencing (Fig. 3C). Control immunoblotting experiments indicated that steady state TbVps23-HA levels were unaltered by TbVps4 silencing (Fig. S2). These results show that disruption of TbVps4 function in BSF trypanosomes leads to significant accumulation of TbVps23 from the cytoplasmic pool to the late endosome, consistent with conservation of function relative to the mammalian ESCRT machinery.
Figure 3. TbVps4 RNAi: TbVps23 localization.
TbVps4 BSF RNAi cells expressing in situ tagged TbVps23-HA were cultured in the absence or presence (20 hr) of tetracycline as in Fig. 2. (A) TbVps23-HA was localized after TbVps4 silencing using anti-HA (red, late endosome) and anti-p67 (green, lysosome) as described in Fig. 1. Representative deconvolved summed stack images are presented. The target reporter proteins are color-coded, and the locations of the nucleus (n), kinetoplast (k), and lysosome (l), and late endosome (le) are indicated (left only). (B) Non-deconvolved images of cells stained for TbVps23-HA (red). Shown are two representative images each of control (tet-) and TbVps4 silenced (tet+) cells. All images were acquired at identical exposure time and were contrast enhanced identically. (C) Quantification of TbVps23-HA fluorescence. Mean fluorescent intensity of the prominent post-nuclear TbVps23-HA signal was measured using NIH ImageJ as described in Methods. All measured images were acquired identically and only raw, unenhanced images were quantified. Data are means ± SEM (n=50 over three biological replicates).
TbVps4 silencing has differential effects on biosynthetic and default lysosomal trafficking
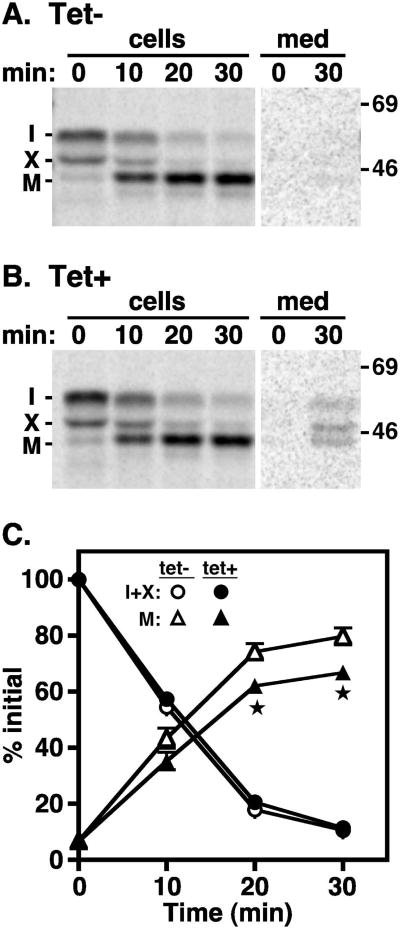
The role of TbVps4 in biosynthetic trafficking to the lysosome was investigated by pulse-chase analysis using soluble TbCatL and membrane-bound p67 as endogenous reporters. As previously shown (25), TbCatL was synthesized in control cells as a 53 kDa precursor that was cleaved over time to a 44 kDa mature form upon lysosomal arrival (Fig. 4A). A similar pattern of processing was seen after TbVps4 silencing (Fig. 4B), although elevated amounts of TbCatL were consistently observed in the final media fraction (∼5%). Quantification (Fig. 4C) revealed that there was a small, but statistically significant reduction in the rate and extent of TbCatL processing corresponding to the increase increase in secreted forms. These results suggest that TbVps4 silencing has a modest but real effect on the biosynthetic sorting of soluble TbCatL in a pre-lysosomal compartment, presumably the late endosome.
Figure 4. TbVps4 RNAi: TbCatL trafficking.
TbVps4 BSF RNAi cells were pulse radiolabeled (10 min) and chased for the indicated times. (A & B) Cell lysates and media fractions were prepared from control (A, Tet-) and silenced (B, Tet+, 20 hr) cells, and TbCatL polypeptides were specifically immunoprecipitated with anti-TbCatL antibodies. Immunoprecipitates were fractionated by SDS-PAGE (107 cell equivalents/lane) and visualized by phosphorimaging. Representative images are shown with mobilities of immature proprotein (I), uncharacterized precursor form (X), and mature lysosomal form (M) indicated on the left. Mobilites of molecular mass markers are indicated on the right (kDa). Individual panels were digitally excised from the same exposure of the same gel. Lanes containing cell fractions were processed identically. Lanes containing media fractions were also processed identically and were deliberately over-contrasted to reveal secreted forms of TbCatL. (C) Quantification of TbCatL turnover. Precursor (I+X) and mature (M) forms are presented as a percentage of initial (I+X). Data are means ± SEM (n=3) with asterisks indicating significance of p<0.05 determined as described in Methods.
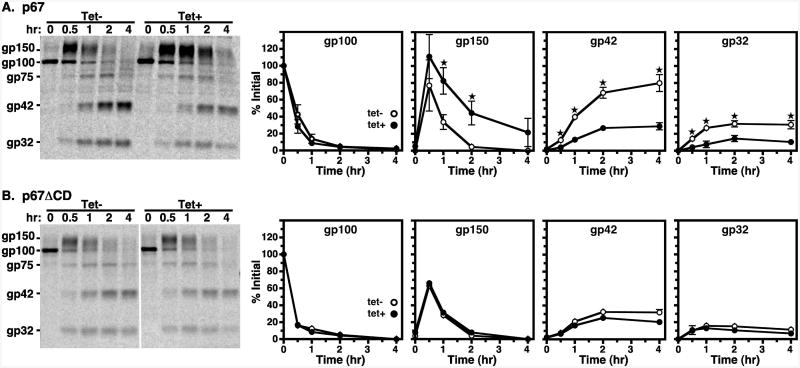
Newly synthesized native p67 is first synthesized as the 100 kDa (gp100) ER glycoform (Fig. 5A, left) in control cells, as previously reported (19, 25). After processing to a 150 kDa Golgi intermediate (gp150), p67 is cleaved into quasi-stable fragments (gp42, gp32) upon arrival in the lysosomal. In TbVps4 silenced cells there was no defect in disappearance of the gp100 ER form or in subsequent appearance of the gp150 Golgi form. However, conversion of gp150 to the lysosomal gp42 and gp32 fragments was significantly delayed. These results show that TbVps4 silencing does not affect p67 trafficking in the early secretory pathway, but does significantly delay lysosomal delivery of p67.
Figure 5. TbVps4 RNAi: p67 and default trafficking.
Turnover of endogenous p67 and the transgenic p67ΔCD-3×HA default reporter in control (tet-, open circles) and TbVps4 silenced (tet+, closed circles) cells were determined by pulse-chase analyses as in Fig 4. (A) Endogenous p67 was immunoprecipitated at the indicated times from TbVps4 RNAi cells with anti-p67. (B) Default trafficking was likewise analyzed in TbVps4 RNAi cells constitutively expressing the transgenic p67ΔCD-3times;HA default trafficking reporter. Immunoprecipitation was performed with anti-HA. (A & B) Immunoprecipitates were fractionated by SDS-PAGE (107 cell equivalents/lane) and visualized by phosphorimaging. Single representative phosphorimages are presented on the left. Mobilities of initial ER gp100, Golgi-modified gp150, and quasi-stable lysosomal gp42 and gp32 glycoforms are indicated. The vertical white line in Panel B indicates irrelevant lanes that were digitally removed after contrast enhancement. Turnover of individual glycoforms was quantified as a percentage of initial gp100 and are presented graphically (to the right). Data are means ± SEM (n=3) with asterisks indicating statistical significance of p<0.05 or greater as determined as described in Methods.
The p67 C-terminal cytoplasmic domain contains two dileucine motifs that are necessary and sufficient for lysosomal delivery in procyclic trypanosome. These signals are completely dispensable for lysosomal targeting in BSF cells (19, 20). This situation implies the existence of a cryptic default pathway to the lysosome operative in BSF trypanosomes. Silencing of TbRab7 has no effect on this pathway suggesting that it may not include the late endosome (25). The possible role of the trypanosomal ESCRT machinery in this default route was investigated by pulse-chase analysis with TbVps4 RNAi cells constitutively expressing modified p67 reporters in which either the cytoplasmic domain (CD), or the transmembrane (TM) and cytoplasmic domains, were replaced with a triple HA tag (p67ΔCD-3×HA and p67ΔTM-3×HA respectively). As expected, in control cells this default reporter was processed in a manner that is indistinguishable from native p67, including the orderly kinetic appearance of all glycoforms (Fig. 5B). Furthermore, as was seen previously with TbRab7 silencing, processing of p67ΔCD-3×HA was completely unaffected by TbVps4 knockdown. Likewise, turnover of the soluble p67ΔTM-3×HA default reporter was unaffected by TbVps4 silencing (Fig. S3). These results imply that the trypanosomal ESCRT machinery does not participate in default lysosomal trafficking of membrane proteins, or alternatively that such trafficking does not proceed via the late endosome. However, the results with native p67 and TbCatL are intriguing. Whereas TbRab7 silencing had no effect on normal trafficking of these proteins (25), TbVps4 knockdown significantly impeded delivery of both to the lysosome. These results suggest that the TbESCRT machinery does function in normal biosynthetic trafficking of endogenous lysosomal proteins.
TbVps4 silencing reduces the kinetics of endocytic trafficking to the lysosome
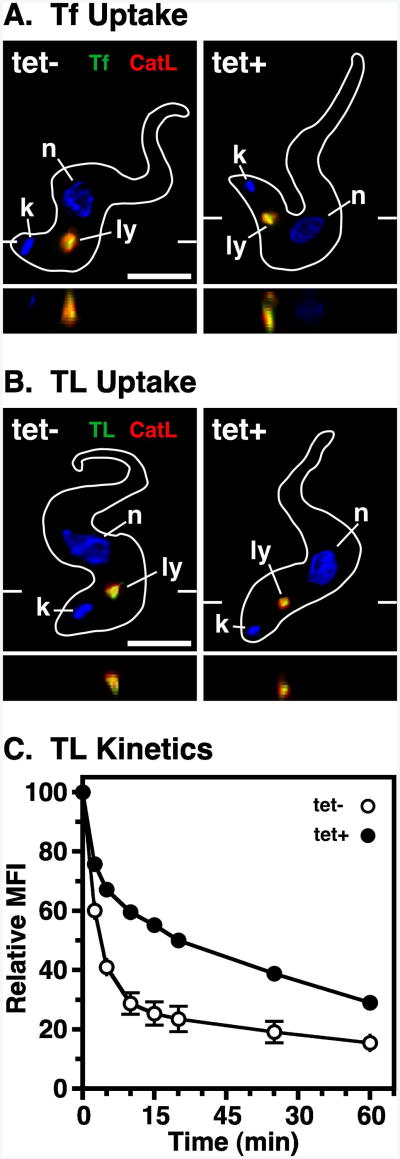
The role of TbVps4 in endocytic trafficking was investigated using tomato lectin (TL) and transferrin (Tf) as markers for receptor-mediated endocytosis. TL binds poly-N-acetyllactosamine (pNAL) structures on flagellar pocket glycoproteins (19, 42) and Tf binds to a unique trypanosomal transferrin receptor (TfR) (43, 44). Both proteins are taken up by clathrin-mediated endocytosis and are delivered to the lysosome via the endosomal system (11, 45). In control and TbVps4 silenced cells, both internalized Tf and TL co-localized prominently with the lysosomal marker p67 (Fig. 6A & B, respectively). To quantitatively investigate the kinetics of uptake and lysosomal delivery, endocytosis of pH-sensitive TL:FITC was monitored by flow cytometry. As seen previously (25), there was a biphasic decrease in fluorescent intensity in control cells as the probe was first rapidly internalized, and then more gradually transited endosomal compartments of decreasing pH, finally reaching the terminal lysosome (Fig. 6C). Silencing of TbVps4 significantly reduced the rate of endocytic trafficking of TL:FITC, most dramatically in the second transitory phase. These results suggest that TbVps4 silencing prevents neither the initial uptake nor the final lysosomal delivery of endocytic cargo, but it does significantly decrease the rate of transport through the endosomal system.
Figure 6. TbVps4 RNAi: endocytosis.
TbVps4 BSF RNAi cell line was cultured (20 hr) without (tet-) or with (tet+) tetracycline resulting in control or TbVps4 silenced cells, respectively. (A) Cells were incubated with Tf:Bio at 37°C for 30 min allowing endocytosis to occur, and then chased for 20 min in fresh media before processing for immunofluorescence microscopy as in Fig. 1. FMK024 (20 μM) was added for 30 minutes preceding and throughout the uptake and chase period to prevent degradation in the lysosome. Cells were probed with anti-TbCatL (red, lysosome) and streptavidin-alexa488 (green, TL). The target reporter proteins are color-coded, and the locations of the nucleus (n), kinetoplast (k), and lysosome (ly) are indicated. Representative deconvolved summed stack images are shown with corresponding z transects through the lysosome. Bar indicates 5 microns (left only). (B) Endocytosis of TL:Bio and subsequent microscopy was performed as above, except that FMK024 was omitted. (C) Kinetics of endocytosis was determined using pH-sensitive TL-FITC. Cells were incubated with TL-FITC at 5°C for 30 min to allow binding and washed into fresh media at 37°C to allow uptake. Mean fluorescence intensity (MFI) was measured over time by flow cytometry. Data are normalized to initial bound TL-FITC and are means ± SEM (n=2).
ESCRT machinery and turnover of ISG65
ISG65 is an endogenous type I membrane glycoprotein that has distinct cell surface and internal endosomal pools (37-39). ISG65 can be modified by ubiquitinylation of defined lysine residues in its cytoplasmic domain and, as in mammalian cells, recognition of these epitopes by the ESCRT machinery is believed to mediate endosomal localization and subsequent turnover. In support of this proposal, mutation of the critical lysine residues blocked ubiquitinylation resulting in increased cell surface localization (39-41), and RNAi knockdown of TbVps23 reduced ISG65 turnover in an acidic compartment (but see below), presumed to be the lysosome (33).
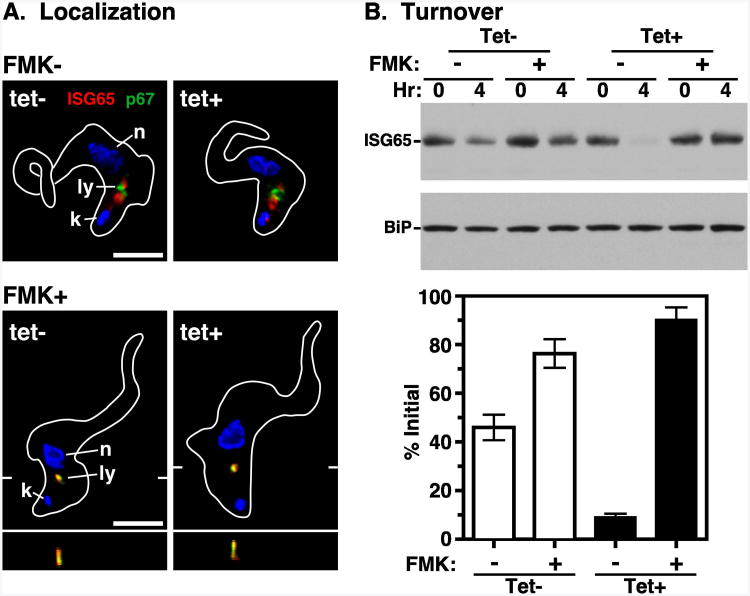
Consistent with these reports we observed prominent ISG65 staining in the post-nuclear endosomal region of permeablized control cells (Fig. 7A, top left). However, following TbVps4 silencing, no gross alteration was seen in ISG65 localization (Fig. 7A, top right), and no apparent increase in cell surface ISG65 was observed in non-permeabilized cells (data not shown). These data suggest that TbVps4 silencing does not qualitatively affect the steady-state location of ISG65, but more qualitative analyses would be required for definitive conclusions.
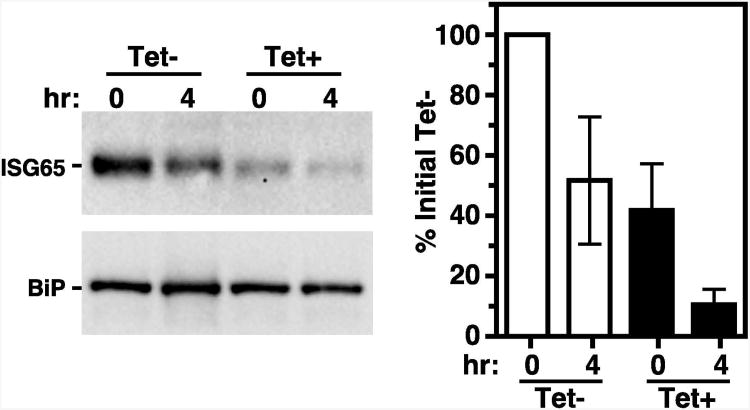
Figure 7. TbVps4 RNAi: ISG65 turnover.
The TbVps4 BSF RNAi cell line was cultured (20 hr) without (tet-) or with (tet+) tetracycline resulting in control or TbVps4 silenced cells, respectively. (A) ISG65 localization was determined in untreated cells (top) or cells co-treated with FMK024 (1 hr, 20 μM) to block degradation in the lysosome (bottom). Staining was performed using anti-p67 (green) and anti-ISG65 (red) as in Fig. 1. Representative images are presented as summed stack projections with corresponding z transects through the lysosome (bottom only). The target reporter proteins are color-coded, and the locations of the nucleus (n), kinetoplast (k), and lysosome (l) are indicated (left only). Bars indicate 5 microns. (B) Inhibition of protein synthesis with cycloheximide (100 μg ml-1) was used to quantify the rate of ISG65 turnover. As indicated, cells were incubated in the absence or presence of FMK024 (20 μM) for 30 min before cycloheximide addition, and continually during the subsequent chase. Whole cell lysates were prepared at the indicated times, fractionated by SDS-PAGE (107 cell equivalents/lane), and transferred to membranes for immunoblotting. Membranes were probed with anti-ISG65, stripped, and reprobed with anti-BiP as a loading control. Representative blots are presented (top). ISG65 signals were quantified using NIH Image J and normalized to the corresponding BiP signals. Turnover is presented graphically as percentage (means ± SEM; n=3) of initial ISG65 (bottom).
The mode of ISG65 turnover was next assessed. In both control and TbVps4 silenced cells, treatment with the lysosomal cysteine protease inhibitor FMK024 led to prominent colocalization of ISG65 with p67 (Fig. 7A, bottom). The apparent loss of signal from the endosomal region is relative only, resulting from the dramatic accumulation of un-degraded ISG65 in the lysosome. Repeated attempts to measure ISG65 turnover by standard pulse-chase analysis failed due to poor signal after immunoprecipitation with anti-ISG65 (data not shown). Therefore, cycloheximide-chase analysis (39) was performed (Fig. 7B). In control cells, after 4 hours of cycloheximide treatment to inhibit protein synthesis, steady state ISG65 was reduced 54.1±5.2%. This turnover was substantially reversed by concurrent treatment with FMK024. However, after TbVps4 silencing, by 4 hours of cycloheximide treatment the ISG65 signal was reduced by 92.8±1.5%. Again turnover was blocked by FMK024. These results confirm that ISG65 is normally turned over in the lysosome in both control and TbVps4 silenced cells. Unexpectedly however, TbVps4 silencing markedly enhanced ISG65 degradation (∼2-fold), suggesting that trafficking to the lysosome from the endosomal pool is actually increased when ESCRT function is blocked.
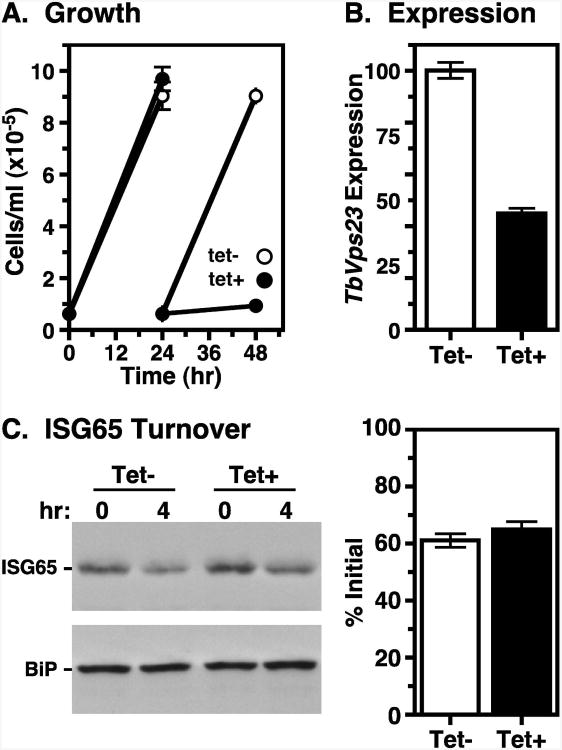
Our finding that disruption of TbVps4 function accelerates ISG65 degradation is contrary to the aforementioned report that silencing of the ESCRT I component TbVps23 blocks ISG65 turnover (33). We therefore reinvestigated TbVps23 function by RNAi silencing. First we precisely replicated the dual opposing promoter construct of Leung et. al (33). Multiple clones were tested without success in reducing cell growth (data not shown). It should be noted that Leung et. al (33) achieved only a transient ∼two-fold decrease in growth rate despite an ∼80% reduction in specific TbVps23 message levels. We next tested an equivalent stem-loop TbVps23 dsRNA construct. In this case, cell growth was completely halted at 24 hour of induction (Fig. 8A), although TbVps23 message levels were only reduced by 55.1±2.0% (Fig 8B). These results indicate that TbVps23 is essential for proliferation of BSF trypanosomes. ISG65 turnover was analyzed at the 24-hour time point because there were no obvious defects in cell morphology as judged by light microscopy (data not shown). Despite the strong growth phenotype, turnover was not affected (Fig. 8C). Collectively, these results strongly indicate that silencing the terminal ESCRT machinery in BSF trypanosomes actually enhances the lysosomal turnover of ubiquitinylated membrane proteins. Possible reasons for the discrepancy of these results with the prior work of Leung et. al (33) are discussed below.
Figure 8. TbVps23 RNAi: ISG65 turnover.
TbVps23 BSF RNAi cells were cultured without (tet-) or with (tet+) tetracycline resulting in control or silenced cells, respectively. (A) Cell densities (means ± SEM; n=3) were measured over time by hemocytometer as in Fig. 2. All subsequent analyses using these cells were performed at 24 hr of induction. (B) TbVps23 message levels in control and silenced cells were determined by qRT-PCR. Normalized data are means ± SEM (n=3). (C) Cycloheximide treatment was used to quantify the rate of ISG65 turnover as in Fig. 7. Representative blots are presented (left). Turnover is quantified (right) as means ± SEM (n=3) and presented graphically as percentage of initial ISG65.
Finally, to further investigate the route of ISG65 trafficking, we followed turnover in a previously characterized TbRab7 RNAi cell line (25). Again ISG65 decreased ∼50% after 4 hours of cycloheximide treatment (Fig. 9). Contrary to expectation however, TbRab7 ablation actually accelerated turnover. The steady state level of ISG65 was reduced to ∼40% of control cells after 28 hr of silencing (T0), and loss of ISG65 during the subsequent ‘chase’ was twice as fast (Tet- ∼2-fold vs Tet+ ∼4-fold decrease at T4). This finding contrasts markedly with the complete disruption of endocytic trafficking to the lysosome caused by TbRab7 silencing (25). Possible explanations are discussed below.
Figure 9. TbRab7 RNAi; ISG65 turnover.
A TbRab7 RNAi cell line (25) was cultured (28 hr) without (tet-) or with (tet+) tetracycline resulting in control or silenced cells, respectively. Cycloheximide treatment was used to quantify the rate of ISG65 turnover as in Fig. 7. Representative blots are presented (left). Turnover is quantified (right) as means ± SEM (n=4) and presented graphically as percentage of initial ISG65 in the control T0 sample.
Discussion
Recent reviews have erroneously placed the small GTPase TbRab7 together with the membrane glycoprotein p67 in the terminal lysosome (34, 35). In fact, the late endosome in trypanosomes is a discrete post-nuclear compartment marked by TbRab7 (14, 25) (this work). It is closely juxtaposed with the lysosome, which is independently marked by both p67 and the soluble hydrolase TbCatL (19, 32) (Fig.10). We have now examined the localization and function of two trypanosomal orthologues of the eukaryotic ESCRT machinery, TbVps23 and TbVps4, in relationship to these two well-defined endocytic organelles. By homology to other systems TbVps23 is an early ESCRT I component, and TbVps4 is the ATPase responsible for disassembly of terminal ESCRT III complexes (26, 27). Both ESCRT proteins localize predominantly to the TbRab7+ late endosome, with no apparent lysosomal presence. These results provide two new validated markers for the late endosome in trypanosomes. Both also have a considerable cytosolic localization, as expected for proteins that are recruited to membranes from soluble pools, and consistent with prior fractionation studies with TbVps23 (33). Likewise, the dramatic accumulation of TbVps23 at the late endosome when TbVps4 is ablated is consistent with a dynamic cytoplasmic pool. This result also confirms the evolutionary conservation of TbVps4 as the terminal ESCRT ATPase. Our localization results differ from Leung et al. (33) who found that TbVps23 partially co-localized with the lysosome (p67), the early endosome (TbRab5), and sites of coated vesicle formation (clathrin light chain). However, these authors used ectopic over-expression of tagged TbVps23, which most likely led to mislocalization. Alternatively, a minor presence of TbVps23 in these compartments may be obscured by the diffuse cytoplasmic pool seen in our images. In any case, it is clear that the main localization of membrane associated TbVps23 and TbVps4 is the late endosome, and this must therefore be the primary focus of ESCRT function(s) in BSF trypanosomes. RNAi silencing of both proteins rapidly blocks cell growth suggesting that these functions are essential. However, in other systems the ESCRT machinery does function in additional cellular processes such as centrosome maintenance and cellular abscission during cytokinesis (46, 47). Consequently, although these processes are markedly different in trypanosomes, we cannot formally rule out that detrimental effects on other ESCRT functions contribute to lethality.
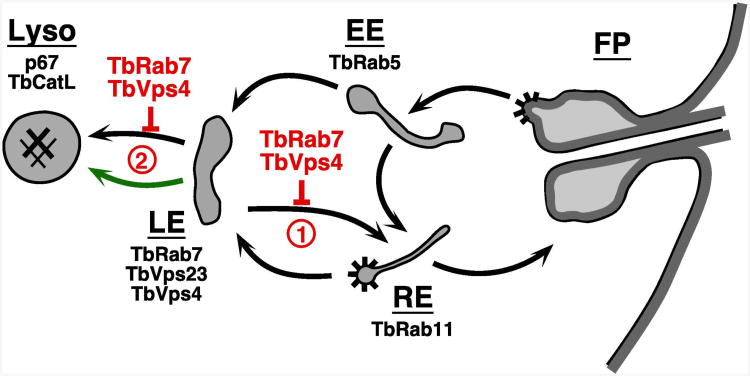
Figure 10. Proposed ISG65 trafficking pathways.
Schematic diagram of the endocytic pathways of trypanosomes. Black arrows indicate documented routes between the flagellar pocket (FP), early endosome (EE), recycling endosome (RE), late endosome (LE), and the terminal lysosome (Lyso). Validated markers for each compartment are: early endosome, TbRab5 (58); recycling endosome, TbRab11 (59); late endosome, TbRab7 (14, 25), TbVps23 and TbVps4 (this work); lysosome, p67 (19, 60), TbCatL (32, 61). Spiny coats indicate known sites of clathrin coated vesicle formation (4, 62). Points at which silencing of TbRab7 and TbVps4 may affect ISG65 trafficking are indicated in red (1 & 2, discussed in text). Green arrow indicates possible alternate accelerated pathway to the lysosome (discussed in text).
With this in mind we have analyzed the effect of TbVps4 ablation on known trafficking routes to the lysosome. Endogenous biosynthetic trafficking was followed with TbCatL and p67. Trafficking of each was negatively impacted, but the effect was greater with p67. These results stand in stark contrast to silencing of TbRab7, which had no effect at all on biosynthetic trafficking of endogenous lysosomal proteins (25). One explanation offered for the previous TbRab7 data is that biosynthetic cargo may actually bypass the late endosome altogether and therefore is not subject to regulation by TbRab7. However, TbVps4 ablation does significantly impact trafficking of biosynthetic cargo suggesting that these cargoes do enter the late endosome en route to the lysosome. Ablation of the ESCRT machinery may disrupt this process, either directly or perhaps indirectly given the modest magnitude of the effect. In contrast, and as found previously for TbRab7 (25), trafficking of the default p67 reporters was unaffected by TbVps4 ablation. This negative result unfortunately provides no additional insight into the routing of this class of cargo to the lysosome. Finally, lysosomal trafficking of endocytic cargo was impaired. Both transferrin and tomato lectin were taken up normally and ultimately delivered to the lysosome. However, the rate at which these cargoes transited the endosomal system, as measured with pH-sensitive TL:FITC, was dramatically retarded. An alternate possibility is that TbVps4 ablation affects trafficking of the vacuolar (H+)ATPase thereby raising pHint throughout the endosomal system. However, extrapolation of the data in Fig. 6 suggests that the pHint of the terminal lysosome in silenced cells is relatively normal arguing against such an effect. In any case, TbRab7 silencing completely blocks lysosomal delivery of endocytic cargo (25), and our data suggest that the ESCRT machinery also influences passage through the late endosome, albeit more subtly.
The most dramatic phenotype seen with TbVps4 silencing was enhanced turnover of ISG65. This type I transmembrane protein is ubiquitinylated on critical lysine residues in the cytoplasmic domain, mutation of which shifts ISG65 from the internal endosomal pool to an external surface location, with a consequent decrease in turnover (39-41). In addition, knockdown of TbVps23 has been reported to negatively impact ISG65 turnover (33). These results suggested that the ESCRT machinery mediates delivery of ISG65 from an endosomal compartmvent to the lysosome for degradation - a model fully consistent with the role of the ESCRT machinery in the turnover of ubiquitinylated membrane proteins in mammalian cells (26-28). In support of this model we find that inhibition of lysosomal proteases blocks ISG65 turnover resulting in dramatic lysosomal accumulation. However, in our hands knockdown of TbVps23 has no effect on ISG65 degradation. This failure can perhaps be explained by redundant function of other ESCRT I components, e.g., TbVps28 [Tb11.01.2510], but TbVps4 knockdown actually gives an ∼2-fold enhancement of turnover, a finding that directly contradicts the prevailing model. We can offer no satisfactory explanation for the discrepancy between our work and that of Leung et al. (33) regarding the role of TbVps23, but several facts argue that our results are correct. First, Leung et al. had only a modest and temporary growth defect (∼2-fold, see Fig. 5 therein) with a dual promoter TbVps23 dsRNA construct, while we see none at all with an identical construct. Second, the error measurements for ISG65 turnover by Leung et al. are probably too large to allow for a meaningful biological effect (see Fig. 9 therein). Third, we find an immediate and complete growth defect with an equivalent TbVps23 stem-loop dsRNA construct, yet ISG65 turnover is completely unimpaired. Finally, and most importantly, Vps4 is the terminal player in the ESCRT cycle and its silencing should be expected to cleanly block ESCRT dependent processes (29). Indeed, we find that TbVps4 silencing gives a rapid and severe growth defect, and under these conditions ISG65 turnover is dramatically accelerated.
Based on these results we propose a new working model for ISG65 trafficking (Fig. 10). ISG65 has a steady state internal pool that is determined by endocytosis and recycling to the cell surface. From this internal pool ISG65 is eventually delivered to the late endosome, from whence if can be recycled to earlier endosomal compartments or delivered to the lysosome These competing processes determines the overall rate of degradation of ISG65, but all ISG65 will eventually be degraded in the lysosome. Within this framework at least two scenarios can account for the observed effects of TbVps4 silencing: 1) TbESCRT mediates ISG65 recycling from the late endosome to earlier endocytic compartments - loss of TbVps4 leads to enhanced delivery to the lysosome. 2) TbESCRT mediates normal ISG65 transport from the late endosome to the lysosome – with ablation of TbVps4 ISG65 enters into an alternate accelerated pathway to the lysosome, perhaps the cryptic default pathway (Fig. 10, green arrow). It must be emphasized that our current data are consistent with either of these scenarios, but we provisionally favor recycling (option #1) since it is the simplest and most direct interpretation. It does at first glance suggest an unconventional ESCRT function in trypanosomes - recycling of cell surface transmembrane proteins. However, the ESCRT machinery is critical for recycling of EGF receptor and claudin-I in some mammalian cell lines so this phenomenon is not without precedent (48, 49).
TbRab7 silencing also accelerated ISG65 turnover; an unexpected finding since delivery of endocytic cargo to the lysosome is critically dependent on TbRab7 (25), and since ISG65 gains access to the lysosome via endosomal compartments. Again, similar scenarios as proposed for TbVsp4 silencing (Fig.10) could account for these results. Whatever the answer our results highlight the complexity (plasticity?) of how lysosomal cargo is handled in the late endosome in trypanosomes. Clearly, more work will be required to illuminate these pathways, but we believe that our model provides a much-needed framework for investigating the role of ESCRT machinery in endocytic trafficking in trypanosomes.
A final comment on the existence of the multivesicular body in trypanosomes is warranted. The acronym ‘MVB’ has been used for many years to label miscellaneous structures in electron micrographs of trypanosomes (3, 30) without resort to currently accepted markers such as TbRab7 and the TbESCRT machinery. More recently, the MVB in trypanosomes has seemingly been elevated to official status based on such markers, but conversely without resort to morphology (33-35). However, the only data cited by these reviews in support of a bona fide MVB structure in trypanosomes are electron micrographs of procyclic cells stressed by RNAi silencing of an AP-1 adaptin subunit (31) (see Fig. 6 therein). We have also observed MVB-like structures in bloodstream form trypanosomes subjected to p67 silencing (32), but such images in stressed cells cannot be used to validate alleged structures in normal cells. Particularly when the target of silencing is not directly involved in ESCRT function. This does not mean that a MVB, defined as a Rab7+ vacuole with intralumenal vesicles, is not present in trypanosomes. Certainly the data presented here and by others (33) suggest that ESCRT driven MVB-like functions exist. The TbRab7+ late endosome in trypanosomes has been characterized as a collection of complex membranous structures with no obvious resemblance to a ‘classic’ MVB (14). But endosomal structures are notoriously sensitive to fixation techniques (36), and perhaps more current methodologies such as high pressure freezing will be helpful in this regard. However, until such a time it seems prudent to avoid using a morphological term for an organelle that is poorly documented in trypanosomes.
Materials and Methods
Parasite cultures
Bloodstream form (BSF) Lister 427 strain T. brucei brucei were grown in HMI-9, as previously described (20, 32). Tetracycline-responsive single-marker BSF cell lines (50) used for inducible expression experiments were maintained in the same media supplemented with tet-free serum (Atlanta Biologicals, Lawrenceville, GA).
Reporter and RNAi constructs
All TbVps4 and TbVps23 constructs are derived from T. b. brucei genes Tb927.3.3280 and Tb11.01.5840, respectively (http://tritrypdb.org). A TbVps4 dsRNA construct was generated using the pLEW100.v5X:Pex11 stem-loop vector (25). Briefly, a 406 bp region (nts 319-724) of the TbVps4 orf, chosen using RNAit (51), was PCR amplified with nested terminal 5' BamHI/XhoI and 3' XbaI/AscI sites. The product was sequentially inserted upstream of the Pex11 stuffer using XhoI/AscI, and then downstream in the opposite orientation using BamHI/XbaI, generating a tetracycline-responsive stem-loop dsRNA plasmid. In an identical manner, a TbVps23 stem-loop construct was created using a 436 bp region (nts 589-1024) of the TbVps23 orf. A second TbVps23 inducible dsRNA construct was created using the dual T7 promoter vector p2T7Ti (52). This construct is identical to that used previously to knockdown TbVps23 (33) and targets the same coding sequence as the above stem-loop construct. The resultant plasmids were linearized with NotI for transfection.
The p67ΔTM-3×HA and p67ΔCD-3×HA default trafficking reporters used here for constitutive over-expression were previously created in pXS6puro (25). These are codons 1-616 (p67ΔTM) and 1-639 (p67ΔCD) of the p67 open reading frame (Tb927.5.1810), each with an in-frame C-terminal 3× HA tag (1×: YPYDVPDYA). These constructs were linearized with NotI for transfection.
An in situ TbVps23 C-terminal 3×HA tagging construct was prepared using the pXS6puro:p67ΔCD-3×HA plasmid. First, the entire TbVps23 ORF was PCR amplified with flanking 5′ HindIII and 3′ XhoI sites and inserted into restricted pXS6:p67ΔCD-3×HA, thereby replacing the p67ΔCD orf with the TbVps23 orf in frame with the C-terminal 3×HA tag. Next, the TbVps23 3′ intergenic region (nts +1 to +448 relative to the TbVps23 stop codon) was PCR amplified with flanking 5' PacI and 3' SacI sites and inserted downstream of the selectable marker. Finally, the entire construct, containing (5′-3′) TbVps23-3×HA, the aldolase intergenic region, the puromycin resistance cassette, and the TbVps23 3′ intergenic region, was excised with HindIII/SacI for transfection. An in situ TbVps4-Ty C-terminal tagging construct was generated using the pXS6neo plasmid. The 5′ targeting sequence was made by PCR amplifying the 3′ end of the TbVps4 ORF (nts 813-1332) with a single in-frame 3′ Ty epitope tag (EVHTNQDPLD) and flanking 5′ ClaI and 3′ XmaI sites. The 3′ targeting sequence was made by PCR amplification of the downstream intragenic region (nts +1 to +556 relative to the TbVps4 stop codon) with flanking 5' PacI and 3′ SacI sites. These fragments were inserted sequentially into pXS6neo using the corresponding restriction sites. The entire construct, containing (5′-3′) TbVps4-Ty, the aldolase intergenic region, the neomycin resistance cassette, and the TbVps4 3′ intergenic region, was excised with ClaI/SacI for transfection. The N-terminal Ty-TbRab7 construct is described elsewhere (25).
All newly constructed plasmids were sequence verified. Linearized plasmids and excised tagging constructs were introduced into the appropriate host cells by electroporation as described previously (53, 54), and clonal cell lines were derived by limiting dilution and selection with the appropriate antibiotic. The TbRab7 RNAi cell line is described elsewhere (25). Induction of TbVps4, TbVps23 and TbRab7 dsRNA in clonal RNAi cell lines was achieved with 1 μg ml-1 of tetracycline.
Antibody, secondary and blotting reagents
Rabbit anti-TbCatL, rabbit anti-BiP, and mouse monoclonal anti-p67 have been described elsewhere (32, 55). Rabbit anti-ISG65 was the kind gift of Professor Mark Carrington (University of Cambridge). It was generated to recombinant protein corresponding to the entire mature extracellular domain (codons 15-355) (56). Mouse anti-Ty was from the UAB Hybridoma Facility (Birmingham, AL) and rabbit anti-HA was from Sigma-Aldrich (St. Louis, MO). Alexa Fluor-conjugated goat secondary antibodies were purchased from Molecular Probes (Eugene, OR). The following ligand conjugates were used for endocytosis assays: transferrin:biotin (Tf:Bio, Molecular Probes); and tomato lectin:biotin and tomato lectin:fluorescein (TL:Bio & TL:FITC, Vector Laboratories, Burlingame, CA). Streptavidin:Alexa Fluor 488 (SA:A488, Molecular Probes) was used for secondary detection of TL:Bio. Horseradish peroxidase (HRP)-conjugated anti-rabbit IgG was from KPL (Gaithersburg, MD).
Radiolabeling and immuoprecipitation
Pulse-chase metabolic radiolabeling with [35S]Met/Cys (Perkin Elmer, Waltham, MA) and subsequent immunoprecipitation of specific radiolabeled proteins from cell lysates were performed as described previously (19). Pulse labeling times were 15 min for p67 and 10 min for TbCatL trafficking; chase times are indicated in the figures. Immunoprecipitations were fractionated by SDS-PAGE and dried gels were analyzed by phosphorimaging using a Typhoon FLA 9000 with native ImageQuant Software (GE Healthcare, Piscataway, NJ). Bands were quantified by volume analysis on areas of interest after subtracting background signal from equivalent unlabeled areas.
ISG65 turnover
Cells were incubated with cycloheximide (100 μg ml-1) to inhibit de novo protein synthesis. As indicated the lyosomal thiol protease inhibitor FMK024 (morpholinourea-phenylalanine-homophenylalanine-fluoromethyl ketone; 20 μM; MP Biomedicals, Aurora, OH) was included prior to and during cycloheximide treatment. Whole cell lysates were fractionated by SDS-PAGE, transferred using an Owl semi dry apparatus (Thermo Fisher Scientific) to an Immobilon-P transfer membrane (Millipore Corp., Bedford, MA), and blocked in a 5% milk solution. Membranes were incubated 1 hr with anti-ISG65 (1/50,000), washed, and incubated with anti-rabbit IgG:HRP (1/10,000, 1 hr). Blots were stripped and reprobed with rabbit anti-BiP. The final washed blots were visualized on X-ray film using Pierce SuperSignal West Pico substrate (Thermo Fisher Scientific), as described elsewhere (57). Film was digitized to 16-bit grayscale using a transparency scanner and bands quantified using the gel volume analysis function of ImageJ 1.46 (NIH, Bethesda, MD).
Quantitative Real Time PCR
The levels of mRNA knockdown during TbVps4 and TbVps23 RNAi were quantified using primers that amplify nts 191-261 of TbVps4 and 324-402 of TbVps23. Single-strand cDNA was synthesized with an anchored oligo(dT) primer using Superscript III Reverse Transcriptase (Invitrogen, Carlsbad, CA) and 10 mg of total RNA as template (Qiagen RNeasy Mini Kit, Valencia, CA). Real time analysis was performed using the StepOne Real-Time PCR System (Applied Biosystems, Carlsbad, CA) with diluted cDNA and the Power SYBR Green PCR Master Mix (Life Technologies, Carlsbad, CA). Post-amplification melting curves confirmed a single dominant product. Specific mRNA levels were determined relative to the reference gene TbGPI8 in triplicate (means ± sem) and were calculated using the StepOne software v2.2.2.
Epifluorescence microscopy
For most immunofluorescence (IFA) microscopy of epitope tags, cells were methanol/acetone-fixed as previously described (32). For imaging with anti-ISG65 cells were formaldehyde fixed/detergent permeablized as described in (12). Cells were stained with primary and secondary antibodies diluted in blocking buffer and mounted as described in (54). Cells were also stained with DAPI (0.5 μg ml-1) to reveal nuclei and kinetoplasts. Serial image stacks (0.2 micron Z-increment) were collected with capture times from 100-500 msec (100× PlanApo, oil immersion, 1.4 na) on a motorized Zeiss Axioimager M2 stand equipped with a rear-mounted excitation filter wheel, a triple pass (DAPI/FITC/Texas Red) emission cube, differential interference contrast (DIC) optics, and an Orca ER CCD camera (Hamamatsu, Bridgewater, NJ). Images were collected with Volocity 6.1 Acquisition Module (Improvision Inc., Lexington, MA) and individual channel stacks were deconvolved by a constrained iterative algorithm, pseudocolored, and merged using Volocity 6.1 Restoration Module. Unless otherwise stated all images presented are summed stack projections of merged channels. The xyz pixel precision of this arrangement has been validated in (54) (see Figure S1 therein). Quantification of TbVps23 fluorescence signal from raw, non-deconvolved images was performed using Image J 1.46. The singular post-nuclear punctate region of TbVps23 signal was selected and the mean gray value of that region was calculated.
Endocytosis assays
Standard binding and uptake assays have been previously described (16). Briefly, for TL uptake washed cells (107 ml-1) were incubated (30 min, 37°C) with TL:Bio (5 μg ml-1) in serum-free HMI9 with 0.5 mg ml-1 BSA, washed, and then incubated in fresh media for 20 min to chase ligand into the terminal lysosome. Tf uptake was performed in the same manner with Tf:Bio (5 μg ml-1), but FMK024 (20 μM) was also included for a 30 min pre-incubation before and during the subsequent uptake and chase periods. In each case cells were processed as described above for IFA and internalized ligands were detected with SA:A488. The kinetics of endocytosis and lysosomal delivery was determined with pH-responsive FITC-conjugated tomato lectin (TL:FITC). Cells were allowed to bind TL:FITC (5 μg ml-1, 5°C, 30 min), washed into fresh medium, and incubated at 37°C to chase bound ligand into internal compartments. At the indicated chase times labeled cells were analyzed on a LSR II flow cytometer (BD Biosciences, San Jose, CA) using native FACSDiva acquisition software and FlowJo analysis software (Tree Star Inc., Ashland, OR), collecting 10,000 events per data point, as previously described (16). Live cells were analyzed with DAPI (5 μg ml-1) staining to exclude dead cells during acquisition.
Statisical analyses
Statistical analyses were performed with Prism 4 software (GraphPad Software, Inc., San Diego CA). Biological replicates (n) are indicated where appropriate.
Supplementary Material
Synopsis.
The role of trypanosomal ESCRT orthologues are investigated in African trypanosomes. TbVps23 (ESCRT I) and TbVps4 (terminal ESCRT ATPase) are essential for in vitro viability. Both are localized to the TbRab7+ late endosome. Knockdown of TbVps4 negatively impacts both biosynthetic and endocytic trafficking to the lysosome, but has no effect on trafficking of default reporters. Turnover of the ubiquitinylated surface protein ISG65 is accelerated in TbVps4 deleted cells. A new model for ESCRT function in trypanosomal late endosomes is proposed.
Acknowledgments
The authors thank Professor Mark Carrington (U. Cambridge) for the generous gift of anti-ISG65. We wish to acknowledge Dr. Shaheen Sutterwala and the students of 2005 Biology of Parasitism Course (Marine Biological Laboratory, Woods Hole, MA) for contributions to the earliest phase of this project. This work was supported by United States Public Health Service Grants R01 AI056866 to JDB. Jason Silverman was supported by NIH “Cellular and Molecular Parasitology” Training Grant (T32 AI07414) to UW-Madison.
Footnotes
The authors have no conflicting interests to report.
References
- 1.Rudenko G. African trypanosomes: the genome and adaptations for immune evasion. Essays Biochem. 2011;51:47–62. doi: 10.1042/bse0510047. [DOI] [PubMed] [Google Scholar]
- 2.Schwede A, Carrington M. Bloodstream form trypanosome plasma membrane proteins: antigenic variation and invariant antigens. Parasitol. 2010;137:2029–2039. doi: 10.1017/S0031182009992034. [DOI] [PubMed] [Google Scholar]
- 3.Langreth SG, Balber AE. Protein uptake and digestion in bloodstream and culture forms of Trypanosoma brucei. J Protozool. 1975;22:40–53. doi: 10.1111/j.1550-7408.1975.tb00943.x. [DOI] [PubMed] [Google Scholar]
- 4.Morgan GW, Allen CL, Jeffries TR, Hollinshead M, Field MC. Developmental and morphological regulation of clathrin-mediated endocytosis in Trypanosoma brucei. J Cell Sci. 2001;114:2605–2015. doi: 10.1242/jcs.114.14.2605. [DOI] [PubMed] [Google Scholar]
- 5.Pamer EG, So M, Davis CE. Identification of a developmentally regulated cysteine protease of Trypanosoma brucei. Mol Biochem Parasitol. 1989;33:27–32. doi: 10.1016/0166-6851(89)90038-8. [DOI] [PubMed] [Google Scholar]
- 6.Balber AE, Bangs JD, Jones SM, Proia RL. Inactivation or elimination of potentially trypanolytic, complement-activating immune complexes by pathogenic trypanosomes. Infect Immun. 1979;24:617–627. doi: 10.1128/iai.24.3.617-627.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barry JD. Capping of variable antigen on Trypanosoma brucei, and its immunological and biological significance. J Cell Sci. 1979;37:287–302. doi: 10.1242/jcs.37.1.287. [DOI] [PubMed] [Google Scholar]
- 8.Engstler M, Pfohl T, Herminghaus S, Boshart M, Wiegertjes G, Heddergott N, Overath P. Hydrodynamic flow-mediated protein sorting on the cell surface of trypanosomes. Cell. 2007;131:505–515. doi: 10.1016/j.cell.2007.08.046. [DOI] [PubMed] [Google Scholar]
- 9.Steverding D, Sexton DW, Wang X, Gehrke SS, Wagner GK, Caffrey CR. Trypanosoma brucei: chemical evidence that cathepsin L is essential for survival and a relevant drug target. Int J Parasitol. 2012;42(5):481–488. doi: 10.1016/j.ijpara.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 10.Engstler M, Bangs JD, Field MC. Intracellular transport systems in trypanosomes: function, evolution and virulence. In: Barry JD, Mottram JC, McCulloch R, Acosta-Serrano A, editors. Trypanosomes - After the Genome. Wymondham, UK: Horizon Scientific Press; pp. 2006pp. 281–317. [Google Scholar]
- 11.Overath P, Engstler M. Endocytosis, membrane recycling and sorting of GPI-anchored proteins: Trypanosoma brucei as a model system. Mol Microbiol. 2004;53:735–744. doi: 10.1111/j.1365-2958.2004.04224.x. [DOI] [PubMed] [Google Scholar]
- 12.Bangs JD. Replication of the ERES:Golgi junction in bloodstream form African trypanosomes. Mol Microbiol. 2011;82:1433–1443. doi: 10.1111/j.1365-2958.2011.07900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Silverman JS, Bangs JD. Form and function in the trypanosomal secretory pathway. Cur Opin Microbiol. 2012;15:463–468. doi: 10.1016/j.mib.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Engstler M, Thilo L, Weise F, Grünfelder CG, Schwarz H, Boshart M, Overath P. Kinetics of endocytosis and recycling of the GPI-anchored variant surface glycoprotein in Trypanosoma brucei. J Cell Sci. 2004;117:1105–1115. doi: 10.1242/jcs.00938. [DOI] [PubMed] [Google Scholar]
- 15.Saftig P, Klumperman J. Lysosome biogenesis and lysosomal membrane proteins: trafficking meets function. Nat Rev Mol Cell Biol. 2009;10(9):623–635. doi: 10.1038/nrm2745. [DOI] [PubMed] [Google Scholar]
- 16.Tazeh NN, Silverman JS, Schwartz KJ, Sevova ES, Sutterwala SS, Bangs JD. The role of AP-1 in developmentally regulated post-Golgi trafficking in Trypanosoma brucei. Euk Cell. 2009;8:1352–1361. doi: 10.1128/EC.00156-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Braulke T, Bonifacino JS. Sorting of lysosomal proteins. Biochim Biophys Acta. 2009;1793(4):605–614. doi: 10.1016/j.bbamcr.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 18.Triggs VP, Bangs JD. Glycosylphosphatidylinositol-dependent protein trafficking in bloodstream stage Trypanosoma brucei. Euk Cell. 2003;2:76–83. doi: 10.1128/EC.2.1.76-83.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alexander DL, Schwartz KJ, Balber AE, Bangs JD. Developmentally regulated trafficking of the lysosomal membrane protein p67 in Trypanosoma brucei. J Cell Sci. 2002;115:3255–3263. doi: 10.1242/jcs.115.16.3253. [DOI] [PubMed] [Google Scholar]
- 20.Tazeh NN, Bangs JD. Multiple signals regulate trafficking of the lysosomal membrane protein p67 in African trypanosomes. Traffic. 2007;8:1007–1017. doi: 10.1111/j.1600-0854.2007.00588.x. [DOI] [PubMed] [Google Scholar]
- 21.Ackers JP, Dhir V, Field MC. A bioinformatic analysis of the RAB genes of Trypanosoma brucei. Mol Biochem Parasitol. 2005;141(141):89–97. doi: 10.1016/j.molbiopara.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 22.Field H, Farjah M, Pal A, Gull K, Field MC. Complexity of trypanosomatid endocytosis pathways revealed by Rab4 and Rab5 isoforms in Trypanosoma brucei. J Biol Chem. 1998;273:32102–32110. doi: 10.1074/jbc.273.48.32102. [DOI] [PubMed] [Google Scholar]
- 23.Pal A, Hall BS, Jefferies TR, Field MC. Rab5 and Rab11 mediate transferrin and anti-variant surface glycoprotein recycling in Trypanosoma brucei. Biochem J. 2003;374:443–451. doi: 10.1042/BJ20030469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hall BS, Smith E, Langer W, Jacobs LA, Goulding D, Field MC. Developmental variation in Rab11-dependent trafficking in Trypanosoma brucei. Euk Cell. 2005;4:971–980. doi: 10.1128/EC.4.5.971-980.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Silverman JS, Schwartz KJ, Hajduk SL, Bangs JD. Late endosomal Rab7 regulates lysosomal trafficking of endocytic but not biosynthetic cargo in Trypanosoma brucei. Mol Microbiol. 2011;82:664–678. doi: 10.1111/j.1365-2958.2011.07842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Henne WM, Buchkovich NJ, Emr SD. The ESCRT pathway. Dev Cell. 2011;21:77–91. doi: 10.1016/j.devcel.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 27.Hanson PI, Cashikar A. Multivesicular body morphogenesis. Annu Rev Cell Dev Biol. 2012;28:337–362. doi: 10.1146/annurev-cellbio-092910-154152. [DOI] [PubMed] [Google Scholar]
- 28.Hagland K, Dikic I. The role of ubiquitylation in receptor endocytosis and endosomal sorting. J Cell Sci. 2012;125:265–275. doi: 10.1242/jcs.091280. [DOI] [PubMed] [Google Scholar]
- 29.Shestakova A, Hanono A, Drosner S, Curtiss M, Davies BA, Katzmann DJ, Babst M. Assembly of the AAA ATPase Vps4 on ESCRT-III. Mol Biol Cell. 2010;21(6):1059–1071. doi: 10.1091/mbc.E09-07-0572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vickerman K. The fine structure of Trypanosoma congolense in its bloodstream phase. J Protozool. 1969;16:54–69. doi: 10.1111/j.1550-7408.1969.tb02233.x. [DOI] [PubMed] [Google Scholar]
- 31.Allen CL, Liao D, Chung WL, Field MC. Dileucine signal-dependent and AP-1-independent targeting of a lysosomal glycoprotein in Trypanosoma brucei. Mol Biochem Parasitol. 2007;156:175–190. doi: 10.1016/j.molbiopara.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 32.Peck RF, Shiflett AM, Schwartz KJ, McCann A, Hajduk SL, Bangs JD. The LAMP-like protein p67 plays an essential role in the lysosome of African trypanosomes. Mol Microbiol. 2008;68:933–946. doi: 10.1111/j.1365-2958.2008.06195.x. [DOI] [PubMed] [Google Scholar]
- 33.Leung KF, Dacks JB, Field MC. Evolution of the multivesicular body ESCRT machinery; retention across the eukaryotic lineage. Traffic. 2008;9:1698–1716. doi: 10.1111/j.1600-0854.2008.00797.x. [DOI] [PubMed] [Google Scholar]
- 34.Field MC, Carrington M. The trypanosome flagellar pocket. Nat Rev Microbiol. 2009;7:775–786. doi: 10.1038/nrmicro2221. [DOI] [PubMed] [Google Scholar]
- 35.Field MC, Lumb JH, Adung'a VO, Jones NG, Engstler M. Macromolecular trafficking and immune evasion in African trypanosomes. Int Rev Cell Mol Biol. 2009;278:1–66. doi: 10.1016/S1937-6448(09)78001-3. [DOI] [PubMed] [Google Scholar]
- 36.Murk JLAN, Posthuma G, Koster AJ, Geuze H, Verkleij AJ, Kleijmeer MJ, Humbel BM. Influence of aldehyde fixation on the morphology of endosomes and lysosomes: quantitative analysis and electron tomography. J Microsc. 2003;212:81–90. doi: 10.1046/j.1365-2818.2003.01238.x. [DOI] [PubMed] [Google Scholar]
- 37.Ziegelbauer K, Multhaup G, Overath P. Molecular characterization of two invariant surface glycoproteins specific for the bloodstream stage of Trypanosoma brucei. J Biol Chem. 1992;267:10797–10803. [PubMed] [Google Scholar]
- 38.Ziegelbauer K, Overath P. Identification of invariant surface glycoproteins in the bloodstream stage of Trypanosoma brucei. J Biol Chem. 1992;267:10791–10796. [PubMed] [Google Scholar]
- 39.Chung WL, Carrington M, Field MC. Cytoplasmic targeting signals in transmembrane invariant surface glycoproteins of trypanosomes. J Biol Chem. 2004;279:54887–54895. doi: 10.1074/jbc.M409311200. [DOI] [PubMed] [Google Scholar]
- 40.Chung WL, Leung KF, Carrington M, Field MC. Ubiquitylation is required for degradation of transmembrane surface proteins in trypanosomes. Traffic. 2008;9(10):1681–1697. doi: 10.1111/j.1600-0854.2008.00785.x. [DOI] [PubMed] [Google Scholar]
- 41.Leung KF, Riley FS, Carrington M, Field MC. Ubiquitylation and developmental regulation of invariant surface protein expression in trypanosomes. Euk Cell. 2011;10(7):916–931. doi: 10.1128/EC.05012-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nolan DP, Geuskens G, Pays E. N-linked glycans containing linear poly-N-acetyllactosamine as sorting signals in endocytosis in Trypanosoma brucei. Curr Biol. 1999;9:1169–1172. doi: 10.1016/S0960-9822(00)80018-4. [DOI] [PubMed] [Google Scholar]
- 43.Ligtenberg MJL, Bitter W, Kieft R, Sterverding D, Janssen H, Calafat J, Borst P. Reconstitution of a surface transferrin binding complex in insect form Trypanosoma brucei. EMBO J. 1994;13:2565–2573. doi: 10.1002/j.1460-2075.1994.tb06546.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Steverding D, Stierhof YD, Chaudri M, Ligtenberg M, Schell D, Beck-Sickinger AG, Overath P. ESAG 6 and 7 products of Trypanosoma brucei form a transferrin binding protein complex. Eur J Cell Biol. 1994;64:78–87. [PubMed] [Google Scholar]
- 45.Allen CL, Goulding D, Field MC. Clathrin-mediated endocytosis is essential in Trypanosoma brucei. EMBO J. 2003;22:4991–5002. doi: 10.1093/emboj/cdg481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morita E, Colf LA, Karren MA, Sandrin V, Rodesch CK, Sundquist WI. Human ESCRT-III and VPS4 proteins are required for centrosome and spindle maintenance. Proc Natl Acad Sci USA. 2010;107:12889–12894. doi: 10.1073/pnas.1005938107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rustin TE, Caccari T, Stenmark H. Shaping development with ESCRTs. Nat Cell Biol. 2012(14):38–45. doi: 10.1038/ncb2381. [DOI] [PubMed] [Google Scholar]
- 48.Baldys A, Raymond Sr., JR Critical role of ESCRT machinery in EGfR recycling. Biochem. 2009;48:9321–9323. doi: 10.1021/bi900865u. [DOI] [PubMed] [Google Scholar]
- 49.Dukes JD, Fish L, Richardson JD, Blaikley E, Burns S, Caunt CJ, Chalmers AD, Whitley P. Functional ESCRT machinery is requird for constitutive recycling of claudin-1 and maintenence of polarity in vertebrate epithelial cells. Mol Biol Cell. 2011;22:3192–3205. doi: 10.1091/mbc.E11-04-0343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wirtz E, Leal S, Ochatt C, Cross G. A tightly regulated inducible expression system for conditional gene knockouts and dominant-negative genetics in Trypanosoma brucei. Mol Biochem Parasitol. 1999;99:89–101. doi: 10.1016/s0166-6851(99)00002-x. [DOI] [PubMed] [Google Scholar]
- 51.Redmond S, Vadivelu J, Field MC. RNAit: an automated web-based tool for the selection of RNAi targets in Trypanosoma brucei. Mol Biochem Parasitol. 2003;128(1):115–118. doi: 10.1016/s0166-6851(03)00045-8. [DOI] [PubMed] [Google Scholar]
- 52.LaCount DJ, Bruse S, Hill KL, Donelson JE. Double-stranded RNA interference in Trypanosoma brucei using head-to-head promoters. Mol Biochem Parasitol. 2000;111:67–76. doi: 10.1016/s0166-6851(00)00300-5. [DOI] [PubMed] [Google Scholar]
- 53.Burkard G, Fragoso CM, Roditi I. Highly efficient stable transformation of bloodstream forms of Trypanosoma brucei. Mol Biochem Parasitol. 2007;153:220–223. doi: 10.1016/j.molbiopara.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 54.Sevova ES, Bangs JD. Streamlined architecture and GPI-dependent trafficking in the early secretory pathway of African trypanosomes. Mol Biol Cell. 2009;20:4739–4750. doi: 10.1091/mbc.E09-07-0542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McDowell MA, Ransom DA, Bangs JD. Glycosyl phosphatidylinositol-dependent secretory transport in Trypanosoma brucei. Biochem J. 1998;335:681–689. doi: 10.1042/bj3350681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ziegelbauer K, Overath P. Organization of two invariant surface glycoproteins in the surface coat of Trypanosoma brucei. Infect Immun. 1993;61:4540–4545. doi: 10.1128/iai.61.11.4540-4545.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gruszynski AE, DeMaster A, Hooper NM, Bangs JD. Surface coat remodeling during differentiation of Trypanosoma brucei. J Biol Chem. 2003;278:24665–24672. doi: 10.1074/jbc.M301497200. [DOI] [PubMed] [Google Scholar]
- 58.Pal A, Hall BS, Nesbeth DN, Field HI, Field MC. Differential endocytic functions of Trypanosoma brucei Rab5 isoforms reveal a glycosylphosphatidylinositol-specific endosomal pathway. J Biol Chem. 2002;277:9529–9539. doi: 10.1074/jbc.M110055200. [DOI] [PubMed] [Google Scholar]
- 59.Jefferies TR, Morgan GW, Field MC. A developmentally regulated rab11 homologue in Trypanosoma brucei is involved in recycling processes. J Biol Chem. 2001;114:2617–2626. doi: 10.1242/jcs.114.14.2617. [DOI] [PubMed] [Google Scholar]
- 60.Brickman MJ, Balber AE. Transport of a lysosomal membrane glycoprotein from the Golgi to endosomes and lysosomes via the cell surface in African trypanosomes. J Cell Sci. 1994;107:3191–3200. doi: 10.1242/jcs.107.11.3191. [DOI] [PubMed] [Google Scholar]
- 61.Mbawa ZR, Webster P, Lonsdale-Eccles JD. Immunolocalisation of a cysteine protease within the lysosomal system of Trypanosoma congolense. Eur J Cell Biol. 1991;56:242–250. [PubMed] [Google Scholar]
- 62.Grünfelder CG, Engstler M, Wieise F, Schwarz H, Stierhof YD, Morgan GW, Field MC, Overath P. Endocytosis of a glycosylphosphatidylinositol-anchored protein via clatherin-coated vesicles, sorting by default in endosomes and exocytosis via RAB11-positive carriers. Mol Biol Cell. 2003;14:2029–2040. doi: 10.1091/mbc.E02-10-0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.