Summary
The bone marrow (BM) microenvironment is composed of multiple niche cells that, by producing angiocrine factors, maintain and regenerate the hematopoietic stem cell (HSC) pool (Morrison and Spradling, 2008). We have previously demonstrated that endothelial cells support the proper regeneration of the hematopoietic system following myeloablation (Butler et al., 2010; Hooper et al., 2009; Kobayashi et al., 2010). Here, we demonstrate that expression of the angiocrine factor Jagged-1, supplied by the BM vascular niche, regulates homeostatic and regenerative hematopoiesis through a Notch-dependent mechanism. Conditional deletion of Jagged-1 in endothelial cells (Jag1(ECKO) mice) results in a profound decrease in hematopoiesis and premature exhaustion of the adult HSC pool, while quantification and functional assays demonstrate that loss ofJagged-1 does not perturb vascular or mesenchymal compartments. Taken together, these data demonstrate that the instructive function of endothelial-specific Jagged-1 is required to support the self-renewal and regenerative capacity of HSCs in the adult BM vascular niche.
Introduction
The bone marrow (BM) microenvironment is a complex system comprised of specialized niche cells that regulate the maintenance of the hematopoietic stem cell (HSC) pool through the production of pro-hematopoietic factors (Morrison and Spradling, 2008). Cross talk among various niche cells maintains and regenerates HSCs. However, the precise mechanism by which niche cells communicate with the HSCs and their progeny to reconstitute hematopoiesis is unknown. Osteoblasts have been shown to sustain the quiescence of HSCs by elaboration of specific growth factors (Adams et al., 2006; Lo Celso et al., 2009; Xie et al., 2009; Yoshihara et al., 2007). It has been strongly implicated that the BM vascular niche, which consists of a vast network of thin-walled, fenestrated sinusoidal endothelial cells and perivascular stromal cells, can provide the proper milieu of pro-hematopoietic factors that are needed to support the HSC pool (Butler et al., 2010; Ding and Morrison, 2013; Ding et al., 2012; Himburg et al., 2010; Hooper et al., 2009; Kobayashi et al., 2010; Mendez-Ferrer et al., 2010; Sugiyama et al., 2006; Yamazaki et al., 2011).
Our group has previously demonstrated that Akt-activated endothelial cells are indispensable for the regeneration of the Notch-dependent HSC pool following hematopoietic insult (Butler et al., 2010). Here, we demonstrate that conditional deletion of Jagged-1 in endothelial cells (Jag1(ECKO)) results in a decrease in phenotypic and functional long-term (LT)-HSCs at steady state, as well as a significant decrease in Notch signaling in hematopoietic stem and progenitor cells (HSPCs). Furthermore, Jag1(ECKO) mice have a profound deficiency in hematopoietic recovery following sublethal irradiation leading to the ultimate demise of half of the mice. Cell cycle analysis of LT-HSCs from Jag1(ECKO) mice revealed that a significant portion of the HSC pool was actively cycling. Serial administration of low-dose chemotherapeutic agents and secondary and tertiary transplantation assays results in the premature exhaustion of the LT-HSC in Jag1(ECKO) mice, confirming that endothelial-specific expression of Jagged-1 maintains the quiescence and self-renewal of LT-HSCs. Therefore, expression of Jagged-1 by the vascular niche supports functional hematopoiesis by preventing premature exhaustion of the HSC pool. Modulating the angiocrine repertoire of endothelium could lead to the discovery of as yet unrecognized instructive factors that augment the use of HSCs for the treatment of hematological disorders.
Results
Maintenance of the HSC pool requires endothelial-specific expression of Jagged-1
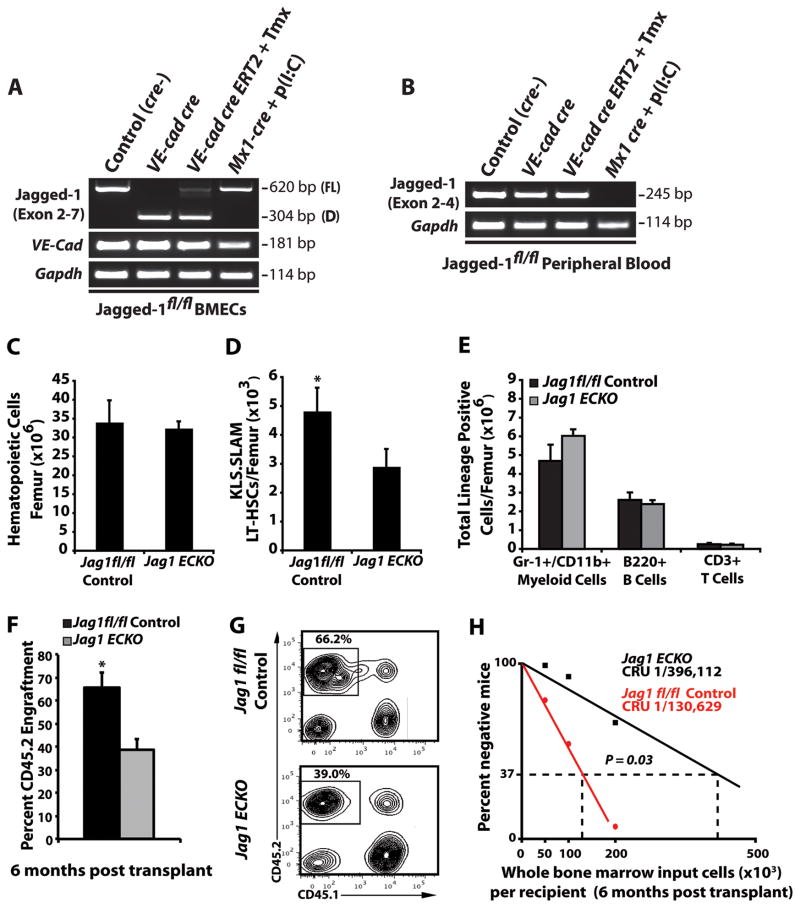
Previous reports have demonstrated that using the conditional Mx1 cre transgene to delete Jagged-1 in the cellular compartments within the BM microenvironment did not result in phenotypic or functional defects in the hematopoietic system (Mancini et al., 2005). In order to determine the most efficient in vivo model system to delete Jagged-1 specifically in endothelial cells, we generated two endothelial cell-specific cre transgenic models. We utilized the constitutive VE-cadherin cre and the inducible VE-cadherin creERT2, and compared these systems to the previously described inducible Mx1 cre system (Figure 1A, B and Supplemental Figure 1). Isolation of BM endothelial cells demonstrated that both the constitutive VE-cadherin cre and the inducible VE-cadherin creERT2 were efficient at deleting floxed Jag1 exons 4–5. However, only the constitutive VE-cadherin cre resulted in complete excision of exons 4–5 in the Jag1 gene. Notably, the inducible Mx1 cre system did not delete Jagged-1 in BM endothelial cells, with transcript levels similar to controls (Figure 1A and Supplemental Figure 1). Analysis of peripheral blood confirmed that induction of Mx1 cre with Poly(I:C) resulted in complete excision of Jag1 exons 4–5, while both VE-cadherin cre systems did not affect Jag1 transcript in hematopoietic cells (Figure 1B and Supplemental Figure 1) as compared to controls. Therefore, these data suggest that the previous report demonstrating that expression of Jagged-1 in the BM microenvironment did not regulate hematopoiesis is due to the inefficient deletion of endothelial-specific Jagged-1.
Figure 1. Maintenance of the HSC pool requires endothelial-specific expression of Jagged-1.
Constitutive VE-cadherin cre transgenic mice promote efficient in vivo deletion of an exon 4–5 floxed Jag1 allele in BM endothelial cells. Jag 1fl/fl conditional mice were crossed with either constitutive VE-cadherin cre, tamoxifen (Tmx) inducible VE-cadherin creERT2, or Poly(I:C) inducible Mx1 cre mice and cDNA was synthesized from either BM endothelial cells sorted to purity (CD45−, Ter119−, VE-Cadherin+, Isolectin GS-IB4+) or peripheral blood. A)RT -PCR using primers spanning conditional Jag1 exons 4–5 were used to assay for the extent of full length (FL) and deleted (D) transcripts in BM ECs. VE-cadherin expression was used to assess for purity. B)RT -PCR analysis of peripheral blood using Jag1 specific primers located within floxed exon 4 demonstrate efficient elimination of exon 4 containing transcripts in Mx1 cre, but not constitutive VE-cadherin cre or inducible VE-cadherin creERT2 mice. C, D) Steady state analysis of control and Jag1(ECKO) mice demonstrates similar levels in BM cellularity C) but a significant increase in the frequency of phenotypic LT-HSC per 106 whole BM cells D) as compared to Jag1fl/fl controls (n=12/cohort). E) Analysis of lineage-committed progenitors shows no significant differences in Jag1(ECKO) mice as compare to Jag1fl/fl controls (n=8 control mice; n=10 Jag1(ECKO) mice). F) To test if whole BM from Jag1(ECKO) mice had decreased engraftment potential, we performed a competitive repopulation assay and demonstrated that whole BM from wild type donors were significantly more efficient at sustaining long-term repopulating ability as compared to Jag1(ECKO) mice. G) Representative contour plots of CD45.1 vs. CD45.2 engraftment 4 months post transplant (n=5/cohort, 3 independent experiments). H) Limiting dilution analysis of the frequency of long-term, multilineage reconstitution cells in Jag1(ECKO) mice as compared to Jag1fl/fl controls (n= 20/cohort, 3 independent experiments). All data represents mean ± s.d. (*P<0.05). See also Figure S1 and Figure S2.
Since constitutive VE-cadherin cre resulted in complete deletion of exons 4–5 of Jag1 in BM endothelial cells (Jag1(ECKO) mice), we analyzed 8–12 week old Jag1(ECKO) miceand they did not exhibit any defects in BM cellularity or the ratios of Lin+ hematopoietic cells (Figure 1C, E). However, endothelial-specific deletion of Jagged-1 resulted in a significant decrease in the number of phenotypic LT-HSCs (Figure 1D). To confirm that the decrease in phenotypic LT-HSCs resulted in a defect in stem cell function, we examined the long-term repopulation capacity of whole BM from control and Jag1(ECKO) mice in BM transplantation assays. We quantified the engraftment potential of whole BM cells from control and Jag1(ECKO) mice by performing a competitive repopulation assay, in which 5 × 105 hematopoietic cells from CD45.2 Jag1fl/fl control and Jag1(ECKO) mice were competed with 5 × 105 hematopoietic cells from CD45.1 mice. These experiments revealed a significant decrease in the engraftment potential of hematopoietic cells isolated from Jag1(ECKO) mice (Figure 1F, G). Additionally, we performed limiting dilution transplantation studies and assessed the long-term, multilineage reconstitution ability of whole BM cells from control and Jag1(ECKO)mice to determine the frequency of functional HSCs. We found that there was a 3-fold decrease in the frequency of long-term, multilineage repopulating cells in Jag1(ECKO) mice (1/130,629 control (Jag1fl/fl control) versus 1/396,112 Jag1(ECKO), respectively) (Figure 1H).
Endothelial-specific deletion of Jagged-1 inteferes with Notch signaling in HSPCs
To date, the in vivo regulation of HSC self-renewal and differentiation by Notch signaling has not been fully determined. The use of a wide array of genetic tools have suggested that Notch signaling is dispensible for the maintenance of the hematopoietic system under steady state conditions (Maillard et al., 2008). However, this study did not demostrate whether there was a significant decrease in Notch downstream target genes following genetic inhibition of canonical Notch signaling in the HSPCs. Nonetheless, studies have demonstrated that a Jagged-1-Notch 2 receptor interaction is efficient to maintain primitive HSPCs (Varnum-Finney et al., 1998) and that Notch 2 dictates the rate of hematopoietic recovery following myeloablative stress (Varnum-Finney et al., 2011), suggesting a role for Notch signaling in homeostatic and regenerative hematopoiesis.
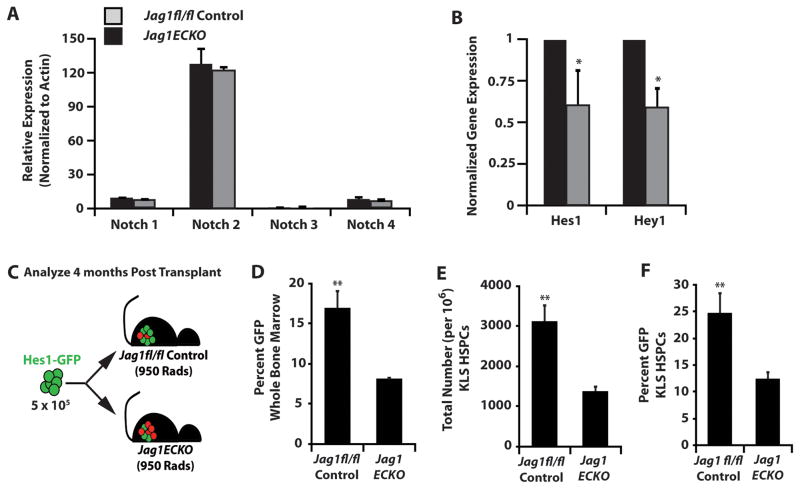
To determine if endothelial-specific deletion of Jagged-1 results in a significant decrease of Notch signaling in HSPCs (cKit+Lineage−Sca-1+, KLS), we first analyzed the expression levels of Notch receptors on HSPCs in Jag1fl/fl control and Jag1(ECKO) mice (Figure 2A). Our data confirms that the primary Notch receptor expressed on HSPCs is Notch 2 (Oh et al., 2013). We next analyzed the expression of downstream Notch target genes and found that there was a significant decrease in Hes1 and Hey1 expression in Jag1(ECKO) HSPCs as compared to Jag1fl/fl control HSPCs (Figure 2B). To formally quantify the decrease in the primary Notch downstream target Hes1 in Jag1(ECKO) HSPCs, we utilized a Hes1-GFP knock-in mouse model (Fre et al., 2011) and transplanted 5 × 105 whole BM cells from Hes1-GFP mice into Jag1fl/fl control and Jag1(ECKO) mice and allowed for the hematopoietic system to fully recover for 4 months (Figure 2C). At 4 months post-transplant, we analyzed the percentage of GFP+ whole BM cells circulating in the peripheral blood and found that there was a 2-fold decrease in GFP expression (Figure 2D). Additionally, we analyzed the number of KLS cells per 106 hematopoietic cells and found that there was a profound decrease in the frequency of KLS cells in Jag1(ECKO) mice, as well as a significant decrease in the percentage of GFP+ cells in the KLS fraction of Jag1(ECKO) mice as compared to Jag1fl/fl controls (Figure 2E, F). Therefore, endothelial-specific deletion of Jagged-1 can directly lead to defects in HSPCs in a Notch-dependent manner.
Figure 2. Endothelial-specific deletion of Jagged-1 interferes with Notch signaling in HSPCs.
A) KLS HSPCs were sorted from control and Jag1(ECKO) mice and analyzed for Notch receptors and B) downstream Notch target genes. There were no significant differences between A) Notch receptors but significant decrease in the B) Notch target genes Hes1 and Hey1 in the Jag1(ECKO) KLS cells. C) Whole BM (5 × 105) from Hes1-GFP knock-in mice was transplanted into lethally irradiated control and Jag1(ECKO) mice and allowed to recover for 4 months. D) Percentage of GFP+ whole bone marrow. E) Total number of KLS cells and F) percentage of GFP+ KLS cells. There was a significant decrease in whole BM and KLS cells positive for Hes1 GFP in Jag1(ECKO) mice.
Endothelial-specific deletion of Jagged-1 impairs ex vivo expansion of Notch+ KLS cells
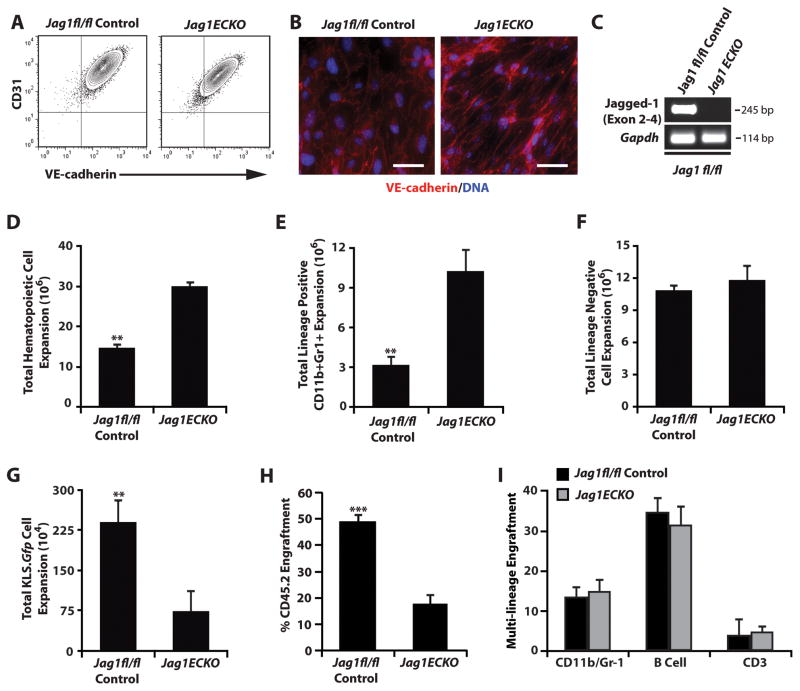
To formally test if expression of Jagged-1 supplied specifically by the endothelial cells is required for the expansion and maintenance of the hematopoietic system, we isolated endothelial cells from Jag1fl/fl control and Jag1(ECKO) mice (Figure 3A–C). Utilizing an endothelial cell/HSC co-culture system previously described in our laboratory (Butler et al., 2010; Kobayashi et al., 2010), we isolated KLS cells from Notch.GFP reporter mice (Duncan et al., 2005) and co-cultured them with endothelial cells isolated from control or Jag1(ECKO) mice in serum-free culture conditions supplemented with 50 ng/mL of murine stem cell factor for 7 days. Notch.GFP reporter mice are transgenic mice that were generated using an EGFP cassette that was placed under the control of the CBF1/RBPJ promoter. Cells that engage in Notch receptor/Notch ligand signaling initiate expression of target genes, as well as expression of EGFP which allows for the accurate monitoring of Notch signaling in our co-culture system. Upon analysis, we found that total hematopoietic expansion was dramatically higher in Jag1(ECKO) culture conditions (Figure 3D), but that total Lineage negative expansion was similar in both control and Jag1(ECKO)co -culture systems (Figure 3F). However, there was a significant decrease in the total number of KLS.GFP+hematopoietic cells when co-cultured with Jag1(ECKO) endothelial cells compared with a Jag1fl/fl control (Figure 3G). Analysis of the Lineage+ fraction demonstrated that the majority of the terminally differentiated cells were CD11b+/Gr-1+ myeloid cells in both co-culture conditions, with co-cultures of KLS cells with Jag1(ECKO) endothelial cells resulting in a significant increase in myeloid cells as compared to Jag1fl/fl control endothelial cells (Figure 3E). Upon transplantation of equal number of KLS cells from each co-culture condition, hematopoietic cells co-cultured with control endothelial cells had a significant advantage in their engraftment potential and similar levels of donor-derived multi-lineage engraftment when compared to hematopoietic cells co-cultured with Jag1(ECKO) endothelial cells (Figure 3H, I).
Figure 3. Endothelial-specific deletion of Jagged-1 impairs ex vivo expansion of Notch+ KLS cells.
A–C) Confirmation of endothelial cell phenotype of isolated endothelium from control and Jag1(ECKO) mice. A) Flow cytometric analysis of cultured endothelial cells based on the co-expression of endothelial-specific markers CD31 and VE-cadherin. B) Fluorescent images demonstrating junctional VE-cadherin staining of cultured endothelial cells. C) Confirmation of Jagged-1 deletion in cultured Jag1(ECKO) endothelium. D–F) 50,000 Lineage-hematopoietic cells from Notch.GFP reporter mice were co-cultured with control and Jag1(ECKO) endothelial cells for 7 days in serum-free culture conditions supplemented with 50 ng/mL of stem cell factor. Hematopoietic cells co-cultured with Jag1(ECKO) endothelial cells resulted in a significant increase in expansion of the total number of hematopoietic cells D) with the majority of the cells being CD11b+Gr1+ Lineage cells E) but with no change in the expansion of Lineage− cells F) when compared to control endothelial cells. However, there was a significant decrease in the total number of KLS cells (data not shown) and KLS cells that were actively signaling through the Notch pathway (GFP+) G). H–I) Competitive repopulation of expanded hematopoietic cells demonstrates that Lineage hematopoietic cells that were co-cultured with control endothelial cells resulted in the expansion of HSCs with a greater H) engraftment potential with similar I) multilineage potential as compared to Jag1(ECKO) endothelial cells. All data represents mean ± s.d. (**P<0.01, **P<0.001); scale bar 50μm.
Endothelial-specific deletion of Jagged-1 inhibits hematopoietic regeneration
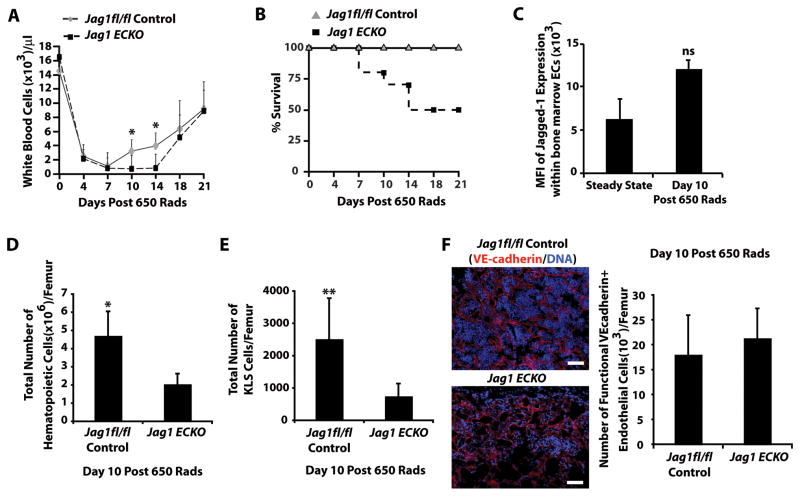
To address the contribution of endothelial-specific expression of Jagged-1 to reconstitution of hematopoiesis, we utilized the aforementioned Jag1(ECKO) mice. To this end, control and Jag1(ECKO) mice were subjected to 650 Rads of sublethal irradiation and recovery of white blood cells and survival of the cohorts were monitored (Figure 4A, B). The difference in survival was significant between the two cohorts, with 50% of the Jag1(ECKO) mice failing to thrive by day 18 post sublethal radiation (Figure 4B). Analysis of Jagged-1 expression in BM endothelial cells at steady state and day 10 post sublethal radiation demonstrated a non-significant increase in Jagged-1 expression following the myelosuppressive insult in control mice (Figure 4C). Since the Jag1(ECKO) mice began dying at day 10 post irradiation, we quantified the total number of hematopoietic and KLS cells at day 10 post irradiation, which exhibited a significant decrease in total and KLS hematopoietic cell number (Figure 4D, E) in the Jag1(ECKO) mice when compared with controls. Upon examination of the diaphysis and trabecular regions of the BM cavity on day 10 post-irradiation, we found that the structural integrity of the BM vascular network was largely intact and most of the hematopoietic cells were found as tightly associated cellular packs within the BM parenchyma in control mice as compared to Jag1(ECKO) mice (Figure 4F). However, it is important to note that there were no differences in the total number of perfusable BM endothelial cells (Figure 4F).
Figure 4. Endothelial-specific deletion of Jagged-1 interferes with hematopoietic regeneration.
A) Analysis of white blood cell recovery in Jag1(ECKO) mice following sublethal irradiation of 650 Rads (n=10/cohort up to day 10, n=6 for duration of analysis, 3 independent experiments). B) Survival curve of control and Jag1(ECKO) mice post sublethal irradiation. All of the control mice fully recover and survived. However, Jag1(ECKO) mice begin succumbing to hematopoietic failure by day 10 and 50% of the cohort has died by day 18 post sublethal irradiation. C) Jagged-1 expression in BM endothelial cells at steady state and day 10 post sublethal irradiation. D, E) Analysis of the BM at day 10 post-sublethal irradiation demonstrated that Jag1(ECKO) mice have a significant decrease in BM cellularity D) and a decrease in KLS HSPCs E) as compared to controls (n=4/cohort, 3 independent experiments). F) Representative fluorescent images of the diaphysis and trabecular regions of Jag1fl/fl and Jag1(ECKO) mice as well as quantification of functional BM endothelial cells. Note the increase in BM cellular integrity in control mice as compared to Jag1(ECKO) mice without any significant changes in vascular density within the BM. All data represents mean ± s.d. (*P<0.05, **P<0.01); scale bar 50μm.
Endothelial-specific deletion of Jagged-1 does not result in postnatal vascular deficiencies
It is plausible that the hematopoietic failure demonstrated in Jag1(ECKO) mice was due to a decrease in the passive function of endothelial cells to deliver the proper nutrients and oxygen necessary to promote efficient regeneration of the hematopoietic system. Indeed, Jagged-1 has been demonstrated to play a major role in vascular integrity during embryonic organogenesis. Global knockout of Jagged-1 leads to embryonic lethality due to vascular abnormalities, including vascular dropout in the vast endothelial network of the yolk sac and forebrain (High et al., 2008; Robert-Moreno et al., 2008; Xue et al., 1999), as well as disrupted vascular smooth muscle cell differentiation. Furthermore, in Jag1(ECKO) mice that were generated using the inducible Pdgfb-iCreERT2 system, one could detect a decreased vascular density in the developing postnatal retina with decreased arterial vascular smooth muscle coverage (Benedito et al., 2009). Utilizing our constitutiveVE -cadherin cre system, we analyzed postnatal (P6) retinal development and found that there was no significant decrease in the vascular density of Jag1(ECKO) mice when compared to control retinas (Figure 5A, B). To assess the integrity of the BM vascular niche during homeostatic hematopoiesis, control and Jag1(ECKO) mice were intravitally injected via the orbital artery with low doses of an Alexa Fluor 647 conjugated VE-cadherin antibody (10 μg/mouse) 10 minutes before sacrifice to visualize the number and morphology of perfused vessels and to analyze the architecture of the hematopoietic compartment (Figure 5C). Subsequently, flow cytometry was used to quantify the number of patent and functional CD45−Ter119−VEcadherin+ BM endothelial cells (Figure 5D). Using this approach, we demonstrated that the total number of functionally perfusable VE-cadherin+ BM endothelial cells that retained their anastomosis to circulation was similar in Jag1(ECKO) and control mice (Figure 5D). Furthermore, we tested whether Jagged-1 modulated the angiocrine profile of BM endothelial cells by disrupting functional capacity of BM endothelial cells to serve as niche cells. To this end, we isolated BM endothelial cells from control and Jag1(ECKO) mice and performed quantitative RT-PCR for known pro-hematopoietic angiocrine factors (Figure 5E and Supplemental Table 1). We found that the majority of the angiocrine factors screened, including Kit-Ligand (Ding et al., 2012) and Sdf-1 (Ding and Morrison, 2013), did not result in significant changes when Jagged-1 was deleted from endothelial cells. However, we did find a small, but significant, increase in Vcam-1 and an upward trend in E-selectin expression in endothelial cells lacking Jagged-1 (Figure 5E, Panel 1). The increase in adhesion molecules led us to hypothesize that there could be a defect in the homing of transplanted HSPCs (Lapidot et al., 2005; Papayannopoulou and Craddock, 1997). To address these concerns, we performed a 16-hour homing assay. We found that there were no significant differences in the homing of HSPCs when assayed in the Jag1(ECKO) background (Figure 5F). These data further support our conclusion that endothelial-specific Jagged-1 directly regulates the maintenance and self-renewal of the HSC pool.
Figure 5. Jag1(ECKO) mice do not manifest postnatal vascular deficiencies that lead to hematopoietic dysfunction.
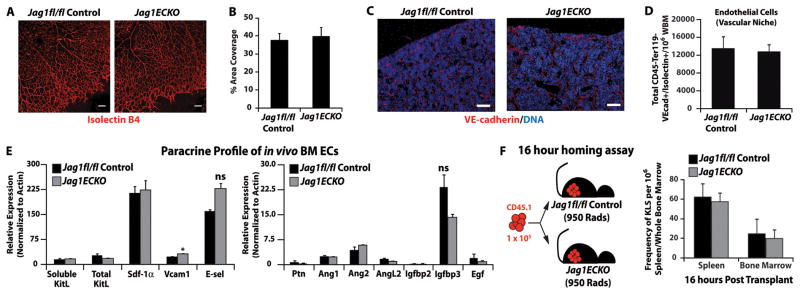
A) Visualization of the retinal vasculature at P6 using isolectin B4 immunofluorescence shows no change in vascular density in Jag1(ECKO) mice, as compared to littermate controls. B) Quantification of vascular parameters at P6 in Jag1fl/fl controls and Jag1(ECKO) retinas. n=3 per group/2 independent experiments. C) Visualization of the BM vascular niche (VE-cadherin-Alexa647/DAPI nuclear counterstain). D) Quantification of the BM vascular niche shows no significant difference between the numbers of functionally patent CD45−Ter119−VEcadherin+Isolectin B4+ BM endothelial cells. N=5 for each experimental group for three independent experiments. E) Bone marrow endothelial cells were isolated and subjected to angiocrine profiling for known pro-hematopoietic angiocrine factors. The angiocrine profile was not significantly different between the Jag1fl/fl and Jag1(ECKO) cohorts except for Vcam1. N=3 for each experiment group. Due to the role of Vcam in homing of HSPCs, we performed a F) 16 hour homing assay where 105 whole BM from CD45.1 mice was transplanted into lethally irradiated Jag1fl/fl controls and Jag1(ECKO) mice and analyzed for donor engraftment 16 hours post transplant. Note that there were no significant differences in the homing of wild type whole BM into control and Jag1(ECKO) recipients. NS=non-significant, scale bar 50μm.
To address whether Notch signaling was directly affected by deletion of Jagged-1 in endothelial cells, we isolated BM endothelial cells from control and Jag1(ECKO) mice and analyzed the expression levels of Notch receptors, Notch ligands, and downstream Notch targets. We found that there were similar levels of Notch receptors and ligands, with the exception of Jagged-1. However, our analysis revealed that there was a significant decrease in Notch downstream targets suggesting that Notch signaling is altered in Jag1(ECKO) BM endothelial cells (Supplemental Figure 2). Although the decrease in endothelial Notch signaling suggests a potential indirect effect that could lead to the hematopoietic defects seen in Jag1(ECKO) mice, our postnatal retinal angiogenic assay suggest that the partial inhibition of Notch signaling in BM endothelial cells does not perturb postnatal vascular integrity that might alter the instructive function of the BM vascular niche in supporting the reconstitution of the hematopoietic system. This data, along with the finding that the angiocrine profiles of control and Jag1(ECKO) mice are similar (Figure 5), suggests that endothelial-specific deletion of Jagged-1 negatively regulates hematopoietic function in a Notch-dependent manner due to the loss of their instructive capacity to support the maintenance and regeneration of the hematopoietic system.
Endothelial-specific Jagged-1 does not alter the function of non-endothelial BM niche cells
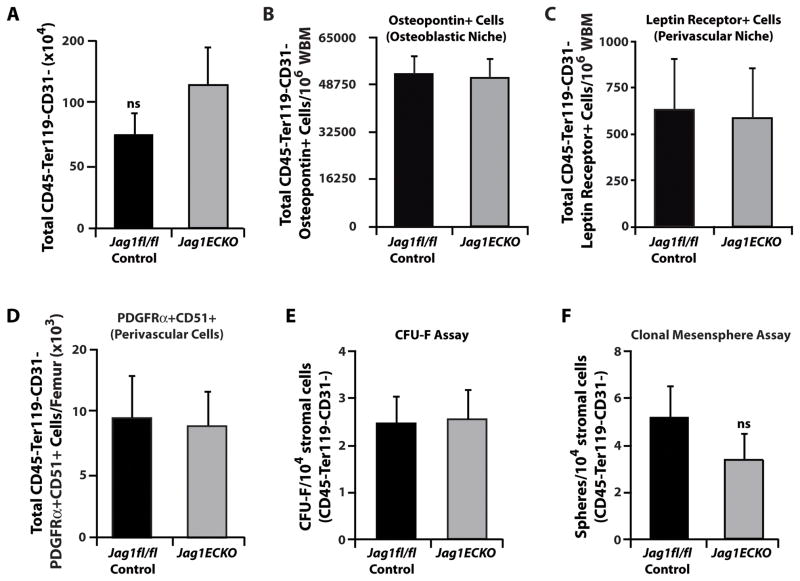
While Notch signaling in HSPCs is inhibited following deletion of EC-specific Jagged-1, it is still plausible that the hematopoietic defects demonstrated in Jag1(ECKO) mice could be due to altered numbers of other BM niche cells or inhibition of proper mesenchymal stem cell (MSC) function (Hilton et al., 2008). To address this issue, we analyzed if non-endothelial BM niche cells were affected by the endothelial-specific deletion of Jagged-1. We found that the total number of non-vascular niche cells, including CD45−Ter119−CD31− stromal cells, Osteopotin+ osteoblasts (Nilsson et al., 2005), and PDGFRα+ CD51+ MSCs (Frenette et al., 2013; Pinho et al., 2013) or Leptin Receptor+ (Ding et al., 2012) perivascular niche cells, were similar between control and Jag1(ECKO) mice (Figure 6A–D). Furthermore, we have isolated CD45−Ter119−CD31− stromal cells from control and Jag1(ECKO) mice and assessed their ability to perform in functional mesenchymal stem/progenitor assays (Figure 6E, F). We performed colony-forming unit-fibroblast assays and clonal mesensphere assays to quantify functional MSCs (Mendez-Ferrer et al., 2010). We found that there were no significant differences in the mesenchymal stem/progenitor functional capacity of stromal CD45−Ter119−CD31− BM cells between control and Jag1(ECKO) mice (Figure 6E, F). Therefore, these data suggests that Jagged-1 deletion in endothelial cells does not alter the function of non-vascular components of the BM microenvironment.
Figure 6. Jag1(ECKO) mice do not manifest alterations to the mesenchymal stem cell or perivascular niches in the BM microenvironment.
A) Quantification of non-vascular stromal cells. B–D) Within the non-vascular niche cell compartment we quantified B) Osteopontin+ osteoblast niche cells and C) Leptin Receptor+ and D) PDGFRα+/CD51+ perivascular niche cells. Note that endothelial-specific deletion of Jagged-1 does not result in dropout of other critical BM niche cells. n=9 per cohort/3 independent experiments. E–F) MSC activity of CD45− Ter119− CD31− stromal cells in Jag1fl/fl and Jag1(ECKO) mice. No differences were detected in the E) number of CFU-Fs and F) clonal mesenspheres derived from 10,000 sorted CD45− Ter119− CD31− bone marrow stromal cells isolated from Jag1fl/fl and Jag1(ECKO) mice. Each functional assay was independently repeated three times with n=3 mice per test. These data suggest that the hematopoietic defect observed in Jag1(ECKO) mice is not an indirect result of alterations to the architectural integrity and geometry of the BM niche, rather a direct instructional role of endothelial cell-specific Jagged-1 expression. N=9 for each experimental group for three independent experiments. All data represents mean ± s.d., ns (statistically non significant).
Endothelial cell Jagged-1 regulates quiescence and self-renewal of HSCs
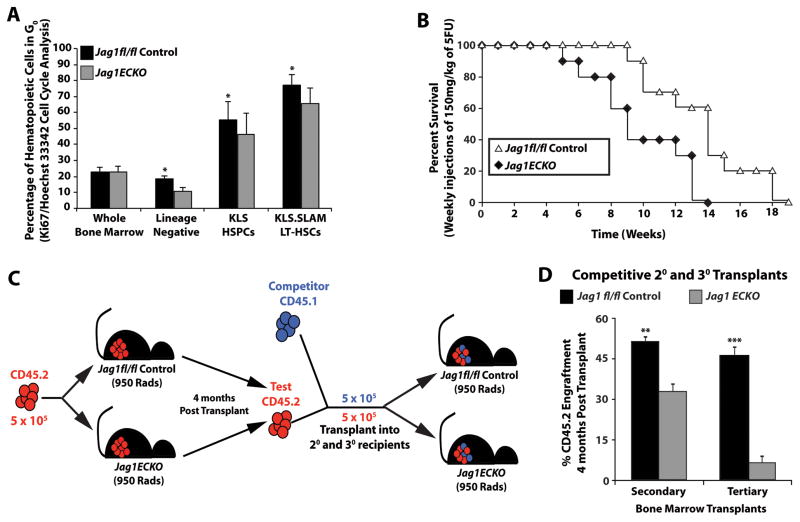
Studies have demonstrated that Jagged-1 produced by osteoblasts can maintain HSCs in a quiescent state (Calvi et al., 2003). Accordingly, endothelial Jagged-1 could potentially control the quiescence state of a subpopulation of the HSC pool during hematopoietic regeneration, ensuring the sustainability of functional hematopoiesis. This notion suggests that the hematopoietic defect seen in Jag1(ECKO) mice could be due to the pre-mature exhaustion of the HSC pool. To test this hypothesis, we analyzed the cell cycle status of control and Jag1(ECKO) mice. We examined the percentage of whole BM, Lineage− hematopoietic progenitors, KLS HSPCs, and KLS.SLAM LT-HSCs that were in G0 by Ki67 and Hoechst 33342 cell cycle staining (Figure 7A). The percentage of quiescent hematopoietic cells in whole BM showed no significant difference between the two cohorts. Upon further analysis of hematopoietic subpopulations enriched for stem cell activity, we found that the control cohort maintained a significant population of HSCs in G0 as compared to Jag1(ECKO) mice in the lineage negative subpopulation (Figure 7A). Analysis of the enriched HSPC and LT-HSC populations demonstrated that Jag1(ECKO)mice had a marked decrease in the percentage of quiescent hematopoietic cells as compared to controls (Figure 7A).
Figure 7. Endothelial-specific deletion of Jagged-1 impairs the self-renewal potential of HSCs.
A) Steady state cell cycle analysis of hematopoietic subpopulations from control and Jag1(ECKO) mice based on Ki67/Hoechst 33342 intracellular staining. Whole BM from both cohorts showed no significant difference in their cell cycle status. However, there was a significant decrease in the percentage of cells that were in G0/quiescence in Jag1(ECKO) mice in the hematopoietic subpopulations (Lineage Negative, KLS HSPCs, and KLS.SLAM) as compared to controls. B) Survival curve monitoring the morbidity/mortality of Jag1fl/fl and Jag1(ECKO) mice following weekly injections of a sublethal dose of 5-FU (150 mg/kg). Jag1(ECKO) mice succumbed to hematopoietic failure by 14 weeks post serial 5-FU injections, whereas, control mice had a significant increase in longevity; with the entire cohort dying at 19 weeks post serial 5-FU injections. C) Schematic depicting transplantation strategy. D) Control and Jag1(ECKO) mice were lethally irradiated (950 Rads) and were transplanted with 5 × 105 whole BM from CD45.2 wild type mice. Four months post transplant, primary CD45.2 cells were competitively transplanted with CD45.1 whole BM into secondary and tertiary Jag1fl/fl and Jag1(ECKO) recipients. CD45.2 engraftment 4 months post secondary transplant demonstrate that endothelial-specific deletion of Jagged-1 results in a significant decrease in engraftment efficiency and self-renewal potential. All data represents mean ± s.d. (*P<0.05, ***P<0.001).
The increased cycling of HSPCs and LT-HSCs in Jag1(ECKO) mice suggest that under regenerative stress, that lack of endothelial Jagged-1 may lead to the premature exhaustion of HSCs. To address this question, control and Jag1(ECKO) mice were treated weekly by administration of the myelosuppressive agent 5-fluorouracil (5-FU; 100 mg/kg) to induce cycling of HSCs and to serially deplete the regenerative capacity of the hematopoietic progenitors (Figure 7B). The rate of hematopoietic recovery and percent survival of each cohort were monitored. Control groups succumbed to hematopoietic failure at the 9th week, with the entire cohort failing to survive by the 19th week (Figure 7B). Endothelial-specific deletion of Jagged-1 significantly reduced the survival rate, with the majority of the deaths detected between the 5th and 14th week (Figure 7B).
To test if endothelial Jagged-1 exerts its biological effect on regulating HSC self-renewal, we transplanted 5 × 105 wild type CD45.2 whole BM into primary control and Jag1(ECKO) recipients and allowed 4 months for recovery and stabilization of the hematopoietic system (Figure 7C, D). We isolated whole BM from the primary transplant recipients and performed a competitive repopulation assay by transplanting 5 × 105 CD45.2 whole BM with 5 × 105 CD45.1 whole BM into secondary and then tertiary control and Jag1(ECKO) recipients (Figure 7C, D). By preconditioning wild type CD45.2 hematopoietic cells in Jag1(ECKO) mice and competitively transplanting the CD45.2 hematopoietic cells back into Jag1(ECKO) mice, we demonstrated that endothelial-specific deletion of Jagged-1 has a negative impact on stem cell engraftment and hematopoietic regeneration. Therefore, endothelial cell-specific expression of the Jagged-1 balances the quiescence and self-renewal of HSCs.
Discussion
Our data demonstrate that endothelial-specific deletion of Jagged-1 results in a decrease in functional hematopoiesis by interfering with Notch signaling in the HSPC pool. Cell cycle and functional transplantation analysis revealed that HSCs from Jag1(ECKO) mice have decreased self-renewal potential and a greater proportion were driven from a quiescent state. Endothelial-specific deletion of Jagged-1 leads to the premature exhaustion of the HSC pool due to the inability of Jagged-1 deficient endothelial cells to maintain the self-renewal ability of the HSC. These data suggests that during steady state hematopoiesis, angiocrine expression of Jagged-1 by the endothelial cells regulate the quiescence and maintenance of the HSC pool as well as lineage-specific differentiation of progenitors. Upon myelosuppressive insult, endothelial-specific Jagged-1 maintains the HSC pool by balancing the rate of self-renewal and lineage-specific differentiation of hematopoietic progenitors, ensuring hematopoietic recovery to homeostatic levels. Therefore, endothelial cells play an instructive role in orchestrating the quiescence, self-renewal, and reconstitution of the hematopoietic compartment.
In this study, we deleted Jagged-1 in endothelial cells by utilizing a constitutive VE-cadherin cre system. One major advantage to utilizing VE-cadherin cre is that it is constitutively expressed during both physiological and pathological conditions in adult endothelial cells (Lambeng et al., 2005). Additionally, as the majority of the endothelial cells in the adult are quiescent during homeostatic conditions, the constitutiveVE -cadherin cre transgenic mouse line provides a completely penetrant and a reproducible system to excise Jag1fl/fl alleles (Figure 1), making it an ideal model to study the role of endothelial-specific expression of Jagged-1 during steady state and regenerative hematopoiesis. Notably, in contrast to the previously published endothelial-specific deletion of Jagged-1, that employed the constitutive Tie2 cre mouse line (High et al., 2008), the deletion of Jagged-1 by the constitutive VE-cadherin cre mouse line does not result in embryonic lethality, likely due to the delayed developmental expression of VE-cadherin (Hofmann et al., 2012).
Notwithstanding the complexities of vascular specific promoters, this model poses two potential caveats. The first being that it is plausible that the hematopoietic defects demonstrated in Jag1(ECKO) mice was due to a decrease in the passive function of ECs to deliver the proper nutrients necessary to promote efficient regeneration of the hematopoietic system. To address this concern, we analyzed Jag1(ECKO) BM endothelial cells for postnatal vascular and angiocrine defects and the effect of endothelial-specific deletion of Jagged-1 on hematopoietic niche cells within the BM microenvironment. We demonstrated that endothelial-specific deletion of Jagged-1 did not disrupt vascular and angiocrine integrity or affect numbers or function of other BM hematopoietic niche cells (Figure 5). The second caveat is that during embryonic development a subset of embryonic hematopoietic cells express VE-cadherin. It has been shown that the majority of the stem cell function can be found in the CD45+VEcadherin+ hematopoietic population up until day E16.5 of fetal liver hematopoiesis (Kim et al., 2005). Therefore, it is possible that the hematopoietic defect we observed in Jag1(ECKO) mice is due to intrinsic effects on the HSC. To address this issue, we isolated E13.5 fetal liver hematopoietic cells from wild type mice using CD45 and VE-cadherin antibodies, demonstrating that the CD45+VE-cadherin− and CD45+VE-cadherin+ hematopoietic populations did not express Jagged-1 protein (Supplemental Figure 3). By contrast, the stromal populations phenotypically identified as CD45−VE -cadherin+ and CD45−VE-cadherin− expressed significant levels of Jagged-1 protein, with the magnitude of Jagged-1 in the endothelial cell population (CD45−VE -cadherin+) being significantly more pronounced than the other cell populations (Supplemental Figure 3). However, it is plausible that endothelial-specific Jagged-1 plays an essential role during development in supporting the establishment and maintenance of the hematopoietic system (Robert-Moreno et al., 2008). Therefore, the hematopoietic defects displayed in adult Jag1(ECKO) mice could reflect a developmental effect of endothelial-specific Jagged-1. Nonetheless, our data indicates that expression of Jagged-1 in endothelial cells provides instructive cues that support the maintenance of the adult HSC and potentially through HSC ontogeny.
Our studies suggest that endothelial cell Jagged-1 plays an essential role in adult hematopoiesis and that the Notch pathway is critical in maintaining homeostatic and regenerative hematopoiesis. However, the role of Notch signaling in the regulation of postnatal hematopoiesis is a debatable subject. In contrast to our studies (Butler et al., 2010; Kobayashi et al., 2010), and many others (Duncan et al., 2005; Karanu et al., 2000; Stier et al., 2002; Varnum-Finney et al., 2011; Varnum-Finney et al., 2000), the Notch pathway has been suggested to be dispensible for adult hematopoiesis (Maillard et al., 2008; Mancini et al., 2005). One explanation for the discrepancy in Maillard et al with our present study is the compensatory roles of other mastermind-like family members (Oyama et al., 2011) in which mastermind-like 3 can compensate for mastermind-like1 in biological functions. Additionally, the deletion of RBPJ removes its function as a natural repressor of canonical Notch signaling (Hsieh et al., 1999; Kao et al., 1998; Lai, 2002; Nagel et al., 2005). Hence, it is conceivable that one could still have basal levels of canonical Notch signaling even in the absence of intracellular Notch/RBPJ-mediated canonical Notch signaling. It has also been suggested that the use of dominant-negative mastermind-like1 mice and retrovirus still result in low levels of canonical Notch signaling (Oyama et al., 2007; Wu et al., 2007). Furthermore, the use of the Mx1 and Vav transgenic promoters to drive Cre expression in the aforementioned studies could have lead to the opposite results in the hematopoietic mouse models employed in these studies. In summary, our data demonstrates that endothelial-specific Jagged-1 is a critical pro-hematopoietic angiocrine factor that fosters the maintenance of the HSC pool. This finding further defines the vascular niche as a crucial cellular hub among other niche cells that sustains the homeostatic and regenerative capacity of the hematopoietic system.
Experimental Procedures
Mice
All animal experiments were performed under the approval of Weill Medical College of Cornell University’s Institutional Animal Care and Use Committee. C57BL/6J (CD45.2) and B6.SJL-Ptprca Pepcb/BoyJ (CD45.1) mice were used at 8–10 weeks of age. VE-cadherin cre mice were obtained from Luisa Iruela-Arispe at UCLA. VE-cadherin creERT2 mice were obtained from Ralf H. Adams. B6.Cg-Tg(Mx1-cre)1Cgn/Jmice were obtained from The Jackson Laboratory. Jag1fl/fl mice were obtained from Jon Epstein at University of Pennsylvania (High et al., 2008) and were generated by Kathleen Loomes and Klaus Kaestner (Loomes et al., 2007). Hes1-GFP knock-in mice were obtained from Iannis Aifantis at New York University. All experimental mice were maintained on the C57BL/6J (CD45.2) background.
Generation of Jag1fl/fl control and Jag1(ECKO) endothelial cells
Lung endothelial cells were isolated from Jag1fl/fl and Jag1(ECKO) mice as previously described (Fehrenbach et al., 2009). Endothelial cells were grown in 1:1 DMEM:Ham’s F-12 supplemented with 20% FBS, 20 mM HEPES (Invitrogen), 100 μg/mL Heparin (Sigma), 100 μg/mL endothelial cell mitogen (Biomedical Technologies, Inc.), MEM nonessential amino acids (Cellgro) and penicillin/streptomycin (Cellgro) and infected with a myristoylated-Akt1 (myrAkt) lentivirus as previously described (Kobayashi et al., 2010). myrAkt expressing cells were selected for one week in X-Vivo 20 media (Lonza) and returned to growth media. Purity of endothelial cells was confirmed by flow cytometry and confocal microscopy. For flow cytometry, cells were stained with antibodies (clone) against CD31 (390) and VE-cadherin (BV13). All cells were blocked with with anantibody against CD16/32 (2.4G2) prior to antibody staining and analyzed using a LSRII SORP (BD Biosciences). For microscopy, cells were blocked with αCD16/32 and stained overnight at 4°C with αVE-cadherin (BV13).
Following staining, cells were washed three times in PBS, pH 7.4, for 5 min each and incubated for 20 min at room temperature with DAPI nuclear counterstain (Molecular Probes, 1:5000). Samples were mounted using VectaShield (Vector Laboratories) and were viewed with appropriate filters. Antibodies were purchased from eBioscience or Biolegend.
Hematopoietic co-culture
Whole BM cells were isolated and enriched by Lineage Cell Depletion (Miltenyi Biotech). 105 Lineage negative cells were plated in one well of a 12-well plate with Jag1fl/fl or Jag1(ECKO) endothelial cells; culture conditions consisted of StemSpan serum-free media (Stem Cell Technologies) supplemented with 50 ng/mL of soluble Kit Ligand (sKitL) (Peprotech). Hematopoietic cells were analyzed using antibodies (clones) recognizing the following surface markers: c-Kit/CD117 (2B8), Sca-1/Ly-6A (D7), CD48 (HM48-1), and CD150 (mShad150). Lineage antibody cocktail included: CD41 (MWReg30), CD3 (145-2C11), CD5 (53–7.3), CD4 (GK1.5), CD8 (53–6.7), Gr-1 (RB6-8C5), CD11b (M1/70), Ter119 (Ter119) and B220 (6B2). All cells were blocked with αCD16/32 (2.4G2) prior to antibody staining and analyzed using a LSRII SORP (BD Biosciences). All antibodies were purchased from BD Pharmingen or eBioscience.
Analysis of steady state hematopoiesis
Syngenic Jag1fl/fl and Jag1(ECKO) mice were subjected to steady state analysis of progenitor activity and phenotypic analysis of differentiated cells and LT-HSCs. Phenotypic analysis of hematopoietic cells was performed by isolating whole BM from femurs by mechanically denuding all muscle and connective tissue and were crushed in a sterile mortar and pestle and digested with 2.5 mg/mL Collagenase A (Roche) and 1 unit/mL Dispase II (Roche) in Hanks Balanced Salt Solution at 37°C for 30 min with gentle agitation. Resulting suspensions were filtered through a 0.45 μm cell strainer (BD Falcon) to a single cell suspension. Antibodies used for lineage and LT-HSC analysis are also described above. Hematopoietic cells were quantified by flow cytometry. For cell cycle analysis, hematopoietic cells were prepared using the Cytofix/Cytoperm kit (BD Biosciences) and stained using Hoechst 33342 and a Ki67 antibody (B56, BD Bioscience). All cells were blocked with αCD16/32 (2.4G2) prior to antibody staining and analyzed using a LSRII SORP (BD Biosciences).
HSC transplantation and LT-HSC repopulation studies
CD45.1 recipients were irradiated (950 Rads) and transplanted with 5 × 105 CD45.2 hematopoietic cells in conjunction with a standard 5 × 105 CD45.1 whole BM competitive dose. Engraftment was monitored at 4, 8, 16, and 24 weeks by FACS analysis of peripheral blood for CD45.2/CD45.1 content (4 independent experiments with n=10 per cohort). For in vivo stem cell function, limiting dilution (Szilvassy et al., 1990) and competitive repopulation assays (Harrison, 1980) were performed in three independent experiments with n=20 per cohort for limiting dilution and n=5 for competitive repopulation. The cell source was either CD45.2 whole BM or KLS hematopoietic cells and cell dose was as indicated in the figures and figure legends. Hematopoietic engraftment was monitored at 4, 8, 16, and 24 weeks by FACS analysis of peripheral blood for CD45.2/CD45.1 content.
Hematopoietic self-renewal assays
Jag1fl/fl control Jag1(ECKO) mice were subjected to serial intraperitoneal administration of the chemotherapeutic agent 5-fluorouracil (100 mg/kg). Mice were monitored for signs of morbidity and sacrificed to ensure that mice did not experience any undo discomfort.
Postnatal retinal analysis
Following sacrifice, eyes were enucleated and fixed in 4% paraformaldehyde for 2 hr at 4°C. Retinas were then dissected out and incubated in 0.5% Triton X-100, 1% BSA, overnight at 4°C. Following washes in PBLEC (1% Triton X-100, 1 mM MgCl2, 1 mM MnCl2, and 1 mM CaCl2 in PBS, pH 6.8), retinas were incubated overnight in PBLEC containing biotinylated isolectin B4 (VectorLabs, 1:50). Alexa Fluor streptavidin conjugated secondary antibodies were used (Invitrogen, 1:500) for detection. A Nikon A1R confocal microscope was used to image the stained and flatmounted retinas. Four 10X stacks were taken of each retina. ImageJ was used for image processing and quantification.
BM niche cell quantification
For quantification of the BM endothelial cells, Osteopontin+ cells, and Nestin/Leptin Receptor+ cells, mice were injected by retro-orbital sinus with 10 μg of Alexa Fluor 647-VE-cadherin (BV13) antibody. After 10 minutes, mice were sacrificed and femurs were denuded of muscle and connective tissue, crushed in a sterile mortar and digested as described above. Niche cells were quantified. The number of functional BM endothelial cells was quantified by analyzing the total number of VE-cadherin+CD45−Ter119− cells per femur. Analysis of expression of Osteopontin was determined by fixing CD45−Ter119−CD31− cells with 2% paraformaldehyde, permeabilized, and stained with Osteopontin (AF497, RnD Systems). Analysis of PDGFRα+ CD51+ (BD Pharmagin) and Leptin Receptor (IC808P, RnD Systems) was performed by cell surface staining of CD45−Ter119−CD31− cells. All cells were blocked with αCD16/32 (2.4G2) prior to antibody staining and analyzed using a LSRII SORP (BD Biosciences).
CFU-F and clonal mesensphere formation assays
BM cells were isolated as previously described (Mendez-Ferrer et al., 2010) with minor modifications. Briefly, cells were gently flushed in L-15 FACS buffer and after erythrocyte lysis, digested with 3 mg/mL Collagenase I (Sigma-Aldrich) and 3 mg/mL Dispase (Gibco) in HBSS with 10% FBS (Hyclone) for 30 min at 37°C. Prior to flow cytometry sorting, cells were enriched by immunomagnetic depletion using αCD45 magnetic beads (Milteyi Biotec), following the manufacturer’s recommendations. CD45−Ter119−CD31− stromal cells were sorted using fluorochrome-conjugated mAbs specific to mouse CD45 (30-F11), Ter119 (Ter-119) from eBioscience and CD31 (MEC13.3) from Biolegend, on a FACSAria (BD Biosciences) to > 95% purity. For clonal sphere formation, cells were plated at clonal density (< 1000 cells/cm2) into ultra-low adherent plates as previously described (Mendez-Ferrer et al., Nature 2010). Cells were kept at 37 °C with 5% CO2 in a water-jacketed incubator and left untouched for one week to prevent cell aggregation. One-half medium changes were performed weekly and spheres counted at day 9. For CFU-F assays, 10,000 sorted cells were seeded per well in 6-well adherent tissue culture plate using phenol-red free α-MEM (Gibco) supplemented with 20% FBS (Hyclone), 10% MesenCult stimulatory supplement (StemCell Technologies) and 0.5% penicillin-streptomycin. One-half of the media was replaced after 7 days and at day 14 cells were stained with Giemsa staining solution (EMD Chemicals) and colonies counted.
Immunostaining of bone sections
Mice were injected by retro-orbital sinus with 10 μg of Alexa Fluor 647-VE-cadherin (BV13). After 10 minutes, bones from control and Jag1(ECKO) mice were fixed overnight in 4°C with 4% paraformaldehyde and were decalcified for 4 days in 10% EDTA (pH 7.2) at room temperature. Femurs were washed with PBS, pH7.4, for 30 min, placed in 30% sucrose at 4°C overnight and were embedded in Tissue-Tek Optimal Cutting Temperature (OCT). Sections were post-fixed for 15 min with 4% paraformaldehyde and washed three times with PBS. Sections were stained for 20 min at room temperature with DAPI nuclear counterstain (Molecular Probes, 1:5000). Samples were mounted using VectaShield (Vector Laboratories).
Peripheral-blood analysis
Retro-orbital blood was collected on indicated days after sublethal irradiation with capillary xpipettes and was analyzed using an automated Advia 120 (Bayer).
Cre recombinase efficiency
To isolate primary BM endothelial cells, mice were injected via retro-orbital sinus with 25 μg of Alexa Fluor 647 conjugated VE-cadherin antibody (BV13) and 50 μg Alexa Fluor 488 conjugated Isolectin GS (Invitrogen). After 10 min, total extracted BM endothelial cells were isolated as described above. Live cells (DAPI−) were gated on Isolectin GS-IB4+/VE-cadherin+ and sorted directly into Trizol (Invitrogen) and total RNA was isolated following the manufacturer’s protocol. All flow sorting was performed on an Aria II SORP (BD Biosciences). To generate cDNA from total BM endothelial cell for RT-PCR analysis was achieved by using the Ovation Pico WTA System V2 (NuGEN Technologies, Inc.) according to the manufacturer’s protocol. Semi-quantitative RT-PCR was performed with 100 ng input cDNA using the PCR Extender System (5 PRIME) at 94°C 2 min – 94°C 30 sec, 58°C 30 sec, 72°C 30 sec (30X) – 72°C 5 min (see Supplemental Table 1 for primer sequences). Products were resolved on 2% agarose/TAE gels and visualized using Ethidium Bromide and the Bio-Rad Universal Hood Imaging System.
Angiocrine profiling of in vivo BM endothelial cells
To generate cDNA for quantitative RT-PCR analysis, total extracted BM endothelial cells were isolated (as described above) and total RNA was converted to cDNA using qScript cDNA Supermix (Quanta) according to the manufacturer’s protocol. Quantitative RT-PCR was carried out using SYBR Green PCR Master Mix (Applied Biosystems) with appropriate primers (see Supplemental Table 1 for primers sequences) on a ViiaA7 Real-Time PCR System (Applied Biosystems) according to the manufacturer’s protocol. Statistics were calculated using Student’s t-test on three biological replicates.
Statistics
Unless otherwise indicated, all data is represented as a mean ± s.d. Log rank and two-tailed Student’s t-tests and 95% confidence intervals were used to determine statistical significance (*P<0.05, **P<0.01, ***P<0.001).
Supplementary Material
Highlights.
Endothelial Jagged-1 maintains the HSC pool by interfering with Notch signaling
Endothelial-specific deletion of Jagged-1 inhibits ex vivo expansion of HSCs
Hematopoietic regeneration following stress is dependent on endothelial Jagged-1
Endothelial Jagged-1 regulates HSC quiescence and self-renewal capacity
Acknowledgments
We would like to thank Kathleen Loomes and Klaus Kaestner for generating and Dr. Jon Epstein for sending us the Jag1fl/fl mice. This work was supported by the Tri-Institutional Stem Cell Initiative (M.G.P. and J.M.B.), the National Institutes of Health (S.R., J.M.B, P.S.F., S.P., N.M.K. and J.K.), Ansary Stem Cell Institute (S.R. and J.M.B), the Howard Hughes Medical Institute (S.R. and I.A.), Empire State Stem Cell Board and New York State Department of Health grants (NYSTEM, C024180, C026438, C026878) (S.R.); NHLBI R01s HL097797 and DK095039 (S.R.); Qatar National Priorities Research Foundation NPRP08-663-3-140 and Qatar foundation BioMedical Research Program (BMRP) (S.R.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams GB, Chabner KT, Alley IR, Olson DP, Szczepiorkowski ZM, Poznansky MC, Kos CH, Pollak MR, Brown EM, Scadden DT. Stem cell engraftment at the endosteal niche is specified by the calcium-sensing receptor. Nature. 2006;439:599–603. doi: 10.1038/nature04247. [DOI] [PubMed] [Google Scholar]
- Benedito R, Roca C, Sorensen I, Adams S, Gossler A, Fruttiger M, Adams RH. The notch ligands Dll4 and Jagged1 have opposing effects on angiogenesis. Cell. 2009;137:1124–1135. doi: 10.1016/j.cell.2009.03.025. [DOI] [PubMed] [Google Scholar]
- Butler JM, Nolan DJ, Vertes EL, Varnum-Finney B, Kobayashi H, Hooper AT, Seandel M, Shido K, White IA, Kobayashi M, et al. Endothelial cells are essential for the self-renewal and repopulation of Notch-dependent hematopoietic stem cells. Cell stem cell. 2010;6:251–264. doi: 10.1016/j.stem.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, Martin RP, Schipani E, Divieti P, Bringhurst FR, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425:841–846. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- Ding L, Morrison SJ. Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature. 2013;495:231–235. doi: 10.1038/nature11885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L, Saunders TL, Enikolopov G, Morrison SJ. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature. 2012;481:457–462. doi: 10.1038/nature10783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan AW, Rattis FM, DiMascio LN, Congdon KL, Pazianos G, Zhao C, Yoon K, Cook JM, Willert K, Gaiano N, et al. Integration of Notch and Wnt signaling in hematopoietic stem cell maintenance. Nature immunology. 2005;6:314–322. doi: 10.1038/ni1164. [DOI] [PubMed] [Google Scholar]
- Fehrenbach ML, Cao G, Williams JT, Finklestein JM, Delisser HM. Isolation of murine lung endothelial cells. American journal of physiology Lung cellular and molecular physiology. 2009;296:L1096–1103. doi: 10.1152/ajplung.90613.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fre S, Hannezo E, Sale S, Huyghe M, Lafkas D, Kissel H, Louvi A, Greve J, Louvard D, Artavanis-Tsakonas S. Notch lineages and activity in intestinal stem cells determined by a new set of knock-in mice. PloS one. 2011;6:e25785. doi: 10.1371/journal.pone.0025785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenette PS, Pinho S, Lucas D, Scheiermann C. Mesenchymal stem cell: keystone of the hematopoietic stem cell niche and a stepping-stone for regenerative medicine. Annual review of immunology. 2013;31:285–316. doi: 10.1146/annurev-immunol-032712-095919. [DOI] [PubMed] [Google Scholar]
- Harrison DE. Competitive repopulation: a new assay for long-term stem cell functional capacity. Blood. 1980;55:77–81. [PubMed] [Google Scholar]
- High FA, Lu MM, Pear WS, Loomes KM, Kaestner KH, Epstein JA. Endothelial expression of the Notch ligand Jagged1 is required for vascular smooth muscle development. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:1955–1959. doi: 10.1073/pnas.0709663105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilton MJ, Tu X, Wu X, Bai S, Zhao H, Kobayashi T, Kronenberg HM, Teitelbaum SL, Ross FP, Kopan R, et al. Notch signaling maintains bone marrow mesenchymal progenitors by suppressing osteoblast differentiation. Nature medicine. 2008;14:306–314. doi: 10.1038/nm1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himburg HA, Muramoto GG, Daher P, Meadows SK, Russell JL, Doan P, Chi JT, Salter AB, Lento WE, Reya T, et al. Pleiotrophin regulates the expansion and regeneration of hematopoietic stem cells. Nature medicine. 2010;16:475–482. doi: 10.1038/nm.2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann JJ, Briot A, Enciso J, Zovein AC, Ren S, Zhang ZW, Radtke F, Simons M, Wang Y, Iruela-Arispe ML. Endothelial deletion of murine Jag1 leads to valve calcification and congenital heart defects associated with Alagille syndrome. Development. 2012;139:4449–4460. doi: 10.1242/dev.084871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper AT, Butler JM, Nolan DJ, Kranz A, Iida K, Kobayashi M, Kopp HG, Shido K, Petit I, Yanger K, et al. Engraftment and reconstitution of hematopoiesis is dependent on VEGFR2-mediated regeneration of sinusoidal endothelial cells. Cell stem cell. 2009;4:263–274. doi: 10.1016/j.stem.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh JJ, Zhou S, Chen L, Young DB, Hayward SD. CIR, a corepressor linking the DNA binding factor CBF1 to the histone deacetylase complex. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:23–28. doi: 10.1073/pnas.96.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao HY, Ordentlich P, Koyano-Nakagawa N, Tang Z, Downes M, Kintner CR, Evans RM, Kadesch T. A histone deacetylase corepressor complex regulates the Notch signal transduction pathway. Genes & development. 1998;12:2269–2277. doi: 10.1101/gad.12.15.2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karanu FN, Murdoch B, Gallacher L, Wu DM, Koremoto M, Sakano S, Bhatia M. The notch ligand jagged-1 represents a novel growth factor of human hematopoietic stem cells. The Journal of experimental medicine. 2000;192:1365–1372. doi: 10.1084/jem.192.9.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim I, Yilmaz OH, Morrison SJ. CD144 (VE-cadherin) is transiently expressed by fetal liver hematopoietic stem cells. Blood. 2005;106:903–905. doi: 10.1182/blood-2004-12-4960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi H, Butler JM, O’Donnell R, Kobayashi M, Ding BS, Bonner B, Chiu VK, Nolan DJ, Shido K, Benjamin L, et al. Angiocrine factors from Akt-activated endothelial cells balance self-renewal and differentiation of haematopoietic stem cells. Nature cell biology. 2010;12:1046–1056. doi: 10.1038/ncb2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai EC. Keeping a good pathway down: transcriptional repression of Notch pathway target genes by CSL proteins. EMBO reports. 2002;3:840–845. doi: 10.1093/embo-reports/kvf170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambeng N, Wallez Y, Rampon C, Cand F, Christe G, Gulino-Debrac D, Vilgrain I, Huber P. Vascular endothelial-cadherin tyrosine phosphorylation in angiogenic and quiescent adult tissues. Circulation research. 2005;96:384–391. doi: 10.1161/01.RES.0000156652.99586.9f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapidot T, Dar A, Kollet O. How do stem cells find their way home? Blood. 2005;106:1901–1910. doi: 10.1182/blood-2005-04-1417. [DOI] [PubMed] [Google Scholar]
- Lo Celso C, Fleming HE, Wu JW, Zhao CX, Miake-Lye S, Fujisaki J, Cote D, Rowe DW, Lin CP, Scadden DT. Live-animal tracking of individual haematopoietic stem/progenitor cells in their niche. Nature. 2009;457:92–96. doi: 10.1038/nature07434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomes KM, Russo P, Ryan M, Nelson A, Underkoffler L, Glover C, Fu H, Gridley T, Kaestner KH, Oakey RJ. Bile duct proliferation in liver-specific Jag1 conditional knockout mice: effects of gene dosage. Hepatology. 2007;45:323–330. doi: 10.1002/hep.21460. [DOI] [PubMed] [Google Scholar]
- Maillard I, Koch U, Dumortier A, Shestova O, Xu L, Sai H, Pross SE, Aster JC, Bhandoola A, Radtke F, et al. Canonical notch signaling is dispensable for the maintenance of adult hematopoietic stem cells. Cell stem cell. 2008;2:356–366. doi: 10.1016/j.stem.2008.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancini SJ, Mantei N, Dumortier A, Suter U, MacDonald HR, Radtke F. Jagged1-dependent Notch signaling is dispensable for hematopoietic stem cell self-renewal and differentiation. Blood. 2005;105:2340–2342. doi: 10.1182/blood-2004-08-3207. [DOI] [PubMed] [Google Scholar]
- Mendez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA, Scadden DT, Ma’ayan A, Enikolopov GN, Frenette PS. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466:829–834. doi: 10.1038/nature09262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SJ, Spradling AC. Stem cells and niches: mechanisms that promote stem cell maintenance throughout life. Cell. 2008;132:598–611. doi: 10.1016/j.cell.2008.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel AC, Krejci A, Tenin G, Bravo-Patino A, Bray S, Maier D, Preiss A. Hairless-mediated repression of notch target genes requires the combined activity of Groucho and CtBP corepressors. Molecular and cellular biology. 2005;25:10433–10441. doi: 10.1128/MCB.25.23.10433-10441.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson SK, Johnston HM, Whitty GA, Williams B, Webb RJ, Denhardt DT, Bertoncello I, Bendall LJ, Simmons PJ, Haylock DN. Osteopontin, a key component of the hematopoietic stem cell niche and regulator of primitive hematopoietic progenitor cells. Blood. 2005;106:1232–1239. doi: 10.1182/blood-2004-11-4422. [DOI] [PubMed] [Google Scholar]
- Oh P, Lobry C, Gao J, Tikhonova A, Loizou E, Manent J, van Handel B, Ibrahim S, Greve J, Mikkola H, et al. In Vivo Mapping of Notch Pathway Activity in Normal and Stress Hematopoiesis. Cell Stem Cell. 2013 doi: 10.1016/j.stem.2013.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyama T, Harigaya K, Muradil A, Hozumi K, Habu S, Oguro H, Iwama A, Matsuno K, Sakamoto R, Sato M, et al. Mastermind-1 is required for Notch signal-dependent steps in lymphocyte development in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:9764–9769. doi: 10.1073/pnas.0700240104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyama T, Harigaya K, Sasaki N, Okamura Y, Kokubo H, Saga Y, Hozumi K, Suganami A, Tamura Y, Nagase T, et al. Mastermind-like 1 (MamL1) and mastermind-like 3 (MamL3) are essential for Notch signaling in vivo. Development. 2011;138:5235–5246. doi: 10.1242/dev.062802. [DOI] [PubMed] [Google Scholar]
- Papayannopoulou T, Craddock C. Homing and trafficking of hemopoietic progenitor cells. Acta haematologica. 1997;97:97–104. doi: 10.1159/000203665. [DOI] [PubMed] [Google Scholar]
- Pinho S, Oliveira A, Costa I, Gouveia CA, Carvalho F, Moreira RF, Dinis-Oliveira RJ. Simultaneous quantification of tramadol and O-desmethyltramadol in hair samples by gas chromatography-electron impact/mass spectrometry. Biomedical chromatography: BMC. 2013 doi: 10.1002/bmc.2894. [DOI] [PubMed] [Google Scholar]
- Robert-Moreno A, Guiu J, Ruiz-Herguido C, Lopez ME, Ingles-Esteve J, Riera L, Tipping A, Enver T, Dzierzak E, Gridley T, et al. Impaired embryonic haematopoiesis yet normal arterial development in the absence of the Notch ligand Jagged1. The EMBO journal. 2008;27:1886–1895. doi: 10.1038/emboj.2008.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stier S, Cheng T, Dombkowski D, Carlesso N, Scadden DT. Notch1 activation increases hematopoietic stem cell self-renewal in vivo and favors lymphoid over myeloid lineage outcome. Blood. 2002;99:2369–2378. doi: 10.1182/blood.v99.7.2369. [DOI] [PubMed] [Google Scholar]
- Sugiyama T, Kohara H, Noda M, Nagasawa T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006;25:977–988. doi: 10.1016/j.immuni.2006.10.016. [DOI] [PubMed] [Google Scholar]
- Szilvassy SJ, Humphries RK, Lansdorp PM, Eaves AC, Eaves CJ. Quantitative assay for totipotent reconstituting hematopoietic stem cells by a competitive repopulation strategy. Proceedings of the National Academy of Sciences of the United States of America. 1990;87:8736–8740. doi: 10.1073/pnas.87.22.8736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varnum-Finney B, Halasz LM, Sun M, Gridley T, Radtke F, Bernstein ID. Notch2 governs the rate of generation of mouse long- and short-term repopulating stem cells. The Journal of clinical investigation. 2011;121:1207–1216. doi: 10.1172/JCI43868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varnum-Finney B, Purton LE, Yu M, Brashem-Stein C, Flowers D, Staats S, Moore KA, Le Roux I, Mann R, Gray G, et al. The Notch ligand, Jagged-1, influences the development of primitive hematopoietic precursor cells. Blood. 1998;91:4084–4091. [PubMed] [Google Scholar]
- Varnum-Finney B, Xu L, Brashem-Stein C, Nourigat C, Flowers D, Bakkour S, Pear WS, Bernstein ID. Pluripotent, cytokine-dependent, hematopoietic stem cells are immortalized by constitutive Notch1 signaling. Nature medicine. 2000;6:1278–1281. doi: 10.1038/81390. [DOI] [PubMed] [Google Scholar]
- Wu L, Maillard I, Nakamura M, Pear WS, Griffin JD. The transcriptional coactivator Maml1 is required for Notch2-mediated marginal zone B-cell development. Blood. 2007;110:3618–3623. doi: 10.1182/blood-2007-06-097030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, Yin T, Wiegraebe W, He XC, Miller D, Stark D, Perko K, Alexander R, Schwartz J, Grindley JC, et al. Detection of functional haematopoietic stem cell niche using real-time imaging. Nature. 2009;457:97–101. doi: 10.1038/nature07639. [DOI] [PubMed] [Google Scholar]
- Xue Y, Gao X, Lindsell CE, Norton CR, Chang B, Hicks C, Gendron-Maguire M, Rand EB, Weinmaster G, Gridley T. Embryonic lethality and vascular defects in mice lacking the Notch ligand Jagged1. Human molecular genetics. 1999;8:723–730. doi: 10.1093/hmg/8.5.723. [DOI] [PubMed] [Google Scholar]
- Yamazaki S, Ema H, Karlsson G, Yamaguchi T, Miyoshi H, Shioda S, Taketo MM, Karlsson S, Iwama A, Nakauchi H. Nonmyelinating Schwann cells maintain hematopoietic stem cell hibernation in the bone marrow niche. Cell. 2011;147:1146–1158. doi: 10.1016/j.cell.2011.09.053. [DOI] [PubMed] [Google Scholar]
- Yoshihara H, Arai F, Hosokawa K, Hagiwara T, Takubo K, Nakamura Y, Gomei Y, Iwasaki H, Matsuoka S, Miyamoto K, et al. Thrombopoietin/MPL signaling regulates hematopoietic stem cell quiescence and interaction with the osteoblastic niche. Cell stem cell. 2007;1:685–697. doi: 10.1016/j.stem.2007.10.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.