Short abstract
A novel, hand-held Reference Point Indentation (RPI) instrument, measures how well the bone of living patients and large animals resists indentation. The results presented here are reported in terms of Bone Material Strength, which is a normalized measure of how well the bone resists indentation, and is inversely related to the indentation distance into the bone. We present examples of the instrument's use in: (1) laboratory experiments on bone, including experiments through a layer of soft tissue, (2) three human clinical trials, two ongoing in Barcelona and at the Mayo Clinic, and one completed in Portland, OR, and (3) two ongoing horse clinical trials, one at Purdue University and another at Alamo Pintado Stables in California. The instrument is capable of measuring consistent values when testing through soft tissue such as skin and periosteum, and does so handheld, an improvement over previous Reference Point Indentation instruments. Measurements conducted on horses showed reproducible results when testing the horse through tissue or on bare bone. In the human clinical trials, reasonable and consistent values were obtained, suggesting the Osteoprobe® is capable of measuring Bone Material Strength in vivo, but larger studies are needed to determine the efficacy of the instrument's use in medical diagnosis.
Keywords: bone, bone fracture, bone mechanical properties, bone material properties
1. Introduction
As people age, bone strength deteriorates and the skeleton becomes more susceptible to fracture [1], which contributes to the morbidity and mortality of osteoporosis. Bone strength is traditionally defined as the integration of bone mass and bone quality [2]. Available techniques for clinical estimation of strength, however, are mainly based on bone mineral density assessments [3] that are reliable but have modest sensitivity and specificity [3,4]. Furthermore, the ability of densitometry to predict the response to a treatment is limited and only a small proportion of treatment related fracture risk reduction is explained by bone mineral density increases [5]. Advanced bone imaging and analysis technologies promise better assessment of bone strength [6] but rely on potentially inaccurate assumptions about the tissue level mechanical properties.
Therefore, there is a critical need to directly quantify bone's ability to resist fracture. The most direct method to determine fracture resistance would be to actually fracture a patient's bone while measuring the difficulty of inducing the fractures. On a large scale, this is clearly impractical; however, on a microscopic scale, one can induce microfractures safely. Recently, a new technique, RPI [7–10], has been reported to quantify the ability of bone to resist indentation in vivo and can also distinguish between the bone of patients with and without fracture [7]. It does so by inducing microfractures in the bone (Fig. 1) while measuring the distance of penetration.
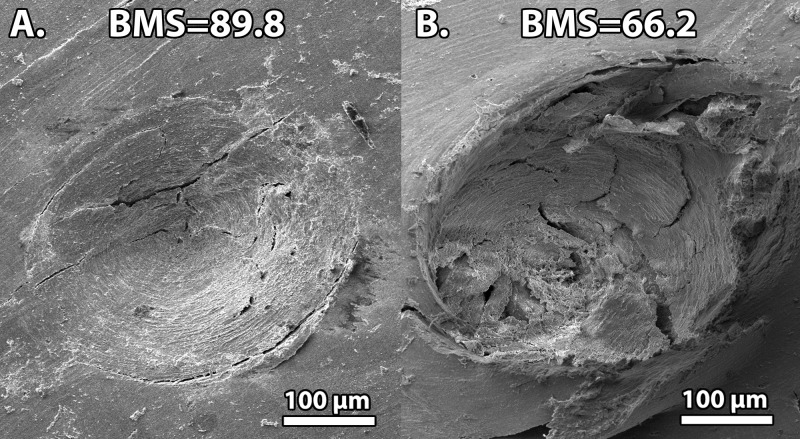
Fig. 1.
Scanning electron microscope images of Osteoprobe indentations in the tibia of two different 83-year-old female donors. These images display the microcracks created by the measurement to determine the BMS. The bone on the left (Sample A) appears to have fewer and shorter microcracks on the bone's surface, which resulted in a lower indentation distance and correspondingly a higher BMS of 89.8. Conversely, the bone on the right (Sample B) appears to have more microcracks, which resulted in a greater indentation distance and a lower BMS of 66.2. Thus, the bone with higher BMS is the bone that is more resistant to local damage from indentation.
There is both clinical and laboratory evidence suggesting that mechanical properties of bone tissue may play a critical role in bone strength [11–13]. One would expect these properties to play a significant role in bone fracture risk; however, it is unclear what mechanical properties are most important [14–17]. In addition, currently available methods for estimates of these mechanical properties require invasive bone sampling [18], making routine clinical use unfeasible. The RPI instrument has the advantage of directly measuring the bone's resistance to fracture, by creating microfractures in a minimally invasive, measured procedure.
Results from a previous RPI instrument that distinguished fracture patients from control patients [7] were acquired from an instrument [9] that required a reference probe, a specially sharpened hypodermic needle. After the initial clinical trials, several improvements were made to the Reference Point Indentation instrument to make the instrument easier to use, less invasive, and more reproducible in a clinical setting, resulting in the Osteoprobe® [19]. The Osteoprobe® is a handheld RPI instrument that does not require a reference probe and is easier to use on human patients and horses. Currently, the Osteoprobe® cannot be used on small animal bones because it requires that the bone have enough mass to avoid being simply pushed away rather than indented during the impact. For these bones, a commercial RPI instrument, such as the BioDent®, can be used.
This paper is a brief presentation of preliminary clinical data obtained with this novel handheld RPI instrument on humans and on horses. This article will focus on the application of the recently introduced Osteoprobe® [19] to measure Bone Material Strength, but as with the other RPI instruments previously described [16–18], it is potentially useful for more general material characterization. It provides a simple, handheld test that is useful in cases where it is inconvenient to specially prepare samples for conventional mechanical testing.
2. Osteoprobe® Operation and Measurements
2.1. Instrument Operation.
The Osteoprobe® is designed to create a microindentation in bone by applying a dynamic impact. A 90 degree conical indenter with a diameter of approximately 380 μm is used. An initial preload on the sample of order 10 N is applied to anchor the indenter into the bone and to ensure it has pierced the periosteum. Once the preload force has been reached, an impact will be initiated, which is the primary force used to create the indentation. This impact generates a peak force of order 40 N and occurs in a fraction of a millisecond. After the impact occurs, the operator will conclude the test or conduct further tests in other locations (at least 2 mm away from previous site).
The primary measurement occurs during the impact cycle where the indentation distance into the sample is measured. This indentation distance cannot be measured absolutely, relative to some external, rigid frame, because of (1) interference from soft tissue on the surface of the bone, (2) the difficulty of keeping a patient or horse absolutely still during measurement, and (3) the bone itself cannot be held fixed relative to the external, rigid frame because it is surrounded by soft tissue including muscles. Consequentially, it is necessary to measure the indentation distance relative to a reference point on the bone itself; thus RPI. The Osteoprobe® eliminates the need for the physical reference probe on the bone, while still maintaining the concept of using a reference point. The reference point is the location where the probe initially contacts the sample just before the impact is triggered. The indentation distance increase from this reference point results from the impact is measured with a custom strain gauge mechanism. This reference point is suitable because the inertia of the body of the instrument keeps it adequately fixed in space during the short duration of the impact. Thus, the distance measured with the strain gauge is the same as the distance that the probe further indents into the sample from the reference point. The elimination of the reference probe has the advantage of simplicity and of removing the possibility of soft tissue buildup and friction between the test probe and the reference probe as in other RPI Devices [7–10]. Further detail of the instrument operation has been reported previously by Bridges et al. [19].
2.2. Bone Material Strength Measurement.
The measurement taken by the Osteoprobe® is a new parameter, called Bone Material Strength (BMS) [19], which quantifies how well a bone resists microindentation. Bone Material Strength is defined as 100 times the ratio of the indentation distance from the impact into a calibration material, PMMA (poly (methyl-methacrylate), divided by the indentation distance from the impact into the bone. As the probe indents, it induces microfractures. The more easily the bone material is fractured, the deeper the probe indents and thus the lower the BMS.
BMS determined from impact microindentation testing has been shown to discriminate patients with and without hip fractures in a case-control study [20]. As a result of these findings, it can be inferred that BMS is a measure of the contribution of bone material properties to whole bone fracture risk.
2.3. Measurement Correlations.
Bone Material Strength, measured with the Osteoprobe®, was correlated with the BioDent® [7–10] and a standard Vickers hardness test. Cadaver samples of cortical bone were excised from the mid diaphysis of the tibia from two 83-year-old female donors. One donor had no history of bone disease (Sample A) and the other donor had Type II Diabetes (Sample B). Ten indentation tests were conducted with each RPI instrument and three Vickers hardness measurements were obtained from each sample. The results are shown in Table 1. The results show a correlation between all three mechanical tests with the same trend. We note, however, that the Vickers hardness measurements are only practical in bone samples from which the soft tissue has been removed, but not in living animals or patients because Vickers hardness measurements depend on imaging the indentation, which would be very difficult even in cases where the bone were surgically exposed.
Table 1.
Results obtained by three different mechanical testers on cortical bone samples from the tibia of two different 83-year-old female donors. All instruments show the trend of Sample A being indented easier than Sample B. Note both the BMS and Vickers Hardness have a positive correlation while the correlation with Total Indentation Distance (TID) is negative. This is due to BMS and Vickers Hardness being inversely related to indentation distance, while the TID does not have this inverse relationship to indentation distance.
| Sample ID | Osteoprobe (BMS) N = 10 | BioDent (TID) N = 10 | Vickers (HV45/30) N = 3 |
|---|---|---|---|
| A | 90.37 ± 4.30 | 98.60 ± 4.39 | 26.68 ± 2.38 |
| B | 73.75 ± 13.24 | 106.33 ± 5.99 | 16.44 ± 1.53 |
Figure 1 shows two Scanning Electron Microscope (SEM) images of an indentation into each of the test samples. Since the SEM image is only of the bone surface, we are unable to quantify fractures completely as it is unknown how the fractures propagate below the surface; however, it appears that more fractures were created on Sample B, which had a BMS of 66.2, compared to Sample A, which appears to resist microfractures and has a BMS of 89.8. These results show a correlation between BMS and the local microscopic damage that contributes to a larger indentation.
3. Human Testing
3.1. Clinical Tests of Living Humans In Vivo.
Human clinical trials were performed in Barcelona, Spain, and in Oregon and at the Mayo Clinic in the United States. The trials in Barcelona involve elderly women over the age of 60 with no history of receiving drug treatment for bone-related conditions. The trials in Oregon involved elderly men. Patients were conscious with only local anesthesia used at the measurement site and no serious complications have been reported. Currently the range in BMS seen in the Barcelona study is 56 to 94 with a mean of 79 and a standard deviation of 8. The range of BMS seen in the Oregon study is 69 to 94 with a mean of 85 and a standard deviation of 9. The similarity of the ranges and standard deviations obtained from these two independent clinical trials reveal that the results obtained from the Osteoprobe® can be highly consistent between different populations of test subjects. In addition, the small variability in measured BMS from user to user highlights its potential wide-spread clinical applicability in assaying fracture risk.
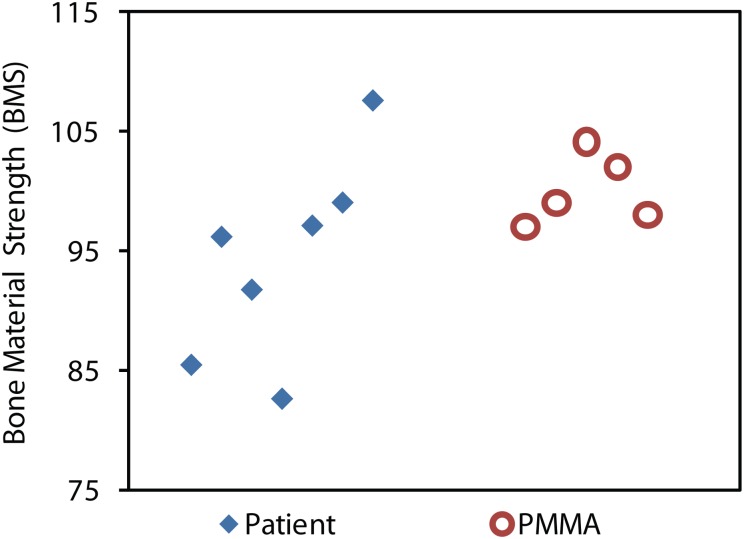
It is important to note that bone is a heterogeneous material; therefore the measurements on a single patient have a larger standard deviation than the measurements on the calibration phantom, poly(methyl methacrylate) (PMMA), which is much more homogeneous (see Fig. 2). This larger standard deviation is not due to the instrument, but rather the natural heterogeneity of bone. For this reason, each patient had at least five measurements taken in one general location. The probe only pierces the skin once, and then is moved incrementally for each of the five measurements around the insertion site, with a separation of at least 2 mm between measurement sites.
Fig. 2.
In vivo testing on a human patient with the calibration phantom (PMMA) test results. The spread of values for the patient, compared to the PMMA Phantom, is larger due to the natural heterogeneity of the bone. This is why at least five tests are conducted in vivo on humans: to reduce the error of the mean below the value that typically separates one patient from another.
At the Mayo Clinic, a recent test was conducted to investigate the reproducibility of the Osteoprobe® measurements. The operator performed ten measurements on a patient, put down the instrument, paused, and then repeated ten additional measurements. For the initial eight patients, the coefficient of determination (R 2) was 0.90 when all ten measurements were used; however, it fell to 0.73 if only the first five measurements were used. These results suggest that ten (or more) measurements should be performed on each patient in future tests. The majority of the time involved in the procedure is spent preparing the patient; therefore performing ten measurements rather than five measurements has a small impact on the duration of the test procedure as each measurement takes only a few seconds.
3.2. Laboratory Tests of Human Donor Samples Through Skin Versus on Bare Bone.
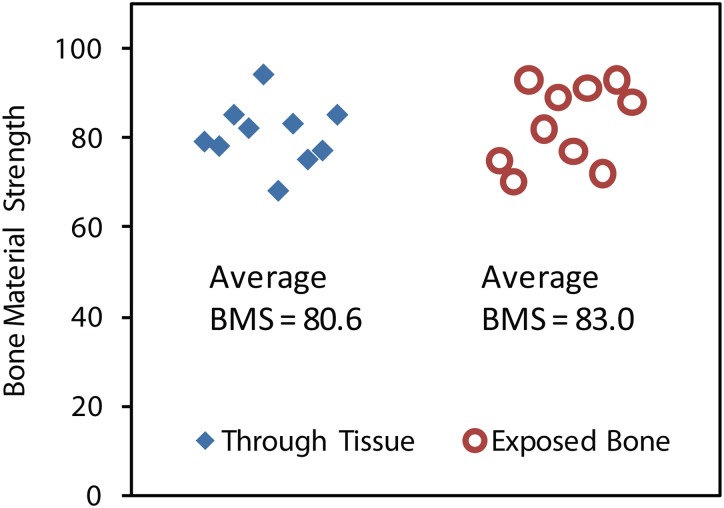
An experiment was conducted to identify potential inconsistencies between data collected from tests performed on exposed bone compared to bone tested through intact tissue overlaying bone. This is a critical investigation because it is a primary difference between clinical in vivo tests and ex vivo tests, typical of a laboratory setting. Two cadaveric samples from the medial section of the right tibia from a female donor (age 83) from the University of California Irvine Health Affairs Willed Body Program were tested while submerged in Hank's buffered saline solution and clamped in place by a mechanical vice. One sample was tested through the local soft tissue, whereas the second was tested after removing all soft tissue, including scraping off the surrounding periosteum. When testing through the soft tissue, the probe was inserted through the skin and periosteum until it was resting on the bone surface. Once on the surface, a measurement was taken. Each sample was tested ten times and the average values of BMS were compared (Fig. 3). This test confirmed that there is not a significant discrepancy in BMS values between testing on exposed bone compared to testing with the presence of overlaying tissue (p > 0.25). These findings are consistent with numerous other previous tests conducted during instrument development to optimize the trigger force and impact force with the goal of having the same reading for both through-tissue and bare-bone parallel measurements. These results verify that this novel instrument is capable of penetrating both the bone's soft tissue and the periosteum, typically the most difficult soft tissue to penetrate between the skin and the bone, which is critical for in vivo use.
Fig. 3.
BMS values of ex vivo human samples comparing through tissue tests to tests performed on exposed bone. The data suggests that there is no significant difference in BMS values between these two methods of indentation (p > 0.25), which is vital because it demonstrates the Osteoprobe®'s consistency between through tissue and exposed bone tests, typical of in vivo and ex vivo testing, respectively.
4. Testing Horses
4.1. Clinical Testing of a Standing Horse In Vivo.
Bone fracture is also a serious problem for horses, especially thoroughbred race horses. There is therefore a need to develop tools for the minimally invasive assay of fracture risk in these animals. In general, it is preferable if measurements can be made on standing horses, with the process being much faster and less invasive. Initial attempts using the earlier version of the RPI instrument on horses yielded little success. The biggest problem was irreproducibility caused by horse movement during the extended (10 s) measurement time required by the previous instrument. The solution to this problem is the drastically decreased 1 ms measurement time of the present instrument. Another related problem was that it was necessary to affix an appliance to hold the previous RPI onto the horse's leg, again because of the prolonged 10 s measurement time. The horse would regard this appliance as an irritation, treating it as something which should be removed by kicking, obviously limiting its usefulness. These problems were eliminated with the present instrument which is capable of very rapid testing (less than 1 ms) while being handheld (Fig. 4). Although the horses required a sedative and local anesthesia at the measurement site, they were conscious. Thus measurements were obtained successfully on standing horses.
Fig. 4.

Bone fracture is a serious problem for horses, especially thoroughbred race horses. Here one of us (DH) at Alamo Pintado stables measures the Bone Material Strength of a young, lame thoroughbred horse. He and (KH) each measured both legs and obtained BMS of 80 ± 13.
4.2. Clinical Trial on Anesthetized Horse Through Skin Versus on Bare Bone.
To verify that the instrument can penetrate horse's periosteum and obtain similar results through tissue and on bare bone, an experiment was conducted on a horse that was previously scheduled to be euthanized at Purdue University. The horse was tested before death through tissue, after death through tissue, and after death on bare bone. The most difficult step in the procedure is penetrating the skin, as it is very tough and a sharp probe is necessary. However, once the probe was on the bone surface it could be moved easily to find a relatively flat surface of the bone that has not been indented without the need to remove the probe between indentations. The results showed that there was only a small difference between the through tissue (mean BMS of 88) and bare bone test (mean BMS of 84). This validates that the Osteoprobe® can penetrate the skin and periosteum for in vivo horse testing and still gives reliable results on the bone itself.
In general, the experience of measuring standing horses was similar to the experience of measuring humans. In both cases, only local anesthesia was used at the measurement site and, for the horses, a sedative. In both cases, the patient was awake. For the case of an unconscious, anesthetized horse, due for euthanasia, it was practical to take many more measurements than on a fully conscious human or horse. From these tests, it can be seen that there is somewhat more scatter in the data on horses compared to data on people. Based on an ANOVA analysis by Morton Brown [9], we had converged on five as an adequate number of tests for a human patient with the conventional RPI instrument. As discussed above, ten tests is better than five with the Osteoprobe®. Since the scatter is more for the horses, a new ANOVA analysis will be necessary to determine the optimal number of tests for a horse patient. Based on these current findings, it would be conservative and safe to perform five measurements in each of the four skin punctures for a total of 20 measurements per horse.
5. Discussion
The Osteoprobe® is an easy-to-use instrument which provides reproducible measurements of the material strength of bone in not only laboratory samples, but also in clinical trials on humans and horses. A novel aspect of this instrument is the method by which it directly measures the indentation resistance in bone, while actually creating fractures. We presented clinical studies on humans that provided reasonable and consistent values. The Osteoprobe® has been shown to successfully obtain BMS measurements through the soft tissue of both horses and humans in vivo. The instrument is able to pierce the soft tissue and periosteum without the need of a reference probe to push the tissue aside. This is an important advancement because it provides for a less invasive procedure compared to previous RPI instruments and does not require extensive training, making the Osteoprobe® a very simple instrument to operate. Further tests will be needed to determine the significance of the measured parameters in animal and human subjects, but initial tests presented here are quite positive.
Acknowledgment
We thank the Fondo de Investigaciones Sanitarias (PI07/90912) and the RETICEF (RD06/0013/1009) of the Instituto Carlos III, Spanish Ministry of Science and Technology, the Mayo Center for Translational Science Activities (UL1 RR024150), and the NIH RO1 GM 065354 for support of this work. The authors wish to thank individuals who donate their bodies and tissues for the advancement of education and research. The measurements reported here were done with prototype instruments, but Active Life Scientific, Inc. may, in the future, produce a commercial version of this instrument if there is demand for it. Four of the authors, P.H., D.B., J.C., and A.P., are members of Active Life Scientific.
Laboratory Study design: CR, DB, PKH. Laboratory Study conduct: CR, DB, SR, HB, PKH. Barcelona Study design: ADP and RGF. Barcelona Study conduct: RGF, ADP, XN, ET, LM. Purdue Study design: TL, SC. Purdue Study conduct: TS, AS, CS, TL. Oregon Study design: EO. Oregon Study conduct: CN, EO. Alamo Pintado Study design and conduct: DH. Mayo Study design: SK. Mayo Study conduct: JNF, LM, SK. SEM imaging: JW. Statistics: HK. Assisted in the organization of studies: DB, AP, JC. Writing and Drafting manuscript: CR, DB, PKH. All authors approve final version of manuscript.
Contributor Information
Daniel Bridges, Department of Physics, University of California, Santa Barbara, CA 93106.
Leonardo Mellibovsky, Hospital del Mar-IMIM-Universitat, Autónoma and RETICEF, Instituto Carlos III, Barcelona 08003, Spain.
Kevin Hoffseth, Department of Mechanical Engineering, University of California, Santa Barbara, CA 93106.
Ananya Srikanth, Materials Engineering, Purdue University, West Lafayette, IN 47907.
James C. Weaver, Wyss Institute for Biologically, Inspired Engineering, Harvard University, Cambridge, MA 02138
Heather Barnard, Department of Physics, University of California, Santa Barbara, CA 93106.
James Candy, Active Life Technologies LLC, Santa Barbara, CA 93101.
Srinivasan Chandrasekar, Materials Engineering, Purdue University, West Lafayette, IN 47907.
Timothy Lescun, Veterinary Clinical Sciences, Purdue University, West Lafayette, IN 47907.
Eric Orwoll, Oregon Health & Science University, Portland, OR 97239.
Doug Herthel, Alamo Pintado Equine Medical Center, Los Olivos, CA 93441.
Hal Kopeikin, Department of Physics, University of California, Santa Barbara, CA 93106.
Henry T. Y. Yang, Department of Mechanical Engineering, University of California, Santa Barbara, CA 93106
Sundeep Khosla, Mayo Clinic, Rochester, MN 55905.
Adolfo Diez-Perez, Hospital del Mar-IMIM-Universitat, Autónoma and RETICEF, Instituto Carlos III, Barcelona 08003, Spain.
Paul K. Hansma, Department of Physics, University of California, Santa Barbara, CA 93106 Active Life Technologies LLC, Santa Barbara, CA 93101.
References
- [1]. Ettinger, M. P. , 2003, “Aging Bone and Osteoporosis: Strategies for Preventing Fractures in the Elderly,” Arch. Intern. Med., 163, pp. 2237–224610.1001/archinte.163.18.2237 [DOI] [PubMed] [Google Scholar]
- [2].NIH Consensus Development Panel, 2001, “Osteoporosis Prevention, Diagnosis, and Therapy,” J. Am. Med. Assoc., 285, pp. 785–79510.1001/jama.285.6.785 [Google Scholar]
- [3]. Rivadeneira, F. , Zillikens, M. C. , Laet, C. E. D. , Hofman, A. , Uitterlinden, A. G. , Beck, T. J. , and Pols, H. A. , 2007, “Femoral Neck BMD is a Strong Predictor of Hip Fracture Susceptibility in Elderly Men and Women Because It Detects Cortical Bone Instability,” J. Bone Miner. Res., 22, pp. 1781–179010.1359/jbmr.070712 [DOI] [PubMed] [Google Scholar]
- [4]. Yang, L. , Peel, N. , Clowes, J. A. , McCloskey E. V., and Eastell, R. , 2009, “Use of DXA-Based Structural Engineering Models of the Proximal Femur to Discriminate Hip Fracture,” J. Bone Miner. Res., 24, pp. 33–4210.1359/jbmr.080906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5]. Cummings, S. R. , Karpf, D. B. , Harris, F. , Genant, H. K. , Ensrud, K. , LaCroix, A. Z. , and Black, D. M. , 2002, “Improvement in Spine Bone Density and Reduction in Risk of Vertebral Fractures During Treatment With Antiresorptive Drugs,” Am. J. Med., 112, pp. 281–28910.1016/S0002-9343(01)01124-X [DOI] [PubMed] [Google Scholar]
- [6]. Boutroy, S. , Rietbergen, B. V. , Sornay-Rendu, E. , Munoz, F. , Bouxsein, M. L. , and Delmas, P. D. , 2008, “Finite Element Analysis Based on In Vivo HR-pQCT Images of the Distal Radius is Associated With Wrist Fracture in Postmenopausal Women,” J. Bone Miner. Res., 23, pp. 392–39910.1359/jbmr.071108 [DOI] [PubMed] [Google Scholar]
- [7]. Diez-Perez, A. , Guerri, R. , Nogues, X. , Caceres, E. , Pena, M. J. , Mellibovsky, L. , Randall, C. , Bridges, D. , Weaver, J. C. , Proctor, A. , Brimer, D. , Koester, K. J. , Ritchie, R. O. , and Hansma, P. K. , 2010, “Microindentation for In Vivo Measurement of Bone Tissue Mechanical Properties in Humans,” J. Bone Miner. Res., 25, pp. 1877–188510.1002/jbmr.73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8]. Hansma, P. , Yu, H. , Schultz, D. , Rodriguez, A. , Yurtsev, E. A. , Orr, J. , Tang, S. , Miller, J. , Wallace, J. , Zok, F. , Li, C. , Souza, R. , Proctor, A. , Brimer, D. , Nogues-Solan, X. , Mellbovsky, L. , Pena, M. J. , Diez-Ferrer, O. , Mathews, P. , Randall, C. , Kuo, A. , Chen, C. , Peters, M. , Kohn, D. , Buckley, J. , Li, X. , Pruitt, L. , Diez-Perez, A. , Alliston, T. , Weaver, V. , and Lotz, J. , 2009, “Tissue Diagnostic Instrument,” Rev. Sci. Instrum., 80, p. 054303.10.1063/1.3127602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9]. Hansma, P. , Turner, P. , Drake, B. , Yurtsev, E. , Proctor, A. , Mathews, P. , Lulejian, J. , Randall, C. , Adams, J. , Jungmann, R. , Garza-de-Leon, F. , Fantner, G. , Mkrtchyan, H. , Pontin, M. , Weaver, A. , Brown, M. B. , Sahar, N. , Rossello, R. , and Kohn, D. , 2008, “The Bone Diagnostic Instrument II: Indentation Distance Increase,” Rev. Sci. Instrum., 79, p. 064303.10.1063/1.2937199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10]. Hansma, P. K. , Turner, P. J. , and Fantner, G. E. , 2006, “Bone Diagnostic Instrument,” Rev. Sci. Instrum., 77, p. 075105.10.1063/1.2221506 [Google Scholar]
- [11]. Chavassieux, P. , Seeman, E. , and Delmas, P. D. , 2007, “Insights into Material and Structural Basis of Bone Fragility From Diseases Associated With Fractures: How Determinants of the Biomechanical Properties of Bone are Compromised by Disease,” Endocr. Rev., 28, pp. 151–16410.1210/er.2006-0029 [DOI] [PubMed] [Google Scholar]
- [12]. Vashishth, D. , 2005, “Age-Dependent Biomechanical Modifications in Bone,” Crit. Rev. Eukar. Gene., 15, pp. 343–35710.1615/CritRevEukarGeneExpr.v15.i4.40 [DOI] [PubMed] [Google Scholar]
- [13]. Currey, J. D. , 1979, “Changes in the Impact Energy Absorption of Bone With Age,” J. Biomech., 12, pp. 459–46910.1016/0021-9290(79)90031-9 [DOI] [PubMed] [Google Scholar]
- [14]. Currey, J. , 2004, “Incompatible Mechanical Properties in Compact Bone,” J. Theor. Biol., 231, pp. 569–58010.1016/j.jtbi.2004.07.013 [DOI] [PubMed] [Google Scholar]
- [15]. Turner, C. H. , 2002, “Biomechanics of Bone: Determinants of Skeletal Fragility and Bone Quality,” Osteop. Int., 13, pp. 97–10410.1007/s001980200000 [DOI] [PubMed] [Google Scholar]
- [16]. Bouxsein, M. L. , 2003, “Bone Quality: Where Do We Go From Here?,” Osteop. Int., 14, pp. S118–S12710.1007/s00198-003-1489-x [DOI] [PubMed] [Google Scholar]
- [17]. Jepsen, K. J. , 2003, “The Aging Cortex: To Crack or Not to Crack,” Osteop. Int., 14, pp. S57–S6210.1007/s00198-003-1475-3 [DOI] [PubMed] [Google Scholar]
- [18]. Seeman, E. , and Delmas, P. D. , 2006, “Bone Quality—The Material and Structural Basis of Bone Strength and Fragility,” New Engl. J. Med., 354, pp. 2250–226110.1056/NEJMra053077 [DOI] [PubMed] [Google Scholar]
- [19]. Bridges, D. , Randall, C. , and Hansma, P. , 2012, “A New Device for Performing Reference Point Indentation Without a Reference Probe,” Rev. Sci. Instrum., 83, p. 044301.10.1063/1.3693085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20]. Fernandez, R. , Diez-Perez, A. , Nogues, X. , Prieto-Alhambra, D. , Mellibovsky, L. , Bridges D., Randall, C. , and Hansma, P. , 2011, “Validation of a Novel Microindenter for Bone Material Strength Measurement,” American Society for Bone and Mineral Research 2011 Annual Meeting, San Diego, CA, September 16–20, http://www.asbmr.org/Meetings/AnnualMeeting/AbstractDetail.aspx?aid=cb0278aa-14cf-47e2-84be-8bf2a7e7896e [Google Scholar]