Abstract
Helminth-induced type 2 immune responses, which are characterized by the T helper 2 cell-associated cytokines interleukin-4 (IL-4) and IL-13, mediate host protection through enhanced tissue repair, the control of inflammation and worm expulsion. In this Opinion article, we consider type 2 immunity in the context of helminth-mediated tissue damage. We examine the relationship between the control of helminth infection and the mechanisms of wound repair, and we provide a new understanding of the adaptive type 2 immune response and its contribution to both host tolerance and resistance.
Vertebrates must be prepared to defend themselves against two distinct types of ‘insult’. In the first type of insult, rapidly replicating microorganisms, such as bacteria, viruses, protozoa and fungi, have the potential to overcome host defences by their sheer numbers. In this situation, an antimicrobial type 1 immune response, which is characterized by the T helper 1 (TH1) cell-associated cytokines interferon-γ and interleukin-12 (IL-12), is invoked; however, this carries the risk of initiating harmful pro-inflammatory responses that could lead to collateral tissue damage. In the second type of insult, the protective barriers of the body are breached by physical trauma. This is the type of insult that occurs when helminths and other metazoan parasites enter, exit or migrate through their host. As these pathogens do not complete their life cycle in the host, the danger of rapid expansion and dissemination is absent. In this context, a type 1 immune response would be either too self-damaging or ineffective, and therefore a distinct type 2 immune response dominates (BOX 1). Animal models have shown that type 2 immunity is essential for helminth control; however, the selection pressures that led to the evolution of the highly complex adaptive type 2 immune response are not yet fully understood and are still being debated1–3.
Box 1. Type 1 versus type 2 immunity.
The body must rapidly assess the nature of any threat, mobilize the appropriate machinery to deal with it and, in the case of infectious agents, activate the relevant adaptive branch of the immune system if the innate immune responses prove insufficient.
The mammalian immune response to rapidly replicating invaders is termed type 1 immunity and involves a vast array of antimicrobial effectors that have a central role in the activation of phagocytic cells, such as macrophages and neutrophils. The antimicrobial function of these innate immune cells is directed and enhanced through cytokines that are produced by T helper 1 (TH1) and TH17 cells of the adaptive immune system. An unwanted side effect of type 1 immunity is that the induction of highly toxic antimicrobial products often has damaging consequences for the host tissue.
Type 2 immunity encompasses the host response to helminth infections and involves an ever-expanding group of innate immune cells, such as basophils, eosinophils, mast cells, M2 macrophages (also known as alternatively activated macrophages) and group 2 innate lymphoid cells45,88, with TH2 cells functioning as the central mediators of the adaptive immune response.
Metazoan parasites can seriously compromise host fitness, as shown by the direct effects of parasites on host fecundity4, energy consumption5 and the reduced ability of some infected mammals to survive the winter6. In humans, helminths not only cause overt morbidity but they also contribute to anaemia and impaired physical and cognitive development, which can result in poor school or work performance7. These fitness effects represent a powerful selection pressure for genes, which minimizes the consequences of macroparasite infection. This protection can occur either through resistance mechanisms that reduce parasite numbers or through tolerance mechanisms that reduce damage to the host without directly affecting parasite numbers8.
Recent literature has shown that there is a close association between type 2 immunity and many aspects of wound repair9–12. As wound repair alleviates the injury that is caused by the infection, rather than directly affecting the pathogen, it is considered to be a tolerance mechanism13. Helminths cause considerable tissue damage and, thus, their association with wound repair may not be surprising. However, building on previous hypotheses1,14, we propose that, in the course of evolution, the adaptive type 2 immune response evolved to direct the wound-healing machinery not only to repair and reconstruct tissue (tolerance) but also to mediate the containment, destruction and expulsion of helminths (resistance); for example, collagen production is involved in both parasite encapsulation and tissue reconstruction, whereas mucus production — a response to injury — promotes the efficient expulsion of worms from the gut. Thus, under the umbrella of type 2 immunity, both wound repair and anti-worm effector pathways have evolved in tandem to mediate host protective responses to helminths. This is in contrast with microbial infection, in which host resistance mechanisms that are mediated by TH1 and TH17 cells are inflammatory and can antagonize many wound repair pathways15; microbial infection therefore shows the more typical trade-off between resistance and tolerance8.
Although type 2 immunity might include responses to a broad range of insults, we propose that it is the ability of helminth parasites to compromise host fitness that is likely to have driven the evolution of the adaptive TH2 cell response in order to both control parasite numbers and to rapidly repair the damage the parasites inflict as they migrate through host tissues. Many aspects of type 2 immunity, from cold adaptation16 to wound repair, suggest that the innate and adaptive type 2 immune responses are primarily focused on maintaining or restoring tissue integrity and homeostasis. Nonetheless, parasite numbers must also be reduced if the worm burden compromises host fitness. Importantly, this does not mean that type 2 immune responses must promote sterile immunity, as low numbers of some helminth species might provide host benefits17,18.
In evolutionary terms, the adaptive immune response, including T cell immunity, evolved to focus effector responses on specific antigens. Thus, TH2 cells provide antigen specificity and memory to innate pathways that can rapidly repair parasite-induced damage, promoting host tolerance. Much of the same machinery contributes to worm killing and expulsion, promoting host resistance. The hypothesis that TH2 cells evolved to direct the innate wound repair machinery does not require innate and/or adaptive type 2 immune responses to be involved in all aspects of repair, nor does it exclude the possibility that wound repair activity is involved in a type 1 immune response. Indeed, transforming growth factor-β1 (TGFβ1) that is produced during both type 1 and type 2 immune responses is an important mediator of fibroblast activation and tissue repair19. Nonetheless, discovering the specific links between type 2 cytokines and wound effector molecules might provide exciting new insights for both helminth immunity and fundamental wound repair pathways.
In this Opinion article, we describe the close relationship that anti-helminth effector immune responses have with wound repair pathways. We explain how the anti-inflammatory activity of the helminth-induced type 2 immune response is directly connected to wound healing and to the attenuation of autoimmune and allergic diseases. We also highlight the damaging consequences of overzealous type 2-mediated wound healing, which has probably contributed to the evolution of the regulatory networks needed to control type 2 immunity.
Injury and type 2 immunity
The signals that induce type 1 immune responses include pathogen-associated molecular patterns (PAMPs), which communicate the presence of a microbial insult to the immune system20. The signals that induce type 2 immunity are fundamentally different and are not as well understood. We propose that tissue damage, in the absence of PAMPs that promote a type 1 immune response, is a potent mechanism that drives type 2 immunity, particularly in the context of helminth infection.
Epithelial alarmins elicit type 2 immunity
Although helminth molecules with direct TH2 cell-inducing capacity might exist17,21, the large size of the parasite and the consequent tissue damage that it causes during invasion might be the most important factor in the initiation of a type 2 immune response. This idea is supported by studies showing that helminth invasion, and the associated tissue damage, induces the release of several epithelium-derived cytokine alarmins that can initiate type 2 immunity22– 24. One of the first such alarmins that was shown to have an important role in helminth-induced type 2 immunity was thymic stromal lymphopoietin (TSLP)25; it was suggested in a recent study that TSLP directs type 2 immunity partly by eliciting a population of functionally distinct IL-4-producing basophils26. TSLP also promotes type 2 immunity by actively suppressing the production of IL-12 by dendritic cells27. The role of TSLP in eliciting type 2 immunity varies in different helminth infections because some parasites bypass the need for TSLP by directly suppressing IL-12 expression27. In these cases, the type 2 immune response might be a default response or it might rely on other alarmins.
IL-33 is a cytokine alarmin that is released from the nucleus of necrotic epithelial cells, endothelial cells and fibroblasts. The IL-33 receptor complex — interleukin-1 receptor-like 1 (IL-1RL1; also known as ST2)–IL-1R accessory protein (IL-1RAP) — has also been shown to initiate type 2 responses following helminth infection28,29. A similar type 2-inducing role has been identified for the IL-17 family cytokine IL-25 (also known as IL-17E)30,31. Thus, multiple cytokine alarmins that are released by epithelial cells and other cells are involved in the initiation of protective type 2 immune responses during macroparasite infection; the relative importance of each mediator is probably dictated by the pathogen and by its mode of entry into the host.
Mechanisms that mimic helminth invasion
Recent studies have shown that type 2 immunity is activated by large inert particulate structures that can cause direct cell and tissue damage. In particular, inert silica32 and titanium microparticles33 induce innate type 2 immune responses and have been successfully used as adjuvants to promote antigen-specific TH2 cell responses through pathways that are independent of Toll-like receptor 4 (TLR4) and the signalling adaptor molecule myeloid differentiation primary-response protein 88 (MYD88)33. One explanation for why these relatively large particulate structures induce type 2 immunity is that they share essential features with helminths. They are both large structures that might induce cell and tissue damage in the absence of type 1-inducing PAMPs; for example, ingested microparticles might induce cellular damage in phagocytes through the release of lysosomal enzymes following lysosome rupture and, consequently, this might activate endogenous danger signals that mediate tissue damage32.
In addition, the TH2-inducing adjuvant aluminium hydroxide (alum) might induce the release of the damage-associated molecular pattern (DAMP) uric acid34. Uric acid crystals that are released from damaged tissues drive type 2 immunity through inflammasome-independent pathways32,34. Furthermore, extracellular ATP, which is presumably released by damaged cells, binds to P2 purinergic receptors that induce the release of IL-33 in the lungs35, resulting in the generation of type 2 immunity. Thus, in this case, the ATP released from damaged tissues functions as a DAMP to induce the secretion of cytokine alarmins that, in turn, stimulate a type 2 immune response. Importantly, no single cellular DAMP has yet been shown to be essential for the development of helminth-induced responses, which suggests that a range of redundant pathways are involved in sensing helminth-induced danger. Indeed, multiple causes of tissue damage, including helminth-derived proteases and insect venoms, might contribute to the activation of type 2 immune responses (BOX 2).
Box 2. Insults and injuries that induce type 2 immune responses.
The stimuli that induce type 2 immune responses are numerous and diverse. They are generally most effective in the absence of a strong interleukin-12 (IL-12) response, which is often induced by potent Toll-like receptor (TLR) signalling. Some helminth-derived factors directly inhibit IL-12 and/or IL-17 production. At the same time, low level TLR signalling can promote a type 2 immune response. It should also be noted that helminths often induce more potent type 2 responses than other stimuli. The stimuli can be divided into several basic categories, which are described below with representative examples.
Active molecules — in particular, enzymes — that mediate tissue damage; for example, venoms, parasite-derived proteases, non-parasite-derived proteases (such as pollen) and the saliva of biting arthropods.
Inactive factors that may still cause tissue damage; for example, small particles such as silica32 and titanium33 and the widely used adjuvant aluminium hydroxide (alum)89.
Factors that downregulate T helper 1 (TH1) and/or TH17 cell responses, thereby promoting type 2 immunity; for example, the helminth excretory–secretory product ES62 (REF. 38), a helminth-derived transforming growth factor-β analogue90, helminth-induced IL-10 production58 and omega 1, which is a component of the schistosome egg antigen39.
Low level TLR signalling or C-type lectin signalling; for example, that induced by low-dose endotoxin91 or house dust mite, which has TLR, C-type lectin and enzyme activity34.
Strong support for the importance of injury alone in the induction of type 2 immunity comes from the evidence that innocuous antigens, when exposed to the immune system in the context of experimentally induced cutaneous tissue damage, can stimulate potent type 2 immune responses36. Therefore, injury and type 2 immunity are closely linked. The capacity of helminths to induce injury might explain an ancient association of metazoan parasites with the type 2 response, leading to the evolution of an adaptive type 2 immune response that protects against the consequences of helminth infection.
Mechanisms that amplify type 2 immunity
The data above show that tissue stress and damage, in the absence of strong TLR signalling, rapidly stimulates type 2 immunity. The combination of tissue stress and damage might be one mechanism by which the host recognizes helminth invasion and responds with an appropriate protective response. The anti-helminth response is further enhanced and polarized by the addition of individual helminth excretory–secretory products that condition dendritic cells to suppress TLR signals and the associated protein machinery that is required to prime TH1 cells37–39. This potent combination might be essential to induce the highly polarized and potent adaptive type 2 immune response that develops following helminth infection.
The cytokine alarmins are important for inducing the recruitment of group 2 innate lymphoid cells (ILC2s), which secrete large quantities of IL-5 and IL-13 (REFS 40–42), that might then activate anti-parasite effector responses43,44. ILC2s, along with multipotent progenitor type 2 cells22, eosinophils, mast cells and basophils, are rapidly recruited to the site of infection and function as important innate sources of IL-4, IL-5 and IL-13. Eosinophils and basophils in particular are important producers of IL-4, which further amplifies and regulates both the innate and adaptive branches of type 2 immunity45,46.
Helminths promote wound repair
Almost all the cell types that are associated with immunity to helminth infections are also implicated in wound healing. These include T cells, eosinophils, M2 macrophages (also known as alternatively activated macrophages) and ILC2s (FIG. 1). TH2 cytokines, including IL-4, IL-5 and IL-13, mediate eosinophil activation and recruitment. Eosinophils store a variety of preformed molecules in intracellular granules, including cytokines, growth factors, matrix metalloproteinases (MMPs), cationic proteins and lipid mediators, that can mediate wound healing, tissue remodelling and myofibroblast differentiation46. The differentiation of M2 macrophages is mostly dependent on the TH2 cytokines IL-4 and IL-13. M2 macrophages characteristically produce resistin-like molecule-α (RELMα), vascular endothelial growth factor (VEGF), arginase 1, YM1, insulin-like growth factor 1 (IGF1), MMPs, triggering receptor expressed on myeloid cells 2 (TREM2), TGFβ and growth factors such as platelet-derived growth factor (PDGF), all of which are closely associated with all stages of the wound-healing response47. Increases in the levels of these mediators have also been observed during helminth infection1,48,49, which suggests that they might contribute to the control of parasite-induced tissue damage.
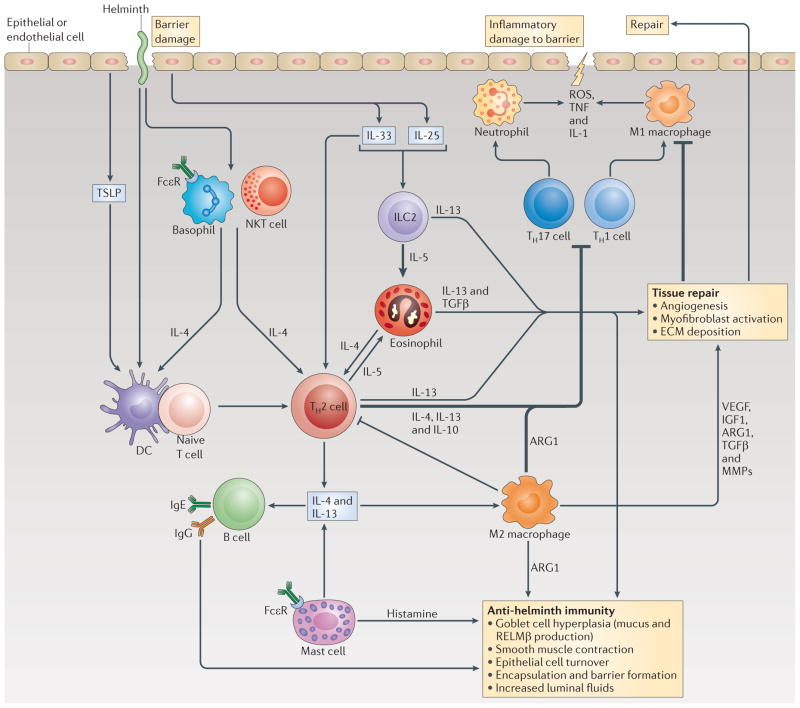
Figure 1. The type 2 immune response promotes tissue repair and immunity against helminths.
When helminth parasites infect their mammalian host, epithelial and endothelial barriers are damaged, which induces a wound repair and an anti-parasite immune response that is driven by the type 2 cytokines interleukin-4 (IL-4), IL-5 and IL-13. This figure is not meant to imply that all of these pathways, cell types and cytokines are crucial to the development of immunity against every helminth parasite; rather, it simply illustrates the many different innate and adaptive mechanisms that have been shown to participate in type 2 immunity, and each parasite is often affected by different components. Dendritic cells (DCs) are the only antigen-presenting cell type that has been shown to be indispensable for the initiation of CD4+ T helper 2 (TH2) cell responses; indeed, basophils, natural killer T (NKT) cells, eosinophils and group 2 innate lymphoid cells (ILC2s) function as accessory cells by producing the key TH2-regulating cytokine IL-4. B cells also participate in secondary type 2 responses by producing parasite-specific IgE, which, following engagement of the high-affinity Fc receptor for IgE (FcεR), can augment the production of IL-4 by basophils and mast cells. Epithelial cells might also help to guide type 2 responses by producing the alarmins thymic stromal lymphopoietin (TSLP), IL-25 and IL-33. TSLP regulates type 2 immunity by suppressing DC-derived IL-12 production, whereas IL-25 and IL-33 primarily target ILC2s, which secrete large quantities of IL-5 and IL-13. Type 2 cytokines in turn target epithelial cells, goblet cells, smooth muscle cells and macrophages, which together coordinate parasite expulsion by increasing fluid and mucus production, encapsulation and barrier formation, epithelial cell turnover, smooth muscle contraction and the production of anti-parasite effector molecules such as resistin-like molecule-β (RELMβ). In addition to activating several anti-parasite effector mechanisms, the type 2 immune response facilitates wound repair, which is important following infection by these large multicellular tissue-invasive organisms. M2 macrophages (also known as alternatively activated macrophages) are intimately involved in this process as they produce matrix metalloproteinases (MMPs), arginase 1 (ARG1), insulin-like growth factor 1 (IGF1), vascular endothelial growth factor (VEGF) and transforming growth factor-β (TGFβ), which together promote myofibroblast activation, angiogenesis, epithelial cell turnover and extracellular matrix (ECM) deposition. The helminth-induced type 2 immune response also promotes effective wound healing by suppressing the pro-inflammatory axis that is mediated by M1 macrophages and TH1 and TH17 cells, which could further exacerbate tissue injury if not quickly controlled. M2 macrophages and IL-10-producing TH2 cells have been shown to have important roles in the suppression of this pro-inflammatory axis and can also control potentially harmful type 2 immune responses. ROS, reactive oxygen species; TNF, tumour necrosis factor.
ILC2s also produce multiple proteins that are associated with wound healing, including the epidermal growth factor receptor ligand amphiregulin10. Indeed, depletion of ILC2s compromises lung epithelial barrier integrity, and barrier function is restored when amphiregulin is administered10.
Memory responses augment type 2 immunity
Effector memory T cells (which rapidly secrete cytokines at high levels) and the production of IgE and IgG by B cells can increase resistance to many helminths50–52. Shortly after infection, many of these parasites migrate through tissues, such as the lungs, where they are actively targeted by immune cells, before they enter the lumen of the small intestines53. Fcε receptors expressed by mast cells bind to parasite-specific IgE, and the subsequent crosslinking of bound IgE by helminth antigens induces degranulation and the release of mediators from mast cells that, in some cases, contribute to parasite expulsion, as has been suggested by the immune response to Trichinella spiralis54. The IgE-mediated rapid degranulation of mast cells might also be important in the defence against other macroparasites such as ticks, as localized tissue oedema that is mediated by the molecules released during degranulation might impede parasite invasion55,56. Amphiregulin is also produced by mast cells following signalling from the high-affinity Fc receptor for IgE (FcεR1)57; this highlights a potential link between the IgE-mediated memory response and wound repair. Thus, a very rapid and sometimes complex immune response is needed to induce expulsion or killing and simultaneously to repair the damage caused by these large macroparasites.
Control of helminth-induced tissue damage
Effective wound repair requires both the direct reconstruction of the injured tissue and the suppression of pro-inflammatory responses15. Recent studies have shown that the type 2 immune response has a direct role in wound healing through the production of mediators that directly enhance the tissue repair process and through the control of inflammation. In the experimental model of schistosomiasis, the adaptive type 2 immune response protects the host during infection by inducing granulomas that sequester the tissue-damaging toxins that are released by parasite eggs. If the CD4+ TH2 cell response is impaired, infected animals develop defective granulomas and quickly succumb to the infection because they develop uncontrolled type 1 and type 17 inflammatory responses in the liver and intestines58,59. The specific blockade of TH2-induced arginase 1 in mice that are severely infected with Schistosoma mansoni caused increased egg-associated intestinal tissue damage, haemorrhaging, and disruption and leakage of the mucosal barrier, all of which were dependent on signalling through IL-12 receptor subunit β1 (IL-12RB1; which is a receptor subunit shared by the cytokines IL-12p40 and IL-23)11. Thus, in this system, arginase 1 production controls the harmful inflammation that is associated with a type 1 immune response, which consequently contributes to tissue repair and to the maintenance of the mucosal barrier.
In another example, infection with the nematode parasite Nippostrongylus brasiliensis induces acute pulmonary haemorrhaging as parasites traffic through the lungs towards to the intestines60,61. This damage is caused by both physical trauma and by IL-17-driven neutrophil inflammation9. The type 2 immune response, which emerges shortly afterwards, reduces the haemorrhaging and the inflammation through multiple IL-4Rα-dependent mechanisms9. In particular, macrophages that have been stimulated through IL-4Rα express IGF1 (REFS 9,62), which has been associated with tissue regeneration, collagen synthesis and fibroblast activation63–65. IGF1 has been shown to be essential for the enhanced acute woundhealing response during parasite migration through the lungs9, which indicates that the helminth-induced type 2 immune response can directly enhance wound healing.
A role for IL-10 and regulatory macrophages
In the N. brasiliensis lung infection model, M2 macrophages engulf and remove damaged red blood cells9; this is consistent with other studies that have implicated M2 macrophages in the phagocytosis and clearance of neutrophils48,66. Although recent in vitro studies have suggested that M2 macrophages might have impaired phagocytosis67, in vivo studies in N. brasiliensis9 suggest that M2 macrophages retain efferocytosis properties, which is a process that is crucial for resolving inflammation and, thus, it is an important aspect of the wound-healing response.
Some studies have suggested that IL-10-secreting macrophages also have an important protective role in the immune response to helminths by suppressing inflammation68–70. However, more recent studies of N. brasiliensis infection have shown that IL-10 is primarily produced by CD4+ T cells rather than by macrophages9, and RNA-sequencing analysis of macrophages during filarial nematode infection showed that there was an absence of IL-10 expression in M2 macrophages62. It is probable that in the absence of additional signals, such as TLR ligands or type I interferons71, helminth-induced M2 macrophages do not secrete substantial quantities of IL-10. These findings are consistent with the proposed wound-healing macrophage phenotype72 and indicate that the majority of macrophages that are induced during these nematode infections may be distinct from the regulatory macrophages that secrete IL-10. Nonetheless, helminthinduced M2 macrophages are fundamentally non-inflammatory; they mediate the IL-4Rα-dependent suppression of all proinflammatory mediators62, in addition to the production of the pro-repair molecules discussed above.
Taken together, these recent studies show that type 2 immunity mediates tissue repair both by limiting inflammation and by directly activating mediators that facilitate wound healing. Thus, as helminths migrate through vital tissues, sometimes causing extensive tissue damage, the potent type 2 immune response that is rapidly evoked by the infection will enhance tolerance to these large multicellular organisms by inducing wound-healing mechanisms that include the suppression of the inflammatory response.
Helminths and disease
Helminths regulate inflammatory disease
The potent anti-inflammatory and direct wound-healing pathways that are induced by helminths might greatly affect the overall immune function. The constant presence of helminths probably promotes a homeostatic set point where immunoregulatory mediators, such as IL-10 and arginase 1, are elevated and are therefore available to control harmful inflammatory responses that might otherwise result in disease (FIG. 2). Indeed, the marked reduction in helminth infections in industrialized countries has been causally linked with the rapid rise in the incidence of many inflammatory diseases; this is hypothesized to be due to a loss of immunoregulatory and wound-healing activity. Consistent with this model, the severity of several inflammatory diseases, including inflammatory bowel disease and type 1 diabetes, is considerably reduced in different experimental models of helminth infection18,73. As a result, much effort has been made to understand helminth-induced immunoregulation, with the aim of therapeutically harnessing these mechanisms of regulation in the future.
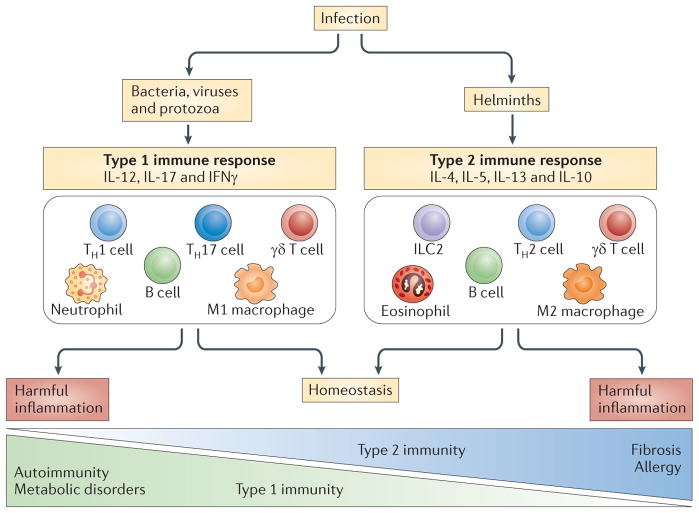
Figure 2. The role of the immune system in homeostasis.
Homeostasis is influenced by the diversity of pathogens that infect host populations during the course of evolution. In the case of vertebrates, frequent exposure to parasites and microorganisms induces regulatory mechanisms, including regulatory T (TReg) cells, which control potentially harmful inflammatory effects (not shown). The absence of these pathogens in the host might result in a dysregulated immune system. In the case of harmful type 1 and type 17 inflammation, autoimmune diseases and metabolic disorders might result. Type 2 inflammation has a particularly important role in preventing the development of these disorders by promoting homeostasis. Conversely, harmful type 2 inflammation might result in the development of fibrosis and allergy. An environment that includes exposure to chronic infections or, perhaps, therapeutic surrogates could therefore promote homeostasis. Allergens seem to be capable of inducing type 2 immunity in the absence of the regulatory networks that are associated with helminth infection, thereby exacerbating the harmful effects of type 2 immunity. In addition, in many cases, the same cell lineages participate in both type 1 and type 2 immune responses but have different activation states. Representative examples are shown. IFNγ, interferon-γ; IL, interleukin; ILC2, group 2 innate lymphoid cell; TH, T helper.
The results so far indicate that the immunomodulatory effects of live helminth infections are superior to those of helminth-derived products. The large structure of a living mobile parasite might be necessary to induce multiple regulatory pathways, particularly those that are associated with suppressing injury-induced inflammatory tissue damage. Indeed, multiple independent regulatory pathways are induced during helminth infection and all of these pathways have been implicated in the downregulation of harmful type 1 inflammation74. Although these mechanisms often work together to suppress tissuedamaging inflammation, recent studies have suggested that helminth-induced IL-10 and TGFβ work independently of conventional TH2 cells and forkhead box P3 (FOXP3)+ regulatory T cells in controlling inflammation and disease severity in non-obese diabetic (NOD) mice75,76. This multicomponent response, which has built-in redundancy, might partly be an explanation for the robustness of the helminth-induced immune response in controlling tissue damage in numerous harmful inflammatory diseases.
Persistent type 2 immunity promotes fibrosis
As described above, type 2 cytokines maintain host fitness during helminth infection by downregulating inflammation, by promoting wound healing and by reducing parasite numbers. However, helminth infection is often chronic with a persistent production of type 2 cytokines. If type 2 immunity itself is not appropriately regulated, it can become pathogenic and can contribute to the development of lethal fibrotic pathology, which results from overzealous or persistent wound-healing responses47,77. In schistosomiasis, hepatic fibrosis leads to the development of portal hypertension, which induces the formation of gastric and oesophageal varices that are prone to rupture78. Indeed, the development of fibrosis and portal hypertension are the major causes of morbidity and mortality in chronic schistosomiasis. Whereas IL-4 was identified as the principal inducer of the protective type 2 response during acute S. mansoni infection59, IL-13 was identified as the key driver of hepatic fibrosis in mice chronically infected with S. mansoni77,79. In mice, the progression of hepatic fibrosis in schistosomiasis correlates with the intensity of the IL-13 response80, and immunological interventions that selectively impair IL-13 activity have been shown to reduce collagen deposition and to improve survival, as long as IL-4 production is preserved77. Thus, type 2 cytokines have both protective and pathogenic activity in chronic schistosome infections, and each infection outcome is directly linked with either beneficial or aberrant wound-healing responses.
Helminth-induced responses control allergy
The need to prevent the consequences of uncontrolled type 2, as well as type 1, immune responses might partly explain the counter-intuitive observation that helminth infection attenuates the harmful type 2 immune responses that are associated with allergy in experimental models81. This is consistent with epidemiological observations that indicate that the incidence of allergy is reduced in helminth-infected individuals2,18.
The findings from both experimental and clinical trials are consistent with a model in which the absence of chronic parasite infections can result in dysregulated immune responses to allergens. However, these data are not consistent with alternative models that suggest that allergic responses might be beneficial because they elicit adverse responses to potentially noxious substances, thereby stimulating avoidance behaviour3. This immunosuppressive effect of helminth infection on allergic responses might be a consequence of evasion mechanisms that have evolved in the parasite that enhance parasite survival not only by impairing type 2 immunity but also by protecting the host from damage caused by overzealous repair. IL-10 — which can be produced by effector T cells, FOXP3+ regulatory T cells, T regulatory type 1 cells and various innate immune cell populations following helminth infection — might be a particularly potent regulator of harmful type 2 immune responses. In addition, a recent study has suggested that basophil-derived IL-4 converts inflammatory monocytes into M2 macrophages that suppress allergic inflammation82. Arginase 1 and RELMα that are produced by M2 macrophages, as well as the IL-13 decoy receptor, are potent negative regulators of type 2 immune responses12,83–85. These helminth-induced regulators of type 2 immunity might therefore control overzealous wound healing and type 2 allergic responses; this would provide a possible explanation for the increase in severe allergic responses that has been observed in industrialized countries. If this hypothesis is correct, it will be important in future studies to elucidate the mechanisms that preferentially induce regulatory molecules during the type 2 immune response to helminths; these mechanisms could then be used to control harmful type 2 inflammation and to promote tissue repair.
Conclusions
In our model, the co-evolution of vertebrates and helminths had a crucial role in shaping a progenitor innate wound-healing response into a host-protective innate and adaptive type 2 immune response that is directed towards metazoan parasites (FIG. 3). Components of the response might also be induced by enzymes, particulates and certain toxins, many of which mimic components or characteristics of helminths and other macroparasites, and each of which elicits type 2 immunity partly through cell and tissue damage. The overall response manifests itself by directly reducing worm burden, by controlling harmful inflammation and by enhancing wound healing. The response needs to rapidly mature to enhance the repair of damaged vital organs, to limit the entry of macroparasites and to promote their expulsion in sensitized individuals at the earliest stages of infection. Rapid tissue repair is vital to survival as it prevents the invasion of bacteria and other pathogenic organisms that could quickly lead to the development of sepsis. The type 2 immune response has multiple components, with individual effector mechanisms tailored to enhance protective immunity against specific species of helminths and other macroparasites. None of these effector mechanisms is currently well understood compared with components of the type 1 immune response but, in the future, these mechanisms could be individually targeted by vaccines that promote tolerance and/or the expulsion of helminth parasites.
Figure 3. Helminth infections as a selective force for evolution of the type 2 immune response.
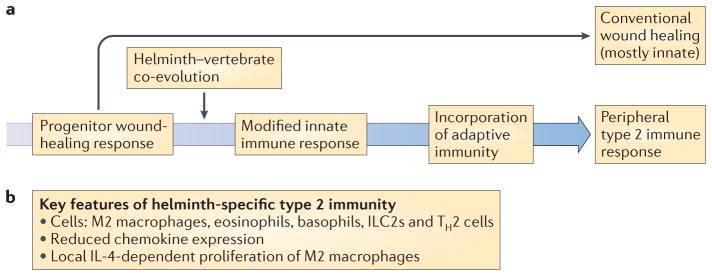
The adaptive type 2 immune response might have originally evolved from a progenitor wound-healing response, with the development of specific adaptations promoting tolerance and resistance during helminth–vertebrate co-evolution. a | After the evolution of adaptive immunity, these innate wound-healing pathways started to be controlled by T helper 2 (TH2) cells, which direct and enhance the responses to specific antigens. b | During a type 2 immune response, damaging inflammation is kept to a minimum through reduced chemokine expression and the local proliferation of macrophages rather than through the recruitment of inflammatory monocytes and neutrophils92. If monocyte recruitment does occur, as would be the case in a gut-dwelling helminth infection, then the presence of interleukin-4 (IL-4) or IL-13 facilitates monocyte conversion to M2 macrophages (also known as alternatively activated macrophages). ILC2s, group 2 innate lymphoid cells.
Several questions emerge from this perspective. It is clear that type 2 immune response-driven wound repair is not restricted to helminth infection. Indeed, signal tranducer and activator of transcription 6 (STAT6)-deficient mice, which lack effector type 2 immune responses, repair punch biopsy wounds more slowly than STAT6-sufficient mice, which provides evidence that TH2 cell-associated cytokines can accelerate wound healing independently of helminth infection86. So, which factors are induced by type 2 immunity that augment tissue repair? From a therapeutic perspective, are there components of the helminth-induced type 2 immune response that could be used to directly promote wound healing or tissue repair in chronic unresolved wounds, such as pressure ulcers or diabetic ulcers? What are the roles of basophils, ILC2s and other innate lymphocytes in the earliest stages of wound repair? In addition, does IgE accelerate or regulate the repair response through receptor expression on mast cells, basophils, dendritic cells and eosinophils?
We propose that adaptive responses to helminths evolved to regulate the wound repair machinery, but are other important homeostatic functions also disrupted by helminth infection? Does this help to explain the recent data showing that type 2 immune responses are important in regulating body temperature16 and host metabolism87? In this context it is fascinating to consider the studies in Soay sheep, in which an interaction involving three factors contributes to their poor survival over the winter: cold, lack of food and nematode infections6. An effective helminth-induced type 2 immune response might promote fitness by promoting adaptation to cold, energy storage and wound repair, as well as by limiting parasite numbers. Finally, as overzealous wound-healing responses can lead to harmful type 2 inflammation, including fibrosis, can helminth-derived products that have evolved to control type 2 responses be used for therapeutic benefit?
Acknowledgments
The authors wish to thank F. Finkelman at the Division of Allergy and Immunology, Cincinnati Children’s Hospital Medical Center, USA, for providing excellent critical comments that strengthened this manuscript. This work was partly supported by the US National Institutes of Health (NIH) grants R01AI031678 and R01AI066188 awarded to W.C.G., and T.A.W. is supported by the Intramural Research Program from the NIH National Institute of Allergy and Infectious Diseases.
Footnotes
Competing interests statement
The authors declare competing financial interests: see Web version for details.
Contributor Information
William C. Gause, Center for Immunity and Inflammation, Department of Medicine, New Jersey Medical School, Rutgers, the State University of New Jersey, Newark, New Jersey 07103–2757, USA
Thomas A. Wynn, Immunopathogenesis Section, Program in Tissue Immunity and Repair and the Laboratory of Parasitic Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland 20892, USA
Judith E. Allen, Centre for Immunity, Infection and Evolution, School of Biological Sciences, University of Edinburgh, Edinburgh EH9 3JT, United Kingdom
References
- 1.Allen JE, Wynn TA. Evolution of Th2 immunity: a rapid repair response to tissue destructive pathogens. PLoS Pathog. 2011;7:e1002003. doi: 10.1371/journal.ppat.1002003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Artis D, Maizels RM, Finkelman FD. Forum: Immunology: Allergy challenged. Nature. 2012;484:458–459. doi: 10.1038/484458a. [DOI] [PubMed] [Google Scholar]
- 3.Palm NW, Rosenstein RK, Medzhitov R. Allergic host defences. Nature. 2012;484:465–472. doi: 10.1038/nature11047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deter J, Cosson JF, Chaval Y, Charbonnel N, Morand S. The intestinal nematode Trichuris arvicolae affects the fecundity of its host, the common vole Microtus arvalis. Parasitol Res. 2007;101:1161–1164. doi: 10.1007/s00436-007-0584-x. [DOI] [PubMed] [Google Scholar]
- 5.Coop RL, Kyriazakis I. Influence of host nutrition on the development and consequences of nematode parasitism in ruminants. Trends Parasitol. 2001;17:325–330. doi: 10.1016/s1471-4922(01)01900-6. [DOI] [PubMed] [Google Scholar]
- 6.Gulland FM. The role of nematode parasites in Soay sheep (Ovis aries L) mortality during a population crash. Parasitology. 1992;105:493–503. doi: 10.1017/s0031182000074679. [DOI] [PubMed] [Google Scholar]
- 7.King CH. Health metrics for helminthic infections. Adv Parasitol. 2010;73:51–69. doi: 10.1016/S0065-308X(10)73003-7. [DOI] [PubMed] [Google Scholar]
- 8.Schneider DS, Ayres JS. Two ways to survive infection: what resistance and tolerance can teach us about treating infectious diseases. Nature Rev Immunol. 2008;8:889–895. doi: 10.1038/nri2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen F, et al. An essential role for TH2-type responses in limiting acute tissue damage during experimental helminth infection. Nature Med. 2012;18:260–266. doi: 10.1038/nm.2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Monticelli LA, et al. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nature Immunol. 2011;12:1045–1054. doi: 10.1031/ni.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herbert DR, et al. Arginase I suppresses IL-12/IL-23p40-driven intestinal inflammation during acute schistosomiasis. J Immunol. 2010;184:6438–6446. doi: 10.4049/jimmunol.0902009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pesce JT, et al. Arginase-1-expressing macrophages suppress Th2 cytokine-driven inflammation and fibrosis. PLoS Pathog. 2009;5:e1000371. doi: 10.1371/journal.ppat.1000371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Read AF, Graham AL, Raberg L. Animal defenses against infectious agents: is damage control more important than pathogen control. PLoS Biol. 2008;6:e4. doi: 10.1371/journal.pbio.1000004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jackson JA, Friberg IM, Little S, Bradley JE. Review series on helminths, immune modulation and the hygiene hypothesis: immunity against helminths and immunological phenomena in modern human populations: coevolutionary legacies? Immunology. 2009;126:18–27. doi: 10.1111/j.1365-2567.2008.03010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eming SA, Krieg T, Davidson JM. Inflammation in wound repair: molecular and cellular mechanisms. J Invest Dermatol. 2007;127:514–525. doi: 10.1038/sj.jid.5700701. [DOI] [PubMed] [Google Scholar]
- 16.Nguyen KD, et al. Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature. 2011;480:104–108. doi: 10.1038/nature10653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hernandez JL, Leung G, McKay DM. Cestode regulation of inflammation and inflammatory diseases. Int J Parasitol. 2012;43:233–243. doi: 10.1016/j.ijpara.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 18.Elliott DE, Weinstock JV. Helminth-host immunological interactions: prevention and control of immune-mediated diseases. Ann NY Acad Sci. 2012;1247:83–96. doi: 10.1111/j.1749-6632.2011.06292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diegelmann RF, Evans MC. Wound healing: an overview of acute, fibrotic and delayed healing. Front Biosci. 2004;9:283–289. doi: 10.2741/1184. [DOI] [PubMed] [Google Scholar]
- 20.Mills KH. TLR-dependent T cell activation in autoimmunity. Nature Rev Immunol. 2011;11:807–822. doi: 10.1038/nri3095. [DOI] [PubMed] [Google Scholar]
- 21.Everts B, Smits HH, Hokke CH, Yazdanbakhsh M. Helminths and dendritic cells: sensing and regulating via pattern recognition receptors, Th2 and Treg responses. Eur J Immunol. 2010;40:1525–1537. doi: 10.1002/eji.200940109. [DOI] [PubMed] [Google Scholar]
- 22.Saenz SA, et al. IL25 elicits a multipotent progenitor cell population that promotes TH2 cytokine responses. Nature. 2010;464:1362–1366. doi: 10.1038/nature08901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramalingam TR, et al. Regulation of helminth-induced Th2 responses by thymic stromal lymphopoietin. J Immunol. 2009;182:6452–6459. doi: 10.4049/jimmunol.0900181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smithgall MD, et al. IL-33 amplifies both Th1- and Th2-type responses through its activity on human basophils, allergen-reactive Th2 cells, iNKT and NK cells. Int Immunol. 2008;20:1019–1030. doi: 10.1093/intimm/dxn060. [DOI] [PubMed] [Google Scholar]
- 25.Zaph C, et al. Epithelial-cell-intrinsic IKK-β expression regulates intestinal immune homeostasis. Nature. 2007;446:552–556. doi: 10.1038/nature05590. [DOI] [PubMed] [Google Scholar]
- 26.Siracusa MC, et al. TSLP promotes interleukin-3-independent basophil haematopoiesis and type 2 inflammation. Nature. 2011;477:229–233. doi: 10.1038/nature10329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Massacand JC, et al. Helminth products bypass the need for TSLP in Th2 immune responses by directly modulating dendritic cell function. Proc Natl Acad Sci USA. 2009;106:13968–13973. doi: 10.1073/pnas.0906367106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Townsend MJ, Fallon PG, Matthews DJ, Jolin HE, McKenzie AN. T1/ST2-deficient mice demonstrate the importance of T1/ST2 in developing primary T helper cell type 2 responses. J Exp Med. 2000;191:1069–1076. doi: 10.1084/jem.191.6.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Humphreys NE, Xu D, Hepworth MR, Liew FY, Grencis RK. IL-33, a potent inducer of adaptive immunity to intestinal nematodes. J Immunol. 2008;180:2443–2449. doi: 10.4049/jimmunol.180.4.2443. [DOI] [PubMed] [Google Scholar]
- 30.Wang YH, et al. IL-25 augments type 2 immune responses by enhancing the expansion and functions of TSLP-DC-activated Th2 memory cells. J Exp Med. 2007;204:1837–1847. doi: 10.1084/jem.20070406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fallon PG, et al. Identification of an interleukin (IL)-25-dependent cell population that provides IL-4, IL-5, and IL-13 at the onset of helminth expulsion. J Exp Med. 2006;203:1105–1116. doi: 10.1084/jem.20051615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuroda E, et al. Silica crystals and aluminum salts regulate the production of prostaglandin in macrophages via NALP3 inflammasomeindependent mechanisms. Immunity. 2011;34:514–526. doi: 10.1016/j.immuni.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 33.Mishra PK, et al. Micrometer-sized titanium particles can induce potent Th2-type responses through TLR4-independent pathways. J Immunol. 2011;187:6491–6498. doi: 10.4049/jimmunol.1101392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kool M, et al. An unexpected role for uric acid as an inducer of T helper 2 cell immunity to inhaled antigens and inflammatory mediator of allergic asthma. Immunity. 2011;34:527–540. doi: 10.1016/j.immuni.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 35.Kouzaki H, Iijima K, Kobayashi T, O’Grady SM, Kita H. The danger signal, extracellular ATP, is a sensor for an airborne allergen and triggers IL-33 release and innate Th2-type responses. J Immunol. 2011;186:4375–4387. doi: 10.4049/jimmunol.1003020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Strid J, Sobolev O, Zafirova B, Polic B, Hayday A. The intraepithelial T cell response to NKG2D-ligands links lymphoid stress surveillance to atopy. Science. 2011;334:1293–1297. doi: 10.1126/science.1211250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carvalho L, et al. Review series on helminths, immune modulation and the hygiene hypothesis: mechanisms underlying helminth modulation of dendritic cell function. Immunology. 2009;126:28–34. doi: 10.1111/j.1365-2567.2008.03008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Puneet P, et al. The helminth product ES-62 protects against septic shock via Toll-like receptor 4-dependent autophagosomal degradation of the adaptor MyD88. Nature Immunol. 2011;12:344–351. doi: 10.1038/ni.2004. [DOI] [PubMed] [Google Scholar]
- 39.Everts B, et al. Schistosome-derived omega-1 drives Th2 polarization by suppressing protein synthesis following internalization by the mannose receptor. J Exp Med. 2012;209:1753–1767. doi: 10.1084/jem.20111381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Neill DR, et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464:1367–1370. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moro K, et al. Innate production of TH2 cytokines by adipose tissue-associated c-Kit+Sca-1+ lymphoid cells. Nature. 2010;463:540–544. doi: 10.1038/nature08636. [DOI] [PubMed] [Google Scholar]
- 42.Price AE, et al. Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proc Natl Acad Sci USA. 2010;107:11489–11494. doi: 10.1073/pnas.1003988107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Allen JE, Maizels RM. Diversity and dialogue in immunity to helminths. Nature Rev Immunol. 2011;11:375–388. doi: 10.1038/nri2992. [DOI] [PubMed] [Google Scholar]
- 44.Wynn TA. IL-13 effector functions. Annu Rev Immunol. 2003;21:425–456. doi: 10.1146/annurev.immunol.21.120601.141142. [DOI] [PubMed] [Google Scholar]
- 45.Pulendran B, Artis D. New paradigms in type 2 immunity. Science. 2012;337:431–435. doi: 10.1126/science.1221064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rosenberg HF, Dyer KD, Foster PS. Eosinophils: changing perspectives in health and disease. Nature Rev Immunol. 2013;13:9–22. doi: 10.1038/nri3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wynn TA, Ramalingam TR. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nature Med. 2012;18:1028–1040. doi: 10.1038/nm.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Loke P, et al. Alternative activation is an innate response to injury that requires CD4+ T cells to be sustained during chronic infection. J Immunol. 2007;179:3926–3936. doi: 10.4049/jimmunol.179.6.3926. [DOI] [PubMed] [Google Scholar]
- 49.Sandler NG, Mentink-Kane MM, Cheever AW, Wynn TA. Global gene expression profiles during acute pathogen-induced pulmonary inflammation reveal divergent roles for Th1 and Th2 responses in tissue repair. J Immunol. 2003;171:3655–3667. doi: 10.4049/jimmunol.171.7.3655. [DOI] [PubMed] [Google Scholar]
- 50.Harris N, Gause WC. To B or not to B: B cells and the Th2-type immune response to helminths. Trends Immunol. 2011;32:80–88. doi: 10.1016/j.it.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Anthony RM, et al. Protective immune mechanisms in helminth infection. Nature Rev Immunol. 2007;7:975–987. doi: 10.1038/nri2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Anthony RM, et al. Memory TH2 cells induce alternatively activated macrophages to mediate protection against nematode parasites. Nature Med. 2006;12:955–960. doi: 10.1038/nm1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Harvie M, et al. The lung is an important site for priming CD4 T-cell-mediated protective immunity against gastrointestinal helminth parasites. Infect Immun. 2010;78:3753–3762. doi: 10.1128/IAI.00502-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gurish MF, et al. IgE enhances parasite clearance and regulates mast cell responses in mice infected with Trichinella spiralis. J Immunol. 2004;172:1139–1145. doi: 10.4049/jimmunol.172.2.1139. [DOI] [PubMed] [Google Scholar]
- 55.Ushio H, Watanabe N, Kiso Y, Higuchi S, Matsuda H. Protective immunity and mast cell and eosinophil responses in mice infested with larval Haemaphysalis longicornis ticks. Parasite Immunol. 1993;15:209–214. doi: 10.1111/j.1365-3024.1993.tb00602.x. [DOI] [PubMed] [Google Scholar]
- 56.Matsuda H, et al. Necessity of IgE antibodies and mast cells for manifestation of resistance against larval Haemaphysalis longicornis ticks in mice. J Immunol. 1990;144:259–262. [PubMed] [Google Scholar]
- 57.Okumura S, Sagara H, Fukuda T, Saito H, Okayama Y. FcεRI-mediated amphiregulin production by human mast cells increases mucin gene expression in epithelial cells. J Allergy Clin Immunol. 2005;115:272–279. doi: 10.1016/j.jaci.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 58.Hoffmann KF, Cheever AW, Wynn TA. IL-10 and the dangers of immune polarization: excessive type 1 and type 2 cytokine responses induce distinct forms of lethal immunopathology in murine schistosomiasis. J Immunol. 2000;164:6406–6416. doi: 10.4049/jimmunol.164.12.6406. [DOI] [PubMed] [Google Scholar]
- 59.Pearce EJ, et al. Schistosoma mansoni in IL-4-deficient mice. Int Immunol. 1996;8:435–444. doi: 10.1093/intimm/8.4.435. [DOI] [PubMed] [Google Scholar]
- 60.Reece JJ, Siracusa MC, Scott AL. Innate immune responses to lung-stage helminth infection induce alternatively activated alveolar macrophages. Infect Immun. 2006;74:4970–4981. doi: 10.1128/IAI.00687-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McNeil KS, Knox DP, Proudfoot L. Anti-inflammatory responses and oxidative stress in Nippostrongylus brasiliensis-induced pulmonary inflammation. Parasite Immunol. 2002;24:15–22. doi: 10.1046/j.0141-9838.2001.00428.x. [DOI] [PubMed] [Google Scholar]
- 62.Thomas GD, et al. The biology of nematode- and IL4Rα-dependent murine macrophage polarization in vivo as defined by RNA-Seq and targeted lipidomics. Blood. 2012;120:e93–e104. doi: 10.1182/blood-2012-07-442640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wynes MW, Frankel SK, Riches DW. IL-4-induced macrophage-derived IGF-I protects myofibroblasts from apoptosis following growth factor withdrawal. J Leukoc Biol. 2004;76:1019–1027. doi: 10.1189/jlb.0504288. [DOI] [PubMed] [Google Scholar]
- 64.Gillery P, Leperre A, Maquart FX, Borel JP. Insulin-like growth factor-I (IGF-I) stimulates protein synthesis and collagen gene expression in monolayer and lattice cultures of fibroblasts. J Cell Physiol. 1992;152:389–396. doi: 10.1002/jcp.1041520221. [DOI] [PubMed] [Google Scholar]
- 65.Toulon A, et al. A role for human skin-resident T cells in wound healing. J Exp Med. 2009;206:743–750. doi: 10.1084/jem.20081787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zeng MY, et al. An efferocytosis-induced, IL-4-dependent macrophage-iNKT cell circuit suppresses sterile inflammation and is defective in murine CGD. Blood. 2013;121:3473–3483. doi: 10.1182/blood-2012-10-461913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Varin A, Mukhopadhyay S, Herbein G, Gordon S. Alternative activation of macrophages by IL-4 impairs phagocytosis of pathogens but potentiates microbialinduced signalling and cytokine secretion. Blood. 2010;115:353–362. doi: 10.1182/blood-2009-08-236711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schopf LR, Hoffmann KF, Cheever AW, Urban JF, Jr, Wynn T. A IL-10 is critical for host resistance and survival during gastrointestinal helminth infection. J Immunol. 2002;168:2383–2392. doi: 10.4049/jimmunol.168.5.2383. [DOI] [PubMed] [Google Scholar]
- 69.Hesse M, et al. Differential regulation of nitric oxide synthase-2 and arginase-1 by type 1/type 2 cytokines in vivo: granulomatous pathology is shaped by the pattern of L-arginine metabolism. J Immunol. 2001;167:6533–6544. doi: 10.4049/jimmunol.167.11.6533. [DOI] [PubMed] [Google Scholar]
- 70.Hesse M, et al. The pathogenesis of schistosomiasis is controlled by cooperating IL-10-producing innate effector and regulatory T cells. J Immunol. 2004;172:3157–3166. doi: 10.4049/jimmunol.172.5.3157. [DOI] [PubMed] [Google Scholar]
- 71.Shirey KA, et al. Control of RSV-induced lung injury by alternatively activated macrophages is IL-4Rα-, TLR4-, and IFN-α-dependent. Mucosal Immunol. 2010;3:291–300. doi: 10.1038/mi.2010.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nature Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cooke A. Review series on helminths, immune modulation and the hygiene hypothesis: how might infection modulate the onset of type 1 diabetes? Immunology. 2009;126:12–17. doi: 10.1111/j.1365-2567.2008.03009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zaccone P, Cooke A. Infectious triggers protect from autoimmunity. Semin Immunol. 2011;23:122–129. doi: 10.1016/j.smim.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 75.Mishra PK, Patel N, Wu W, Bleich D, Gause WC. Prevention of type 1 diabetes through infection with an intestinal nematode parasite requires IL-10 in the absence of a Th2-type response. Mucosal Immunol. 2012;6:297–308. doi: 10.1038/mi.2012.71. [DOI] [PubMed] [Google Scholar]
- 76.Hubner MP, et al. Helminth protection against autoimmune diabetes in nonobese diabetic mice is independent of a type 2 immune shift and requires TGF-β. J Immunol. 2012;188:559–568. doi: 10.4049/jimmunol.1100335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mentink-Kane MM, et al. Accelerated and progressive and lethal liver fibrosis in mice that lack interleukin (IL)-10, IL-12p40, and IL-13Rα2. Gastroenterology. 2011;141:2200–2209. doi: 10.1053/j.gastro.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wilson MS, et al. Immunopathology of schistosomiasis. Immunol Cell Biol. 2007;85:148–154. doi: 10.1038/sj.icb.7100014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chiaramonte MG, Donaldson DD, Cheever AW, Wynn TA. An IL-13 inhibitor blocks the development of hepatic fibrosis during a T-helper type 2-dominated inflammatory response. J Clin Invest. 1999;104:777–785. doi: 10.1172/JCI7325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chiaramonte MG, Cheever AW, Malley JD, Donaldson DD, Wynn TA. Studies of murine schistosomiasis reveal interleukin-13 blockade as a treatment for established and progressive liver fibrosis. Hepatology. 2001;34:273–282. doi: 10.1053/jhep.2001.26376. [DOI] [PubMed] [Google Scholar]
- 81.Wilson MS, et al. Suppression of allergic airway inflammation by helminth-induced regulatory T cells. J Exp Med. 2005;202:1199–1212. doi: 10.1084/jem.20042572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Egawa M, et al. Inflammatory monocytes recruited to allergic skin acquire an anti-inflammatory M2 phenotype via basophil-derived interleukin-4. Immunity. 2013;38:570–580. doi: 10.1016/j.immuni.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 83.Nair MG, et al. Alternatively activated macrophagederived RELM-α is a negative regulator of type 2 inflammation in the lung. J Exp Med. 2009;206:937–952. doi: 10.1084/jem.20082048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pesce JT, et al. Retnlα (relmα/fizz1) suppresses helminth-induced Th2-type immunity. PLoS Pathog. 2009;5:e1000393. doi: 10.1371/journal.ppat.1000393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wilson MS, et al. IL-13Rα2 and IL-10 coordinately suppress airway inflammation, airway-hyperreactivity, and fibrosis in mice. J Clin Invest. 2007;117:2941–2951. doi: 10.1172/JCI31546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Seno H, et al. Efficient colonic mucosal wound repair requires Trem2 signaling. Proc Natl Acad Sci USA. 2009;106:256–261. doi: 10.1073/pnas.0803343106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wu D, et al. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science. 2011;332:243–247. doi: 10.1126/science.1201475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wills-Karp M, Finkelman FD. Innate lymphoid cells wield a double-edged sword. Nature Immunol. 2011;12:1025–1027. doi: 10.1038/ni.2142. [DOI] [PubMed] [Google Scholar]
- 89.Kool M, Fierens K, Lambrecht BN. Alum adjuvant: some of the tricks of the oldest adjuvant. J Med Microbiol. 2012;61:927–934. doi: 10.1099/jmm.0.038943-0. [DOI] [PubMed] [Google Scholar]
- 90.Grainger JR, et al. Helminth secretions induce de novo T cell Foxp3 expression and regulatory function through the TGF-β pathway. J Exp Med. 2010;207:2331–2341. doi: 10.1084/jem.20101074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Piggott DA, et al. MyD88-dependent induction of allergic Th2 responses to intranasal antigen. J Clin Invest. 2005;115:459–467. doi: 10.1172/JCI22462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jenkins SJ, et al. Local macrophage proliferation, rather than recruitment from the blood, is a signature of TH2 inflammation. Science. 2011;332:1284–1288. doi: 10.1126/science.1204351. [DOI] [PMC free article] [PubMed] [Google Scholar]