Abstract
The release and uptake of neurotransmitters by synaptic vesicles is a tightly controlled process that occurs in response to diverse stimuli at morphologically disparate synapses. To meet these architectural and functional synaptic demands, it follows that there should be diversity in the mechanisms that control their secretion and retrieval and possibly in the composition of synaptic vesicles within the same terminal. Here we pay particular attention to areas where such diversity is generated, such as the variance in exocytosis/endocytosis coupling, SNAREs defining functionally diverse synaptic vesicle populations and the adaptor-dependent sorting machineries capable of generating vesicle diversity. We argue that there are various synaptic vesicle recycling pathways at any given synapse and discuss several lines of evidence that support the role of the endosome in synaptic vesicle recycling.
Multiple vesicle species (e.g., defined by different SNAREs) contribute to chemical neurotransmission. Endocytosis and endosome-retrieval mechanisms may generate these diverse vesicle pools.
Chemical synapses contain discrete numbers of synaptic vesicles, which are capable of sustaining neurotransmitter release. Sustained neurotransmission occurs despite the secretory demands imposed by persistent and diverse patterns of neuronal electrical activity. Maintaining synaptic vesicle numbers requires local mechanisms to regenerate these vesicles to prevent their exhaustion, preserve plasma membrane surface area, and to maintain the molecularly distinct identity of a vesicle versus plasma membrane. Rizzoli and Betz (2005) eloquently draw a parallel between chemical neurotransmission with synapse chatter saying that some synapses “whisper,” whereas others “shout.” The “louder” the synapse, the more synaptic vesicles are required, extending from a few hundred vesicles (whisperers) to nearly thousands (shouters). This beautiful analogy implies that every synapse has just one “voice” or species of vesicle. Here we will present the case that synapses are more like choirs in which multiple vesicle species or “voices” contribute to the “pianissimo” or “fortissimo” parts of chemical neurotransmission.
Synaptic terminals show a range of structural and functional differences in distinct regions of the brain, suggesting that the mechanisms for exocytosis/endocytosis coupling, as well as local vesicle recycling, may also be diverse. On one side, the Calyx of Held nerve terminal participates in fast and sustained synaptic transmission at high frequency (800 Hz), which is crucial for sound localization in the auditory brainstem (Taschenberger and von Gersdorff 2000; Borst and Soria van Hoeve 2012). The Calyx of Held houses ∼70,000 synaptic vesicles with nearly 3000 vesicles docked per Calyx terminal. These docked vesicles are distributed across the ∼500 active zones that exist per Calyx where vesicle fusion occurs (Satzler et al. 2002). On the other hand, hippocampal synapses fire action potentials at ∼0.5 Hz in bursts (Dobrunz and Stevens 1999). This synapse contains ∼200 synaptic vesicles and one active zone with ∼10 vesicles docked (Schikorski and Stevens 1997). With such a wide functional and structural gamut of synapses, it is reasonable to hypothesize that synaptic vesicles may differ in their retrieval mechanisms, not just at the rate at which the process occurs but also in the molecular pathways used.
Two synaptic vesicle retrieval mechanisms, namely clathrin/AP-2/dynamin-dependent biogenesis and kiss-and-run, have been summarized in outstanding recent reviews (see, for example, Augustine et al. 2006; Rizzoli and Jahn 2007; Smith et al. 2008; Royle and Lagnado 2010; Ferguson and De Camilli 2012; Saheki and De Camilli 2012). Therefore, here we focus on the coupling of secretion and membrane retrieval, as well as endosome sorting. We will discuss new developments supporting the existence of diverse functional and molecular pools of synaptic vesicles and how endocytosis and endosome retrieval mechanisms may generate these vesicle pools.
NOT ALL VOICES ARE CREATED EQUAL: MOLECULAR DIVERSITY OF SYNAPTIC VESICLES
Functionally defined pools of synaptic vesicles presume molecular differences in vesicle composition (Rizzoli and Betz 2005; Denker and Rizzoli 2010). However, until recently the model has been that synaptic vesicle components are homogenously distributed in vesicles except for limited differences in neurotransmitter transporters (Gronborg et al. 2010). Morgenthaler et al. (2003) pioneered the notion that there is heterogeneity in the molecular composition of synaptic vesicles in the same neuron. These authors observed that synapses from the same neuron contain different amounts of synaptic vesicle markers (Morgenthaler et al. 2003). The vesicle heterogeneity concept has received further support from biochemical, electrophysiological, and electron microscopy studies, all of which suggest molecular heterogeneity among synaptic vesicles that carry the zinc transporter 3 (ZnT3) within the same terminal (Salazar et al. 2004; Lavoie et al. 2011). Similarly, immunoelectron microscopy of the vesicular glutamate transporter 2 (VGLUT2) and the vesicular GABA transporter (VGAT) shows that within the same terminals, both transporters localize to distinct populations of synaptic vesicles (Boulland et al. 2009).
The study of vesicular SNAREs (v-SNAREs) VAMP2, VAMP4, VAMP7, and Vti1a provides new ways to examine molecularly and functionally distinct synaptic vesicle pools in the same nerve terminals. In studying VAMP7’s role in spontaneous vesicle release, Scheuber et al. brought these “noncanonical” synaptic vesicle v-SNAREs to the spotlight (Scheuber et al. 2006). The participation of these “noncanonical” synaptic vesicle v-SNAREs is also founded on synaptic phenotypes of mouse and flies lacking the “canonical” synaptic vesicle v-SNARE, VAMP2. VAMP2-null synapses possess severely impaired stimulus-evoked neurotransmitter secretion, yet other forms of neurotransmission such as spontaneous neurotransmitter release and hypertonic sucrose-evoked responses remain mostly unaffected (Broadie et al. 1995; Schoch et al. 2001). The concept emerging now from “noncanonical” SNARE studies is that different SNAREs define functionally distinct synaptic vesicle populations: VAMP2 marks vesicles destined for stimulus-evoked synchronous neurotransmitter secretion (Broadie et al. 1995; Schoch et al. 2001). VAMP4 defines vesicles that undergo stimulus evoked asynchronous neurotransmitter release (Raingo et al. 2012), and vesicles undergoing spontaneous release are marked by the presence of Vti1a and/or VAMP7 (Scheuber et al. 2006; Hua et al. 2011; Raingo et al. 2012). Whether “noncanonical” v-SNAREs coexist with VAMP2 needs thorough experimental analysis. However, VAMP2 and VAMP7 are present in a common population of synaptic vesicles, suggesting that vesicles may differ in the relative v-SNARE concentration per vesicle rather than by absolute segregation of v-SNAREs among vesicles (Newell-Litwa et al. 2009). This idea predicts that VAMP2 may be present at different ratios with other v-SNAREs in individual vesicles, as suggested by single vesicle quantification of VAMP2 content (Mutch et al. 2011).
The hypothesis of functional and molecular heterogeneity of synaptic vesicles within the same nerve terminal makes at least two predictions. First, it predicts diverse molecular mechanisms controlling the coupling between exocytosis and vesicle retrieval and, second, it envisions sorting mechanisms and organelles capable of assembling vesicles of different composition, such as endosomes. We discuss the evidence supporting these predictions in the following sections.
COUPLING BETWEEN EXOCYTOSIS AND ENDOCYTOSIS
Endocytic membrane retrieval at synapses is tightly coupled to exocytosis to maintain proper vesicle pools, and also to retain plasma membrane integrity (Heuser and Reese 1973; Ryan 2006; Haucke et al. 2011; Koch and Holt 2012). This concept is nicely illustrated by membrane capacitance measurements, where after an exocytic-dependent increase in capacitance, the capacitance trace reliably decays back to baseline, indicating that the amount of endocytosed membrane is equal to that previously exocytosed (Wu et al. 2007; Smith et al. 2008). The development of a pH-sensitive GFP, pHlorin, was a major breakthrough for the field, because it allows for direct visualization of exocytosis and endocytosis in living nerve terminals (Miesenbock et al. 1998). In some cases, the pHlorins have revealed two modes of endocytosis, fast and slow (Gandhi and Stevens 2003; Zhu et al. 2009), whereas in other cases the existence of a separate, fast endocytosis is disputed (Granseth et al. 2006; Balaji and Ryan 2007). One recent study using an improved, brighter pHlorin reported the surprising finding that two vesicles are recaptured after exocytosis of a single vesicle when cells are stimulated at low frequency (Zhu et al. 2009). However, as stimulation frequency increases, the number of endocytosed vesicles precisely matches the number of vesicles previously exocytosed (Zhu et al. 2009). Thus, during stimulated release there is tight coupling between exocytosis and endocytosis, although this can break down under low frequency stimulation in which single vesicle fusion events can be resolved. The question arises, then, what are the molecular mechanisms that ensure such tight coupling between exocytosis and endocytosis? And, how is this tight coupling achieved, given that synapses express different modes of exocytosis (e.g., evoked, spontaneous, asynchronous) and endocytosis (e.g., fast, slow)?
A growing body of evidence indicates that calcium influx provides one of the critical molecular links between exocytosis and endocytosis. It is well established that calcium influx through voltage-dependent calcium channels (VDCCs) serves as the trigger for evoked synaptic vesicle exocytosis and neurotransmitter release (Katz and Miledi 1967; Augustine 2001; Jahn and Fasshauer 2012; Sudhof 2012). In general, calcium positively regulates the initiation, speed, and amount of vesicle endocytosis in a range of central and peripheral synapses in vertebrates and invertebrates (von Gersdorff and Matthews 1994; Gad et al. 1998; Ales et al. 1999; Neves et al. 2001; Sankaranarayanan and Ryan 2001; Kuromi and Kidokoro 2005; Wu et al. 2005, 2009; Balaji et al. 2008; Yamashita 2012; Yao et al. 2012b). For a long time, it was assumed that the calcium influx needed for synaptic vesicle endocytosis is through VDCCs on the presynaptic plasma membrane. Indeed a recent study at the Calyx of Held synapse provides strong evidence that this is the case (Xue et al. 2012). However, another study reported a potential role for Flower, a synaptic vesicle-associated calcium channel, in linking exocytosis and endocytosis (Yao et al. 2009), a finding that has since been challenged (Xue et al. 2012). Regardless of the source of calcium, its influx seems to be a requirement for vesicle recycling at most synapses, providing a strong link with exocytic mechanisms.
Downstream from calcium influx, several endocytic calcium sensors and effectors have been identified (Yamashita et al. 2010). Calmodulin, a calcium-binding protein, has been described as the calcium sensor for synaptic vesicle endocytosis at several synapses, including the Calyx of Held and chromaffin cells (Artalejo et al. 1996; Wu et al. 2009; Yao and Sakaba 2012). Calcineurin, a Ca2+/calmodulin-dependent protein phosphatase, has also been implicated. Upon synaptic activity, calcineurin dephosphorylates a suite of proteins involved in clathrin-mediated synaptic vesicle recycling (e.g., dynamin, amphiphysin, synaptojanin), thus triggering protein–protein interactions that drive endocytosis (Marks and McMahon 1998; Cousin and Robinson 2001; Sun et al. 2010). Synaptotagmin is another calcium-binding protein that serves as a dual calcium sensor for both exocytosis and endocytosis (Jahn and Fasshauer 2012; Sudhof 2012; Yao et al. 2012a,b). During endocytosis, synaptotagmin interacts with another set of proteins involved in clathrin-mediated synaptic vesicle recycling, including AP-2 and stonin 2 (Ullrich et al. 1994; Jarousse and Kelly 2001; Diril et al. 2006). Finally, calcium regulates the phosphorylation and activity of the major PI(4,5)P2-synthesizing enzyme in brain (PIP kinase type 1γ), which generates a pool of PI(4,5)P2 required for recruitment of clathrin adaptors and nucleation of actin (Wenk et al. 2001; Di Paolo et al. 2004; Morgan et al. 2004). These studies suggest a model whereby calcium-calmodulin-calcineurin-dependent mechanisms trigger the concerted assembly of the clathrin machinery necessary for synaptic vesicle endocytosis.
This model presents a circumscribed set of molecular mechanisms coupling exocytosis to endocytosis. However, a careful examination of the literature indicates that the story is much more complex. In fact, the requirements for calcium-calmodulin during membrane retrieval are modulated by the stimulation frequency, developmental state, and anatomical location of a synapse. For example, the role of calmodulin at the Calyx of Held depends on the stimulation paradigm: at high levels of activity, calmodulin is required for synaptic vesicle endocytosis, whereas at low levels of activity it is not (Yao and Sakaba 2012). At hippocampal synapses, calcium and calcineurin paradoxically slow the retrieval of single synaptic vesicles (Leitz and Kavalali 2011). Calcineurin also inhibits vesicle endocytosis at the Drosophila neuromuscular junction (Kuromi et al. 1997). The developmental stage of a synapse also influences calcium-calmodulin requirements for membrane retrieval. Synaptic vesicle recycling becomes independent of the calcium-calmodulin-calcineurin pathway upon maturation of the Calyx of Held, (Yamashita et al. 2010), whereas the opposite is true at cerebellar granule cells (Smillie et al. 2005). Still other studies implicate the existence of an extracellular sensor for calcium in synaptic vesicle endocytosis (Gad et al. 1998; Teng and Wilkinson 2003). Thus, the most likely scenario is that synapses use multiple mechanisms for coupling exocytosis to endocytosis, depending on the level of synaptic activity, mode of vesicle retrieval, and age of the synapse. Indeed, the newly discovered molecular diversity of synaptic vesicles, described in the previous section, almost necessitates that synapses use multiple mechanisms for coupling exocytosis to endocytosis. A recent review suggests that the calcium-dependent regulation of endocytosis, like exocytosis, is coordinated through synaptic activity, intracellular calcium levels, and the spatial diffusion of calcium, providing a nice guideline for how the field might approach this problem in future studies (Yamashita 2012). An interesting possibility is that the five synaptotagmin isoforms present in the synaptic vesicle proteome may define vesicle pools that respond to different calcium thresholds (Takamori et al. 2006).
ENDOSOMES AND SYNAPTIC VESICLE RECYCLING: REVISION OF A “CONTROVERSY”
After vesicle internalization, a growing body of evidence suggests that cargo and vesicular contents are sorted through a local presynaptic endosomal pathway. Endosomes support multiple essential cell functions such as nutrient uptake, intracellular signaling, morphogenesis during development, defense against pathogens, and membrane recycling at the nerve terminal. Endosomes are acidic membrane bound organelles accessible from the extracellular milieu by internalized tracers, yet discontinuous with the cell surface. This functional and elemental definition has been refined by the time an extracellular tracer takes to reach an endosome (early versus late endosomes), by the endosome localization within cells with respect to the nucleus, by ultrastructural morphological signatures, and by a growing list of molecular markers which include membrane and lumenal protein markers, rab GTPases, SNAREs, and proteins that regulate rabs and SNARE functional states (Table 1) (Mukherjee et al. 1997; Conner and Schmid 2003; Maxfield and McGraw 2004; van Meel and Klumperman 2008; Huotari and Helenius 2011). The centrality of endosomes is highlighted by the observation that nearly a quarter of a mammalian genome encodes proteins that phenotypically modify endosomes as determined in genome wide siRNA screens (Collinet et al. 2010). Whereas the role of endosomes in synaptic vesicle membrane retrieval is controversial, we argue below that cumulated functional, ultrastructural, and genetic evidence, as well as the presence of diverse endosome molecular markers at the nerve terminal (Table 1), substantially favors endosome participation in synaptic vesicle biogenesis. Moreover, we offer a reassessment of a cornerstone study challenging the role of endosomes in retrieval.
Table 1.
Endosome markers detected in purified synaptic vesicles
| Category | Protein |
|---|---|
| Rab GTPases | Rab4 |
| Rab5 | |
| Rab7 | |
| Rab11 | |
| Rab23 | |
| Rab32 | |
| SNAREs | VAMP3 |
| VAMP4 | |
| VAMP7 | |
| Syntaxin6 | |
| Syntaxin7 | |
| Syntaxin13 | |
| Vti1a | |
| SNAP29 | |
| Sec1-Munc18 (SM) proteins | Vps33b |
| Vps45 | |
| Transmembrane | LAMP1 |
| Synaptogyrin | |
| SCAMP3-5 | |
| Coats | Adaptor complex 1 (AP-1) |
| Adaptor complex 3 (AP-3) | |
| Coat binding | BLOC-1 (dysbindin) |
| BLOC-1 (pallidin) | |
| BLOC-1 (SNAPIN) | |
| Lipid raft | Flotillin |
| Thy1 | |
| Lipid binding/modification | PI4KIIα |
| Snx5 |
The table lists molecules present in synaptic vesicles that also have been documented in endosomes or shown to affect endosome-morphology function in non-neuronal cells. Molecules were collated from Takamori et al. (2006).
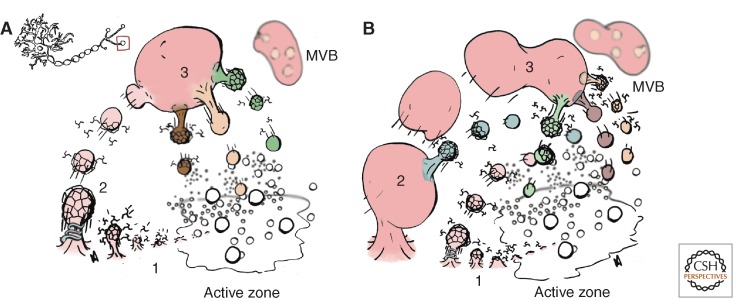
Forty years ago Heuser and Reese and Ceccarelli independently studied vesicle recycling at frog neuromuscular junctions using extracellularly applied tracers. Their findings created two nonexclusive models of membrane retrieval at the nerve terminal: one in which endosomes are intermediaries in vesicle recycling (Fig. 1A,B), and a second model in which vesicles bypass the need for an endosome intermediary (Ceccarelli et al. 1973; Heuser and Reese 1973; Morgan et al. 2002). Heuser and Reese reported that after high frequency electrical stimulation (10 Hz for 1–15 min) synaptic vesicles acquire an extracellular tracer, whereas vesicle content per terminal is reduced. These two events happen concomitantly with the appearance of large membranous cisternae (Heuser and Reese 1973). Some of these cisternae are deep infolds of the cell surface (Fig. 1B, step 2), whereas others may be disconnected from the plasma membrane, suggestive of endosomes (Fig. 1B, step 3). The formation of these cisternae under high-frequency electrical stimulation is known as activity-dependent bulk endocytosis (Cousin 2009). Importantly, synaptic vesicle depletion after electrical stimulation is reversed by resting the nerve terminal, a process that occurs at the expense of cisternae. These observations led to a model in which cisternae are the precursor organelles of synaptic vesicles (Heuser and Reese 1973). The observation that clathrin-coated pits and vesicles are frequently observed budding from cisternae supports a model in which endosomes are intermediaries in vesicle recycling (Fig. 1B, steps 2 and 3) (Heuser and Reese 1973; Miller and Heuser 1984). In contrast, Ceccarelli’s terminals were stimulated at low frequency (2 Hz for up to 4 h), a key difference between these studies (Ceccarelli et al. 1973). Ceccarelli observed minimal modification in the number of synaptic vesicles and an absence of cisternae and coated membranes even though extracellular tracer was captured into synaptic vesicles. Ceccarelli’s findings suggest rapid vesicle retrieval directly from the plasma membrane after vesicle fusion. If vesicles do not collapse into the plasma membrane, rapid reversal is achieved by the closure of transient opening pore. Such a mechanism is designated “kiss-and-run” (Fesce et al. 1994). Alternatively, vesicles may fully collapse into the plasma membrane and then be rapidly retrieved by clathrin-mediated endocytosis (Fig. 1B, step 1). The speed and/or low frequency of these retrieval events would make them difficult to identify by transmission electron microscopy. In this process, synaptic vesicle recycling could involve a single vesicle budding step mediated by clathrin, bypassing the need for an endosome intermediary (Takei et al. 1996; Granseth et al. 2006). Thus, nerve terminal endosomes may appear under high frequency electrical activity of a synapse rather than being preexisting and static organelles (Fig. 1B, step 3). Alternatively, endosomes may preexist, yet they are rather small in size and numbers in resting synapses or under low frequency activity, making them anatomically difficult to distinguish from the pool of synaptic vesicles (Fig. 1A, step 1).
Figure 1.
Synaptic vesicle recycling mechanisms and the origins of synaptic vesicle species. (A,B) Mechanisms of vesicle recycling at the presynaptic terminal. Note that vesicles have different sizes only as a way to add depth to the diagram. Kiss-and-run retrieval of vesicle membrane has been omitted for simplicity. The insert in A denotes the region of the neuron magnified in both diagrams. A and B differ in the level of activity of the synapse. A represents a resting synapse in which spontaneous fusion of vesicles occurs at the active zone. Membrane is retrieved by clathrin-mediated endocytosis (steps 1–2) to an early endosome (step 3). This endosome is capable of sorting synaptic membrane proteins into different vesicles that repopulate the synaptic vesicle pool located at the active zone. B represents a synapse undergoing evoked activity. Pathway 1 operates at low frequency and route 2–3 is recruited under high-frequency stimulation. Synaptic vesicle membrane is retrieved by clathrin-dynamin-mediated endocytosis directly from the plasma membrane adjacent to active zones in one step (step 1), or from deep plasma membrane invaginations or cisternae (step 2). These cisternae are also capable of sorting vesicle proteins into clathrin-dynamin-mediated budding profiles (step 2). Plasma-membrane-connected invaginations (step 2) give rise to endosomes (step 3) where membrane proteins get packed into different synaptic vesicle pools located at the active zone. Vesicle colors indicate different vesicular composition. Multivesicular body (MVB) accommodates targeting of presynaptic proteins to cell body lysosomes/degradative organelles as suggested by recent studies (Uytterhoeven et al. 2011; Maday et al. 2012).
The main challenge to the participation of endosomes in synaptic vesicle recycling comes from measurements of the FM1–43 dye content during the life cycle of a synaptic vesicle. The key argument is that the amount of dye taken up per vesicle by endocytosis equals the amount of dye a vesicle releases on exocytosis, thus internalized vesicles do not communicate with intermediate endosomes where dye would be diluted during the recycling process (Murthy and Stevens 1998; Zenisek et al. 2000). This model rests on the following assumptions. First, the model considers that there is a pool of preexisting dye-free endosomes at nerve terminals whose volume is large enough to dilute any dye brought by incoming vesicles. Second, the model assumes that endosomes are obligate intermediaries for all recycling synaptic vesicles; and third, it considers all synaptic vesicles to be biochemically identical and that FM1–43 labels all recycling vesicles. Endosomes exist in vivo as determined by immunoelectron microscopy and three-dimensional electron microscopy reconstruction of neuromuscular junctions, as well as the Calyx of Held synapse in rats (Wucherpfennig et al. 2003; Teng et al. 2007; Uytterhoeven et al. 2011; Korber et al. 2012). However, they are low in number and are dynamic structures that form and disappear at either low or high frequency stimulation (Teng et al. 2007). This dynamic behavior is common to nonneuronal endosomes whose appearance in cells requires continuous supply of vesicular membrane from the cell surface (Collinet et al. 2010; Scita and Di Fiore 2010; Zeigerer et al. 2012). In fact, the presence of synaptic endosomes is sensitive to the Drosophila dynamin mutant allele, shibire (Wucherpfennig et al. 2003). Thus it is possible that, like in nonneuronal cells, endosomes form by homotypic fusion of incoming vesicles (Collinet et al. 2010; Scita and Di Fiore 2010; Zeigerer et al. 2012). In such a scenario FM1–43 dye concentration in vesicles and endosomes would be similar, precluding a dilution of vesicle dye (Richards et al. 2000, 2003). Two predictions of this model are experimentally supported. First, FM1–43 dye accumulates in cisternae induced by high frequency stimulation arguing against this compartment as a dye dilution stage (Richards et al. 2000, 2003). Second, synaptic vesicles can fuse homotypically in vitro and possibly in vivo (Shimizu et al. 2003; Rizzoli et al. 2006; He et al. 2009). The use of FM dyes to track vesicles in combination with powerful genetic tools in Drosophila or the use of transmembrane proteins that selectively tag synaptic vesicle pools in vertebrate neurons is offering new insights into the role of endosomes in synaptic vesicle retrieval.
BIOCHEMICAL, FUNCTIONAL, AND GENETIC EVIDENCE FAVOR ENDOSOMES AS SYNAPTIC VESICLE RECYCLING STATIONS
The search for insight into the function, origin, and fate of an organelle often begins with its isolation and characterization of its components. This is the case for synaptic vesicles, which can be purified to a high degree (Carlson et al. 1978; Wagner et al. 1978; Huttner et al. 1983; Craige et al. 2004). We have a detailed and stoichiometric understanding of synaptic vesicle composition as a result of quantitative organelle proteomics. These studies indicate that synaptic vesicles are enriched, or at least possess, a plethora of molecules that are bona-fide endosome resident proteins (Table 1) (Takamori et al. 2000, 2006; Burre and Volknandt 2007, 2011). Some of these proteins are integral membrane proteins, which concentrate in synaptic vesicles. Thus, we can infer the existence of mechanisms actively sorting these membrane cargoes into vesicles.
The list of endosome markers is extensive and includes rab GTPases from diverse types of endosomes, endosomal v- and t-SNARES, endosome localized munc18/nsec1-like proteins, and late endosome-lysosome membrane proteins (Table 1) (Chen et al. 1985; Antonin et al. 2000; Balla et al. 2002; Guo et al. 2003; Muzerelle et al. 2003; Pan et al. 2005; Rizzoli et al. 2006; Scheuber et al. 2006; Craige et al. 2008; Morrison et al. 2008; Hoopmann et al. 2010; Pavlos et al. 2010; Chan et al. 2011; Kang et al. 2011; Sato et al. 2011; Uytterhoeven et al. 2011; Zlatic et al. 2011; Raingo et al. 2012). Importantly, coats involved in vesicle biogenesis from endosomes, such as the cytosolic clathrin-binding adaptors AP-1, ubiquitous AP-3, and neuronal AP-3 complexes copurify with synaptic vesicles (Robinson 2004; Takamori et al. 2006; Newell-Litwa et al. 2007; Gronborg et al. 2010) The presence of these proteins in synaptic vesicles suggests that endosomes and their vesicle biogenesis machineries could be intermediaries in vesicle cycling (Fig. 2). In fact, we will discuss below how perturbation of some of these endosomal molecules can modify the structure and composition of synaptic vesicles, and how some of these organelle modifications impact chemical neurotransmission.
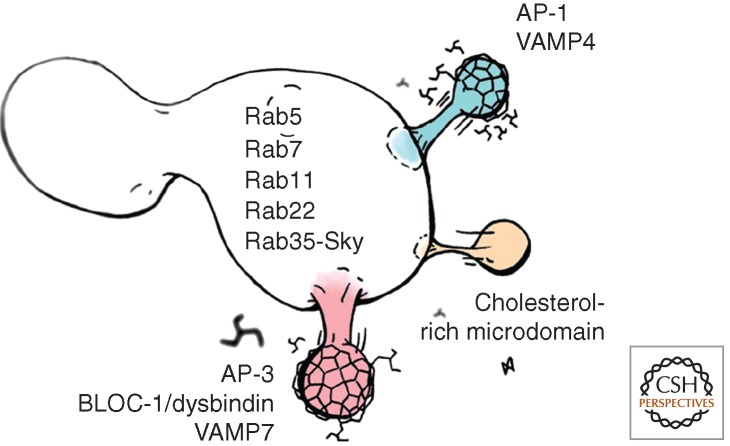
Figure 2.
Endosome-sorting mechanisms at the nerve terminal. The image depicts a presynaptic early endosome as those presented in step 3 in Figure 1A or B. The proposed rab composition of the endosome is highlighted in one endosome yet different rabs could define distinct endosome populations. The endosome depicts three budding profiles with their proposed clathrin-adaptor-sorting mechanism, AP-1 and AP-3-BLOC-1, and a cholesterol-rich microdomain-dependent-sorting mechanism. The v-SNAREs VAMP4 and VAMP7 have been paired with adaptors known to bind these SNAREs. The degree to which these endosome-sorting mechanisms operate in diverse functional statuses of a synapse, namely, resting synapses, synchronous versus asynchronous evoked released, and low- versus high-frequency stimulation release, is discussed in the text.
Rab5a localizes to nerve terminals, and its overexpression impairs synaptic vesicle recycling in mammalian cells by an undetermined mechanism (de Hoop et al. 1994; Star et al. 2005). In Drosophila, rab5 and PIP3, a rab5 effector, are present in synaptic bouton organelles that by electron microscopy are distinct from synaptic vesicles and are suggestive of endosomes. These endosomes are sensitive to the loss of rab5 and dynamin activity (Wucherpfennig et al. 2003). The dynamin sensitivity indicates that bouton endosomes are dynamically maintained by membrane inflow from the plasma membrane, rather than existing as static organelles. Importantly, a rab5 dominant negative mutant impairs synaptic vesicle recycling, affecting both the release and retrieval of vesicles (Wucherpfennig et al. 2003). These phenotypes correlate with the appearance of large membranous organelles in terminals and impaired neurotransmission because of decreased quantal content, a reflection of the vesicle pool available to fuse with the plasma membrane (Shimizu et al. 2003; Wucherpfennig et al. 2003). These rab5 phenotypes predict that molecules regulating rab5 or the cycle of other endosomal rab GTPases should also affect neurotransmission. One such factor was recently identified in a screen for defects in synaptic transmission in Drosophila (Verstreken et al. 2009). A loss of function mutant of a neuronal rab GTPase activating protein (GAP), Skywalker (Sky), enhances neuronal transmission by facilitating endosomal trafficking of synaptic vesicles at Drosophila neuromuscular junction boutons (Uytterhoeven et al. 2011). Skywalker resides in boutons and activates the GTPase activity of rab35, and to a lesser extent rab23, but not rab5 (Uytterhoeven et al. 2011). Skywalker mutants increase the quantal content twofold, a phenomenon that is phenocopied by constitutively active rab5, rab23, or rab35 mutants. However, genetic interactions are restricted to Skywalker and rab35 (Uytterhoeven et al. 2011). These results suggest that rab5 and rab35 operate in parallel pathways that modify neurotransmission at the nerve terminal (Fig. 2). Rab regulated-traffic at the nerve terminal is likely to be more complex because, in addition to constitutively active rab5 and rab35 pathways, constitutively active rab7 and rab11 mutants also perturb synaptic transmission at the neuromuscular junction (Fig. 2). The results by Uytterhoeven raise several important questions. First, do individual synaptic vesicles and presynaptic endosomes carry multiple rabs or just one (Fig. 2)? Does a vesicle retain or exchange its rabs? Rab exchange is known as maturation, a process in which the same endosome membrane exchanges one rab GTPase, such as rab5, for another rab, such as rab7 (Huotari and Helenius 2011). How does nerve terminal activity regulate rab-mediated membrane fusion? An interesting possibility is that tetanic stimulation, such as the one that induces the appearance of endosomes in nerve terminals, may favor homotypic fusion of vesicles just originated from the plasma membrane, therefore carrying extracellular tracers, rather than heterotypic fusion with the plasma membrane. The presence of multiple rabs in synaptic vesicles and the phenotypes associated with rab function perturbation suggest that in addition to the multiple molecularly distinct synaptic vesicle pools, multiple recycling/sorting pathways may also exist in the nerve terminal.
MOLECULARLY HETEROGENOUS SYNAPTIC VESICLES REQUIRE DIVERSE SORTING MACHINERIES
Different coats assemble molecularly distinct vesicles (Bonifacino and Glick 2004; Faini et al. 2013). Among these coats, clathrin binding adaptors participate in membrane protein sorting and vesicle budding events in compartments relevant to the nerve terminal: endosomes and the plasma membrane (Robinson 2004; Canagarajah et al. 2013). The adaptor complex AP-2 sorts membrane proteins into vesicle buds that pinch off at the plasma membrane, whereas the adaptors complexes 1 and 3 (AP-1 and AP-3) do so from endosomes (Robinson 2004; Newell-Litwa et al. 2007). AP-3 was the first non-AP-2 adaptor identified with a possible role at the synapse (Newman et al. 1995; Faundez et al. 1998; Kantheti et al. 1998). The function of AP-2 at the nerve terminal has been the subject of great, recent reviews (see, for example, Royle and Lagnado 2010; Saheki and De Camilli 2012). Thus, we focus here on sorting machinery distinct from AP-2. We propose that diverse sorting mechanisms at the nerve terminal offer alternative retrieval routes in addition to AP-2-clathrin-dependent mechanisms. This proposition is compatible with evidence indicating that synaptic vesicle recycling can proceed even in the absence of AP-2 (Gu et al. 2008; Kim and Ryan 2009a,b; Willox and Royle 2012).
The adaptor complexes AP-1 and AP-3 localize to nerve terminals and copurify with synaptic vesicles, suggesting that these complexes participate in their life cycle (Salazar et al. 2005; Seong et al. 2005; Takamori et al. 2006; Newell-Litwa et al. 2009; Glyvuk et al. 2010; Gronborg et al. 2010; Newell-Litwa et al. 2010). Moreover, AP-3 decorates synaptic vesicles by immunoelectron microscopy (Newman et al. 1995; Newell-Litwa et al. 2010). Predictably, AP-1 and AP-3 null mice are characterized by one or more of the following phenotypes: alterations in synaptic vesicle size, composition or acidification; impaired intraterminal trafficking of neurotransmitter transporters; and defective neurotransmission (Blumstein et al. 2001; Nakatsu et al. 2004; Salazar et al. 2004; Seong et al. 2005; Voglmaier et al. 2006; Newell-Litwa et al. 2009, 2010; Glyvuk et al. 2010). AP-3 functions in the generation of vesicles that undergo spontaneous fusion, as well as in the generation of synaptic vesicles from activity-dependent bulk endocytosis-generated endosomes, but not from the plasma membrane (Fig. 1A and B, step 3 and Fig. 2) (Scheuber et al. 2006; Cheung and Cousin 2012). AP-1 also participates in the generation of synaptic vesicles from activity-dependent bulk endocytosis-generated endosomes in a pathway likely parallel to AP-3 (Fig. 1B, step 3 and Fig. 2) (Cheung and Cousin 2012). An intriguing possibility is that vSNAREs and adaptor pairs could define molecularly and functionally distinct synaptic vesicle pools (Fig. 2). VAMP7 is targeted by AP-3 complexes by direct binding to AP-3 (Martinez-Arca et al. 2003; Salazar et al. 2006; Newell-Litwa et al. 2009, 2010; Kent et al. 2012) and could define synaptic vesicles that undergo spontaneous fusion or are derived from bulk endocytosis-generated endosomes. VAMP4 and AP-1 could define activity-dependent, asynchronously released vesicles because VAMP-4 directly binds to AP-1 (Peden et al. 2001; Ren et al. 2013). This model would predict that VAMP4 and VAMP7 would enrich into distinct vesicle populations at the nerve terminal (Fig. 1B, step 3, and Fig. 2).
What is remarkable of AP-1 and AP-3 is that neuronal enriched isoforms of these adaptors participate in synaptic vesicle formation (Newell-Litwa et al. 2007; Glyvuk et al. 2010). Genetic defects in these neuronal subunits cause profound neurological and behavioral phenotypes in mice (Nakatsu et al. 2004; Seong et al. 2005; Newell-Litwa et al. 2009, 2010; Glyvuk et al. 2010). Mutations in the neuronal enriched isoform of AP-1, encoded by the gene AP1S2, cause syndromic and nonsyndromic intellectual disability in humans (Tarpey et al. 2006; Saillour et al. 2007; Borck et al. 2008; Ballarati et al. 2012). Single copy loss or mutation of the neuronal AP-3 subunit (AP3B2) is associated with schizophrenia and autism spectrum disorders (Gokhale et al. 2012; O’Roak et al. 2012). It is important to emphasize that, although a loss of local presynaptic function of these adaptors could explain disease and mouse phenotypes, AP-1 and AP-3 adaptor function in the cell body could also contribute to them. In fact, impaired AP-1 or AP-3 activity prevents delivery of membrane proteins from the cell body to dendrites (AP-1), axons, and presynaptic terminals (AP-3) (Dwyer et al. 2001; Seong et al. 2005; Larimore et al. 2011; Farias et al. 2012).
There are two additional mechanisms that may participate in synaptic vesicle protein sorting at the nerve terminal. One requires the biogenesis of lysosomes complex 1, BLOC-1, the other cholesterol-rich membranes rafts (Fig. 2). Cholesterol-rich domains may contribute to synaptic vesicle protein segregation and, ultimately, to sorting in endosomes as suggested by a cholesterol sensitive clustering of synaptic vesicle membrane proteins in endosomes (Hoopmann et al. 2010). The BLOC-1 complex is a cytosolic octamer present in early endosomes and involved in the targeting of selected membrane proteins from endosomes to lysosome-related organelles and synaptic vesicles (Ghiani and Dell’Angelica 2011; Mullin et al. 2011). Two of its subunits, dysbindin and pallidin, are found in the presynaptic terminal as determined by immunoelectron microscopy (Talbot et al. 2006; Larimore and Faundez, submitted). Null alleles of BLOC-1 subunits alter synaptic vesicle composition, suggesting that BLOC-1 participates in synaptic vesicle retrieval at the nerve terminal (Newell-Litwa et al. 2009, 2010; Larimore et al. 2011). The precise role of BLOC-1 in mammalian cells is not yet defined but it could work as an accessory adaptor protein by virtue of its binding to AP-3 or, in a nonexclusive mechanism, BLOC-1 could target membrane proteins independent of AP-3, as it does in nonneuronal cells (Di Pietro et al. 2006; Setty et al. 2008; Larimore et al. 2011; Gokhale et al. 2012). BLOC-1 binds SNAREs and regulates either SNAREs’ subcellular localization or content in nonneuronal cells. Some of these SNAREs, such as VAMP7 and syntaxin13, are present in synaptic vesicles (Huang et al. 1999; Salazar et al. 2006; Ghiani et al. 2009; Newell-Litwa et al. 2009, 2010; Gokhale et al. 2012). A soluble syntaxin13 fragment reduces synaptic vesicle retrieval by inhibiting endosomal recycling (Hoopmann et al. 2010). Thus, it is interesting to speculate that some of the neurological phenotypes observed in BLOC-1 deficient mice may result from impaired targeting of syntaxin13 at nerve terminal endosomes (Ghiani and Dell’Angelica 2011; Mullin et al. 2011).
CONCLUSION AND FUTURE DIRECTIONS
No single model can on its own account for most observations on endo- and exocytosis, how these processes are coupled, and how synaptic vesicle proteins are sorted and recycled. The complexity of these mechanisms is underscored by their diversity in every synapse type throughout an array of functional and developmental states. As we gain a greater understanding of the molecular composition of synaptic vesicles, we have also established new tools with which to study the mechanisms underlying their recycling at synapses. Increasing evidence suggests that SNAREs, clathrin-adaptors, and rabs may define distinct synaptic vesicle populations. Indeed, these proteins and, in particular, SNAREs will be useful for biochemical, functional, and microscopic discrimination of vesicles subpopulations. However, remaining questions about the diversity of vesicles at the nerve terminal and their recycling mechanisms necessitate a combined approach of genetic or acute molecular manipulations of synapses coupled to high-resolution microscopy. The synapse inaccessibility to optical approaches as a result of the very constrained space where organelles are packed may be breached by breakthroughs in imaging, such as super-resolution microscopy and genetically encoded probes for correlative light-electron microscopy. As an example, super-resolution microscopy recently allowed for tracking of synaptic vesicle multiprotein complexes containing synaptotagmin (Willig et al. 2006; Opazo et al. 2010), which were originally described biochemically (Bennett et al. 1992). These microscopy findings indicate that synaptotagmin remains clustered in the membrane after exocytosis (Willig et al. 2006; Opazo et al. 2010). These observations raise important questions as to how clustering may contribute to exocytosis and endocytosis coupling and to what extent membrane protein clustering may render dispensable a retrieval mechanism that requires concentrative sorting by adaptors. Super-resolution microscopy, optical tags in combination with genetics augur a prosperous future for synaptic vesicles, organelles that in two years will reach their 60th birthday (De Robertis and Bennett 1955).
ACKNOWLEDGMENTS
This work is supported by grants from the National Institutes of Health (GM077569), Emory University Research Committee, and Emory Parkinson’s Disease Collaborative Environmental Research Center (PD-CERC) 5P01ES016731 to V.F. J.R.M. is supported by a grant from the National Institutes of Health (NIH RO1 NS078165). M.C. is a recipient of the Woodruff Fellowship from Emory University School of Medicine. The authors are indebted to Gene H. LePere for her contributions.
Footnotes
Editors: Sandra L. Schmid, Alexander Sorkin, and Marino Zerial
Additional Perspectives on Endocytosis available at www.cshperspectives.org
REFERENCES
- Ales E, Tabares L, Poyato JM, Valero V, Lindau M, Alvarez de Toledo G 1999. High calcium concentrations shift the mode of exocytosis to the kiss-and-run mechanism. Nat Cell Biol 1: 40–44 [DOI] [PubMed] [Google Scholar]
- Antonin W, Riedel D, von Mollard GF 2000. The SNARE Vti1a-β is localized to small synaptic vesicles and participates in a novel SNARE complex. J Neurosci 20: 5724–5732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artalejo CR, Elhamdani A, Palfrey HC 1996. Calmodulin is the divalent cation receptor for rapid endocytosis, but not exocytosis, in adrenal chromaffin cells. Neuron 16: 195–205 [DOI] [PubMed] [Google Scholar]
- Augustine GJ 2001. How does calcium trigger neurotransmitter release? Curr Opin Neurobiol 11: 320–326 [DOI] [PubMed] [Google Scholar]
- Augustine GJ, Morgan JR, Villalba-Galea CA, Jin S, Prasad K, Lafer EM 2006. Clathrin and synaptic vesicle endocytosis: Studies at the squid giant synapse. Biochem Soc Trans 34: 68–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaji J, Ryan TA 2007. Single-vesicle imaging reveals that synaptic vesicle exocytosis and endocytosis are coupled by a single stochastic mode. Proc Natl Acad Sci 104: 20576–20581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaji J, Armbruster M, Ryan TA 2008. Calcium control of endocytic capacity at a CNS synapse. J Neurosci 28: 6742–6749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balla A, Tuymetova G, Barshishat M, Geiszt M, Balla T 2002. Characterization of type II phosphatidylinositol 4-kinase isoforms reveals association of the enzymes with endosomal vesicular compartments. J Biol Chem 277: 20041–20050 [DOI] [PubMed] [Google Scholar]
- Ballarati L, Cereda A, Caselli R, Maitz S, Russo S, Selicorni A, Larizza L, Giardino D 2012. Deletion of the AP1S2 gene in a child with psychomotor delay and hypotonia. Euro J Med Genet 55: 124–127 [DOI] [PubMed] [Google Scholar]
- Barth J, Volknandt W 2011. Proteomic investigations of the synaptic vesicle interactome. Expert Rev Proteomics 8: 211–220 [DOI] [PubMed] [Google Scholar]
- Bennett MK, Calakos N, Kreiner T, Scheller RH 1992. Synaptic vesicle membrane proteins interact to form a multimeric complex. J Cell Biol 116: 761–775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumstein J, Faundez V, Nakatsu F, Saito T, Ohno H, Kelly RB 2001. The neuronal form of adaptor protein-3 is required for synaptic vesicle formation from endosomes. J Neurosci 21: 8034–8042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifacino JS, Glick BS 2004. The mechanisms of vesicle budding and fusion. Cell 116: 153–166 [DOI] [PubMed] [Google Scholar]
- Borck G, Molla-Herman A, Boddaert N, Encha-Razavi F, Philippe A, Robel L, Desguerre I, Brunelle F, Benmerah A, Munnich A, et al. 2008. Clinical, cellular, and neuropathological consequences of AP1S2 mutations: Further delineation of a recognizable X-linked mental retardation syndrome. Hum Mutat 29: 966–974 [DOI] [PubMed] [Google Scholar]
- Borst JG, Soria van Hoeve J 2012. The calyx of held synapse: From model synapse to auditory relay. Ann Rev Physiol 74: 199–224 [DOI] [PubMed] [Google Scholar]
- Boulland JL, Jenstad M, Boekel AJ, Wouterlood FG, Edwards RH, Storm-Mathisen J, Chaudhry FA 2009. Vesicular glutamate and GABA transporters sort to distinct sets of vesicles in a population of presynaptic terminals. Cereb Cortex 19: 241–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadie K, Prokop A, Bellen HJ, O’Kane CJ, Schulze KL, Sweeney ST 1995. Syntaxin and synaptobrevin function downstream of vesicle docking in Drosophila. Neuron 15: 663–673 [DOI] [PubMed] [Google Scholar]
- Burre J, Volknandt W 2007. The synaptic vesicle proteome. J Neurochem 101: 1448–1462 [DOI] [PubMed] [Google Scholar]
- Canagarajah BJ, Ren X, Bonifacino JS, Hurley JH 2013. The clathrin adaptor complexes as a paradigm for membrane-associated allostery. Protein Sci 22: 517–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson SS, Wagner JA, Kelly RB 1978. Purification of synaptic vesicles from elasmobranch electric organ and the use of biophysical criteria to demonstrate purity. Biochemistry 17: 1188–1199 [DOI] [PubMed] [Google Scholar]
- Ceccarelli B, Hurlbut WP, Mauro A 1973. Turnover of transmitter and synaptic vesicles at the frog neuromuscular junction. J Cell Biol 57: 499–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CC, Scoggin S, Wang D, Cherry S, Dembo T, Greenberg B, Jin EJ, Kuey C, Lopez A, Mehta SQ, et al. 2011. Systematic discovery of Rab GTPases with synaptic functions in Drosophila. Curr Biol 21: 1704–1715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JW, Murphy TL, Willingham MC, Pastan I, August JT 1985. Identification of two lysosomal membrane glycoproteins. J Cell Biol 101: 85–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung G, Cousin MA 2012. Adaptor protein complexes 1 and 3 are essential for generation of synaptic vesicles from activity-dependent bulk endosomes. J Neurosci 32: 6014–6023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collinet C, Stoter M, Bradshaw CR, Samusik N, Rink JC, Kenski D, Habermann B, Buchholz F, Henschel R, Mueller MS, et al. 2010. Systems survey of endocytosis by multiparametric image analysis. Nature 464: 243–249 [DOI] [PubMed] [Google Scholar]
- Conner SD, Schmid SL 2003. Regulated portals of entry into the cell. Nature 422: 37–44 [DOI] [PubMed] [Google Scholar]
- Cousin MA 2009. Activity-dependent bulk synaptic vesicle endocytosis—A fast, high capacity membrane retrieval mechanism. Mol Neurobiol 39: 185–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousin MA, Robinson PJ 2001. The dephosphins: Dephosphorylation by calcineurin triggers synaptic vesicle endocytosis. Trend Neurosci 24: 659–665 [DOI] [PubMed] [Google Scholar]
- Craige B, Salazar G, Faundez V 2004. Isolation of synaptic vesicles. Curr Protoc Cell Biol 10.1002/0471143030.cb0312s25 [DOI] [PubMed] [Google Scholar]
- Craige B, Salazar G, Faundez V 2008. Phosphatidylinositol-4-kinase type II α contains an AP-3-sorting motif and a kinase domain that are both required for endosome traffic. Mol Biol Cell 19: 1415–1426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Hoop MJ, Huber LA, Stenmark H, Williamson E, Zerial M, Parton RG, Dotti CG 1994. The involvement of the small GTP-binding protein Rab5a in neuronal endocytosis. Neuron 13: 11–22 [DOI] [PubMed] [Google Scholar]
- De Robertis ED, Bennett HS 1955. Some features of the submicroscopic morphology of synapses in frog and earthworm. J Biophys Biochem Cytol 1: 47–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denker A, Rizzoli SO 2010. Synaptic vesicle pools: An update. Front Synaptic Neurosci 2: 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Paolo G, Moskowitz HS, Gipson K, Wenk MR, Voronov S, Obayashi M, Flavell R, Fitzsimonds RM, Ryan TA, De Camilli P 2004. Impaired PtdIns(4,5)P2 synthesis in nerve terminals produces defects in synaptic vesicle trafficking. Nature 431: 415–422 [DOI] [PubMed] [Google Scholar]
- Di Pietro SM, Falcon-Perez JM, Tenza D, Setty SR, Marks MS, Raposo G, Dell’Angelica EC 2006. BLOC-1 interacts with BLOC-2 and the AP-3 complex to facilitate protein trafficking on endosomes. Mol Biol Cell 17: 4027–4038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diril MK, Wienisch M, Jung N, Klingauf J, Haucke V 2006. Stonin 2 is an AP-2-dependent endocytic sorting adaptor for synaptotagmin internalization and recycling. Dev Cell 10: 233–244 [DOI] [PubMed] [Google Scholar]
- Dobrunz LE, Stevens CF 1999. Response of hippocampal synapses to natural stimulation patterns. Neuron 22: 157–166 [DOI] [PubMed] [Google Scholar]
- Dwyer ND, Adler CE, Crump JG, L’Etoile ND, Bargmann CI 2001. Polarized dendritic transport and the AP-1 μ1 clathrin adaptor UNC-101 localize odorant receptors to olfactory cilia. Neuron 31: 277–287 [DOI] [PubMed] [Google Scholar]
- Faini M, Beck R, Wieland FT, Briggs JA 2013. Vesicle coats: Structure, function, and general principles of assembly. Trend Cell Biol 23: 279–288 [DOI] [PubMed] [Google Scholar]
- Farias GG, Cuitino L, Guo X, Ren X, Jarnik M, Mattera R, Bonifacino JS 2012. Signal-mediated, AP-1/clathrin-dependent sorting of transmembrane receptors to the somatodendritic domain of hippocampal neurons. Neuron 75: 810–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faundez V, Horng JT, Kelly RB 1998. A function for the AP3 coat complex in synaptic vesicle formation from endosomes. Cell 93: 423–432 [DOI] [PubMed] [Google Scholar]
- Ferguson SM, De Camilli P 2012. Dynamin, a membrane-remodelling GTPase. Nat Rev Mol Cell Biol 13: 75–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fesce R, Grohovaz F, Valtorta F, Meldolesi J 1994. Neurotransmitter release: Fusion or “kiss-and-run?” Trend Cell Biol 4: 1–4 [DOI] [PubMed] [Google Scholar]
- Gad H, Low P, Zotova E, Brodin L, Shupliakov O 1998. Dissociation between Ca2+-triggered synaptic vesicle exocytosis and clathrin-mediated endocytosis at a central synapse. Neuron 21: 607–616 [DOI] [PubMed] [Google Scholar]
- Gandhi SP, Stevens CF 2003. Three modes of synaptic vesicular recycling revealed by single-vesicle imaging. Nature 423: 607–613 [DOI] [PubMed] [Google Scholar]
- Ghiani CA, Dell’Angelica EC 2011. Dysbindin-containing complexes and their proposed functions in brain: From zero to (too) many in a decade. ASN Neuro 3: e00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiani CA, Starcevic M, Rodriguez-Fernandez IA, Nazarian R, Cheli VT, Chan LN, Malvar JS, de Vellis J, Sabatti C, Dell’Angelica EC 2009. The dysbindin-containing complex (BLOC-1) in brain: Developmental regulation, interaction with SNARE proteins and role in neurite outgrowth. Mol Psychiatry 15: 204–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glyvuk N, Tsytsyura Y, Geumann C, D’Hooge R, Huve J, Kratzke M, Baltes J, Boening D, Klingauf J, Schu P 2010. AP-1/σ1B-adaptin mediates endosomal synaptic vesicle recycling, learning and memory. EMBO J 29: 1318–1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gokhale A, Larimore J, Werner E, So L, Moreno-De-Luca A, Lese-Martin C, Lupashin VV, Smith Y, Faundez V 2012. Quantitative proteomic and genetic analyses of the schizophrenia susceptibility factor dysbindin identify novel roles of the biogenesis of lysosome-related organelles complex 1. J Neurosci 32: 3697–3711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granseth B, Odermatt B, Royle SJ, Lagnado L 2006. Clathrin-mediated endocytosis is the dominant mechanism of vesicle retrieval at hippocampal synapses. Neuron 51: 773–786 [DOI] [PubMed] [Google Scholar]
- Gronborg M, Pavlos NJ, Brunk I, Chua JJ, Munster-Wandowski A, Riedel D, Ahnert-Hilger G, Urlaub H, Jahn R 2010. Quantitative comparison of glutamatergic and GABAergic synaptic vesicles unveils selectivity for few proteins including MAL2, a novel synaptic vesicle protein. J Neurosci 30: 2–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu M, Schuske K, Watanabe S, Liu Q, Baum P, Garriga G, Jorgensen EM 2008. μ2 adaptin facilitates but is not essential for synaptic vesicle recycling in Caenorhabditis elegans. J Cell Biol 183: 881–892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J, Wenk MR, Pellegrini L, Onofri F, Benfenati F, De Camilli P 2003. Phosphatidylinositol 4-kinase type IIα is responsible for the phosphatidylinositol 4-kinase activity associated with synaptic vesicles. Proc Natl Acad Sci 100: 3995–4000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haucke V, Neher E, Sigrist SJ 2011. Protein scaffolds in the coupling of synaptic exocytosis and endocytosis. Nat Rev Neurosci 12: 127–138 [DOI] [PubMed] [Google Scholar]
- He L, Xue L, Xu J, McNeil BD, Bai L, Melicoff E, Adachi R, Wu LG 2009. Compound vesicle fusion increases quantal size and potentiates synaptic transmission. Nature 459: 93–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser JE, Reese TS 1973. Evidence for recycling of synaptic vesicle membrane during transmitter release at the frog neuromuscular junction. J Cell Biol 57: 315–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoopmann P, Punge A, Barysch SV, Westphal V, Buckers J, Opazo F, Bethani I, Lauterbach MA, Hell SW, Rizzoli SO 2010. Endosomal sorting of readily releasable synaptic vesicles. Proc Natl Acad Sci 107: 19055–19060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua Z, Leal-Ortiz S, Foss SM, Waites CL, Garner CC, Voglmaier SM, Edwards RH 2011. v-SNARE composition distinguishes synaptic vesicle pools. Neuron 71: 474–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L, Kuo YM, Gitschier J 1999. The pallid gene encodes a novel, syntaxin 13-interacting protein involved in platelet storage pool deficiency. Nat Genet 23: 329–332 [DOI] [PubMed] [Google Scholar]
- Huotari J, Helenius A 2011. Endosome maturation. EMBO J 30: 3481–3500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttner WB, Schiebler W, Greengard P, De Camilli P 1983. Synapsin I (protein I), a nerve terminal-specific phosphoprotein. III. Its association with synaptic vesicles studied in a highly purified synaptic vesicle preparation. J Cell Biol 96: 1374–1388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn R, Fasshauer D 2012. Molecular machines governing exocytosis of synaptic vesicles. Nature 490: 201–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarousse N, Kelly RB 2001. The AP2 binding site of synaptotagmin 1 is not an internalization signal but a regulator of endocytosis. J Cell Biol 154: 857–866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J, Bai Z, Zegarek MH, Grant BD, Lee J 2011. Essential roles of snap-29 in C. elegans. Dev Biol 355: 77–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantheti P, Qiao X, Diaz ME, Peden AA, Meyer GE, Carskadon SL, Kapfhamer D, Sufalko D, Robinson MS, Noebels JL, et al. 1998. Mutation in AP-3 δ in the mocha mouse links endosomal transport to storage deficiency in platelets, melanosomes, and synaptic vesicles. Neuron 21: 111–122 [DOI] [PubMed] [Google Scholar]
- Katz B, Miledi R 1967. Ionic requirements of synaptic transmitter release. Nature 215: 651. [DOI] [PubMed] [Google Scholar]
- Kent HM, Evans PR, Schafer IB, Gray SR, Sanderson CM, Luzio JP, Peden AA, Owen DJ 2012. Structural basis of the intracellular sorting of the SNARE VAMP7 by the AP3 adaptor complex. Dev Cell 22: 979–988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Ryan TA 2009a. A distributed set of interactions controls μ2 functionality in the role of AP-2 as a sorting adaptor in synaptic vesicle endocytosis. J Biol Chem 284: 32803–32812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Ryan TA 2009b. Synaptic vesicle recycling at CNS snapses without AP-2. J Neurosci 29: 3865–3874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch M, Holt M 2012. Coupling exo- and endocytosis: An essential role for PIP2 at the synapse. Biochim Biophys Acta 1821: 1114–1132 [DOI] [PubMed] [Google Scholar]
- Korber C, Horstmann H, Satzler K, Kuner T 2012. Endocytic structures and synaptic vesicle recycling at a central synapse in awake rats. Traffic 13: 1601–1611 [DOI] [PubMed] [Google Scholar]
- Kuromi H, Kidokoro Y 2005. Exocytosis and endocytosis of synaptic vesicles and functional roles of vesicle pools: Lessons from the Drosophila neuromuscular junction. Neuroscientist 11: 138–147 [DOI] [PubMed] [Google Scholar]
- Kuromi H, Yoshihara M, Kidokoro Y 1997. An inhibitory role of calcineurin in endocytosis of synaptic vesicles at nerve terminals of Drosophila larvae. Neurosci Res 27: 101–113 [DOI] [PubMed] [Google Scholar]
- Larimore J, Tornieri K, Ryder PV, Gokhale A, Zlatic SA, Craige B, Lee JD, Talbot K, Pare JF, Smith Y, et al. 2011. The schizophrenia susceptibility factor dysbindin and its associated complex sort cargoes from cell bodies to the synapse. Mol Biol Cell 22: 4854–4867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavoie N, Jeyaraju DV, Peralta MR 3rd, Seress L, Pellegrini L, Toth K 2011. Vesicular zinc regulates the Ca2+ sensitivity of a subpopulation of presynaptic vesicles at hippocampal mossy fiber terminals. J Neurosci 31: 18251–18265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitz J, Kavalali ET 2011. Ca2+ influx slows single synaptic vesicle endocytosis. J Neurosci 31: 16318–16326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maday S, Wallace KE, Holzbaur EL 2012. Autophagosomes initiate distally and mature during transport toward the cell soma in primary neurons. J Cell Biol 196: 407–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks B, McMahon HT 1998. Calcium triggers calcineurin-dependent synaptic vesicle recycling in mammalian nerve terminals. Curr Biol 8: 740–749 [DOI] [PubMed] [Google Scholar]
- Martinez-Arca S, Rudge R, Vacca M, Raposo G, Camonis J, Proux-Gillardeaux V, Daviet L, Formstecher E, Hamburger A, Filippini F, et al. 2003. A dual mechanism controlling the localization and function of exocytic v-SNAREs. Proc Natl Acad Sci 100: 9011–9016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxfield FR, McGraw TE 2004. Endocytic recycling. Nat Rev Mol Cell Biol 5: 121–132 [DOI] [PubMed] [Google Scholar]
- Miesenbock G, De Angelis DA, Rothman JE 1998. Visualizing secretion and synaptic transmission with pH-sensitive green fluorescent proteins. Nature 394: 192–195 [DOI] [PubMed] [Google Scholar]
- Miller TM, Heuser JE 1984. Endocytosis of synaptic vesicle membrane at the frog neuromuscular junction. J Cell Biol 98: 685–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan JR, Augustine GJ, Lafer EM 2002. Synaptic vesicle endocytosis: The races, places, and molecular faces. Neuromolecular Med 2: 101–114 [DOI] [PubMed] [Google Scholar]
- Morgan JR, Di Paolo G, Werner H, Shchedrina VA, Pypaert M, Pieribone VA, De Camilli P 2004. A role for talin in presynaptic function. J Cell Biol 167: 43–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgenthaler FD, Knott GW, Floyd Sarria JC, Wang X, Staple JK, Catsicas S, Hirling H 2003. Morphological and molecular heterogeneity in release sites of single neurons. Euro J Neurosci 17: 1365–1374 [DOI] [PubMed] [Google Scholar]
- Morrison HA, Dionne H, Rusten TE, Brech A, Fisher WW, Pfeiffer BD, Celniker SE, Stenmark H, Bilder D 2008. Regulation of early endosomal entry by the Drosophila tumor suppressors Rabenosyn and Vps45. Mol Biol Cell 19: 4167–4176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee S, Ghosh RN, Maxfield FR 1997. Endocytosis. Physiol Rev 77: 759–803 [DOI] [PubMed] [Google Scholar]
- Mullin AP, Gokhale A, Larimore J, Faundez V 2011. Cell biology of the BLOC-1 complex subunit dysbindin, a schizophrenia susceptibility gene. Mol Neurobiol 44: 53–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy VN, Stevens CF 1998. Synaptic vesicles retain their identity through the endocytic cycle. Nature 392: 497–501 [DOI] [PubMed] [Google Scholar]
- Mutch SA, Kensel-Hammes P, Gadd JC, Fujimoto BS, Allen RW, Schiro PG, Lorenz RM, Kuyper CL, Kuo JS, Bajjalieh SM, et al. 2011. Protein quantification at the single vesicle level reveals that a subset of synaptic vesicle proteins are trafficked with high precision. J Neurosci 31: 1461–1470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzerelle A, Alberts P, Martinez-Arca S, Jeannequin O, Lafaye P, Mazie JC, Galli T, Gaspar P 2003. Tetanus neurotoxin-insensitive vesicle-associated membrane protein localizes to a presynaptic membrane compartment in selected terminal subsets of the rat brain. Neuroscience 122: 59–75 [DOI] [PubMed] [Google Scholar]
- Nakatsu F, Okada M, Mori F, Kumazawa N, Iwasa H, Zhu G, Kasagi Y, Kamiya H, Harada A, Nishimura K, et al. 2004. Defective function of GABA-containing synaptic vesicles in mice lacking the AP-3B clathrin adaptor. J Cell Biol 167: 293–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neves G, Gomis A, Lagnado L 2001. Calcium influx selects the fast mode of endocytosis in the synaptic terminal of retinal bipolar cells. Proc Natl Acad Sci 98: 15282–15287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell-Litwa K, Seong E, Burmeister M, Faundez V 2007. Neuronal and non-neuronal functions of the AP-3 sorting machinery. J Cell Sci 120: 531–541 [DOI] [PubMed] [Google Scholar]
- Newell-Litwa K, Salazar G, Smith Y, Faundez V 2009. Roles of BLOC-1 and adaptor protein-3 complexes in cargo sorting to synaptic vesicles. Mol Biol Cell 20: 1441–1453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell-Litwa K, Chintala S, Jenkins S, Pare JF, McGaha L, Smith Y, Faundez V 2010. Hermansky-Pudlak protein complexes, AP-3 and BLOC-1, differentially regulate presynaptic composition in the striatum and hippocampus. J Neurosci 30: 820–831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman LS, McKeever MO, Okano HJ, Darnell RB 1995. β-NAP, a cerebellar degeneration antigen, is a neuron-specific vesicle coat protein. Cell 82: 773–783 [DOI] [PubMed] [Google Scholar]
- O’Roak BJ, Vives L, Girirajan S, Karakoc E, Krumm N, Coe BP, Levy R, Ko A, Lee C, Smith JD, et al. 2012. Sporadic autism exomes reveal a highly interconnected protein network of de novo mutations. Nature 485: 246–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opazo F, Punge A, Buckers J, Hoopmann P, Kastrup L, Hell SW, Rizzoli SO 2010. Limited intermixing of synaptic vesicle components upon vesicle recycling. Traffic 11: 800–812 [DOI] [PubMed] [Google Scholar]
- Pan PY, Cai Q, Lin L, Lu PH, Duan S, Sheng ZH 2005. SNAP-29-mediated modulation of synaptic transmission in cultured hippocampal neurons. J Biol Chem 280: 25769–25779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlos NJ, Gronborg M, Riedel D, Chua JJ, Boyken J, Kloepper TH, Urlaub H, Rizzoli SO, Jahn R 2010. Quantitative analysis of synaptic vesicle Rabs uncovers distinct yet overlapping roles for Rab3a and Rab27b in Ca2+-triggered exocytosis. J Neurosci 30: 13441–13453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peden AA, Park GY, Scheller RH 2001. The Di-leucine motif of vesicle-associated membrane protein 4 is required for its localization and AP-1 binding. J Biol Chem 276: 49183–49187 [DOI] [PubMed] [Google Scholar]
- Raingo J, Khvotchev M, Liu P, Darios F, Li YC, Ramirez DM, Adachi M, Lemieux P, Toth K, Davletov B, et al. 2012. VAMP4 directs synaptic vesicles to a pool that selectively maintains asynchronous neurotransmission. Nat Neurosci 15: 738–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren X, Farias GG, Canagarajah BJ, Bonifacino JS, Hurley JH 2013. Structural basis for recruitment and activation of the AP-1 clathrin adaptor complex by Arf1. Cell 152: 755–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards DA, Guatimosim C, Betz WJ 2000. Two endocytic recycling routes selectively fill two vesicle pools in frog motor nerve terminals. Neuron 27: 551–559 [DOI] [PubMed] [Google Scholar]
- Richards DA, Guatimosim C, Rizzoli SO, Betz WJ 2003. Synaptic vesicle pools at the frog neuromuscular junction. Neuron 39: 529–541 [DOI] [PubMed] [Google Scholar]
- Rizzoli SO, Betz WJ 2005. Synaptic vesicle pools. Nat Rev Neurosci 6: 57–69 [DOI] [PubMed] [Google Scholar]
- Rizzoli SO, Jahn R 2007. Kiss-and-run, collapse and “readily retrievable” vesicles. Traffic 8: 1137–1144 [DOI] [PubMed] [Google Scholar]
- Rizzoli SO, Bethani I, Zwilling D, Wenzel D, Siddiqui TJ, Brandhorst D, Jahn R 2006. Evidence for early endosome-like fusion of recently endocytosed synaptic vesicles. Traffic 7: 1163–1176 [DOI] [PubMed] [Google Scholar]
- Robinson MS 2004. Adaptable adaptors for coated vesicles. Trend Cell Biol 14: 167–174 [DOI] [PubMed] [Google Scholar]
- Royle SJ, Lagnado L 2010. Clathrin-mediated endocytosis at the synaptic terminal: Bridging the gap between physiology and molecules. Traffic 11: 1489–1497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan TA 2006. A pre-synaptic to-do list for coupling exocytosis to endocytosis. Curr Opin Cell Biol 18: 416–421 [DOI] [PubMed] [Google Scholar]
- Saheki Y, De Camilli P 2012. Synaptic vesicle endocytosis. Cold Spring Harbor Perspect Biol 4: a005645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saillour Y, Zanni G, Des Portes V, Heron D, Guibaud L, Iba-Zizen MT, Pedespan JL, Poirier K, Castelnau L, Julien C, et al. 2007. Mutations in the AP1S2 gene encoding the σ 2 subunit of the adaptor protein 1 complex are associated with syndromic X-linked mental retardation with hydrocephalus and calcifications in basal ganglia. J Med Genet 44: 739–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar G, Love R, Werner E, Doucette MM, Cheng S, Levey A, Faundez V 2004. The zinc transporter ZnT3 interacts with AP-3 and it is preferentially targeted to a distinct synaptic vesicle subpopulation. Mol Biol Cell 15: 575–587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar G, Craige B, Wainer BH, Guo J, De Camilli P, Faundez V 2005. Phosphatidylinositol-4-kinase type II α is a component of adaptor protein-3-derived vesicles. Mol Biol Cell 16: 3692–3704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar G, Craige B, Styers ML, Newell-Litwa KA, Doucette MM, Wainer BH, Falcon-Perez JM, Dell’Angelica EC, Peden AA, Werner E, et al. 2006. BLOC-1 complex deficiency alters the targeting of adaptor protein complex-3 cargoes. Mol Biol Cell 17: 4014–4026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankaranarayanan S, Ryan TA 2001. Calcium accelerates endocytosis of vSNAREs at hippocampal synapses. Nat Neurosci 4: 129–136 [DOI] [PubMed] [Google Scholar]
- Sato M, Saegusa K, Sato K, Hara T, Harada A, Sato K 2011. Caenorhabditis elegans SNAP-29 is required for organellar integrity of the endomembrane system and general exocytosis in intestinal epithelial cells. Mol Biol Cell 22: 2579–2587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satzler K, Sohl LF, Bollmann JH, Borst JG, Frotscher M, Sakmann B, Lubke JH 2002. Three-dimensional reconstruction of a calyx of Held and its postsynaptic principal neuron in the medial nucleus of the trapezoid body. J Neurosci 22: 10567–10579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheuber A, Rudge R, Danglot L, Raposo G, Binz T, Poncer JC, Galli T 2006. Loss of AP-3 function affects spontaneous and evoked release at hippocampal mossy fiber synapses. Proc Natl Acad Sci 103: 16562–16567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schikorski T, Stevens CF 1997. Quantitative ultrastructural analysis of hippocampal excitatory synapses. J Neurosci 17: 5858–5867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoch S, Deak F, Konigstorfer A, Mozhayeva M, Sara Y, Sudhof TC, Kavalali ET 2001. SNARE function analyzed in synaptobrevin/VAMP knockout mice. Science 294: 1117–1122 [DOI] [PubMed] [Google Scholar]
- Scita G, Di Fiore PP 2010. The endocytic matrix. Nature 463: 464–473 [DOI] [PubMed] [Google Scholar]
- Seong E, Wainer BH, Hughes ED, Saunders TL, Burmeister M, Faundez V 2005. Genetic analysis of the neuronal and ubiquitous AP-3 adaptor complexes reveals divergent functions in brain. Mol Biol Cell 16: 128–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setty SR, Tenza D, Sviderskaya EV, Bennett DC, Raposo G, Marks MS 2008. Cell-specific ATP7A transport sustains copper-dependent tyrosinase activity in melanosomes. Nature 454: 1142–1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu H, Kawamura S, Ozaki K 2003. An essential role of Rab5 in uniformity of synaptic vesicle size. J Cell Sci 116: 3583–3590 [DOI] [PubMed] [Google Scholar]
- Smillie KJ, Evans GJ, Cousin MA 2005. Developmental change in the calcium sensor for synaptic vesicle endocytosis in central nerve terminals. J Neurochem 94: 452–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Renden R, von Gersdorff H 2008. Synaptic vesicle endocytosis: Fast and slow modes of membrane retrieval. Trend Neurosci 31: 559–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Star EN, Newton AJ, Murthy VN 2005. Real-time imaging of Rab3a and Rab5a reveals differential roles in presynaptic function. J Physiol 569: 103–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudhof TC 2012. Calcium control of neurotransmitter release. Cold Spring Harbor Perspect Biol 4: a011353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun T, Wu XS, Xu J, McNeil BD, Pang ZP, Yang W, Bai L, Qadri S, Molkentin JD, Yue DT, et al. 2010. The role of calcium/calmodulin-activated calcineurin in rapid and slow endocytosis at central synapses. J Neurosci 30: 11838–11847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamori S, Riedel D, Jahn R 2000. Immunoisolation of GABA-specific synaptic vesicles defines a functionally distinct subset of synaptic vesicles. J Neurosci 20: 4904–4911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamori S, Holt M, Stenius K, Lemke EA, Gronborg M, Riedel D, Urlaub H, Schenck S, Brugger B, Ringler P, et al. 2006. Molecular anatomy of a trafficking organelle. Cell 127: 831–846 [DOI] [PubMed] [Google Scholar]
- Takei K, Mundigl O, Daniell L, De Camilli P 1996. The synaptic vesicle cycle: A single vesicle budding step involving clathrin and dynamin. J Cell Biol 133: 1237–1250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot K, Cho DS, Ong WY, Benson MA, Han LY, Kazi HA, Kamins J, Hahn CG, Blake DJ, Arnold SE 2006. Dysbindin-1 is a synaptic and microtubular protein that binds brain snapin. Hum Mol Genet 15: 3041–3054 [DOI] [PubMed] [Google Scholar]
- Tarpey PS, Stevens C, Teague J, Edkins S, O’Meara S, Avis T, Barthorpe S, Buck G, Butler A, Cole J, et al. 2006. Mutations in the gene encoding the σ 2 subunit of the adaptor protein 1 complex, AP1S2, cause X-linked mental retardation. Am J Hum Genet 79: 1119–1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taschenberger H, von Gersdorff H 2000. Fine-tuning an auditory synapse for speed and fidelity: Developmental changes in presynaptic waveform, EPSC kinetics, and synaptic plasticity. J Neurosci 20: 9162–9173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng H, Wilkinson RS 2003. “Delayed” endocytosis is regulated by extracellular Ca2+ in snake motor boutons. J Physiol 551: 103–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng H, Lin MY, Wilkinson RS 2007. Macroendocytosis and endosome processing in snake motor boutons. J Physiol 582: 243–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich B, Li C, Zhang JZ, McMahon H, Anderson RG, Geppert M, Sudhof TC 1994. Functional properties of multiple synaptotagmins in brain. Neuron 13: 1281–1291 [DOI] [PubMed] [Google Scholar]
- Uytterhoeven V, Kuenen S, Kasprowicz J, Miskiewicz K, Verstreken P 2011. Loss of skywalker reveals synaptic endosomes as sorting stations for synaptic vesicle proteins. Cell 145: 117–132 [DOI] [PubMed] [Google Scholar]
- van Meel E, Klumperman J 2008. Imaging and imagination: Understanding the endo-lysosomal system. Histochem Cell Biol 129: 253–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstreken P, Ohyama T, Haueter C, Habets RL, Lin YQ, Swan LE, Ly CV, Venken KJ, De Camilli P, Bellen HJ 2009. Tweek, an evolutionarily conserved protein, is required for synaptic vesicle recycling. Neuron 63: 203–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voglmaier SM, Kam K, Yang H, Fortin DL, Hua Z, Nicoll RA, Edwards RH 2006. Distinct endocytic pathways control the rate and extent of synaptic vesicle protein recycling. Neuron 51: 71–84 [DOI] [PubMed] [Google Scholar]
- von Gersdorff H, Matthews G 1994. Inhibition of endocytosis by elevated internal calcium in a synaptic terminal. Nature 370: 652–655 [DOI] [PubMed] [Google Scholar]
- Wagner JA, Carlson SS, Kelly RB 1978. Chemical and physical characterization of cholinergic synaptic vesicles. Biochemistry 17: 1199–1206 [DOI] [PubMed] [Google Scholar]
- Wenk MR, Pellegrini L, Klenchin VA, Di Paolo G, Chang S, Daniell L, Arioka M, Martin TF, De Camilli P 2001. PIP kinase Iγ is the major PI(4,5)P2 synthesizing enzyme at the synapse. Neuron 32: 79–88 [DOI] [PubMed] [Google Scholar]
- Willig KI, Rizzoli SO, Westphal V, Jahn R, Hell SW 2006. STED microscopy reveals that synaptotagmin remains clustered after synaptic vesicle exocytosis. Nature 440: 935–939 [DOI] [PubMed] [Google Scholar]
- Willox AK, Royle SJ 2012. Stonin 2 is a major adaptor protein for clathrin-mediated synaptic vesicle retrieval. Curr Biol 22: 1435–1439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W, Xu J, Wu XS, Wu LG 2005. Activity-dependent acceleration of endocytosis at a central synapse. J Neurosci 25: 11676–11683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LG, Ryan TA, Lagnado L 2007. Modes of vesicle retrieval at ribbon synapses, calyx-type synapses, and small central synapses. J Neurosci 27: 11793–11802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu XS, McNeil BD, Xu J, Fan J, Xue L, Melicoff E, Adachi R, Bai L, Wu LG 2009. Ca2+ and calmodulin initiate all forms of endocytosis during depolarization at a nerve terminal. Nat Neurosci 12: 1003–1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wucherpfennig T, Wilsch-Brauninger M, Gonzalez-Gaitan M 2003. Role of Drosophila Rab5 during endosomal trafficking at the synapse and evoked neurotransmitter release. J Cell Biol 161: 609–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue L, Zhang Z, McNeil BD, Luo F, Wu XS, Sheng J, Shin W, Wu LG 2012. Voltage-dependent calcium channels at the plasma membrane, but not vesicular channels, couple exocytosis to endocytosis. Cell Rep 1: 632–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita T 2012. Ca2+-dependent regulation of synaptic vesicle endocytosis. Neurosci Res 73: 1–7 [DOI] [PubMed] [Google Scholar]
- Yamashita T, Eguchi K, Saitoh N, von Gersdorff H, Takahashi T 2010. Developmental shift to a mechanism of synaptic vesicle endocytosis requiring nanodomain Ca2+. Nat Neurosci 13: 838–844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao L, Sakaba T 2012. Activity-dependent modulation of endocytosis by calmodulin at a large central synapse. Proc Natl Acad Sci 109: 291–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao CK, Lin YQ, Ly CV, Ohyama T, Haueter CM, Moiseenkova-Bell VY, Wensel TG, Bellen HJ 2009. A synaptic vesicle-associated Ca2+ channel promotes endocytosis and couples exocytosis to endocytosis. Cell 138: 947–960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao J, Kwon SE, Gaffaney JD, Dunning FM, Chapman ER 2012a. Uncoupling the roles of synaptotagmin I during endo- and exocytosis of synaptic vesicles. Nat Neurosci 15: 243–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao LH, Rao Y, Varga K, Wang CY, Xiao P, Lindau M, Gong LW 2012b. Synaptotagmin 1 is necessary for the Ca2+ dependence of clathrin-mediated endocytosis. J Neurosci 32: 3778–3785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeigerer A, Gilleron J, Bogorad RL, Marsico G, Nonaka H, Seifert S, Epstein-Barash H, Kuchimanchi S, Peng CG, Ruda VM, et al. 2012. Rab5 is necessary for the biogenesis of the endolysosomal system in vivo. Nature 485: 465–470 [DOI] [PubMed] [Google Scholar]
- Zenisek D, Steyer JA, Almers W 2000. Transport, capture and exocytosis of single synaptic vesicles at active zones. Nature 406: 849–854 [DOI] [PubMed] [Google Scholar]
- Zhu Y, Xu J, Heinemann SF 2009. Two pathways of synaptic vesicle retrieval revealed by single-vesicle imaging. Neuron 61: 397–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlatic SA, Tornieri K, L’Hernault SW, Faundez V 2011. Clathrin-dependent mechanisms modulate the subcellular distribution of class C Vps/HOPS tether subunits in polarized and nonpolarized cells. Mol Biol Cell 22: 1699–1715 [DOI] [PMC free article] [PubMed] [Google Scholar]