Background: Start codon selection requires eIF1 dissociation from its 40 S-binding site.
Results: eIF1 residues in β-hairpin loop-1 and helix α1 make functionally critical contacts with the 40 S subunit.
Conclusion: Direct 40 S contacts of eIF1 regulate the rate of Met-tRNAi recruitment and block non-AUG recognition.
Significance: eIF1's direct contacts with the 40 S subunit are crucial for AUG recognition in vivo.
Keywords: Ribosome Function, Ribosomes, Transfer RNA (tRNA), Translation, Translation Initiation Factors, AUG Recognition, eIF1, eIF2
Abstract
Recognition of the translation initiation codon is thought to require dissociation of eIF1 from the 40 S ribosomal subunit, enabling irreversible GTP hydrolysis (Pi release) by the eIF2·GTP·Met-tRNAi ternary complex (TC), rearrangement of the 40 S subunit to a closed conformation incompatible with scanning, and stable binding of Met-tRNAi to the P site. The crystal structure of a Tetrahymena 40 S·eIF1 complex revealed several basic amino acids in eIF1 contacting 18 S rRNA, and we tested the prediction that their counterparts in yeast eIF1 are required to prevent premature eIF1 dissociation from scanning ribosomes at non-AUG triplets. Supporting this idea, substituting Lys-60 in helix α1, or either Lys-37 or Arg-33 in β-hairpin loop-1, impairs binding of yeast eIF1 to 40 S·eIF1A complexes in vitro, and it confers increased initiation at UUG codons (Sui− phenotype) or lethality, in a manner suppressed by overexpressing the mutant proteins or by an eIF1A mutation (17–21) known to impede eIF1 dissociation in vitro. The eIF1 Sui− mutations also derepress translation of GCN4 mRNA, indicating impaired ternary complex loading, and this Gcd− phenotype is likewise suppressed by eIF1 overexpression or the 17–21 mutation. These findings indicate that direct contacts of eIF1 with 18 S rRNA seen in the Tetrahymena 40 S·eIF1 complex are crucial in yeast to stabilize the open conformation of the 40 S subunit and are required for rapid TC loading and ribosomal scanning and to impede rearrangement to the closed complex at non-AUG codons. Finally, we implicate the unstructured N-terminal tail of eIF1 in blocking rearrangement to the closed conformation in the scanning preinitiation complex.
Introduction
In eukaryotic translation initiation, the AUG start codon is generally identified by a scanning mechanism in which the 43 S preinitiation complex (PIC),3 harboring the eIF2·GTP·Met-tRNAiMet TC and several other initiation factors, binds to the capped 5′ end of the mRNA and inspects the 5′-untranslated region (5′UTR) base-by-base for complementarity with the anticodon of Met-tRNAiMet. The factors eIF1 and eIF1A function cooperatively within the PIC to stabilize an open conformation of the 40 S subunit that is conducive to scanning with TC bound in a metastable state capable of sampling successive triplets as they enter the P site. In this scanning PIC, eIF5 stimulates hydrolysis of the GTP bound to eIF2 to produce an internal equilibrium between GTP and GDP·Pi, but release of Pi is impeded. Base-pairing of the Met-tRNAiMet anticodon with an AUG in suitable sequence context evokes dissociation of eIF1, enabling Pi release from eIF2·GDP·Pi, rearrangement of the 40 S subunit to a closed conformation incompatible with scanning, and tighter binding of Met-tRNAiMet in the P site. Subsequent joining of the 60 S subunit produces an 80 S initiation complex competent for the elongation phase of protein synthesis (1, 2).
Several lines of evidence suggest that eIF1's functions are reversed on AUG recognition by its dissociation from the 40 S subunit. In vitro experiments revealed that the rate constants for release of eIF1 and Pi from reconstituted 43 S·mRNA PICs are similar in magnitude and greater for mRNAs containing AUG versus non-AUG start codons, suggesting that AUG recognition triggers eIF1 dissociation and attendant Pi release. Supporting this, an eIF1 substitution (G107R) that reduced the rate of eIF1 dissociation similarly decreased the rate of Pi release at AUG codons (3). Furthermore, certain “Sui−” substitutions in yeast eIF1, which elevate recognition of near-cognate start codons (e.g. UUG) in vivo, were shown to decrease eIF1 affinity for 40 S subunits (4, 5). One such Sui− substitution of residues 93/96/97 (sui1-93-97) moderately reduced 40 S binding affinity and accelerated eIF1 dissociation on start codon recognition from reconstituted yeast PICs (5). Furthermore, alanine substitutions of residues 17–21 of the eIF1A N-terminal tail (tif11-17-21), which suppresses UUG initiation in various Sui− mutants (the Ssu− phenotype) (6, 7), decreased the off-rate of eIF1 in reconstituted PICs (5). These findings, plus genetic observations that overexpressing wild-type (WT) eIF1 reduces UUG initiation in Sui− mutants (4, 6–8), provided evidence that inappropriate eIF1 dissociation from the 40 S subunit enables aberrant initiation at near-cognate start codons.
Biochemical and structural analyses of reconstituted yeast PICs indicated that the TC binds more rapidly to the open conformation of the 40 S subunit, stabilized by eIF1 and eIF1A, even though TC is bound more tightly to the closed conformation containing eIF1A alone, which would prevail following eIF1 dissociation on AUG recognition (9). It was also shown that residues in the unstructured C-terminal tail (CTT) of eIF1A, comprising the scanning enhancer (SEs) elements, stabilize the open conformation of the 40 S subunit and enhance TC binding, presumably in the conformation conducive for sampling P-site triplets during scanning (dubbed POUT) (7). The SEs also likely impede isomerization of TC to the higher affinity state of the closed complex required for AUG recognition (dubbed PIN), as mapping of the eIF1A CTT in reconstituted mammalian PICs predicts a location that would obstruct canonical P site-binding of the anticodon stem-loop (ASL) of Met-tRNAiMet (10). Accordingly, nonlethal substitutions of the SE elements have two related consequences. First, by destabilizing the open 40 S/POUT conformation, they reduce the rate of TC binding to the 43 S·mRNA PIC, with attendant derepression of GCN4 mRNA translation in vivo (the Gcd− phenotype) (11). Second, by facilitating rearrangement to the closed/PIN conformation, SE mutations allow initiation to occur inappropriately at UUG codons, conferring the Sui− phenotype. Both of these defects are suppressed by the 17–21 substitution in eIF1A mentioned earlier, which disables the “scanning inhibitor” (SI) element of the NTT, leading to the model that the SI element antagonizes the open 40 S/POUT conformation to promote rearrangement to the closed/PIN state on AUG recognition (Fig. 1A) (7).
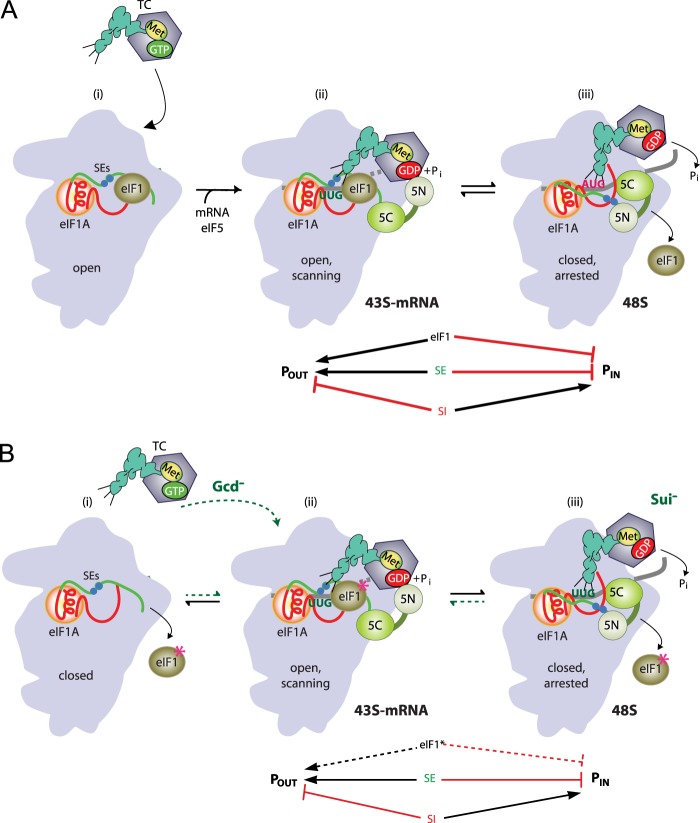
FIGURE 1.
A, hypothetical model describing the roles of eIF1 and eIF1A in start codon recognition. eIF1 is depicted in olive green, eIF1A in orange with the CTT in green (harboring the SE elements as blue spheres), and the helical domain and NTT (harboring the SI elements) shown in red. i, eIF1 and the scanning enhancer elements in the CTT of eIF1A stabilize an open conformation of the 40 S subunit that rapidly loads the TC. ii, following attachment to mRNA and eIF5, the 43 S PIC in the open conformation scans the mRNA for the start codon with Met-tRNAi anchored in the POUT state. GAP domain in the eIF5-NTD (5N) stimulates GTP hydrolysis by the TC to produce an equilibrium between GTP and GDP·Pi, but release of Pi is impeded. iii, on AUG recognition, the Met-tRNAi moves from the POUT to PIN state, and eIF1 dissociates from its 40 S-binding site, and the eIF1A SE elements are displaced from the P site. eIF5-NTD dissociates from eIF2 and moves toward the eIF1A CTT, facilitating Pi release. The 48 S complex reflects the stable PIC produced after eIF1 dissociation and Pi release. eIF1 and the eIF1A SE elements promote POUT and impede the transition to the PIN state, whereas the SI elements in the NTT and helical domain of eIF1A stabilize the PIN state. (Adapted from Ref. 26, 27.) B, hypothetical effects on TC loading and start codon recognition of eIF1 mutations that weaken its binding to the 40 S subunit. i, mutations in helix α1 and loop-1 of eIF1 are predicted to impair its binding to the 40 S subunits and destabilize the POUT state, reducing the rate of TC loading and thereby conferring the Gcd− phenotype. ii and iii, once the TC is bound to the PIC, these eIF1 mutations would also allow inappropriate dissociation of eIF1 from the 40 S subunit and attendant transition to the PIN state to occur more readily at UUG codons, producing the Sui− phenotype. Mutations in the eIF1A SE elements also destabilize the POUT state conferring Gcd− and Sui− phenotypes, whereas mutations in eIF1A SI elements suppress the effect of such eIF1A SE mutations, rescuing rapid TC binding to POUT and suppressing UUG initiation, by stabilizing the POUT state (Ssu− phenotype). By enhancing 40 S binding of eIF1, SI mutations in eIF1A are predicted to mitigate the Gcd− and Sui− phenotypes of helix α1 and loop-1 mutations that weaken eIF1 binding to the 40 S subunit.
In the model described above, both eIF1 and eIF1A SE elements must be antagonized to access the closed/PIN state for AUG recognition. Presumably, the eIF1A SE elements are displaced from their location in the P site to accommodate canonical P site binding of Met-tRNAiMet (10). Interestingly, it was predicted from the crystal structure of the Tetrahymena thermophila 40 S·eIF1 complex (12) that eIF1 will also hinder P site-binding of Met-tRNAiMet, with the β1-β2 hairpin loop of eIF1 (henceforth loop-1) clashing with the ASL of Met-tRNAiMet. Considering that a basic residue in loop-1 also directly contacts the 18 S rRNA, it might be expected that the duplex formed between the anticodon of Met-tRNAiMet and AUG in the P site would perturb loop-1 interaction with the 40 S subunit and accelerate eIF1 dissociation. Conserved lysine residues in helix-1 (α1) of eIF1 are predicted to make additional contacts with rRNA (12). Accordingly, it can be anticipated that mutations in the equivalent basic residues in either loop-1 or helix α1 of yeast eIF1 would weaken its binding to the 40 S platform and destabilize the open/POUT conformation of the PIC. This should reduce the rate of TC loading and confer the Gcd− phenotype and also enable isomerization to the closed/PIN conformation at UUG codons to produce the Sui− phenotype (Fig. 1B). As for Gcd−/Sui− mutations in eIF1A SE elements, both phenotypes should be suppressed by the 17–21 mutation in the eIF1A SI element or by overexpressing the mutant eIF1 proteins, as the result of stabilizing the eIF1-bound open conformation of the PIC (Fig. 1B).
It is noteworthy that most of the existing Sui− substitutions in yeast eIF1 (5, 13) alter residues mapping outside of the two surfaces of the protein (helix α1 and loop-1) predicted to interact directly with the 40 S subunit (12, 14). Thus, previous studies have implicated nonbasic residues located in helix α2 or β-strand 5 (β5) at the extreme C terminus of yeast eIF1 in both 40 S binding and accuracy of start codon recognition (4, 5, 13, 15). As the corresponding amino acids are exposed to solvent in the Tetrahymena 40 S·eIF1 crystal structure (12), it remains unclear how altering these residues affects yeast eIF1 binding to the 40 S. Moreover, in the case of eIF1 substitution G107R, the Sui− phenotype actually results from an excessively tight interaction with the PIC that delays eIF1 dissociation specifically at AUG start codons (15). Previously, we described site-directed substitutions of two basic residues in helix α1 that conferred a Sui− phenotype, but the effects of these mutations on TC recruitment and eIF1 binding to the 40 S subunit were unknown (16).
In this study, we have exploited the Tetrahymena 40 S·eIF1 crystal structure to thoroughly investigate whether the conserved basic residues in loop-1 or helix α1 predicted to mediate direct eIF1 contacts with the 40 S subunit are indeed important for yeast eIF1 function in vivo and for its 40 S binding affinity in vitro. The results of these analyses provide strong support for our model concerning the dual role of eIF1 in promoting TC recruitment and blocking non-AUG recognition, and they imply that the Tetrahymena 40 S·eIF1 crystal structure depicts a physiologically crucial mode of eIF1 binding in the scanning PIC. Our findings further suggest that an extended basic surface of loop-1 contacts the phosphate backbone of 18 S rRNA in the exit channel of the 40 S subunit presumably to stabilize eIF1–40 S interaction and also impede Met-tRNAi binding in the PIN state at non-AUG codons.
EXPERIMENTAL PROCEDURES
Yeast Strain Constructions
Derivatives of strain JCY03 (MATa ura3-52 leu2-3 leu2-112 trp1Δ-63 his4-301(ACG) sui1Δ::hisG p1200 (sc URA3 SUI1)) were constructed by transforming JCY03 to Leu+ with single copy (sc), low copy (lc), or high copy (hc) LEU2 plasmids harboring the appropriate SUI1 alleles (indicated in supplemental Table S2) on synthetic complete medium lacking leucine (SC-Leu), and the resident SUI1+ URA3 plasmid (p1200) was evicted by selecting for growth on 5-FOA medium, except for lethal mutations unable to lose the SUI1+ URA3 plasmid.
Strain PMY03 (MATa ura3-52 leu2-3 leu2-112 trp1Δ-63 his4-301(ACG) sui1Δ::hisG kanMX6:PGAL1-TIF11 p1200 (sc URA3 SUI1)) was transformed with a sc TRP1 plasmid containing TIF11 (pPMB76) or tif11-17-21 (pPMB77) on SC medium lacking tryptophan (SC-Trp) to generate PMY212 and PMY214. To obtain strains PMY236 through PMY239 and PMY243 through PMY245, PMY212 was transformed with sc LEU2 plasmids harboring the appropriate SUI1 alleles on SC lacking leucine and tryptophan (SC-LW), and p1200 was evicted on 5-FOA medium. To generate PMY246 through PMY249, PMY253 through PMY255, and pMY263 through PMY267, PMY214 was transformed with sc LEU2 plasmids harboring the appropriate SUI1 alleles on SC-LW, and p1200 was evicted on 5-FOA medium except for lethal mutations unable to lose the SUI1+ URA3 plasmid.
Derivatives of strain CHY01 (MATa ura3-52 leu2-3 leu2-11 trp1Δ63 gcn2Δ sui1Δ::hisG (TRP1 GCN4-lacZ) p1200 (sc URA3 SUI1)) were constructed by transforming CHY01 to Leu+ with sc LEU2 plasmids harboring the appropriate SUI1 alleles, as indicated in supplemental Table S2, on SC-LW medium, and the resident SUI1+ URA3 plasmid (p1200) was evicted by selecting for growth on 5-FOA medium.
Plasmid Constructions
The QuikChange® site-directed mutagenesis system (Stratagene) was employed with primers indicated in supplemental Table S3 to generate all of the corresponding plasmids shown in supplemental Table S1 using as templates plasmids pJCB101, pCFB03, pET-SUI1-His, or pTYB2-eIF1, whereas pPMB35A (harboring sui1-I3N) was obtained by random mutagenesis of SUI1 as described previously (16). The relevant hc plasmid versions were created by inserting a 1.6-kb HindIII-SacI fragment containing the appropriate SUI1 alleles from the corresponding sc plasmids into HindIII and SacI sites of YCplac181. pPMB35 was generated by introducing a 1.6-kb HindIII-SacI fragment containing sui1-I3N from pPMB35A into the corresponding sites of pRS315. pPMB76 and pPMB77 were constructed by inserting the TIF11 WT or the TIF11-NDSDG17–21AAAAA mutant alleles from plasmids pDSO9 and p4552, respectively, between the EcoRI and SalI sites of YCplac22.
Biochemical Assays in Whole Cell Extracts (WCEs)
Assays of β-galactosidase activity in WCEs were performed as described previously (17). For Western analysis, WCEs were prepared by trichloroacetic acid extraction, as described previously (18), and immunoblot analysis was conducted as described (15) using antibodies against eIF1, eIF2Bϵ/Gcd6, or the His6 epitope.
Biochemical Assays in the Reconstituted in Vitro System
Buffers and Reagents
For all experiments, the reaction buffer was 30 mm Hepes-KOH (pH 7.4), 100 mm potassium acetate (pH 7.4), 3 mm magnesium acetate, and 2 mm dithiothreitol. The composition of the enzyme buffer was 20 mm Hepes-KOH (pH 7.4), 100 mm KOAc (pH 7.4), 2 mm DTT, and 10% glycerol.
Initiation factors eIF1A and eIF1 WT and mutant variants of this protein were purified using the IMPACT system (New England Biolabs) as described previously (19). eIF1 WT and mutant proteins were labeled at their C termini with cysteine-lysine-fluorescein dipeptide, using the expressed protein ligation system as described previously (20). His-tagged eIF2 was overexpressed in yeast and purified as described (19). 40 S subunits were purified as described previously (19). The sequences of the model mRNAs were 5′-GGAA(UC)7UAUG(CU)10C-3′ and 5′-GGAA(UC)7UUUG(CU)10C-3′. Yeast tRNAiMet was synthesized from a hammerhead fusion template using T7 polymerase transcription and charged with [35S]Met or unlabeled methionine as described previously (19).
Fluorescence Anisotropy Experiments
Fluorescence anisotropy measurements of equilibrium binding constants (Kd) were performed using a T-format Spex Fluorolog-3 (J. Y. Horiba) as described previously (20). The excitation and emission wavelengths were 497 and 520 nm, respectively. The data were fit with a hyperbolic binding equation describing the binding of fluorescently labeled eIF1 mutants to 40 S subunits to give Kd values (20). In competition experiments with unlabeled eIF1, the data were fit with a quadratic equation describing the competitive binding of two ligands to a receptor (20).
Ternary Complex Binding Experiments
Measurement of kinetics of TC binding to 40 S subunits was monitored using a native gel shift assay as described previously (9, 19, 21). TC assembled with 0.5–1 nm [35S]Met-tRNAi was mixed with 10–40 nm 40 S subunits, eIF1 (1 μm), eIF1A (1 μm), and mRNA (10 μm). Binding of labeled TC was stopped by adding a chase of excess (≥300-fold) unlabeled TC at different times. The kobs values were calculated by fitting the data with a single exponential equation. To determine the association rate constants (kon), the kobs values were measured at different 40 S concentrations; the slope of the plot of kobs versus [40 S] yielded kon (21).
To determine rate constants for TC dissociation from PICs, 43 S·mRNA complexes were formed using [35S]Met-tRNAi as described above. A chase of excess (≥300-fold) unlabeled TC was added, and the fraction of bound and labeled TC was monitored over time on native gels. Curves were fit to a single exponential equation, to determine the dissociation rate constant (koff).
Purification of eIF1 Proteins and NMR Experiments
NMR experiments were performed as described previously (22, 23). HSQC spectra were recorded at 298 K on a Bruker 500 MHz spectrometer. The WT and mutant yeast eIF1 proteins were overexpressed in E. coli using minimal media (M9) containing [15N]ammonium chloride. Cells were lysed, and proteins were purified via nickel affinity and gel filtration column chromatography. Samples for NMR measurements (HSQC spectra) contained 200 μm protein in 200 mm NaCl, 20 mm Tris-HCl, 2 mm DTT, 1 mm EDTA, and 10% D2O (pH 7.2). The backbone resonance assignments of yeast eIF1 (24) were used to reference the HSQC spectra and confirm the location of the point mutants.
RESULTS
Genetic Evidence That Conserved Basic Residues in Helix α1 of Yeast eIF1 Mediate Critical Contacts with the 40 S Subunit in Vivo
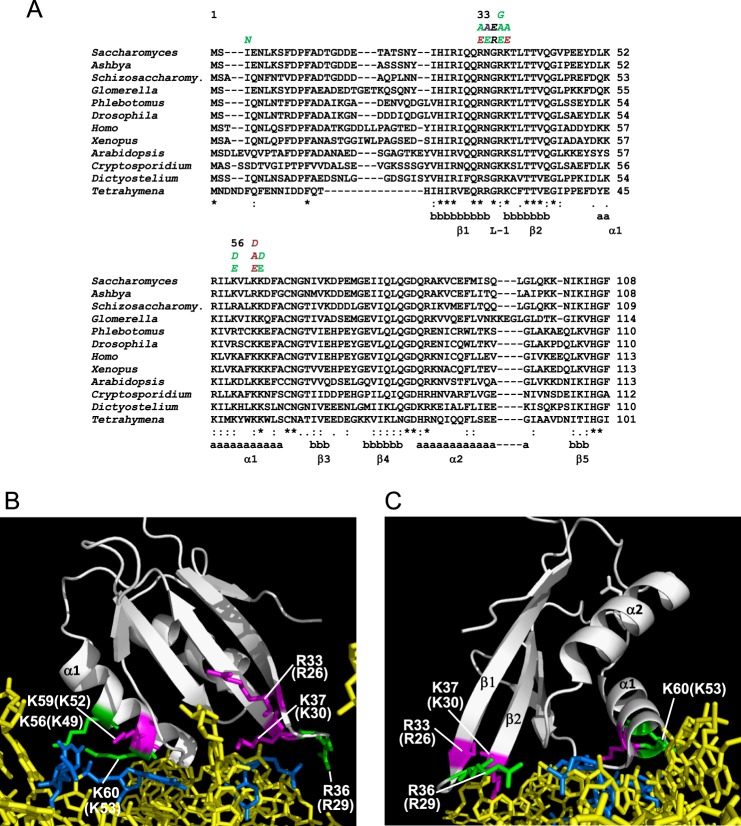
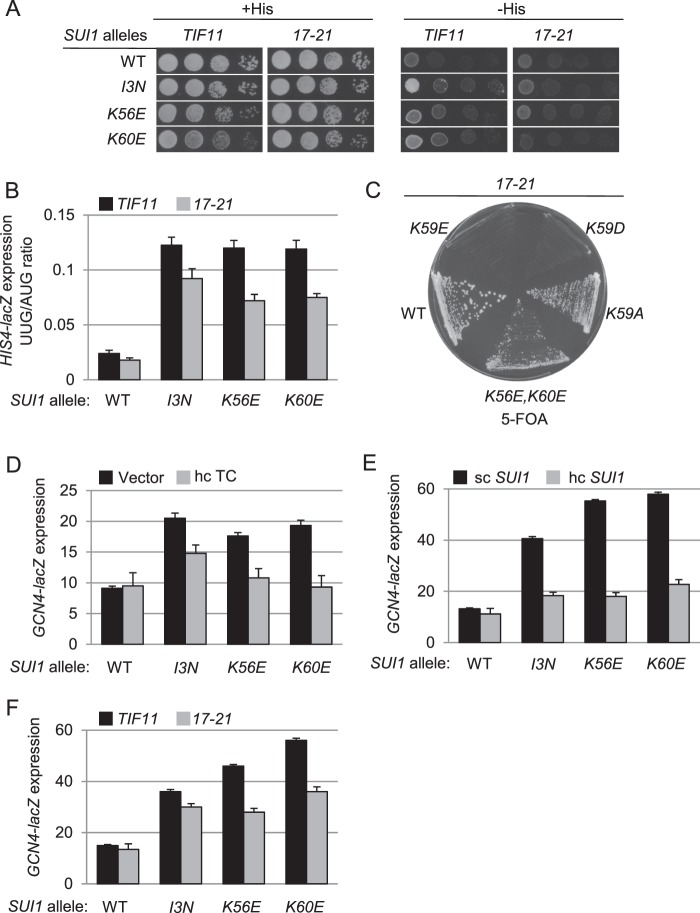
The crystal structure of the Tetrahymena 40 S subunit bound to eIF1 identified three basic residues making direct contacts with 18 S rRNA residues, namely Arg-29 in loop-1 and Lys-52 and Lys-53 in helix α1 (12). Consistent with critical roles in eIF1 binding to the ribosome, these basic residues and surrounding amino acids are among the most highly conserved residues in eIF1 from diverse eukaryotes (Fig. 2A). Their locations in the 40 S·eIF1 complex are depicted in Fig. 2B, labeled with their amino acid positions in Saccharomyces cerevisiae eIF1, i.e. Arg-36, Lys-59, and Lys-60. We predicted that altering these basic residues in yeast eIF1 to negatively charged Asp or Glu residues would weaken 40 S binding by eIF1 and confer a Sui− phenotype. Indeed, we previously reported a Sui− phenotype for the K60E substitution in α1 (16). Here, we extended our analysis to include other substitutions of Lys-60 and mutations of the adjacent, invariant Lys residue at position 59.
FIGURE 2.
A, alignment of eIF1 sequences from diverse eukaryotes. Secondary structures are indicated as a (α-helix) and b (β-sheet). Residues substituted by site-directed or random mutagenesis are indicated above, with green and purple designating Sui− and lethal phenotypes, respectively, and black indicating no detectable Slg− or Sui− phenotypes. B and C, ribbon depiction of T. thermophila eIF1 bound to the 40 S subunit (PDB file 2XZM). 18 S rRNA phosphate backbone is shown in yellow with bases predicted to contact eIF1 residues in blue. Highlighted are residues in yeast eIF1 that when substituted confer Sui− (in green) or lethal (in purple) phenotypes, and in parentheses are the corresponding residues in Tetrahymena.
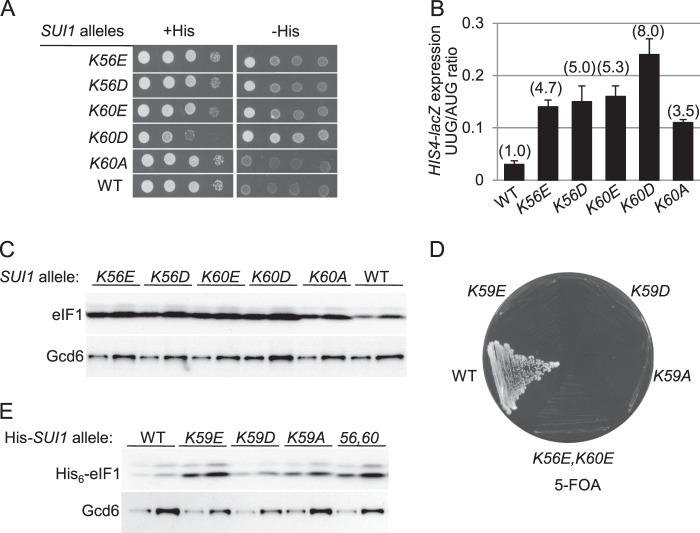
To this end, the appropriate mutations were generated in a SUI1 allele (encoding yeast eIF1) under its native promoter on a single copy LEU2 plasmid; the resulting plasmids were introduced into a his4-301 sui1Δ strain containing WT SUI1 on a URA3 plasmid, and the latter SUI1+ plasmid was evicted by counter-selection on medium containing 5-FOA (25). Because the mutant his4-301 mRNA lacks an AUG start codon, the parental SUI1+ his4-301 strain cannot grow on medium lacking histidine (or containing only 1% of the normal histidine supplement), whereas Sui− mutations restore growth on −His media by increasing initiation at the third (UUG) codon in his4-301 mRNA. Compared with the K60E substitution analyzed previously (16), we found that the K60D substitution confers an even stronger growth defect on histidine-replete (+His) medium and more pronounced growth on −His medium in the his4-301 strain; however, the K60A substitution did not alter growth on either medium (Fig. 3A). Phenotypes indistinguishable from K60E were observed for Glu and Asp substitutions at residue Lys-56 (Fig. 3A), located one helical turn upstream of Lys-59 and Lys-60 in helix α1. Although Lys-56 is not predicted to make direct contacts with the 40 S, it lies on the same face of α1 and is close to the predicted eIF1:40 S interface (Fig. 2B) (12). The K60D, K60E, K56D, and K56E substitutions all conferred an increased frequency of UUG initiation, detected by assaying matched HIS4-lacZ fusions harboring AUG or UUG start codons, demonstrating that they are bona fide Sui− mutants. Consistent with the His+ phenotypes (Fig. 3A), the largest increase in the UUG/AUG initiation ratio (8-fold) was observed for K60D (Fig. 3B) (16). Although the K60A substitution did not confer a His+ phenotype (Fig. 3A), it produced a significant increase in the UUG/AUG initiation ratio, albeit of smaller degree than for the other variants. Hence, removing a positive charge at residue 60 is sufficient to reduce initiation accuracy, and introducing a negative charge evokes a similar defect of greater magnitude.
FIGURE 3.
Substitutions of lysines 56, 60, and 59 in helix α1 confer Sui− or lethal phenotypes. A, Slg− and His+/Sui− phenotypes of Lys-56 and Lys-60 substitutions. 10-Fold serial dilutions of derivatives of sui1Δ his4-301 strain JCY03 with the indicated SUI1 alleles on sc plasmids were spotted on synthetic complete medium lacking leucine (SC-Leu) supplemented with 0.3 mm histidine (+His) and on SC-Leu plus 0.003 mm His (−His) media and cultured at 30 °C for 2 or 7 days, respectively. B, quantification of Sui− phenotypes. Strains from A also harboring plasmids p367 or p391 containing HIS4-lacZ reporters with an AUG or UUG start codon, respectively, were cultured in synthetic dextrose minimal medium (SD) supplemented with His and Trp at 30 °C to A600 of ∼1.0, and β-galactosidase activities were measured in WCEs. The ratio of expression of the UUG to AUG reporter was calculated for replicate experiments, and the mean and S.E. were plotted. Numbers in parentheses are the means normalized to the WT value. C, derivatives of sui1Δ strain JCY03 containing the indicated SUI1 alleles were cultured in SD supplemented with His, Trp, and uracil at 30 °C to an A600 of ∼1.0, and WCEs were subjected to Western analysis using antibodies against eIF1/Sui1 or eIF2Bϵ/Gcd6 (used as loading control). Two different amounts of each extract differing by a factor of 2 were loaded in successive lanes. Data in A–C on mutants K56E and K60E were published previously (16). D, JCY03 derivatives containing SUI1+ WT and a His6-tagged version of the indicated SUI1 alleles (His-SUI1) were streaked on SC-Leu and supplemented with 5-FOA and incubated at 30 °C for 4 days. E, same strains as in D were cultured and subjected to Western analysis as in C using antibodies against His6 epitope to detect His6-eIF1 proteins.
Western analysis of WCEs revealed that these sui1 mutants all contain higher than WT levels of eIF1 (normalized to the eIF2Bϵ subunit Gcd6) (Fig. 3C). The acidic substitutions of Lys-56 or Lys-60 evoked ∼4–5-fold increases, whereas the relatively weaker Sui− variant K60A produced only an ∼3-fold increase in eIF1 expression. Previously, we established that the elevated expression of Sui− eIF1 mutants results from diminished discrimination against the poor sequence context of the SUI1 AUG start codon with attendant increased translation of SUI1 mRNA (16). Considering that overexpression of eIF1 does not confer a Sui− phenotype, and actually suppresses the Sui− phenotypes of mutations in other initiation factors (2), we conclude that the Lys-56 and Lys-60 substitutions confer Sui− phenotypes by altering eIF1 function rather than its expression.
Interestingly, combining the K56E and K60E mutations (K56E,K60E) was lethal, as was substitution of highly conserved Lys-59 with Glu, Asp, or Ala, as indicated by the fact that the SUI1+ URA3 plasmid could not be evicted from transformants harboring these sui1 alleles to permit growth on 5-FOA medium (Fig. 3D). Western analysis of His6-tagged versions of these eIF1 proteins in viable cells that additionally harbor untagged SUI1+ (to maintain viability) revealed that the lethal mutations did not reduce eIF1 expression below the WT level (Fig. 3E). Thus, eliminating the positively charged side chain of Lys-59, or introducing negatively charged side chains at both Lys-56 and Lys-60 simultaneously, confers a lethal reduction in eIF1 function. These findings support the idea that Lys-59 and Lys-60 of helix α1 make critical contacts with 18 S rRNA (Fig. 2B) and suggest that Lys-59 is more important than Lys-60 for 40 S interaction with yeast eIF1, as even the Ala substitution at that position is lethal.
Genetic Evidence That the Sui− and Lethal eIF1 Mutations in Helix α1 Provoke Inappropriate Dissociation of eIF1 from 40 S Subunits
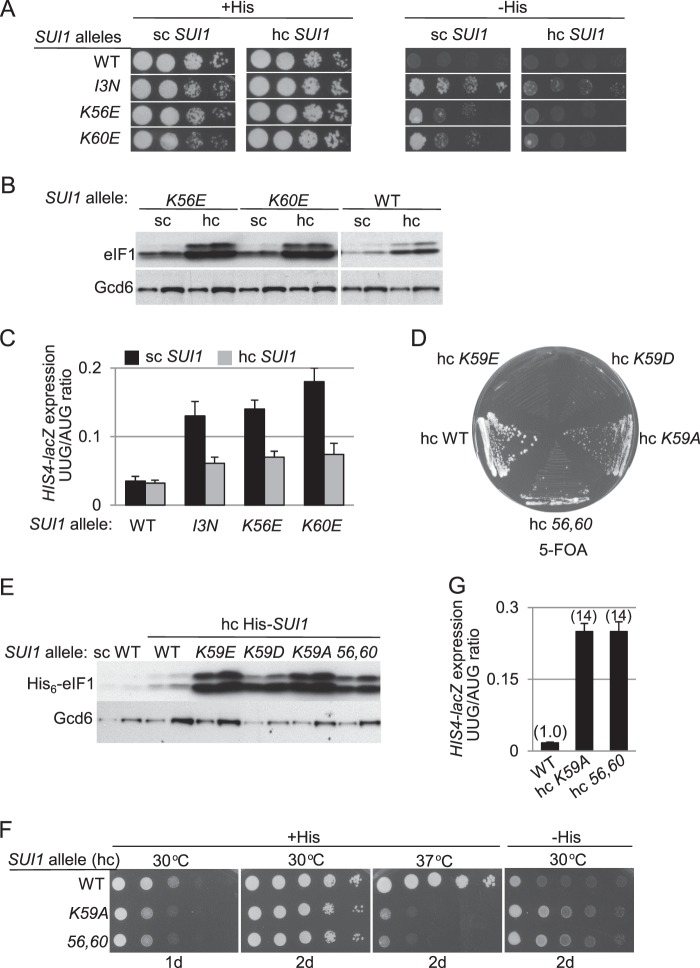
We reasoned that if the lethality or Sui− phenotypes of substitutions of Lys residues in α1 result from reduced eIF1 binding to the 40 S subunit and inappropriate eIF1 dissociation from the PIC at non-AUG codons, these phenotypes should be suppressed by overexpressing the mutant eIF1 proteins to restore high level 40 S occupancy by mass action (5). Consistent with this prediction, overexpressing the eIF1-K56E and eIF1-K60E variants from hc plasmids diminished the Slg− phenotypes on +His medium and His+/Sui− phenotypes on −His medium that they confer when expressed from sc plasmids (Fig. 4A, +His; −His). Similar results were observed for another Sui− eIF1 substitution we obtained by random mutagenesis of SUI1, I3N, which alters a single residue in the unstructured NTT (Fig. 4A). (Fig. 4A reveals that overexpressing WT eIF1 confers a modest Slg− phenotype on +His medium not seen on overexpression of the I3N, K60E, or K56E variants. Presumably, an excess of WT eIF1 interferes with some aspect of initiation in a manner eliminated by these loss-of-function substitutions.) Overexpression of the mutant eIF1 proteins also partially suppressed the elevated UUG/AUG initiation ratios of HIS4-lacZ expression conferred by the sui1 mutations (Fig. 4C). Western analysis showed that the I3N variant is expressed at WT, or slightly less than WT, levels in different transformants harboring the sc sui1-I3N plasmid (data not shown) and that all three eIF1 mutant proteins were overproduced in yeast when expressed from hc plasmids (Fig. 4B and data not shown).
FIGURE 4.
Overexpression of mutant eIF1 proteins suppresses their Sui− or lethal phenotypes. A, JCY03 derivatives harboring the indicated SUI1 alleles in sc, low copy (I3N), or hc plasmids were analyzed on +His and −His media as in Fig. 3A. B, same strains as in A were cultured and subjected to Western analysis as in Fig. 3C. C, transformants of the strains from A containing the AUG or UUG HIS4-lacZ reporters were analyzed as in Fig. 3B. D, JCY03 derivatives containing SUI1+ and the indicated His-SUI1 alleles in hc plasmids were streaked on SC-Leu + 5-FOA and incubated at 30 °C for 5 days (d). E, same strains as in D were cultured and subjected to Western analysis as in Fig. 3E. F, JCY03 derivatives containing the indicated hc His-SUI1 alleles (after selection on SC-Leu + 5-FOA) were analyzed on +His and −His media for the indicated days as in Fig. 3A. G, transformants of the strains from F containing the AUG or UUG HIS4-lacZ reporters were analyzed as in Fig. 3B.
Interestingly, overexpressing the sui1 alleles K59A and K56E,K60E overcame their lethality (Fig. 4, D and E), whereas the mutations that replace the crucial positive charge at Lys-59 with negatively charged Asp or Glu (K59E and K59D) remained lethal despite high level overexpression (Fig. 4, D and E). Although cells overexpressing the K59A and K56E,K60E mutants are viable, they display residual growth defects on +His medium, particularly at 37 °C, and His+/Sui− phenotypes on −His medium (Fig. 4F); and they also elevated UUG/AUG initiation ratios (Fig. 4G). We presume that high level expression of these variants does not restore a WT level of 40 S binding. The results of the dosage suppression experiments in Fig. 4 support the idea that the eIF1 substitutions provoke inappropriate eIF1 dissociation from the 40 S subunit in a manner that elevates recognition of near-cognate UUG codons and, in the case of Lys-59 substitutions, provokes a lethal reduction in canonical AUG initiation as well.
We showed previously that Ala substitution of residues 17–21 in the NTT of eIF1A confers an Ssu− phenotype in vivo, partially suppressing the Sui− and Slg− phenotypes of Sui− mutations affecting eIF2β (SUI3–2), eIF5 (SUI5), and eIF1A itself (6, 7). Interestingly, the 17–21 substitution also reduces the rate of eIF1 dissociation from reconstituted PICs in vitro (5). Hence, we reasoned that the 17–21 mutation should suppress the His+/Sui− and Slg− phenotypes of the eIF1 mutations described above if they provoke abnormally rapid dissociation of eIF1 from PICs in vivo. To test this prediction, we generated sui1Δ strains harboring the chromosomal gene encoding eIF1A (TIF11+) placed under the GAL1 promoter, plasmid-borne tif11-17-21 or TIF11+, and mutant or WT sui1 alleles on a separate plasmid. When cultured continuously on glucose medium, where the GAL1 promoter is repressed, the plasmid-borne tif11-17-21 or TIF11+ allele provides the only source of eIF1A in the cell (16).
In accordance with our prediction, the tif11-17-21 mutation partially suppresses both the Slg− and His+/Sui− phenotypes conferred by the viable K60E, K56E, and I3N mutations in eIF1 (Fig. 5A). (Note that suppression of growth on −His for the K56E mutant is obscured by the improved growth on +His medium afforded by the 17–21 mutation in this double mutant.) The 17–21 mutation also diminishes the UUG/AUG initiation ratios conferred by these four Sui− mutations, although the I3N substitution is inefficiently suppressed compared with the others (Fig. 5B). Strikingly, the lethality of K59A and the K56E,K60E double mutation is also suppressed by the 17–21 mutation (Fig. 5C), supporting the notion that, in otherwise WT cells, these mutations confer an intolerable reduction in eIF1 binding to the 40 S subunit, which can be overcome by stabilizing the open conformation of the PIC by an eIF1A NTT mutation. The fact that 17–21 is less effective than overexpressing the mutant eIF1 proteins in suppressing the elevated UUG/AUG ratios conferred by the viable substitutions (cf. Figs. 4C and 5B) might be explained by considering our previous finding that 17–21 exacerbates the effect of poor context at the SUI1 AUG start codon and reduces eIF1 expression (16). Reducing the abundance of the eIF1 mutant proteins would be expected to exacerbate their Sui− phenotypes and thereby dampen the suppressor activity of 17–21.
FIGURE 5.
A–C, tif11-17-21 Ssu− mutation in the NTT of eIF1A partially suppresses the Sui− phenotypes and the lethality of mutations in eIF1. A, 10-fold serial dilutions of derivatives of sui1Δ his4-301 PGAL-TIF11 strain PMY03 containing plasmid-borne TIF11+ (pPMB76) or tif11-17-21 (pPMB77) with the indicated SUI1 alleles on sc or lc (I3N) plasmids were analyzed as in Fig. 3A except using SC-Leu-Trp instead of SC-Leu. B, strains from A containing the AUG or UUG HIS4-lacZ reporters were cultured in SD + His, and β-galactosidase activities were measured in WCEs. C, derivatives of sui1Δ his4-301 PGAL-TIF11 strain PMY03 containing tif11-17-21 (pPMB77) with the indicated sc SUI1 alleles were streaked on SC-Leu-Trp supplemented with 5-FOA. D, derivatives of sui1Δ gcn2Δ strain CHY01 harboring the indicated SUI1 alleles in sc or lc (I3N) plasmids and either vector or hc TC plasmid p1780-IMT were cultured in SD + His, and β-galactosidase activities were measured in WCEs. E, derivatives of sui1Δ his4-301 strain JCY03 harboring the indicated SUI1 alleles in sc, lc (I3N), or hc plasmids and plasmid p180 were cultured in SD + His, Trp, and analyzed as in D. F, strains from A containing p180 were analyzed as in B.
Genetic Evidence That Mutations That Weaken 40 S Binding of eIF1 Decrease the Rate of TC Recruitment in Vivo
Previous biochemical findings led to the suggestion that eIF1 stabilizes the open conformation of the 40 S as a means of stimulating the rate of TC loading on the PIC (9). We reasoned that if this mechanism prevails in vivo then the Sui− mutations described above, which appear to weaken 40 S binding by eIF1, should decrease the fraction of 40 S subunits in the open conformation and thereby decrease the rate at which TC re-binds to 40 S subunits engaged in reinitiation on GCN4 mRNA. This would suppress reinitiation at the inhibitory uORFs 2–4 and allow reinitiation further downstream at the GCN4 AUG codon, derepressing GCN4 translation in the absence of eIF2α phosphorylation by Gcn2 (the Gcd− phenotype). Moreover, this Gcd− defect should be overcome by increasing the cellular concentration of TC achieved by overexpressing all three eIF2 subunits and tRNAiMet from a single hc plasmid (hc TC) (11).
Supporting our predictions, the sui1 mutations I3N, K56E, and K60E all evoked derepression of a GCN4-lacZ reporter harboring all four uORFs in a manner diminished by overexpressing TC (Fig. 5D), as would be expected if this Gcd− phenotype results at least partly from a reduced rate of TC binding to reinitiating 40 S subunits that can be rescued at high TC levels by mass action.
If the eIF1 mutations reduce the rate of TC loading by destabilizing the open conformation of the PIC, then their Gcd− phenotypes should be suppressed by overexpressing eIF1 or by the eIF1A 17–21 substitution (7). Consistent with this prediction, overexpression of the I3N, K56E, and K60E mutants from hc plasmids efficiently suppressed the derepression of GCN4-lacZ expression that was evoked by each mutation when expressed from an sc plasmid (Fig. 5E). Moreover, the 17–21 eIF1A mutation also reduced the extent of GCN4-lacZ derepression in cells containing these same eIF1 mutants (Fig. 5F), although not to the same extent observed on their overexpression (Fig. 5E). The relatively greater suppression of the Gcd− phenotypes afforded by overexpressing the eIF1 variants mirrors the findings above that mutant overexpression was more effective than 17–21 in reducing the elevated UUG/AUG ratios. Together, the genetic data support the model that mutations that weaken eIF1 binding to the 40 S subunit destabilize the open PIC conformation in vivo, reducing the rate of TC loading during reinitiation on GCN4 mRNA and also increasing the probability of inappropriate rearrangement to the closed PIC conformation at near-cognate (UUG) codons.
Genetic Evidence That Residues in eIF1 Loop-1 Are Crucial for 40 S Binding by eIF1 in Vivo
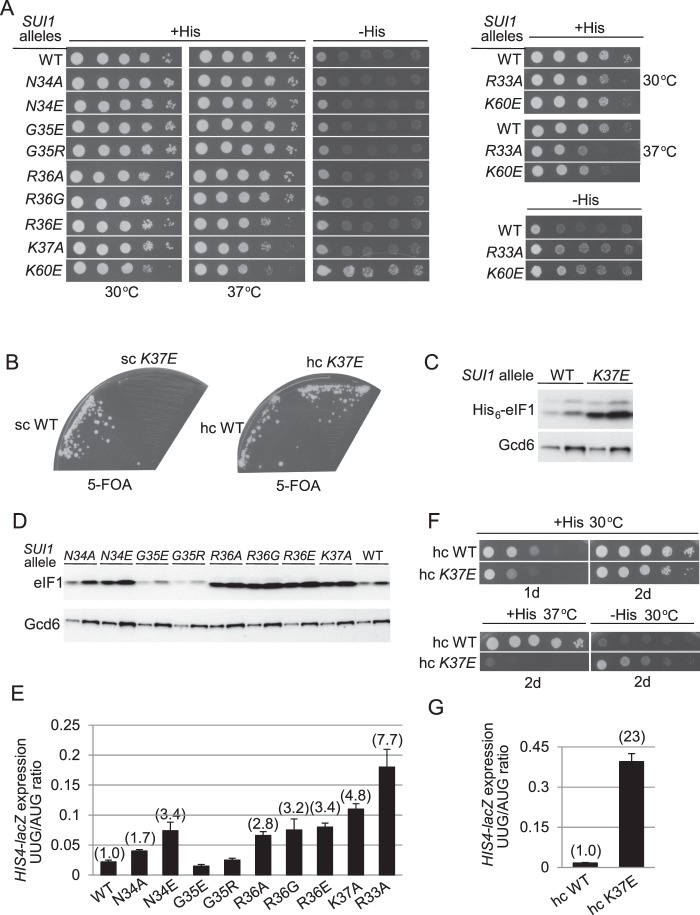
As noted above, Arg-36 corresponds to the residue in loop-1 of Tetrahymena eIF1 (Arg-29) that directly contacts 18 S rRNA in the eIF1·40 S complex (12) (Fig. 2, B and C); however, no substitutions affecting loop-1 have been described. Based on findings above, we predicted that eliminating the positively charged side chain in Arg-36 would impair cell growth and confer a Sui− phenotype. Surprisingly, however, although replacing Arg-36 with acidic Asp produced a modest reduction in growth at 37 °C, the mutant grew indistinguishably from WT on −His medium (suggesting the absence of a Sui− phenotype), and the R36G and R36A substitutions had no mutant phenotypes whatsoever (Fig. 6A, left panel). The same results were obtained for substitutions in residues Asn-34 and Gly-35 located immediately N-terminal of Arg-36, including N34A, N34E, G35R, and G35E (Fig. 6A, left panel). Similarly, Ala substitution of the adjacent basic residue Lys-37 produced a noticeable growth defect at 37 °C, but was His− (Fig. 6A, left panel). However, the K37E replacement is lethal (Fig. 6B, left panel), even though Western analysis of His6-tagged eIF1-K37E revealed a higher than WT level of its expression (Fig. 6C), indicating that Lys-37 carries out an essential eIF1 function.
FIGURE 6.
Substitutions of residues in β-hairpin loop-1 confer Sui− or lethal phenotypes. A, derivatives of sui1Δ his4-301 strain JCY03 with the indicated sc SUI1 alleles were analyzed on +His and −His media and cultured at 30 or 37 °C for 2 days (+His) and at 30 °C for 7 days (−His). B, JCY03 derivatives containing SUI1+ and the indicated His-SUI1 alleles in sc or hc plasmids were streaked on SC-Leu + 5-FOA and incubated at 30 °C for 4 or 5 days. C, JCY03 derivatives containing SUI1+ and the indicated His -SUI1 alleles in sc plasmids were cultured and subjected to Western analysis as in Fig. 3E. D, same strains as in A were cultured and subjected to Western analysis as in Fig. 3C. E, strains from A containing the AUG or UUG HIS4-lacZ reporters were cultured and analyzed as in Fig. 3B. F, derivatives of strain JCY03 with the indicated hc SUI1 alleles analyzed on +His and −His media and cultured at 30 or 37 °C for the indicated days. G, strains from F containing the AUG or UUG HIS4-lacZ reporters were cultured and analyzed as in Fig. 3B.
Interestingly, although none of the substitutions of Arg-36 or nearby residues conferred His+ phenotypes, we found that all but the G35E and G35R variants evoke higher than WT eIF1 protein expression, with the smallest increase observed for the N34A mutant (Fig. 6D). As overexpression is a hallmark of eIF1 Sui− mutants (16), we analyzed the UUG/AUG initiation ratios for these mutants. Except for G35E and G35R, we observed significant increases in UUG initiation for all the loop-1 substitutions (Fig. 6E), which were generally of lesser magnitude than those given by the Lys-56 and Lys-60 substitutions discussed above (Fig. 3B). Moreover, overexpressing the K37E protein restored cell viability (Fig. 6B, right panel), but the mutant cells displayed residual Slg−, temperature sensitivity, and His+/Sui− phenotypes (Fig. 6F), plus a dramatically elevated UUG/AUG initiation ratio (Fig. 6G). These findings support the possibility that reducing the positive charge, or adding a negative charge, in loop-1 decreases 40 S binding of yeast eIF1, with Lys-37 substitutions having the greatest impact.
Although the residue corresponding to Lys-37 of yeast eIF1 is located in strand β2 rather than loop-1 of Tetrahymena eIF1 (residue Lys-30, Fig. 2C) (12), Lys-37 resides within loop-1 of yeast eIF1, which encompasses residues 32QRNGRKT38 (24) and is thus five residues larger than the two-residue loop-1 of Tetrahymena eIF1. Noting that Arg-33 is a third basic residue in loop-1 of yeast eIF1, we extended our analysis to include Ala or Glu substitutions of this residue. Interestingly, the Ala substitution confers strong Slg− and His+/Sui− phenotypes (Fig. 6A, right panel) and a markedly increased UUG/AUG initiation ratio (Fig. 6E). The R33E substitution by contrast is lethal, despite being expressed at higher than WT levels, and further overexpressing it does not restore cell viability (data not shown). Thus, considering that both R33E and K37E are lethal, whereas R36E is viable, it is possible that Arg-33 or Lys-37 carries out the critical role of Arg-29 in Tetrahymena eIF1 of contacting a specific uridine residue in helix 44 of 18 S rRNA (12). Alternatively, examination of the Tetrahymena 40 S·eIF1 structure suggests that all three of these residues could make separate contacts with adjoining rRNA residues (Fig. 2C) (12), in which case contacts made by Arg-33 and Lys-37 of yeast eIF1 could be more critical than that involving Arg-36 (see below).
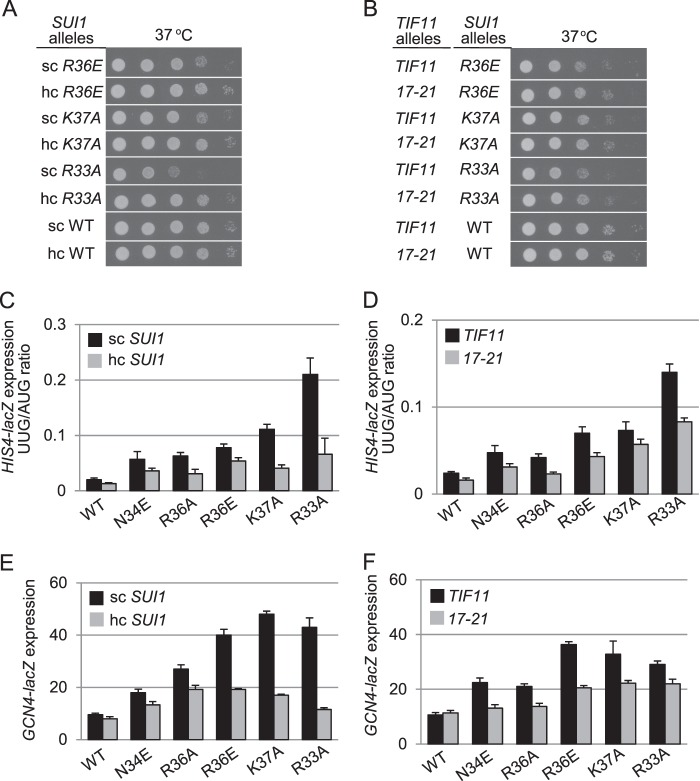
Evidence that altering the loop-1 residues confers Slg− and Sui− phenotypes by weakening 40 S binding by eIF1 came from our finding that overexpression diminished the Slg− phenotypes at 37 °C conferred by the R36E, K37A, and R33A mutations (Fig. 7A) and reduced the elevated UUG/AUG initiation ratios conferred by all of the loop-1 substitutions that confer increased UUG initiation (Fig. 7C). We verified that all of the loop-1 mutant proteins were overexpressed from the hc plasmids (data not shown). Similar results were obtained when the eIF1 mutations were combined with the 17–21 mutation (Fig. 7, B and D), except that suppression by 17–21 was relatively less complete, as observed above for other Sui− mutations. As would be expected for mutations that reduce eIF1 binding to the 40 S subunit, all of the loop-1 mutations also confer Gcd− phenotypes, derepressing GCN4-lacZ expression to an extent that generally correlates with the degree to which they increase the UUG/AUG ratio (cf. Fig. 7, C and E, sc SUI1 lanes), and this phenotype is likewise suppressed by overexpressing the mutant proteins and by the 17–21 mutation (Fig. 7, E and F). These findings provide strong evidence that the basic residues of β-hairpin loop-1 stabilize eIF1 binding to the 40 S subunit in vivo and thereby promote TC recruitment and accurate start codon selection.
FIGURE 7.
Overexpression of the eIF1 mutant protein and the tif11-17-21 mutation in eIF1A reduce the Slg−, Sui−, and Gcd− phenotypes in SUI1 mutant cells. A, derivatives of sui1Δ his4-301 strain JCY03 with the indicated sc or hc SUI1 alleles were grown at 37 °C for 2 days on SC-Leu. B, derivatives of sui1Δ his4-301 PGAL-TIF11 strain PMY03 containing TIF11+ (pPMB76) or tif11-17-21 (pPMB77) with the indicated sc SUI1 alleles were grown at 37 °C for 2 days on SC-Leu-Trp. C and D, strains from A (C) and from B (D) containing the AUG or UUG HIS4-lacZ reporters were cultured and analyzed as in Figs. 3B and 5B. E and F, strains from A (E) and from B (F) harboring plasmid p180 were cultured and analyzed as in Fig. 5, E and F.
Basic Residues in Helix α1 and Loop-1 Are Crucial for Stable eIF1 Binding to 40 S Subunits in Vitro
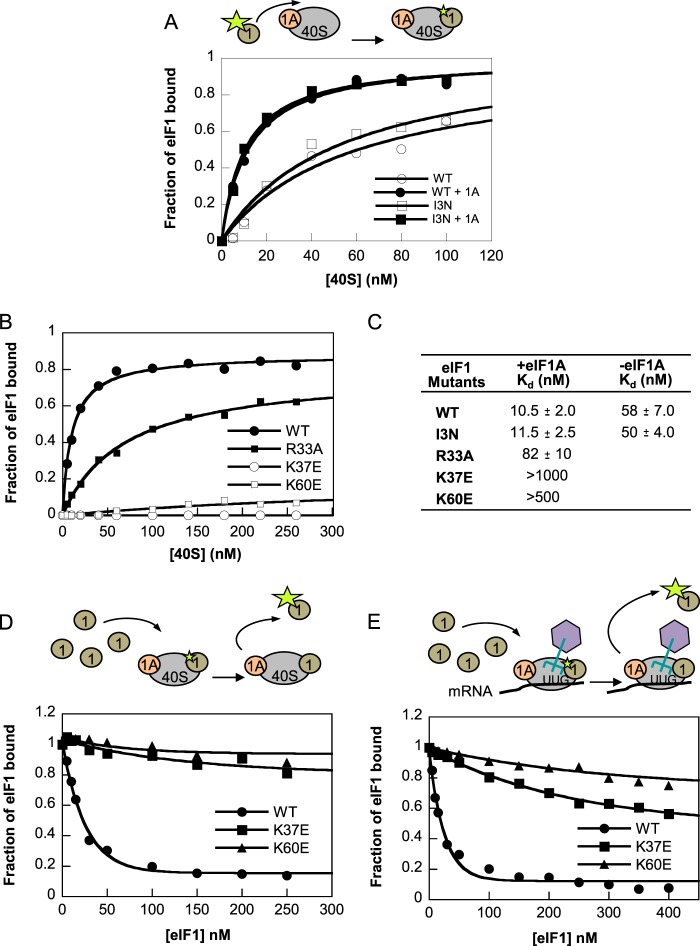
To provide biochemical evidence supporting the conclusion that basic residues in α1 and loop-1 mediate direct binding of yeast eIF1 to 40 S subunits, we measured the 40 S binding affinity of purified, recombinant forms of the eIF1 variants.
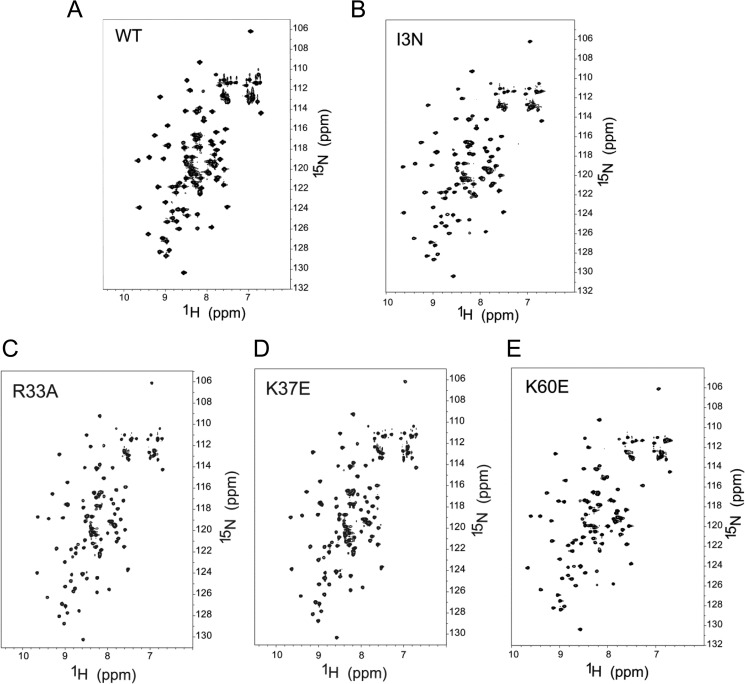
We first assessed whether the mutations perturbed the folding of eIF1 by comparing the dispersion of the chemical shifts of the fingerprint HSQC spectra between WT eIF1 and the mutants I3N, R33A, K37E, and K60E. The spectra for WT and the four eIF1 mutants exhibit very similar 1H and 15N chemical shift patterns, confirming that the tertiary folds of these proteins are nearly identical (Fig. 8, A–E). These results indicate that none of the substitutions produces a notable alteration of the tertiary structure of eIF1.
FIGURE 8.
1H-15N HSQC spectra of 0.2 mm15N-labeled WT (A), I3N (B), R33A (C), K37E (D), and K60E (E) eIF1proteins.
The eIF1 variants were fluorescently labeled on their C termini with fluorescein, and the fraction of eIF1FL bound to 40 S subunits alone or in the presence of a saturating concentration of eIF1A was measured at different concentrations of 40 S subunits by monitoring the change in fluorescence anisotropy. In agreement with previous results, the affinity of WT eIF1 for 40 S subunits was increased by the presence of eIF1A, lowering the Kd value into the low nanomolar range, because of thermodynamic coupling of their association with ribosomes (Fig. 9A) (20). The I3N substitution in the NTT did not significantly alter eIF1 binding to 40 S·eIF1A complexes (Fig. 9A), whereas the R33A mutation in loop-1 increased the Kd value of eIF1 for 40 S subunits in the presence of eIF1A by ∼8-fold (Fig. 9, B and C). The K60E and K37E substitutions in α1 and loop-1 more dramatically reduced the affinity of eIF1 for the 40 S·eIF1A complex, increasing the Kd values in the presence of eIF1A to >500 nm and >1 μm, respectively (Fig. 9, B and C).
FIGURE 9.
Sui− mutations in helix α1 and loop-1 impair binding to 40 S subunits in vitro. A, fluorescein-labeled WT or I3N mutant eIF1 protein was mixed with increasing concentrations of 40 S subunits in the presence or absence of 1 μm eIF1A, and the increase in fluorescence anisotropy was measured. B, binding of fluorescein-labeled WT or the indicated mutant eIF1 proteins to 40 S subunits in the presence of 1 μm eIF1A was measured as in A monitored using fluorescence anisotropy. C, Kd values from A and B calculated from fitting with hyperbolic curves. D, fluorescein-labeled WT eIF1 (5 nm) was pre-bound to 15 nm 40 S subunits in the presence of 1 μm eIF1A, mixed with increasing concentrations of unlabeled WT or mutant eIF1, and the change in anisotropy was measured. E, fluorescein-labeled WT eIF1 (15 nm) was mixed with 120 nm 40 S, 1 μm eIF1A, 150 nm pre-formed TC complex (300 nm eIF2, 150 nm tRNAi, and 1 mm GDPNP), and 10 μm mRNA with UUG start codon, to form 43 S·mRNA (UUG) complexes. Increasing concentrations of unlabeled WT or mutant eIF1 were added, and the change in anisotropy was measured.
To confirm these findings with unlabeled variants, we conducted competition binding assays wherein 40 S·eIF1A·eIF1FL complexes formed with WT eIF1FL were challenged with increasing concentrations of unlabeled WT or mutant eIF1, and the fraction of eIF1FL bound to 40 S·eIF1A complexes was measured at each concentration of competitor. In accordance with the direct binding assays, even at very high concentrations, the K60E and K37E variants were unable to compete with WT eIF1FL for binding to 40 S·eIF1A complexes (Fig. 9D). Finally, we conducted competition binding assays using WT eIF1FL bound to 43 S·mRNA (UUG) complexes, formed with 40 S subunits, eIF1FL, eIF1A, TC, and an unstructured model mRNA with a UUG start codon. In lacking an AUG codon, this 43 S·mRNA (UUG) complex is considered to be a model of the scanning PIC. The results showed that the K60E and K37E mutants could not compete effectively with WT eIF1FL for binding to 43 S·mRNA (UUG) PICs (Fig. 9E). We conclude that conserved basic residues in helix α1 and loop-1 are crucial for stable binding of eIF1 to a reconstituted complex representing the scanning PIC.
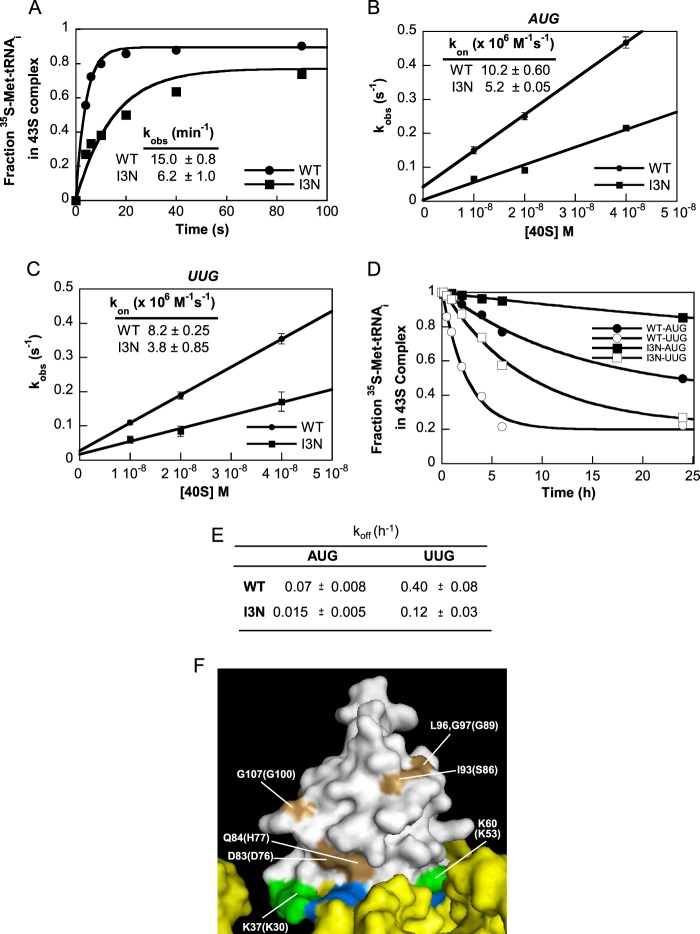
I3N Substitution Impairs Effector Functions of the eIF1 NTT in TC Recruitment and Start Codon Selection
Having found that the I3N substitution does not affect the affinity of eIF1 for the 40 S subunit, we hypothesized that it impaired an important function of the NTT in regulating start codon recognition. We first investigated the effect of I3N on the rate of TC binding to PICs reconstituted with eIF1A, 40 S subunits, a model mRNA with AUG or UUG start codon, and saturating amounts of either WT or mutant eIF1. The TC was assembled with [35S]Met-tRNAiMet, and its binding to the 40 S subunit was monitored by electrophoretic mobility shift (21). As shown in Fig. 10A, the I3N substitution produced an obvious reduction in the observed rate of TC binding to complexes formed with the AUG mRNA, and similar results were observed for the UUG mRNA (data not shown). By measuring the kobs at different 40 S concentrations, we determined that I3N decreased the kon by a factor of 2 for mRNAs containing either AUG or UUG start codons (Fig. 10, B and C). This reduced rate of TC binding is consistent with the Gcd− phenotype of the I3N substitution in vivo.
FIGURE 10.
I3N mutation in the NTT of eIF1 impairs TC recruitment and start codon selection in vitro. A, binding of TC to the 40 S subunits as a function of time measured by a native gel assay as the fraction of [35S]Met-tRNAiMet associated with 40 S subunits in the presence of saturating amounts of eIF1 WT or mutant, eIF1A, and mRNA with an AUG start codon. Values are the averages of three independent experiments. B and C, kinetics of TC binding at several concentrations of 40 S subunit in the presence of mRNA with an AUG (B) or UUG (C) start codons was measured to obtain observed rate constants. The kobs were plotted against the concentration of 40 S subunits and fit linearly to obtain the rate constant of TC binding to 40 S subunits. The kon is the slope of the line. D and E, rate of TC dissociation from 43 S·mRNA complexes formed with [35S]Met-tRNAiMet was determined by adding a chase of excess (≥300-fold) unlabeled TC, and the fraction of labeled TC bound to the 40 S subunits was monitored over time in native gels. Values are the averages of two or three independent experiments. F, surface representation of Tetrahymena eIF1 (shown in gray) bound to the 40 S subunit with the 18 S rRNA shown as a yellow surface and bases that contact eIF1 colored in blue (constructed from PDB file 2XZM). Highlighted in pale orange are residues corresponding to previously analyzed Sui− substitutions, with the Tetrahymena residues listed in parentheses, including substitutions Q84P and D83G (13), G107R,G107K (15, 28), and the I93A,L96A,G97A substitutions in mutant 93–97 (5). Colored green at the 40 S interface are the basic residues in helix α1 and loop-1 analyzed here.
We also examined the stability of TC binding to the PIC by measuring the rate of dissociation of 35S-labeled TC from pre-formed 43 S·mRNA complexes using an excess of unlabeled TC as a “chase.” Consistent with previous results (21), the rate of TC dissociation is substantially lower with an AUG versus UUG start codon in the mRNA, with koff values of 0.07 and 0.40 h−1 at AUG and UUG, respectively (Fig. 10, D and E). This difference is thought to reflect the greater stability of the PIN state of TC binding with an AUG versus the near-cognate UUG start codon. Interestingly, I3N considerably decreases koff for both start codons (Fig. 10, D and E). The increased stability of the PIN state at the UUG codon inferred from these data is likely instrumental in the increased UUG initiation rate conferred by the I3N substitution in vivo. Perhaps the increased stability of the AUG complex for the I3N mutant would not similarly increase the initiation rate at AUG codons because the PIN state at AUG for WT eIF1 is already optimal for efficient initiation.
DISCUSSION
In this study, we have exploited the crystal structure of the Tetrahymena 40 S·eIF1 complex (12) to conduct a structure-guided analysis of eIF1's contacts with the 40 S subunit. The results identify three lysines on the same face of helix α1 and three basic residues in β-hairpin loop-1 as being crucial for eIF1 function in vivo and for robust 40 S binding by eIF1 in vitro. Our analysis further indicates that tight eIF1 binding to the 40 S subunit mediated by these conserved residues is required for both rapid recruitment of TC by the PIC and to prevent inappropriate eIF1 dissociation and rearrangement to the closed PIC conformation at non-AUG codons. These results provide strong support for the dual function of eIF1 in promoting PIC assembly and accurate start codon recognition, and they indicate that the 40 S contacts observed in the Tetrahymena eIF1·40 S crystal structure are critical in vivo for eIF1's key activities in translation initiation.
Surprisingly, there was little previous evidence that these highly conserved basic amino acids are important for eIF1 function, as most studies had implicated residues in helix α2 or the extreme C terminus of yeast eIF1 in 40 S binding and start codon recognition in vivo (Fig. 10F) (4, 5, 13, 15). However, it was reported that simultaneously substituting all five basic residues in helix α1 with alanines is lethal and reduces eIF1 association with native PICs (24), and we had shown that individually substituting two of these residues, Lys-56 and Lys-60, with glutamate confers a Sui− phenotype in vivo (16). Here, we thoroughly examined the consequences of various substitutions of Lys-59 and Lys-60, whose counterparts in Tetrahymena eIF1 (Lys-52 and Lys-53) make direct contacts with rRNA in the 40 S·eIF1 complex (Fig. 2, B and C), as well as mutations in the nearby α1 residue Lys-56. Substitutions of Lys-56 or Lys-60 with Glu or Asp and Lys-60 with Ala confer Slg− and/or Sui− phenotypes that can be mitigated by overexpressing the mutant proteins or by the 17–21 substitution in eIF1A, shown previously to reduce the rate of eIF1 dissociation on start codon recognition in reconstituted PICs (5). (See Table 1 for a summary of genetic and biochemical analyses of representative eIF1 variants.) Furthermore, a K56E,K60E double substitution is lethal, and although cell growth is rescued by overexpressing the double mutant or by eIF1A 17–21, the viable cells display residual strong Slg− and Sui− phenotypes. In vitro analysis provided direct evidence that the K60E substitution dramatically decreased the affinity of recombinant eIF1 for 40 S·eIF1A and 43 S·mRNA (UUG) complexes (Table 1), the latter representing a model of the scanning PIC. Interestingly, substitutions of Lys-59 with Glu, Asp, or even Ala are lethal, despite higher than WT expression levels, and only the K59A variant could be rescued by overexpression or by the eIF1A 17–21 suppressor in a manner associated with residual Slg− and Sui− phenotypes. These results indicate that Lys-56, Lys-59, and Lys-60 all participate in physiologically important interactions of eIF1 helix α1 with the 40 S subunit, with Lys-59 apparently making the most important contact.
TABLE 1.
Summary of results of genetic and biochemical assays of representative eIF1 mutants
| eIF1 variant | Location of substitution | Growth |
Increase in UUG/AUG initiation (Sui−)a | Suppression of Sui− by |
Increase in GCN4-lacZ expression (Gcd−)b | Suppression of Gcd− by |
Binding to 40 S, Kd (nm) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| +His | −His | hc mutant | tif11-17–21 | hc mutant | tif11-17–21 | |||||
| WT | NAc | + | − | 1 | NA | NA | 1 | NA | NA | 10.5 |
| I3N | NTT | Slg− | + | ∼4.0 | ∼50% | ∼25% | ∼3.0 | ∼55% | ∼20% | 11.5 |
| K60E | Helix α1 | Slg− | + | ∼5.5 | ∼60% | ∼40% | ∼4.5 | ∼60% | ∼40% | >500 |
| R33A | Loop-1 | Slg− | + | ∼7.5 | ∼70% | ∼40% | ∼4.5 | ∼70% | ∼25% | 82 |
| K37E | Loop-1 | Leth. | Leth. | NDd | ND | ND | ND | ND | ND | >1000 |
a Values are means normalized to the WT value obtained by assaying matched HIS4-lacZ fusions harboring AUG or UUG start codons.
b Values are means normalized to the WT value.
c NA means not applicable.
d ND means not determined, as K37E is lethal.
Our analysis of conserved basic residues in loop-1 provides the first evidence that this β-hairpin provides a second critical contact between yeast eIF1 and the 40 S subunit and is crucial for eIF1's functions in vivo. We were surprised to find that even the Glu substitution of Arg-36, whose counterpart in Tetrahymena eIF1 (Arg-29) contacts rRNA (Fig. 2, B and C), did not produce a strong Slg− or Sui− phenotype and that Ala, Gly, and Glu substitutions at this residue all conferred a similar moderate (∼3-fold) increase in the UUG/AUG initiation ratio and elevated expression of eIF1. However, when we expanded the mutagenesis to include other loop-1 residues, we found that Glu substitutions of the adjacent amino acid Lys-37 and nearby residue Arg-33 are lethal and that even the Ala substitution of Arg-33 confers Slg− and strong His+/Sui− phenotypes. Moreover, overexpressing the K37E variant rescues its lethality but leaves intact residual Slg− and Sui− phenotypes. Our findings that the Slg− and elevated UUG/AUG ratios conferred by various substitutions in Arg-33, Arg-36, and Lys-37 are diminished by overexpressing the eIF1 mutants and by the eIF1A-17–21 variant indicate that they too derive from reduced 40 S binding of the eIF1 variants in vivo (Table 1). Supporting this interpretation, K37E and R33A impaired binding of recombinant eIF1 to reconstituted 40 S·eIF1A complexes in vitro. Hence, we propose that a positively charged surface comprised of all three basic residues in the seven-residue loop-1 of yeast eIF1 interacts with 18 S rRNA and that Arg-33 and Lys-37 make more critical 40 S contacts than does Arg-36.
Considering that loop-1 of Tetrahymena eIF1 is predicted to clash with the ASL of Met-tRNAiMet when the latter is base-paired with AUG in the P-site (12), it is possible that the Ala substitutions of Arg-33, Arg-36, or Lys-37 diminish this clash. This would contribute to the Sui− phenotypes of these variants by removing an impediment to the PIN state at UUG codons. Consistent with this possibility, the R33A substitution in loop-1 evokes a greater increase in UUG/AUG ratio than does K60E in α1 even though the latter produces a much larger reduction in eIF1 binding to 40 S·eIF1A complexes in vitro (Table 1). Hence, we propose that Arg-33 both supports eIF1 binding to the 40 S subunit and also impedes canonical binding of Met-tRNAiMet to the P site at non-AUG codons.
Biochemical analysis in the reconstituted system suggested that TC binds rapidly only to the open conformation of the PIC stabilized by eIF1 but that eIF1 dissociation on AUG recognition allows the TC to rearrange from a less stable (POUT) mode of 40 S binding to a more stable state with the ASL more fully accommodated in the P site (PIN) (7, 9). Our genetic analysis provides strong support for this dual function of eIF1, as α1 and loop-1 substitutions that weaken eIF1 contacts with the 40 S subunit in vitro also reduce the rate of TC loading on 40 S subunits in vivo, evoking the Gcd− phenotype in a manner roughly correlated with the increased UUG initiation they confer in yeast cells. The decreased rate of TC loading is the predicted consequence of reduced eIF1 occupancy of 40 S subunits and the attendant destabilization of the open conformation to which TC most rapidly binds. Furthermore, the observed co-suppression of the Gcd− and Sui− phenotypes of these eIF1 mutants by their overexpression, or by the 17–21 substitution in the eIF1A NTT, is the predicted consequence from our model of restoring higher 40 S occupancy for these eIF1 variants (5, 6).
We found that the I3N substitution in the eIF1 NTT confers both Sui− and Gcd− phenotypes. Hydrophobic residues in the eIF1 NTT have been implicated in eIF1 binding to segments of eIF5 and eIF2β (24), and NTT substitutions have been shown to confer a Gcd− phenotype by reducing the rate of TC loading in vitro (5), as observed here for I3N, or by reducing AUG recognition during reinitiation (24). However, our results are the first to implicate the eIF1 NTT in controlling the fidelity of start codon recognition. Both the Sui− and Gcd− phenotypes of I3N are diminished by overexpressing the mutant protein or by introducing eIF1A-17–21, as would be expected from reduced eIF1 affinity for the 40 S subunit; however, eIF1-I3N shows no defect in binding to 40 S·eIF1A complexes in vitro (Table 1). This suggests that the unstructured eIF1 NTT might perform an “effector” function on the 40 S subunit analogous to that of the unstructured CTT of eIF1A, stabilizing the open conformation and blocking rearrangement to the closed state of the PIC (7). The I3N substitution would weaken this function and destabilize the open conformation, reducing the rate of TC binding, while also allowing transition to the closed state at UUG codons.
Supporting this last interpretation, we found that the eIF1-I3N variant at saturating concentrations decreases the kon for TC in reconstituted 43 S·mRNA PICs, in accordance with its Gcd− phenotype. It also decreases the off-rate of TC from reconstituted PICs (koff), indicating stabilization of the PIN conformation, consistent with its Sui− phenotype. Presumably, these defects can be mitigated at least partially by increasing the concentration of eIF1-I3N in the cell or by stabilizing its 40 S interaction by eIF1A-17–21, to produce abnormally high 40 S occupancy of this eIF1 variant. This would be akin to the fact that overexpressing WT eIF1 can suppress Sui− mutations of various other initiation factors (1, 2) and reduce derepression of GCN4 translation in WT cells evoked by eIF2α phosphorylation and diminished TC assembly.4
It is intriguing that the structured portion of the eIF1 NTT visible in the 40 S·eIF1 crystal structure (residues 13–18 of Tetrahymena eIF1, Fig. 2A) is also predicted to clash with the acceptor helix of Met-tRNAi when bound to the 40 S subunit in its canonical P site orientation (12).5 Although I3N alters a nonstructured (flexible) portion of the eIF1 NTT not visible in the crystal structure, it seems possible that this substitution alters the conformation of the NTT in a manner that reduces the clash with Met-tRNAi as a way of facilitating the rearrangement from POUT to PIN at UUG codons, just as we proposed above for the R33A substitution in loop-1.
Supplementary Material
Acknowledgments
We thank Tom Dever for many helpful suggestions during the course of this work, and we are grateful to Felix Voigts-Hoffmann and Nenad Ban for providing important details about the predicted clash between eIF1 and Met-tRNAi in the PIC.
This work was supported, in whole or in part, by National Institutes of Health Grants GM62128 (to J. R. L.) and CA068262 (to G. W.) and by the Intramural Research Program (to P. M. M. and A. G. H.).
This article was selected as a Paper of the Week.

This article contains supplemental Tables S1–S3 and additional references.
P. Martin-Marcos and A. G. Hinnebusch, unpublished observations.
F. Voigts-Hoffmann and N. Ban, personal communication.
- PIC
- preinitiation complex
- TC
- ternary complex
- WCE
- whole cell extract
- CTT
- C-terminal tail
- NTT
- N-terminal tail
- SE
- scanning enhancer
- 5-FOA
- 5-fluoroorotic acid
- ASL
- anticodon stem-loop
- SI
- scanning inhibitor
- sc
- single copy
- lc
- low copy
- hc
- high copy
- HSQC
- heteronuclear single quantum coherence
- GDPNP
- guanosine 5′-[β,γ-imido]triphosphate.
REFERENCES
- 1. Pestova T. V., Lorsch J. R., Hellen C. U. T. (2007) in Translational Control in Biology and Medicine (Mathews M., Sonenberg N., Hershey J. W., eds) pp. 87–128, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 2. Hinnebusch A. G. (2011) Molecular mechanism of scanning and start codon selection in eukaryotes. Microbiol. Mol. Biol. Rev. 75, 434–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Algire M. A., Maag D., Lorsch J. R. (2005) Pi release from eIF2, not GTP hydrolysis, is the step controlled by start-site selection during eukaryotic translation initiation. Mol. Cell 20, 251–262 [DOI] [PubMed] [Google Scholar]
- 4. Valásek L., Nielsen K. H., Zhang F., Fekete C. A., Hinnebusch A. G. (2004) Interactions of eukaryotic translation initiation factor 3 (eIF3) subunit NIP1/c with eIF1 and eIF5 promote preinitiation complex assembly and regulate start codon selection. Mol. Cell. Biol. 24, 9437–9455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cheung Y. N., Maag D., Mitchell S. F., Fekete C. A., Algire M. A., Takacs J. E., Shirokikh N., Pestova T., Lorsch J. R., Hinnebusch A. G. (2007) Dissociation of eIF1 from the 40 S ribosomal subunit is a key step in start codon selection in vivo. Genes Dev. 21, 1217–1230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fekete C. A., Mitchell S. F., Cherkasova V. A., Applefield D., Algire M. A., Maag D., Saini A. K., Lorsch J. R., Hinnebusch A. G. (2007) N- and C-terminal residues of eIF1A have opposing effects on the fidelity of start codon selection. EMBO J. 26, 1602–1614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Saini A. K., Nanda J. S., Lorsch J. R., Hinnebusch A. G. (2010) Regulatory elements in eIF1A control the fidelity of start codon selection by modulating tRNAiMet binding to the ribosome. Genes Dev. 24, 97–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Alone P. V., Cao C., Dever T. E. (2008) Translation initiation factor 2γ mutant alters start codon selection independent of Met-tRNA binding. Mol. Cell. Biol. 28, 6877–6888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Passmore L. A., Schmeing T. M., Maag D., Applefield D. J., Acker M. G., Algire M. A., Lorsch J. R., Ramakrishnan V. (2007) The eukaryotic translation initiation factors eIF1 and eIF1A induce an open conformation of the 40 S ribosome. Mol. Cell 26, 41–50 [DOI] [PubMed] [Google Scholar]
- 10. Yu Y., Marintchev A., Kolupaeva V. G., Unbehaun A., Veryasova T., Lai S. C., Hong P., Wagner G., Hellen C. U., Pestova T. V. (2009) Position of eukaryotic translation initiation factor eIF1A on the 40 S ribosomal subunit mapped by directed hydroxyl radical probing. Nucleic Acids Res. 37, 5167–5182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hinnebusch A. G. (2005) Translational regulation of GCN4 and the general amino acid control of yeast. Annu. Rev. Microbiol. 59, 407–450 [DOI] [PubMed] [Google Scholar]
- 12. Rabl J., Leibundgut M., Ataide S. F., Haag A., Ban N. (2011) Crystal structure of the eukaryotic 40 S ribosomal subunit in complex with initiation factor 1. Science 331, 730–736 [DOI] [PubMed] [Google Scholar]
- 13. Yoon H. J., Donahue T. F. (1992) The sui1 suppressor locus in Saccharomyces cerevisiae encodes a translation factor that functions during tRNAiMet recognition of the start codon. Mol. Cell. Biol. 12, 248–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lomakin I. B., Kolupaeva V. G., Marintchev A., Wagner G., Pestova T. V. (2003) Position of eukaryotic initiation factor eIF1 on the 40 S ribosomal subunit determined by directed hydroxyl radical probing. Genes Dev. 17, 2786–2797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nanda J. S., Cheung Y. N., Takacs J. E., Martin-Marcos P., Saini A. K., Hinnebusch A. G., Lorsch J. R. (2009) eIF1 controls multiple steps in start codon recognition during eukaryotic translation initiation. J. Mol. Biol. 394, 268–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Martin-Marcos P., Cheung Y. N., Hinnebusch A. G. (2011) Functional elements in initiation factors 1, 1A, and 2β discriminate against poor AUG context and non-AUG start codons. Mol. Cell. Biol. 31, 4814–4831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Moehle C. M., Hinnebusch A. G. (1991) Association of RAP1-binding sites with stringent control of ribosomal protein gene transcription in Saccharomyces cerevisiae. Mol. Cell. Biol. 11, 2723–2735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Reid G. A., Schatz G. (1982) Import of proteins into mitochondria. Yeast cells grown in the presence of carbonyl cyanide m-chlorophenylhydrazone accumulate massive amounts of some mitochondrial precursor polypeptides. J. Biol. Chem. 257, 13056–13061 [PubMed] [Google Scholar]
- 19. Acker M. G., Kolitz S. E., Mitchell S. F., Nanda J. S., Lorsch J. R. (2007) Reconstitution of yeast translation initiation. Methods Enzymol. 430, 111–145 [DOI] [PubMed] [Google Scholar]
- 20. Maag D., Lorsch J. R. (2003) Communication between eukaryotic translation initiation factors 1 and 1A on the yeast small ribosomal subunit. J. Mol. Biol. 330, 917–924 [DOI] [PubMed] [Google Scholar]
- 21. Kolitz S. E., Takacs J. E., Lorsch J. R. (2009) Kinetic and thermodynamic analysis of the role of start codon/anticodon base pairing during eukaryotic translation initiation. RNA 15, 138–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Marintchev A., Frueh D., Wagner G. (2007) NMR methods for studying protein-protein interactions involved in translation initiation. Methods Enzymol. 430, 283–331 [DOI] [PubMed] [Google Scholar]
- 23. Luna R. E., Arthanari H., Hiraishi H., Nanda J., Martin-Marcos P., Markus M. A., Akabayov B., Milbradt A. G., Luna L. E., Seo H. C., Hyberts S. G., Fahmy A., Reibarkh M., Miles D., Hagner P. R., O'Day E. M., Yi T., Marintchev A., Hinnebusch A. G., Lorsch J. R., Asano K., Wagner G. (2012) The C-terminal domain of eukaryotic initiation factor 5 promotes start codon recognition by its dynamic interplay with eIF1 and eIF2β. Cell Rep. 1, 689–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Reibarkh M., Yamamoto Y., Singh C. R., del Rio F., Fahmy A., Lee B., Luna R. E., Ii M., Wagner G., Asano K. (2008) Eukaryotic initiation factor (eIF) 1 carries two distinct eIF5-binding faces important for multifactor assembly and AUG selection. J. Biol. Chem. 283, 1094–1103 [DOI] [PubMed] [Google Scholar]
- 25. Boeke J. D., Trueheart J., Natsoulis G., Fink G. R. (1987) 5-Fluoroorotic acid as a selective agent in yeast molecular genetics. Methods Enzymol. 154, 164–175 [DOI] [PubMed] [Google Scholar]
- 26. Hinnebusch A. G., Lorsch J. R. (2012) The mechanism of eukaryotic translation initiation: new insights and challenges. Cold Spring Harbor Perspect. Biol. 4, a011544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nanda J. S., Saini A. K., Muñoz A. M., Hinnebusch A. G., Lorsch J. R. (2013) Coordinated movements of eukaryotic translation initiation factors eIF1, eIF1A, and eIF5 trigger phosphate release from eIF2 in response to start codon recognition by the ribosomal pre-initiation complex. J. Biol. Chem. 288, 5316–5329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cui Y., Dinman J. D., Kinzy T. G., Peltz S. W. (1998) The Mof2/Sui1 protein is a general monitor of translational accuracy. Mol. Cell. Biol. 18, 1506–1516 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.