Abstract
Only a few decades ago, students of the pathophysiology of cardiovascular disease paid little heed to the involvement of inflammation and immunity. Multiple lines of evidence now point to the participation of innate and adaptive immunity and inflammatory signaling in a variety of cardiovascular conditions. Hence, interest has burgeoned in this intersection. This review will focus on the contribution of innate immunity to both acute injury to the heart muscle itself, notably myocardial infarction, and to chronic inflammation in the artery wall, namely atherosclerosis, the cause of most myocardial infarctions. Our discussion of the operation of innate immunity in cardiovascular diseases will focus on functions of the mononuclear phagocytes with special attention to emerging data regarding the participation of different functional subsets of these cells in cardiovascular pathophysiology.
Keywords: myocardial infarction, atherosclerosis, acute coronary syndromes, inflammation, mononuclear phagocytes, innate immunity
Monocyte heterogeneity and the response to acute myocardial injury
Myocardial infarction is the most frequent cause of acute myocardial injury. This disease has taken on global proportions with increased longevity in the developing world and an augmented prevalence of risk factors for developing coronary heart disease worldwide [1, 2]. Myocardial infarction results from ischemic injury, most often due to interruption of blood supply to the heart muscle due to thrombotic or embolic occlusion of coronary arteries. The myocardium has particular susceptibility to ischemic injury due to the high oxygen demands occasioned by its incessant beating and preference for fatty acid substrates for energy metabolism.
According to traditional concepts, the interruption of coronary blood flow unleashes an inevitable cascade of events leading to death of the tissue perfused by the affected segment of the coronary arterial bed. We now recognize that the consequence of a given coronary occlusion is not “all or nothing” but can vary in ways that correlate with clinical outcomes [3]. Expansive remodeling of the left ventricle predicts poor long-term outcomes [4]. Such remodeled ventricles often pump ineffectively, lead to functional mitral regurgitation, and to the syndrome of heart failure, a major impediment to quality of life and drain on healthcare resources. Recognition of mutability of responses to myocardial ischemic injury has heightened focus on the healing response that occurs after the initial ischemic insult.
In a pioneering description of the sequence of events occurring in infarcted myocardium, Mallory and colleagues described an early polymorphonuclear leukocyte infiltrate occurring one to two days following onset of the symptoms of acute myocardial infarction [5]. They described a second phase in the ensuing days of mononuclear phagocyte infiltration, which preceded the phase of formation of granulation tissue. The key characteristics of granulation tissue formation include fibrosis, notably deposition of interstitial collagen, and new formation of microvessels in the healing myocardium. This predictable sequence of events prevailed as the major model of myocardial healing from the 1930s until a few years ago.
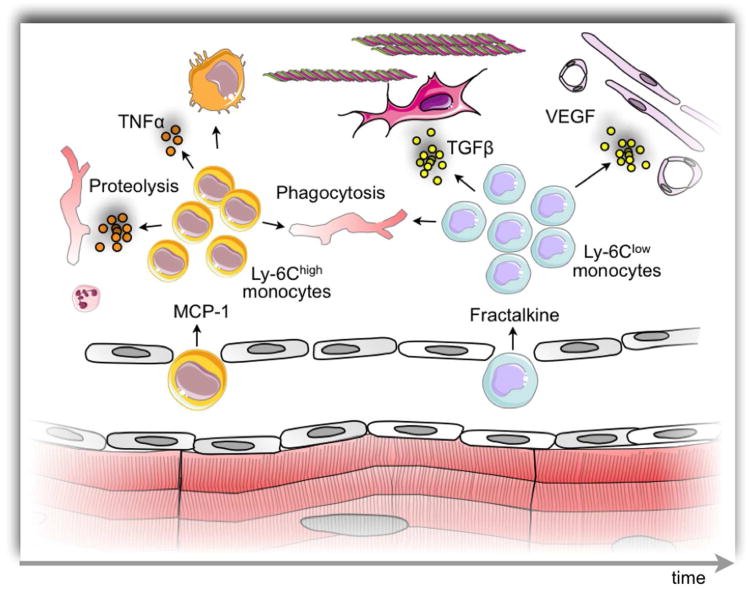
With the emerging recognition of monocyte heterogeneity, our group tested the hypothesis that different phases of the leukocytic infiltration in response to coronary artery occlusion would involve different functional subtypes of mononuclear phagocytes [6]. Indeed, after coronary artery occlusion in mice, we noted a first wave of accumulation of particularly pro-inflammatory monocytes identified by expression of high levels of the surface marker Ly-6C (sometimes referred to as Gr-1, which recognizes Ly-6C and the granulocytic marker Ly-6G), followed by a second wave of less inflammatory monocytes expressing low levels of Ly-6C (Fig. 1). We focused on the functional characteristics of these monocytes in the context of myocardial healing. The Ly-6Chigh subset expressed high levels of protease activity and phagocytosis. These pro-inflammatory monocytes also elaborated high levels of pro-inflammatory cytokines such as tumor necrosis factor-alpha (TNF-α) that could sustain and amplify inflammatory responses by recruiting more leukocytes to the healing myocardium via heightened expression of adhesion molecules and chemokines. The proteolytic enzymes released by the pro-inflammatory monocytes could catabolize contractile proteins and other contents of dead cells in the infarcted myocardium. The phagocytic capacity of this monocyte subset could promote the clearance of the debris that accumulates due to myocardial cell death [6].
Figure 1.
Biphasic monocyte response after myocardial ischemic injury. The cartoon depicts temporally resolved recruitment of monocyte subsets. First, on the left side of the cartoon, Ly-6Chigh monocytes are recruited to the infarct via the MCP-1/CCR2 axis. These cells are inflammatory, have a high TNFα produciton and predominantly support removal of debris using their proteolytic and phagocytic capabilities. These cells may also differentiate into macrophages. Several days later, the infarct tissue switches to secreting fractalkine, which attracts Ly-6Clow monocytes. This monocyte subset is also phagocytic but less inflammatory, and supports repair processes such as angiogenesis and new extracellular matrix production.
The subsequent wave of the reparative Ly-6Clow monocytes also exhibits functions that could prove critical in determining the healing response in the infarcted myocardium. This lessinflammatory subset shows lower expression of pro-inflammatory cytokines than their Ly-6Chigh counterparts. Thus, the positive stimulus to amplification of the local inflammatory response by recruitment of additional monocytes would decline. Moreover, monocytes of the Ly-6Clow subset elaborate higher levels of transforming growth factor-beta (TGF-β) family members, mediators known to attenuate inflammatory responses in the myocardium. TGF-β also stimulates fibrosis by strongly augmenting collagen production by myocardial fibroblasts. Generation of interstitial collagen could influence the healing response in important ways. Enhanced collagen production could yield a more durable scar with augmented tensile strength that could resist ventricular septal defects, pseudo-aneurysm formation, or rupture of the free wall, much feared complications of myocardial infarction. Moreover, a more robust fibrotic response could limit adverse geometric remodeling of the left ventricle characterized by scar expansion. Such expansive remodeling associates with the development of heart failure and poor clinical prognosis as mentioned above. The Ly-6Clow monocyte subset also expresses high levels of angiogenic growth factors such as vascular endothelial growth factor (VEGF). Local VEGF expression could promote the proliferation of neovessels, a defining constituent of granulation tissue that characterizes the intermediate phase of healing of injured tissues and wounds including the infarcted myocardium [6].
The biphasic recruitment of monocyte subsets to the infarcted myocardium raises the question whether monocyte subsets differentiate to distinct macrophage populations. In vitro, so-called classical activation of macrophages involves the stimulation of bone marrow cells with IFNγ or LPS. Macrophages derived in such a way secrete inflammatory mediators and have been termed M1. Macrophages generated with combinations of IL-4, IL-10, or IL-13 exhibit less inflammatory functions. This “alternative activation” strategy, a term coined by Siamon Gordon, yields so-called M2 macrophages [7, 8]. Many extrapolate this simple demarcation to what may occur in vivo. As Mosser and Edwards [9] and many others have correctly pointed out, in vivo macrophage biology likely involves a continuum that spans the M1 and M2 extremes (and “M3”, “4”, “5” macrophages may also exist). Macrophages are highly adaptable cells that respond to a multitude of stimuli and, in principle, may fall into as many “subsets” as combinations of stimuli. That said, the question remains whether Ly-6Chigh monocytes preferentially give rise to M1-enriched macrophages while Ly-6Clow monocytes preferentially develop into M2-type macrophages in the infarcted myocardium. Although available data do not illuminate what happens in the heart, other models of injury provide clues. The emerging picture positions Ly-6Chigh monocytes as the precursors of macrophages that can polarize toward different functions in the tissue depending on the timing of their accumulation and tissue context. Ly-6Clow monocytes may either remain as monocytes or differentiate to specific resident macrophages that re-populate the tissue after inflammation subsides [10-12]. The macrophage response in the infarcted myocardium, which also starts with an inflammatory M1 macrophage phenotype and evolves over time towards an M2 phenotype [13, 14], likely reflects a combination of sequential monocyte subset accumulation and in situ macrophage polarization.
The accumulation of the pro-inflammatory subset of monocytes in the first days following coronary occlusion begs the question of their origin. Monocytes classically arise in the bone marrow from their hematopoietic progenitors. The rapid appearance of monocytes shortly after myocardial infarction suggested that either the bone marrow dramatically heightens its monocyte output or that reservoirs of ready-made monocytes exist that can supplement the increased demand. The spleen serves as such a reservoir in mice [15]. The subcapsular pulp of the mouse spleen contains a population of monocytes that resemble circulating monocytes phenotypically and morphologically. The spleen, a secondary lymphoid organ and a filter of blood, contains various sessile macrophage and dendritic cell populations. The identification of undifferentiated monocytes suggested a reservoir that could be rapidly mobilized to sites of distant injury or infection. Indeed, this pre-existing pool mobilizes in response to coronary artery ligation. Intravital microscopy provided direct evidence for departure of these cells. Experimental depletion of the splenic pool compromised myocardial healing in mice. While the bone marrow remains the dominant source of monocytes, the splenic reservoir, these studies showed, can contribute a sizeable population of inflammatory monocytes.
Studies aiming at identifying a mechanism by which the reservoir is mobilized focused on angiotensin II [16]. The hormone, which is released after MI, activates splenic monocytes via the A1 receptor [15]. Activation of this receptor renders splenic monocytes more motile, and thus more likely to exit the splenic parenchyma and enter the circulation [15]. This observation has particular clinical importance because treatment with inhibitors of angiotensin converting enzyme (ACE), the major generator of active angiotensin II in vivo, can attenuate expansive remodeling of the myocardium in rodents and in humans. Large-scale clinical trials have demonstrated improved outcomes in patients treated with ACE inhibitors following myocardial infarction [17, 18]. Thus, our experimental observations in mice [16] focusing on monocytes in the healing of acute myocardial infarctions provide new mechanistic insight into the clinical benefits of this therapeutic intervention. Moreover, this example provides an illustration of targeting supply or functional attributes of monocytes in therapeutic manipulation of the tissue response to myocardial ischemia. Of note, it is currently unclear if ACE inhibitors target all monocyte subsets indiscriminately. If prolonged recruitment of inflammatory monocytes impairs resolution of inflammation and infarct healing, as shown in mice with atherosclerosis and blood monocytosis [19], then targeting the inflammatory monocyte subset could prove beneficial. Because the recruitment of inflammatory monocytes depends on interaction of MCP-1 with CCR2 [6, 20], this axis has interest as a therapeutic target for dampening inflammation in the cardiac wound. Indeed, silencing CCR2 in monocytes with nanoparticle-enabled in vivo RNAi reduces their recruitment after ischemia-reperfusion injury and into atherosclerotic lesions [21]. Future studies should explore whether modulation of monocyte subset recruitment or macrophage polarization can promote beneficial infarct healing.
The existence of the preformed pool of pro-inflammatory leukocytes in the spleen raises the question of their origin. In the steady state, the de novo generation of monocytes in the bone marrow continuously replenishes this splenic reservoir. Bone marrow “monocytes” (identified as CD11b+CD115+F4/80low cells) are almost exclusively Ly-6Chigh. Many of these cells proliferate, however, and tend to have a band-shaped, rather than a kidney or horseshoe-shaped nucleus. In these ways, bone marrow monocytes display features of their precursors, and may not yet be “true” monocytes; CD11b+CD115+F4/80low cells become monocytes when they enter the blood, and can retain this status when they settle in the spleen. In the steady state, at any given time, the spleen contains substantially more monocytes than are found in the blood [15]. The question then arises: what happens to the reservoir once it is depleted after myocardial infarction? Experiments have shown that local hematopoiesis in the spleen largely mediates replenishment of this reservoir after MI [22]. In response to MI, hematopoietic stem cell progenitors mobilize from the bone marrow, seed the spleen, and give rise to monocytes and neutrophils. The process likely requires a large number of mediators, IL-1β among them [22]. This repopulation of the splenic reservoir goes beyond simple monocyte replenishment but also contributes to the accumulation of monocytes in the myocardium later in the response. The high turnover of monocytes in the myocardium causes a high demand. Thus, extramedullary hematopoiesis not only provides a mechanism by which the reservoir replenishes, but also contributes essentially to meeting the heightened leukocyte demand during inflammation.
Monocyte heterogeneity in chronic cardiovascular disease, the case of atherosclerosis
Atherosclerotic cardiovascular disease causes an increasing burden of morbidity and mortality worldwide, one of the major causes of loss of useful life years globally. Atherosclerosis causes most myocardial infarctions, as described above. In addition, atherosclerosis causes many ischemic strokes, events that can impair communication, mobility, and the ability to live independently, hence constituting a major clinical challenge. Atherosclerosis of the peripheral arteries commonly causes debilitating intermittent claudication and limb ischemia that can lead to gangrene and commonly require amputations, particularly in people with diabetes.
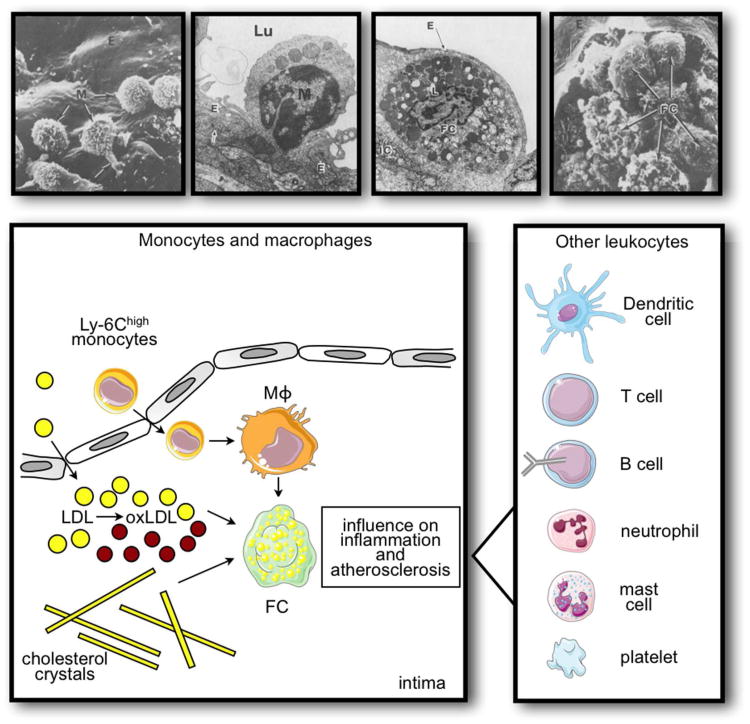
Initially conceived of as a passive accumulation of waxy cholesterol deposits in the arterial wall, we now recognize the participation of both innate and adaptive immune responses in this disease. Mononuclear phagocytes comprise the vast majority of the inflammatory cells in the atherosclerotic plaques. These cells imbibe lipids, become engorged with lipid droplets, and form foam cells, the hallmark of the atherosclerotic lesion (Fig. 2). Among the risk factors for atherosclerosis, hypercholesterolemia has an indubitable causal role.
Figure 2.
Leukocytes in atherosclerotic plaque. The scanning electron and transmission electron microscope images show, from left to right, monocytes (M) adhering to the endothelium (E), monocytes extending into lumen (Lu) trapped in junctions of the endothelium, endothelium overlying foam cells (FC) in intima, and ruptured endothelium revealing numerous foam cells. (Images reproduced with permission from: Gerrity, R. G. 1981. The role of the monocyte in atherogenesis: I. Transition of blood-borne monocytes into foam cells in fatty lesions. Am J Pathol 103:181-190; Gerrity, R. G. 1981. The role of the monocyte in atherogenesis: II. Migration of foam cells from atherosclerotic lesions. Am J Pathol 103:191-200.) The cartoon shows the role of monocytes in lesion development and complication. Activated endothelium preferably recruits inflammatory monocytes from the blood stream, which differentiate to macrophages and foam cells. These cells ingest LDL and cholesterol crystals, which may augment their inflammatory, plaque destabilizing action. Other leukocytes, shown in the panel on the right, contribute to the inflammatory milieu in plaque, often via interaction with monocytes and macrophages.
Mice with genetically determined susceptibility to diet-induced atherosclerosis exhibit a striking dimorphism in circulating monocytes as shown by work simultaneously conducted by our group and that led by Gwendalyn Randolph. We found a profound shift in blood monocytes from low monocyte levels approximately equally distributed between Ly-6Chigh and Ly-6Clow populations, to a marked monocytosis characterized by an excess of Ly-6Chigh monocytes [23, 24]. Our previous work implicated MCP-1 signaling through the chemokine receptor CCR2 as a contributor to early lesion formation in the arteries of hypercholesterolemic mice. CCR2 appears causally related to the preferential accumulation of Ly-6Chigh monocytes in the nascent atherosclerotic plaque of hypercholesterolemic mice [25, 26]. Ly-6Chigh monocytes also adhere more avidly to the surface of TNF-α-stimulated endothelial cells in vitro than their Ly-6Clow counterparts [23]. The enhanced proteolytic potential of Ly-6Chigh monocytes may participate in their migration, and in remodeling of the arterial extracellular matrix during atherogenesis. As discussed above, Ly-6Chigh monocytes may indeed give rise preferentially to macrophages expressing the M1 functions associated with promotion of inflammation, especially in this setting of nonresolving inflammation.
The identification of the splenic pool of pro-inflammatory monocytes suggested the hypothesis that this organ provided a source of Ly-6Chigh monocytes not only for mobilization to sites of acutely injured tissue such as infarcting myocardium but also to sites of chronic inflammation such at the atherosclerotic plaque. Indeed, in experimental atherosclerosis in mice, spleenderived monocytes give rise to a substantial fraction of the mononuclear phagocytes in the evolving lesion, as shown by spleen transplantation [27]. Thus, the splenic precursor pool of pro-inflammatory monocytes serves as a reservoir for providing rapidly mobilized monocytes not only to sites of acute tissue injury such as infarcting myocardium as discussed above, but also to chronic lesions such as the evolving atherosclerotic plaque. GM-CSF and IL-3 promoted extramedullary hematopoiesis and the expansion of the monocyte reservoir in atherosclerotic lesions because antibodies against GM-CSF and IL-3 attenuated the phenomenon [27]. The newly described innate response activator (IRA) B cells, which arise in experimental sepsis and selectively produce GM-CSF [28, 29], might drive extramedullary hematopoiesis in atherosclerosis. It is likely that combination of extrinsic factors such as growth factor production [27] and bone marrow mobilization [30], as well as cell intrinsic factors involving cholesterol efflux pathways [31, 32] contribute to the monocytosis and extramedullary hematopoiesis that characterizes experimental atherosclerosis.
Monocyte heterogeneity, and the amplification of chronic regional innate immune responses by systemic inflammation
Atherosclerotic plaques exemplify local chronic sites of inflammation. Mononuclear phagocytes accumulate in these lesions. Clinical considerations suggest that lesion progression and complication does not occur continuously. Rather, episodes of rapid change may punctuate prolonged periods of relative quiescence. Serial angiographic studies suggest a discontinuous evolution of coronary arterial stenoses in time [33, 34]. Moreover, the thrombotic complications of atherosclerosis such as the acute coronary syndromes often occur unheralded, causing a nearly instantaneous transition from chronic stable lesions that may preexist for decades to a dramatic thrombotic occlusion of an artery yielding tissue injury.
We formulated a construct to account for this discontinuity in the evolution of atherosclerotic plaques. We postulated that episodes of systemic inflammation could boost local inflammation at sites of regional innate immune responses such as the atheromatous plaque. To test this hypothesis we mimicked a systemic inflammatory response in rabbits by intravenous injection of Gram negative bacterial endotoxin. We found that indeed the systemic stimulus evoked local overexpression of the pro-inflammatory cytokine interleukin-1 (IL-1) in atherosclerotic rabbit aortae [35]. We then produced graded degrees of atherosclerosis in rabbit aortae by feeding atherogenic diets enriched with increasing levels of cholesterol ranging from 0.1 % to 0.9 % of the diet on a weight basis. One hour after intravenous injection of Gram-negative bacterial endotoxin (a time shown in prior experiments to be optimum), we documented evoked overexpression of both isoforms of IL-1 and TNF-α messenger RNA. We found higher levels of expression of these pro-inflammatory cytokines in the aortae affected by greater degrees of atherosclerosis, characterized by mononuclear phagocyte accumulation in the arterial intima [36]. These experiments furnished the basis for understanding how episodic bouts of lesion activation could reflect a systemic inflammatory response.
We further postulated that remote bacterial or viral infections, through release of bacterial products or stimulation of a systemic inflammatory response could promote the development of atherosclerosis and atherosclerotic lesion complication [37]. We informally denoted this concept, the “echo” phenomenon, indicating the relationship between systemic inflammatory stimuli and the local evoked responses at the level of the artery wall.
Our recent experiments revisited this concept in the context of acute myocardial infarction as a clinically relevant form of inflammation remote from the artery wall that might evoke a bout of intensified inflammation in the regional innate immune process chronically smoldering in established atherosclerotic plaques. This concept has considerable clinical relevance as individuals who have sustained myocardial injury due to an acute coronary syndrome have an increased risk for early recurrent events [38, 39]. We thus hypothesized that myocardial infarction could elicit a systemic inflammatory response that could aggravate inflammation locally in atheromata, and contribute to activation of plaques, and precipitation of early recurrent events.
To test this hypothesis we ligated coronary arteries in atherosclerotic mice to precipitate an acute myocardial infarction. Indeed, in the immediate aftermath of experimental myocardial infarction, remote atherosclerotic plaques displayed enhanced inflammatory activation as evidenced by increased activity of proteolytic enzymes. Moreover, the number of pro-inflammatory monocytes marked by high levels of Ly-6C increased in the preformed atherosclerotic lesions following coronary ligation. Likewise, levels of pro-inflammatory cytokines such as TNF-α and IL-1β rose following experimental myocardial infarction in mice [40].
Further experiments sought a mechanistic explanation for the enhanced accumulation of pro-inflammatory monocytes in atherosclerotic plaques following myocardial infarction. Based on previous observations we focused on splenic extramedullary hematopoiesis and the relocation of hematopoietic stem and progenitor cells from the bone marrow to the spleen. Acute myocardial infarction in humans elicits a strong sympathetic nervous system response due to pain and anxiety. Work from Frenette's group suggested that adrenergic stimuli, in particular beta-3 adrenergic stimulation, could mobilize leukocyte precursor cells from bone marrow niches [41, 42]. Subsequent experiments demonstrated that recruitment of the Ly-6Chigh monocytes to the atherosclerotic plaques in mice undergoing experimental myocardial infarction depended on beta-3 adrenergic signaling [40]. Tyrosine hydroxylase, the rate-limiting enzyme for the synthesis of catecholamines, the mediators of the effects of sympathetic nerve activation, rose in the microvessels in bone marrow stroma following myocardial infarction. During experimental acute myocardial infarction, stem cells departed from bone marrow niches in a beta-3 adrenergic dependent manner as shown by intravital microscopic monitoring. Thus, the insult resulting from myocardial injury mobilized monocyte precursors from the bone marrow, promoted their expansion in the spleen, and enhanced their recruitment and accumulation in atherosclerotic lesions.
The burst of pro-inflammatory cytokine expression in response to myocardial infarction could amplify the regional innate immune response accelerating the evolution of atherosclerotic plaques. Local overexpression of proteinases in plaques could promote weakening of the extracellular matrix that renders plaques susceptible to rupture and precipitation of acute thrombotic complications [43]. Moreover, the local inflammatory stimulation in plaques can enhance tissue factor production, increasing the thrombogenicity of plaques. As the systemic acute phase response to myocardial infarction can also increase fibrinogen, the precursor of clots, and plasminogen activator inhibitor-1, the major endogenous inhibitor of thrombolysis. A conjunction of increased activation of the “solid state” of the atheroma itself and increased coagulability and impaired fibrinolysis in “fluid phase” of blood provoked by acute myocardial infarction could set the stage for increased susceptibility to occlusive thrombi precipitating recurrent acute coronary syndromes [44].
Human observations support the concept that myocardial infarction can effect monocyte biology in patients. Interrogation of large clinical trial data sets indicate that individuals who underwent acute coronary syndromes who took beta adrenergic blocking agents before the acute event had lower levels of circulating monocytes that beta blocker-naïve individuals undergoing acute coronary syndromes [40]. Moreover, observation on autopsy specimens of spleens showed an increase in c-kit positive cells in the spleen, representing leukocyte precursors, which also bore markers of proliferation (Ki-67), indicating that humans who have sustained an acute myocardial infarction have increased splenic hematopoiesis [40].
This amalgam of well defined animal experimentation and observations on humans illustrates the practical and clinical implications of innate immune responses, mononuclear phagocyte heterogeneity, and trafficking of innate immune cells in human cardiovascular disease. Monocyte biology provides a mechanistic link between ischemic myocardial tissue injury and “echoes” at the level of the atherosclerotic plaque. The acute myocardial infarction can awaken dormant plaques through evoking a round of inflammatory activation enhancing their propensity to precipitate another ischemic event.
Translation to humans of the biology of monocyte heterogeneity of cardiovascular disease
The burgeoning data regarding monocyte heterogeneity in experimental cardiovascular disease in mice has heightened interest in clinical translation of these advances in monocyte biology and innate immune responses. The markers that serve so well in mice for delineating pro-inflammatory and less inflammatory subsets of mononuclear phagocytes lack clear human counterparts. Investigators have used surface expression of markers such as CD14 and CD16 to distinguish human monocyte subsets that functionally mirror the Ly6Chigh/Gr-1+ and Ly6Clow/Gr-1− monocyte populations in mice. Monocytes commonly called “classical” dominate in human blood and display surface markers denoted as CD14high CD16−. These monocytes phenotypically resemble mouse Ly-6Chigh monocytes because they express high levels of CCR2, CD62L, CD64, and low levels of CX3CR1. The second, CD14dim CD16+ population resembles most closely Ly-6Clow monocytes both phenotypically and functionally: it expresses low levels of CCR2, high levels of CX3CR1, and exhibits patrolling behavior. A third CD14+CD16+ population secretes TNFα in response to LPS. Cluster analysis indicates that this CD14+CD16+ population tracks with CD16− monocytes and resembles Ly-6Chigh more than Ly-6Clow monocytes (Table) [45-48].
Table. The phenotype of mouse and human monocyte subsets.
| Mouse | Human | ||||
|---|---|---|---|---|---|
|
|
|
||||
| Ly-6Chigh | Ly-6Clow | CD16-CD14high | CD16+ CD14+ | CD16+ CD14dim | |
|
|
|||||
| CCR2 | ++ | − | ++ | + | − |
|
|
|||||
| CX3CRI | + | +++ | + | ++ | +++ |
|
|
|||||
| CCR5 | + | + | + | + | − |
|
|
|||||
| CD11b | ++ | ++ | ++ | ++ | + |
|
|
|||||
| CD62L | ++ | − | ++ | − | − |
|
|
|||||
| PSGL-1 | +++ | + | +++ | + | + |
|
|
|||||
| CD11a | + | ++ | ++ | + | + |
|
|
|||||
| CD11c | − | + | − | + | + |
|
|
|||||
| F4/80 | + | + | ND | ND | ND |
|
|
|||||
| CD36 | − | + | ++ | + | + |
|
|
|||||
| CD64 | + | + | ++ | + | − |
|
|
|||||
| MHCII | − | + | ND | ND | ND |
|
|
|||||
| HLA-DR | ND | ND | − | ++ | + |
|
|
|||||
| CD11a | ++ | ++ | ++ | ++ | ++ |
|
|
|||||
No fewer than a dozen, mostly small, observational association studies have evaluated human monocyte subsets in diseases related to the cardiovascular system either directly or indirectly [49-62]. Most studies have observed a higher proportion of CD16+ monocytes with disease progression. One notable recent study using 700 patients has shown that CD16− monocytes can predict cardiovascular events independently of other risk factors in a randomly selected population [63]. In contrast, another recent study using 951 patients concluded that the CD14++ CD16+ subset independently predicts cardiovascular events [64]. The debate therefore continues. Ever more sophisticated tools will no doubt sharpen our understanding of monocyte subset biology in human cardiovascular disease, while mouse studies will continue to inform on the basic biology.
Unanswered questions and future goals of research into monocyte heterogeneity in cardiovascular disease
Beyond continued efforts to translate the mouse experiments to humans, the practical implications of monocyte heterogeneity in cardiovascular disease require intense further investigation. In the context of acute myocardial infarction, could manipulation of the recruitment of monocyte subsets be used to advantage to promote appropriate tissue healing and prevent adverse myocardial remodeling? The early wave of pro-inflammatory monocytes recruited to infarcting myocardium presumably has an important function in clearing debris of dead and dying cells and paves the way for tissue repair. Finding the optimum time of activation of these pro-inflammatory monocytes following myocardial infarction could permit manipulation to optimize their contribution to appropriate tissue healing.
Likewise, could an earlier arrival of less inflammatory monocytes, functionally poised to promote fibrosis and angiogenesis favor healthy repair? Could the recruitment and kinetics of retention of various monocyte subclasses in the infarcting myocardium respond to therapeutic manipulation through targeting chemokines and chemokine receptors? Experimental observations already indicate roles for pharmacologic agents commonly used in patients suffering acute myocardial infarction such as angiotensin converting enzyme inhibitors and statins as modulators of monocyte biology. Could novel anti-inflammatory agents currently under investigation such as cochicine [65], methotrexate [66], or anti-cytokine strategies [67] selectively manipulate the biology of monocyte subpopulations in a way that could improve long-term clinical outcomes?
In the context of the atherosclerotic plaque, we know that statins can experimentally decrease levels of Ly-6Chigh monocytes in the blood. Whether various established or emerging interventions that target lipoprotein metabolism could likewise exert beneficial effects on monocyte populations in the context of the evolution and complication of atherosclerotic lesions requires further investigation.
In conclusion, the recognition of monocyte heterogeneity has opened new fields of inquiry in cardiovascular disease, chronic as well as acute, and affecting the heart muscle itself as well as the blood vessels. Progress in this field has increased mechanistic insights into the pathogenesis of cardiovascular diseases. Harnessing this progress in understanding the basic biology could lead to clinical advances in the future to address the increasing burden of cardiovascular worldwide.
References
- 1.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Barker-Collo S, Bartels DH, Bell ML, Benjamin EJ, Bennett D, Bhalla K, Bikbov B, Bin Abdulhak A, Birbeck G, Blyth F, Bolliger I, Boufous S, Bucello C, Burch M, Burney P, Carapetis J, Chen H, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahodwala N, De Leo D, Degenhardt L, Delossantos A, Denenberg J, Des Jarlais DC, Dharmaratne SD, Dorsey ER, Driscoll T, Duber H, Ebel B, Erwin PJ, Espindola P, Ezzati M, Feigin V, Flaxman AD, Forouzanfar MH, Fowkes FG, Franklin R, Fransen M, Freeman MK, Gabriel SE, Gakidou E, Gaspari F, Gillum RF, Gonzalez-Medina D, Halasa YA, Haring D, Harrison JE, Havmoeller R, Hay RJ, Hoen B, Hotez PJ, Hoy D, Jacobsen KH, James SL, Jasrasaria R, Jayaraman S, Johns N, Karthikeyan G, Kassebaum N, Keren A, Khoo JP, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Lipnick M, Lipshultz SE, Ohno SL, Mabweijano J, MacIntyre MF, Mallinger L, March L, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGrath J, Mensah GA, Merriman TR, Michaud C, Miller M, Miller TR, Mock C, Mocumbi AO, Mokdad AA, Moran A, Mulholland K, Nair MN, Naldi L, Narayan KM, Nasseri K, Norman P, O'Donnell M, Omer SB, Ortblad K, Osborne R, Ozgediz D, Pahari B, Pandian JD, Rivero AP, Padilla RP, Perez-Ruiz F, Perico N, Phillips D, Pierce K, Pope CAr, Porrini E, Pourmalek F, Raju M, Ranganathan D, Rehm JT, Rein DB, Remuzzi G, Rivara FP, Roberts T, De Leon FR, Rosenfeld LC, Rushton L, Sacco RL, Salomon JA, Sampson U, Sanman E, Schwebel DC, Segui-Gomez M, Shepard DS, Singh D, Singleton J, Sliwa K, Smith E, Steer A, Taylor JA, Thomas B, Tleyjeh IM, Towbin JA, Truelsen T, Undurraga EA, Venketasubramanian N, Vijayakumar L, Vos T, Wagner GR, Wang M, Wang W, Watt K, Weinstock MA, Weintraub R, Wilkinson JD, Woolf AD, Wulf S, Yeh PH, Yip P, Zabetian A, Zheng ZJ, Lopez AD, Murray CJ. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bloom DE, Cafiero ET, Jane-Llopis E, Abrahams-Gessel S, Bloom LR, Fathima S, Feigl AB, Gaziano T, Mowafi M, Pandya A, Prettner K, Rosenberg L, Seligman B, Stein AZ, Weinstein C. The Global Economic Burden of Noncommunicable Diseases 2011 [Google Scholar]
- 3.Braunwald E, Maroko PR, Libby P. Reduction of infarct size following coronary occlusion. Circ Res. 1974;35(3):192–201. [PubMed] [Google Scholar]
- 4.Pfeffer MA, Braunwald E. Ventricular remodeling after myocardial infarction. Experimental observations and clinical implications. Circulation. 1990;81:1161–1172. doi: 10.1161/01.cir.81.4.1161. [DOI] [PubMed] [Google Scholar]
- 5.Mallory GK, White PD, Salcedo-Salga J. The speed of healing of myocardial infarction: a study of the pathologic anatomy in 72 cases. Am Heart J. 1939;18:647–671. [Google Scholar]
- 6.Nahrendorf M, Swirski FK, Aikawa E, Stangenberg L, Wurdinger T, Figueiredo JL, Libby P, Weissleder R, Pittet MJ. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med. 2007;204:3037–3047. doi: 10.1084/jem.20070885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 8.Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122:787–795. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arnold L, Henry A, Poron F, Baba-Amer Y, van Rooijen N, Plonquet A, Gherardi RK, Chazaud B. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J Exp Med. 2007;204:1057–1069. doi: 10.1084/jem.20070075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramachandran P, Pellicoro A, Vernon MA, Boulter L, Aucott RL, Ali A, Hartland SN, Snowdon VK, Cappon A, Gordon-Walker TT, Williams MJ, Dunbar DR, Manning JR, van Rooijen N, Fallowfield JA, Forbes SJ, Iredale JP. Differential Ly-6C expression identifies the recruited macrophage phenotype, which orchestrates the regression of murine liver fibrosis. Proc Natl Acad Sci U S A. 2012;109:E3186–95. doi: 10.1073/pnas.1119964109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yona S, Kim KW, Wolf Y, Mildner A, Varol D, Breker M, Strauss-Ayali D, Viukov S, Guilliams M, Misharin A, Hume DA, Perlman H, Malissen B, Zelzer E, Jung S. Fate Mapping Reveals Origins and Dynamics of Monocytes and Tissue Macrophages under Homeostasis. Immunity. 2013;38:79–91. doi: 10.1016/j.immuni.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Troidl C, Mollmann H, Nef H, Masseli F, Voss S, Szardien S, Willmer M, Rolf A, Rixe J, Troidl K, Kostin S, Hamm C, Elsasser A. Classically and alternatively activated macrophages contribute to tissue remodelling after myocardial infarction. J Cell Mol Med. 2009;13:3485–3496. doi: 10.1111/j.1582-4934.2009.00707.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma Y, Halade GV, Zhang J, Ramirez TA, Levin D, Voorhees AP, Jin YF, Han HC, Manicone AM, Lindsey M. Matrix Metalloproteinase-28 Deletion Exacerbates Cardiac Dysfunction and Rupture Following Myocardial Infarction in Mice by Inhibiting M2 Macrophage Activation. Circ Res. 2012 doi: 10.1161/CIRCRESAHA.111.300502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Swirski FK, Nahrendorf M, Etzrodt M, Wildgruber M, Cortez-Retamozo V, Panizzi P, Figueiredo JL, Kohler RH, Chudnovskiy A, Waterman P, Aikawa E, Mempel TR, Libby P, Weissleder R, Pittet MJ. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science. 2009;325:612–616. doi: 10.1126/science.1175202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leuschner F, Panizzi P, Chico-Calero I, Lee WW, Ueno T, Cortez-Retamozo V, Waterman P, Gorbatov R, Marinelli B, Iwamoto Y, Chudnovskiy A, Figueiredo JL, Sosnovik DE, Pittet MJ, Swirski FK, Weissleder R, Nahrendorf M. Angiotensin-converting enzyme inhibition prevents the release of monocytes from their splenic reservoir in mice with myocardial infarction. Circ Res. 2010;107:1364–1373. doi: 10.1161/CIRCRESAHA.110.227454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ambrosioni E, Borghi C, Magnani B. The effect of the angiotensin-converting-enzyme inhibitor zofenopril on mortality and morbidity after anterior myocardial infarction. The Survival of Myocardial Infarction Long-Term Evaluation (SMILE) Study Investigators. N Engl J Med. 1995;332:80–85. doi: 10.1056/NEJM199501123320203. [DOI] [PubMed] [Google Scholar]
- 18.Pfeffer MA, Braunwald E, Moye LA, Basta L, Brown EJ, Jr, Cuddy TE, Davis BR, Geltman EM, Goldman S, Flaker GC, et al. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction. Results of the survival and ventricular enlargement trial. The SAVE Investigators. N Engl J Med. 1992;327:669–677. doi: 10.1056/NEJM199209033271001. [DOI] [PubMed] [Google Scholar]
- 19.Panizzi P, Swirski FK, Figueiredo JL, Waterman P, Sosnovik DE, Aikawa E, Libby P, Pittet M, Weissleder R, Nahrendorf M. Impaired infarct healing in atherosclerotic mice with Ly-6C(hi) monocytosis. J Am Coll Cardiol. 2010;55:1629–1638. doi: 10.1016/j.jacc.2009.08.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dewald O, Zymek P, Winkelmann K, Koerting A, Ren G, Abou-Khamis T, Michael LH, Rollins BJ, Entman ML, Frangogiannis NG. CCL2/Monocyte Chemoattractant Protein-1 regulates inflammatory responses critical to healing myocardial infarcts. Circ Res. 2005;96:881–889. doi: 10.1161/01.RES.0000163017.13772.3a. [DOI] [PubMed] [Google Scholar]
- 21.Leuschner F, Dutta P, Gorbatov R, Novobrantseva TI, Donahoe JS, Courties G, Lee KM, Kim JI, Markmann JF, Marinelli B, Panizzi P, Lee WW, Iwamoto Y, Milstein S, Epstein-Barash H, Cantley W, Wong J, Cortez-Retamozo V, Newton A, Love K, Libby P, Pittet MJ, Swirski FK, Koteliansky V, Langer R, Weissleder R, Anderson DG, Nahrendorf M. Therapeutic siRNA silencing in inflammatory monocytes in mice. Nat Biotechnol. 2011;29:1005–1010. doi: 10.1038/nbt.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leuschner F, Rauch PJ, Ueno T, Gorbatov R, Marinelli B, Lee WW, Dutta P, Wei Y, Robbins C, Iwamoto Y, Sena B, Chudnovskiy A, Panizzi P, Keliher E, Higgins JM, Libby P, Moskowitz MA, Pittet MJ, Swirski FK, Weissleder R, Nahrendorf M. Rapid monocyte kinetics in acute myocardial infarction are sustained by extramedullary monocytopoiesis. J Exp Med. 2012;209:123–137. doi: 10.1084/jem.20111009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Swirski FK, Libby P, Aikawa E, Alcaide P, Luscinskas FW, Weissleder R, Pittet MJ. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J Clin Invest. 2007;117:195–205. doi: 10.1172/JCI29950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tacke F, Alvarez D, Kaplan TJ, Jakubzick C, Spanbroek R, Llodra J, Garin A, Liu J, Mack M, van Rooijen N, Lira SA, Habenicht AJ, Randolph GJ. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J Clin Invest. 2007;117:185–194. doi: 10.1172/JCI28549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Combadiere C, Potteaux S, Rodero M, Simon T, Pezard A, Esposito B, Merval R, Proudfoot A, Tedgui A, Mallat Z. Combined inhibition of CCL2, CX3CR1, and CCR5 abrogates Ly6C(hi) and Ly6C(lo) monocytosis and almost abolishes atherosclerosis in hypercholesterolemic mice. Circulation. 2008;117:1649–1657. doi: 10.1161/CIRCULATIONAHA.107.745091. [DOI] [PubMed] [Google Scholar]
- 26.Saederup N, Chan L, Lira SA, Charo IF. Fractalkine deficiency markedly reduces macrophage accumulation and atherosclerotic lesion formation in CCR2-/- mice: evidence for independent chemokine functions in atherogenesis. Circulation. 2008;117:1642–1648. doi: 10.1161/CIRCULATIONAHA.107.743872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robbins CS, Chudnovskiy A, Rauch PJ, Figueiredo JL, Iwamoto Y, Gorbatov R, Etzrodt M, Weber GF, Ueno T, van Rooijen N, Mulligan-Kehoe MJ, Libby P, Nahrendorf M, Pittet MJ, Weissleder R, Swirski FK. Extramedullary hematopoiesis generates Ly-6C(high) monocytes that infiltrate atherosclerotic lesions. Circulation. 2012;125:364–374. doi: 10.1161/CIRCULATIONAHA.111.061986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rauch PJ, Chudnovskiy A, Robbins CS, Weber GF, Etzrodt M, Hilgendorf I, Tiglao E, Figueiredo JL, Iwamoto Y, Theurl I, Gorbatov R, Waring MT, Chicoine AT, Mouded M, Pittet MJ, Nahrendorf M, Weissleder R, Swirski FK. Innate response activator B cells protect against microbial sepsis. Science. 2012;335:597–601. doi: 10.1126/science.1215173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robbins CS, Swirski FK. Newly discovered innate response activator B cells: crucial responders against microbial sepsis. Expert Rev Clin Immunol. 2012;8:405–407. doi: 10.1586/eci.12.32. [DOI] [PubMed] [Google Scholar]
- 30.Westerterp M, Gourion-Arsiquaud S, Murphy AJ, Shih A, Cremers S, Levine RL, Tall AR, Yvan-Charvet L. Regulation of hematopoietic stem and progenitor cell mobilization by cholesterol efflux pathways. Cell Stem Cell. 2012;11:195–206. doi: 10.1016/j.stem.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murphy AJ, Akhtari M, Tolani S, Pagler T, Bijl N, Kuo CL, Wang M, Sanson M, Abramowicz S, Welch C, Bochem AE, Kuivenhoven JA, Yvan-Charvet L, Tall AR. ApoE regulates hematopoietic stem cell proliferation, monocytosis, and monocyte accumulation in atherosclerotic lesions in mice. J Clin Invest. 2011;121:4138–4149. doi: 10.1172/JCI57559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yvan-Charvet L, Pagler T, Gautier EL, Avagyan S, Siry RL, Han S, Welch CL, Wang N, Randolph GJ, Snoeck HW, Tall AR. ATP-binding cassette transporters and HDL suppress hematopoietic stem cell proliferation. Science. 2010;328:1689–1693. doi: 10.1126/science.1189731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bruschke AV, Kramer JRJ, Bal ET, Haque IU, Detrano RC, Goormastic M. The dynamics of progression of coronary atherosclerosis studied in 168 medically treated patients who underwent coronary arteriography three times. Am Heart J. 1989;117:296–305. doi: 10.1016/0002-8703(89)90772-2. [DOI] [PubMed] [Google Scholar]
- 34.Yokoya K, Takatsu H, Suzuki T, Hosokawa H, Ojio S, Matsubara T, Tanaka T, Watanabe S, Morita N, Nishigaki K, Takemura G, Noda T, Minatoguchi S, Fujiwara H. Process of progression of coronary artery lesions from mild or moderate stenosis to moderate or severe stenosis: A study based on four serial coronary arteriograms per year. Circulation. 1999;100:903–909. doi: 10.1161/01.cir.100.9.903. [DOI] [PubMed] [Google Scholar]
- 35.Clinton SK, Fleet JC, Loppnow H, Salomon RN, Clark BD, Cannon JG, Shaw AR, Dinarello CA, Libby P. Interleukin-1 gene expression in rabbit vascular tissue in vivo. Am J Pathol. 1991;138:1005–1014. [PMC free article] [PubMed] [Google Scholar]
- 36.Fleet JC, Clinton SK, Salomon RN, Loppnow H, Libby P. Atherogenic diets enhance endotoxin-stimulated interleukin-1 and tumor necrosis factor gene expression in rabbit aortae. J Nutr. 1992;122:294–305. doi: 10.1093/jn/122.2.294. [DOI] [PubMed] [Google Scholar]
- 37.Libby P, Egan D, Skarlatos S. Roles of infectious agents in atherosclerosis and restenosis: an assessment of the evidence and need for future research. Circulation. 1997;96:4095–4103. doi: 10.1161/01.cir.96.11.4095. [DOI] [PubMed] [Google Scholar]
- 38.Goldstein JA, Demetriou D, Grines CL, Pica M, Shoukfeh M, O'Neill WW. Multiple complex coronary plaques in patients with acute myocardial infarction. N Engl J Med. 2000;343:915–922. doi: 10.1056/NEJM200009283431303. [DOI] [PubMed] [Google Scholar]
- 39.Milonas C, Jernberg T, Lindback J, Agewall S, Wallentin L, Stenestrand U. Effect of Angiotensin-converting enzyme inhibition on one-year mortality and frequency of repeat acute myocardial infarction in patients with acute myocardial infarction. Am J Cardiol. 2010;105:1229–1234. doi: 10.1016/j.amjcard.2009.12.032. [DOI] [PubMed] [Google Scholar]
- 40.Dutta P, Courties G, Wei Y, Leuschner F, Gorbatov R, Robbins CS, Iwamoto Y, Thompson B, Carlson AL, Heidt T, Majmudar MD, Lasitschka F, Etzrodt M, Waterman P, Waring MT, Chicoine AT, van der Laan AM, Niessen HW, Piek JJ, Rubin BB, Butany J, Stone JR, Katus HA, Murphy SA, Morrow DA, Sabatine MS, Vinegoni C, Moskowitz MA, Pittet MJ, Libby P, Lin CP, Swirski FK, Weissleder R, Nahrendorf M. Myocardial infarction accelerates atherosclerosis. Nature. 2012;487:325–329. doi: 10.1038/nature11260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mendez-Ferrer S, Lucas D, Battista M, Frenette PS. Haematopoietic stem cell release is regulated by circadian oscillations. Nature. 2008;452:442–447. doi: 10.1038/nature06685. [DOI] [PubMed] [Google Scholar]
- 42.Scheiermann C, Kunisaki Y, Lucas D, Chow A, Jang JE, Zhang D, Hashimoto D, Merad M, Frenette PS. Adrenergic nerves govern circadian leukocyte recruitment to tissues. Immunity. 2012;37:290–301. doi: 10.1016/j.immuni.2012.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Libby P. The molecular mechanisms of the thrombotic complications of atherosclerosis. J Intern Med. 2008;263:517–527. doi: 10.1111/j.1365-2796.2008.01965.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Libby P, Theroux P. Pathophysiology of coronary artery disease. Circulation. 2005;111:3481–3488. doi: 10.1161/CIRCULATIONAHA.105.537878. [DOI] [PubMed] [Google Scholar]
- 45.Cros J, Cagnard N, Woollard K, Patey N, Zhang SY, Senechal B, Puel A, Biswas SK, Moshous D, Picard C, Jais JP, D'Cruz D, Casanova JL, Trouillet C, Geissmann F. Human CD14dim monocytes patrol and sense nucleic acids and viruses via TLR7 and TLR8 receptors. Immunity. 2010;33:375–386. doi: 10.1016/j.immuni.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ingersoll MA, Spanbroek R, Lottaz C, Gautier EL, Frankenberger M, Hoffmann R, Lang R, Haniffa M, Collin M, Tacke F, Habenicht AJ, Ziegler-Heitbrock L, Randolph GJ. Comparison of gene expression profiles between human and mouse monocyte subsets. Blood. 2010;115:e10–9. doi: 10.1182/blood-2009-07-235028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Skrzeczynska-Moncznik J, Bzowska M, Loseke S, Grage-Griebenow E, Zembala M, Pryjma J. Peripheral blood CD14high CD16+ monocytes are main producers of IL-10. Scand J Immunol. 2008;67:152–159. doi: 10.1111/j.1365-3083.2007.02051.x. [DOI] [PubMed] [Google Scholar]
- 48.Wong KL, Tai JJ, Wong WC, Han H, Sem X, Yeap WH, Kourilsky P, Wong SC. Gene expression profiling reveals the defining features of the classical, intermediate, and nonclassical human monocyte subsets. Blood. 2011;118:e16–31. doi: 10.1182/blood-2010-12-326355. [DOI] [PubMed] [Google Scholar]
- 49.Engstrom G, Melander O, Hedblad B. Leukocyte count and incidence of hospitalizations due to heart failure. Circ Heart Fail. 2009;2:217–222. doi: 10.1161/CIRCHEARTFAILURE.108.827071. [DOI] [PubMed] [Google Scholar]
- 50.Heine GH, Ulrich C, Seibert E, Seiler S, Marell J, Reichart B, Krause M, Schlitt A, Kohler H, Girndt M. CD14(++)CD16+ monocytes but not total monocyte numbers predict cardiovascular events in dialysis patients. Kidney Int. 2008;73:622–629. doi: 10.1038/sj.ki.5002744. [DOI] [PubMed] [Google Scholar]
- 51.Hristov M, Leyendecker T, Schuhmann C, von Hundelshausen P, Heussen N, Kehmeier E, Krotz F, Sohn HY, Klauss V, Weber C. Circulating monocyte subsets and cardiovascular risk factors in coronary artery disease. Thromb Haemost. 2010;104:412–414. doi: 10.1160/TH10-01-0069. [DOI] [PubMed] [Google Scholar]
- 52.Imanishi T, Ikejima H, Tsujioka H, Kuroi A, Ishibashi K, Komukai K, Tanimoto T, Ino Y, Takeshita T, Akasaka T. Association of monocyte subset counts with coronary fibrous cap thickness in patients with unstable angina pectoris. Atherosclerosis. 2010;212:628–635. doi: 10.1016/j.atherosclerosis.2010.06.025. [DOI] [PubMed] [Google Scholar]
- 53.Kashiwagi M, Imanishi T, Tsujioka H, Ikejima H, Kuroi A, Ozaki Y, Ishibashi K, Komukai K, Tanimoto T, Ino Y, Kitabata H, Hirata K, Akasaka T. Association of monocyte subsets with vulnerability characteristics of coronary plaques as assessed by 64-slice multidetector computed tomography in patients with stable angina pectoris. Atherosclerosis. 2010;212:171–176. doi: 10.1016/j.atherosclerosis.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 54.Liu Y, Imanishi T, Ikejima H, Tsujioka H, Ozaki Y, Kuroi A, Okochi K, Ishibashi K, Tanimoto T, Ino Y, Kitabata H, Akasaka T. Association between circulating monocyte subsets and in-stent restenosis after coronary stent implantation in patients with ST-elevation myocardial infarction. Circ J. 2010;74:2585–2591. doi: 10.1253/circj.cj-10-0544. [DOI] [PubMed] [Google Scholar]
- 55.Poitou C, Dalmas E, Renovato M, Benhamo V, Hajduch F, Abdennour M, Kahn JF, Veyrie N, Rizkalla S, Fridman WH, Sautes-Fridman C, Clement K, Cremer I. CD14dimCD16+ and CD14+CD16+ monocytes in obesity and during weight loss: relationships with fat mass and subclinical atherosclerosis. Arterioscler Thromb Vasc Biol. 2011;31:2322–2330. doi: 10.1161/ATVBAHA.111.230979. [DOI] [PubMed] [Google Scholar]
- 56.Rogacev KS, Seiler S, Zawada AM, Reichart B, Herath E, Roth D, Ulrich C, Fliser D, Heine GH. CD14++CD16+ monocytes and cardiovascular outcome in patients with chronic kidney disease. Eur Heart J. 2011;32:84–92. doi: 10.1093/eurheartj/ehq371. [DOI] [PubMed] [Google Scholar]
- 57.Rothe G, Herr AS, Stohr J, Abletshauser C, Weidinger G, Schmitz G. A more mature phenotype of blood mononuclear phagocytes is induced by fluvastatin treatment in hypercholesterolemic patients with coronary heart disease. Atherosclerosis. 1999;144:251–261. doi: 10.1016/s0021-9150(99)00061-1. [DOI] [PubMed] [Google Scholar]
- 58.Schlitt A, Heine GH, Blankenberg S, Espinola-Klein C, Dopheide JF, Bickel C, Lackner KJ, Iz M, Meyer J, Darius H, Rupprecht HJ. CD14+CD16+ monocytes in coronary artery disease and their relationship to serum TNF-alpha levels. Thromb Haemost. 2004;92:419–424. doi: 10.1160/TH04-02-0095. [DOI] [PubMed] [Google Scholar]
- 59.Timmerman KL, Flynn MG, Coen PM, Markofski MM, Pence BD. Exercise training-induced lowering of inflammatory (CD14+CD16+) monocytes: a role in the anti-inflammatory influence of exercise? J Leukoc Biol. 2008;84:1271–1278. doi: 10.1189/jlb.0408244. [DOI] [PubMed] [Google Scholar]
- 60.Tsujioka H, Imanishi T, Ikejima H, Kuroi A, Takarada S, Tanimoto T, Kitabata H, Okochi K, Arita Y, Ishibashi K, Komukai K, Kataiwa H, Nakamura N, Hirata K, Tanaka A, Akasaka T. Impact of heterogeneity of human peripheral blood monocyte subsets on myocardial salvage in patients with primary acute myocardial infarction. J Am Coll Cardiol. 2009;54:130–138. doi: 10.1016/j.jacc.2009.04.021. [DOI] [PubMed] [Google Scholar]
- 61.van der Laan AM, Hirsch A, Robbers LF, Nijveldt R, Lommerse I, Delewi R, van der Vleuten PA, Biemond BJ, Zwaginga JJ, van der Giessen WJ, Zijlstra F, van Rossum AC, Voermans C, van der Schoot CE, Piek JJ. A proinflammatory monocyte response is associated with myocardial injury and impaired functional outcome in patients with ST-segment elevation myocardial infarction: monocytes and myocardial infarction. Am Heart J. 2012;163:57–65.e2. doi: 10.1016/j.ahj.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 62.Wildgruber M, Lee H, Chudnovskiy A, Yoon TJ, Etzrodt M, Pittet MJ, Nahrendorf M, Croce K, Libby P, Weissleder R, Swirski FK. Monocyte subset dynamics in human atherosclerosis can be profiled with magnetic nano-sensors. PLoS One. 2009;4:e5663. doi: 10.1371/journal.pone.0005663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Berg KE, Ljungcrantz I, Andersson L, Bryngelsson C, Hedblad B, Fredrikson GN, Nilsson J, Bjorkbacka H. Elevated CD14++CD16- monocytes predict cardiovascular events. Circ Cardiovasc Genet. 2012;5:122–131. doi: 10.1161/CIRCGENETICS.111.960385. [DOI] [PubMed] [Google Scholar]
- 64.Rogacev KS, Cremers B, Zawada AM, Seiler S, Binder N, Ege P, Grosse-Dunker G, Heisel I, Hornof F, Jeken J, Rebling NM, Ulrich C, Scheller B, Bohm M, Fliser D, Heine GH. CD14++CD16+ monocytes independently predict cardiovascular events: a cohort study of 951 patients referred for elective coronary angiography. J Am Coll Cardiol. 2012;60:1512–1520. doi: 10.1016/j.jacc.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 65.Nidorf SM, Eikelboom JW, Budgeon CA, Thompson PL. Low-dose colchicine for secondary prevention of cardiovascular disease. J Am Coll Cardiol. 2013;61:404–410. doi: 10.1016/j.jacc.2012.10.027. [DOI] [PubMed] [Google Scholar]
- 66.Ridker PM. Testing the inflammatory hypothesis of atherothrombosis: scientific rationale for the cardiovascular inflammation reduction trial (CIRT) J Thromb Haemost. 2009;7(1):332–339. doi: 10.1111/j.1538-7836.2009.03404.x. [DOI] [PubMed] [Google Scholar]
- 67.Ridker PM, Thuren T, Zalewski A, Libby P. Interleukin-1beta inhibition and the prevention of recurrent cardiovascular events: rationale and design of the Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS) Am Heart J. 2011;162:597–605. doi: 10.1016/j.ahj.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 68.Gerrity RG. The role of the monocyte in atherogenesis: I. Transition of blood-borne monocytes into foam cells in fatty lesions. Am J Pathol. 1981;103:181–190. [PMC free article] [PubMed] [Google Scholar]
- 69.Gerrity RG. The role of the monocyte in atherogenesis: II. Migration of foam cells from atherosclerotic lesions. Am J Pathol. 1981;103:191–200. [PMC free article] [PubMed] [Google Scholar]