Abstract
BK polyomavirus (BKV) causes significant urinary tract pathogenesis in immunosuppressed individuals, including kidney and bone marrow transplant recipients. It is currently unclear whether BKV-neutralizing antibodies can moderate or prevent BKV disease. We developed reporter pseudoviruses based on seven divergent BKV isolates and performed neutralization assays on sera from healthy human subjects. The results demonstrate that BKV genotypes I, II, III, and IV are fully distinct serotypes. While nearly all healthy subjects had BKV genotype I-neutralizing antibodies, a majority of subjects did not detectably neutralize genotype III or IV. Surprisingly, BKV subgenotypes Ib1 and Ib2 can behave as fully distinct serotypes. This difference is governed by as few as two residues adjacent to the cellular glycan receptor-binding site on the virion surface. Serological analysis of mice given virus-like particle (VLP)-based BKV vaccines confirmed these findings. Mice administered a multivalent VLP vaccine showed high-titer serum antibody responses that potently cross-neutralized all tested BKV genotypes. Interestingly, each of the neutralization serotypes bound a distinct spectrum of cell surface receptors, suggesting a possible connection between escape from recognition by neutralizing antibodies and cellular attachment mechanisms. The finding implies that different BKV genotypes have different cellular tropisms and pathogenic potentials in vivo. Individuals who are infected with one BKV serotype may remain humorally vulnerable to other BKV serotypes after implementation of T cell immunosuppression. Thus, prevaccinating organ transplant recipients with a multivalent BKV VLP vaccine might reduce the risk of developing posttransplant BKV disease.
INTRODUCTION
BK polyomaviruses (BKVs or BKPyVs) are a species of icosahedral, nonenveloped, double-stranded DNA viruses. The genomes of all known full-length isolates of BKV can be categorized into four discrete genotypes based on analyses of nucleotide sequences (1). The prevalence and sequence characteristics of each genotype are thought to vary within different human populations worldwide (2).
Studies indicate that 83 to 98% of individuals show serum antibody responses to the BKV genotype I (BKV-I) major capsid protein, VP1, by the time they are 21 years of age (3). The virus is thought to establish a lifelong chronic infection in the urinary epithelium, and virions are periodically shed at low levels in the urine of 5 to 27% of healthy adults (4, 5). Although BKV-I can cause malignancy in animal model systems, conclusive evidence showing a causal connection between BKVs and human cancer is still lacking (reviewed in reference 6). While it remains uncertain whether BKVs cause pathology in healthy individuals, it is clear that certain T cell-immunosuppressed individuals, such as recipients of kidney or bone marrow transplants, are at risk of developing uncontrolled BKV replication that leads to nephropathy or hemorrhagic cystitis, respectively (reviewed in reference 7). Currently available antiviral drugs are not highly effective for treating these conditions, and patients are managed by reducing the level of immunosuppressive therapy in response to the detection of BKV viremia. Although reductions in T cell-immunosuppressive therapy typically restore immunological control of BKV replication, this approach must be balanced against the increased risk of immunological rejection of the engrafted kidney or graft versus host disease in bone marrow transplant recipients (reviewed in reference 8).
The failure of anti-BKV capsid antibodies to protect transplant recipients against the development of uncontrolled BKV replication has been a long-standing puzzle. When considering this puzzle, it is important to note that nearly all studies of BKV seroepidemiology have relied on assays, such as enzyme-linked immunosorbent assays (ELISAs), that measure antibody binding. A drawback of this approach is that it detects any antibody capable of binding the capsid, including antibodies that do not functionally neutralize the infectivity of the virus. Using reporter pseudoviruses based on individual representatives of BKV genotypes I and IV, we have recently shown that persons with readily detectable BKV-I ELISA reactivity fail to neutralize the infectivity of a BKV-IV pseudovirus (9). This result suggests a model in which individuals who are robustly seropositive in BKV-I-based ELISAs are humorally vulnerable to de novo BKV-IV infection after the implementation of T cell immunosuppression.
The initial goals of the current study were to extend our previous findings to a comprehensive panel of BKV reporter pseudoviruses representing all known clades of BKV and to develop a virus-like particle (VLP) vaccine that might be capable of eliciting broad-spectrum neutralizing antibody responses effective against all BKV serotypes. For other viral families, mutations that drive escape from antibody-mediated neutralization are sometimes accompanied by a shift in the use of cellular receptors for infectious entry (10, 11). To address this possibility, we performed an additional set of analyses examining the binding characteristics and cellular entry tropism of each of the BKV serotypes.
MATERIALS AND METHODS
Ethics statement.
A previously described set of anonymized human serum samples (12) were purchased from Equitech-Bio, Inc., and Innovative Research, Inc. Ethics assurances are posted on the supplier websites.
Animal blood samples were purchased from Lampire Biological Laboratories, with ethical assurance from the supplier. Human red blood cells (RBCs) were collected by finger prick under the approval of the National Cancer Institute (NCI) Institutional Review Board.
Mouse experiments were performed at NCI facilities under the approval of the Animal Care and Use Committee and according to the guidelines of the Association for Assessment and Accreditation of Laboratory Animal Care International. Procedures were carried out in accordance with the eighth edition of the National Research Council of the National Academies' Guide for the Care and Use of Laboratory Animals. All efforts were made to minimize animal suffering.
Phylogenetic analysis.
All nonredundant BKV VP1 protein sequences in GenBank were obtained using a BLASTP search via the U.S. National Library of Medicine. Sequences were aligned using MUSCLE (13) with MacVector version 12.5 default settings. A phylogenetic reconstruction of the alignment was generated using the neighbor-joining method (14) with uncorrected “p” in best tree mode. The tree was displayed using FigTree software (http://tree.bio.ed.ac.uk/software/figtree/). Bootstrap values were 99 for the BKV-I branch, 88 for the BKV-IV branch, 93 for the common BKV-II and BKV-III branch, and 50 or less for the BKV-I subgenotypes, BKV-IV subgenotypes, and the BKV-II and BKV-III branches.
VLP and pseudovirus production.
Based on initial phylogenetic analyses, we chose BKV representatives from each of the major branches of the tree, specifically BKV-Ia (isolate name BK-D; accession number JF894228), BKV-Ib1 (KOM-5; AB211374) (15), BKV-Ib2 (PittVR2; DQ989796) (16), BKV-Ic (RYU-2; AB211377) (15), BKV-II (GBR-12; AB263920) (17), BKV-III (KOM-3; AB211386) (15), BKV-IVb1 (THK-8; AB211390) (15), and BKV-IVc2 (A-66H; AB369093) (2, 9). A codon-modified version of the VP1 gene of each variant was designed according to a previously reported algorithm (18), with the exception of variant KOM-5, which was expressed from an unmodified late region fragment that includes the native agnoprotein and VP2/3 genes (19). The KOM-5 isolate encodes a VP1 protein sequence identical to that of the laboratory strain BK-D. Although the two BKV isolates are formally designated subtype Ib1 and Ia, respectively, these designations are based on nucleotide sequence variations outside the VP1 gene; therefore, they are irrelevant with respect to the present study. The BK-D construct was used to generate VLPs representing both subtypes Ia and Ib1. There was no discernible difference between neutralization assays using the KOM-5 pseudovirus or the codon-modified BK-D-based pseudovirus (unpublished results). This indicates that the two pseudovirus production systems generate pseudovirions that are functionally equivalent in terms of neutralization serology.
Codon-modified versions of the selected BKV variants were synthesized by BlueHeron Biotech. Gateway (Invitrogen) recombination was used to transfer the codon-modified open reading frames into expression plasmid pGwf (12). The codon-modified VP2/VP3 minor capsid protein genes based on BKV isolate A-66H were used for production of all codon-modified BKV pseudoviruses. Since the minor capsid proteins are not exposed on the surface of the virion, they were assumed not to be targets of antibody-mediated neutralization. Plasmid maps and detailed pseudovirus production methods can be found on our laboratory website (http://home.ccr.cancer.gov/LCO/).
VLPs and pseudovirions were generated as previously described (12, 20), with minor modifications. Briefly, 293TT cells (21) were cotransfected with expression plasmids for VP1, VP2, and VP3 together with a reporter plasmid encoding Gaussia luciferase (phGluc). For VLP production, 293TT cells were transfected only with the relevant VP1 plasmid. Roughly 48 h after transfection, pseudovirions or VLPs were harvested by trypsinizing cells and resuspending them at a concentration of >100 million/ml in phosphate-buffered saline (PBS). Neuraminidase V (Sigma) was added to a final concentration of 1 U/ml, and the cell suspension was incubated for 15 min at 37°C. The cells were then lysed by the addition of 0.5% Triton X-100 (Sigma) and incubated at 37°C for an additional 15 min. The lysates were buffered with a final concentration of 25 mM ammonium sulfate. For pseudovirus production, the lysate was treated with 0.1% RNase A/T1 cocktail (Ambion). For VLP production, the lysate was treated with 0.1% benzonase (Sigma) and 0.1% Plasmid-Safe (exonuclease V; Epicentre). Capsid maturation was allowed to proceed overnight at 37°C. Lysates were clarified by centrifugation at 5,000 × g for 10 min and purified through a 27 to 33 to 39% iodixanol step gradient (Optiprep; Sigma).
The VP1 concentration of VLP stocks was determined by comparison to a bovine serum albumin standard (Bio-Rad) in Coomassie-stained SDS-PAGE gels. The VP1 content of pseudovirus preparations was estimated by quantitative Western blot comparison to BKV-Ib1 VLP stock using pooled plasma from mice immunized with all 7 variants of BKV (see below).
Mice and immunization.
Eight-week-old female BALB/cAnNCr mice were immunized intramuscularly with 2 μg of VP1-only BKV-VLPs adsorbed to 50 μg of aluminum hydroxide (Alum; InvivoGen). Eight groups of mice were primed as follows. Seven groups of five mice received one of the individual BKV variants described above (Ia/Ib1, Ib2, Ic, II, III, IVb1, or IVc2); the remaining group of five mice received 2 μg of each of the seven variants mixed together. Four weeks after the priming immunization, submandibular bleeds were performed and plasma was obtained using Microtainer lithium-heparin tubes (Becton, Dickinson). One month after priming, mice were administered an intramuscular booster of the same alum-adsorbed VLP preparation as that used for priming. The mice were euthanized, and heparinized blood was collected by heart puncture 2 months after the initial priming dose.
Neutralization assays.
Neutralization assays were performed as described previously (12). Briefly, 293TT cells were preplated for several hours at a density of 3 × 104 cells per well in 96-well plates. Pseudovirion preparations were diluted according to their infectivity in 293TT cells. Pseudovirion doses ranged from 2 to 90 pg of VP1 per well. Human serum and mouse plasma samples were heat inactivated at 56°C for 30 min. Diluted pseudovirion stocks were combined with 4-fold serial dilutions of serum or plasma and incubated on ice for 1 h before adding the combination to cells for 72 h. Culture supernatants were harvested, and the presence of secreted Gaussia luciferase (Gluc) reporter protein was assayed with a BioLux kit (New England BioLabs) as directed by the manufacturer. Relative light units (RLUs) were read on a POLARstar Optima microplate luminometer (BMG Labtech). The reciprocal 50% neutralizing dilution (EC50) for each serum was calculated using Prism Software (GraphPad) by fitting a variable-slope sigmoidal dose-response curve for each serum/plasma dilution series.
Hemagglutination (HA).
Sodium citrate-preserved animal blood was purchased from Lampire Biological Laboratories. Human red blood cells were collected by finger prick (without preservatives) immediately prior to use. Red blood cells were rinsed in PBS without calcium or magnesium by centrifugation at 2,000 × g for 5 min twice. The pelleted cells were suspended in PBS at 0.5% (vol/vol) and distributed into round-bottom 96-well plates. Three-fold serial dilutions of VP1-only VLPs spanning a range of 10,000 ng/ml to 56 pg/ml from each of the BKV variants were mixed with the diluted RBCs, and the suspension was allowed to settle at 4°C for 3 h.
Cell culture and infectivity assays.
293TT cells (21) were cultured in Dulbecco's modified essential medium (DMEM) with 10% fetal bovine serum (FBS), Glutamax-I, and 250 μg/ml hygromycin (Invitrogen). GM95 cells (Riken Resource Center, Japan) were cultured in the same medium without hygromycin. ART cells were cultured in RPMI medium containing 10% FBS, Glutamax-I, and 170 μg/ml of hygromycin. ART cells were generated by stable transfection of the ovarian cancer line NCI/ADR-RES (Developmental Therapeutics Program [DTP]; National Cancer Institute, NIH) with the simian virus 40 (SV40) large T antigen (LT) expression plasmid pTIH (21). SFT cells were derived from the gliosarcoma SF-539 cell line (DTP) by stable transfection with the pTIH plasmid, and the line was maintained with 50 μg/ml of hygromycin in RPMI with 5% FBS. A549 cells (DTP) were cultured in RPMI with 5% FBS.
For infectivity assays, cells were seeded at 2,000 to 30,000 cells per well in 96-well plates several hours prior to infection in their respective media and in the presence of 100 μg/ml of Primocin antibiotic (Invivogen). For 293TT, ART, SFT, and A549 cells, 50 pg of BKV pseudovirions was added to the wells and incubated for 72 h before assaying for Gaussia luciferase expression in the culture supernatant. For GM95 cells, 1.3 ng of pseudovirions was used. Experiments using GM95 cells were repeated using serum-free medium, with similar results (data not shown).
RESULTS
BKV serotyping analysis using sera from naturally infected humans.
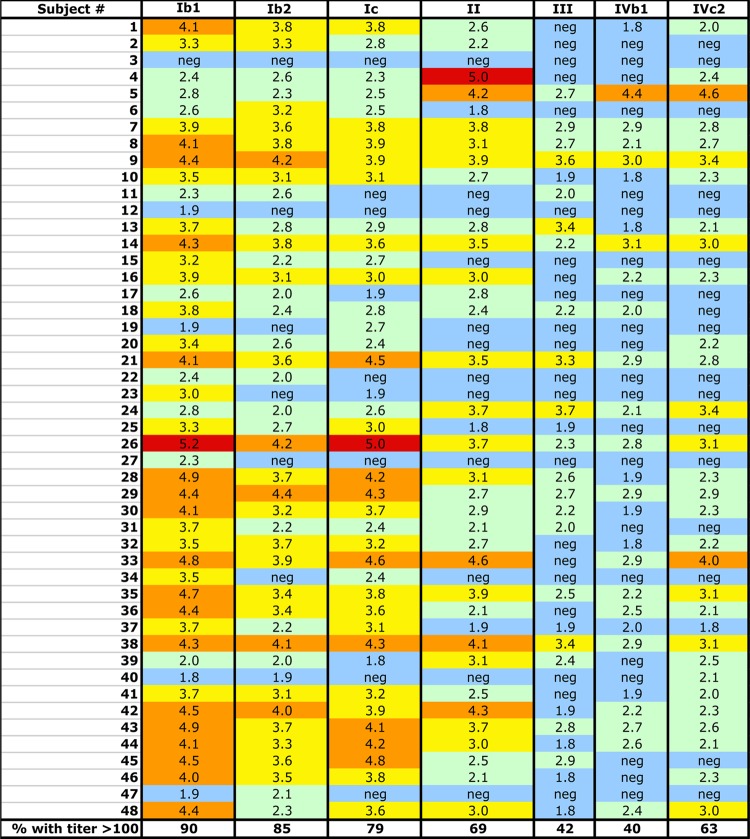
To comprehensively address the relationship between BKV genotypes and serotypes, we developed reporter pseudoviruses based on the VP1 major capsid proteins of seven distinct BKV primary isolates from each major arm of a VP1 protein-based phylogenetic tree (Fig. 1; also see Fig. S1 in the supplemental material). The seven reporter pseudoviruses were used to perform neutralization assays on a panel of sera from 48 healthy human subjects. The results (50% neutralizing titers [EC50], expressed in reciprocal log values) are summarized in Fig. 2.
Fig 1.
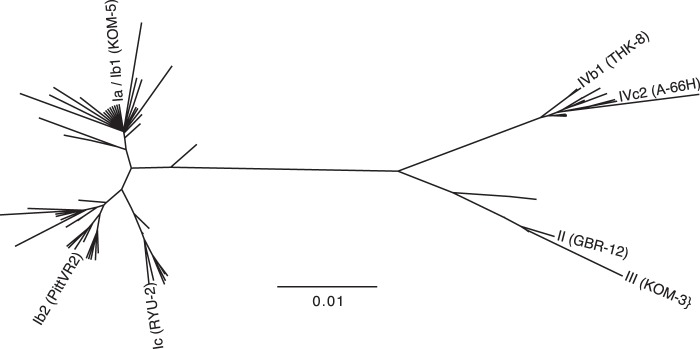
Phylogenetic tree of BKV variants. The tree illustrates the relationship between the VP1 proteins of the seven tested BKV isolates (labeled branches) and other unique full-length BKV VP1 sequences found in GenBank (unlabeled branches). BKV genotypes Ia and Ib1 are indistinguishable on the basis of VP1 protein sequence.
Fig 2.
BKV-neutralizing titers of human sera. The chart shows the neutralizing titer (displayed as log10 values) of the specified BKV variant (columns) by serum samples from individual human subjects (rows). Log titers of 5 and above are shown in red, 4 to 4.9 in orange, 3 to 3.9 in yellow, 2 to 2.9 in green, and negative (undetectable to 1.9) in blue.
The definition of a serotype varies depending on the viral family. For rotaviruses, a 20-fold difference in neutralizing titer between cognate and noncognate types is sufficient to apply the distinction (22), while for adenovirus, 8- to 16-fold differences define serotypes (23). For BKV, a very wide range of titers has been used to define serotypes (4- to 100-fold difference) (9, 24). For this study, we chose a conservative definition of at least a 100-fold difference in neutralizing titer between two BKV variants for at least one human serum sample. By this definition, genotypes I, II, III, and IV can be considered distinct serotypes in at least one tested individual. In contrast, the two BKV-IV subtypes (IVb1 and IVc2) showed similar neutralizing titers, suggesting that all members of BKV genotype IV occupy a single serotype. All of the human sera showed similar neutralizing titers against the Ib2 and Ic pseudoviruses, indicating a single serotype. The serum of subject 48 indicated that subtypes Ib1 and Ib2 could be considered distinct serotypes by our conservative definition. An additional eight individuals showed Ib1 versus Ib2 titer differences ranging from 16- to 32-fold. In the cases of subjects 23, 27, and 34, the Ib1 pseudovirus was neutralized with EC50 titers ranging from 200 to 3,200, but these individuals showed no detectable neutralization of the Ib2 pseudovirus at the 1:50 dilution. This leaves open the possibility that the true Ib2 EC50 titer is much less than 50, meeting the 100-fold difference definition. The results indicate that the seven tested BKV isolates can be divided into at least five serotypes.
PCR-based prevalence studies have suggested that infection with BKV-II or -III is rare in all human populations worldwide (17, 25). In contrast to these prior PCR-based prevalence studies, our serological analysis indicates that 33/48 (69%) members of this group of subjects are BKV-II seropositive (defined by EC50 titers of 100 or greater). The seroprevalence of BKV-III is also much higher than expected (42%).
Confirmatory serotyping in BKV VLP-vaccinated mice.
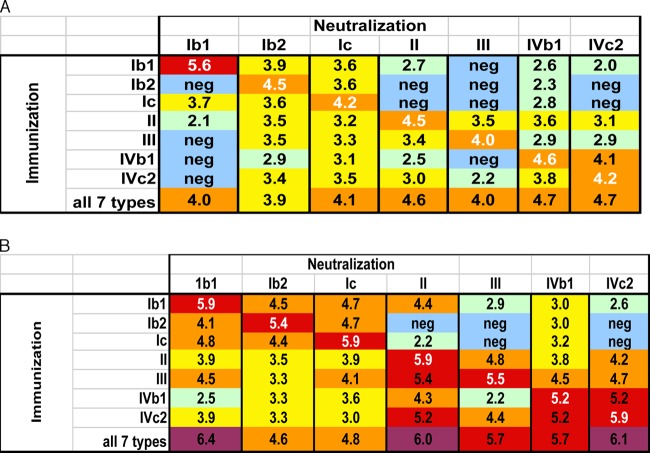
A few of the human sera detectably neutralized all tested BKV pseudoviruses. However, a complicating factor in analysis of sera from naturally infected humans is that some individuals may be simultaneously coinfected with multiple BKV types. Thus, it is unclear whether broadly neutralizing human sera reflect coinfection with multiple BKV subtypes or a single-serotype infection that has generated an unusually broad degree of cross-neutralizing antibodies. To address this issue, we confirmed the human serotyping results using plasma from mice that were experimentally vaccinated with BKV VLP-based vaccines. Separate groups of mice were immunized with recombinant VLPs based on each of the seven BKV subtypes. An eighth group of mice was given a combination of all seven BKV VLP subtypes. Four weeks after priming the mice, samples from each group were pooled and tested for neutralizing activity against the cognate or noncognate (i.e., homologous and heterologous) BKV pseudoviruses.
Mice primed with VLPs from individual BKV subtypes generated high-titer cognate responses after priming (Fig. 3A), with neutralizing EC50s as high as 400,000 (Ib1 immunization versus Ib1 neutralization). Robust cross-neutralization was also observed for most closely related pairs of subtypes. For example, Ib2-primed mice neutralized the cognate Ib2 pseudovirus with a titer of 32,000 and cross-neutralized the Ic pseudovirus with a titer of 4,000. In contrast, the same Ib2 sample failed to detectably neutralize the Ib1 pseudovirus. This result confirms the observation that subtypes Ib1 and Ib2 can behave as distinct serotypes.
Fig 3.
BKV-neutralizing responses in mice. The chart shows the neutralizing titer (log10) of the specified BKV variant (column) by pooled plasma samples from mice (5 animals per group) immunized with VP1-only VLPs based on specified BKV variants (rows). (A) Samples taken 4 weeks after initial VLP priming. (B) Samples taken 4.5 weeks after administration of a booster dose of VLPs.
Testing of mouse samples also confirmed that the two BKV-IV subtypes are not serologically distinct from one another. Puzzlingly, results for the primed mice failed to confirm the BKV-II versus BKV-III difference that was clearly evident in several human serum samples.
The group of mice that was primed with the mixture of all seven VLP types had neutralizing titers against all subtypes, with titers ranging from 8,000 (BKV-Ib2) to 50,000 (BKV-IVb1 and BKV-IVc2). This shows that a single 2-μg dose of a multivalent VLP vaccine administered intramuscularly in alum can elicit antibody responses capable of potently neutralizing all known BKV isolates.
After booster immunization of the primed mice, cognate neutralizing responses increased by 2- to 16-fold (Fig. 3B). Cross-neutralization was also markedly improved. For example, mice primed with BKV-Ib2, -III, -IVb1, and -IVc2, which had undetectable anti-BKV-Ib1 neutralizing titers after priming, showed anti-Ib1 titers as high as 13,000 after boosting. Despite the overall increase in reactivity, some of the viruses remained refractory to antibody-mediated neutralization in complex nonreciprocal patterns. For example, the Ib2 VLP immunization did not elicit detectable neutralization of BKV-II, -III, or -IVc2, while all seven individual VLP vaccines elicited neutralizing antibody responses against BKV-Ib2 with titers of at least 2,000.
Fine mapping of VP1 residues responsible for Ib1/Ib2 serotype differences.
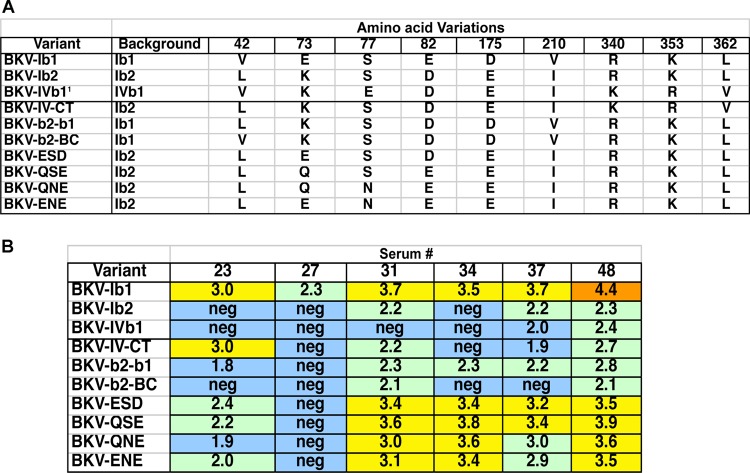
The BKV Ib1 and Ib2 isolates that we have analyzed differ from one another by only five amino acid residues (Fig. 4A; also see Fig. S1 in the supplemental material). It is unexpected that this small number of changes can result in escape from neutralization by polyclonal antibody responses in both mice and humans. To explore which specific residues are essential for defining these two serotypes, we generated a panel of intersubtype chimeric VP1 constructs based on amino acid variations found in different isolates of BKV-Ib1 and -Ib2 (Fig. 4A).
Fig 4.
Identification of amino acids responsible for serotypes. (A) Amino acid variations in natural BKV variants and mutants created for this work. (B) Neutralizing titers (log10) of six normal human sera against various VP1 mutant pseudoviruses. All sera on this panel showed >10-fold differences between BKV-Ib1 and Ib2 in initial testing (Fig. 1).
Not all BKV-Ib2 isolates have identical VP1 proteins. Relative to Ib1 and the Ib2 isolate we used, some also carry substitutions near the VP1 C terminus. These chemically subtle substitutions include R340K, K353R, and L362V (see Fig. S1 in the supplemental material). Coincidentally, the BKV-IVb1 isolate we used carries all three substitutions. We therefore generated a chimeric Ib2 VP1 carrying the C terminus of IVb1. Reporter pseudoviruses assembled from the IV-CT chimeric VP1 were tested against a panel of six human sera that had shown significant titer differences between Ib1 and Ib2 neutralization. The human sera generally neutralized the chimeric virus in a manner similar to that of the unmodified Ib2 pseudovirus, indicating that C-terminal variations do not play a major role in defining the observed Ib1/Ib2 serotype difference (Fig. 4B).
An additional pseudovirus, b2-b1, was constructed in which the N-terminal third of VP1 was derived from Ib2 (containing three of five amino acid differences) and the remainder was derived from Ib1. This chimeric pseudovirus was neutralized in a manner similar to that for Ib2. A separate pseudovirus carrying the BC loop of Ib2 (containing two of five amino acid differences) in the Ib1 background (mutant b2-BC) also behaved like the Ib2 parent in neutralization assays. The results map the serological phenotype of Ib2 to the BC loop, which contains E73K and E82D transitions relative to Ib1. A panel of additional mutants manipulating residues 73, 77, and 83 suggests that the identity of residue 73 is of particular importance. This result is consistent with previous study of BKV's close relative, SV40, showing that positions 73 and 77 (within the BC2 loop) are crucial for escape from a neutralizing monoclonal antibody (26). The Ib1/Ib2 serotyping results were confirmed by neutralization serology using sera from vaccinated mice (data not shown).
Different BKV serotypes show different entry tropisms.
Alignments of divergent BKV VP1 proteins show a hypervariable region spanning residues 61 to 82 (see Fig. S1 in the supplemental material). These residues map to the BC loop on the outer surface of the VP1 capsomer knob. Interestingly, previous studies (27) have shown that the BC and HI loops of BKV VP1 play a crucial role in binding to its known cell surface receptors (ganglioside GT1b or GD1b) (28). We therefore wondered whether the different BKV serotypes engage different spectra of cell surface attachment receptors.
HA of human red blood cells by BKV has long been used as a surrogate measure of viral binding to cell surface receptor glycans during the infectious entry process (29). We therefore tested the HA of RBCs from humans and other animal species with the different BKV variants to see if their binding patterns vary. All BKV variants were able to hemagglutinate human RBCs when the virus was added at concentrations ranging from 1.5 to 41 ng/ml, except BKV-II, which required 7,000 ng/ml of VLPs to hemagglutinate these RBCs (Fig. 5). For each of the BKV variants, the HA patterns for RBCs from different animal species varied dramatically. These data suggest that each BKV variant binds a distinct spectrum of cell surface attachment moieties on red blood cells.
Fig 5.
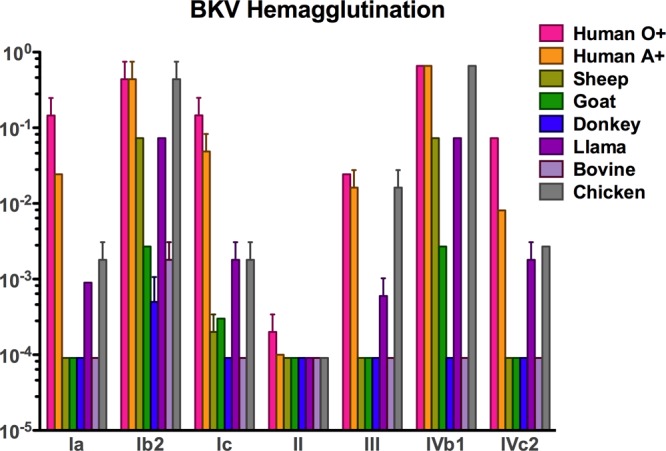
Hemagglutination by BKV variants. Bar graphs express the inverse of the last amount (in ng/ml) of VP1 VLPs that was able to hemagglutinate a 0.5% solution of red blood cells from the animal species indicated.
We next used each of the seven BKV reporter pseudoviruses to transduce four different cell lines. Several cell lines were chosen based on their relatively high transducibility with the BKV-IVc2 pseudovirus (30). The human embryonic kidney-based cell line 293TT expresses high levels of the SV40 large T antigen (LT), which drives replication of the incoming reporter pseudogenome, thereby increasing the expression of the Gluc reporter protein. This allows the use of lower, more physiologically relevant doses of pseudovirions. Two additional cell lines, NCI/ADR-RES and SF-539 (ovarian cancer and gliosarcoma, respectively), were modified to stably express SV40 LT. The resulting lines were named ART and SFT, respectively. A549 cells (non-small-cell lung carcinoma), which do not express LT, were also transduced.
The transduction results show that different BKV pseudovirus types have distinct entry tropism patterns on the four tested cell lines (Fig. 6). In order to assess transduction on a per-cell basis, green fluorescent protein (GFP) was used as the reporter gene, and similar results were observed (data not shown). Remarkably, the HA patterns observed for the different BKV variants do not correlate with the infectivity data. This suggests that the attachment receptors used during transduction of cultured cell lines are not the same as the attachment moieties engaged during red blood cell agglutination. This apparent discrepancy is reminiscent of misleading results encountered by researchers using HA assays to analyze the neutralization of influenza viruses (reviewed in reference 31). The results suggest that traditional HA assays are not an appropriate proxy for BKV infectious entry.
Fig 6.
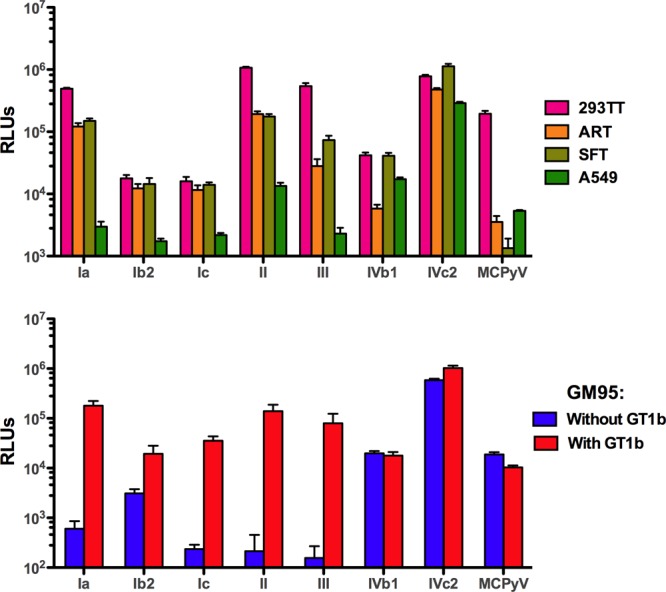
Transduction efficiency of BKV variants. BKV variants were normalized by VP1 content and tested for transduction of various human cell lines (top panel) or the ganglioside-deficient murine cell line, GM95 (bottom panel). GM95 cells were preloaded with the ganglioside GT1b or mock loaded. Error bars represent the standard deviations from quadruplicate testing. Similar results were observed in three independent experiments.
BKV-Ia is known to use GT1b or GD1b gangliosides as infectious entry receptors (28). To examine whether other BKV genotypes require gangliosides for infectious entry, we transduced murine GM95 cells, which lack gangliosides due to a defect in ceramide glucosyltransferase activity (32). GM95 cells were transduced with each BKV variant with or without supplementation with exogenous ganglioside GT1b. Merkel cell polyomavirus (MCPyV), which does not use gangliosides for infectious entry (33), was used as a control. As expected, BKV-Ib1 infectivity was highly sensitive to the presence of gangliosides, showing a 300-fold improvement in transduction when GT1b was present (Fig. 6B). The infectivity of BKV-Ic, -II, and -III pseudoviruses was also highly responsive to ganglioside supplementation. Variant BKV-Ib2 showed only a modest (6-fold) improvement in transduction with the presence of GT1b. This was largely attributable to moderate levels of transduction of the GM95 cells in the absence of GT1b. Surprisingly, the two representatives of the BKV-IV genotype (IVb1 and IVc2) behaved like the MCPyV control, robustly transducing the GM95 cells in the absence of exogenous gangliosides, and supplementation with GT1b had no effect. Similar results were obtained when GD1a or GD1b was used as the supplemented ganglioside (data not shown).
The results show that BKV-IV isolates can transduce the murine GM95 cell line via a ganglioside-independent pathway. This contrasts with our previous data showing that BKV-IV cannot efficiently transduce ganglioside-deficient Chinese hamster ovary (CHO) cells unless GT1b is added exogenously (33). To examine whether BKV-IV can transduce cell lines other than GM95 via a ganglioside-independent pathway, we performed a series of experiments in which ganglioside expression was knocked down on human A549 or ART cells using short interfering RNA targeting GM3 synthase (34). The results showed that GM3 synthase knockdown inhibited BKV-IV transduction (data posted at http://home.ccr.cancer.gov/Lco/BKV.asp). Thus, BKV-IV transduction appears to follow different entry pathways in different cell lines. Specifically, the pseudovirus can use a ganglioside-independent pathway for transduction of GM95, while the presence of complex gangliosides is required for efficient transduction of other cell lines, including CHO, A549, and ART.
DISCUSSION
Although antibodies can neutralize viral infectivity through a variety of mechanisms, a classic mechanism is antibody occlusion of virion surface epitopes that mediate attachment of the virus to cell surface receptor moieties. Viruses are confronted by the evolutionary problem that any mutations that prevent recognition by this type of antibody must also preserve the function of underlying receptor-binding motifs required for the initial interaction with host cells during the infectious entry process.
Members of many different viral families use sialylated glycans present on the cell surface as attachment receptors or coreceptors for infectious entry (reviewed in reference 35). Vertebrate cells express a wide range of distinct sialylated glycans; thus, viruses may be presented with a wide range of glycan choices that could serve for the attachment step of the infectious entry process. Therefore, one potential mechanism of escape from receptor-binding site-occluding antibodies is through viral mutations that simultaneously prevent antibody binding and also alter the sialylated glycan receptor specificity of the virion. An apparent correlation between altered sialylated glycan usage and escape from neutralization by an antibody has been recently documented for influenza A virus (36). Interestingly, this study showed that the antibody-driven alteration of receptor usage resulted in a more virulent phenotype in experimentally infected animals. In experiments using adeno-associated virus (AAV), Li and colleagues have likewise shown that small numbers of amino acid changes in the viral capsid protein can simultaneously shift cellular tropism and also promote escape from antibody-mediated neutralization in experimentally vaccinated animals (10). A recent study of porcine rotavirus also provides an example of apparent antibody escape variations clustered around the viral receptor-binding site (11).
Using polyclonal sera from naturally infected humans and experimentally vaccinated mice, we have shown that the VP1 capsid proteins of each of the major BKV genotypes can reciprocally escape from neutralization by antibodies raised against the other types. Surprisingly, even the closely related BKV subgenotypes Ib1 and Ib2 represent at least partially distinct neutralization serotypes. The serotype-defining differences between these two subgenotypes were mapped to only two amino acid differences within the BC2 surface loop of VP1.
Our data show that each of the neutralization serotypes appears to engage a distinct spectrum of cell surface attachment receptors. This is consistent with past observations showing that the BC2 loop, where at least some of the antibody escape mutations were mapped, is critically involved in engagement of the sialylated glycan headgroup of the gangliosides required for BKV-I infectious entry. Our findings raise the interesting question of whether different BKV genotypes or subgenotypes exhibit altered tissue tropism or different levels of virulence in vivo. Although previous studies have surveyed BKV genotypes found in nephropathic lesions (15, 16, 37–39), these studies have relied on BKV-I-biased PCR primers that underestimate the abundance of BKV genotypes II, III, and IV (40). The possibility that commonly used PCR primers do not robustly detect non-genotype I BKVs (particularly in the case of individuals coinfected with multiple BKV genotypes) is consistent with our observation that BKV-II seroprevalence (Fig. 2) is about 20-fold higher than prior PCR-based prevalence estimates (17, 25). A recent report by Chehadeh and Nampoory may also illustrate the problem of older BKV-I-biased PCR results (41). This study of serum and urine samples from kidney transplant recipients used PCR primers targeting highly conserved sequences within the VP1 genes of all currently known BKV and JCV isolates. Although a majority of the BKV amplicons were identical to previously identified genotypes, a number of the reported amplicon sequences showed entirely novel amino acid changes clustered in the VP1 BC loop (data posted at http://home.ccr.cancer.gov/Lco/BKV.asp). The result raises the possibility of the existence of additional, previously undetected BKV serotypes. In light of these uncertainties, the issue of whether certain BKV genotypes are more often correlated with nephropathic lesions or hemorrhagic cystitis should be considered an important unresolved question.
We have previously suggested a model in which kidney transplant recipients who lack antibodies capable of neutralizing one or more BKV serotypes are at risk of de novo infection with those serotypes after the implementation of T cell-immunosuppressive therapy to prevent graft rejection (9). Such a de novo infection might arise either from the transplanted kidney or from environmental exposure to the virus. Our finding that very small numbers of mutations in VP1 can permit escape from antibody-mediated neutralization raises the third possibility that spontaneous mutations arising from a preexisting BKV infection allow the virus to escape from some recipients' neutralizing antibody repertoires.
It seems likely that a vaccine capable of eliciting neutralizing antibodies against all BKV serotypes prior to the implementation of immunosuppressive therapy could protect kidney transplant recipients against diseases caused by BKV replication. It is encouraging that mice given BKV VLP vaccines displayed high-titer serum antibody responses capable of neutralizing all known BKV serotypes. However, it will be important to investigate whether BKV VLP vaccines can elicit comparable broadly cross-neutralizing responses in humans, who have typically already been exposed to one or more BKV genotypes.
Although a multivalent vaccine containing a mixture of BKV genotypes was most effective in the murine model, monovalent prime-boost vaccination with some individual genotypes elicited responses capable of neutralizing all tested BKV serotypes with at least moderate titers. A possible explanation for the much broader cross-neutralizing responses observed in boosted mice could be that the boosting effectively restimulated a broad range of memory B cells to become antibody-secreting plasma cells (42). A minority of such restimulated cells would presumably secrete antibodies that fortuitously cross-neutralize a range of different BKV serotypes. If this model is correct, then humans infected with only one BKV serotype might already harbor a subset of memory B cells with antibodies capable of cross-neutralizing other BKV serotypes. Thus, VLP-based vaccination might induce broadly cross-neutralizing serum antibody responses simply by stimulating a broader range of existing memory B cells to become plasma cells.
Supplementary Material
ACKNOWLEDGMENTS
This work was by the Intramural Research Program of the NIH, with support from the Center for Cancer Research and the NCI Director's Intramural Innovation Award Program.
Authors C.B.B. and D.V.P. are coinventors on U.S. patent application no. 61/508,897. All other authors declare no conflict of interest.
Footnotes
Published ahead of print 10 July 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JVI.01189-13.
REFERENCES
- 1. Luo C, Bueno M, Kant J, Martinson J, Randhawa P. 2009. Genotyping schemes for polyomavirus BK, using gene-specific phylogenetic trees and single nucleotide polymorphism analysis. J. Virol. 83:2285–2297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhong S, Randhawa PS, Ikegaya H, Chen Q, Zheng HY, Suzuki M, Takeuchi T, Shibuya A, Kitamura T, Yogo Y. 2009. Distribution patterns of BK polyomavirus (BKV) subtypes and subgroups in American, European and Asian populations suggest co-migration of BKV and the human race. J. Gen. Virol. 90:144–152 [DOI] [PubMed] [Google Scholar]
- 3. Stolt A, Sasnauskas K, Koskela P, Lehtinen M, Dillner J. 2003. Seroepidemiology of the human polyomaviruses. J. Gen. Virol. 84:1499–1504 [DOI] [PubMed] [Google Scholar]
- 4. Zhong S, Zheng HY, Suzuki M, Chen Q, Ikegaya H, Aoki N, Usuku S, Kobayashi N, Nukuzuma S, Yasuda Y, Kuniyoshi N, Yogo Y, Kitamura T. 2007. Age-related urinary excretion of BK polyomavirus by nonimmunocompromised individuals. J. Clin. Microbiol. 45:193–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Husseiny MI, Anastasi B, Singer J, Lacey SF. 2010. A comparative study of Merkel cell, BK and JC polyomavirus infections in renal transplant recipients and healthy subjects. J. Clin. Virol. 49:137–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Abend JR, Jiang M, Imperiale MJ. 2009. BK virus and human cancer: innocent until proven guilty. Semin. Cancer Biol. 19:252–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dalianis T, Hirsch HH. 2013. Human polyomaviruses in disease and cancer. Virology 437:63–72 [DOI] [PubMed] [Google Scholar]
- 8. Kasiske BL ZM, Craig JC, Ekberg H, Garvey CA, Green MD, Jha V, Josephson MA, Kiberd BA, Kreis HA, McDonald RA, Newmann JM, Obrador GT, Chapman JR, Vincenti FG, Balk EM, Wagner M, Raman G, Earley A, Abariga S. 2009. KDIGO clinical practice guideline for the care of kidney transplant recipients. Am. J. Transplant 9 (Suppl. 3):S1–S155 [DOI] [PubMed] [Google Scholar]
- 9. Pastrana DV, Brennan DC, Cuburu N, Storch GA, Viscidi RP, Randhawa PS, Buck CB. 2012. Neutralization serotyping of BK polyomavirus infection in kidney transplant recipients. PLoS Pathog. 8:e1002650. 10.1371/journal.ppat.1002650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li C, Diprimio N, Bowles DE, Hirsch ML, Monahan PE, Asokan A, Rabinowitz J, Agbandje-McKenna M, Samulski RJ. 2012. Single amino acid modification of adeno-associated virus capsid changes transduction and humoral immune profiles. J. Virol. 86:7752–7759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ciarlet M, Hoshino Y, Liprandi F. 1997. Single point mutations may affect the serotype reactivity of serotype G11 porcine rotavirus strains: a widening spectrum? J. Virol. 71:8213–8220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pastrana DV, Tolstov YL, Becker JC, Moore PS, Chang Y, Buck CB. 2009. Quantitation of human seroresponsiveness to Merkel cell polyomavirus. PLoS Pathog. 5:e1000578. 10.1371/journal.ppat.1000578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Edgar RC. 2004. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32:1792–1797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Saitou N, Nei M. 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4:406–425 [DOI] [PubMed] [Google Scholar]
- 15. Nishimoto Y, Takasaka T, Hasegawa M, Zheng HY, Chen Q, Sugimoto C, Kitamura T, Yogo Y. 2006. Evolution of BK virus based on complete genome data. J. Mol. Evol. 63:341–352 [DOI] [PubMed] [Google Scholar]
- 16. Sharma PM, Gupta G, Vats A, Shapiro R, Randhawa P. 2006. Phylogenetic analysis of polyomavirus BK sequences. J. Virol. 80:8869–8879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zheng HY, Nishimoto Y, Chen Q, Hasegawa M, Zhong S, Ikegaya H, Ohno N, Sugimoto C, Takasaka T, Kitamura T, Yogo Y. 2007. Relationships between BK virus lineages and human populations. Microbes Infect. 9:204–213 [DOI] [PubMed] [Google Scholar]
- 18. Pastrana DV, Buck CB, Pang YY, Thompson CD, Castle PE, FitzGerald PC, Kruger Kjaer S, Lowy DR, Schiller JT. 2004. Reactivity of human sera in a sensitive, high-throughput pseudovirus-based papillomavirus neutralization assay for HPV16 and HPV18. Virology 321:205–216 [DOI] [PubMed] [Google Scholar]
- 19. Nakanishi A, Chapellier B, Maekawa N, Hiramoto M, Kuge T, Takahashi RU, Handa H, Imai T. 2008. SV40 vectors carrying minimal sequence of viral origin with exchangeable capsids. Virology 379:110–117 [DOI] [PubMed] [Google Scholar]
- 20. Buck CB, Thompson CD. 2007. Production of papillomavirus-based gene transfer vectors. Curr. Protoc. Cell Biol. Chapter 26:Unit 26.21. 10.1002/0471143030.cb2601s37 [DOI] [PubMed] [Google Scholar]
- 21. Buck CB, Pastrana DV, Lowy DR, Schiller JT. 2004. Efficient intracellular assembly of papillomaviral vectors. J. Virol. 78:751–757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wyatt RG, Greenberg HB, James WD, Pittman AL, Kalica AR, Flores J, Chanock RM, Kapikian AZ. 1982. Definition of human rotavirus serotypes by plaque reduction assay. Infect. Immun. 37:110–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fauquet C, Mayo MA, Maniloff J, Desselberger U, Ball LA. (ed). 2005. Virus taxonomy: eighth report of the International Committee on Taxonomy of Viruses. Elsevier/Academic Press, Amsterdam, the Netherlands [Google Scholar]
- 24. Knowles WA, Gibson PE, Gardner SD. 1989. Serological typing scheme for BK-like isolates of human polyomavirus. J. Med. Virol. 28:118–123 [DOI] [PubMed] [Google Scholar]
- 25. Yogo Y, Sugimoto C, Zhong S, Homma Y. 2009. Evolution of the BK polyomavirus: epidemiological, anthropological and clinical implications. Rev. Med. Virol. 19:185–199 [DOI] [PubMed] [Google Scholar]
- 26. Murata H, Teferedegne B, Sheng L, Lewis AM, Jr, Peden K. 2008. Identification of a neutralization epitope in the VP1 capsid protein of SV40. Virology 381:116–122 [DOI] [PubMed] [Google Scholar]
- 27. Dugan AS, Gasparovic ML, Tsomaia N, Mierke DF, O'Hara BA, Manley K, Atwood WJ. 2007. Identification of amino acid residues in BK virus VP1 that are critical for viability and growth. J. Virol. 81:11798–11808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Low JA, Magnuson B, Tsai B, Imperiale MJ. 2006. Identification of gangliosides GD1b and GT1b as receptors for BK virus. J. Virol. 80:1361–1366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sinibaldi L, Viti D, Goldoni P, Cavallo G, Caroni C, Orsi N. 1987. Inhibition of BK virus haemagglutination by gangliosides. J. Gen. Virol. 68(Part 3):879–883 [DOI] [PubMed] [Google Scholar]
- 30. Schowalter RM, Reinhold WC, Buck CB. 2012. Entry tropism of BK and Merkel cell polyomaviruses in cell culture. PLoS One 7:e42181. 10.1371/journal.pone.0042181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hoag H. 2013. A universal problem. Nat. Med. 19:12–14 [DOI] [PubMed] [Google Scholar]
- 32. Ichikawa S, Nakajo N, Sakiyama H, Hirabayashi Y. 1994. A mouse B16 melanoma mutant deficient in glycolipids. Proc. Natl. Acad. Sci. U. S. A. 91:2703–2707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schowalter RM, Pastrana DV, Buck CB. 2011. Glycosaminoglycans and sialylated glycans sequentially facilitate Merkel cell polyomavirus infectious entry. PLoS Pathog. 7:e1002161. 10.1371/journal.ppat.1002161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Magaldi TG, Buch MH, Murata H, Erickson KD, Neu U, Garcea RL, Peden K, Stehle T, DiMaio D. 2012. Mutations in the GM1 binding site of simian virus 40 VP1 alter receptor usage and cell tropism. J. Virol. 86:7028–7042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Neu U, Bauer J, Stehle T. 2011. Viruses and sialic acids: rules of engagement. Curr. Opin. Struct. Biol. 21:610–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. O'Donnell CD, Vogel L, Wright A, Das SR, Wrammert J, Li GM, McCausland M, Zheng NY, Yewdell JW, Ahmed R, Wilson PC, Subbarao K. 2012. Antibody pressure by a human monoclonal antibody targeting the 2009 pandemic H1N1 virus hemagglutinin drives the emergence of a virus with increased virulence in mice. mBio 3:e00120–12. 10.1128/mBio.00120-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hoffman NG, Cook L, Atienza EE, Limaye AP, Jerome KR. 2008. Marked variability of BK virus load measurement using quantitative real-time PCR among commonly used assays. J. Clin. Microbiol. 46:2671–2680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jin L, Gibson PE, Knowles WA, Clewley JP. 1993. BK virus antigenic variants: sequence analysis within the capsid VP1 epitope. J. Med. Virol. 39:50–56 [DOI] [PubMed] [Google Scholar]
- 39. Krumbholz A, Zell R, Egerer R, Sauerbrei A, Helming A, Gruhn B, Wutzler P. 2006. Prevalence of BK virus subtype I in Germany. J. Med. Virol. 78:1588–1598 [DOI] [PubMed] [Google Scholar]
- 40. Randhawa P, Kant J, Shapiro R, Tan H, Basu A, Luo C. 2011. The impact of genomic sequence variability on quantitative polymerase chain reaction assays for the diagnosis of polyomavirus BK infection. J. Clin. Microbiol. 49:4072–4076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chehadeh W, Nampoory MR. 2013. Genotypic diversity of polyomaviruses circulating among kidney transplant recipients in Kuwait. J. Med. Virol. [Epub ahead of print.] 10.1002/jmv.23639 [DOI] [PubMed] [Google Scholar]
- 42. Purtha WE, Tedder TF, Johnson S, Bhattacharya D, Diamond MS. 2011. Memory B cells, but not long-lived plasma cells, possess antigen specificities for viral escape mutants. J. Exp. Med. 208:2599–2606 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.