Abstract
Changes in mRNA stability and translation are critical control points in the regulation of gene expression, particularly genes encoding growth factors, inflammatory mediators, and proto-oncogenes. Adenosine and uridine (AU)-rich elements (ARE), often located in the 3′ untranslated regions (3′UTR) of mRNAs, are known to target transcripts for rapid decay. They are also involved in the regulation of mRNA stability and translation in response to extracellular cues. This review focuses on one of the best characterized ARE binding proteins, tristetraprolin (TTP), the founding member of a small family of CCCH tandem zinc finger proteins. In this survey, we have reviewed the current status of TTP interactions with mRNA and proteins, and discussed current thinking about TTP's mechanism of action to promote mRNA decay. We also review the proposed regulation of TTP's functions by phosphorylation. Finally, we have discussed emerging evidence for TTP operating as a translational regulator.
Introduction
Gene expression is a highly regulated process that begins with transcriptional initiation and ends with translation of a mature mRNA into protein. In between these two points are a linked series of events including processing and splicing of the pre-mRNA, export of the message from the nucleus to the cytoplasm, quality control assessment of the mRNA through the pioneer round of translation, message decay and stabilization, and translational repression and de-repression. All of these events from initiation of transcription by transcription factors to the stability of the message to effective translation of the message are controlled by the presence of specific nucleotide sequences which are bound by specific trans-acting proteins. As a message is transcribed, proteins bind to form a messenger ribonucleoprotein complex (mRNP) and the composition of the mRNP controls all aspects of the life of the mRNA, from pre-mRNA processing to mRNA localization to translation and degradation. Transitions between these events are accompanied by mRNP remodeling and exchange of mRNP proteins.
Posttranscriptional Regulation
Posttranscriptional control of gene expression, particularly mRNA stability and translation, allows for rapid changes in mRNA levels. Dysregulated mRNA stability and translation underlies a number of diseases, directly contributing to the overexpression of many genes encoding growth factors, inflammatory cytokines, and proto-oncogenes [1,2,3,4]. Among the best studied cis-elements regulating posttranscriptional control are the adenosine and uridine (AU)-rich elements (ARE), located in the 3′ untranslated region (3′UTR) of many unstable mRNAs. AREs are most often arranged in repeated and/or overlapping pentamers of the sequence AUUUA, although there can be variability [5]. The relevance of these conserved cis-acting sequence elements is apparent in their frequency, currently estimated at approximately 8% of the human transcriptome [6,7]. AREs target mRNAs for rapid decay but also allow the stability and translation of mRNAs to be regulated in response to extracellular cues. These effects of an ARE on the posttranscriptional fate of a message depend on the interaction of an ever increasing list of trans-acting RNA binding proteins [4,8]. The focus of this review is on one of the best characterized ARE binding proteins, tristetraprolin (TTP).
Tristetraprolin
TTP, also known as Nup475, G0S24, and TIS11, is the founding member of a small family of proteins containing tandem CCCH zinc fingers. One of the major purposes of this review was to survey the current literature for reports of mRNA binding “targets” for TTP. As described below, we tried to include as many reports as we could find, but we did not attempt to determine the likelihood that a given report described a “true”, physiologically relevant interaction. Accordingly, we performed a literature search on August 15, 2012, searching PubMed, Scopus, and Web of Science for the terms tristetraprolin, G0S24, and Nup475, and tris-tetraprolin; and we also searched with the combined terms “TTP and RNA”. After the removal of abstracts, duplicates and papers on unrelated subjects, this search yielded 293 papers under those search terms.
The original description of full-length TTP was in 1990 [9], which describes the cloning of the mouse cDNA from insulin-stimulated 3T3-L1 fibroblasts; this was followed within six weeks by the description of the same sequence as Nup475, cloned from serum-stimulated fibroblasts [10]. A partial fusion sequence had been published earlier from phorbol ester-stimulated fibroblasts [11]; this sequence was later corrected [12]. These initial descriptions were followed by descriptions of the human sequence [13]; in a later description, this was labeled G0S24 [14]. These represent the earliest descriptions of what we now know as tristetraprolin or TTP.
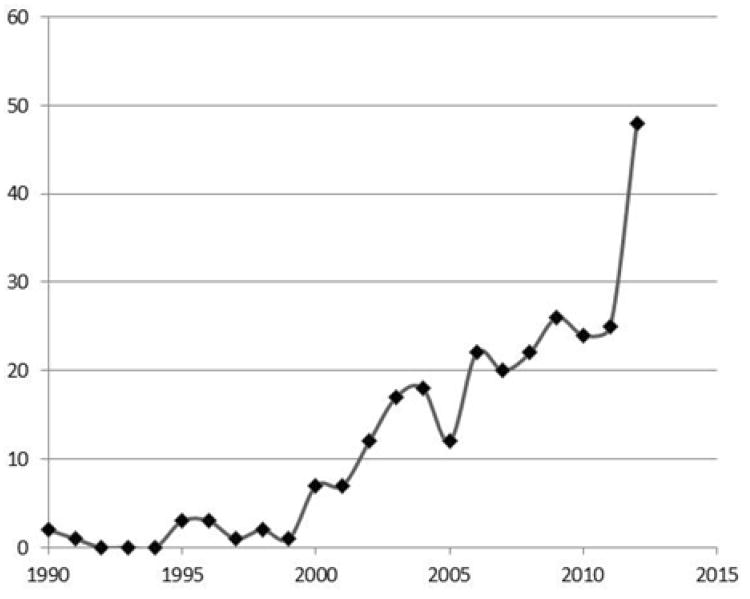
Fig. 1 shows the number of publications from the above search and their year of publication. It shows that there was very little activity for several years following the initial cloning of the human and mouse cDNAs, although the laboratory of one of us (PJB) made contributions during that time on the hormone and growth factor stimulated phosphorylation of the protein [15], the hormone and growth factor stimulated nuclear export of the protein [16], the influence of metal ions on its mRNA stability [17], and characteristics of the Zfp36 promoter from mice and man [18].
Fig. 1. Papers published on TTP since 1990.
The figures shows the results of the literature search described in the text; abstracts were not included. The asterisk for 2012 indicates that papers not picked up in the search on August 15, 2012, were not included.
Tristetraprolin and Tumor Necrosis Factor Alpha (TNF)
The modern era of TTP research can be said to have begun with the development and evaluation of a TTP knockout mouse, which exhibited a severe syndrome of growth retardation, cachexia, arthritis, inflammation and autoimmunity [19]. Most importantly, this paper established the first connection between TTP and the potent pro-inflammatory cytokine, tumor necrosis factor alpha (TNF). Treatment of the TTP-deficient mice, soon after birth, with anti-TNF antibodies prevented the development of virtually all aspects of the TTP deficiency syndrome.
This observation was followed by the demonstration that the syndrome could be transplanted with whole bone marrow into lethally irradiated immunodeficient mice. A lag of several months was necessary for disease development, suggesting that hematopoietic cells were required but that lymphocytes were unlikely to be the major sources of excess TNF; rather myeloid cells were the most likely source of TNF [20]. This paper also showed that TNF mRNA accumulated abnormally in the TTP KO macrophages, isolated from several body sites, after stimulation with various concentrations of lipopolysaccharide (LPS), and that TNF protein was hypersecreted from the same cells after LPS stimulation.
Finally, a physical connection between TTP protein and the TNF mRNA was made in a subsequent paper [21]. This work showed that the TNF mRNA decayed more slowly in the TTP KO macrophages after stimulation with LPS and inhibition of transcription with actinomycin D; in these experiments, conducted after four hours of LPS stimulation, the half-life of the TNF mRNA was increased from about 39 minutes to about 85 minutes. These experiments demonstrated for the first time that TTP protein could bind directly to the TNF mRNA, as assayed by RNA gel shift experiments. Thus, in the absence of TTP, the TNF mRNA accumulated abnormally due to its increased stability, leading in turn to enhanced TNF secretion, and presumably elevated local and systemic levels of the TNF protein.
Although a detailed discussion of this and other animal models is beyond the scope of this review, there are many important questions regarding the role of TTP in the intact mouse that remain unanswered. For example, in the “TNF delta ARE” mice described by Kontoyiannis et al [22], the mice not only develop the cachexia and arthritis characteristic of the TTP KO mice, but also severe colitis; this has not been seen in the TTP KO mice to our knowledge. One possible explanation is the presence of the other TTP family members in the cells of the intestine, possibly regulating locally produced TNF or other cytokines, but this remains to be determined. Another interesting model is the myeloid-specific TTP KO mouse, which does not exhibit cachexia or arthritis, but is hypersensitive to low doses of LPS [23,24]. These data suggest that TTP-dependent pathways need to be disregulated in one or more cells types other than myeloid cells to produce the full TTP-deficiency phenotype, but the identity of these cells is currently unknown.
TTP Binding to AU-Rich Elements
Subsequent work characterized the binding sites on the TNF mRNA as one of several conserved instances of the simple linear RNA motif, UUAUUUAUU, in the 3′UTR of the message [25]. A similar conclusion had been reached from a completely different avenue of experimentation, using different selection procedures, but arriving at the same optimal binding site [26]. The site of RNA binding was the central tandem zinc finger domain (TZF), and it was striking that a single mutation of any of the eight cysteines or histidines in the TZF domain completely abrogated RNA binding [27]. Quantitative analysis of TZF-RNA interaction was later performed using fluorescence anisotropy, in a solution with pure components, in which the TZF domain was represented by a pure 75 amino acid totally synthetic peptide derived from the human TTP TZF domain sequence, and the “targets” were fluorescently labeled RNA oligonucleotides [28]. A selection of the dissociation constants from the interactions described in that paper is shown in Table 1. One of the key features of this interaction is that the previously determined optimal binding sequence, UUAUUUAUU, bound to the synthetic TZF peptide with a Kd of 3.0-3.2 nM at room temperature. This compares, for example, to a Kd of 280 nM for a polyU sequence, or 56 nM for polyU containing a single A residue. However, one point that remains a subject of ongoing research is the physiological importance of different numbers of U residues between the two As. As shown in the table, increasing this number to four increases the Kd to 6.4 nM, and increasing the internal Us to five increases the Kd to 17 nM. Conversely, decreasing the number of Us to two increases the Kd to 18 nM, while decreasing the number of Us to one increases the Kd to 160 nM. Even more radically, replacing one of the A residues with G, leaving three internal Us, results in Kds of 28 nM, regardless of which A is replaced. Since some of these Kds are still quite low, it is possible that they are physiologically significant binding sites in some circumstances; this is one of the particularly interesting aspects of new target identification, the major subject of this review.
Table 1. Binding of fluorescent ARE probes to a 75 amino acid synthetic TZF domain peptide from human TTP.
Taken from fluorescence anisotropy data described in [28]. A residues are in red; sequences discussed in the text are highlighted in bold.
| Probe sequence | Kd at 25°C (nM) |
|---|---|
| Fl-AUUUAUUUAUUUA | 3.6 |
| Fl-UUAUUUAUU | 3.0 |
| Fl-UAUUUAU | 19 |
| Fl-UUUUUUUUUUUUU | 280 |
| Fl-UUUUAUUUAUUUU | 3.2 |
| Fl-UUUUAUUUUUUUU | 56 |
| Fl-UUUUAUUUCUUUU | 83 |
| Fl-UUUUAUUUGUUUU | 28 |
| Fl-UUUUUUUUAUUUU | 54 |
| Fl-UUUUCUUUAUUUU | 93 |
| Fl-UUUUGUUUAUUUU | 28 |
| Fl-UUAUUUUUU | 130 |
| Fl-UUUUUUAUU | 120 |
| Fl-UUUUAUAUUUU | 160 |
| Fl-UUUUAUUAUUUU | 18 |
| Fl-UUUUAUUUAUUUU | 3.2 |
| Fl-UUUUAUUUUAUUUU | 6.4 |
| Fl-UUUUAUUUUUAUUUU | 17 |
Later studies demonstrated that the other TTP family members could bind to the same optimal RNA sequences, and that they could promote mRNA decay in a similar manner to TTP [27]. In structural terms, a critical observation was made by Hudson et al [29], in which a TZF domain peptide from the TTP family member ZFP36L2 (TIS11D, ERF2, BRF2) was used to determine an NMR structure to the same nine base oligonucleotide described above, UUAUUUAUU. This paper described many of the crucial interactions that lead to the high affinity binding, including electrostatic interactions and stacking interactions between some of the RNA bases and the rings of the aromatic amino acids in the TZF domain. It also illustrates two key features of the structure: First, that the RNA in its bound form is totally without secondary structure, raising the possibility that secondary structure is not only not necessary for binding, but might indeed interfere with binding; and second, that the RNA occupies a single “face” of the TZF domain structure, leaving open the possibility that other proteins or small molecules could interact with other faces of the TZF domain and perhaps affect RNA binding affinity. A final interesting feature about the structure is that the first and ninth bases are more or less left dangling in the breeze; this is the basis for the use of the “core 7-mer” binding site concept that has been used to inform bioinformatics searches of binding sites.
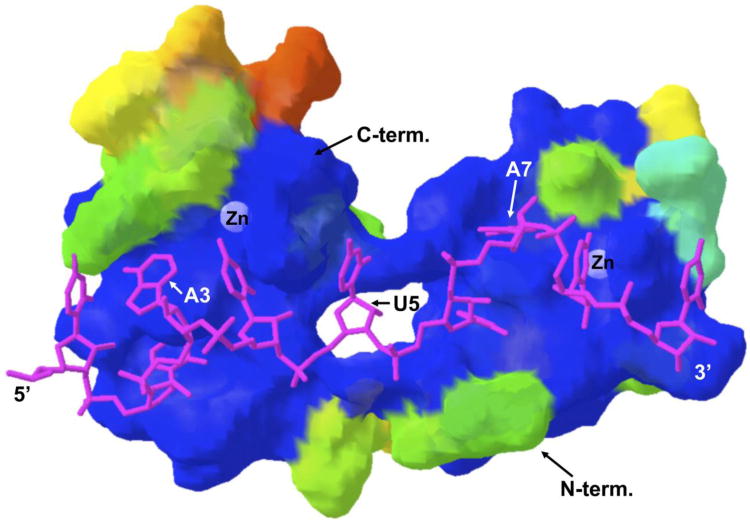
Another consideration concerns the nature of TTP specific residues within the TZF domain. We have previously modeled the human TTP TZF domain bound to the 9-mer RNA oligonucleotide [30], with the perfectly conserved residues among the family members represented in blue, and the extent of the chemical deviation of the TTP amino acids from the original ZFP36L2 residues illustrated in the movement from blue to warmer colors of the amino acid representations (Fig. 2). This analysis points to the importance of highly conserved or identical residues at the actual sites of contact with the RNA, as well as with the zinc ions, and more protein-specific residues on faces of the TZF domain that are not interfacing directly with RNA. This leaves open the possibility of proteins and small molecules that could bind to other faces of the TZF domain, potentially in a TTP-specific manner.
Fig. 2. Model of the human TTP TZF domain bound to the 9-mer sequence UUAUUUAUU.
The figure shows a model of the interaction between the TZF domain of human TTP and the 9-mer UUAUUUAUU, based on the original structure for human ZFP36L2 bound to the same oligonucleotide [29]. In this view, the amino-terminal zinc finger is on the right, the C-terminal zinc finger is on the left. The RNA oligonucleotide runs from left to right in the 5′ to 3′ orientation. The zinc residues are highlighted, as are certain bases in the RNA. The color is meant to demonstrate amino acid identities between this TTP sequence and the original ZFP36L2 (TIS11D) TZF domain that was used to form the original structure. As the colors become “warmer” from dark blue, they represent progressively greater chemical differences between the TTP and ZFP36L2 amino acids. Taken from [30], with permission.
Other TTP Family Members
In phylogenetic terms, TTP family member proteins have been identified in organisms as diverse as humans through yeasts. Most mammals express three family members, with rodents expressing a fourth. In the mouse, the official gene names are Zfp36, encoding TTP; Zfp36l1, encoding the ZFP36L1 (also known as cMG1, BRF1, ERF1, and TIS11b); Zfp36l2, encoding ZFP36L2 (also known as TIS11D, BRF2, ERF2); and Zfp36l3, encoding ZFP36L3. Although the focus of this review is on TTP itself, we will use the Zfp36 nomenclature when necessary to refer to other mammalian family members. The first three family members are widely expressed in the mouse; the fourth family member, ZFP36L3, is expressed in placenta and yolk sac of rodents but not, to our knowledge, in other tissues or animal species [31]. In amphibians, orthologues of the three core family members TTP, ZFP36L1 and ZFP36L2 are present [32]; in addition, in Xenopus laevis and Silurana tropicalis a fourth family member is present, which contains a second degenerate TZF domain and has been implicated in meiotic metaphase arrest [33]. In insects, in general only a single family member is expressed; the lengths of the two isoforms of TIS11 protein in Drosophila melanogaster are 408 and 436 amino acids (GenBank accession numbers NP_727633.1 and NP_511141.2, respectively), and to date the shortest insect family member to be identified is from the red flour beetle, Tribolium castaneum, at 250 amino acids (XP_968440.1). In most yeast species, only a single family member is expressed; however, two are expressed in S. cerevesiae (Cth1p, NP_010435.1, and Cth2p, NP_013237.1). The shortest family member to be identified in yeast, or indeed in any organism to our knowledge, is in the yeast Meyerozyma guilliermondii, in which the full length predicted protein sequence is 156 amino acids (M.L. Wells and P.J. Blackshear, unpublished data); in this predicted protein, the TZF domain represents the extreme C-terminal 65 amino acids. Although target sequences have not been analyzed in detail for these non-mammalian proteins, recent studies with the yeast and fly proteins suggest that they can bind to essentially identical RNA sequences as the mammalian proteins.
Thus, the available data support the concept that the TZF domains from all of these proteins binds to the optimum sequence UUAUUUAUU. In most cases, quantitative binding of the type used by Brewer et al [28] has not generally been performed with TZF domains from other proteins, so that minor changes in affinity are still possible among the family members from a given animal species, and among orthologues of a single protein among different animal species. An interesting exception to the general rules cited above is seen in C. elegans, in which a large number of proteins are expressed that contain variants of the core, TTP-like TZF domain [34]. Some of these conform to the sequence conservation and spacing rules for the TTP-like TZF domains, but others vary widely in internal spacing, conserved residues, etc. Discussion of these proteins is beyond the scope of this review, but they represent interesting outliers in the overall discussion of structure vs. function for this class of proteins.
Methods of Determining Whether a Given Transcript is a TTP “Target”
The early data demonstrating the role of TTP in regulating the stability of the TNF mRNA exemplify the types of evidence that can be assembled in increasing the probability that a given mRNA is a true physiological target of TTP or its family members. We have not tried to weight these criteria in attempting to determine a formal probability, but the more criteria that are satisfied for a given proposed interaction, the more likely that the interaction is of physiological relevance. In the case of TNF, investigators were forced to work “backwards” from the phenotype of the TTP KO mice, with the clinical criteria driving the hypothesis that TNF excess was contributing to the phenotype. The initial test of the hypothesis used treatment with anti-TNF antibodies to prevent the development of the phenotype in the TTP KO mice [19]. Subsequent genetic work, in which the TTP KO animals were crossed with mice deficient in one or both types of TNF receptors, definitively established the role of TNF overexpression in the phenotype [35]. In both cases, most if not all of the TTP-deficiency phenotype was corrected by the anti-TNF treatments. This allowed the Blackshear group to demonstrate, sequentially, the overproduction of TNF from macrophages from the TTP KO mice; the association of this overproduction with elevated levels of TNF mRNA in the same cells; and the association of these elevated mRNA levels with increased stability of the mRNA in the KO cells. Thus, in our view, one of the most important criteria in determining whether a given mRNA is a target for TTP is the demonstration that its mRNA is stabilized in cells isolated from TTP KO mice. It is obviously possible that such stabilization can occur in mRNAs that are not true targets, by various types of secondary effects; however, when this analysis is combined with various induction strategies that ensure that TTP protein is present in high concentrations, as with LPS stimulation of macrophages, or serum stimulation of fibroblasts, then the likelihood of a direct TTP effect is enhanced considerably.
However, there are obvious problems with this approach. One is that KO mice are expensive to maintain, and since the homozygous TTP KO mice are essentially infertile, the colonies need to be maintained as heterozygotes, increasing colony size and complexity. A second problem is that it is essentially impossible to isolate 100 % homogeneous cultured cells from mice, given contamination with other cell types. When this has been accomplished, as in fibroblasts maintained for more than 200 passages to complete homogeneity [36], there is the likelihood that novel mutations will have arisen during this extended period in culture. Third, the situation in cultured cells may bear a limited relationship to the situation in an organ in situ. Fourth, as illustrated by the cell-free binding data of Brewer et al [28], it is possible to demonstrate RNA binding of TTP TZF domains to probable non-physiological target sequences of much lower affinity, simply by increasing concentrations of the binding peptide or protein; this can easily be accomplished using transfection methods in cultured cells, where TTP overexpression can lead to a triphasic dose response curve of mRNA decay. Finally, it is relatively easy to demonstrate non-specific squelching of gene transcription using TTP overexpression in transfection experiments. All of these problems limit the usefulness of co-transfection assays as means of determining the likelihood that a given mRNA is a true, physiologically relevant target of TTP.
However, if these limitations are borne in mind, then other types of evidence can be useful in assembling an argument in favor of the physiological relevance of a given interaction. These types of evidence can include, in no particular order of importance: 1. Demonstration of one or more ideal TTP binding site sequences in the putative target mRNA (the online databases ARED and AREScore can be helpful in identifying these sites (http://brp.kfshrc.edu.sa/ARED/) (http://arescore.dkfz.de/arescore.pl)); 2. Demonstration of binding, using RNA gel shift analyses or other methods for measuring RNA-protein interactions; 3. Use of co-transfection assays to demonstrate an effect of WT TTP to promote the decay of a putative target transcript, and demonstration that this effect is lost with one or more TZF domain mutants, or target sequence mutants; 4. Demonstration of TTP-dependent target deadenylation, either in intact cells or in cell free deadenylation systems (see below for a discussion of the phenomenon in intact cells); 5. Demonstration of co-immunoprecipitation of the target mRNA with TTP itself, preferably using endogenous rather than transfected-expressed protein; 6. Sequence alignments demonstrating phylogenetic conservation of the binding sites. In the case of the TNF mRNA, every one of these additional criteria has been satisfied, making it the “gold standard” for demonstrating the physiological importance of a proposed interaction. The TTP interaction with the TNF mRNA has the additional advantage over other proposed interactions in that it is physiologically very obvious, i.e., the animals get very sick when TTP is absent, but their disease is prevented when TNF is neutralized with antibody or genetic ablation of TNF receptors. Given the overwhelming dependence of the TTP deficiency phenotype on TNF, it seems unfair to subject other molecules to this standard. However, the other molecules identified below as likely TTP targets may well contribute to some aspects of the overall TTP deficiency phenotype, or to other facets of mouse physiology that are not apparent in the mice severely affected by TTP-dependent TNF excess. It may be possible to decipher some of these roles in the TTP-TNFR1-TNFR2 “triple KO” mice, which exhibit a minimal phenotype [35]
TTP and GM-CSF
After the discovery of TNF mRNA as the first identified TTP target, and one that explained the phenotype of TTP deficiency in the mouse, other potential targets were explored that took advantage of the previously described consensus TTP binding sequence. One of the best known ARE-containing mammalian mRNAs is that encoding granulocyte-macrophage colony-stimulating factor (GM-CSF), a potent growth and migration factor for cells of the myeloid lineage. This growth factor is of significant clinical interest in its therapeutic use to stimulate the production of white blood cells following chemotherapy. In addition, GM-CSF deficiency is associated with pulmonary alveolar proteinosis in mice. GM-CSF mRNA was initially used as the primary example of the demonstration that AU-rich elements can confer instability on otherwise stable mRNAs [37]. Mammalian GM-CSF mRNA orthologues contain a highly conserved ARE that contains many repeats of the optimal UUAUUUAUU TTP binding sequence, although some of these are overlapping (Table 2).
Table 2. Alignments of selected ARE regions from selected mammalian species for TNF, GM-CSF and IL3.
The numbers refer to the base number in the RefSeq from GenBank for each species. The core 7-mer TTP binding site sequences are highlighted in red.
| TNF | ||
|---|---|---|
| Human | 1307 | GCCAG-CTCCCTC-TATTTATGTTTGCACTTG-------TGATTATTTATTATTTATTTA |
| Orangutan | 1308 | GCCAG-CTCCCTC-TATTTATGTTTGCACTTG-------TGATTATTTATTATTTATTTA |
| Gibbon | 1307 | GCCAG-CTCCCTC-TATTTATGTTTGCACTTG-------CGATTATTTATTATTTATTTA |
| Squirrel monk | 1313 | GCCAG-C-CCCTC-TATTTATGTTTGCACCTG -------TGATTATTTATTATTTATTTA |
| Pig | 1315 | GCCAG-CTCCCTCTTATTTATATTTGCACTTG-------GCATTATTATTTATTTATTTA |
| Cow | 1326 | GCCAA-CTCCCTC-TGTTTATGTTTGCACTTG-------TGATTATTTATTATTTATTTA |
| Sheep | 1302 | GCCAA-CTCCCTC-TGTTTATGTTTGCACTTA-------TGATTATTTATTATTTATTTA |
| Rabbit | 1294 | GCAGG-GCCCCTC-TATTTATAGTTGCACTGGTGATTATTGATTATTTATTATTTATTTA |
| Mouse | 1271 | GCCAGCCCCCCTC-TATTTATATTTGCACTT----------ATTATTTATTATTTATTTA |
| Rat | 1303 | ACC-----CCCTC-TATTTATAATTGCACCTG-------TGACTATTTATTTATTATTTA |
| Human | 1358 | TTATTTATTTATTTACAGATGAATG--TATTTATTTGGGAGACCGGGGTATCCTGGGGGA |
| Orangutan | 1359 | TTATTTATTTATTTACCGATGAATG--TATTTATTTGAGAGGTCGGGGTATCCTGGGGGA |
| Gibbon | 1358 | TTATTTATTTATTTACCGATGAATG--TATTTATTTGGGAGGTCGGGGTATCCTGGGGGA |
| Squirrel monk | 1363 | TTATTTATTTATTTACCGACGAATGTATATTTATTTAAGAGGTCGAGGCATCCCGAGGGA |
| Pig | 1367 | TTTATTATTTATTTACTAGTGAATG--TATTTATTCAGGAGGGCGAGGTGTCCTGGGAGA |
| Cow | 1377 | TTATTTATTTATTTACTAATGAATG--TATTTATTCAGGAGGTCAAGGTGTCCTGGGAGA |
| Sheep | 1353 | TTATTTATTTATTTACTAATGAATG--TATTTATTCAGGAGGTCAAGGTGTCCTGGGAGA |
| Rabbit | 1352 | ATATTTATTTATTTGCCGATGAATG--TATTTATTTGGAAGCTCAGCGCATCCTGGGGTA |
| Mouse | 1320 | TTATTTATTTATTTGCTTATGAATG--TATTTATTTGGAAGGCCGGGGTGTCCTGGAGGA |
| Rat | 1350 | TTATTTATTTATTTGCTTATGAATG--TATTTATTTGGAAGGCCGGGGCGTCCTGGAGGA |
| GM-CSF | ||
| Human | 675 | AT----ATATTTATAT-TTTTAAAA----------TATTTATTTATTTATTTATTTAAGT |
| Chimp | 669 | AT----ATATTTATAT-TTTTAAAA----------TATTTATTTATTTATTTATTTAAGT |
| Gibbon | 669 | AT----ATATTTATAT-TTTTAAAG----------TATTTATTTATTTATTTATTTAAGT |
| Orangutan | 669 | AT----ATATTTATAT-TTTTCAAA----------TATTTATTTATTTATTTATTTAAGT |
| Squirrel monk | 664 | AT----ATATTTATAT-TTTTAAAA------------------TATTTATTTATTTAAGT |
| Horse | 658 | AT----ATATTTATGT-ATTT-TAA----------TATTTATTTATTTATTTATTTAAGC |
| Pig | 681 | ATTAACCTATTTATGTATTTTAATATTTATTTATTTATTTATCTATTTATTTATTTAAGC |
| Cow | 669 | AT----ATATTTATGT-ATTT-TAA----------TATTTATTTATTTATTTATTTAAAC |
| Dog | 694 | AT----ATATTTATGT-ATTT-TAA----------TATTTATTTATTTATTTATTTAAGA |
| Mouse | 933 | AT----ATATTTATAT-TTTTTAAA----------TATTTATTTATTTATTTATTTATTT |
| Human | 720 | T--CATATTCCATATTTATTCAAGATGTTTTACCGTAATAAT-TATTAT--TAAA-AAT- |
| Chimp | 714 | T--CATATTCCATATTTATTCAAGATGTTTTACCGTAATAAT-TATTAT--TAAA-AAT- |
| Gibbon | 714 | T--CATACTCCATATTTATTCAAGATGTTTTACCGTAATAAT-TATTAT--TAAA-AAT- |
| Orangutan | 714 | T--CATACTCCATATTTATTCAAGATGTTTTACCGTAATAAT-TATTAT--TAAA-AAT- |
| Squirrel monk | 701 | T--TTTACTCCATATTTATTCAAGATGTTTTACTGTAATAAT-AATTAT--TAAA-AAT- |
| Horse | 702 | T--CATACTCCATATTTATTCAAGATGTTTTACCATTAGAATAAATTAT--TAAA-A |
| Pig | 741 | T--TGAACTTCATATTTATTCAAGATGTTTTACCATAATAATAAATTAT--TTAA-AAT- |
| Cow | 713 | T--CATACCCCATATTTATTCAAGATGTTTTTCTATAATAATAAATTAT--TCAA-A |
| Dog | 738 | T--CATACTCTGTATTTATTCAAGACATTTTACTATTATAATAAATTAT--TAAA-A--- |
| Mouse | 978 | TTGCA-ACTC--TATTTATTGAGAATGTCTTACCAGAATAATAAATTAT--TAAA-A |
| IL3 | ||
| Human | 686 | TTTTTATCCCATTGAGAC-TATTTATTTA----TGTATGTATGTATTTATTTATTTATTGC |
| Pygmy chimp | 686 | TTTTTATCCCATTGAGAC-TATTTATTTA----T----GTATTTATTTATTTATTTATTGC |
| Gibbon | 688 | TTTTTATCCCATTGAGAC-TATTTATGTATGTCTGTATTTATTTATTTATTTATTTATTGC |
| Orangutan | 682 | TTTTTATCCCATTGAGAC-TATTTATT--------TATGTATTTATTTATTTATTTATTGC |
| Horse | 662 | TTTTTATCCTATTGAGGC-TATTTATTTA----TTTATGTATTTATTTATTACCTTGT-GC |
| Dog | 657 | TTTTTATC---------C-TATTTATTTA----TTTATGTATGTTTTTATTTA-TTACCAT |
| Cow | 622 | TTTTTAATCTATTGAGACTTATTTATTTA----TGTATGTATTTATTTATTACCTTGT-GC |
| Mouse | 679 | TATTTTATTCCATTAAGGCTATTTATTTATGTATTTATGTATTTATTTATTTATTGCCTTC |
Based on the presence of these potential binding motifs alone, Carballo et al [38] chose a cell type that expressed both TTP and GM-CSF, bone marrow derived stromal cells, and examined these cells from TTP WT and KO mice to determine whether TTP deficiency resulted in stabilization of this transcript after LPS stimulation. The results were unequivocally positive – that is, in the normal cells under these conditions, GM-CSF mRNA decayed with a half-life of approximately 99 min, whereas in the TTP KO cells, there was effectively no decay during the several hour period examined after LPS stimulation. In the same cells under the same conditions, TNF mRNA was also stabilized in the absence of TTP, making these stromal cells the second cell type in which TTP had an effect on TNF mRNA stability.
One of the most important aspects of this paper was the finding that, in the normal stromal cells, GM-CSF mRNA was expressed on northern blots as two bands of approximately equal intensity that differed in size by approximately 200 nucleotides. In the normal cells, these decayed more or less in parallel after actinomycin D treatment. However, in the KO cells, not only did the mRNA not decay after actinomycin D, but it was present almost entirely as the larger form of the transcript. This was demonstrated by RNAse H experiments to be the primary spliced and polyadenylated transcript, whereas the lower molecular weight species turned out to be a largely deadenylated transcript. In other words, TTP deficiency was not only stabilizing the mRNA, but preventing its initial deadenylation, then as now thought to be the rate limiting step in mRNA decay in eukaryotes.
This serendipitous finding of a deadenylated intermediate expressed in normal cells therefore led to the concept that the primary effect of TTP, after binding to AU-rich elements in RNA, was to promote processive removal of the polyA tail, or deadenylation. To our knowledge, this fortunate finding of a stable deadenylated intermediate has not been repeated during the identification of other TTP binding targets, but was extremely useful in our understanding of TTP's mode of action. In situations in which it has been tested, the effects of other TTP family members, including the other mammalian proteins and those from non-vertebrate organisms, act in similar ways, i.e., RNA binding followed by deadenylation. This can be mimicked in cell free deadenylation assays [39], and TTP can potentiate the effect of certain deadenylases such as PARN to promote deadenylation of ARE-containing target transcripts [40]. A detailed mechanism of how TTP family members may promote deadenylation is presented below; ultimately, a complete understanding of TTP mediated mRNA decay rests on the continued identification of TTP-associated proteins and other factors.
TTP mRNA Targets
By the year 2000, the TNF and GM-CSF mRNAs were the only established, apparently physiologically relevant TTP targets. Both of these transcripts were characterized by multiple, closely spaced (and in many cases overlapping) 7, 8 or 9-mer consensus sequences that were highly conserved among mammals (Table 2). In both cases, the sequence conservation outside of the ARE was considerably poorer than within the ARE, supporting the idea that these were sequence elements important throughout mammalian evolution. It therefore seemed reasonable to search for other important transcripts that shared these characteristics, and test them as likely TTP targets. One example chosen by one of us (PJB) was the mRNA encoding interleukin 3 (IL3), an important cytokine involved in hematopoiesis. As shown in Table 2, in which ARE sequences from several disparate mammals for TNF, GM-CSF and IL3 are described, the IL3 AREs from many organisms contain what would appear to be ideal TTP binding sites that are conserved among species. It seemed reasonable to investigate the possibility that this transcript would be a TTP target in a physiological cell system.
Bone marrow derived mast cells were chosen as a cell type that expressed both TTP and IL3. However, despite ready demonstrations of induction of IL3 and TTP mRNAs by ionophore, similar actinomycin D decay curves in WT and TTP KO cells did not demonstrate any apparent effect of TTP deficiency on IL3 mRNA decay (E. Carballo and P.J. Blackshear, unpublished data). This experiment highlights a remaining significant conundrum in this field, i.e., why do some apparently “perfect” target transcripts not respond in the expected way to the presence and absence of TTP? Various explanations can be proposed, including prior occupancy of the AREs by protective proteins; differences in intracellular localization between the TTP protein and the IL3 mRNA; cell-type specific effects on either the protein or the transcript that would not hold true in other types of cells; and many other possibilities. Resolution of these mysteries will considerably increase our understanding of TTP physiology.
Part of this failure to identify IL3 mRNA as a TTP target in mast cells may be the choice of cell type, although the mechanism of this failure remains unexplained. For example, Stoecklin et al [41] described imaginative experiments in HT1080 cells in which they examined the effects of mutagenesis on a GFP fusion transcript linked to the IL3 mRNA 3′UTR. They identified three mutant cell lines in which this transcript was stabilized; two of these could be complemented by the expression of TTP. However, the stability of TNF mRNA was not affected in these cell lines. A later study by the same group supported these findings [42], using co-transfection and over-expression of TTP to promote IL3 mRNA turnover.
That same year, studies in purified T cells identified interleukin 2 (IL2) as a potential binding target for TTP, as well as supporting binding interactions with TNF,GM-CSF, c-Fos, and IL3 mRNAs [43]. These studies were performed by immunoprecipitating TTP and investigating candidate mRNAs by real-time RT-PCR, and did not involve studies of mRNA decay.
Since the description of TNF and GM-CSF as the first two physiologically relevant TTP targets, there have been numerous descriptions of other mRNAs that have been proposed and, to some extent, validated as targets in various systems. These findings will be summarized in the paragraphs that follow. Because of space limitations, we will not attempt to describe targets for the other TTP family members in vertebrates, nor the targets for TTP family members in non-vertebrate systems like insects and yeasts.
After the year 2000, descriptions of TTP target identification began to be published frequently. The starting database for the present compilation were the 293 papers that resulted from the search described at the top of this section; we regret any that we have missed because of this possibly not all-encompassing search. In Table 3, we have included all identified targets from this literature search in roughly chronological order, along with the cell types in which the studies were performed, if possible. The official mouse or human gene symbols are included in the first column, and the description of the protein or its common name were included in the second column. For TNF and GM-CSF, we have not included all the additional citations after the first descriptions mentioned above. We will try to maintain this as an “updatable” table, so authors are encouraged to contact the authors regarding mistakes, updates, etc. – please be polite!
Table 3.
Identified TTP mRNA targets from the literature through August 15, 2012.
| Gene symbol | Common or protein name | Cell type | References |
|---|---|---|---|
| Tnf | Tumor necrosis factor | Mouse macrophages | [19,21] |
| Csf2 | Granulocyte-macrophage colony-stimulating factor | Mouse bone-marrow derived stromal cells | [38] |
| Il3 | Interleukin-3 | Misc. cells | [41,42,129,130] |
| Ptgs2 | Cyclooxygenase 2 | Misc. cells | [110,130,131,132,133,134,135,136,137] |
| Pitx2 | Pituitary homeobox 2 | Mouse pituitary alpha T3-1 cells | [138] |
| SERPINB2 | Plasminogen activator inhibitor type 2 | Human HEK293 cells | [139] |
| Zfp36 | Tristetraprolin | Mouse RAW 264.7 cells | [77,78] |
| Nos2 | Inducible nitric oxide synthase | Human colon carcinoma DLD1 cells | [52,53] |
| Il2 | Interleukin-2 | Mouse splenocytes | [140] |
| Il1b | Interleukin-1 beta | Mouse RAW264.7 cells | [141] |
| Il12 | Interleukin-12 | Mouse J774 macrophages | [142] |
| Cxcl2 | C-X-C motif chemokine ligand 2 | Mouse J774 macrophages | [142] |
| Ccl20 | C-C motif chemokine ligand 20 | Mouse J774 macrophages | [142] |
| Il6 | Interleukin-6 | Misc. cells | [135,142,143,144,145,146,147] |
| Ier3 | Immediate early response 3 | Mouse fibroblasts | [36] |
| Lif | Leukemia inhibitory factor | Mouse fibroblasts | [36] |
| Plk3 | Polo-like kinase 3 | Mouse fibroblasts | [36,148] |
| HIV-1 | HIV-1 | Genomic RNA | [51] |
| Ccnd1 | Cyclin D1 | Mouse fibroblasts | [73] |
| Myc | c-Myc protooncogene | Mouse fibroblasts, human liver cancer cells | [73][149] |
| Ccl2 | C-C motif chemokine ligand 2 | Mouse macrophages, fibroblasts | [143] |
| Ccl3 | C-C motif chemokine ligand 3 | Mouse macrophages, fibroblasts | [143][150] |
| Vegfa | Vascular endothelial growth factor | Misc cells | [137,151,152,153][75] |
| Tcf3 | E47 | Mouse B cells | [154] |
| Il8 | Interleukin-8 | Misc. cells | [152,155,156,157,158,159,160] |
| Cxcl1 | Growth-regulated alpha protein precursor | Mouse macrophages | [161] |
| Il10 | Interleukin-10 | Mouse macrophages | [79,162,163] |
| Clmp | CXADR-like membrane protein precursor | Sertoli TM4 cells | [164] |
| Ifng | Interferon gamma precursor | Mouse T cells | [165] |
| UBE3A | Ubiquitin-protein ligase E3A isoform 1 | Human cervical cancer cells | [166] |
| Il1a | Interleukin-1 alpha precursor | Mouse macrophages | [162,167] |
| TLR4 | Toll-like receptor 4 | Human THP-1 cells | [168] |
| PLAU | Urokinase-type plasminogen activator | Human breast cancer cells | [136] |
| PLAUR | Urokinase plasminogen activator surface receptor | Human breast cancer cells | [136] |
| MMP1 | Interstitial collagenase isoform 1 preproprotein | Human breast cancer cells | [136] |
| ECSCR | Endothelial cell surface expressed chemotaxis and apoptosis regulator | HeLa cells | [169] |
| Hif1a | Hypoxia-inducible factor 1-alpha | Endothelial cells? | [170,171] |
| LATS2 | Serine/threonine-protein kinase LATS2 | Human A549 lung cancer cells | [172] |
| MYC | c-Myc proto-oncogene | Human liver cancer cells | [149] |
| Dusp6 | Dual specificity protein phosphatase 6 | HEK293, A375 | [173] |
| SLC10A2 | Ileal sodium/bile acid cotransporter | Human Caco-2 cells | [174] |
| Thbd | Thrombomodulin | Rabbit monocytes | [175] |
| Il23a | Interleukin-23 subunit alpha precursor | Mouse dendritic cells, macrophages | [176] |
| Cdkn1a | Cyclin-dependent kinase inhibitor 1 | Mouse fibroblasts | [177] |
| IL17 | Interleukin-17 | Human T cell lines | [178] |
| Pim1 | Serine/threonine-protein kinase pim-1 | Mouse fibroblasts | [179] |
| SERPINH1 | Serpin H1 precursor | Human hepatoma cells | [180] |
| Many | Many transcripts | Mouse fibroblasts | [36] |
| Many | Many transcripts | Mouse fibroblasts | [181] |
| Many | Many transcripts | Human dendritic cells | [182] |
| Many | Many transcripts | Mouse dendritic cells | [183] |
| Many | Many transcripts | Mouse macrophages | [24] |
A final caveat is that we did not make an attempt to go through each of these papers and determine the probability that the described experiments establish a given transcript as a physiologically relevant TTP target. We hope that interested readers will turn to the original publications and make up their own minds as to the validity of the conclusions.
There are several papers in the literature that describe unusual aspects of these interactions, or interactions with DNA or the transcription apparatus. In one example, the authors were not able to detect an effect of TTP on TNF mRNA stability, but instead demonstrated a potent effect of TTP to suppress TNF transcription [44]. Interested readers should refer to the original article, but it is worth noting here that TTP has been shown to non-specifically “squelch” many promoters, and frequently causes cell death when overexpressed. Nonetheless, the question of TTP's nuclear role, if any, is an important one that remains to be adequately explained. A few years later, the same group published a fascinating paper in which they demonstrated a role for the microRNA miR16 in ARE-dependent mRNA turnover, apparently through an indirect interaction between the microRNA and TTP, mediated by various members of the RISC complex [45]. They concluded that “…the cooperation of miRNA and ARE binding proteins, like TTP, appears to be essential in ARE-mediated mRNA degradation”. This group followed up on some aspects of these studies in further work [46]. In contrast, recent work from another group [47] showed that ARE mediated mRNA decay can occur independently of the miR machinery. Further work is required to clarify this area of TTP biology.
Other papers that describe possible nuclear functions for TTP include a study showing that TTP can negatively regulate NF-kB signaling at the transcriptional co-repressor level” [48], a second study from the same year showing that TTP can interfere with NF-kB activity, at least in part, by interfering with the nuclear translocation of p65 [49], and, most recently, a study demonstrating that siRNA knockdown of TTP can interfere with hepatic 3-hydroxy-3-methylglutaryl coenzyme A reductase promoter activity, both with and without insulin stimulation [50]. This last result is quite remarkable; since one of us (PJB) has recently developed a hepatocyte-specific TTP KO mouse, it should be possible to probe this finding further using this genetic model.
Two other types of findings deserve further highlighting, in our view. One is the report describing direct binding of TTP to the HIV-1 genomic RNA, apparently resulting in inhibited virus production in CD4+ T cells [51]. To our knowledge, this interesting and provocative finding has not been followed up on. A second, unrelated group of reports describes an activity of TTP to promote inducible nitric oxide mRNA decay, not by direct RNA binding but by interacting with a second RNA binding protein, KSRP [52,53].
TTP Interacting Proteins
We have compiled a list of TTP interacting proteins (Table 4). These were identified by searching Entrez for “tristetraprolin immunoprecipitation”, “tristetraprolin interactor”, “tristetraprolin yeast” (for yeast two hybrid), and “tristetraprolin kinase assay”. The searches resulted in a net of 107 individual manuscripts. As above, we have not attempted to determine the likelihood that a given report described a “true”, physiologically relevant interaction. However, we did exclude proteins from the list that were only identified as “co-localizing” with TTP, for example by immunocytochemistry. We regret any that we have missed because of this possibly not all-encompassing search.
Table 4.
Proposed TTP Interacting Proteins
| Interacting Proteins | ID Method | TTP Interacting Domain | Reference | ||||
|---|---|---|---|---|---|---|---|
| Deadenylation | |||||||
| CAF1 | OE-IP | Carboxyl Term Domain | [113] | ||||
| Ccr4 | OE-IP | Amino Term Domain | [114] | ||||
| Not1 | OE-IP | Carboxyl Term Domain | [113] | ||||
| Decapping | |||||||
| hDcp1a | IP, Y2H | Carboxyl Term Domain | [114,130] | ||||
| hDcp2 | OE-IP | Amino Term Domain | [114,120] | ||||
| hEdc3 | OE-IP | Amino Term Domain | [120] | ||||
| Hedls/hEdc4 | OE-IP | Amino Term Domain | [120] | ||||
| Exosome Proteins | |||||||
| Rrp4 | OE-IP | Amino Term Domain | [114] | ||||
| Rrp45 | IP | [114] | |||||
| Rrp43 | Y2H | [184] | |||||
| Mtr3 | Y2H | [184] | |||||
| Histone Deacetylases | |||||||
| HDAC1 | OE-IP | [48] | |||||
| HDAC3 | OE-IP | [48] | |||||
| HDAC7 | OE-IP | [48] | |||||
| Micro-RNA Pathway | |||||||
| hAgo2/eIF2C2 | OE-IP | [45] | |||||
| hAgo4/eIF2C4 | OE-IP | [45] | |||||
| Nuclear | |||||||
| PABPN1 | OE-IP | TTP Zinc Finger | [185] | ||||
| PAP | OE-IP | TTP Zinc Finger | [185] | ||||
| Hsp40 | Y2H | Carboxl Term Domain | [130] | ||||
| Nucleolin | Y2H, OE-IP | Zn-Finger Domain | [56,186] | ||||
| Transportin | OE-IP | [187] | |||||
| Nup214 | Y2H, OE-IP | [188] | |||||
| Proteasome-related | |||||||
| Hsp70 | Y2H, OE-IP | Zn-Finger Domain | [56,186] | ||||
| RNases | |||||||
| hXrn1 | IP, Y2H IP | Amino Term, Carboxyl Term Domain | [114,130,184] | ||||
| RNA Surveillance | |||||||
| Upf1 | Y2H | [184] | |||||
| Upf2 | Y2H | [184] | |||||
| RNA Helicases | |||||||
| DDX5 | OE-IP | [189] | |||||
| DDX17 | Y2H, OE-IP Zn-Finger Domain | [189,190] | |||||
| DHX9 | OE-IP | [189] | |||||
| RHAU | Y2H | Carboxyl Term Domain | [130] | ||||
| RNA Binding Proteins | |||||||
| AUF1 | OE-IP | [189] | |||||
| AUF1 p45 | OE-IP | Zn-Finger Domain | [191] | ||||
| CRD-BP/IGF2BP1 | OE-IP | [189] | |||||
| HuR | OE-IP | [189] | |||||
| KSRP | IP | [192] | |||||
| PABPC1/PABP1 | Y2H, OE-IP | [56,70,114,186,189] | |||||
| PABP4 | OE-IP | [189] | |||||
| TIAR | OE-IP | [189] | |||||
| hnRNP A1 | OE-IP | [114] | |||||
| hnRNPs A/B, A1, A2/B, A3, | F, G, H, H′, K, L, M, R and U | ||||||
| OE-IP | [189] | ||||||
| Signaling Proteins | |||||||
| MK2 | KA | [61] | |||||
| MK3 | Mouse-KO | [118] | |||||
| ERK2 | KA | [15] | |||||
| JNK | KA | [58] | |||||
| p38 | KA | [58] | |||||
| PP2A | IP, OE-IP | Carboxyl Term Domain, pSer178 | [63,193] | ||||
| MEKK1 | IP | [194] | |||||
| TRAF2 | IP | [194] | |||||
| Protor-2 | Y2H | Carboxyl Term Domain | [130] | ||||
| Transcription Factors | |||||||
| Stat2 | Y2H | [195] | |||||
| p65/NFκB | OE-IP | [48] | |||||
| Phospho-TTP Binding Proteins | |||||||
| 14-3-3β | OE-IP | pSer52, pSer178 | [60,66,196] | ||||
| 14-3-3η | Y2H, OE-IP | [196] | |||||
| 14-3-3ζ | OE-IP | [196,197] | |||||
| 14-3-3γ, ε, σ and τ | OE-IP | [196] | |||||
| Miscellaneous | |||||||
| CIN 85 | Y2H, OE-IP | Carboxyl Term Domain | [186] | ||||
| Tax/p40 | Y2H, OE-IP | [198] | |||||
| DIPA/Ccdc85B | Y2H | [199] | |||||
| HmgB1 | OE-IP | [200] | |||||
IP - Immunprecipitation
OE-IP - Overexpression Immunoprecipitation
Y2H - Yeast two hybrid
KA - In Vitro Kinase Assay
When a TTP domain was examined, it is indicated; otherwise the entire protein was employed.
Phosphorylation of TTP
The TTP protein is subject to extensive posttranslational modification, particularly phosphorylation (for review see [54]), and several reports have also described its ubiquitination [55,56,57]. A number of signaling pathways have been reported to target TTP, including the ERK MAPK, p38 MAPK, JNK, GSK3β, PKA, PKB/AKT, and PKC pathways [54,58,59,60,61]. All three broadly expressed TTP family member proteins contain amino acid sequences that are well conserved at specific phosphorylation sites, and all are assumed to be similarly phosphorylated [62]. While extensive TTP phosphorylation has been mapped, only the serine-threonine phosphatase PP2A has been established as promoting TTP dephosphorylation [63]. Indirectly, the casein kinase 2 (CK2) has been shown to enhance TTP-mediated mRNA decay by phosphorylating MKP-1 (DUSP1), which in turn can dephosphorylate p38 MAPK [64].
The p38 MAPK pathway represents a critical controller of TTP function. TTP can be phosphorylated by MAPK-activated protein kinase 2 (MK2), a downstream target of p38, at serines 52 and 178 in mouse, 60 and 186 in humans [60,65,66]. Phosphorylation of TTP at these sites was initially hypothesized as regulating TTP ARE binding, the initial event leading to TTP activity [56,59,67]. However, data indicating that TTP is present on the polysomes [65,68] required rethinking of the hypothesis. Subsequent work by several groups reported that TTP mRNA binding activity was not affected by phosphorylation occurring through ERK MAPK, p38 MAPK/MK2, or JNK kinase activity [58,60,66,69,70], and this finding has been extended by reports indicating that phosphorylation of ZFP36L1 does not affect the ability of this TTP family member to bind AREs [71,72]. Numerous publications reported that, in response to p38 phosphorylation, TTP can form complexes with the multifunctional adaptor 14-3-3 proteins[60,63,66,70,71,73], an interaction that reportedly inhibits TTP mediated mRNA decay and protects TTP proteins from proteasomal degradation [55,63,74].
Completing the signaling pathway circuit, PP2A has been reported to mediate dephosphorylation of TTP, thus promoting decay of proinflammatory cytokine mRNAs that are stabilized through activation of p38/MK2 mediated phosphorylation of TTP [63]. More recent work implicates the ERK pathway as regulating TTP protein stability [57,75,76]. These findings are consistent with earlier work reporting that p38 activation, while necessary to inhibit TTP function, was not sufficient by itself to inhibit TTP function. Rather, the combined activation of both ERK and p38 was needed to inhibit TTP mediated TNF ARE decay [57]. It is also clear that our current understanding of TTP phosphorylation and the impact of phosphorylation events on TTP function is incomplete. For example, TTP is still capable of promoting decay of the TNF transcript following four hours of LPS stimulation, a time point at which the TTP protein is fully induced and phosphorylated [21]. Thus, at least in some physiological situations, apparently full phosphorylation of TTP does not abrogate its mRNA destabilizing capability.
A final component in discussing the regulation of TTP function must mention reports that TTP mRNA stability is controlled through an auto-regulatory negative feedback loop. Several studies have reported that TTP protein can bind to the AREs within its own 3′UTR, thus promoting decay [77,78]. In these experiments, the TTP message is quite labile, yielding half-lives of 17-22 min in unstimulated cells and about 45 min in stimulated cells [77,78]. One of us (PJB) has recently developed a knock-in mouse in which a single base change has been knocked into the Zfp36 locus, resulting in a mutation of one of the cysteines in the TZF domain, which produces a protein incapable of binding mRNA (W.S. Lai and P.J. Blackshear, unpublished data). This mouse should be very useful in dissecting the effects of TTP to regulate the stability of its own mRNA in a physiological setting. Both ZFP36L1 and ZFP36L2 mRNAs also contain AREs in their respective 3′UTRs [6]. While cross regulation of TTP, ZFP36L1 and ZFP36L2 has not to our knowledge been demonstrated, both family member mRNAs are found to be part of the TTP-associated mRNA pool [79], suggesting the possibility of cross-regulation between TTP and its family members.
Cytoplasmic mRNA Degradation
Upon export from the nucleus to the cytoplasm, mRNAs undergo a pioneer round of translation which is initiated by the nuclear cap-binding CBP20–CBP80 protein complex. The pioneer round of translation serves as a surveillance mechanism ensuring that the mRNA is properly spliced, contains an appropriate stop codon, and does not contain sequences resulting in ribosomal stalling. Aberrant messages are rapidly degraded via nonsense-mediated decay [80,81], non-stop mediated decay [82,83], or no-go mediated decay [84]. The pioneering round of translation results in a dramatic remodeling of the mRNP, stripping off nuclear bound proteins, including the exon junction complexes, and replacing the CBP20–CBP80 complex with the cytoplasmic cap-binding protein eukaryotic translation initiation factor 4E (eIF4E) [85]. The remodeled mRNP can then i) recruit the translation initiation complex to the mRNA through eIF4E, resulting in protein production, ii) target the mRNA for degradation or, iii) target the mRNA for storage, where at some future point it can be translated or degraded.
Deadenylation is thought to be the initial step in mRNA decay in all eukaryotes. Vertebrates express at least three deadenylation complexes: the Ccr4/Caf1/Not complex, the Pan2/Pan3 complex, and the poly A-specific ribonuclease (PARN) complex [86,87,88]. Following deadenylation, transcripts can be rapidly degraded via the 5′-3′ Xrn1 exonuclease pathway or the 3′-5′ exosome pathway [89,90].
In the 5′-to-3′ decay pathway, the mRNP rearrangement that is brought about by poly(A) tail shortening facilitates the recruitment of the decapping enzymes Dcp1 and Dcp2 to the cap structure, resulting in message decapping [91,92]. Once decapped, the transcript is degraded by the Xrn1 5′-to-3′ exonuclease. 5′-to-3′ decay components, including Dcp1, Dcp2, and Xrn1, are concentrated in cytoplasmic granules called processing bodies (P-bodies) [93]; however, microscopically visible P-body formation is not essential for 5′-to-3′ decay [94]. Experiments using translational inhibitors indicate that mRNAs need to be released from polysomes prior to their localization in P-bodies [95,96,97]. In addition, the presence of an RNA hairpin in the 5′ UTR, which represses translation initiation, does not enhance localization of ARE-containing mRNAs to P-bodies [95]. These data suggest that ARE-binding proteins may stimulate polysome release. However, TTP and ZFP36L1 tethered to a message are reportedly less effective at localizing mRNA to P-bodies than messages containing AREs, implying the involvement of other ARE proteins, perhaps TIA-1 and TIAR [98,99,100,101,102]. Interestingly, TTP has been described in P-bodies irrespective of its phosphorylation status [103].
In the 3′-to-5′ decay pathway, transcripts are fully deadenylated and then degraded by the exosome 3′-to-5′ exonuclease complex [104,105]. Recent data indicate co-localization of Rrp44 (Dis3) and Rrp6 (Pm/Scl-100), the exosome specific RNase subunits, with Xrn1 as well as other P-body and exosome components [68,106]. ARE-dependent mRNA decay can utilize both the 5′-3′ and 3′-5′ decay pathways [107] and TTP, as well as other TTP family members, can associate with both exosomes [53,57,107,108] and P-bodies [66,103,109,110]. The mechanism(s) underlying targeting of a TTP-bound message to one pathway or the other are at present unclear, but the current consensus is that the 5′-3′ decay pathway predominates for most mRNA decay and most ARE-dependent mRNA decay [92,93,111].
Finally, TTP family members can also localize to stress granules (SG) [66,103,109]. Heat shock, energy deprivation and other stressors induce the shutdown of bulk mRNA translation, resulting in the formation of granules containing untranslated mRNAs together with stalled translation initiation factors [112]. Unlike the situation with P-bodies, TTP co-localizes to SG only in the absence of phosphorylation at serines 52 and 178 [103]. It is possible that access to the pool of translationally stalled mRNAs may be an important function of TTP family proteins, allowing for a return to cellular homeostasis once the stressing stimuli are removed.
TTP Mediated mRNA Decay
So where does TTP specifically fit into the process of mRNA decay? As discussed above, TTP has a high affinity for the nonamer sequence UUAUUUAUU, and promotes the shortening of the poly(A) tail of GM-CSF transcripts [38]. Recent work indicates that TTP can specifically recruit the Ccr4/Caf1/Not deadenylase complex [69,70,113,114]. TTP (and ZFP36L1) can associate with Ccr4, and this interaction results in the deadenylation and decay of ARE-containing reporter mRNAs [69,70,113]. Ccr4 recruitment appears dependent on the interaction of Not1 with the C-terminal domain of TTP [113] and depletion of Caf1 or Ccr4 inhibits TTP-directed deadenylation, resulting in stabilization of ARE-containing transcripts [69,70,113].
Deadenylase recruitment by TTP appears to be regulated by the p38-MK2 signaling axis, with phosphorylation of TTP by MK2 reported to inhibit TTP-mediated deadenylation of ARE-containing transcripts [69,70]. This is consistent with the observation that p38 activity regulates the poly(A) tail length of endogenous TNF mRNA [70]. However, the specific role of 14-3-3 proteins in this process remains ambiguous. Recall that earlier work identified 14-3-3 binding to TTP when it is phosphorylated at serines 52 and 178, and that this interaction both inhibits TTP-mediated mRNA decay and protects the TTP protein from degradation [55,56,63,76]. Recent work by Clement et al. found that 14-3-3 binding to TTP directly inhibited deadenylase recruitment [69]. In contrast, Marchese et al. found that recruitment of CAF1 to TTP was inhibited by MK2, but this inhibition did not require 14-3-3 [70]. Clearly, further work is needed to clarify this issue.
Several additional points remain unresolved. The p38-MK2 axis does not appear to be the only signaling pathway contributing to the recruitment of deadenylases. A non-MK2 phosphorylatable TTP mutant (TTP-AA), in which serines 52 and 78 are mutated to alanines, is thought to exhibit enhanced ARE-mediated decay. However, the TTP-AA mutant exhibits a partial reduction in deadenylase affinity in the presence of okadaic acid, which inhibits PP2A, even though the p38/MK2 phosphorylation sites cannot be phosphorylated [69]. These data support the hypothesis that phosphorylation events other than those mediated by MK2 also contribute to the interaction of TTP with the deadenylation complex. Additionally, proteasome antagonists inhibit TTP function, implying that proteasome-mediated turnover of TTP protein is involved in ARE-mediated mRNA decay [57]. However, data with the TTP-AA mutant demonstrate that proteasome blockade does not completely inhibit the function of TTP-AA [55]. Given earlier work indicating TTP protein stability and function are regulated in part by ERK [57,75,76], it seems plausible that ERK-mediated phosphorylation is involved.
Taken together, the literature described above support the following model of TTP-mediated, ARE-containing mRNA decay. TTP target binding likely occurs in the cytoplasm following the pioneer round of translation. When p38 activity is low and TTP is relatively unphosphorylated at serines 52 and 178, TTP can bind to Not1, recruiting the Ccr4/Caf1/Not deadenylation complex. This results in shortening of the poly(A) tail and in decay of the TTP bound mRNA. The TTP protein itself is unstable in the dephosphorylated form and is turned over by the proteasome. Proteasome inhibition inhibits TTP mediated decay, but the precise relationship between TTP protein turnover and mRNA decay is unclear at this time.
Upon signal-dependent activation of p38 and ERK, TTP proteins are phosphorylated, inhibiting the interaction of TTP with Not1, and thus recruitment of the Ccr4/Caf1/Not deadenylation complex. The TTP-ARE containing mRNP is resistant to decay and the TTP protein is resistant to proteasome degradation. As the activating signal wanes, the phosphatase PP2A promotes dephosphorylation of TTP, allowing resumption in decay activity.
TTP and mRNA Translation
Translation in eukaryotes is regulated primarily at the level of initiation, and the efficiency of translation initiation determines the rate of protein synthesis (for review, see [115]). Several studies have linked the p38-MK2 signaling axis with ARE-mediated translation [56,116,117,118], but the proteins involved in the process have remained elusive. Three recent manuscripts appear to shed light on this process and both involve TTP.
In the first report, Cul4B, a scaffolding component of cullin-ring finger ubiquitin ligases (CRL4B), was identified as present in a TNF 3′UTR mRNP in the context of TTP expression, but not in the in the absence of TTP expression [55]. When Cul4B expression was reduced by shRNA, TNF mRNA levels and stability were comparable to control shRNA expressing macrophages in the absence of activation. Following LPS stimulation, cells with reduced Cul4B expression failed to fully stabilize the TNF message and exhibited enhanced TTP-mediated ARE decay. Indeed, reduced Cul4B expression increased the activity of the TTP-AA mutant, while constitutive activation of ERK and p38, which completely inhibited wild-type TTP function in control shRNA cells, only partially inhibited TTP mediated mRNA decay in Cul4B shRNA cells. Importantly, TTP protein levels were reduced in Cul4B shRNA cells, likely via the same translational mechanism, and TTP phosphorylation and ubiquitination were comparable in Control and Cul4B shRNA cell, making it unlikely that TTP itself is the direct target of Cul4B. Examining the polysome distribution of endogenous TNF mRNA revealed that reduced Cul4B levels resulted in reduced TNF polysome loading. It was concluded that in response to LPS stimulation, Cul4B promoted polysome loading of the TTP-bound TNF mRNP. Reduction in Cul4B levels reduced TNF mRNA polysome loading, shunting TNF mRNA back to the TTP mRNA decay pathway.
In the second paper, careful polysome profiling analysis following TTP knockdown and with TTP tethered to non-ARE containing mRNA further indicate a role for TTP as a translational regulator [119]. Qi et al. also report that silencing RCK/p54 resulted in a 1.5 fold increase in protein expression from a GM-CSF 3′UTR luciferase reporter, with a modest effect on mRNA levels [119]. Similar effects were seen with endogenous TNF in the context of RCK/p54 knockdown. Enhanced translation with RCK/p54 knockdown was observed even if TTP was overexpressed, indicating the involvement of RCK/p54 and TTP in the translational regulation of endogenous TNF mRNA. Thus, depleting RCK/p54 inhibited TTP-mediated repression of ARE-containing reporters and endogenous TNF. These results are consistent with some of the initial findings in the TTP knockout animal, in which TTP-deficient macrophages secreted about 5-fold more TNF, accompanied by a 2-fold increase in TNF mRNA levels, compared to wild-type macrophages [19].
It does not appear that TTP and RCK/p54 directly interact but rather are both members of larger protein assemblies [120]. Importantly, unlike TTP, RCK/p54 is not present on the polysomes [121], but it appears that translation initiation is required for translational repression mediated by Dhht1, the yeast homologue of RCK/p54 [122]. Previous work has shown that conditions which increase decapping or mRNA decay rates decrease translational initiation, while conditions that stabilize mRNAs within the polysome pool or promote translation decrease mRNA decapping, decay, and P-body formation [97,114,123,124,125,126]. Thus, there is a reciprocal relationship between mRNA translation and mRNA degradation, consistent with the findings that RCK/p54 overexpression inhibits translation and knockdown enhances translation.
Finally, a recent paper suggests that the ARE binding protein HuR needs to replace TTP in order for TNF translation to occur [127]. Working in immortalized macrophages double negative for MK2 and MK3 which were subsequently reconstituted with either MK2 or GFP (Control), the authors demonstrated that much of the translating TNF mRNA localizes to the endoplasmic reticulum, and that this localization is enhanced by MK2. In their hands, the endoplasmic reticulum-specific polysome pool contained HuR but not TTP, whereas the cytoplasmic polysome pool contained both HuR and TTP. In vitro binding assays demonstrated that HuR was able to displace TTP from an ARE-containing RNA probe when TTP was phosphorylated by MK2. The authors also showed that HuR immunoprecipitations captured about 2% of the TNF mRNA in the MK2 reconstituted, LPS-stimulated macrophages. An examination of TTP-bound TNF mRNA was not performed due to difficulties in effectively clearing the TTP protein, a familiar problem due to the high concentrations of TTP protein in activated macrophages. While the precise percentage of polysome-localized TNF mRNA associated with TTP protein is difficult to determine, TTP does appear to associate with the TNF mRNA on the polysomes, consistent with the presence of TTP protein in polysomes found by several groups [65,68,127]. The authors propose the interesting idea that the critical transition involving TTP and HuR is not translation/no translation, but rather cytoplasmic polysome localization to endoplasmic reticulum polysomes.
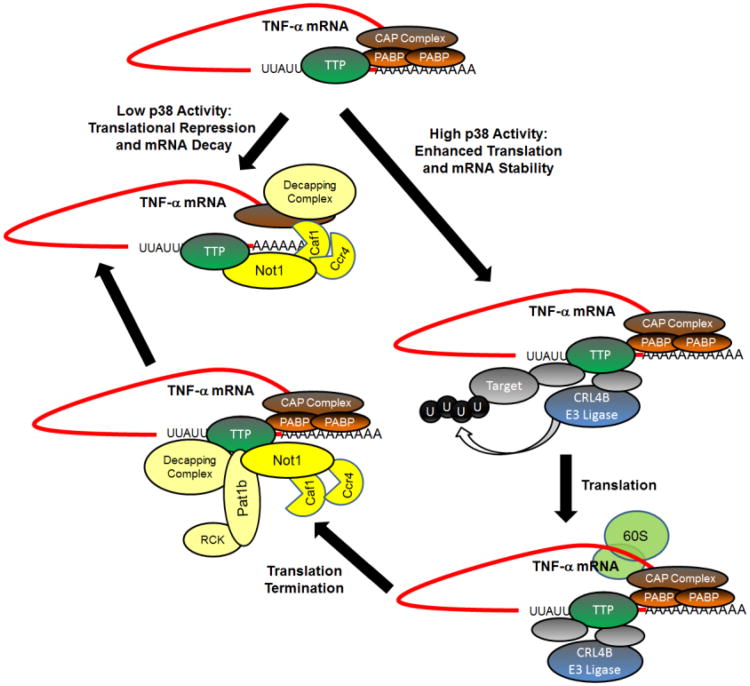
These data imply that TTP, rather than functioning exclusively as an mRNA decay promoting protein that is turned on and off, rather functions as a signal integrating switch, serving as the “link” between decay and translation of its mRNA ligands (Fig. 3). In this model, under conditions of low p38 activity, TTP promotes ARE-mediated mRNA decay and ARE-mediated translational repression. Increased p38 activity switches TTP function so that not only is the ARE-transcript stabilized, but its translation is enhanced. The finding that RCK/p54 acts through TTP to regulate GM-CSF and TNF translation implies that RCK is acting after translation initiation to shut off protein production after it has been induced [122]. For Cul4B, one can imagine CRL4B ubiquitination of its target protein, resulting in protein degradation or conformation change, operating to enhance the formation of a translationally competent mRNP or allowing a transition from cytoplasmic to endoplasmic reticulum translation. It is conceivable that translation of the signal sequence, necessary for localization to the endoplasmic reticulum for translation, is involved in regulating Cul4B activity. Alternatively, one could imagine the CRL4B targeting RCK/p54 and inhibiting translational termination. It remains unresolved precisely how mRNAs shift from the polysomes to translationally repressed mRNPs that are targets for decay or P-body accumulation. Further work is required to clarify these questions.
Fig. 3. Model of posttranscriptional regulation by TTP.
This schematic figure is intended to illustrate some of the elements of TTP function. Many protein interactions are not presented, and the specifics of the portrayed protein-protein interactions are necessarily simplistic. TTP cycles with its mRNA ligand between the translating and non-translating pool of messages [65,68,95,97]. Under conditions of low p38 activation, TTP is dephosphorylated at serines 52 and 178 (mouse) [60,66] which recruits Not1 [113]to the carboxyl end of the protein resulting in message deadenylation by the Ccr4/Caf1/Not deadenylation complex [69,70,113]. The amino terminal end of TTP recruits the decapping complex through an interaction with Dcp2 and Edc3 [120], but the specific phosphorylation status of TTP regarding this interaction has not been determined. Following p38 phosphorylation, TTP is unable to interact with Not1, and the TTP containing mRNP moves onto the polysomes. This process reportedly involves the CRL4B ubiquitin ligase component Cul4B [55]. The presence of Cul4B on the TTP containing mRNP is not dependent on p38 activity [55]. While not indicated in the figure, the translation competent TNF polysome complex may localize to the endoplasmic reticulum following signal sequence translation, and this process appears to involve HuR [127]. Through an as yet undetermined process, translation of the TTP containing mRNA ceases, and this process is reported to involve the translational repressor RCK/p54 [119]. RCK/p54 is not present on the polysomes [121] and appears to require translation initiation to operate as a translational repressor [122]. RCK/p54 interacts with a scaffolding protein, Pat1b, which also interacts with Not1 and, through Dcp1, Dcp2 [201]. Thus a deadenylation/decapping complex can form the TTP containing mRNP.
Conclusion
Since its discovery as a regulator of TNF mRNA stability more than 15 years ago, TTP has become one of the best understood posttranscriptional regulators and ARE binding proteins. Still a great deal of work remains for a complete understanding of the regulation of TTP function and its targets. As technology advances, particularly ribosome profiling and deep sequencing, we will likely be able to more clearly identify true TTP mRNA ligands and the functional consequences of TTP binding. While clearly an mRNA stability regulator, recent work indicating that TTP also functions as a translational regulator represents an exciting new avenue of research. Validation of this hypothesis requires additional work to establish the precise mechanism of action, similar to the work indicating that TTP mediated mRNA decay is initiated by recruitment of the Ccr4/Caf1/Not deadenylation complex. Finally, establishing the regulation of TTP expression, subcellular localization, and function in normal versus diseased tissues should enable a transition from the laboratory to bedside applications.
Highlights for review.
We provide an up to date list of TTP mRNA ligands described in the literature.
We describe a list of TTP interacting proteins published to date.
This review discusses how phosphorylation has been proposed to regulate TTP function
A model of TTP mediated mRNA decay is provided.
A model is discussed of the proposed role of TTP to regulate mRNA translation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lopez de Silanes I, Quesada MP, Esteller M. Aberrant regulation of messenger RNA 3′-untranslated region in human cancer. Cell Oncol. 2007;29:1–17. doi: 10.1155/2007/586139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benjamin D, Moroni C. mRNA stability and cancer: an emerging link? Expert Opin Biol Ther. 2007;7:1515–1529. doi: 10.1517/14712598.7.10.1515. [DOI] [PubMed] [Google Scholar]
- 3.Hitti E, Khabar KS. Sequence variations affecting AU-rich element function and disease. Front Biosci. 2012;17:1846–1860. doi: 10.2741/4023. [DOI] [PubMed] [Google Scholar]
- 4.Schott J, Stoecklin G. Networks controlling mRNA decay in the immune system. Wiley Interdiscip Rev RNA. 2010;1:432–456. doi: 10.1002/wrna.13. [DOI] [PubMed] [Google Scholar]
- 5.Beisang D, Bohjanen PR. Perspectives on the ARE as it turns 25 years old. Wiley Interdiscip Rev RNA. 2012;3:719–731. doi: 10.1002/wrna.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bakheet T, Williams BR, Khabar KS. ARED 3.0: the large and diverse AU-rich transcriptome. Nucleic Acids Res. 2006;34:D111–114. doi: 10.1093/nar/gkj052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Halees AS, El-Badrawi R, Khabar KS. ARED Organism: expansion of ARED reveals AU-rich element cluster variations between human and mouse. Nucleic Acids Res. 2008;36:D137–140. doi: 10.1093/nar/gkm959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mittal N, Roy N, Babu MM, Janga SC. Dissecting the expression dynamics of RNA-binding proteins in posttranscriptional regulatory networks. Proc Natl Acad Sci U S A. 2009;106:20300–20305. doi: 10.1073/pnas.0906940106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lai WS, Stumpo DJ, Blackshear PJ. Rapid insulin-stimulated accumulation of an mRNA encoding a proline-rich protein. The Journal of biological chemistry. 1990;265:16556–16563. [PubMed] [Google Scholar]
- 10.DuBois RN, McLane MW, Ryder K, Lau LF, Nathans D. A growth factor-inducible nuclear protein with a novel cysteine/histidine repetitive sequence. The Journal of biological chemistry. 1990;265:19185–19191. [PubMed] [Google Scholar]
- 11.Varnum BC, Lim RW, Sukhatme VP, Herschman HR. Nucleotide sequence of a cDNA encoding TIS11, a message induced in Swiss 3T3 cells by the tumor promoter tetradecanoyl phorbol acetate. Oncogene. 1989;4:119–120. [PubMed] [Google Scholar]
- 12.Ma Q, Herschman HR. A corrected sequence for the predicted protein from the mitogen-inducible TIS11 primary response gene. Oncogene. 1991;6:1277–1278. [PubMed] [Google Scholar]
- 13.Taylor GA. The Human TTP protein: Sequence, alignment with related proteins, and chromosomal localization of the mouse and human genes. Nucleic Acids Res. 1991;19:3454. doi: 10.1093/nar/19.12.3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heximer SP, Forsdyke DR. A human putative lymphocyte G0/G1 switch gene homologous to a rodent gene encoding a zinc-binding potential transcription factor. DNA and cell biology. 1993;12:73–88. doi: 10.1089/dna.1993.12.73. [DOI] [PubMed] [Google Scholar]
- 15.Taylor GA, Thompson MJ, Lai WS, Blackshear PJ. Phosphorylation of tristetraprolin, a potential zinc finger transcription factor, by mitogen stimulation in intact cells and by mitogen-activated protein kinase in vitro. J Biol Chem. 1995;270:13341–13347. doi: 10.1074/jbc.270.22.13341. [DOI] [PubMed] [Google Scholar]
- 16.Taylor GA, Thompson MJ, Lai WS, Blackshear PJ. Mitogens stimulate the rapid nuclear to cytosolic translocation of tristetraprolin, a potential zinc-finger transcription factor. Molecular endocrinology (Baltimore, Md) 1996;10:140–146. doi: 10.1210/mend.10.2.8825554. [DOI] [PubMed] [Google Scholar]
- 17.Taylor GA, Blackshear PJ. Zinc inhibits turnover of labile mRNAs in intact cells. Journal of cellular physiology. 1995;162:378–387. doi: 10.1002/jcp.1041620310. [DOI] [PubMed] [Google Scholar]
- 18.Lai WS, Thompson MJ, Taylor GA, Liu Y, Blackshear PJ. Promoter analysis of Zfp-36, the mitogen-inducible gene encoding the zinc finger protein tristetraprolin. J Biol Chem. 1995;270:25266–25272. doi: 10.1074/jbc.270.42.25266. [DOI] [PubMed] [Google Scholar]
- 19.Taylor GA, Carballo E, Lee DM, Lai WS, Thompson MJ, Patel DD, Schenkman DI, Gilkeson GS, Broxmeyer HE, Haynes BF, Blackshear PJ. A pathogenetic role for TNF alpha in the syndrome of cachexia, arthritis, and autoimmunity resulting from tristetraprolin (TTP) deficiency. Immunity. 1996;4:445–454. doi: 10.1016/s1074-7613(00)80411-2. [DOI] [PubMed] [Google Scholar]
- 20.Carballo E, Gilkeson GS, Blackshear PJ. Bone marrow transplantation reproduces the tristetraprolin-deficiency syndrome in recombination activating gene-2 (-/-) mice. Evidence that monocyte/macrophage progenitors may be responsible for TNFalpha overproduction. The Journal of clinical investigation. 1997;100:986–995. doi: 10.1172/JCI119649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carballo E, Lai WS, Blackshear PJ. Feedback inhibition of macrophage tumor necrosis factor-alpha production by tristetraprolin. Science (New York, NY) 1998;281:1001–1005. doi: 10.1126/science.281.5379.1001. [DOI] [PubMed] [Google Scholar]
- 22.Kontoyiannis D, Pasparakis M, Pizarro TT, Cominelli F, Kollias G. Impaired on/off regulation of TNF biosynthesis in mice lacking TNF AU-rich elements: implications for joint and gut-associated immunopathologies. Immunity. 1999;10:387–398. doi: 10.1016/s1074-7613(00)80038-2. [DOI] [PubMed] [Google Scholar]
- 23.Qiu LQ, Stumpo DJ, Blackshear PJ. Myeloid-specific tristetraprolin deficiency in mice results in extreme lipopolysaccharide sensitivity in an otherwise minimal phenotype. J Immunol. 2012;188:5150–5159. doi: 10.4049/jimmunol.1103700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kratochvill F, Machacek C, Vogl C, Ebner F, Sedlyarov V, Gruber AR, Hartweger H, Vielnascher R, Karaghiosoff M, Rulicke T, Muller M, Hofacker I, Lang R, Kovarik P. Tristetraprolin-driven regulatory circuit controls quality and timing of mRNA decay in inflammation. Mol Syst Biol. 2011;7:560. doi: 10.1038/msb.2011.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blackshear PJ, Lai WS, Kennington EA, Brewer G, Wilson GM, Guan X, Zhou P. Characteristics of the interaction of a synthetic human tristetraprolin tandem zinc finger peptide with AU-rich element-containing RNA substrates. J Biol Chem. 2003;278:19947–19955. doi: 10.1074/jbc.M301290200. [DOI] [PubMed] [Google Scholar]
- 26.Worthington MT, Pelo JW, Sachedina MA, Applegate JL, Arseneau KO, Pizarro TT. RNA binding properties of the AU-rich element-binding recombinant Nup475/TIS11/tristetraprolin protein. J Biol Chem. 2002;277:48558–48564. doi: 10.1074/jbc.M206505200. [DOI] [PubMed] [Google Scholar]
- 27.Lai WS, Carballo E, Thorn JM, Kennington EA, Blackshear PJ. Interactions of CCCH zinc finger proteins with mRNA. Binding of tristetraprolin-related zinc finger proteins to Au-rich elements and destabilization of mRNA. J Biol Chem. 2000;275:17827–17837. doi: 10.1074/jbc.M001696200. [DOI] [PubMed] [Google Scholar]
- 28.Brewer BY, Malicka J, Blackshear PJ, Wilson GM. RNA sequence elements required for high affinity binding by the zinc finger domain of tristetraprolin: conformational changes coupled to the bipartite nature of Au-rich MRNA-destabilizing motifs. J Biol Chem. 2004;279:27870–27877. doi: 10.1074/jbc.M402551200. [DOI] [PubMed] [Google Scholar]
- 29.Hudson BP, Martinez-Yamout MA, Dyson HJ, Wright PE. Recognition of the mRNA AU-rich element by the zinc finger domain of TIS11d. Nat Struct Mol Biol. 2004;11:257–264. doi: 10.1038/nsmb738. [DOI] [PubMed] [Google Scholar]
- 30.Carrick DM, Lai WS, Blackshear PJ. The tandem CCCH zinc finger protein tristetraprolin and its relevance to cytokine mRNA turnover and arthritis. Arthritis research & therapy. 2004;6:248–264. doi: 10.1186/ar1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blackshear PJ, Phillips RS, Ghosh S, Ramos SB, Richfield EK, Lai WS. Zfp36l3, a rodent X chromosome gene encoding a placenta-specific member of the Tristetraprolin family of CCCH tandem zinc finger proteins. Biology of reproduction. 2005;73:297–307. doi: 10.1095/biolreprod.105.040527. [DOI] [PubMed] [Google Scholar]
- 32.De J, Lai WS, Thorn JM, Goldsworthy SM, Liu X, Blackwell TK, Blackshear PJ. Identification of four CCCH zinc finger proteins in Xenopus, including a novel vertebrate protein with four zinc fingers and severely restricted expression. Gene. 1999;228:133–145. doi: 10.1016/s0378-1119(98)00617-9. [DOI] [PubMed] [Google Scholar]
- 33.Belloc E, Mendez R. A deadenylation negative feedback mechanism governs meiotic metaphase arrest. Nature. 2008;452:1017–1021. doi: 10.1038/nature06809. [DOI] [PubMed] [Google Scholar]
- 34.Kaymak E, Wee LM, Ryder SP. Structure and function of nematode RNA-binding proteins. Current opinion in structural biology. 2010;20:305–312. doi: 10.1016/j.sbi.2010.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carballo E, Blackshear PJ. Roles of tumor necrosis factor-alpha receptor subtypes in the pathogenesis of the tristetraprolin-deficiency syndrome. Blood. 2001;98:2389–2395. doi: 10.1182/blood.v98.8.2389. [DOI] [PubMed] [Google Scholar]
- 36.Lai WS, Parker JS, Grissom SF, Stumpo DJ, Blackshear PJ. Novel mRNA targets for tristetraprolin (TTP) identified by global analysis of stabilized transcripts in TTP-deficient fibroblasts. Mol Cell Biol. 2006;26:9196–9208. doi: 10.1128/MCB.00945-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shaw G, Kamen R. A conserved AU sequence from the 3′ untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell. 1986;46:659–667. doi: 10.1016/0092-8674(86)90341-7. [DOI] [PubMed] [Google Scholar]
- 38.Carballo E, Lai WS, Blackshear PJ. Evidence that tristetraprolin is a physiological regulator of granulocyte-macrophage colony-stimulating factor messenger RNA deadenylation and stability. Blood. 2000;95:1891–1899. [PubMed] [Google Scholar]
- 39.Lai WS, Carballo E, Strum JR, Kennington EA, Phillips RS, Blackshear PJ. Evidence that tristetraprolin binds to AU-rich elements and promotes the deadenylation and destabilization of tumor necrosis factor alpha mRNA. Mol Cell Biol. 1999;19:4311–4323. doi: 10.1128/mcb.19.6.4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lai WS, Kennington EA, Blackshear PJ. Tristetraprolin and its family members can promote the cell-free deadenylation of AU-rich element-containing mRNAs by poly(A) ribonuclease. Mol Cell Biol. 2003;23:3798–3812. doi: 10.1128/MCB.23.11.3798-3812.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stoecklin G, Ming XF, Looser R, Moroni C. Somatic mRNA turnover mutants implicate tristetraprolin in the interleukin-3 mRNA degradation pathway. Mol Cell Biol. 2000;20:3753–3763. doi: 10.1128/mcb.20.11.3753-3763.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ming XF, Stoecklin G, Lu M, Looser R, Moroni C. Parallel and independent regulation of interleukin-3 mRNA turnover by phosphatidylinositol 3-kinase and p38 mitogen-activated protein kinase. Molecular and cellular biology. 2001;21:5778–5789. doi: 10.1128/MCB.21.17.5778-5789.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Raghavan A, Robison RL, McNabb J, Miller CR, Williams DA, Bohjanen PR. HuA and tristetraprolin are induced following T cell activation and display distinct but overlapping RNA binding specificities. J Biol Chem. 2001;276:47958–47965. doi: 10.1074/jbc.M109511200. [DOI] [PubMed] [Google Scholar]
- 44.Zhu W, Brauchle MA, Di Padova F, Gram H, New L, Ono K, Downey JS, Han J. Gene suppression by tristetraprolin and release by the p38 pathway. Am J Physiol Lung Cell Mol Physiol. 2001;281:L499–508. doi: 10.1152/ajplung.2001.281.2.L499. [DOI] [PubMed] [Google Scholar]
- 45.Jing Q, Huang S, Guth S, Zarubin T, Motoyama A, Chen J, Di Padova F, Lin SC, Gram H, Han J. Involvement of microRNA in AU-rich element-mediated mRNA instability. Cell. 2005;120:623–634. doi: 10.1016/j.cell.2004.12.038. [DOI] [PubMed] [Google Scholar]
- 46.Mols J, van den Berg A, Otsuka M, Zheng M, Chen J, Han J. TNF-alpha stimulation inhibits siRNA-mediated RNA interference through a mechanism involving poly-(A) tail stabilization. Biochimica et biophysica acta. 2008;1779:712–719. doi: 10.1016/j.bbagrm.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Helfer S, Schott J, Stoecklin G, Forstemann K. AU-rich element-mediated mRNA decay can occur independently of the miRNA machinery in mouse embryonic fibroblasts and Drosophila S2-cells. PLoS One. 2012;7:e28907. doi: 10.1371/journal.pone.0028907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liang J, Lei T, Song Y, Yanes N, Qi Y, Fu M. RNA-destabilizing factor tristetraprolin negatively regulates NF-kappaB signaling. J Biol Chem. 2009;284:29383–29390. doi: 10.1074/jbc.M109.024745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schichl YM, Resch U, Hofer-Warbinek R, de Martin R. Tristetraprolin impairs NF-kappaB/p65 nuclear translocation. J Biol Chem. 2009;284:29571–29581. doi: 10.1074/jbc.M109.031237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ness GC, Edelman JL, Brooks PA. Involvement of tristetraprolin in transcriptional activation of hepatic 3-hydroxy-3-methylglutaryl coenzyme A reductase by insulin. Biochem Biophys Res Commun. 2012;420:178–182. doi: 10.1016/j.bbrc.2012.02.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maeda M, Sawa H, Tobiume M, Tokunaga K, Hasegawa H, Ichinohe T, Sata T, Moriyama M, Hall WW, Kurata T, Takahashi H. Tristetraprolin inhibits HIV-1 production by binding to genomic RNA. Microbes Infect. 2006;8:2647–2656. doi: 10.1016/j.micinf.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 52.Fechir M, Linker K, Pautz A, Hubrich T, Forstermann U, Rodriguez-Pascual F, Kleinert H. Tristetraprolin regulates the expression of the human inducible nitric-oxide synthase gene. Molecular pharmacology. 2005;67:2148–2161. doi: 10.1124/mol.104.008763. [DOI] [PubMed] [Google Scholar]
- 53.Linker K, Pautz A, Fechir M, Hubrich T, Greeve J, Kleinert H. Involvement of KSRP in the post-transcriptional regulation of human iNOS expression-complex interplay of KSRP with TTP and HuR. Nucleic Acids Res. 2005;33:4813–4827. doi: 10.1093/nar/gki797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cao H, Deterding LJ, Blackshear PJ. Phosphorylation site analysis of the anti-inflammatory and mRNA-destabilizing protein tristetraprolin. Expert Rev Proteomics. 2007;4:711–726. doi: 10.1586/14789450.4.6.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pfeiffer JR, Brooks SA. Cullin 4B is recruited to tristetraprolin-containing messenger ribonucleoproteins and regulates TNF-alpha mRNA polysome loading. J Immunol. 2012;188:1828–1839. doi: 10.4049/jimmunol.1102837. [DOI] [PubMed] [Google Scholar]
- 56.Hitti E, Iakovleva T, Brook M, Deppenmeier S, Gruber AD, Radzioch D, Clark AR, Blackshear PJ, Kotlyarov A, Gaestel M. Mitogen-activated protein kinase-activated protein kinase 2 regulates tumor necrosis factor mRNA stability and translation mainly by altering tristetraprolin expression, stability, and binding to adenine/uridine-rich element. Mol Cell Biol. 2006;26:2399–2407. doi: 10.1128/MCB.26.6.2399-2407.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Deleault KM, Skinner SJ, Brooks SA. Tristetraprolin regulates TNF TNF-alpha mRNA stability via a proteasome dependent mechanism involving the combined action of the ERK and p38 pathways. Mol Immunol. 2008;45:13–24. doi: 10.1016/j.molimm.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 58.Cao H, Dzineku F, Blackshear PJ. Expression and purification of recombinant tristetraprolin that can bind to tumor necrosis factor-alpha mRNA and serve as a substrate for mitogen-activated protein kinases. Arch Biochem Biophys. 2003;412:106–120. doi: 10.1016/s0003-9861(03)00012-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Carballo E, Cao H, Lai WS, Kennington EA, Campbell D, Blackshear PJ. Decreased sensitivity of tristetraprolin-deficient cells to p38 inhibitors suggests the involvement of tristetraprolin in the p38 signaling pathway. J Biol Chem. 2001;276:42580–42587. doi: 10.1074/jbc.M104953200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chrestensen CA, Schroeder MJ, Shabanowitz J, Hunt DF, Pelo JW, Worthington MT, Sturgill TW. MAPKAP kinase 2 phosphorylates tristetraprolin on in vivo sites including Ser178, a site required for 14-3-3 binding. J Biol Chem. 2004;279:10176–10184. doi: 10.1074/jbc.M310486200. [DOI] [PubMed] [Google Scholar]
- 61.Mahtani KR, Brook M, Dean JL, Sully G, Saklatvala J, Clark AR. Mitogen-activated protein kinase p38 controls the expression and posttranslational modification of tristetraprolin, a regulator of tumor necrosis factor alpha mRNA stability. Mol Cell Biol. 2001;21:6461–6469. doi: 10.1128/MCB.21.9.6461-6469.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Clark A, Dean J, Tudor C, Saklatvala J. Post-transcriptional gene regulation by MAP kinases via AU-rich elements. Front Biosci. 2009;14:847–871. doi: 10.2741/3282. [DOI] [PubMed] [Google Scholar]
- 63.Sun L, Stoecklin G, Van Way S, Hinkovska-Galcheva V, Guo RF, Anderson P, Shanley TP. Tristetraprolin (TTP)-14-3-3 complex formation protects TTP from dephosphorylation by protein phosphatase 2a and stabilizes tumor necrosis factor-alpha mRNA. J Biol Chem. 2007;282:3766–3777. doi: 10.1074/jbc.M607347200. [DOI] [PubMed] [Google Scholar]
- 64.Lee WH, Lee HH, Vo MT, Kim HJ, Ko MS, Im YC, Min YJ, Lee BJ, Cho WJ, Park JW. Casein kinase 2 regulates the mRNA-destabilizing activity of tristetraprolin. J Biol Chem. 2011;286:21577–21587. doi: 10.1074/jbc.M110.201137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rigby WF, Roy K, Collins J, Rigby S, Connolly JE, Bloch DB, Brooks SA. Structure/function analysis of tristetraprolin (TTP): p38 stress-activated protein kinase and lipopolysaccharide stimulation do not alter TTP function. J Immunol. 2005;174:7883–7893. doi: 10.4049/jimmunol.174.12.7883. [DOI] [PubMed] [Google Scholar]
- 66.Stoecklin G, Stubbs T, Kedersha N, Wax S, Rigby WF, Blackwell TK, Anderson P. MK2-induced tristetraprolin:14-3-3 complexes prevent stress granule association and ARE-mRNA decay. Embo J. 2004;23:1313–1324. doi: 10.1038/sj.emboj.7600163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cao H. Expression, purification, and biochemical characterization of the anti-inflammatory tristetraprolin: a zinc-dependent mRNA binding protein affected by posttranslational modifications. Biochemistry. 2004;43:13724–13738. doi: 10.1021/bi049014y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pfeiffer JR, McAvoy BL, Fecteau RE, Deleault KM, Brooks SA. CARHSP1 is required for effective tumor necrosis factor alpha mRNA stabilization and localizes to processing bodies and exosomes. Mol Cell Biol. 2011;31:277–286. doi: 10.1128/MCB.00775-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Clement SL, Scheckel C, Stoecklin G, Lykke-Andersen J. Phosphorylation of tristetraprolin by MK2 Impairs AU-rich element mRNA decay by preventing deadenylase recruitment. Mol Cell Biol. 2011;31:256–266. doi: 10.1128/MCB.00717-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Marchese FP, Aubareda A, Tudor C, Saklatvala J, Clark AR, Dean JL. MAPKAP kinase 2 blocks tristetraprolin-directed mRNA decay by inhibiting CAF1 deadenylase recruitment. J Biol Chem. 2010;285:27590–27600. doi: 10.1074/jbc.M110.136473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schmidlin M, Lu M, Leuenberger SA, Stoecklin G, Mallaun M, Gross B, Gherzi R, Hess D, Hemmings BA, Moroni C. The ARE-dependent mRNA-destabilizing activity of BRF1 is regulated by protein kinase B. Embo J. 2004;23:4760–4769. doi: 10.1038/sj.emboj.7600477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Maitra S, Chou CF, Luber CA, Lee KY, Mann M, Chen CY. The AU-rich element mRNA decay-promoting activity of BRF1 is regulated by mitogen-activated protein kinase-activated protein kinase 2. Rna. 2008;14:950–959. doi: 10.1261/rna.983708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Marderosian M, Sharma A, Funk AP, Vartanian R, Masri J, Jo OD, Gera JF. Tristetraprolin regulates Cyclin D1 and c-Myc mRNA stability in response to rapamycin in an Akt-dependent manner via p38 MAPK signaling. Oncogene. 2006;25:6277–6290. doi: 10.1038/sj.onc.1209645. [DOI] [PubMed] [Google Scholar]
- 74.Benjamin D, Schmidlin M, Min L, Gross B, Moroni C. BRF1 protein turnover and mRNA decay activity are regulated by protein kinase B at the same phosphorylation sites. Mol Cell Biol. 2006;26:9497–9507. doi: 10.1128/MCB.01099-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Essafi-Benkhadir K, Pouyssegur J, Pages G. Implication of the ERK pathway on the post-transcriptional regulation of VEGF mRNA stability. Methods Mol Biol. 2010;661:451–469. doi: 10.1007/978-1-60761-795-2_28. [DOI] [PubMed] [Google Scholar]
- 76.Brook M, Tchen CR, Santalucia T, McIlrath J, Arthur JS, Saklatvala J, Clark AR. Posttranslational regulation of tristetraprolin subcellular localization and protein stability by p38 mitogen-activated protein kinase and extracellular signal-regulated kinase pathways. Mol Cell Biol. 2006;26:2408–2418. doi: 10.1128/MCB.26.6.2408-2418.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Brooks SA, Connolly JE, Rigby WF. The role of mRNA turnover in the regulation of tristetraprolin expression: evidence for an extracellular signal-regulated kinase-specific, AU-rich element-dependent, autoregulatory pathway. J Immunol. 2004;172:7263–7271. doi: 10.4049/jimmunol.172.12.7263. [DOI] [PubMed] [Google Scholar]
- 78.Tchen CR, Brook M, Saklatvala J, Clark AR. The stability of tristetraprolin mRNA is regulated by mitogen-activated protein kinase p38 and by tristetraprolin itself. J Biol Chem. 2004;279:32393–32400. doi: 10.1074/jbc.M402059200. [DOI] [PubMed] [Google Scholar]
- 79.Stoecklin G, Tenenbaum SA, Mayo T, Chittur SV, George AD, Baroni TE, Blackshear PJ, Anderson P. Genome-wide analysis identifies interleukin-10 mRNA as target of tristetraprolin. J Biol Chem. 2008;283:11689–11699. doi: 10.1074/jbc.M709657200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Silva AL, Romao L. The mammalian nonsense-mediated mRNA decay pathway: to decay or not to decay! Which players make the decision? FEBS Lett. 2009;583:499–505. doi: 10.1016/j.febslet.2008.12.058. [DOI] [PubMed] [Google Scholar]
- 81.Neu-Yilik G, Kulozik AE. NMD: multitasking between mRNA surveillance and modulation of gene expression. Adv Genet. 2008;62:185–243. doi: 10.1016/S0065-2660(08)00604-4. [DOI] [PubMed] [Google Scholar]
- 82.van Hoof A, Frischmeyer PA, Dietz HC, Parker R. Exosome-mediated recognition and degradation of mRNAs lacking a termination codon. Science. 2002;295:2262–2264. doi: 10.1126/science.1067272. [DOI] [PubMed] [Google Scholar]
- 83.Frischmeyer PA, van Hoof A, O'Donnell K, Guerrerio AL, Parker R, Dietz HC. An mRNA surveillance mechanism that eliminates transcripts lacking termination codons. Science. 2002;295:2258–2261. doi: 10.1126/science.1067338. [DOI] [PubMed] [Google Scholar]
- 84.Harigaya Y, Parker R. No-go decay: a quality control mechanism for RNA in translation. Wiley Interdiscip Rev RNA. 2010;1:132–141. doi: 10.1002/wrna.17. [DOI] [PubMed] [Google Scholar]
- 85.Moore MJ. From birth to death: the complex lives of eukaryotic mRNAs. Science. 2005;309:1514–1518. doi: 10.1126/science.1111443. [DOI] [PubMed] [Google Scholar]
- 86.Schwede A, Ellis L, Luther J, Carrington M, Stoecklin G, Clayton C. A role for Caf1 in mRNA deadenylation and decay in trypanosomes and human cells. Nucleic Acids Res. 2008;36:3374–3388. doi: 10.1093/nar/gkn108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yamashita A, Chang TC, Yamashita Y, Zhu W, Zhong Z, Chen CY, Shyu AB. Concerted action of poly(A) nucleases and decapping enzyme in mammalian mRNA turnover. Nat Struct Mol Biol. 2005;12:1054–1063. doi: 10.1038/nsmb1016. [DOI] [PubMed] [Google Scholar]
- 88.Parker R, Song H. The enzymes and control of eukaryotic mRNA turnover. Nat Struct Mol Biol. 2004;11:121–127. doi: 10.1038/nsmb724. [DOI] [PubMed] [Google Scholar]
- 89.Eulalio A, Behm-Ansmant I, Izaurralde E. P bodies: at the crossroads of post-transcriptional pathways. Nat Rev Mol Cell Biol. 2007;8:9–22. doi: 10.1038/nrm2080. [DOI] [PubMed] [Google Scholar]
- 90.Garneau NL, Wilusz J, Wilusz CJ. The highways and byways of mRNA decay. Nat Rev Mol Cell Biol. 2007;8:113–126. doi: 10.1038/nrm2104. [DOI] [PubMed] [Google Scholar]
- 91.Parker R, Sheth U. P bodies and the control of mRNA translation and degradation. Mol Cell. 2007;25:635–646. doi: 10.1016/j.molcel.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 92.Franks TM, Lykke-Andersen J. The control of mRNA decapping and P-body formation. Mol Cell. 2008;32:605–615. doi: 10.1016/j.molcel.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Anderson P, Kedersha N. RNA granules: post-transcriptional and epigenetic modulators of gene expression. Nat Rev Mol Cell Biol. 2009;10:430–436. doi: 10.1038/nrm2694. [DOI] [PubMed] [Google Scholar]
- 94.Eulalio A, Behm-Ansmant I, Schweizer D, Izaurralde E. P-body formation is a consequence, not the cause, of RNA-mediated gene silencing. Mol Cell Biol. 2007;27:3970–3981. doi: 10.1128/MCB.00128-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Franks TM, Lykke-Andersen J. TTP and BRF proteins nucleate processing body formation to silence mRNAs with AU-rich elements. Genes Dev. 2007;21:719–735. doi: 10.1101/gad.1494707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cougot N, Babajko S, Seraphin B. Cytoplasmic foci are sites of mRNA decay in human cells. J Cell Biol. 2004;165:31–40. doi: 10.1083/jcb.200309008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Teixeira D, Sheth U, Valencia-Sanchez MA, Brengues M, Parker R. Processing bodies require RNA for assembly and contain nontranslating mRNAs. Rna. 2005;11:371–382. doi: 10.1261/rna.7258505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dember LM, Kim ND, Liu KQ, Anderson P. Individual RNA recognition motifs of TIA-1 and TIAR have different RNA binding specificities. J Biol Chem. 1996;271:2783–2788. doi: 10.1074/jbc.271.5.2783. [DOI] [PubMed] [Google Scholar]
- 99.Gueydan C, Droogmans L, Chalon P, Huez G, Caput D, Kruys V. Identification of TIAR as a protein binding to the translational regulatory AU-rich element of tumor necrosis factor alpha mRNA. J Biol Chem. 1999;274:2322–2326. doi: 10.1074/jbc.274.4.2322. [DOI] [PubMed] [Google Scholar]
- 100.Piecyk M, Wax S, Beck AR, Kedersha N, Gupta M, Maritim B, Chen S, Gueydan C, Kruys V, Streuli M, Anderson P. TIA-1 is a translational silencer that selectively regulates the expression of TNF-alpha. Embo J. 2000;19:4154–4163. doi: 10.1093/emboj/19.15.4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lopez de Silanes I, Galban S, Martindale JL, Yang X, Mazan-Mamczarz K, Indig FE, Falco G, Zhan M, Gorospe M. Identification and functional outcome of mRNAs associated with RNA-binding protein TIA-1. Mol Cell Biol. 2005;25:9520–9531. doi: 10.1128/MCB.25.21.9520-9531.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mazan-Mamczarz K, Lal A, Martindale JL, Kawai T, Gorospe M. Translational repression by RNA-binding protein TIAR. Mol Cell Biol. 2006;26:2716–2727. doi: 10.1128/MCB.26.7.2716-2727.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kedersha N, Stoecklin G, Ayodele M, Yacono P, Lykke-Andersen J, Fritzler MJ, Scheuner D, Kaufman RJ, Golan DE, Anderson P. Stress granules and processing bodies are dynamically linked sites of mRNP remodeling. J Cell Biol. 2005;169:871–884. doi: 10.1083/jcb.200502088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lykke-Andersen S, Brodersen DE, Jensen TH. Origins and activities of the eukaryotic exosome. J Cell Sci. 2009;122:1487–1494. doi: 10.1242/jcs.047399. [DOI] [PubMed] [Google Scholar]
- 105.Ibrahim H, Wilusz J, Wilusz CJ. RNA recognition by 3′-to-5′ exonucleases: the substrate perspective. Biochim Biophys Acta. 2008;1779:256–265. doi: 10.1016/j.bbagrm.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Brooks SA. Functional interactions between mRNA turnover and surveillance and the ubiquitin proteasome system. WIREs RNA. 2010;1 doi: 10.1002/wrna.11. epub May 14, 2010. [DOI] [PubMed] [Google Scholar]
- 107.Stoecklin G, Mayo T, Anderson P. ARE-mRNA degradation requires the 5′-3′ decay pathway. EMBO Rep. 2006;7:72–77. doi: 10.1038/sj.embor.7400572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chen CY, Gherzi R, Ong SE, Chan EL, Raijmakers R, Pruijn GJ, Stoecklin G, Moroni C, Mann M, Karin M. AU binding proteins recruit the exosome to degrade ARE-containing mRNAs. Cell. 2001;107:451–464. doi: 10.1016/s0092-8674(01)00578-5. [DOI] [PubMed] [Google Scholar]
- 109.Kedersha N, Anderson P. Stress granules: sites of mRNA triage that regulate mRNA stability and translatability. Biochem Soc Trans. 2002;30:963–969. doi: 10.1042/bst0300963. [DOI] [PubMed] [Google Scholar]
- 110.Phillips K, Kedersha N, Shen L, Blackshear PJ, Anderson P. Arthritis suppressor genes TIA-1 and TTP dampen the expression of tumor necrosis factor alpha, cyclooxygenase 2, and inflammatory arthritis. Proc Natl Acad Sci U S A. 2004;101:2011–2016. doi: 10.1073/pnas.0400148101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Buchan JR, Parker R. Eukaryotic stress granules: the ins and outs of translation. Mol Cell. 2009;36:932–941. doi: 10.1016/j.molcel.2009.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Anderson P, Kedersha N. Stressful initiations. J Cell Sci. 2002;115:3227–3234. doi: 10.1242/jcs.115.16.3227. [DOI] [PubMed] [Google Scholar]
- 113.Sandler H, Kreth J, Timmers HT, Stoecklin G. Not1 mediates recruitment of the deadenylase Caf1 to mRNAs targeted for degradation by tristetraprolin. Nucleic Acids Res. 2011;39:4373–4386. doi: 10.1093/nar/gkr011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lykke-Andersen J, Wagner E. Recruitment and activation of mRNA decay enzymes by two ARE-mediated decay activation domains in the proteins TTP and BRF-1. Genes Dev. 2005;19:351–361. doi: 10.1101/gad.1282305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sonenberg N, Hinnebusch AG. Regulation of translation initiation in eukaryotes: mechanisms and biological targets. Cell. 2009;136:731–745. doi: 10.1016/j.cell.2009.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kotlyarov A, Gaestel M. Is MK2 (mitogen-activated protein kinase-activated protein kinase 2) the key for understanding post-transcriptional regulation of gene expression? Biochem Soc Trans. 2002;30:959–963. doi: 10.1042/bst0300959. [DOI] [PubMed] [Google Scholar]
- 117.Neininger A, Kontoyiannis D, Kotlyarov A, Winzen R, Eckert R, Volk HD, Holtmann H, Kollias G, Gaestel M. MK2 targets AU-rich elements and regulates biosynthesis of tumor necrosis factor and interleukin-6 independently at different post-transcriptional levels. J Biol Chem. 2002;277:3065–3068. doi: 10.1074/jbc.C100685200. [DOI] [PubMed] [Google Scholar]
- 118.Ronkina N, Kotlyarov A, Dittrich-Breiholz O, Kracht M, Hitti E, Milarski K, Askew R, Marusic S, Lin LL, Gaestel M, Telliez JB. The mitogen-activated protein kinase (MAPK)-activated protein kinases MK2 and MK3 cooperate in stimulation of tumor necrosis factor biosynthesis and stabilization of p38 MAPK. Molecular and cellular biology. 2007;27:170–181. doi: 10.1128/MCB.01456-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Qi MY, Wang ZZ, Zhang Z, Shao Q, Zeng A, Li XQ, Li WQ, Wang C, Tian FJ, Li Q, Zou J, Qin YW, Brewer G, Huang S, Jing Q. AU-rich-element-dependent translation repression requires the cooperation of tristetraprolin and RCK/P54. Mol Cell Biol. 2012;32:913–928. doi: 10.1128/MCB.05340-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Fenger-Gron M, Fillman C, Norrild B, Lykke-Andersen J. Multiple processing body factors and the ARE binding protein TTP activate mRNA decapping. Mol Cell. 2005;20:905–915. doi: 10.1016/j.molcel.2005.10.031. [DOI] [PubMed] [Google Scholar]
- 121.Holetz FB, Correa A, Avila AR, Nakamura CV, Krieger MA, Goldenberg S. Evidence of P-body-like structures in Trypanosoma cruzi. Biochem Biophys Res Commun. 2007;356:1062–1067. doi: 10.1016/j.bbrc.2007.03.104. [DOI] [PubMed] [Google Scholar]
- 122.Coller J, Parker R. General translational repression by activators of mRNA decapping. Cell. 2005;122:875–886. doi: 10.1016/j.cell.2005.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Schwartz DC, Parker R. mRNA decapping in yeast requires dissociation of the cap binding protein, eukaryotic translation initiation factor 4E. Mol Cell Biol. 2000;20:7933–7942. doi: 10.1128/mcb.20.21.7933-7942.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Schwartz DC, Parker R. Mutations in translation initiation factors lead to increased rates of deadenylation and decapping of mRNAs in Saccharomyces cerevisiae. Mol Cell Biol. 1999;19:5247–5256. doi: 10.1128/mcb.19.8.5247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sheth U, Parker R. Decapping and decay of messenger RNA occur in cytoplasmic processing bodies. Science. 2003;300:805–808. doi: 10.1126/science.1082320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yamasaki S, Stoecklin G, Kedersha N, Simarro M, Anderson P. T-cell intracellular antigen-1 (TIA-1)-induced translational silencing promotes the decay of selected mRNAs. J Biol Chem. 2007;282:30070–30077. doi: 10.1074/jbc.M706273200. [DOI] [PubMed] [Google Scholar]
- 127.Tiedje C, Ronkina N, Tehrani M, Dhamija S, Laass K, Holtmann H, Kotlyarov A, Gaestel M. The p38/MK2-driven exchange between tristetraprolin and HuR regulates AU-rich element-dependent translation. PLoS Genet. 2012;8:e1002977. doi: 10.1371/journal.pgen.1002977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ostareck DH, Ostareck-Lederer A, Shatsky IN, Hentze MW. Lipoxygenase mRNA silencing in erythroid differentiation: The 3′UTR regulatory complex controls 60S ribosomal subunit joining. Cell. 2001;104:281–290. doi: 10.1016/s0092-8674(01)00212-4. [DOI] [PubMed] [Google Scholar]
- 129.Stoecklin G, Gross B, Ming XF, Moroni C. A novel mechanism of tumor suppression by destabilizing AU-rich growth factor mRNA. Oncogene. 2003;22:3554–3561. doi: 10.1038/sj.onc.1206418. [DOI] [PubMed] [Google Scholar]
- 130.Holmes B, Artinian N, Anderson L, Martin J, Masri J, Cloninger C, Bernath A, Bashir T, Benavides-Serrato A, Gera J. Protor-2 interacts with tristetraprolin to regulate mRNA stability during stress. Cell Signal. 2012;24:309–315. doi: 10.1016/j.cellsig.2011.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Boutaud O, Dixon DA, Oates JA, Sawaoka H. Tristetraprolin binds to the COX-2 mRNA 3′ untranslated region in cancer cells. Advances in experimental medicine and biology. 2003;525:157–160. doi: 10.1007/978-1-4419-9194-2_32. [DOI] [PubMed] [Google Scholar]
- 132.Sawaoka H, Dixon DA, Oates JA, Boutaud O. Tristetraprolin binds to the 3′-untranslated region of cyclooxygenase-2 mRNA. A polyadenylation variant in a cancer cell line lacks the binding site. J Biol Chem. 2003;278:13928–13935. doi: 10.1074/jbc.M300016200. [DOI] [PubMed] [Google Scholar]
- 133.Sully G, Dean JL, Wait R, Rawlinson L, Santalucia T, Saklatvala J, Clark AR. Structural and functional dissection of a conserved destabilizing element of cyclo-oxygenase-2 mRNA: evidence against the involvement of AUF-1 [AU-rich element/poly(U)-binding/degradation factor-1], AUF-2, tristetraprolin, HuR (Hu antigen R) or FBP1 (far-upstream-sequence-element-binding protein 1) Biochem J. 2004;377:629–639. doi: 10.1042/BJ20031484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lin NY, Lin CT, Chang CJ. Modulation of immediate early gene expression by tristetraprolin in the differentiation of 3T3-L1 cells. Biochem Biophys Res Commun. 2008;365:69–74. doi: 10.1016/j.bbrc.2007.10.119. [DOI] [PubMed] [Google Scholar]
- 135.Young LE, Sanduja S, Bemis-Standoli K, Pena EA, Price RL, Dixon DA. The mRNA binding proteins HuR and tristetraprolin regulate cyclooxygenase 2 expression during colon carcinogenesis. Gastroenterology. 2009;136:1669–1679. doi: 10.1053/j.gastro.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Al-Souhibani N, Al-Ahmadi W, Hesketh JE, Blackshear PJ, Khabar KS. The RNA-binding zinc-finger protein tristetraprolin regulates AU-rich mRNAs involved in breast cancer-related processes. Oncogene. 2010;29:4205–4215. doi: 10.1038/onc.2010.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Cha HJ, Lee HH, Chae SW, Cho WJ, Kim YM, Choi HJ, Choi DH, Jung SW, Min YJ, Lee BJ, Park SE, Park JW. Tristetraprolin downregulates the expression of both VEGF and COX-2 in human colon cancer. Hepatogastroenterology. 2011;58:790–795. [PubMed] [Google Scholar]
- 138.Briata P, Ilengo C, Corte G, Moroni C, Rosenfeld MG, Chen CY, Gherzi R. The Wnt/beta-catenin-->Pitx2 pathway controls the turnover of Pitx2 and other unstable mRNAs. Molecular cell. 2003;12:1201–1211. doi: 10.1016/s1097-2765(03)00407-6. [DOI] [PubMed] [Google Scholar]
- 139.Yu H, Stasinopoulos S, Leedman P, Medcalf RL. Inherent instability of plasminogen activator inhibitor type 2 mRNA is regulated by tristetraprolin. J Biol Chem. 2003;278:13912–13918. doi: 10.1074/jbc.M213027200. [DOI] [PubMed] [Google Scholar]
- 140.Ogilvie RL, Abelson M, Hau HH, Vlasova I, Blackshear PJ, Bohjanen PR. Tristetraprolin down-regulates IL-2 gene expression through AU-rich element-mediated mRNA decay. J Immunol. 2005;174:953–961. doi: 10.4049/jimmunol.174.2.953. [DOI] [PubMed] [Google Scholar]
- 141.Chen YL, Huang YL, Lin NY, Chen HC, Chiu WC, Chang CJ. Differential regulation of ARE-mediated TNFalpha and IL-1beta mRNA stability by lipopolysaccharide in RAW264.7 cells. Biochemical and biophysical research communications. 2006;346:160–168. doi: 10.1016/j.bbrc.2006.05.093. [DOI] [PubMed] [Google Scholar]
- 142.Jalonen U, Nieminen R, Vuolteenaho K, Kankaanranta H, Moilanen E. Down-regulation of tristetraprolin expression results in enhanced IL-12 and MIP-2 production and reduced MIP-3alpha synthesis in activated macrophages. Mediators Inflamm. 2006;2006:40691. doi: 10.1155/MI/2006/40691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sauer I, Schaljo B, Vogl C, Gattermeier I, Kolbe T, Muller M, Blackshear PJ, Kovarik P. Interferons limit inflammatory responses by induction of tristetraprolin. Blood. 2006;107:4790–4797. doi: 10.1182/blood-2005-07-3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Patil CS, Liu M, Zhao W, Coatney DD, Li F, VanTubergen EA, D'Silva NJ, Kirkwood KL. Targeting mRNA stability arrests inflammatory bone loss. Molecular therapy: the journal of the American Society of Gene Therapy. 2008;16:1657–1664. doi: 10.1038/mt.2008.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Van Tubergen E, Vander Broek R, Lee J, Wolf G, Carey T, Bradford C, Prince M, Kirkwood KL, D'Silva NJ. Tristetraprolin regulates interleukin-6, which is correlated with tumor progression in patients with head and neck squamous cell carcinoma. Cancer. 2011;117:2677–2689. doi: 10.1002/cncr.25859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zhao W, Liu M, D'Silva NJ, Kirkwood KL. Tristetraprolin regulates interleukin-6 expression through p38 MAPK-dependent affinity changes with mRNA 3′ untranslated region. J Interferon Cytokine Res. 2011;31:629–637. doi: 10.1089/jir.2010.0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Brahma PK, Zhang H, Murray BS, Shu FJ, Sidell N, Seli E, Kallen CB. The mRNA-binding protein Zfp36 is upregulated by beta-adrenergic stimulation and represses IL-6 production in 3T3-L1 adipocytes. Obesity (Silver Spring) 2012;20:40–47. doi: 10.1038/oby.2011.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Horner TJ, Lai WS, Stumpo DJ, Blackshear PJ. Stimulation of polo-like kinase 3 mRNA decay by tristetraprolin. Mol Cell Biol. 2009;29:1999–2010. doi: 10.1128/MCB.00982-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Sohn BH, Park IY, Lee JJ, Yang SJ, Jang YJ, Park KC, Kim DJ, Lee DC, Sohn HA, Kim TW, Yoo HS, Choi JY, Bae YS, Yeom YI. Functional switching of TGF-beta1 signaling in liver cancer via epigenetic modulation of a single CpG site in TTP promoter. Gastroenterology. 2010;138:1898–1908. doi: 10.1053/j.gastro.2009.12.044. [DOI] [PubMed] [Google Scholar]
- 150.Kang JG, Amar MJ, Remaley AT, Kwon J, Blackshear PJ, Wang Py, Hwang PM. Zinc Finger Protein Tristetraprolin Interacts with CCL3 mRNA and Regulates Tissue Inflammation. Journal of Immunology. 2011;187:2696–2701. doi: 10.4049/jimmunol.1101149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Essafi-Benkhadir K, Onesto C, Stebe E, Moroni C, Pages G. Tristetraprolin inhibits Ras-dependent tumor vascularization by inducing vascular endothelial growth factor mRNA degradation. Mol Biol Cell. 2007;18:4648–4658. doi: 10.1091/mbc.E07-06-0570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Suswam E, Li Y, Zhang X, Gillespie GY, Li X, Shacka JJ, Lu L, Zheng L, King PH. Tristetraprolin down-regulates interleukin-8 and vascular endothelial growth factor in malignant glioma cells. Cancer Res. 2008;68:674–682. doi: 10.1158/0008-5472.CAN-07-2751. [DOI] [PubMed] [Google Scholar]
- 153.Lee HH, Son YJ, Lee WH, Park YW, Chae SW, Cho WJ, Kim YM, Choi HJ, Choi DH, Jung SW, Min YJ, Park SE, Lee BJ, Cha HJ, Park JW. Tristetraprolin regulates expression of VEGF and tumorigenesis in human colon cancer. Int J Cancer. 2010;126:1817–1827. doi: 10.1002/ijc.24847. [DOI] [PubMed] [Google Scholar]
- 154.Frasca D, Landin AM, Alvarez JP, Blackshear PJ, Riley RL, Blomberg BB. Tristetraprolin, a negative regulator of mRNA stability, is increased in old B cells and is involved in the degradation of E47 mRNA. J Immunol. 2007;179:918–927. doi: 10.4049/jimmunol.179.2.918. [DOI] [PubMed] [Google Scholar]
- 155.Winzen R, Thakur BK, Dittrich-Breiholz O, Shah M, Redich N, Dhamija S, Kracht M, Holtmann H. Functional analysis of KSRP interaction with the AU-rich element of interleukin-8 and identification of inflammatory mRNA targets. Molecular and cellular biology. 2007;27:8388–8400. doi: 10.1128/MCB.01493-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Balakathiresan NS, Bhattacharyya S, Gutti U, Long RP, Jozwik C, Huang W, Srivastava M, Pollard HB, Biswas R. Tristetraprolin regulates IL-8 mRNA stability in cystic fibrosis lung epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2009;296:L1012–1018. doi: 10.1152/ajplung.90601.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Bhattacharyya S, Gutti U, Mercado J, Moore C, Pollard HB, Biswas R. MAPK signaling pathways regulate IL-8 mRNA stability and IL-8 protein expression in cystic fibrosis lung epithelial cell lines, American journal of physiology. Lung cellular and molecular physiology. 2011;300:L81–87. doi: 10.1152/ajplung.00051.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Bourcier C, Griseri P, Grepin R, Bertolotto C, Mazure N, Pages G. Constitutive ERK activity induces downregulation of tristetraprolin, a major protein controlling interleukin8/CXCL8 mRNA stability in melanoma cells. Am J Physiol Cell Physiol. 2011;301:C609–618. doi: 10.1152/ajpcell.00506.2010. [DOI] [PubMed] [Google Scholar]
- 159.Al Ghouleh I, Magder S. NADPH oxidase-derived superoxide destabilizes lipopolysaccharide-induced interleukin 8 mRNA via p38, extracellular signal-regulated kinase mitogen-activated protein kinase, and the destabilizing factor tristetraprolin. Shock. 2012;37:433–440. doi: 10.1097/SHK.0b013e31824582e6. [DOI] [PubMed] [Google Scholar]
- 160.Galbiati V, Carne A, Mitjans M, Galli CL, Marinovich M, Corsini E. Isoeugenol destabilizes IL-8 mRNA expression in THP-1 cells through induction of the negative regulator of mRNA stability tristetraprolin. Arch Toxicol. 2012;86:239–248. doi: 10.1007/s00204-011-0758-2. [DOI] [PubMed] [Google Scholar]
- 161.Datta S, Biswas R, Novotny M, Pavicic PG, Jr, Herjan T, Mandal P, Hamilton TA. Tristetraprolin regulates CXCL1 (KC) mRNA stability. J Immunol. 2008;180:2545–2552. doi: 10.4049/jimmunol.180.4.2545. [DOI] [PubMed] [Google Scholar]
- 162.Tudor C, Marchese FP, Hitti E, Aubareda A, Rawlinson L, Gaestel M, Blackshear PJ, Clark AR, Saklatvala J, Dean JL. The p38 MAPK pathway inhibits tristetraprolin-directed decay of interleukin-10 and pro-inflammatory mediator mRNAs in murine macrophages. FEBS Lett. 2009;583:1933–1938. doi: 10.1016/j.febslet.2009.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Gaba A, Grivennikov SI, Do MV, Stumpo DJ, Blackshear PJ, Karin M. Cutting Edge: IL-10-Mediated Tristetraprolin Induction Is Part of a Feedback Loop That Controls Macrophage STAT3 Activation and Cytokine Production. Journal of immunology (Baltimore, Md : 1950) 2012 doi: 10.4049/jimmunol.1201126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Sze KL, Lui WY, Lee WM. Post-transcriptional regulation of CLMP mRNA is controlled by tristetraprolin in response to TNFalpha via c-Jun N-terminal kinase signalling. Biochem J. 2008;410:575–583. doi: 10.1042/BJ20070901. [DOI] [PubMed] [Google Scholar]
- 165.Ogilvie RL, Sternjohn JR, Rattenbacher B, Vlasova IA, Williams DA, Hau HH, Blackshear PJ, Bohjanen PR. Tristetraprolin mediates interferon-gamma mRNA decay. J Biol Chem. 2009;284:11216–11223. doi: 10.1074/jbc.M901229200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Sanduja S, Kaza V, Dixon DA. The mRNA decay factor tristetraprolin (TTP) induces senescence in human papillomavirus-transformed cervical cancer cells by targeting E6-AP ubiquitin ligase. Aging (Albany NY) 2009;1:803–817. doi: 10.18632/aging.100086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Schaljo B, Kratochvill F, Gratz N, Sadzak I, Sauer I, Hammer M, Vogl C, Strobl B, Muller M, Blackshear PJ, Poli V, Lang R, Murray PJ, Kovarik P. Tristetraprolin is required for full anti-inflammatory response of murine macrophages to IL-10. J Immunol. 2009;183:1197–1206. doi: 10.4049/jimmunol.0803883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Tsai CS, Chen DL, Lin SJ, Tsai JC, Lin TC, Lin CY, Chen YH, Huang GS, Tsai HY, Lin FY, Li CY. TNF-alpha inhibits toll-like receptor 4 expression on monocytic cells via tristetraprolin during cardiopulmonary bypass. Shock. 2009;32:40–48. doi: 10.1097/SHK.0b013e318199608d. [DOI] [PubMed] [Google Scholar]
- 169.Kim CW, Kim HK, Vo MT, Lee HH, Kim HJ, Min YJ, Cho WJ, Park JW. Tristetraprolin controls the stability of cIAP2 mRNA through binding to the 3′UTR of cIAP2 mRNA. Biochem Biophys Res Commun. 2010;400:46–52. doi: 10.1016/j.bbrc.2010.07.136. [DOI] [PubMed] [Google Scholar]
- 170.Kim TW, Yim S, Choi BJ, Jang Y, Lee JJ, Sohn BH, Yoo HS, Yeom YI, Park KC. Tristetraprolin regulates the stability of HIF-1alpha mRNA during prolonged hypoxia. Biochem Biophys Res Commun. 2010;391:963–968. doi: 10.1016/j.bbrc.2009.11.174. [DOI] [PubMed] [Google Scholar]
- 171.Chamboredon S, Ciais D, Desroches-Castan A, Savi P, Bono F, Feige JJ, Cherradi N. Hypoxia-inducible factor-1alpha mRNA: a new target for destabilization by tristetraprolin in endothelial cells. Mol Biol Cell. 2011;22:3366–3378. doi: 10.1091/mbc.E10-07-0617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Lee HH, Vo MT, Kim HJ, Lee UH, Kim CW, Kim HK, Ko MS, Lee WH, Cha SJ, Min YJ, Choi DH, Suh HS, Lee BJ, Park JW, Cho WJ. Stability of the LATS2 tumor suppressor gene is regulated by tristetraprolin. J Biol Chem. 2010;285:17329–17337. doi: 10.1074/jbc.M109.094235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Bermudez O, Jouandin P, Rottier J, Bourcier C, Pages G, Gimond C. Post-transcriptional regulation of the DUSP6/MKP-3 phosphatase by MEK/ERK signaling and hypoxia. Journal of cellular physiology. 2011;226:276–284. doi: 10.1002/jcp.22339. [DOI] [PubMed] [Google Scholar]
- 174.Chen F, Shyu AB, Shneider BL. Hu antigen R and tristetraprolin: counter-regulators of rat apical sodium-dependent bile acid transporter by way of effects on messenger RNA stability. Hepatology. 2011;54:1371–1378. doi: 10.1002/hep.24496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Lin FY, Tsai YT, Lee CY, Lin CY, Lin YW, Li CY, Shih CM, Huang CY, Chang NC, Tsai JC, Chen TL, Tsai CS. TNF-alpha-decreased thrombomodulin expression in monocytes is inhibited by propofol through regulation of tristetraprolin and human antigen R activities. Shock. 2011;36:279–288. doi: 10.1097/SHK.0b013e3182236e7e. [DOI] [PubMed] [Google Scholar]
- 176.Qian X, Ning H, Zhang J, Hoft DF, Stumpo DJ, Blackshear PJ, Liu J. Posttranscriptional regulation of IL-23 expression by IFN-gamma through tristetraprolin. J Immunol. 2011;186:6454–6464. doi: 10.4049/jimmunol.1002672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Al-Haj L, Blackshear PJ, Khabar KS. Regulation of p21/CIP1/WAF-1 mediated cell-cycle arrest by RNase L and tristetraprolin, and involvement of AU-rich elements. Nucleic Acids Res. 2012 doi: 10.1093/nar/gks545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Lee HH, Yoon NA, Vo MT, Kim CW, Woo JM, Cha HJ, Cho YW, Lee BJ, Cho WJ, Park JW. Tristetraprolin down-regulates IL-17 through mRNA destabilization. FEBS Lett. 2012;586:41–46. doi: 10.1016/j.febslet.2011.11.021. [DOI] [PubMed] [Google Scholar]
- 179.Mahat DB, Brennan-Laun SE, Fialcowitz-White EJ, Kishor A, Ross CR, Pozharskaya T, Rawn JD, Blackshear PJ, Hassel BA, Wilson GM. Coordinated expression of tristetraprolin post-transcriptionally attenuates mitogenic induction of the oncogenic Ser/Thr kinase Pim-1. PLoS One. 2012;7:e33194. doi: 10.1371/journal.pone.0033194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Novakovic D, Kuo AC, Lin JH, Koschinsky ML, Boffa MB. Identification of tristetraprolin as a factor that modulates the stability of the TAFI transcript through binding to the 3′-untranslated region. Journal of thrombosis and haemostasis: JTH. 2012;10:887–894. doi: 10.1111/j.1538-7836.2012.04689.x. [DOI] [PubMed] [Google Scholar]
- 181.Ishmael FT, Fang X, Galdiero MR, Atasoy U, Rigby WF, Gorospe M, Cheadle C, Stellato C. Role of the RNA-binding protein tristetraprolin in glucocorticoid-mediated gene regulation. J Immunol. 2008;180:8342–8353. doi: 10.4049/jimmunol.180.12.8342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Emmons J, Townley-Tilson WH, Deleault KM, Skinner SJ, Gross RH, Whitfield ML, Brooks SA. Identification of TTP mRNA targets in human dendritic cells reveals TTP as a critical regulator of dendritic cell maturation. RNA. 2008;14:888–902. doi: 10.1261/rna.748408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Bros M, Wiechmann N, Besche V, Art J, Pautz A, Grabbe S, Kleinert H, Reske-Kunz AB. The RNA binding protein tristetraprolin influences the activation state of murine dendritic cells. Mol Immunol. 2010;47:1161–1170. doi: 10.1016/j.molimm.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 184.Lehner B, Sanderson CM. A protein interaction framework for human mRNA degradation. Genome Res. 2004;14:1315–1323. doi: 10.1101/gr.2122004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Su YL, Wang SC, Chiang PY, Lin NY, Shen YF, Chang GD, Chang CJ. Tristetraprolin inhibits poly(A)-tail synthesis in nuclear mRNA that contains AU-rich elements by interacting with poly(A)-binding protein nuclear 1. PLoS One. 2012;7:e41313. doi: 10.1371/journal.pone.0041313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Kedar VP, Darby MK, Williams JG, Blackshear PJ. Phosphorylation of human tristetraprolin in response to its interaction with the Cbl interacting protein CIN85. PLoS One. 2010;5:e9588. doi: 10.1371/journal.pone.0009588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Chang WL, Tarn WY. A role for transportin in deposition of TTP to cytoplasmic RNA granules and mRNA decay. Nucleic Acids Res. 2009;37:6600–6612. doi: 10.1093/nar/gkp717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Carman JA, Nadler SG. Direct association of tristetraprolin with the nucleoporin CAN/Nup214. Biochem Biophys Res Commun. 2004;315:445–449. doi: 10.1016/j.bbrc.2004.01.080. [DOI] [PubMed] [Google Scholar]
- 189.Rowlett RM, Chrestensen CA, Schroeder MJ, Harp MG, Pelo JW, Shabanowitz J, DeRose R, Hunt DF, Sturgill TW, Worthington MT. Inhibition of tristetraprolin deadenylation by poly(A) binding protein. Am J Physiol Gastrointest Liver Physiol. 2008;295:G421–430. doi: 10.1152/ajpgi.00508.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Chen G, Guo X, Lv F, Xu Y, Gao G. p72 DEAD box RNA helicase is required for optimal function of the zinc-finger antiviral protein. Proc Natl Acad Sci U S A. 2008;105:4352–4357. doi: 10.1073/pnas.0712276105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Kedar VP, Zucconi BE, Wilson GM, Blackshear PJ. Direct binding of specific AUF1 isoforms to tandem zinc finger domains of tristetraprolin (TTP) family proteins. J Biol Chem. 2012;287:5459–5471. doi: 10.1074/jbc.M111.312652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Linker K, Pautz A, Fechir M, Hubrich T, Greeve J, Kleinert H. Involvement of KSRP in the post-transcriptional regulation of human iNOS expression-complex interplay of KSRP with TTP and HuR. Nucleic acids research. 2005;33:4813–4827. doi: 10.1093/nar/gki797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Frasca D, Romero M, Landin AM, Diaz A, Riley RL, Blomberg BB. Protein phosphatase 2A (PP2A) is increased in old murine B cells and mediates p38 MAPK/tristetraprolin dephosphorylation and E47 mRNA instability. Mech Ageing Dev. 2010;131:306–314. doi: 10.1016/j.mad.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Schichl YM, Resch U, Lemberger CE, Stichlberger D, de Martin R. Novel phosphorylation-dependent ubiquitination of tristetraprolin by mitogen-activated protein kinase/extracellular signal-regulated kinase kinase kinase 1 (MEKK1) and tumor necrosis factor receptor-associated factor 2 (TRAF2) J Biol Chem. 2011;286:38466–38477. doi: 10.1074/jbc.M111.254888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Papin J, Subramaniam S. Bioinformatics and cellular signaling. Curr Opin Biotechnol. 2004;15:78–81. doi: 10.1016/j.copbio.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 196.Johnson BA, Stehn JR, Yaffe MB, Blackwell TK. Cytoplasmic localization of tristetraprolin involves 14-3-3-dependent and -independent mechanisms. J Biol Chem. 2002;277:18029–18036. doi: 10.1074/jbc.M110465200. [DOI] [PubMed] [Google Scholar]
- 197.Lin NY, Lin TY, Yang WH, Wang SC, Wang KT, Su YL, Jiang YW, Chang GD, Chang CJ. Differential expression and functional analysis of the tristetraprolin family during early differentiation of 3T3-L1 preadipocytes. Int J Biol Sci. 2012;8:761–777. doi: 10.7150/ijbs.4036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Twizere JC, Kruys V, Lefebvre L, Vanderplasschen A, Collete D, Debacq C, Lai WS, Jauniaux JC, Bernstein LR, Semmes OJ, Burny A, Blackshear PJ, Kettmann R, Willems L. Interaction of retroviral Tax oncoproteins with tristetraprolin and regulation of tumor necrosis factor-alpha expression. J Natl Cancer Inst. 2003;95:1846–1859. doi: 10.1093/jnci/djg118. [DOI] [PubMed] [Google Scholar]
- 199.Rual JF, Venkatesan K, Hao T, Hirozane-Kishikawa T, Dricot A, Li N, Berriz GF, Gibbons FD, Dreze M, Ayivi-Guedehoussou N, Klitgord N, Simon C, Boxem M, Milstein S, Rosenberg J, Goldberg DS, Zhang LV, Wong SL, Franklin G, Li S, Albala JS, Lim J, Fraughton C, Llamosas E, Cevik S, Bex C, Lamesch P, Sikorski RS, Vandenhaute J, Zoghbi HY, Smolyar A, Bosak S, Sequerra R, Doucette-Stamm L, Cusick ME, Hill DE, Roth FP, Vidal M. Towards a proteome-scale map of the human protein-protein interaction network. Nature. 2005;437:1173–1178. doi: 10.1038/nature04209. [DOI] [PubMed] [Google Scholar]
- 200.Dintilhac A, Bernues J. HMGB1 interacts with many apparently unrelated proteins by recognizing short amino acid sequences. J Biol Chem. 2002;277:7021–7028. doi: 10.1074/jbc.M108417200. [DOI] [PubMed] [Google Scholar]
- 201.Ozgur S, Chekulaeva M, Stoecklin G. Human Pat1b connects deadenylation with mRNA decapping and controls the assembly of processing bodies. Mol Cell Biol. 2010;30:4308–4323. doi: 10.1128/MCB.00429-10. [DOI] [PMC free article] [PubMed] [Google Scholar]