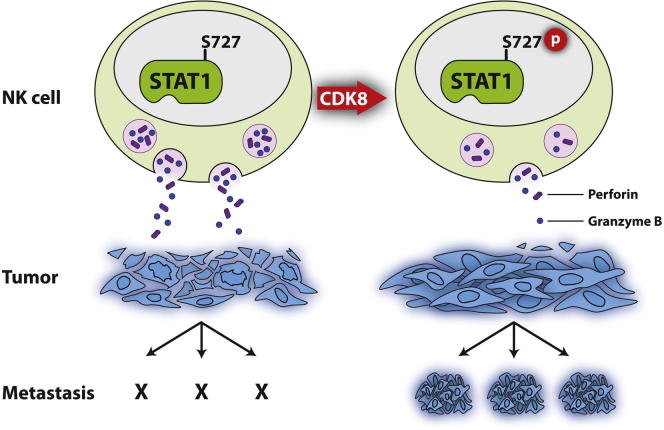
Summary
The transcription factor STAT1 is important in natural killer (NK) cells, which provide immediate defense against tumor and virally infected cells. We show that mutation of a single phosphorylation site (Stat1-S727A) enhances NK cell cytotoxicity against a range of tumor cells, accompanied by increased expression of perforin and granzyme B. Stat1-S727A mice display significantly delayed disease onset in NK cell-surveilled tumor models including melanoma, leukemia, and metastasizing breast cancer. Constitutive phosphorylation of S727 depends on cyclin-dependent kinase 8 (CDK8). Inhibition of CDK8-mediated STAT1-S727 phosphorylation may thus represent a therapeutic strategy for stimulating NK cell-mediated tumor surveillance.
Graphical Abstract
Highlights
-
•
Constitutive STAT1-S727 phosphorylation inhibits NK cell cytotoxicity
-
•
Stat1-S727A NK cells are highly cytotoxic against tumor cells
-
•
Stat1-S727A mice are more resistant to NK cell-surveilled tumors
-
•
CDK8 phosphorylates STAT1-S727 in NK cells and restrains their cytotoxicity
The transcription factor STAT1 mediates pivotal signals in NK cells. In this study, Sexl and colleagues report a role of STAT1-S727 phosphorylation, which is constitutively present in NK cells independent of cytokine stimulation. NK cells with a phosphorylation-dead STAT1 mutant (Stat1-S727A) display signs of hyperactivity resulting in enhanced tumor surveillance in vivo. Correspondingly, the authors identify the upstream kinase as CDK8, and they find that targeting this kinase significantly enhances NK cell cytotoxicity. Targeting CDK8 may thus constitute a promising tumor therapy.
Introduction
Signal transducer and activator of transcription 1 (STAT1) is activated downstream of interferons (IFNs) and transmits antiviral and antimicrobial signals from the cell surface to the nucleus (Stark and Darnell, 2012). Mice with complete or tissue-specific loss of Stat1 are prone to succumb to infections and to develop tumors (Kaplan et al., 1998; Klover et al., 2010; Schneckenleithner et al., 2011; Koromilas and Sexl, 2013). STAT1 activity is regulated posttranscriptionally, e.g., by JAK-mediated phosphorylation of tyrosine701 (Y701), which allows STAT1 dimerization and nuclear translocation. Various kinases phosphorylate STAT1’s transactivation domain at serine727 (S727) (Kovarik et al., 1999; Nair et al., 2002; Deb et al., 2003; Bancerek et al., 2013). IFN-induced S727 phosphorylation occurs after STAT1 dimers bind to DNA (Sadzak et al., 2008) and modulates transcription (Wen et al., 1995). Knockin mice with point-mutated STAT1-S727 (Stat1-S727A) display reduced antimicrobial activity, emphasizing the importance of the phosphorylation site and suggesting that the S727A mutation generates a hypomorphic Stat1 allele (Varinou et al., 2003; Pilz et al., 2009).
Natural killer (NK) cells are important in the defense against virally infected and malignant cells (Vivier et al., 2011). Their development, activation, and cytotoxic function are tightly regulated by cytokines, which induce the JAK/STAT signaling pathway. Interleukins 2 and 15 (IL-2 and IL-15) drive NK cell development, proliferation, and homeostasis and signal predominantly via STAT5 (Lodolce et al., 1998). STAT1, STAT3, and STAT4 are downstream of IL-12, mediating NK cell activation and cytotoxicity and triggering the transcription of IFN-γ, granzymes, and perforin (Watford et al., 2004). IFN type I induces NK cell cytotoxicity in viral infections and stimulates NK cell proliferation and survival via activation of dendritic cells (DCs) (Nguyen et al., 2002; Lucas et al., 2007).
Although STAT1 regulates many aspects of NK cell biology (Lee et al., 2000; Kovacic et al., 2006), the role of STAT1-S727 phosphorylation in NK cells is unknown. We show that in Stat1-S727A mutant mice, NK cells display higher cytotoxicity toward a broad panel of target cells, leading to more efficient tumor clearance. The upstream kinase is CDK8, which offers a therapeutic opportunity to stimulate NK cell activity.
Results
High Cytotoxic Activity of Stat1-S727A NK Cells
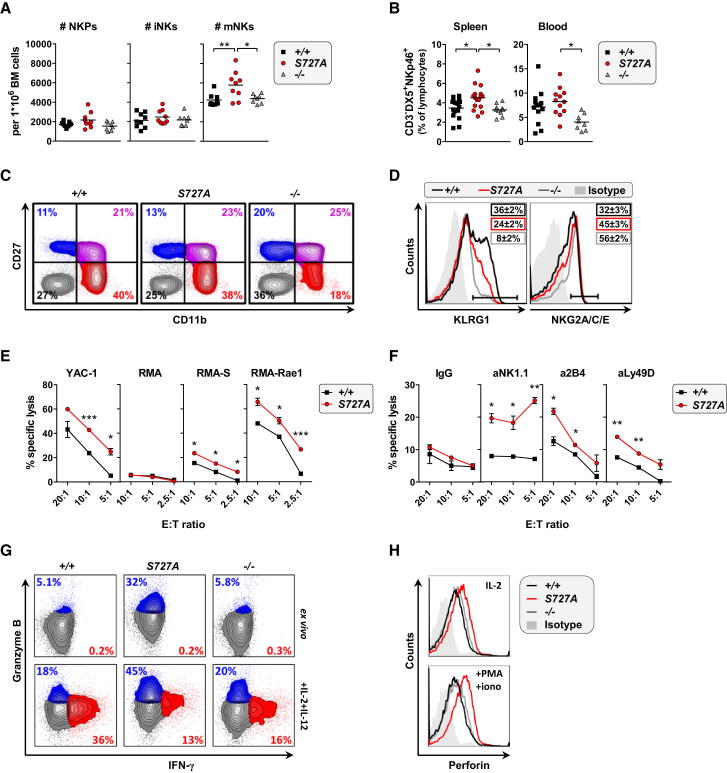
Stat1-S727A mice exhibit slightly elevated numbers of mature NK cells (mNKs) in bone marrow, spleen, and blood (Figures 1A and 1B) not paralleled by enhanced numbers of NK cell precursors (Figure 1A) nor by increased proliferation (data not shown). Unlike the situation in Stat1−/− NK cells, maturation of Stat1-S727A NK cells is unaffected (Figure 1C). Consistent with previous findings by Robbins et al. (2005), Stat1−/− peripheral NK cells have an immature phenotype (Figures 1C and 1D; Table S1). In contrast, changes on Stat1-S727A cells are minor: only the relative increase of NKG2A/C/E+ and a reduction of KLRG1+ NK cells (Figure 1D; Table S1) are consistently observed despite normal levels of MHC class I (data not shown).
Figure 1.
Presence, Development, and Cytotoxic Function of Stat1-S727A NK Cells
(A) Bone marrow was investigated for NK cell precursors (NKPs, CD3−Ter-119−GR1−B220−CD122+NK1.1−DX5−), immature NK cells (iNKs, CD3−Ter-119−GR1−B220−CD122+NK1.1+DX5−), and mNKs (CD3−Ter-119−GR1−B220−CD122+NK1.1+DX5+). Each symbol represents an individual mouse; small horizontal lines indicate the mean (∗p < 0.05, ∗∗p < 0.01; one-way ANOVA and Tukey’s post hoc test).
(B) Number of CD3−DX5+NKp46+ NK cells, given as percentages of spleen and blood lymphocytes, is shown. Each symbol represents an individual mouse; horizontal lines indicate the mean (∗p < 0.05; one-way ANOVA and Tukey’s post hoc test).
(C) NK cell maturation profiles represented by the expression of CD27 and CD11b on CD3−NK1.1+ splenocytes are shown. Data are representative for at least five independent experiments with n > 10 for each genotype.
(D) Expression of KLRG1 and NKG2A/C/E on CD3−DX5+NK1.1+NKp46+ splenocytes is shown. Numbers indicate the percentage of gate+ (horizontal line) NK cells ± SEM; n ≥ 9 for each genotype.
(E) Cytotoxicity of wild-type versus Stat1-S727A NK cells against YAC-1, RMA, RMA-S, and RMA-Rae1 tumor targets at given effector-to-target (E:T) ratios, measured in a 4 hr [51Cr]-release cytotoxicity assay is shown. Symbols represent means, and error bars indicate SEM of triplicates (∗p < 0.05, ∗∗∗p < 0.001; unpaired t test). Data are representative of three independent experiments.
(F) R-ADCC against FcR+ Daudi target cells crosslinked with antibodies against NK1.1, 2B4, and Ly49D is shown. Symbols represent means, and error bars indicate SEM of triplicates (∗p < 0.05, ∗∗p < 0.01; unpaired t test). Data are representative of two independent experiments.
(G) IFN-γ and granzyme B protein expression in splenic NK cells (gated on CD3−NKp46+) ex vivo and after 4 hr stimulation with IL-2 and IL-12 is presented.
(H) LAK cells stained intracellularly for perforin under normal culturing conditions and after 4 hr treatment with PMA and ionomycin are shown.
Unexpectedly, purified and in vitro-expanded NK cells derived from Stat1-S727A mice show significantly higher cytotoxicity toward YAC-1, RMA-S, and RMA-Rae1 target cells (Figure 1E). Redirected antibody-dependent cellular cytotoxicity (R-ADCC) against the receptors NK1.1, 2B4, and Ly49D confirmed this finding (Figure 1F).
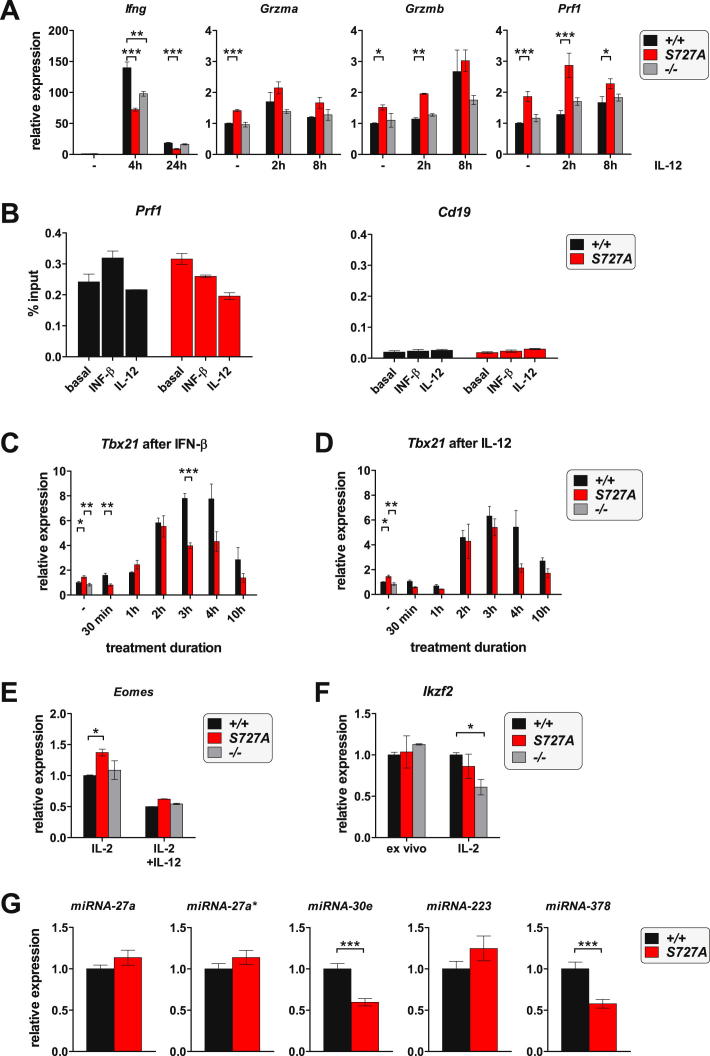
IL-12 is a potent activator of NK cell cytotoxicity and induces the transcription of granzymes, perforin, and IFN-γ (Watford et al., 2004; Figure S1). Il12p40 expression is enhanced in Stat1-S727A bone marrow-derived macrophages upon CpG treatment (Schroder et al., 2007). We failed to detect any differences in Il12p40 expression under basal conditions. Stimulation with LPS increased Il12p40 expression in Stat1-S727A splenocytes less than in wild-type cells (Figure S2), showing that enhanced NK cell cytotoxicity in Stat1-S727A mice is not caused by higher IL-12 levels. IL-12-dependent STAT activation is comparable in wild-type and Stat1-S727A NK cells (Figures 2 and S2). Surprisingly, we found reduced IFN-γ production upon IL-12 stimulation in Stat1−/− and Stat1-S727A NK cells (Figures 1G and S1): Stat1-S727A mutation can thus phenocopy some aspects of Stat1 deficiency. In contrast, granzyme B expression is significantly enhanced in primary Stat1-S727A NK cells, both without and after stimulation by IL-2 and IL-12 (Figure 1G). Levels of perforin are enhanced under standard culturing conditions and especially after stimulation with PMA and ionomycin (Figure 1H). The increased amounts of granzyme B and perforin correlate with changes in the levels of microRNA-378 and -30e, which posttranscriptionally regulate the two enzymes, respectively (Wang et al., 2012) (see Extended Results; Figure S1).
Figure S1.
Effect of Stat1-S727A Mutation on the Transcription of Effector Molecules, Related to Figure 1 and Table S2
(A) mRNA expression of Ifng (IFN-γ), Grzma (granzyme A), Grzmb (granzyme B) and Prf1 (perforin) were measured in LAK cells derived from wild-type, Stat1-S727A and Stat1−/− animals under standard culturing conditions and after IL-12 stimulation (∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001; one-way ANOVA and Tukey’s post hoc test). The summary of at least 3 independent LAK cell preparations is depicted; all values were normalized to untreated wild-type LAK cells.
(B) The recruitment of polymerase II to the Prf1 gene was investigated via ChIP in wild-type and Stat1-S727A LAK cells under standard culturing conditions (basal) and after 30 min stimulation with 100 U/ml IFN-β or 5 ng/ml IL-12 (left panel). Polymerase II binding to the B lymphocyte antigen gene Cd19 was determined as negative control (right panel). Plotted are means ± SEM.
(C and D) In vitro expanded NK cells were stimulated for the indicated periods with 100 U/ml IFN-β (C) or 5 ng/ml IL-12 (D) and the expression level of Tbx21 (T-bet) was determined via semiquantitative real time PCR relative to Gapdh (∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001; one-way ANOVA and Tukey's post hoc test).
(E) The mRNA level of Eomes (Eomesodermin) was measured in expanded NK cells and after 8 hr stimulation with 5 ng/ml IL-12 (∗p < 0.05; one-way ANOVA and Tukey's post hoc test).
(F) Ikzf2 (Helios) mRNA was quantified in freshly isolated splenic NK cells and after 7 days in culture. All values are normalized to untreated wild-type NK cells. Depicted is the summary of at least two independent experiments (∗p < 0.05; one-way ANOVA followed by Tukey’s post hoc test).
(G) The expression levels of miRNAs involved in the posttranscriptional regulation of perforin and granzyme B were determined in freshly isolated NK cells derived from wild-type and Stat1-S727A mice (n ≥ 3 per genotype, ∗∗∗p < 0.001; unpaired t test). Plotted are means ± SEM.
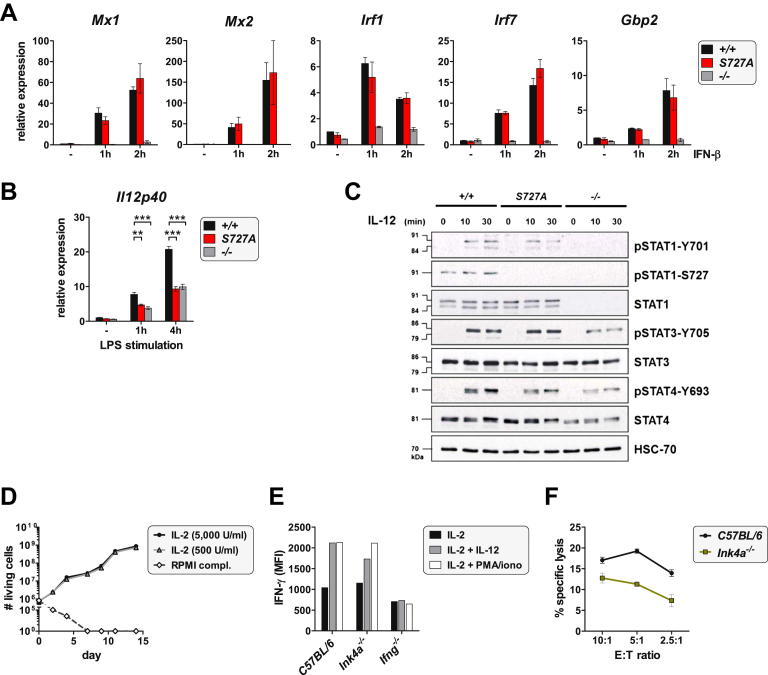
Figure S2.
IL-12 and IFN-β Signaling and NK Cell Line Characterization, Related to Figure 2 and Table S2
(A) Transcription of Mx1, Mx2, Irf1, Irf7 and Gbp2 was investigated in isolated, cultivated LAK cells 1 and 2 hr after the treatment with 100 U/ml IFN-β. mRNA levels were measured via semiquantitative real time PCR relative to Gapdh. All values were normalized to untreated wild-type LAK cells. Stat1−/− LAK cells failed to induce appreciable mRNA levels upon IFN-β stimulation, whereas Stat1-S727A LAK cells did not show any difference compared to wild-type. Presented is the summary of 2 independent experiments with 3-6 replicates per group. Plotted are means ± SEM.
(B) Splenocytes from wild-type, Stat1-S727A and Stat1−/− mice (2 per group) were prepared and subjected to LPS stimulation (100 ng/ml, 1 hr and 4 hr). Il12p40 mRNA expression was measured via semiquantitative real time PCR relative to Gapdh. All values were normalized to untreated wild-type LAK cells; shown are means ± SEM (∗∗p < 0.01, ∗∗∗p < 0.001; one-way ANOVA followed by Tukey’s post hoc test).
(C) IL-12-induced STAT1, STAT3 and STAT4 activation in LAK cells derived from wild-type, Stat1-S727A and Stat1−/− animals. HSC-70 served as loading control.
(D) Ink4a−/− splenic NK cells were MACS (DX5) purified, sorted for CD3-NK1.1+ expression and cultivated in vitro under standard conditions (5,000 U/ml IL-2) for several months. Proliferation assay of an immortal NK cell line cultivated under standard conditions, low dose of IL-2 (500 U/ml) and deprived from IL-2 (RPMI compl.). One week after IL-2 deprivation all NK cells underwent apoptosis.
(E) Intracellular FACS staining for IFN-γ production in an Ink4a−/− NK cell line under normal culturing conditions (IL-2) or after stimulation for 4 hr with IL-12 or PMA and ionomycin, respectively. C57BL/6 LAK cells served as positive control, Ifng−/− LAK cells as negative control.
(F) Cytotoxic capacity of an Ink4a−/− NK cell line against YAC-1 target cells measured in a FACS-based cytotoxicity assay. Ink4a−/− NK cell lines retained some cytotoxic capacity, although diminished compared to primary wild-type NK cells after 7 days in vitro cultivation. Symbols represent means, and error bars indicate SEM of triplicates.
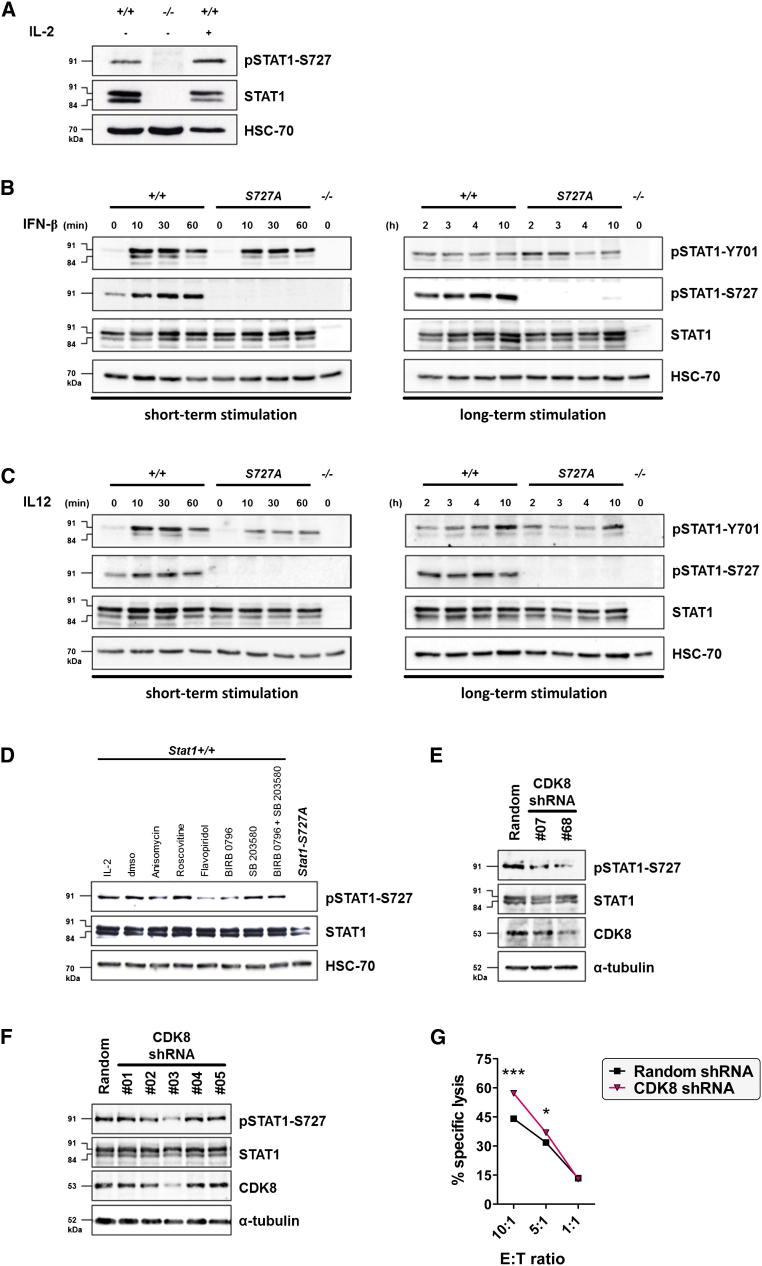
Figure 2.
Constitutive Basal STAT1-S727 Phosphorylation in NK Cells Is Mediated by CDK8
(A) Western blot of freshly isolated DX5+ splenocytes from wild-type animals shows constitutive basal phosphorylation on STAT1-S727 in vivo.
(B and C) In vitro-expanded LAK cells from wild-type and Stat1-S727A mice were treated with or without (B) 100 U/ml IFN-β or (C) 5 ng/ml IL-12 for the given times, and the phosphorylation on STAT1-Y701 and STAT1-S727 was investigated by western blot. Stat1−/− LAK cells served as negative control and HSC-70 as loading control.
(D) Wild-type LAK cells were treated with different kinase inhibitors targeting CDKs (roscovitine and flavopiridol) or MAPK p38 (BIRB 0796 and SB 203580), and the phosphorylation status of STAT1-S727 was determined in a western blot.
(E) CDK8 knockdown in primary NK cells with two hairpins led to reduced STAT1-S727 phosphorylation. A random hairpin served as control.
(F) Knockdown of CDK8 with five hairpins in an immortalized Ink4a−/− NK cell line is shown. A random hairpin served as control.
(G) FACS-based cytotoxicity assay of immortalized NK cells transduced with a random hairpin or a hairpin against CDK8 (#3) against YAC-1 target cells is presented. Symbols represent mean, and error bars indicate SEM of triplicates (∗p < 0.05, ∗∗∗p < 0.001; unpaired t test).
See also Figure S2.
Extended Results.
Stat1-S727A NK cells are characterized by an increased cytotoxic capacity, which is accompanied by enhanced levels of granzyme B and perforin under steady-state conditions as well as upon stimulation (Figures 1G and 1H). Interestingly, the enhanced cytotoxicity is independent of IFN-γ, as Stat1-S727A NK cells produce less IFN-γ assessed in intracellular cytokine stainings (Figure 1G). To test whether these differences were caused by altered transcriptional regulation we performed qPCR experiments. As expected, Ifng levels were strongly reduced in Stat1−/− as well as in Stat1-S727A NK cells. Interestingly, only small but consistent and significant differences in mRNA levels of granzyme B (Grzmb) and perforin (Prf1) were detectable in Stat1-S727A NK cells compared to wild-type (Figure S1A). In line with the minor alterations of mRNA levels also upon IL-12 stimulation, we did not detect any significant changes when we analyzed the recruitment of polymerase II to the perforin gene via chromatin immunoprecipitation (ChIP) (Figure S1B). Moreover, the transcriptional upstream regulators of granzyme B and perforin, T-bet (Tbx21) and Eomesodermin (Eomes) (Pearce et al., 2003; Townsend et al., 2004), were expressed at comparable levels in Stat1-S727A and wild-type NK cells (Figures S1C–S1E). Similarly, we failed to detect any changes in the expression of the transcription factor Helios (Ikzf2) that was recently described as a modulator of NK cell reactivity (Narni-Mancinelli et al., 2012) (Figure S1F).
The fact that we observed significant changes in protein expression accompanied by less dramatic alterations in mRNA levels and comparable polymerase II binding suggested the involvement of microRNAs (miRNAs). Recent reports have unraveled a key role for miRNAs as posttranslational regulators of perforin and granzyme B (Fehniger et al., 2010; Kim et al., 2011; Leong et al., 2012; Wang et al., 2012). When we tested the expression of miRNAs in NK cells ex vivo we indeed found a statistically significant downregulation of miRNA-30e and miRNA-378 in Stat1-S727A NK cells compared to wild-type NK cells (Figure S1G). These findings propose that STAT1-S727 phosphorylation regulates miRNA levels. In the absence of STAT1-S727 phosphorylation less miRNAs are available to suppress the transcription of perforin and granzyme B, which ultimately results in enhanced NK cell cytotoxicity.
Constitutive Basal Phosphorylation on STAT1-S727 Is Mediated by CDK8
In contrast to Y701, S727 is constitutively phosphorylated in unstimulated wild-type NK cells (Figures 2A–2C). In lymphokine-activated killer (LAK) cells, STAT1-S727 phosphorylation is enhanced by IFN-β or IL-12 (Figures 2B and 2C). In line with previous reports by Bromberg et al. (1996) and Horvath and Darnell (1996), lack of S727 phosphorylation does not interfere with IFN-β-mediated transcription of typical STAT1 target genes (Figure S2).
Web-based inquiries to identify the STAT1-S727 kinase gave the highest support vector machine (SVM) scores for cyclin-dependent kinases (CDKs) and mitogen-activated protein kinases (MAPKs). The pan-CDK inhibitor flavopiridol strongly reduces STAT1-S727 phosphorylation, whereas roscovitine, which inhibits CDK1, CDK2, CDK5, CDK7, and CDK9, has no impact. Similarly, the p38 MAPK inhibitor BIRB 0796 reduces STAT1-S727 phosphorylation, but the prototypic p38 inhibitor SB 203580 does not (Figure 2D). We performed knockdown of CDK8, a kinase targeted by both flavopiridol and BIRB 0796 (Davis et al., 2011), in primary NK cells and found a reduction of STAT1-S727 phosphorylation (Figure 2E). The lifespan of primary NK cells is limited, so we generated immortalized primary murine NK cells from mice with a disrupted Ink4a tumor suppressor locus (Serrano et al., 1996). The immortalized NK cells retain their growth dependence on IL-2 and their functionality with respect to IFN-γ production and cytotoxicity for several months (Figure S2). CDK8 knockdown in the Ink4a−/− NK cells verified the link between CDK8, STAT1-S727 phosphorylation and enhanced cytotoxicity (Figures 2F and 2G). Attempts to inhibit CDK8 in primary NK cells and to test the resulting cytotoxicity were inconclusive because there is still no inhibitor specific for CDK8. Inhibitors target multiple kinases, and this renders an interpretation of the assays impossible.
Stat1-S727A Mice Are Less Susceptible to NK Cell-Surveilled Tumors
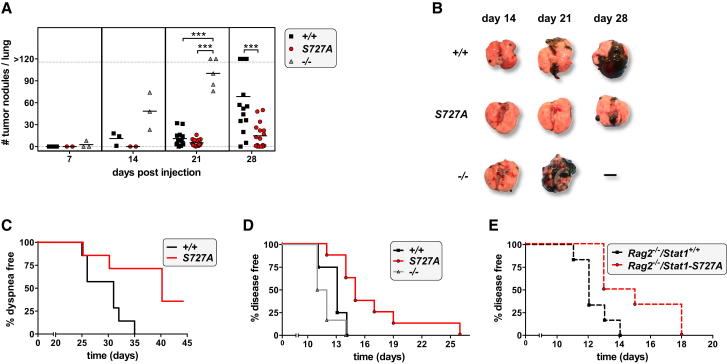
To examine whether the increased cell-mediated cytotoxicity associated with the Stat1-S727A mutation confers enhanced protection against NK cell-surveilled tumors, we used B16F10 melanoma cells, which form tumor nodules in the lung upon intravenous injection. In Stat1−/− animals, the first nodules were detected as early as 7 days postinjection. Wild-type animals showed the first tumors after 14 days, whereas Stat1-S727A mice remained free of visible tumor nodules until day 21 (Figures 3A and 3B). The enhanced resistance of Stat1-S727A mice to B16F10 tumor formation led to a significant increase in overall survival (Figure 3C).
Figure 3.
Stat1-S727A Mice Are Less Susceptible to B16F10 Melanoma and v-abl+ Leukemic Cells
(A) Wild-type, Stat1-S727A, and Stat1−/− mice were injected intravenously with B16F10 melanoma cells. At the indicated time points, mice were sacrificed, and tumor nodules visible on the lung surface were counted. Each symbol represents an individual mouse; horizontal lines indicate the mean (∗∗∗p < 0.001, one-way ANOVA and Tukey’s post hoc test on day 21, unpaired t test on day 28).
(B) Representative lung photographs at the indicated time points after injection of B16F10 melanoma cells are shown.
(C) Kaplan-Meier plot of wild-type (n = 7) and Stat1-S727A (n = 7) mice intravenously injected with B16F10 melanoma cells is illustrated. Log rank testing revealed a significant difference in the onset of dyspnea (p = 0.027; MST wild-type = 31 days; MST Stat1-S727A = 40 days).
(D) Kaplan-Meier plot of wild-type (n = 8), Stat1-S727A (n = 8), and Stat1−/− (n = 6) recipient animals inoculated intravenously with v-abl+ leukemic cells is presented. Stat1-S727A mice succumb to leukemia with an increased latency (MST = 15 days) than wild-type (MST = 13 days) and Stat1−/− (MST = 11.5 days) animals. Log rank test was corrected with Bonferroni for multiple testing and revealed a significant difference between Stat1-S727A and wild-type (p = 0.0053) and between Stat1-S727A and Stat1−/− animals (p = 0.0023).
(E) Kaplan-Meier plot of Rag2−/−/Stat1+/+ (n = 6) and Rag2−/−/Stat1-S727A (n = 6) animals injected intravenously with v-abl+ leukemic cells is shown. Rag2−/−/Stat1-S727A mice succumb to leukemia with increased latency (MST = 14 days) than Rag2−/−/Stat1+/+ (MST = 12 days) animals. Log rank test was statistically significant (p = 0.017).
See also Figure S3.
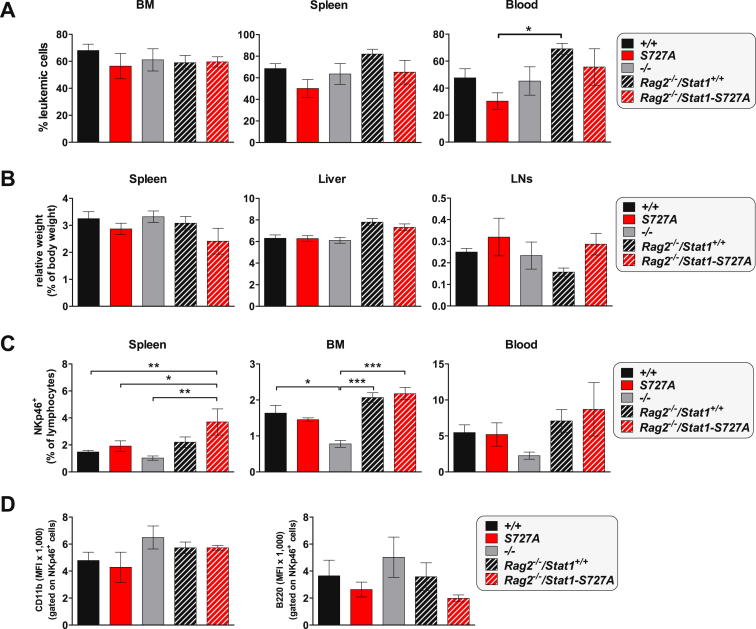
We also injected wild-type, Stat1−/−, and Stat1-S727A mice intravenously with v-abl-transformed leukemic cells, which are eradicated by NK cells (Stoiber et al., 2004). Stat1−/− mice rapidly succumb to leukemia, but Stat1-S727A animals are significantly less susceptible (Figure 3D). As a control, we used Rag2−/−/Stat1-S727A mice that lack B and T lymphocytes (Figure 3E). The survival curves of Rag2−/− and Rag2−/−/Stat1-S727A mice are superimposable with those of wild-type and Stat1-S727A mice. Disease severity at the day of sacrifice was comparable (Figure S3).
Figure S3.
Characterization of Diseased Mice after v-abl+ Leukemic Cell Transplantation, Related to Figure 3
Wild-type (n = 8), Stat1-S727A (n = 8), Stat1−/− (n = 6), Rag2−/− (n = 6) and Rag2−/−/Stat1-S727A mice (n = 6) were injected intravenously with 105v-abl+ leukemic cells. The mice were checked daily and sacrificed at the first sign of disease. As shown in Figure 3Stat1-S727A and Rag2−/−/Stat1-S727A mice survived the leukemic challenge with the longest latency.
(A) At the respective day of sacrifice, all mice were investigated for the infiltration of CD19+CD43+B220+ leukemic tumor cells into bone marrow (BM), spleen and blood. Disease severity was comparable in all mice at the day of sacrifice. Depicted are means ± SEM (∗p < 0.05, one-way ANOVA and Tukey’s post hoc test).
(B) Spleen, liver and lymph node (LN) weight, depicted relatively to the body weight, was measured and showed no difference. Plotted are means ± SEM.
(C) Numbers of NKp46+ NK cells were measured in spleen, BM and blood of all animals. Depicted are means ± SEM (∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001; one-way ANOVA followed by Tukey’s post hoc test).
(D) To measure NK cell maturity and activation, the expression of CD11b (left hand) and B220 (right hand) was determined on NKp46+ NK cells, respectively. There were no significant differences detectable. Depicted are means ± SEM. This experiment was conducted with 3 different v-abl+ cell lines with comparable results.
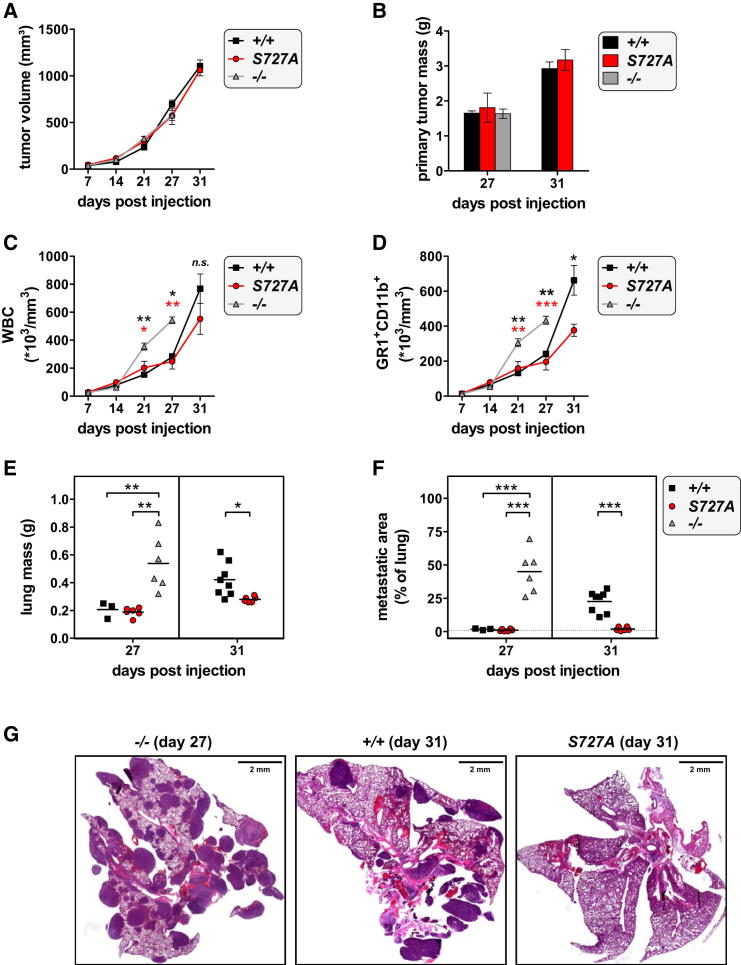
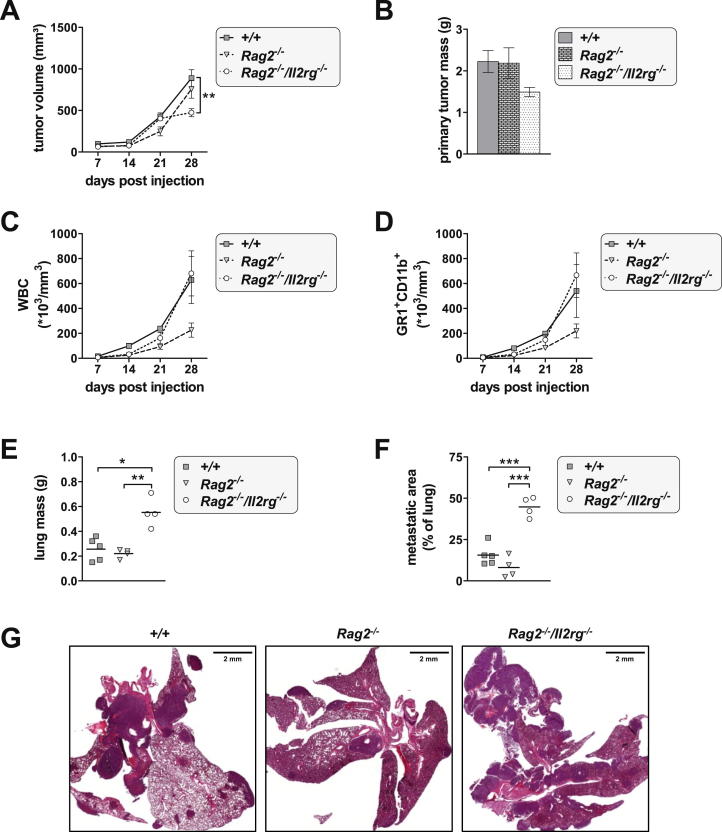
Metastasis represents a major problem in cancer therapy. In the 4T1-induced cancer model, the growth of the primary breast tumor is NK cell independent, whereas its ability to metastasize is restricted by NK cell-mediated tumor surveillance. Wild-type mice, Stat1−/− mice, and mice with the Stat1-S727A mutation showed no differences in volume or weight of primary tumors (Figures 4A and 4B). We also monitored white blood cell (WBC) counts and the appearance of myeloid-derived suppressor cells (MDSCs, defined as GR1+CD11b+) in the blood, surrogate markers of disease severity. Despite comparable growth rates of the primary tumors, WBC and MDSC counts were significantly enhanced in Stat1−/− mice from day 21. After 31 days, WBC and MDSC counts markedly increased in wild-type mice but stayed clearly lower in Stat1-S727A mice (Figures 4C and 4D). The lungs of all diseased mice were investigated for metastatic infiltrates. As expected, Stat1−/− mice had dense tumor cell infiltration in their lungs. At day 31 postinoculation, wild-type animals had significantly enhanced lung weights and severe metastatic burden, whereas Stat1-S727A mice were completely metastasis-free (Figures 4E–4G). To confirm the impact of NK cell-mediated tumor surveillance of 4T1 metastasis formation, we transplanted 4T1 cells into Rag2−/− and Rag2−/−/Il2rg−/− recipients. Twenty-eight days postinoculation, Rag2−/−/Il2rg−/− mice showed increased metastatic lung infiltrates compared to wild-type and Rag2−/− animals (Figure S4).
Figure 4.
Stat1-S727A Mice Are Resistant to 4T1 Breast Cancer Metastasis into Lungs
Wild-type (n = 11), Stat1-S727A (n = 12), and Stat1−/− (n = 6) animals received orthotopical transplants of 4T1 breast cancer cells.
(A) Primary tumor volumes were measured weekly. Symbols represent mean, and error bars indicate SEM.
(B) Some recipients were sacrificed at day 27 (wild-type n = 3, Stat1-S727A n = 6, Stat1−/− n = 6) and remaining recipients at day 31, then the primary tumors were weighed. Bar graph depicts mean ± SEM.
(C and D) Recipient mice were bled weekly, and (C) WBCs and (D) MDSCs (GR1+CD11b+) were counted (∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001; one-way ANOVA and Tukey’s post hoc test on days 21 and 27; unpaired t test on day 31). Symbols represent means, and error bars indicate SEM. n.s., not significant.
(E and F) After termination of the experiment, (E) lungs were weighed, and (F) the percentage (%) of metastatic area in the lung was determined histochemically (∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001; one-way ANOVA and Tukey’s post hoc test on day 27; unpaired t test on day 31). Each symbol represents an individual mouse; horizontal lines indicate the mean.
(G) Representative H&E-stained lung sections of Stat1−/− animals at day 27 and wild-type and Stat1-S727A animals at day 31 post transplantation are shown.
See also Figure S4.
Figure S4.
4T1 Breast Cancer Metastasis Formation in Immune-Compromised Rag2−/− and Rag2−/−/Il2rg−/− Animals, Related to Figure 4
BALB/c wild-type (n = 5), Rag2−/− (n = 4) and Rag2−/−/Il2rg−/− (n = 4) animals received orthotopical transplants of 4T1 breast cancer cells.
(A) Breast tumor volumes were measured weekly. Symbols represent means and error bars indicate SEM. Rag2−/−/Il2rg−/− animals seemed to develop smaller breast tumors, which was due to increased intra-tumoral necrosis (∗∗p < 0.01; one-way ANOVA and Tukey's post hoc test).
(B–D) After sacrifice at day 28 post transplantation, primary tumors were prepared and weighed. Plotted are means ± SEM. No significant differences in primary tumor weight were detected. Recipient mice were bled weekly and (C) white blood cell (WBC) counts and (D) the numbers of myeloid-derived suppressor cells (MDSCs, GR1+CD11b+) were determined as surrogate marker of disease progression. Rag2−/− animals showed substantially lower WBC and MDSC counts at day 28 post injection. Symbols represent means and error bars indicate SEM.
(E and F) After termination of the experiment, (E) lungs were weighed and (F) the percentage of metastatic area in the lung was determined histochemically (∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001; one-way ANOVA and Tukey’s post hoc test). Each symbol represents an individual mouse; small horizontal lines indicate the mean.
(G) Representative H&E-stained lung sections of wild-type, Rag2−/− and Rag2−/−/Il2rg−/− animals.
Discussion
Our study reveals an inhibitory role of STAT1-S727 phosphorylation for NK cell cytotoxicity. STAT1-S727 is constitutively phosphorylated in unstimulated NK cells, where it modulates their activity.
The transactivation domain of all STAT proteins except STAT2 harbors at least one conserved serine-phosphorylation site. Experiments in Stat1-S727A-mutated NK cells show that STAT1-S727 phosphorylation is a prerequisite for full IFN-γ induction, although it decreases granzyme B and perforin transcription. Perforin function shows a pronounced gene-dosage effect: Prf1+/− NK cells display reduced cytotoxicity (Lowin et al., 1994). Higher perforin levels may thus lead to higher cytotoxicity, as we observed in Stat1-S727A NK cells. Cytotoxicity might also be affected by additional mechanisms such as a reduction of the inhibitory KLRG1 or an increase in expression of partially activating NKG2A/C/E.
Several kinases phosphorylate STAT1-S727 upon stimulation, depending on cell type and stimulus (Kovarik et al., 1999; Nair et al., 2002; Deb et al., 2003; Bancerek et al., 2013). The transactivation domain of STAT1 is also phosphorylated on S708, S744, and S747. Phosphorylation on S708 has been attributed to IKKε and is indispensable for antiviral IFN signaling (Tenoever et al., 2007).
CDK8 has recently been identified as a major kinase in the response to IFN signaling mediating STAT1-S727 phosphorylation (Bancerek et al., 2013). In fibroblasts and macrophages, STAT1-S727 phosphorylation depends on stimulation by cytokines. We now report an additional role for STAT1-S727 phosphorylation, which is observed in NK cells even in the absence of cytokines and with no concomitant tyrosine (Y701) phosphorylation. We show that S727 phosphorylation exerts a negative influence and show that a biological response is restricted by STAT1-S727 phosphorylation independent of IFN signaling. The Stat1-S727A mutant acts as a hypermorphic rather than a hypomorphic allele of Stat1 in NK cells. Knockdown of CDK8 verified its essential role for basal STAT1-S727 phosphorylation in NK cells and significantly enhanced cytotoxicity. NK cells derived from Stat1-S727A mice are exceptionally cytotoxic and display signs of hyperactivity. Stat1-S727A mice are less susceptible to B16F10 melanoma and v-abl+ leukemia and are even resistant to 4T1 breast cancer metastasis. Hence, specific CDK8 kinase inhibitors may be useful in tumor therapy. A combination of classical pharmacological target intervention and novel immune cell-based strategies may be able to address the complex nature of cancer immunology. The ability to generate NK cells primed to kill more efficiently raises the hope of enhancing clinical efficacy and outcome.
Experimental Procedures
Mice
BALB/c and C57BL/6 wild-type(+/+), Stat1−/− (Durbin et al., 1996), Stat1-S727A (Varinou et al., 2003), Rag2−/− (Shinkai et al., 1992), Rag2−/−/Stat1-S727A, Rag2−/−/Il2rg−/− (Cao et al., 1995), Ifng−/− (Dalton et al., 1993), and Ink4a−/− (Serrano et al., 1996) mice were maintained under pathogen-free conditions. The in vitro and B16F10 in vivo experiments were performed with mice on C57BL/6 background. For v-abl+ and 4T1 tumor models, mice on BALB/c background were used. All experiments were carried out with gender- and age-matched 6- to 12-week-old mice, were approved by the institutional ethics committee, and conform to Austrian laws (license 66.009/0019-II/10b/2010 14.1.10).
Inhibitor Studies and shRNA Knockdown
Web-based inquiries, e.g., Group-based Prediction System (GPS; http://gps.biocuckoo.org/) and KinasePhos 2.0 (http://kinasephos2.mbc.nctu.edu.tw/index.html), were performed. For inhibitor studies, LAK cells were deprived of IL-2 for 3 hr prior to 4 hr treatment with 30 μM DMSO (Carl Roth), 100 ng/ml anisomycin (Sigma-Aldrich), 5 μM roscovitine (Calbiochem), 0.5 μM flavopiridol (Sigma-Aldrich), 1 μM BIRB 0796 (Böhringer Ingelheim), or 25 μM SB 203580 (Sigma-Aldrich).
For shRNA-mediated knockdown of CDK8, five pGIPZ clones (RMM4532-NM_001260) and two TRC clones (TRCN0000023107 and TRCN0000023268) were obtained from Open Biosystems. The best knockdown efficiency was shown by clone 3 for pGIPZ (V2LMM_192695) and clone TRCN0000023268 for TRC. A clone containing a validated nonsilencing shRNA (RHS4346 for pGIPZ and RHS4080 for TRC) served as control. VSV-G-pseudotyped lentiviruses were produced as described previously (Hoermann et al., 2011). Recombinant lentiviruses were produced in HEK293T cells with pGIPZ vectors and plasmids encoding GAG-POL and VSV-G. NK cells were transduced by spin infection (800 × g, 90 min, 32°C) in the presence of 7 μg/ml Polybrene (Sigma-Aldrich) and selected with 2 μg/ml puromycin (Invivogen/Eubio) for 5 days.
In Vitro Cytotoxicity Assay
Standard 4 hr [51Cr]-release assays and flow cytometry-based cytotoxicity assays (Zebedin et al., 2008; Schuster et al., 2011) are described in detail in the Extended Experimental Procedures.
Extended Experimental Procedures.
Cell Culture
B16F10 melanoma and A010 A-MuLV producer cells were maintained in DMEM high glucose (PAA) supplemented with 10% fetal calf serum (FCS, PAA), 50 μM 2-mercaptoethanol (Sigma-Aldrich), 100 U/ml penicillin and 100 μg/ml streptomycin (Life Technologies). YAC-1, RMA, RMA-S, RMA-Rae1, Daudi and v-abl+ leukemic cells were maintained in RPMI-1640 medium containing L-glutamine (PAA), 10% FCS, 50 μM 2-mercaptoethanol, 100 U/ml penicillin and 100 μg/ml streptomycin. 4T1 cells were cultivated in EAGLE medium (house intern media kitchen), supplemented with 2 mM L-glutamine (Sigma-Aldrich), 20 mM HEPES buffer (Sigma-Aldrich), 0.02 mg/l Ciproxin (Bayer), 1 U/ml penicillin and 1 μg/ml streptomycin (Sigma-Aldrich). NK cells were isolated from splenocytes using the MACS® NK cell separation kit (anti-DX5 microbeads, Miltenyi Biotec). In order to obtain LAK cultures, the MACS®-purified NK cells were plated for 5-7 days in RPMI-1640 containing L-glutamine, 10% FCS, 50 μM 2-mercaptoethanol, 100 U/ml penicillin, 100 μg/ml streptomycin and 5,000 U/ml rhIL-2 (Proleukin®, Novartis). The purity of LAK cells was routinely checked before the experiments (%NK1.1+CD3- cells > 90%).
NK Cell Stimulation
Freshly purified NK or LAK cells were harvested and stimulated with 5,000 U/ml rhIL-2 (Novartis), 100 U/ml rmIFN-β (AbD Serotec), 5 ng/ml rmIL-12 (R&D Systems), or 10 ng/ml phorbol 12-myristate 13-acetate (PMA, Sigma-Aldrich) and 250 ng/ml ionomycin (Sigma-Aldrich).
Antibodies and Flow Cytometric Analysis
Following antibodies (clones) were purchased from BD Biosciences: B220 (RA3-6B2), CD3ε (145-2C11), CD11b (M1/70), CD19 (1D3), CD27 (LG.3A10), CD43 (S7), CD122 (TM-Beta 1), CD244.2 (2B4), IFN-γ (XMG1.2), KLRG1 (2F1), Ly49A (A1), Ly49C/I (5E6), Ly49D (4E5), NKG2A/C/E (20d5) and Ter-119 (TER-119). Following antibodies were purchased from eBioscience: CD3ε (17A2, 145-2C11), CD16/CD32 (93), CD49b (DX5), CD51 (RMV-7), CD226 (10E5), granzyme B (NGZB), NK1.1 (PK136), NKG2D (CX5), NKp46 (29A1.4) and perforin (eBioOMAK-D). Whole blood and splenocytes were depleted of erythrocytes prior to staining. Purified anti-CD16/CD32 was added to avoid nonspecific binding. Intracellular staining for perforin was performed with the Fixation/Permeabilization kit (eBioscience, #88-8823-88), granzyme B and IFN-γ were determined with the Foxp3/Transcription factor staining buffer set (eBioscience, #00-5523-00) after the application of a fixable viability dye eFluor® 450 (eBioscience). All samples were recorded on a FACSCanto™ II flow cytometer (BD Biosciences, Heidelberg, Germany) and analyzed with BD FACSDiva software version 6.1.2 or with FlowJo software version 7.6.1 (Tree Star).
Antibodies and Western Blot
106 purified NK cells were boiled (95°C) for 20 min in 150 μl SDS-sample buffer [5% SDS (Biomol), 5% glycerol (Merck), 2.5% 2-mercaptoethanol and a trace amount of bromophenol blue sodium salt (Merck) in 375 mM Tris/HCl (pH 6.8)]. Equal amounts of proteins were separated by SDS/PAGE and transferred to a nitrocellulose membrane (Whatman® Protran®). After blocking with 5% BSA in pY-TBST buffer (10 mM Tris/HCl pH 7.4, 75 mM NaCl, 1 mM EDTA, 0.1% Tween-20), membranes were probed with antibodies against α-tubulin (sc-32293), HSC-70 (sc-7298) and STAT1 (sc-592) (Santa Cruz Technology), CDK8 (CS#4101), pSTAT1-Y701 (CS#9171), pSTAT1-S727 (CS#9177), STAT3 (CS#9132), pSTAT3-Y705 (CS#9131), STAT4 (CS#2653) (Cell Signaling) and pSTAT4-Y693 (BD 612738, BD Biosciences). Immunoreactive bands were visualized by chemiluminescent detection (LumiGLO®, Cell Signaling; and ImmunStar™ WesternC™ chemiluminescent kit, Bio-Rad) by the ChemiDoc MP Imaging System (Bio-Rad Laboratories, CA).
Semiquantitative Real-Time PCR
RNA was prepared from freshly isolated splenic lymphocytes or from purified and in vitro expanded NK cells using peqGOLD TriFast reagent (PEQLAB). 1 μg of RNA was reversely transcribed by the iSCRIPT cDNA synthesis kit (Bio-Rad). Real-time PCR was performed on a MyiQ2 cycler (Bio-Rad Laboratories, CA) with SsoFast™ EvaGreen®Supermix (Bio-Rad). The applied primer sets are described in Table S2. The Ikzf2 (Helios) QuantiTect primer assay was purchased from QIAGEN. Target gene expression was normalized to Gapdh.
miRNA Quantification
DX5+ NK cells were isolated from whole splenocytes (MACS® NK cell separation kit, Miltenyi Biotec) and lysed in peqGOLD TriFast reagent (PEQLAB). Total RNA including miRNA was purified with the miRNeasy Mini Kit (QIAGEN). cDNA was generated with the miScript II RT Kit (QIAGEN) and for qPCR the miScript SYBR Green PCR Kit (QIAGEN) and the following miScript primer assays (QIAGEN) were used: Mm_miR-27a∗_1, Mm_miR-27a_1, Mm_miR-30e_2, Mm_miR-223_2, Mm_miR-378_2, Hs_SNORD61_1. qPCR was performed as described in the standard protocol provided by the supplier and miRNA expression levels were normalized to Snord61.
ChIP Assay
ChIP assays were performed as described previously (Farlik et al., 2010) with slight modifications. In brief, 1∗107 LAK cells were either left untreated or stimulated for 30 min with 100 U/ml IFN-β or 5 ng/ml IL-12 prior to formaldehyde-crosslinking for 10 min at room temperature. After the addition of 0.5 M glycine, nuclei were prepared and lysed overnight in the presence of complete protease inhibitor cocktail (Roche). The chromatin was sonicated for 7 cycles (30 s on, 30 s off) with a Bioruptor Plus (Diagenode, Liège, Belgium), which resulted in the formation of chromatin fragments 300bp – 1kbp in size. After overnight incubation with 4 μg of anti-polymerase II antibody (Santa Cruz sc-899), the IP was conducted with Dynabeads Protein G (Invitrogen). Following elution and reverse crosslinking overnight, the chromatin was purified by phenol/chloroform/isoamyl alcohol extraction and ethanol precipitation. The transcription start site of the murine perforin gene was amplified with the primers 5′- TGGTGGGACTTCAGGTAAGGA-3′ and 5′- CAACTCTCTTTCCCCAGGGT-3′ and compared to the irrelevant region 188 bps 3′-downstream of CD19 5′-CCCTCTTCTCATTCGTTTTCCA-3′ and 5′- CCAGGAAAGAATTTGAGAAAAATCA-3′.
In Vitro Cytotoxicity Assays
FACS-based cytotoxicity assays were performed as follows: MACS®-purified LAK cells were co-cultured with 5∗104 CFSE-stained (2.5 μM; CellTrace CFSE Cell Proliferation Kit, Molecular Probes) target cells at different effector-to-target (E:T) ratios in triplicates. After 4-6 hr co-incubation all samples were subjected to 0.1 μg 7-aminoactinomycin D (7-AAD, eBioscience) for 5 min and analyzed by flow cytometry: % specific lysis = [% 7-AAD+CFSE+ cells after co-incubation with NK cells] – [% 7-AAD+CFSE+ cells without addition of NK cells].
Standard 4 hr [51Cr]-release assays were performed labeling target cells with 100 μCi of Na51CrO4 (PerkinElmer) at 37°C for 1 hr. 104 labeled target cells were added to titrated numbers of LAK cells. Supernatants were collected after 4 hr and radioactivity was determined in a γ-counter (Packard, Meriden, CT): % specific lysis = [NK-induced release (cpm) − spontaneous release (cpm)]/[maximum release (cpm) − spontaneous release (cpm)] × 100. For redirected antibody-dependent cell-mediated cytotoxicity (R-ADCC) LAK cells were incubated with 20 μg/ml mAbs specific for NK1.1 (PK136), Ly49D (4E5), CD244 (2B4) or isotype controls for 20 min at 4°C prior to the 4 hr [51Cr]-release assay using Daudi target cells.
B16F10 Tumor Model
Mice were injected via tail vein with 5 × 104 B16F10 melanoma cells and sacrificed after 7, 14, 21, or 28 days (time course) or at the first sign of dyspnea (Kaplan-Meier). Stat1−/− mice were included only until day 21. Lungs were removed, weighed, and captured on camera, and the visible tumor nodules on their surface were counted.
Leukemia Model
Single-cell suspensions from BALB/c bone marrow were infected with A-MuLV retrovirus in the presence of 7 μg/ml Polybrene (Sigma-Aldrich) and 10 ng/ml rmIL-7 (PeproTech) to establish stable cell lines. After intravenous injection of 105 v-abl+ leukemic cells, mice were checked daily for the onset of leukemia and sacrificed at the first sign of disease. They were analyzed for spleen and liver weight and the presence of leukemic cells (CD19+CD43+B220+) in bone marrow, spleen, and blood.
4T1 Tumor Model
A total of 5 × 105 tumor cells/30 μl PBS was injected in the fat pad of the fifth pair of mammary glands of sedated mice. Once a week, tumor volumes were measured (tumor volume = (width2 × length)/2) and WBCs determined in an animal blood counter (Scil Animal Care). GR1+CD11b+ MDSCs in the blood were assessed via flow cytometry. At the indicated time points, lungs were excised, paraformaldehyde fixed, and paraffin embedded, and six to ten sections (3 μm) were stained with hematoxylin and eosin (H&E, Microm HMS 740 Robot Stainer; Thermo Scientific) and scanned with a Zeiss MIRAX scanner. The percentage of metastatic lung area was quantified by Definiens Analyst software.
Statistical Analysis
Statistical analysis was performed using GraphPad Prism version 5.00 for Windows (GraphPad Software; http://www.graphpad.com). Where ANOVA showed a statistical difference, Tukey’s multiple comparison testing was applied. Comparison of more than two survival curves was made by the Bonferroni correction of log rank tests. The α level for all tests was set to 0.05, and p values were two tailed. The significance level is indicated for each experiment (in general: ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001).
For further details, see the Extended Experimental Procedures and Table S2.
Acknowledgments
We are indebted to S. Fajmann, S. Wienerroither, M. Parrini, K. Schelch, M. Mair, M. Prchal-Murphy, and B. Strobl for support and scientific input and G. Tebb for careful reading and revision of the manuscript. We are grateful to the mouse facility. The work was supported by the Austrian Academy of Science DOC-fFORTE fellowship (to E.M.P.; http://stipendien.oeaw.ac.at/en), the Austrian Science Fund FWF grant SFB F28 (to M.M., T.D., and V.S.; http://www.fwf.ac.at/en/projects/sfb.html), and the GEN-AU program “Austromouse” of the Austrian Federal Ministry of Science and Research (to M.M.; http://www.gen-au.at/projekt.jsp?projektId=110&lang=en).
Published: August 8, 2013
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
Supplemental Information includes Extended Results, Extended Experimental Procedures, four figures, and two tables and can be found with this article online at http://dx.doi.org/10.1016/j.celrep.2013.07.012.
Web Resources
The URLs for data presented herein are as follows:
Austrian Academy of Science DOC-fFORTE fellowship, http://stipendien.oeaw.ac.at/en
Austrian Science Fund FWF grant SFB F28, http://www.fwf.ac.at/en/projects/sfb.html
GEN-AU program “Austromouse” of the Austrian Federal Ministry of Science and Research, http://www.gen-au.at/projekt.jsp?projektId=110&lang=en
GraphPad Software, http://www.graphpad.com
KinasePhos 2.0, http://kinasephos2.mbc.nctu.edu.tw/index.html
Supplemental Information
Primer pairs used in this paper are from Putz et al., 2012; Sadzak et al., 2008; Tayade et al., 2005; Mizutani et al., 2012; Pilz et al., 2003; Kamezaki et al., 2004; Gratz et al., 2008; and Alcaide et al., 2007.
References
- Bancerek J., Poss Z.C., Steinparzer I., Sedlyarov V., Pfaffenwimmer T., Mikulic I., Dölken L., Strobl B., Müller M., Taatjes D.J., Kovarik P. CDK8 kinase phosphorylates transcription factor STAT1 to selectively regulate the interferon response. Immunity. 2013;38:250–262. doi: 10.1016/j.immuni.2012.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromberg J.F., Horvath C.M., Wen Z., Schreiber R.D., Darnell J.E., Jr. Transcriptionally active Stat1 is required for the antiproliferative effects of both interferon alpha and interferon gamma. Proc. Natl. Acad. Sci. USA. 1996;93:7673–7678. doi: 10.1073/pnas.93.15.7673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X., Shores E.W., Hu-Li J., Anver M.R., Kelsall B.L., Russell S.M., Drago J., Noguchi M., Grinberg A., Bloom E.T. Defective lymphoid development in mice lacking expression of the common cytokine receptor gamma chain. Immunity. 1995;2:223–238. doi: 10.1016/1074-7613(95)90047-0. [DOI] [PubMed] [Google Scholar]
- Dalton D.K., Pitts-Meek S., Keshav S., Figari I.S., Bradley A., Stewart T.A. Multiple defects of immune cell function in mice with disrupted interferon-gamma genes. Science. 1993;259:1739–1742. doi: 10.1126/science.8456300. [DOI] [PubMed] [Google Scholar]
- Davis M.I., Hunt J.P., Herrgard S., Ciceri P., Wodicka L.M., Pallares G., Hocker M., Treiber D.K., Zarrinkar P.P. Comprehensive analysis of kinase inhibitor selectivity. Nat. Biotechnol. 2011;29:1046–1051. doi: 10.1038/nbt.1990. [DOI] [PubMed] [Google Scholar]
- Deb D.K., Sassano A., Lekmine F., Majchrzak B., Verma A., Kambhampati S., Uddin S., Rahman A., Fish E.N., Platanias L.C. Activation of protein kinase C delta by IFN-gamma. J. Immunol. 2003;171:267–273. doi: 10.4049/jimmunol.171.1.267. [DOI] [PubMed] [Google Scholar]
- Durbin J.E., Hackenmiller R., Simon M.C., Levy D.E. Targeted disruption of the mouse Stat1 gene results in compromised innate immunity to viral disease. Cell. 1996;84:443–450. doi: 10.1016/s0092-8674(00)81289-1. [DOI] [PubMed] [Google Scholar]
- Hoermann G., Cerny-Reiterer S., Perné A., Klauser M., Hoetzenecker K., Klein K., Müllauer L., Gröger M., Nijman S.M.B., Klepetko W. Identification of oncostatin M as a STAT5-dependent mediator of bone marrow remodeling in KIT D816V-positive systemic mastocytosis. Am. J. Pathol. 2011;178:2344–2356. doi: 10.1016/j.ajpath.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath C.M., Darnell J.E., Jr. The antiviral state induced by alpha interferon and gamma interferon requires transcriptionally active Stat1 protein. J. Virol. 1996;70:647–650. doi: 10.1128/jvi.70.1.647-650.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan D.H., Shankaran V., Dighe A.S., Stockert E., Aguet M., Old L.J., Schreiber R.D. Demonstration of an interferon gamma-dependent tumor surveillance system in immunocompetent mice. Proc. Natl. Acad. Sci. USA. 1998;95:7556–7561. doi: 10.1073/pnas.95.13.7556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klover P.J., Muller W.J., Robinson G.W., Pfeiffer R.M., Yamaji D., Hennighausen L. Loss of STAT1 from mouse mammary epithelium results in an increased Neu-induced tumor burden. Neoplasia. 2010;12:899–905. doi: 10.1593/neo.10716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koromilas A.E., Sexl V. The tumor suppressor function of STAT1 in breast cancer. JAKSTAT. 2013;2:1–5. doi: 10.4161/jkst.23353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacic B., Stoiber D., Moriggl R., Weisz E., Ott R.G., Kreibich R., Levy D.E., Beug H., Freissmuth M., Sexl V. STAT1 acts as a tumor promoter for leukemia development. Cancer Cell. 2006;10:77–87. doi: 10.1016/j.ccr.2006.05.025. [DOI] [PubMed] [Google Scholar]
- Kovarik P., Stoiber D., Eyers P.A., Menghini R., Neininger A., Gaestel M., Cohen P., Decker T. Stress-induced phosphorylation of STAT1 at Ser727 requires p38 mitogen-activated protein kinase whereas IFN-gamma uses a different signaling pathway. Proc. Natl. Acad. Sci. USA. 1999;96:13956–13961. doi: 10.1073/pnas.96.24.13956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C.K., Rao D.T., Gertner R., Gimeno R., Frey A.B., Levy D.E. Distinct requirements for IFNs and STAT1 in NK cell function. J. Immunol. 2000;165:3571–3577. doi: 10.4049/jimmunol.165.7.3571. [DOI] [PubMed] [Google Scholar]
- Lodolce J.P., Boone D.L., Chai S., Swain R.E., Dassopoulos T., Trettin S., Ma A. IL-15 receptor maintains lymphoid homeostasis by supporting lymphocyte homing and proliferation. Immunity. 1998;9:669–676. doi: 10.1016/s1074-7613(00)80664-0. [DOI] [PubMed] [Google Scholar]
- Lowin B., Beermann F., Schmidt A., Tschopp J. A null mutation in the perforin gene impairs cytolytic T lymphocyte- and natural killer cell-mediated cytotoxicity. Proc. Natl. Acad. Sci. USA. 1994;91:11571–11575. doi: 10.1073/pnas.91.24.11571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas M., Schachterle W., Oberle K., Aichele P., Diefenbach A. Dendritic cells prime natural killer cells by trans-presenting interleukin 15. Immunity. 2007;26:503–517. doi: 10.1016/j.immuni.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair J.S., DaFonseca C.J., Tjernberg A., Sun W., Darnell J.E., Jr., Chait B.T., Zhang J.J. Requirement of Ca2+ and CaMKII for Stat1 Ser-727 phosphorylation in response to IFN-gamma. Proc. Natl. Acad. Sci. USA. 2002;99:5971–5976. doi: 10.1073/pnas.052159099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen K.B., Salazar-Mather T.P., Dalod M.Y., Van Deusen J.B., Wei X.Q., Liew F.Y., Caligiuri M.A., Durbin J.E., Biron C.A. Coordinated and distinct roles for IFN-alpha beta, IL-12, and IL-15 regulation of NK cell responses to viral infection. J. Immunol. 2002;169:4279–4287. doi: 10.4049/jimmunol.169.8.4279. [DOI] [PubMed] [Google Scholar]
- Pilz A., Kratky W., Stockinger S., Simma O., Kalinke U., Lingnau K., von Gabain A., Stoiber D., Sexl V., Kolbe T. Dendritic cells require STAT-1 phosphorylated at its transactivating domain for the induction of peptide-specific CTL. J. Immunol. 2009;183:2286–2293. doi: 10.4049/jimmunol.0901383. [DOI] [PubMed] [Google Scholar]
- Robbins S.H., Tessmer M.S., Van Kaer L., Brossay L. Direct effects of T-bet and MHC class I expression, but not STAT1, on peripheral NK cell maturation. Eur. J. Immunol. 2005;35:757–765. doi: 10.1002/eji.200425797. [DOI] [PubMed] [Google Scholar]
- Sadzak I., Schiff M., Gattermeier I., Glinitzer R., Sauer I., Saalmüller A., Yang E., Schaljo B., Kovarik P. Recruitment of Stat1 to chromatin is required for interferon-induced serine phosphorylation of Stat1 transactivation domain. Proc. Natl. Acad. Sci. USA. 2008;105:8944–8949. doi: 10.1073/pnas.0801794105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneckenleithner C., Bago-Horvath Z., Dolznig H., Neugebauer N., Kollmann K., Kolbe T., Decker T., Kerjaschki D., Wagner K.-U., Müller M. Putting the brakes on mammary tumorigenesis: loss of STAT1 predisposes to intraepithelial neoplasias. Oncotarget. 2011;2:1043–1054. doi: 10.18632/oncotarget.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroder K., Spille M., Pilz A., Lattin J., Bode K.A., Irvine K.M., Burrows A.D., Ravasi T., Weighardt H., Stacey K.J. Differential effects of CpG DNA on IFN-beta induction and STAT1 activation in murine macrophages versus dendritic cells: alternatively activated STAT1 negatively regulates TLR signaling in macrophages. J. Immunol. 2007;179:3495–3503. doi: 10.4049/jimmunol.179.6.3495. [DOI] [PubMed] [Google Scholar]
- Schuster C., Berger A., Hoelzl M.A., Putz E.M., Frenzel A., Simma O., Moritz N., Hoelbl A., Kovacic B., Freissmuth M. The cooperating mutation or “second hit” determines the immunologic visibility toward MYC-induced murine lymphomas. Blood. 2011;118:4635–4645. doi: 10.1182/blood-2010-10-313098. [DOI] [PubMed] [Google Scholar]
- Serrano M., Lee H., Chin L., Cordon-Cardo C., Beach D., DePinho R.A. Role of the INK4a locus in tumor suppression and cell mortality. Cell. 1996;85:27–37. doi: 10.1016/s0092-8674(00)81079-x. [DOI] [PubMed] [Google Scholar]
- Shinkai Y., Rathbun G., Lam K.P., Oltz E.M., Stewart V., Mendelsohn M., Charron J., Datta M., Young F., Stall A.M. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell. 1992;68:855–867. doi: 10.1016/0092-8674(92)90029-c. [DOI] [PubMed] [Google Scholar]
- Stark G.R.R., Darnell J.E.E., Jr. The JAK-STAT pathway at twenty. Immunity. 2012;36:503–514. doi: 10.1016/j.immuni.2012.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoiber D., Kovacic B., Schuster C., Schellack C., Karaghiosoff M., Kreibich R., Weisz E., Artwohl M., Kleine O.C., Müller M. TYK2 is a key regulator of the surveillance of B lymphoid tumors. J. Clin. Invest. 2004;114:1650–1658. doi: 10.1172/JCI22315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenoever B.R., Ng S.L., Chua M.A., McWhirter S.M., García-Sastre A., Maniatis T. Multiple functions of the IKK-related kinase IKKepsilon in interferon-mediated antiviral immunity. Science. 2007;315:1274–1278. doi: 10.1126/science.1136567. [DOI] [PubMed] [Google Scholar]
- Varinou L., Ramsauer K., Karaghiosoff M., Kolbe T., Pfeffer K., Müller M., Decker T. Phosphorylation of the Stat1 transactivation domain is required for full-fledged IFN-gamma-dependent innate immunity. Immunity. 2003;19:793–802. doi: 10.1016/s1074-7613(03)00322-4. [DOI] [PubMed] [Google Scholar]
- Vivier E., Raulet D.H., Moretta A., Caligiuri M.A., Zitvogel L., Lanier L.L., Yokoyama W.M., Ugolini S. Innate or adaptive immunity? The example of natural killer cells. Science. 2011;331:44–49. doi: 10.1126/science.1198687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P., Gu Y., Zhang Q., Han Y., Hou J., Lin L., Wu C., Bao Y., Su X., Jiang M. Identification of resting and type I IFN-activated human NK cell miRNomes reveals microRNA-378 and microRNA-30e as negative regulators of NK cell cytotoxicity. J. Immunol. 2012;189:211–221. doi: 10.4049/jimmunol.1200609. [DOI] [PubMed] [Google Scholar]
- Watford W.T., Hissong B.D., Bream J.H., Kanno Y., Muul L., O’Shea J.J. Signaling by IL-12 and IL-23 and the immunoregulatory roles of STAT4. Immunol. Rev. 2004;202:139–156. doi: 10.1111/j.0105-2896.2004.00211.x. [DOI] [PubMed] [Google Scholar]
- Wen Z., Zhong Z., Darnell J.E., Jr. Maximal activation of transcription by Stat1 and Stat3 requires both tyrosine and serine phosphorylation. Cell. 1995;82:241–250. doi: 10.1016/0092-8674(95)90311-9. [DOI] [PubMed] [Google Scholar]
- Zebedin E., Simma O., Schuster C., Putz E.M., Fajmann S., Warsch W., Eckelhart E., Stoiber D., Weisz E., Schmid J.A. Leukemic challenge unmasks a requirement for PI3Kdelta in NK cell-mediated tumor surveillance. Blood. 2008;112:4655–4664. doi: 10.1182/blood-2008-02-139105. [DOI] [PubMed] [Google Scholar]
Supplemental References
- Alcaide P., Jones T.G., Lord G.M., Glimcher L.H., Hallgren J., Arinobu Y., Akashi K., Paterson A.M., Gurish M.A., Luscinskas F.W. Dendritic cell expression of the transcription factor T-bet regulates mast cell progenitor homing to mucosal tissue. J. Exp. Med. 2007;204:431–439. doi: 10.1084/jem.20060626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farlik M., Reutterer B., Schindler C., Greten F., Vogl C., Müller M., Decker T. Nonconventional initiation complex assembly by STAT and NF-kappaB transcription factors regulates nitric oxide synthase expression. Immunity. 2010;33:25–34. doi: 10.1016/j.immuni.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehniger T.A., Wylie T., Germino E., Leong J.W., Magrini V.J., Koul S., Keppel C.R., Schneider S.E., Koboldt D.C., Sullivan R.P. Next-generation sequencing identifies the natural killer cell microRNA transcriptome. Genome Res. 2010;20:1590–1604. doi: 10.1101/gr.107995.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratz N., Siller M., Schaljo B., Pirzada Z.A., Gattermeier I., Vojtek I., Kirschning C.J., Wagner H., Akira S., Charpentier E., Kovarik P. Group A streptococcus activates type I interferon production and MyD88-dependent signaling without involvement of TLR2, TLR4, and TLR9. J. Biol. Chem. 2008;283:19879–19887. doi: 10.1074/jbc.M802848200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamezaki K., Shimoda K., Numata A., Matsuda T., Nakayama K.I., Harada M. The role of Tyk2, Stat1 and Stat4 in LPS-induced endotoxin signals. Int. Immunol. 2004;16:1173–1179. doi: 10.1093/intimm/dxh118. [DOI] [PubMed] [Google Scholar]
- Kim T.D., Lee S.U., Yun S., Sun H.-N., Lee S.H., Kim J.W., Kim H.M., Park S.-K., Lee C.W., Yoon S.R. Human microRNA-27a∗ targets Prf1 and GzmB expression to regulate NK-cell cytotoxicity. Blood. 2011;118:5476–5486. doi: 10.1182/blood-2011-04-347526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong J.W., Sullivan R.P., Fehniger T.A. Natural killer cell regulation by microRNAs in health and disease. J. Biomed. Biotechnol. 2012;2012:632329. doi: 10.1155/2012/632329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizutani T., Neugebauer N., Putz E.M., Moritz N., Simma O., Zebedin-Brandl E., Gotthardt D., Warsch W., Eckelhart E., Kantner H.-P. Conditional IFNAR1 ablation reveals distinct requirements of Type I IFN signaling for NK cell maturation and tumor surveillance. OncoImmunology. 2012;1:1027–1037. doi: 10.4161/onci.21284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narni-Mancinelli E., Jaeger B.N., Bernat C., Fenis A., Kung S., De Gassart A., Mahmood S., Gut M., Heath S.C., Estellé J. Tuning of natural killer cell reactivity by NKp46 and Helios calibrates T cell responses. Science. 2012;335:344–348. doi: 10.1126/science.1215621. [DOI] [PubMed] [Google Scholar]
- Pearce E.L., Mullen A.C., Martins G.A., Krawczyk C.M., Hutchins A.S., Zediak V.P., Banica M., DiCioccio C.B., Gross D.A., Mao C.A. Control of effector CD8+ T cell function by the transcription factor Eomesodermin. Science. 2003;302:1041–1043. doi: 10.1126/science.1090148. [DOI] [PubMed] [Google Scholar]
- Pilz A., Ramsauer K., Heidari H., Leitges M., Kovarik P., Decker T. Phosphorylation of the Stat1 transactivating domain is required for the response to type I interferons. EMBO Rep. 2003;4:368–373. doi: 10.1038/sj.embor.embor802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putz E.M., Prchal-Murphy M., Simma O.A., Forster F., Koenig X., Stockinger H., Piekorz R.P., Freissmuth M., Müller M., Sexl V., Zebedin-Brandl E. PI3Kδ is essential for tumor clearance mediated by cytotoxic T lymphocytes. PLoS ONE. 2012;7:e40852. doi: 10.1371/journal.pone.0040852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tayade C., Fang Y., Black G.P., v A P., Jr., Erlebacher A., Croy B.A. Differential transcription of Eomes and T-bet during maturation of mouse uterine natural killer cells. J. Leukoc. Biol. 2005;78:1347–1355. doi: 10.1189/jlb.0305142. [DOI] [PubMed] [Google Scholar]
- Townsend M.J., Weinmann A.S., Matsuda J.L., Salomon R., Farnham P.J., Biron C.A., Gapin L., Glimcher L.H. T-bet regulates the terminal maturation and homeostasis of NK and Valpha14i NKT cells. Immunity. 2004;20:477–494. doi: 10.1016/s1074-7613(04)00076-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Primer pairs used in this paper are from Putz et al., 2012; Sadzak et al., 2008; Tayade et al., 2005; Mizutani et al., 2012; Pilz et al., 2003; Kamezaki et al., 2004; Gratz et al., 2008; and Alcaide et al., 2007.