Abstract
Chronic immune activation is a major complication of antiretroviral therapy (ART) for HIV infection and can cause a devastating immune reconstitution inflammatory syndrome (IRIS) in the brain. The mechanism of T-cell activation in this population is not well understood. We found HIV-Tat protein and IL-17–expressing mononuclear cells in the brain of an individual with IRIS. Tat was also present in the CSF of individuals virologically controlled on ART. Hence we examined if Tat protein could directly activate T cells. Tat transcriptionally dysregulated 94 genes and induced secretion of 11 cytokines particularly activation of IL-17 signaling pathways supporting the development of a proinflammatory state. Tat increased IL-17 transcription and secretion in T cells. Tat entered the T cells rapidly by clathrin-mediated endocytosis and localized to both the cytoplasm and the nucleus. Tat activated T cells through a nonclassical pathway dependent upon vascular endothelial growth factor receptor-2 and downstream secondary signaling pathways but independent of the T-cell receptor. However, Tat stimulation of T cells did not induce T-cell proliferation but increased viral infectivity. This study demonstrates Tat’s role as a virulence factor, by driving T-cell activation and contributing to IRIS pathophysiology. This supports the necessity of an anti-Tat therapy in conjunction with ART and identifies multiple targetable pathways to prevent Tat-mediated T-cell activation.
Keywords: CNS inflammation, chronic inflammation, lymphocyte, central nervous system, chromatin
Antiretroviral therapy (ART) has transformed HIV infection from a fatal illness to a chronic condition by controlling viral replication. However, despite adequate viral control, chronic inflammation persists in ART-treated individuals with 20–35% developing a severe inflammation resulting in immune reconstitution inflammatory syndrome (IRIS) (1, 2). IRIS is the most recognizable form of HIV-associated inflammation and, if it involves the central nervous system (CNS), it may result in death or permanent disability (3).
Although HIV associated inflammation is well recognized in the HIV managed population, the origin of T-cell activation in individuals treated with ART is unclear. Possibilities suggested include response to opportunistic infections (OI) (1), residual HIV (4), autoantigens (5), or activation by translocation of lipopolysaccharide (LPS) (6). However, CNS inflammation with T-cell activation persists in the absence of OI even in virologically well controlled individuals (2), and treatment intensification with CNS penetrating ART has no effect on CNS inflammation indicating that viral replication may not be driving the observed inflammation (7). These data do not eliminate the possibility that HIV proteins produced during ART contribute to the inflammation.
Tat, a viral protein crucial for HIV replication, is produced in infected cells and secreted into the extracellular space. More than 65% of the Tat protein produced in HIV-infected cells is secreted (8) where it enters surrounding cells via clathrin-mediated endocytosis and modulates signaling pathways potentially contributing to immunopathologies (9). Tat binds to several host proteins and receptors, including the vascular endothelial growth factor receptor-2 (VEGFR2) (10). Recent studies have demonstrated that VEGFR2 is expressed on human lymphocytes and that this receptor can induce PI3K/AKT signaling pathways (11). Tat is known to alter lymphocyte signaling, although the underlying mechanism remains elusive.
Here we report the histopathological findings of an individual with CNS-IRIS in the absence of an OI. Tat-expressing infiltrates and IL-17+ cells were detected in the absence of viral replication. Tat was also detected in the cerebrospinal fluid (CSF) of virologically controlled individuals. We categorized gene transcription changes in T cells in response to exogenous Tat and show that Tat induced global changes in histone acetylation and gene expression in uninfected T cells in an antigen-independent manner. Notably, Tat induced the secretion of IL-17 from T cells and increased the percentage of IL-17+ T cells without inducing proliferation. T-cell activation was independent of the T-cell receptor (TCR) but dependent on endocytosis of Tat and activation of VEGFR2. Tat also increased HIV infection in nonreplicating T cells. Thus, Tat-induced T-cell activation may play a critical role in mediating the inflammation associated with HIV infection.
Results
Histopathological Findings in CNS-IRIS.
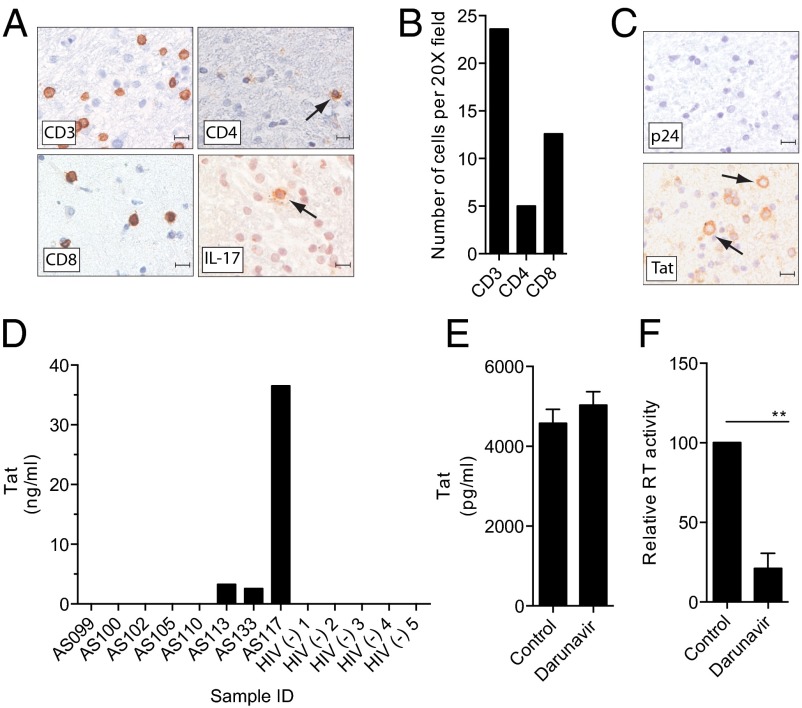
CNS-IRIS in the absence of an OI is rare and poorly characterized (3). However, examination of severe inflammation in the absence of an OI can elucidate key pathophysiological mechanisms. Therefore, we evaluated a brain biopsy from such an individual with CNS-IRIS. Magnetic resonance imaging showed diffuse high signal intensity lesions in the white matter (SI Appendix). The brain biopsy specimen showed mononuclear perivascular and scattered parenchymal inflammatory infiltrates in the white matter (Fig. 1A). The invading cells were primarily CD8+ T cells with occasional IL-17+ T cells (Fig. 1 A and B). Previously, elevated IL-17 levels have been detected in the plasma of patients with IRIS (12, 13). We also observed the presence of Tat in a large fraction of infiltrating mononuclear cells, whereas p24 antigen was undetectable (Fig. 1C). This finding suggested that Tat was produced within the CNS compartment despite successful ART.
Fig. 1.
Detection of Tat in CSF and characterization of immune infiltrates and HIV antigens in CNS-IRIS. (A and B) Immunohistochemical analysis of brain tissue from an individual with CNS-IRIS showed infiltrates of CD3 + T cells which were predominantly CD8+ with few CD4+ cells and occasional IL-17+ cells (arrow). Images shown are 40×. (Scale bar: 10 µm.) (B) Cell counts were performed on three 20× fields. (C) There was no detectable p24 antigen but robust cytoplasmic production of Tat was seen in infiltrating mononuclear cells (arrows). (D) Three of eight CSF samples from HIV-infected individuals controlled on ART were positive for Tat by ELISA. (E and F) Tat was consistently produced by HIV-infected PBMC despite treatment with darunavir (E), whereas viral production was inhibited as measured by product enhanced reverse transcriptase assay (F). Data represent mean ± SD from three independent experiments, *P < 0.05 by paired Student t test.
Detection of Tat in CSF of ART-Treated Individuals.
To determine whether Tat was produced within the CNS during ART, we examined the CSF in a cohort of HIV-infected individuals on ART. Tat was detected in the CSF of three of eight individuals with undetectable (<50 RNA copies per mL) HIV viral load in the blood and CSF and in zero of five CSF samples from HIV negative individuals with pseudotumor cerebri (Fig. 1D). To confirm that Tat was produced during ART, we infected peripheral blood mononuclear cells (PBMCs) with HIVSF162 and treated the cultures with the protease inhibitor, Darunavir. Cells showed an inhibition of viral replication (Fig. 1E) (P < 0.05) but Tat production was not impacted (Fig. 1F). These data provide evidence that Tat is produced and secreted in patients well controlled on ART and thereby may contribute to chronic inflammation even in the absence of viral replication.
Tat Induces Proinflammatory Gene Expression in T Cells.
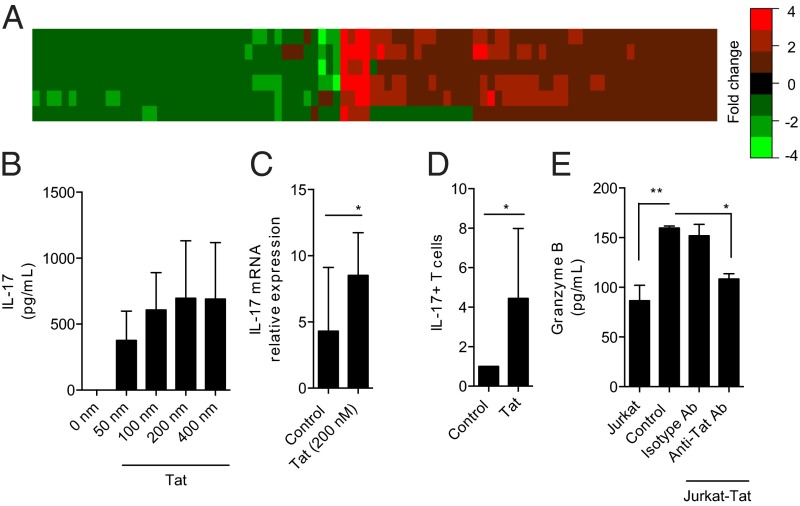
To determine changes in gene transcription induced by extracellular Tat in uninfected cells, expression profiling was performed in human primary T cells exposed to Tat by microarray. One hundred gene fragments, representing 94 known genes, were identified as dysregulated in response to Tat (Fig. 2A and SI Appendix). Up-regulated genes included proinflammatory cytokines such as IL1B and IL8. Furthermore, pathway enrichment analysis showed significant dysregulation of IL-17 signaling (SI Appendix). Importantly, this dysregulation occurred without any changes in IL21 or IL23 (SI Appendix). These results were confirmed by immunoaffinity array. Specifically, higher levels of secreted proinflammatory cytokines from PBMCs were observed post Tat exposure including IL-17 (SI Appendix). Because IL-17 can propagate inflammation (14), we determined whether Tat could induce IL-17 secretion in an antigen-independent manner. Tat induced the secretion of both IL-17 (Fig. 2B) and granzyme B (SI Appendix) in a dose-dependent manner. Tat increased IL-17 transcription (Fig. 2C) and also increased the fraction of IL-17 positive T cells (Fig. 2D) predominantly IL-17+/CD4+ cells (SI Appendix). The specificity of the Tat response in primary human T cells was validated following transwell coculture with a Tat secreting, Jurkat–Tat cell line in the absence or presence of Tat neutralizing antibodies (Fig. 2E).
Fig. 2.
Tat induces proinflammatory gene expression changes and induces a Th17 phenotype. (A) Heat map depicting expression differences between primary T cells exposed to Tat for 24 h versus 0 h for six separate pairs of cells (y axis) for 94 genes (x axis). Data are fold change units, with magnitude and direction described using color. Each of the 94 genes has an absolute difference in means ≥ 1.25-fold and a P < 0.05 by paired Student t test. (B) Treatment of primary human T cells for 72 h with Tat showed a dose-dependent release of IL-17 secretion (n = 3). (C) T cells were treated with Tat for 6 h and IL-17 transcripts were quantified by PCR. *P < 0.05 by paired Student t test (n = 8). (D) IL-17–expressing T cells are increased after Tat treatment, *P < 0.05 by two-way paired Student t test (n = 5). (E) Tat released by Jurkat–Tat cells is capable of activating T cells. Primary T cells were cocultured with Jurkat or Jurkat–Tat cells in transwells for 72 h in the presence or absence of anti-Tat antibody (Ab) or irrelevant isotype Ab. Significantly increased levels of granzyme B were released when cocultured with Jurkat–Tat cells, which was blocked by anti-Tat Ab but not isotype Ab. Data represent mean ± SD from three independent experiments and were analyzed by ANOVA and Bonferroni’s multiple comparison test, *P < 0.05, **P < 0.001.
Mechanism of T-Cell Activation by Tat Is Antigen Independent.
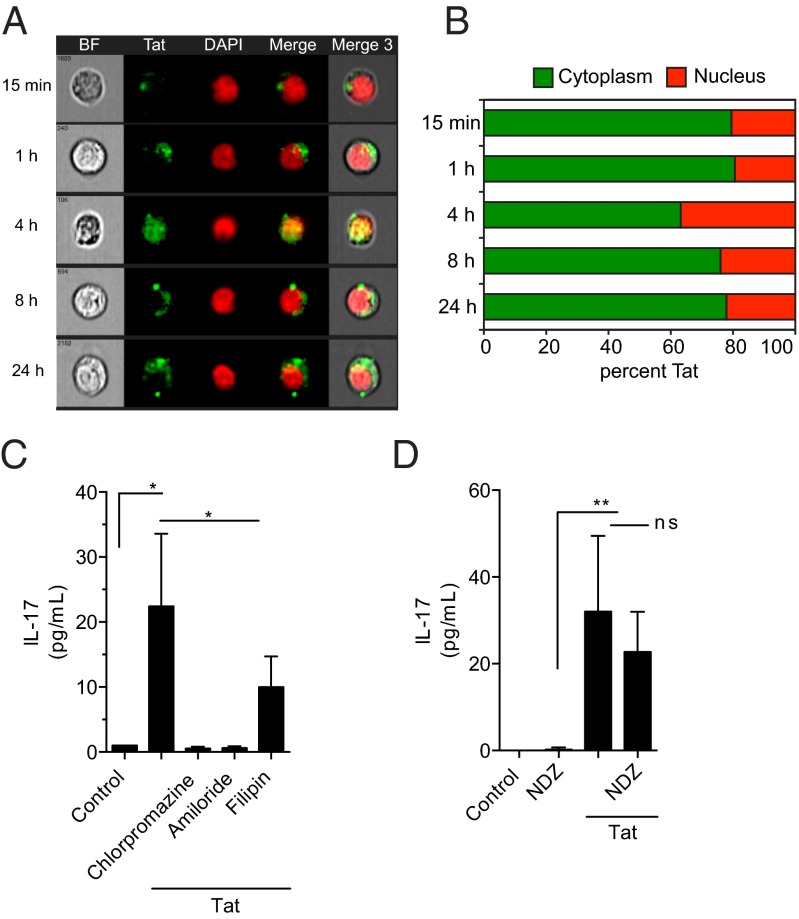
It has been previously established that Tat is taken up by cells through endocytosis (9). To define the kinetics of Tat uptake and its subcellular localization, ImageStream analysis was performed on T cells exposed to fluorescently labeled Tat at different time intervals. Tat was rapidly taken up by cells and diffusely spread throughout the cytoplasm with ∼25% of endocytosed Tat entering the nucleus (Fig. 3 A and B). Inhibition of clathrin-mediated endocytosis by chlorpromazine or amiloride resulted in complete inhibition of Tat induced IL-17 induction, whereas the caveolae inhibitor filipin did not significantly inhibit T-cell activation (Fig. 3C and SI Appendix). To determine whether Tat was driving T-cell activation in an antigen-independent manner, T cells were treated with nocodazole (NDZ) to inhibit Lck phosphorylation and downstream signaling from the TCR. Interestingly, NDZ did not inhibit Tat-mediated T-cell activation, suggesting that no additional antigenic stimulus was required to activate T cells in the presence of Tat (Fig. 3D).
Fig. 3.
T-cell stimulation by Tat is mediated by endocytosis and is independent of antigen recognition. (A) T cells were incubated with Tat-venus (green) for various time periods; nuclei were stained with DAPI (red) and analyzed by ImageStream. Data shown include bright field (BF), Tat-Venus, DAPI, and merged images. One representative image from each time point demonstrates the uptake and localization of Tat. (B) Graph illustrates the percentage of Tat present in the cytoplasm versus the nucleus for each time point. (C) T-cell stimulation by Tat as measured by IL-17 in culture supernatants was completely blocked by chlorpromazine and amiloride but not by filipin. (D) Pretreatment with NDZ to block TCR signaling had no effect on Tat-mediated T-cell stimulation. Data in C and D are mean ± SD from three to five independent experiments compared by ANOVA with Bonferroni’s multiple comparison,*P < 0.05, **P < 0.001; ns, not significant.
Tat-Mediated T-Cell Activation Is Dependent upon VEGFR2.
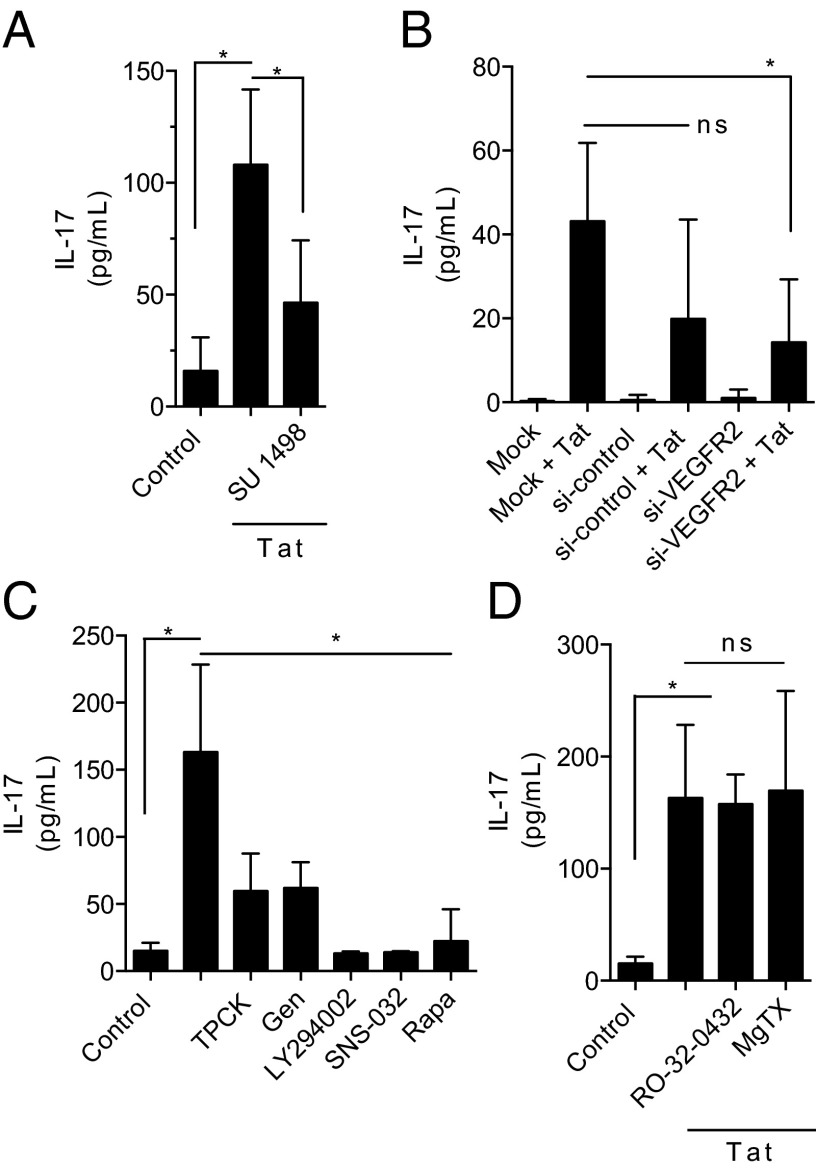
To elucidate the mechanism of TCR-independent T-cell activation by Tat, we determined the subcellular signaling pathway involved. Gene expression analysis and immunoaffinity array showed that IL-8 and IL-1β (Fig. 2A and SI Appendix) were strongly up-regulated by Tat. Both IL-8 and IL-1β activate VEGFR2 (KDR) gene expression (15). Ingenuity analysis of the VEGFR2 pathway showed an up-regulation of NME2 and PIM1, VEGFR2 responsive genes, suggesting that VEGFR2 was functionally active (SI Appendix). VEGFR2 is expressed on T cells constitutively at low levels (16, 17); however the function of VEGFR2 in lymphocytes is not well understood. To investigate the role of VEGFR2 in Tat induced T-cell activation, T cells were treated with the VEGFR2-specific inhibitor SU1498, which dose-dependently inhibited IL-17 production (Fig. 4A and SI Appendix). This inhibition was confirmed by siRNA-mediated knockdown of VEGFR2 (Fig. 4B and SI Appendix). T-cell activation by Tat was dependent on known VEGFR2 downstream signaling pathways including PI3 kinase, NF-κB, CDKs, and mTOR (Fig. 4C). This activation was independent of PKC and Kv1.3 channel (Fig. 4D), supporting VEGFR2 as the primary signaling pathway. None of the inhibitors alone affected T-cell activation or viability (SI Appendix).
Fig. 4.
T-cell activation by Tat is dependent upon VEGFR2 and downstream signals. (A) SU 1498, a VEGFR2 inhibitor, inhibited Tat mediated T-cell stimulation. (B) T cells were transfected without RNA (mock) nonspecific siRNA (si-control) or siRNA to VEGFR2 (si-VEGFR2) for 24 h and then exposed to Tat for 24 h. (C) Pretreatment of T cells with TPCK (NFκB inhibitor), genistein (Gen; TK inhibitor), LY294002 (PI3 kinase inhibitor), SNS-032 (CDK 2, 7, and 9 inhibitor) or with rapamycin (Rapa; mTOR inhibitor) inhibited Tat-mediated T-cell stimulation. (D) Pretreatment of T cells with RO-32-0432 (PKC inhibitor) or margatoxin (MgTX; Kv1.3 inhibitor) had no effect on Tat-mediated T-cell stimulation. Data are mean ± SD from three to five independent experiments compared by ANOVA with Bonferroni’s multiple comparison, *P < 0.05, **P < 0.001; ns, not significant.
Tat Induces Chromatin Remodeling.
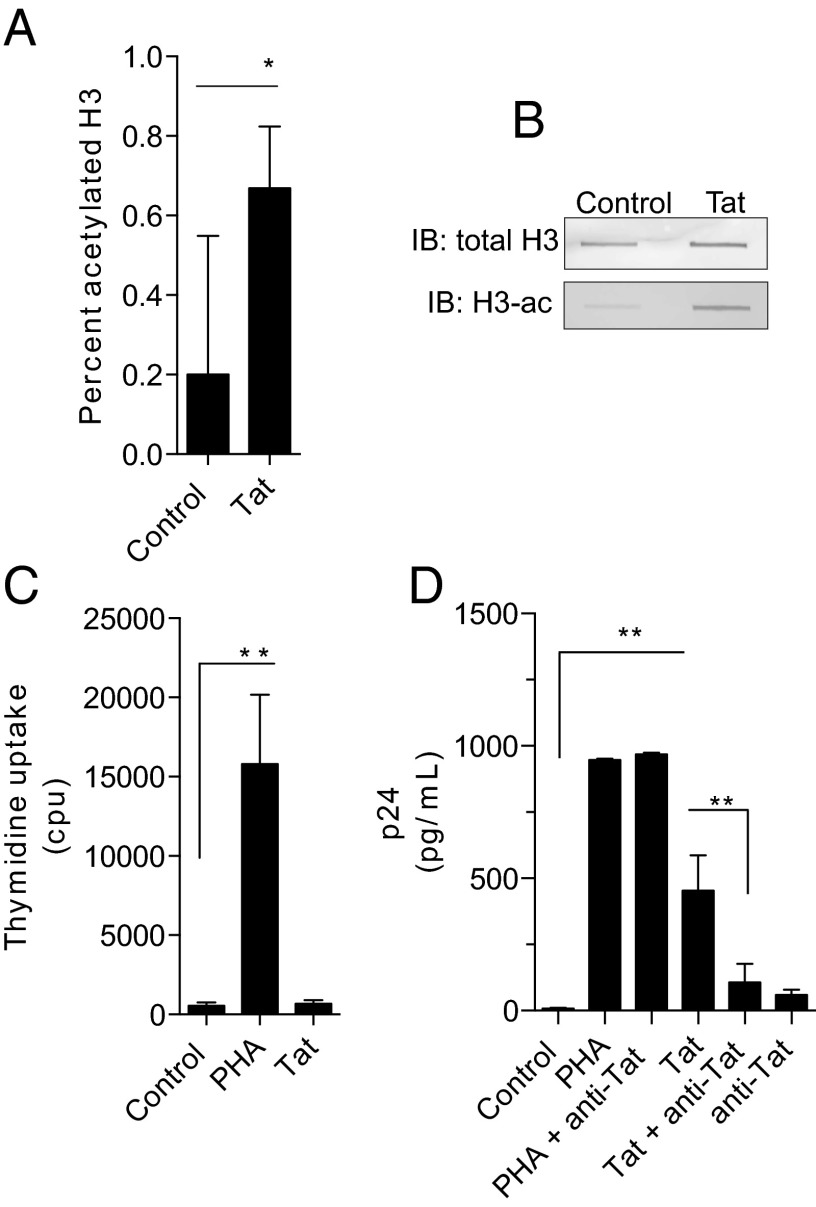
The chromatin of resting T cells is densely packaged and heterochromatic, limiting active transcription (18). To assess the role of Tat in remodeling the epigenetic landscape of T cells, global levels of acetylated histone H3 (H3ac), a marker for euchromatin associated with actively transcribed genes, were measured. A profound increase of global H3ac levels in T cells treated with Tat was observed indicating that Tat changes the global chromatin structure in T cells (Fig. 5 A and B).
Fig. 5.
T-cell stimulation by Tat enhances histone acetylation and viral infection. (A) Tat stimulation of T cells for 24 h increases acetylated histone H3 in nuclear extracts. Data are mean ± SD from three independent experiments compared by two-way paired T test, *P < 0.05. (B) Immunoblots on the same extracts confirm a corresponding increase in acetylated histone H3 (H3-ac) with unchanged total H3 levels. (C) T cells were left unstimulated (control) or treated for 5 d with either Tat or PHA. For the last 18 h, cells were pulsed with [3H]thymidine. No significant (ns) uptake was seen in the Tat-treated cells. Data represent mean ± SD from three independent experiments and were analyzed by ANOVA with Bonferroni’s multiple comparison test, **P < 0.001. (D) T cells were stimulated with PHA or Tat in the presence or absence of anti-Tat antibody and infected with HIV, and p24 antigen was measured in culture supernatants 1 wk later. Tat-stimulated T cells showed increased HIV replication, which was blocked by anti-Tat antibody. Data are mean ± SD from seven independent experiments compared by ANOVA with Bonferroni’s multiple comparison test, **P < 0.001.
Virologic Consequence of Tat Activation of T Cells.
Classically, it has been thought that only activated T cells are capable of being infected (19). We therefore determined the effect of Tat on T-cell proliferation by measuring both thymidine incorporation and carboxyfluorescein succinimidyl ester (Fig. 5C and SI Appendix). Remarkably, no T-cell proliferation was observed. We further investigated whether Tat-induced T-cell activation may allow for enhanced proviral integration in the absence of proliferation.
To investigate this hypothesis, T cells were pretreated with Tat and then infected with HIV. Viral replication was determined by measuring p24 antigen in the supernatant (Fig. 5D). Prior exposure to exogenous Tat strongly increased HIV replication. This effect of Tat was blocked by the addition of neutralizing anti-Tat antibodies, confirming that Tat can increase HIV virulence in an autocrine and paracrine manner.
Discussion
Chronic immune activation in HIV-infected individuals remains a serious complication of ART particularly in the CNS. Following the paradigm of studying extreme manifestations to gain insight into key pathophysiological mechanisms, we investigated CNS-IRIS. CNS-IRIS represents the most fulminate and devastating form of HIV induced inflammation. Here, we provide evidence that the secreted HIV protein Tat is produced within the ART treated population. We show that Tat is detectable in the CSF of a subset of virologically controlled individuals and in the brain of a single individual with CNS-IRIS without an OI. It is worth noting that the constellation of CNS-IRIS in a patient without a confounding OI is very rare. In this individual, IRIS was confirmed by a typical clinical course and presence of T cells in the biopsy without any evidence of an opportunistic infection (20). In the pre-ART era, Tat was detected in the brain of HIV-infected individuals in association with infiltrating macrophages, viral replication, and dementia (21). We now show that Tat is present at reasonably high levels in the CSF and brain in the absence of detectable viral replication. In vitro experiments confirmed that Tat could be produced from HIV-infected cells even when HIV replication was controlled by a protease inhibitor. Currently, protease inhibitors are the only class of drugs available that can inhibit HIV replication once proviral DNA is formed; however, these drugs act downstream of Tat production (22). Characterization of the inflammatory infiltrates in the biopsy tissue showed presence of both CD4+ and CD8+ T cells as well as the presence of IL-17+ T cells. Although the exact role of IL-17 in neuroinflammation remains unclear, T helper (Th) 17 cells interact directly with neurons and can mediate neuronal injury (23). Extrapolation of the role of IL-17 in IRIS must be limited however as these data are from a brain biopsy from one individual. Likewise, further longitudinal CSF studies are needed. Despite these limitations, these clinical data suggest that T-cell activation by Tat may be contributing to chronic inflammation seen in individuals despite adequate treatment with ART (7).
Our in vitro studies support the clinical findings that Tat may be contributing to HIV inflammation and specifically to IL-17 production. Using an unbiased approach, we determined that Tat could drive T cells toward a proinflammatory state by altering gene transcription and protein secretion in T cells. Specifically, Tat drove the production of several cytokines including IL-17 and pathway analysis indicated a significant enrichment of canonical IL-17 signaling. Additionally, we detected an increase in IL-17+ cells after Tat exposure, indicating that this viral protein is capable of driving cells toward a Th17 phenotype in the absence of IL-23 production. A previous study showed that HIV-infected individuals with increased Th17 responses are predisposed to developing IRIS (12). IL-17 is a cytokine produced predominantly by Th17 cells and has been implicated in the pathophysiology of autoimmune diseases. Anti–IL-17 approaches are being developed for treatment of these diseases (24); a similar approach could be considered in treatment of IRIS.
To investigate the mechanism of Tat mediated T-cell activation, we imaged the interactions of fluorescently labeled Tat with T cells. Tat was taken up by these cells rapidly and localized to both the nucleus and the cytoplasm. The intracellular fate of endocytosed Tat is unknown. Further studies to address these questions are necessary to completely understand the effects of exogenous Tat. Tat uptake occurred via clatherin-mediated endocytosis as reported (9), suggesting an alternative mechanism of T-cell activation. We further confirmed that Tat is capable of activating T cells through a mechanism independent of the TCR as Tat-mediated T-cell activation could not be inhibited with NDZ. NDZ inhibits downstream signaling from the TCR; therefore, these data suggest that Tat is capable of activating T cells directly, without additional antigenic stimulus. Antigen-independent T-cell activation has been described and is thought to support the production of nonspecific T cells, contributing to autoimmune processes (25, 26). Further investigation demonstrated that Tat-mediated T-cell activation was dependent upon VEGFR2 and that inhibition of VEGFR2 prevented Tat driven T-cell activation. Inhibition of signaling pathways downstream of VEGFR2 also blocked Tat-mediated T-cell activation. In contrast, blockers of other pathways of T-cell activation, such as PKC and the Kv1.3 channel, did not prevent T-cell activation by Tat. Together, these observations suggest a VEGFR2-dependent pathway of nonclassical T-cell activation which may have clinical relevance in the treatment of IRIS and HIV-associated inflammation. There are several VEGFR inhibitors approved for treatment of cancer (27), which may be useful in preventing Tat-mediated inflammation. The clinical validity of this hypothesis needs to be further evaluated.
To further confirm the activation status of Tat-stimulated T cells, we investigated the chromatin acetylation level of Tat treated T cells. Tat induced marked increase in histone acetylation. Previous studies have examined the role of Tat on chromatin structure in cell lines (28), on specific target genes in infected cells (29), or in uninfected cells (30) and have found both increased and decreased levels of acetylation suggesting the effects of Tat on chromatin structure are cell type and infection status dependent. Our data demonstrate that Tat can induce de novo global chromatin remodeling in T cells in the absence of virus or other stimulation. Although we limited our study to examination of histone H3 acetylation, further studies may examine the effects of Tat on other known chromatin modifications.
Tat-treated T cells did not proliferate yet had enhanced infection with HIV, indicating that Tat rendered nonproliferating T cells permissive to HIV infection. HIV is not capable of infecting quiescent T cells (19). For HIV to integrate into CD4+ T cells, the cells must progress from the G0/1a phase into the G1b phase of the cell cycle, characterized by high levels of RNA synthesis in the absence of DNA synthesis (31, 32). T-cell activation must occur before viral exposure; activation after exposure does not rescue the ability of the virus to establish infection (33). Our data suggest that Tat released from infected cells is capable of activating T cells, and we speculate that Tat is driving cells into the G1b phase of the cell cycle before viral exposure, priming T cells for HIV infection. Through this mechanism, Tat secretion and entry into uninfected T cells is advantageous for the virus, providing evidence that Tat is a virulence factor and not solely a mediator of viral transcription. This finding suggests that activation of T cells to prime cells for infection may be the selection pressure driving the secretion of a viral protein from infected cells where it is required for viral transcription.
Here we show that immune activation associated with HIV infection may be driven by the HIV-Tat protein. Chronic immune activation with HIV infection despite ART has been associated with a number of systemic complications and accelerated aging (reviewed in ref. 34). Currently, there are several pharmacological inhibitors available to control inflammation; however, the use of corticosteroids in patients with prior immune suppression is limited to the most severe CNS-associated episodes. Ideally, the inhibition of nonbeneficial global inflammation should be controlled without impairing the normal restoration and function of the immune system. To this end, this study supports the necessity of an anti-Tat therapy in conjunction with ART. Current ART includes compounds that bind to and inhibit HIV proteins, but this strategy may not work for Tat because Tat is capable of delivering a wide variety of compounds into cells (35, 36). Another approach might be the development of Tat antisense therapy or the use of the recently developed Tat vaccine (37) as a therapeutic vaccine. Thus, the next generation of compounds for treating HIV infection should target the Tat protein to prevent CNS and systemic complications due to the inflammatory response in this population.
Methods
Cell Isolation, Culture, and Stimulation.
T cells were isolated from healthy donors using a Pan T-cell II isolation kit (Miltenyl Biotec) Primary cells, Jurkat E6.1, and Jurkat–Tat cells and were maintained in Iscove’s modified Dulbecco’s media with 10% (vol/vol) human serum supplemented with 1% (vol/vol) antibiotic/antimycotic solution at 37 °C with 5% (vol/vol) CO2. A total of 5 × 105 T cells were seeded challenged with no peptide (negative control), 200 nM Tat peptide, or 1 μg/mL phytohemagglutinin (PHA) (Invitrogen). Endotoxin-free recombinant Tat was produced and functionally characterized as described (38). The following pharmacological inhibitors were used for mapping of pathways: Tosyl phenylalanyl chloromethyl ketone (Sigma, 10 µM), RO-32–0432 (Sigma, 100 nM), Margatoxin (Santa Cruz, 100 nM), Genistein (Sigma, 100 µM), LY294002 (Cell Signaling, 100 µM), SNS-032 (Selleck, 0.5 µM), Rapamycin (Selleck, 5 nM), Nocodazole (Sigma, 5 mg/mL), SU 1498 (Invitrogen, 2 µM), Chlorpromazine (Sigma, 30 µM), Amiloride (Sigma catalog, 500 µM), and Filipin (Sigma, 3 µg/mL).
Microarray.
Sample preparation, hybridization, and CEL file creation were performed using Human GeneChip 1.0 ST Array (Affymetrix) following manufacturer’s guidelines. Conversion of CEL files to normalized gene fragment expression data were accomplished using Affymetrix’s Expression Console with the “RMA Sketch” option. Statistical testing of the data by paired t test was accomplished in R. Gene fragments with a test P value < 0.05 and an absolute difference of means ≥ 1.25 fold were deemed differentially expressed. Subsequent gene fragment annotation, network analysis and pathway enrichment analysis was accomplished using IPA.
Flow Cytometry and Cell Imaging.
T cells treated with Tat (200 nM) or unstimulated were fixed and permeabilized before staining with anti–IL-17–PerCPCy5.5 (Biolegend, 1 μL per 106 cells). Analysis was performed on a BD Biosciences LSR II at the Flow Cytometry Core Facility, Johns Hopkins Medical Institute. Flow cytometry coupled with confocal imaging was performed on the Amnis Image Stream Flow Cytometer at the National Institute of Neurological Disorders and Stroke Flow Cytometry Core Facility, National Institutes of Health. For Tat uptake studies, T cells were treated with 200 nM Tat–Venus.
Protein Arrays.
PBMCs were isolated and 1 × 107 cells were untreated or stimulated with Tat for 24 or 72 h. After incubation, supernatants were analyzed by cytokine array (R&D Systems, Human cytokine array, Panel A). Equivalent amounts of protein were applied to the arrays. The intensity of the signal was visualized and interpreted as present (+) or absent (−).
Coculture Stimulation of T Cells.
A total of 1 × 106 T cells were plated in 500 μL in the bottom of a 24-well transwell plate and pretreated for one hour with either anti-Tat polyclonal antibody (Abcam, Ab43014) or an antibody isotype. A total of 1 × 105 Jurkat or Jurkat–Tat cells were plated in 250 μL in the transwell insert. Cells were incubated for 72 h, and supernatants were assayed for granzyme B.
ELISAs.
Concentration of granzyme B (Cell Sciences), IL-17 A/F kit (Cell Sciences), p24 (ZeptoMetrix Retrotek), histone H3 (Abcam), and acetylated histone H3 (Abcam) were measured using ELISA kits according to manufactures instructions. For quantification of Tat by ELISA monoclonal anti-Tat (1D9, 1:500) was used as the capture antibody. This reagent was obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health: HIV-1 Tat monoclonal antibody (1D9) from Dr. Dag E. Helland (39). The detection antibody was a rabbit polyclonal anti-Tat (Abcam Ab43015, 1:500). Signal was amplified with strepavidin–HRP and developed colorimetrically. The Tat ELISA is capable of detecting Tat from clades A and B (range = 100,000–100 pg/mL; fractioned recovery 74.5%).
Immunoblotting.
To visualize histone H3, acetlated histone H3, VEGFR2 and β-actin, protein from nuclear extracts or cell lysates were immobilized on Immobilon-FL membranes and incubated with primary antibodies (histone H3 and acetyl histone H3, Cell Signaling, 1:1,000; VEGFR2, Abcam, 1 µg/ ml; β-actin, Sigma, 1:5,000). Blots were visualized via chemiluminescence following detection with horseradish peroxidase-conjugated secondary antibodies (Cell Signaling, 1:5,000) on Proteinsimple FlourChem M Imager.
Immunohistochemisty.
Standard immunohistochemical methods with Powervision-HRP secondary antibodies were used to determine the subset of T cells present in paraffin embedded, and to determine the presence of HIV p24 (DAKO, 1:10) and Tat (Abcam, 1:500) in serial sections. Heat-mediated antigen retrieval was performed. Sections from an HIV encephalitis patient served as positive controls and sections from HIV (−) autopsy patients served as a negative control for p24. Sections from Jurkat and Jurkat–Tat cell pellets were used as a positive and negative control for Tat.
Knockdown of VEGFR2.
A total of 2 × 107 T cells were transfected with 100 µM RNA of Silencer Select siRNA to VEGFR2 (Life Technologies) or Silencer Select negative control with Lipofectamine 2000 reagent following manufacturers protocol. Control cells were treated with Lipofectamine reagent in the absence of RNA.
Proliferation Assays.
For the carboxyfluorescein succinimidyl ester (CFSE) assay, T cells were labeled with CFSE using a Molecular Probes Cell Trace kit (Invitrogen). Labeled cells were treated with Tat (200 nM), PHA (1 μg/mL) or left unstimulated for 72 h and then analyzed by flow cytometry at 500 nm excitation and 520 nm emission filters. For the thymidine uptake assay, T cells were isolated and treated with Tat (200 nM), PHA (1 μg/mL) or left unstimulated for 5 d. For the last 18 h, cells were pulsed with [3H]thymidine and thymidine uptake was quantified.
Infection of Primary T Cells.
A total of 2.5 × 106 T cells were pretreated for 72 h with either PHA (1 μg/mL) or Tat (200 nM). Cells were then collected and washed, and 2 × 106 cells were infected with 500 TCID50 of HIV-NL4-3 for 24 h. One week after infection, supernatants were collected and analyzed for Tat and product enhanced reverse transcriptase (40).
Supplementary Material
Acknowledgments
We thank Richa Tyagi, Lee Blosser, Caroline Anderson, Lena Hu, and Abdel Elkahloun for technical assistance; Abhik Ray-Chaudhury for interpretation of histopathological findings; Scott Letendre for providing CSF samples; and Pablo Okhuysen for requesting brain biopsy specimen. We thank Michael C. Haffner for assistance in manuscript preparation and useful discussions. This work was supported by National Institutes of Health Grants R01NS039253, R01NS056884, and F31NS063855 and intramural National Institute of Neurological Disorders and Stroke funds.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
Data deposition: The data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo (accession no. GSE44460).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1308673110/-/DCSupplemental.
References
- 1.Shelburne SA, et al. Incidence and risk factors for immune reconstitution inflammatory syndrome during highly active antiretroviral therapy. AIDS. 2005;19(4):399–406. doi: 10.1097/01.aids.0000161769.06158.8a. [DOI] [PubMed] [Google Scholar]
- 2.Sinclair E, et al. Antiretroviral treatment effect on immune activation reduces cerebrospinal fluid HIV-1 infection. J Acquir Immune Defic Syndr. 2008;47(5):544–552. doi: 10.1097/QAI.0b013e318162754f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson T, Nath A. Immune reconstitution inflammatory syndrome and the central nervous system. Curr Opin Neurol. 2011;24(3):284–290. doi: 10.1097/WCO.0b013e328346be57. [DOI] [PubMed] [Google Scholar]
- 4.Miller RF, et al. Cerebral CD8+ lymphocytosis in HIV-1 infected patients with immune restoration induced by HAART. Acta Neuropathol. 2004;108(1):17–23. doi: 10.1007/s00401-004-0852-0. [DOI] [PubMed] [Google Scholar]
- 5.Berger JR, et al. Relapsing and remitting human immunodeficiency virus-associated leukoencephalomyelopathy. Ann Neurol. 1992;31(1):34–38. doi: 10.1002/ana.410310107. [DOI] [PubMed] [Google Scholar]
- 6.Brenchley JM, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12(12):1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 7.Dahl V, et al. Raltegravir treatment intensification does not alter cerebrospinal fluid HIV-1 infection or immunoactivation in subjects on suppressive therapy. J Infect Dis. 2011;204(12):1936–1945. doi: 10.1093/infdis/jir667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rayne F, et al. Phosphatidylinositol-(4,5)-bisphosphate enables efficient secretion of HIV-1 Tat by infected T cells. EMBO J. 2010;29(8):1348–1362. doi: 10.1038/emboj.2010.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vendeville A, et al. HIV-1 Tat enters T cells using coated pits before translocating from acidified endosomes and eliciting biological responses. Mol Biol Cell. 2004;15(5):2347–2360. doi: 10.1091/mbc.E03-12-0921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Albini A, et al. The angiogenesis induced by HIV-1 tat protein is mediated by the Flk-1/KDR receptor on vascular endothelial cells. Nat Med. 1996;2(12):1371–1375. doi: 10.1038/nm1296-1371. [DOI] [PubMed] [Google Scholar]
- 11.Basu A, et al. Cutting edge: Vascular endothelial growth factor-mediated signaling in human CD45RO+ CD4+ T cells promotes Akt and ERK activation and costimulates IFN-gamma production. J Immunol. 2010;184(2):545–549. doi: 10.4049/jimmunol.0900397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boulware DR, et al. Clinical features and serum biomarkers in HIV immune reconstitution inflammatory syndrome after cryptococcal meningitis: A prospective cohort study. PLoS Med. 2010;7(12):e1000384. doi: 10.1371/journal.pmed.1000384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grant PM, et al. Elevated interleukin 8 and T-helper 1 and T-helper 17 cytokine levels prior to antiretroviral therapy in participants who developed immune reconstitution inflammatory syndrome during ACTG A5164. J Infect Dis. 2012;206(11):1715–1723. doi: 10.1093/infdis/jis604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Langrish CL, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201(2):233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Petreaca ML, Yao M, Liu Y, Defea K, Martins-Green M. Transactivation of vascular endothelial growth factor receptor-2 by interleukin-8 (IL-8/CXCL8) is required for IL-8/CXCL8-induced endothelial permeability. Mol Biol Cell. 2007;18(12):5014–5023. doi: 10.1091/mbc.E07-01-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Edelbauer M, et al. Effect of vascular endothelial growth factor and its receptor KDR on the transendothelial migration and local trafficking of human T cells in vitro and in vivo. Blood. 2010;116(11):1980–1989. doi: 10.1182/blood-2009-11-252460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suzuki H, et al. VEGFR2 is selectively expressed by FOXP3high CD4+ Treg. Eur J Immunol. 2010;40(1):197–203. doi: 10.1002/eji.200939887. [DOI] [PubMed] [Google Scholar]
- 18.Roh TY, Cuddapah S, Zhao K. Active chromatin domains are defined by acetylation islands revealed by genome-wide mapping. Genes Dev. 2005;19(5):542–552. doi: 10.1101/gad.1272505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chou CS, Ramilo O, Vitetta ES. Highly purified CD25- resting T cells cannot be infected de novo with HIV-1. Proc Natl Acad Sci USA. 1997;94(4):1361–1365. doi: 10.1073/pnas.94.4.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Venkataramana A, et al. Immune reconstitution inflammatory syndrome in the CNS of HIV-infected patients. Neurology. 2006;67(3):383–388. doi: 10.1212/01.wnl.0000227922.22293.93. [DOI] [PubMed] [Google Scholar]
- 21.Hudson L, et al. Detection of the human immunodeficiency virus regulatory protein tat in CNS tissues. J Neurovirol. 2000;6(2):145–155. doi: 10.3109/13550280009013158. [DOI] [PubMed] [Google Scholar]
- 22.Arts EJ, Hazuda DJ. HIV-1 antiretroviral drug therapy. Cold Spring Harb Perspect Med. 2012;2(4):a007161. doi: 10.1101/cshperspect.a007161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Siffrin V, et al. In vivo imaging of partially reversible th17 cell-induced neuronal dysfunction in the course of encephalomyelitis. Immunity. 2010;33(3):424–436. doi: 10.1016/j.immuni.2010.08.018. [DOI] [PubMed] [Google Scholar]
- 24.Kim JS, Jordan MS. Diversity of IL-17-producing T lymphocytes. Cell Mol Life Sci. 2012;70(13):2271–2290. doi: 10.1007/s00018-012-1163-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramanathan S, et al. Cytokine synergy in antigen-independent activation and priming of naive CD8+ T lymphocytes. Crit Rev Immunol. 2009;29(3):219–239. doi: 10.1615/critrevimmunol.v29.i3.30. [DOI] [PubMed] [Google Scholar]
- 26.Martino G, et al. Proinflammatory cytokines regulate antigen-independent T-cell activation by two separate calcium-signaling pathways in multiple sclerosis patients. Ann Neurol. 1998;43(3):340–349. doi: 10.1002/ana.410430312. [DOI] [PubMed] [Google Scholar]
- 27.Sonpavde G, et al. Venous thromboembolic events with vascular endothelial growth factor receptor tyrosine kinase inhibitors: A systematic review and meta-analysis of randomized clinical trials. Crit Rev Oncol Hematol. 2013;87(1):80–89. doi: 10.1016/j.critrevonc.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 28.Jarboui MA, et al. Nucleolar protein trafficking in response to HIV-1 Tat: Rewiring the nucleolus. PLoS ONE. 2012;7(11):e48702. doi: 10.1371/journal.pone.0048702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gadad SS, et al. HIV-1 infection induces acetylation of NPM1 that facilitates Tat localization and enhances viral transactivation. J Mol Biol. 2011;410(5):997–1007. doi: 10.1016/j.jmb.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 30.Saiyed ZM, et al. HIV-1 Tat upregulates expression of histone deacetylase-2 (HDAC2) in human neurons: Implication for HIV-associated neurocognitive disorder (HAND) Neurochem Int. 2011;58(6):656–664. doi: 10.1016/j.neuint.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Korin YD, Zack JA. Progression to the G1b phase of the cell cycle is required for completion of human immunodeficiency virus type 1 reverse transcription in T cells. J Virol. 1998;72(4):3161–3168. doi: 10.1128/jvi.72.4.3161-3168.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou Y, Zhang H, Siliciano JD, Siliciano RF. Kinetics of human immunodeficiency virus type 1 decay following entry into resting CD4+ T cells. J Virol. 2005;79(4):2199–2210. doi: 10.1128/JVI.79.4.2199-2210.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vatakis DN, Bristol G, Wilkinson TA, Chow SA, Zack JA. Immediate activation fails to rescue efficient human immunodeficiency virus replication in quiescent CD4+ T cells. J Virol. 2007;81(7):3574–3582. doi: 10.1128/JVI.02569-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deeks SG. Immune dysfunction, inflammation, and accelerated aging in patients on antiretroviral therapy. Top HIV Med. 2009;17(4):118–123. [PubMed] [Google Scholar]
- 35.Jones AT, Sayers EJ. Cell entry of cell penetrating peptides: Tales of tails wagging dogs. J Controlled Release. 2012;161(2):582–591. doi: 10.1016/j.jconrel.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 36.Schmidt N, Mishra A, Lai GH, Wong GC. Arginine-rich cell-penetrating peptides. FEBS Lett. 2010;584(9):1806–1813. doi: 10.1016/j.febslet.2009.11.046. [DOI] [PubMed] [Google Scholar]
- 37.Ensoli B, et al. Therapeutic immunization with HIV-1 Tat reduces immune activation and loss of regulatory T cells and improves immune function in subjects on HAART. PLoS ONE. 2010;5(11):e13540. doi: 10.1371/journal.pone.0013540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hollman AM, et al. Selective isolation and purification of tat protein via affinity membrane separation. Biotechnol Prog. 2005;21(2):451–459. doi: 10.1021/bp049804z. [DOI] [PubMed] [Google Scholar]
- 39.Valvatne H, Szilvay AM, Helland DE. A monoclonal antibody defines a novel HIV type 1 Tat domain involved in trans-cellular trans-activation. AIDS Res Hum Retroviruses. 1996;12(7):611–619. doi: 10.1089/aid.1996.12.611. [DOI] [PubMed] [Google Scholar]
- 40.Vermeire J, et al. Quantification of reverse transcriptase activity by real-time PCR as a fast and accurate method for titration of HIV, lenti- and retroviral vectors. PLoS ONE. 2012;7(12):e50859. doi: 10.1371/journal.pone.0050859. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.