Abstract
Activated leukocyte cell adhesion molecule (ALCAM) has been implicated in tumorigenesis. Our goal was to examine the levels of ALCAM, in addition to the classical breast cancer tumor markers carbohydrate antigen 15-3 (CA15-3) and carcinoembryonic antigen (CEA), in serum by quantitative ELISA for diagnosis in breast cancer patients. The three proteins were measured in serum of 100 healthy women, 50 healthy men and 150 breast carcinoma patients. The diagnostic sensitivity and specificity of the tests were calculated and the association of serum marker concentrations with various clinicopathologic variables was examined using nonparametric Kruskal-Wallis tests. Receiver operating characteristic (ROC) curves were used to evaluate the diagnostic performance of the biomarkers. ALCAM, with area under the curve (AUC) of 0.78 [95% CI: 0.73, 0.84] outperformed CA15-3 (AUC= 0.70 [95% CI: 0.64, 0.76]) and CEA (AUC= 0.63 [95% CI: 0.56, 0.70]). The incremental values of AUC for ALCAM over that for CA15-3 were statistically significant (Delong test, p <0.05). Combining CA15-3 and ALCAM yielded a ROC curve with an AUC of 0.81 (95% CI [0.75, 0.87]). Serum ALCAM appears to be a new biomarker for breast cancer and may have value for disease diagnosis.
Keywords: ALCAM, breast cancer, immunoassay, immunoglobulin superfamily, tumor marker
Introduction
Over one million new breast cancer cases are diagnosed each year1. It is a heterogeneous disease with a wide range of histological, clinical and molecular presentations. Unfortunately, other than definitive diagnosis by biopsy and histopathology, no diagnostic or screening test is presently suitable for early detection of breast cancer2. The ability to detect human malignancy via a simple blood test has long been a major objective in medical screening. Carbohydrate antigen 15-3 (CA15-3) and carcinoembryonic antigen (CEA), discovered more than two and four decades ago, respectively, are the most commonly used tumor markers for breast cancer3-5. CA15-3 and CEA levels in serum are recommended for monitoring therapy of advanced breast cancer2. However, these cancer biomarkers have proven to be ineffective in detecting the early stages of the disease due to low diagnostic sensitivity and specificity6-8. Clearly, new tumor markers are urgently needed for screening, diagnosis, prognosis or selection of therapy of breast cancer patients.
We have previously performed an extensive proteomic analysis of tissue culture supernatants from three different breast cancer cell lines9. Over 1,000 proteins were identified in our proteomic analysis. Based on literature searches, from the top 100 differentially expressed proteins (as defined by spectral counting), 46 of the molecules had been previously examined as a serological breast cancer marker. The remaining proteins were scrutinized to select proteins for further investigation that was found only in the conditioned media of breast cancer cell lines and absent in MCF-10A (semi-normal breast epithelial cell line). This filtering criterion resulted in 30 candidates for further analysis. Of these 30 candidates, 11 of them had reagents available to develop an immunoassay to measure the levels of these proteins in biological fluids. Analyzing the serum of healthy individuals and patients with breast cancer resulted in one promising candidate molecule: activated leukocyte cell adhesion molecule (ALCAM) (Supplementary Figure 1).
Cell adhesion molecules are cell surface receptors that mediate cell-cell and cell-substrate interactions. These molecules can be grouped into four families: integrins, cadherins, selectins and the immunoglobulin superfamily (Ig-SF)10. Alterations in cellular adhesion and communication can contribute to uncontrolled cell growth11. In this respect, ALCAM is a type 1 transmembrane glycoprotein of the Ig-SF12. The molecular weight of ALCAM is 65kDa but with N-glycosylation at 8 putative sites, the mature ALCAM molecule has a molecular weight of 110kDa13. Five extracellular Ig domains, a transmembrane region and a short cytoplasmic tail make up the ALCAM protein that resembles E-cadherin in motif-arrangement12. ALCAM mediates both heterophilic (ALCAM-CD6) and homophilic (ALCAM-ALCAM) cell-cell interactions14. The extracellular structures of ALCAM provide two structurally and functionally distinguishable modules, one involved in ligand binding (to CD6)15 and the other in avidity14, 16. Physiologically, ALCAM is expressed in activated leukocytes and neural, epithelial and hematopoietic progenitor cells17. Functionally, ALCAM may act as a cell surface sensor to register local growth saturation and to regulate cellular signaling and dynamic responses18. ALCAM-CD6 interaction is required for optimal activation of T-cells suggesting a possible ALCAM involvement in the immunologic response to tumor cells19. ALCAM may favor interactions between tumor and endothelial cells18.
The aim of our study was to investigate if ALCAM, either alone, or in combination with the classical breast cancer biomarkers (CA15-3 and CEA) represent a new strategy for breast cancer diagnosis with high sensitivity and specificity in serum, using quantitative methodologies. The association between serum marker concentrations with various clinicopathologic parameters was also examined.
Materials and Methods
Patients and Specimens
The clinical material used consisted of 150 serum samples from breast cancer patients (ages 34 to 82 years; median, 62 years), 100 serum samples from normal, apparently healthy women (ages 24 to 56 years; median, 40 years), and as an additional control, 50 serum samples from normal healthy men (ages 23 to 61 years; median, 48 years). Serum samples from healthy subjects were obtained through the Preventive Medical Unit at Venice General Hospital. The subjects were asked to donate blood samples for studies on tumor biomarkers and then asked to fill out a complete patient form. Any subjects that had a history of any serious or chronic illnesses or neoplasms were eliminated from the sample collection. The breast carcinoma serum samples were obtained on the morning prior to surgery and prior to any treatment. Peripheral blood samples were drawn by venopuncture after overnight fasting between 8 and 8:30 AM. Serum was collected in a Becton Dickinson vacutainer, code n°367896, containing a coagulation coactivator and kept at ambient temperature (20-25°C) before centrifugation. They were centrifuged at 1500g at ambient temperature (20-25°C) after 45 minutes and within 2 hours were aliquoted and snap frozen in liquid nitrogen and immediately stored at -80°C. Of the 150 breast carcinoma patients, 32 were stage 1, 57 were stage 2A or 2B, 27 were stage 3A or 3B and stage information was not available for the remaining 34. Supplementary Table 1 contains detailed information on the 150 breast cancer patient samples including their histology, grading (Elston-Ellis), diameter (cm), pT, pTNM, stage and age. Clinical grades 1, 2 and 3, corresponding to 26, 62 and 56 patients, respectively, were included in this study. The clinical characteristics of the breast cancer patients are described later. Serum samples, obtained from Venice, Italy, from all patients were stored at -80°C until further analysis. Our protocols have been approved by the review boards of the participating institutions.
Measurement of ALCAM, CA15-3 and CEA in serum
The concentration of ALCAM in serum was measured by using a highly sensitive and specific non-competitive “sandwich-type” ELISA, developed in our laboratory. The assay is based on mouse monoclonal antibody capture and biotinylated mouse monoclonal detection antibody (both obtained from R&D Systems, Minneapolis, MN). The assay has a detection limit of 0.05μg/L and a dynamic range of up to 10μg/L. Precision was less than 10% within the measurement range. Serum samples were analyzed in triplicate with inclusion of two quality control samples in every run. In addition, CA15-3 and CEA were measured using a commercially available automated ELISA kit (Elecsys CA15-3 and CEA Immunoassay, respectively; Roche Diagnostics, Indianapolis, IN). The upper limit of normal for CA15-3 for this method is 30 U/mL and for CEA is 5 ng/mL.
Data analysis and statistics
The relationships between biomarkers and patient and tumor characteristics were examined with the Kruskal-Wallis test, a nonparametric method for examining differences among multiple groups. Spearman's rank correlation coefficient was used to assess the correlations among biomarkers. Logistic regression was performed to calculate the odds ratio (OR) that defines the relation between biomarkers and case or control status. OR were calculated on log-transformed biomarkers and were represented with their 95% confidence interval (95% CI) and two-sided p-values.
To further evaluate the diagnostic usefulness of the markers for dichotomous classification, we considered receiver operating characteristic (ROC) curve analysis. For each ROC curve, we calculated the area under the curve (AUC). Bootstrap method was used to calculate the confidence intervals for AUC. The ROC analysis was first conducted on individual markers and then in combination, to explore the potential that a marker panel can lead to improved performance. We considered an algorithm that renders a single composite score using the linear predictor fitted from a binary regression model. This algorithm has been justified to be optimal under the linearity assumption20 in the sense that ROC curve is maximized (i.e., best sensitivity) at every threshold value. Since an independent validation series was not available for this study, the predictive accuracy of the composite scores was evaluated based on re-sampling of the original data. All analyses were performed using Splus 8.0 software (Insightful Corp., Seattle WA).
Results
ALCAM ELISA Assay Development
To ensure that the immunoassay was suitable for measuring clinical serum samples, the recovery, reproducibility, linearity, cross-reactivity and serum sample stability were examined. Recombinant human ALCAM protein was added into the general diluent (control), normal serum (male and female) and into serum of breast cancer patients at different concentrations, and measured with the ALCAM immunoassay. A recovery of 90-100% was observed in these samples. The assay also showed negligible cross-reactivity to another adhesion molecule of the Ig-SF, B-cell adhesion molecule14, displayed excellent linearity with serial dilutions and showed < 10% CV for intra- and inter-assay variability studies (Supplementary Figure 2). Finally, the design of the stability study consisted of collecting serum at different time points (2 weeks, 4 weeks and fresh samples) and storing them at 4°C, −20°C and −80°C. ALCAM levels were measured in these samples using the immunoassay. No difference was observed among the samples stored at the different temperature conditions and among the different time point collections, compared to the freshly obtained samples.
Association of Biomarkers with Age
Since cases and controls were not matched for age, we first explored if marker values differed by age. The comparisons between cases and controls were based on data from females only. While no change with age was observed for CA15-3 concentrations, the level of CEA appeared to increase with age for both cases and controls. With respect to ALCAM, there was a trend for marker level to increase with age for cases but not for controls (See Supplementary Figure 3).
Correlations Among Biomarkers
Spearman's rank correlation coefficients were used to assess the correlations among markers for female controls and cases, respectively, and the results are listed in Table 1. CEA appeared to be weakly correlated with ALCAM in both cases (Spearman r = 0.371, p < 0.001) and controls (Spearman r = 0.348, p = 0.001), whereas CA15-3 was weakly correlated with ALCAM among cases only (Spearman r = 0.2, p = 0.015).
Table 1. Spearman's rank correlation coefficients among 3 markers for female controls and cases.
| Female Controls | Cases | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| CA15-3 | CEA | ALCAM | CA15-3 | CEA | ALCAM | |
| CA15-3 | 1 | 1 | ||||
| CEA | -0.091 | 1 | 0.161* | 1 | ||
| ALCAM | 0.082 | 0.348* | 1 | 0.2* | 0.371* | 1 |
p < 0.05
Association of Biomarkers with Tumor Characteristics for Cases
The association of ALCAM, CA15-3 and CEA with patient and tumor characteristics such as age, tumor diameter, ER and PgR status, grade, histology, ratio of lymph node positive (lpos) and total lymph nodes (ltot), menopausal status, and stage were examined. A significant association was obtained for the following clinicopathologic variables: age (<=50, 51-60, 61-70 and >70), menopausal status (pre- and post-menopausal), and stage (I, II, III). The distributions of each marker in cases for these variables are given in Table 2. Post-menopausal women displayed higher values of CEA and ALCAM (all p < 0.001). As well, levels of ALCAM were not significantly associated with stage whereas CEA and CA15-3 were. Finally, while a statistically significant p-value was not obtained for an association between ALCAM values and tumor grade, a general trend was observed with elevated ALCAM levels corresponding to increased tumor grade (Supplementary Figure 4).
Table 2. Marker distributions by tumor characteristics for cases.
| # Of Patients | CA15-3 Median | Q31* | CEA Median | Q31* | ALCAM Median | Q31* | |
|---|---|---|---|---|---|---|---|
| Age | |||||||
| <=50 | 36 | 20.36 | 5.11 | 1.59 | 0.59 | 66.00 | 7.00 |
| 51-60 | 34 | 21.78 | 6.06 | 1.82 | 0.99 | 77.00 | 11.50 |
| 61-70 | 40 | 18.90 | 4.34 | 2.02 | 0.75 | 75.00 | 9.00 |
| 70+ | 40 | 22.92 | 6.12 | 2.62 | 0.96 | 82.00 | 8.25 |
| p value** | 0.31 | 0.01 | <0.001 | ||||
| Menopausal status | |||||||
| pre | 30 | 21.30 | 5.35 | 1.03 | 0.56 | 66.00 | 6.00 |
| post | 103 | 20.61 | 6.06 | 2.14 | 0.98 | 78.00 | 9.50 |
| p value** | 0.92 | <0.001 | <0.001 | ||||
| Stage | |||||||
| I | 32 | 17.20 | 5.93 | 1.48 | 0.61 | 72.00 | 11.25 |
| II | 57 | 19.46 | 4.66 | 1.75 | 0.86 | 74.00 | 8.00 |
| III | 27 | 23.40 | 10.32 | 2.47 | 1.10 | 72.00 | 11.50 |
| p value** | 0.003 | 0.004 | 0.88 | ||||
| ER | |||||||
| negative | 21.34 | 5.36 | 1.70 | 1.07 | 72.00 | 8.75 | |
| positive | 21.48 | 6.20 | 2.00 | 0.84 | 76.00 | 9.50 | |
| p value | 0.55 | 0.4 | 0.64 | ||||
| PgR | |||||||
| Negative 1 | 9 | 21.45 | 8.12 | 1.67 | 1.08 | 84.00 | 8.50 |
| positive 1 | 31 | 21.46 | 5.43 | 1.97 | 0.86 | 74.00 | 9.50 |
| p value | 0.27 | 0.72 | 0.09 |
Q31, semi-interquartile range: computed as one half the difference between the 75th percentile (Q3) and the 25th percentile (Q1)
p value: computed from global nonparametric Kruskal-Wallis test for testing the association between a marker and a clinical variable
Association of Biomarkers with Breast Cancer
The distributions of the 3 markers, as measured by immunoassays, in cases and controls, are shown in Figure 1. Distributions of the patients with breast cancer differed from controls (female or male) for ALCAM, but to a lesser degree for the other two markers. The median values of males and females were similar for all 3 markers. When comparing the ALCAM values between normal women (n = 100) and patients with breast cancer (n = 150) by the non-parametric Mann Whitney test (two-tailed), the medians were significantly different (median normals = 60 μg/L; median cancer = 74 μg/L; P<0.0001). For CA15-3, the medians were significantly different (median normals = 15 units/mL; median cancer = 21 units/mL; P<0.0001). Lastly for CEA, the medians were different (median normals = 1.3 μg/L; median cancer = 1.9 μg/L; P = 0.0003). The association of the markers with cancer was further considered with linear regression models of logarithm-transformed marker values as a function of clinical status (cancer vs non-cancer; females only) and age. Adjusting for age, the mean levels of log(CA15-3) and log(ALCAM) were significantly higher in cancer; levels of log(CEA) did not differ between cancer and controls.
Figure 1.
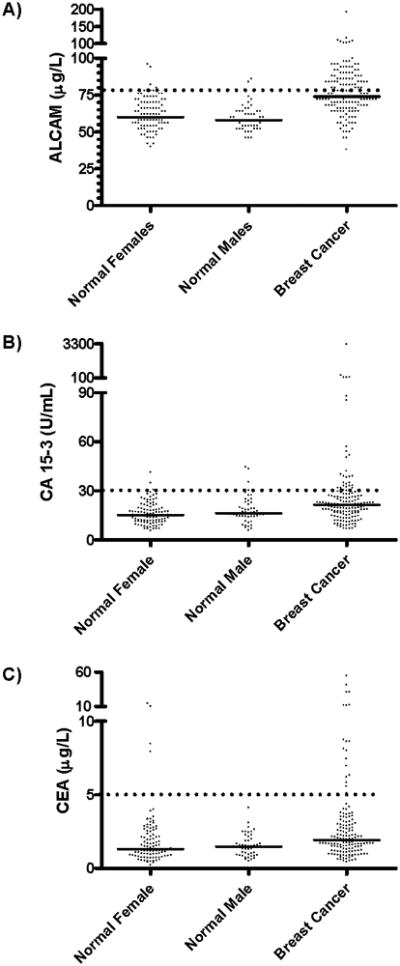
Distribution of markers A) ALCAM, B) CA15-3 and C) CEA in the three groups (normal female, normal male and breast carcinoma) examined by an immunoassay specific to the molecule. The solid horizontal line indicates the median value for each of the groups. The dotted horizontal line indicates the cut-off values to discriminate cancer from control subjects A) ALCAM: 78 μg/L, 95% specificity cut-off; B) CA15-3: 30 U/mL and C) CEA: 5 ng/mL.
We also considered logistic regression models to further characterize the associations between markers and breast cancer, adjusting for age. Similar to the results from linear regression, we found that two individual markers, CA15-3 (OR=1.12, 95% CI [1.04,1.19]) and ALCAM (OR=1.42, 95% CI [1.14,1.77]) univariately predicted breast cancer, but this was not the case for CEA (OR=0.99, 95% CI [0.95,1.05]). In a logistic regression model, which included age and all three markers, we found that CA15-3 and ALCAM independently predicted breast cancer. Results from the logistic regression models are given in Table 3.
Table 3. Results from logistic regression models.
| Univariate * | Multivariate** | |||
|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | |
| Marker | ||||
| CA15-3 | 1.12 | (1.04,1.19) | 1.09 | (1.02,1.18) |
| CEA | 0.99 | (0.95,1.05) | 0.94 | (0.89,1.00) |
| ALCAM | 1.42 | (1.14,1.77) | 1.39 | (1.09,1.78) |
logistic model with logarithm of the marker and age as predictors.
logistic model with logarithm of all three markers and age as predictors.
OR, odds ratio; CI, confidence interval.
The Diagnostic Values of the Three Markers
ROC curve analysis (Figure 2) was used to quantify the diagnostic value of the three markers. All three markers have AUC significantly better than 0.5, with ALCAM having the best performance (AUC=0.78, 95% CI [0.73,0.84]). The superiority of ALCAM over the other two markers was also evident when we considered sensitivities at fixed values of 90% and 80% specificities, respectively (Table 4). For example, at specificity of 80%, ALCAM yielded a sensitivity of 60%, compared with 48% for CA15-3. Likewise, at 90% specificity, ALCAM displayed higher sensitivity than CA15-3 and CEA. Combining CA15-3 and ALCAM, based on the linear predictors from a logistic regression model, yielded a ROC curve with an AUC of 0.81 (bootstrap 95% CI [0.75, 0.87]). Combining CA15-3, ALCAM and CEA did not result in any improvement in ROC curves compared to CA15-3 and ALCAM. Re-sampling methods which aimed to adjust for over-fitting21 did not yield substantially different results.
Figure 2.
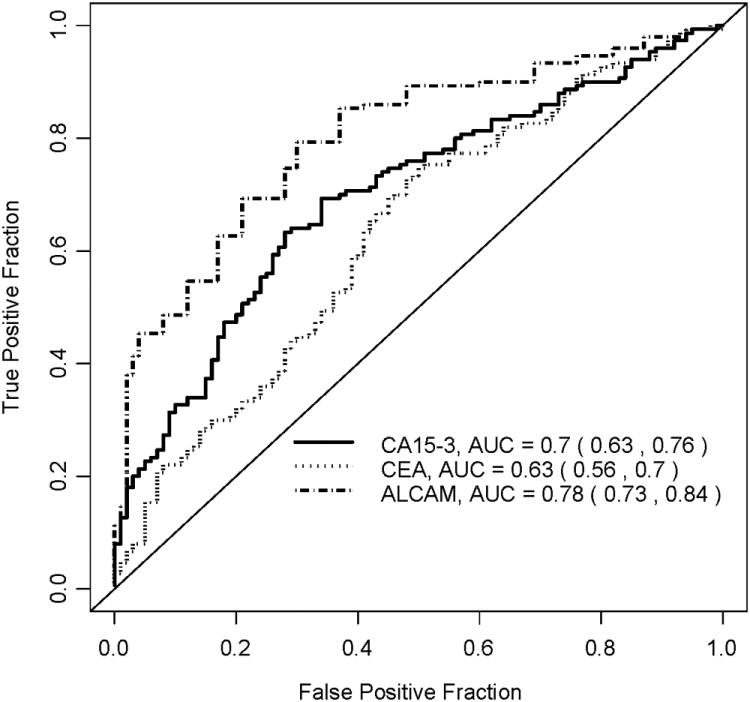
ROC curves for the three markers (CA15-3, CEA, ALCAM).
Table 4. ROC analysis for biomarkers.
| Sensitivity | ||||||
|---|---|---|---|---|---|---|
|
|
||||||
| AUC | 95% CI | 90% Specificity | 95% CI | 80% Specificity | 95% CI | |
| Marker | ||||||
| CA15-3 | 0.70 | (0.64,0.76) | 0.32 | (0.19,0.44) | 0.48 | (0.32,0.63) |
| CEA | 0.63 | (0.56,0.70) | 0.22 | (0.12,0.31) | 0.32 | (0.22,0.41) |
| ALCAM | 0.78 | (0.73,0.84) | 0.47 | (0.38,0.57) | 0.60 | (0.48,0.73) |
| Combined* | 0.81 | (0.75,0.87) | 0.52 | (0.39,0.64) | 0.67 | (0.54,0.80) |
Combined: linear combination of CA15-3 and ALCAM
Discussion
Most primary cancers show loss of expression of adhesion molecules to allow for a critical step in metastasis to occur: detachment of the invading cell from its neighbors 22, 23. However, a number of potential reasons exist for observing elevated levels of adhesion molecules such as ALCAM in cancer patients versus normal individuals. First, increased homotypic intercellular adhesion (due to elevated levels of these molecules) may favor the metastatic process since cell aggregates, rather than single cells breaking away from the primary tumor, have a greater chance of survival in the circulation and of lodging in other organs24. Second, it is known that cell adhesion is necessary for the metastatic spread of cancer cells to new organs (secondary tumor establishment)23. As well, overproduction of adhesion molecules may disrupt the normally operative intercellular adhesion forces, allowing more cell movement and the adoption of a less ordered tissue architecture25. An illustration of this is CEA, a member of the Ig-SF that is expressed in a variety of secretory tissues26, 27. Interestingly, expression of CEA is increased in colon carcinomas and it may be important to processes of intercellular recognition28, 29. It has been suggested that this might either result in disturbance of normal intercellular adhesion or provide advantages in further steps of metastasis25 such as conceivably facilitating establishment of a secondary tumor23, 30.
ALCAM expression has been explored in a number of different tumor types displaying a clear upregulation in some tumors and downregulation in others. In addition, variable levels of ALCAM expression have been found at different stages of tumor development in the same type of malignancies. In melanoma, ALCAM has been suggested to exhibit a role in cell invasion and neoplastic progression31. In prostate carcinoma, ALCAM gene was found upregulated in high Gleason grade prostate cancers compared to benign prostatic hyperplasia cases32. However, one study observed an upregulation of ALCAM in low-grade tumors 33. Yet, another study on prostate cancer found ALCAM to predict prostate-specific antigen (PSA) relapse17. In colon cancer, using IHC, no significant correlation with patient age, tumor grade, stage or nodal status and ALCAM expression was observed, but membranous ALCAM expression correlated significantly with shortened patient survival34.
There have been a few studies investigating ALCAM expression in breast cancer. Low levels of ALCAM mRNA correlated with nodal involvement, high grade and worse prognosis35, 36. At the protein level, laser scanning cytometry and confocal microscopy showed that high levels of ALCAM correlated with small tumor diameter, low grade and the presence of hormone receptors, which supported the view that this adhesion molecule is a tumor suppressor with prognostic significance 12. However, an IHC analysis showed that high cytoplasmic ALCAM expression was associated with shortened patient disease-free survival37. Yet a further study found that ALCAM-ALCAM interactions between breast cancer cells were important for survival in the primary tumor and that a loss of ALCAM was associated with programmed cell death38. Finally, Ihnen et al discovered that patients with high ALCAM mRNA expression who did not receive chemotherapy tended to have a worse prognosis, suggesting that high ALCAM expression levels may be a marker for prediction of the response to adjuvant chemotherapy in breast cancer39. Indeed, the discordant data between RNA and protein levels of ALCAM in breast cancer and even discordance among different protein expression studies suggest the need for additional research to evaluate the role of ALCAM in breast cancer.
The finding of decreased levels of ALCAM in breast cancer tissue compared to normal breast tissue is not contradictory to our results of elevated levels of ALCAM in serum of breast cancer patients. It is possible that ALCAM levels decrease in tissue but is elevated in serum. For example, although PSA gene transcription is down-regulated in prostate cancer, PSA protein levels in the circulation of prostate cancer patients increase due to disruption of the anatomic barriers between the glandular lumen and capillaries. In addition, our data of increased ALCAM levels in cancer versus healthy controls is in line with data about ALCAM expression in prostate and colon cancer and melanoma. It should also be noted that we are the first to report presence of ALCAM in serum of breast cancer patients. Until now, all studies regarding ALCAM expression have been performed at the transcript level or using IHC or confocal microscopy. In this study, we developed a robust and highly sensitivity immunoassay to measure ALCAM in biological fluids.
It is generally agreed upon that no single cancer biomarker will provide all necessary information for optimal cancer diagnosis. The current trend is to focus on the identification of multiple biomarkers that can be used in combination. The present data provides evidence that serum ALCAM represents a novel biomarker for breast cancer. This biomarker displays higher diagnostic sensitivity for breast cancer than the currently used tumor markers CA15-3 and CEA (Table 4). Moreover, among the 120/150 cancer patients examined who displayed normal levels of CA15-3 (< 30U/mL), 48 of them (40%) had elevated levels of ALCAM (values of 78 μg/L or greater; the cut-off for 95% specificity). For this reason, CA15-3 measurements will benefit from combining ALCAM measurements, to increase the diagnostic sensitivity of each of the markers alone. As well, assuming a 95% specificity, the statistical power of our study (n = >100 for both control and cases) will allow the detection of a 20% difference between mean values of ALCAM levels in breast cancer patients and controls. The difference between the ALCAM means in this study was >20%, within the power of our study. In addition, a correlation of elevated ALCAM levels with increasing age was observed in breast carcinoma patients (Table 2). However, there was no correlation between ALCAM levels and age of normal women (data not shown). This suggests that the difference in age between cases and controls is not a confounding factor in this study.
In conclusion, we show evidence that serum ALCAM concentration represents a novel biomarker for breast carcinoma, which has potential utility as a diagnostic tool. The combination of ALCAM with CA15-3 improved the diagnostic sensitivity. It is important to note that both CA 15-3 and CEA levels in serum are related to tumour size and nodal involvement and are recommended by international bodies such as ASCO (American Society of Clinical Oncology) for monitoring therapy of advanced breast cancer; it is not recommended as a diagnostic marker for breast cancer. However, when evaluating the potential of a new marker such as the one presented in this study for breast cancer, a benchmark molecule is needed for comparison. Since the only markers available for breast cancer were CA 15-3 and CEA, both markers were used to compare the diagnostic discriminatory ability. Moreover, the availability of a reliable immunoassay, such as the one developed in this study, for measuring serum ALCAM may facilitate further studies to establish the clinical usefulness of this marker in breast cancer. For example, examining the levels of ALCAM in other serum samples such as those obtained from patients pre- and post-surgery as well as serial serum samples collected from patients undergoing therapy may be beneficial in evaluating the biomarker potential of ALCAM as a prognostic or predictive marker of therapy. Further validation studies that integrate ALCAM with imaging modalities, namely mammography, may reveal potential clinical utility of ALCAM for breast cancer.
Supplementary Material
Acknowledgments
This work was supported by a collaborative research and development grant from the Natural Sciences and Engineering Research Council of Canada and Proteomic Methods Inc., and from a grant from the Ontario Institute for Cancer Research. In addition, this work was partly supported by ABO Project 2001, Italy.
Non-Standard Abbreviations
- ALCAM
activated leukocyte cell adhesion molecule
- AUC
area under the curve
- CI
confidence interval
- CV
coefficient of variation
- ELISA
Enzyme-linked immunosorbent assay
- ER
estrogen receptor
- Ig-SF
immunoglobulin superfamily
- OR
odds ratio
- PgR
progesterone receptor
- PSA
prostate-specific antigen
- ROC
receiver operating characteristic
References
- 1.Parkin DM, Pisani P, Ferlay J. Estimates of the worldwide incidence of 25 major cancers in 1990. Int J Cancer. 1999;80(6):827–841. doi: 10.1002/(sici)1097-0215(19990315)80:6<827::aid-ijc6>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 2.Harris L, Fritsche H, Mennel R, Norton L, Ravdin P, Taube S, Somerfield MR, Hayes DF, Bast RC., Jr American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol. 2007;25(33):5287–5312. doi: 10.1200/JCO.2007.14.2364. [DOI] [PubMed] [Google Scholar]
- 3.Kufe D, Inghirami G, Abe M, Hayes D, Justi-Wheeler H, Schlom J. Differential reactivity of a novel monoclonal antibody (DF3) with human malignant versus benign breast tumors. Hybridoma. 1984;3(3):223–232. doi: 10.1089/hyb.1984.3.223. [DOI] [PubMed] [Google Scholar]
- 4.Gold P, Freedman SO. Specific carcinoembryonic antigens of the human digestive system. J Exp Med. 1965;122(3):467–481. doi: 10.1084/jem.122.3.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hilkens J, Buijs F, Hilgers J, Hageman P, Calafat J, Sonnenberg A, van der V. Monoclonal antibodies against human milk-fat globule membranes detecting differentiation antigens of the mammary gland and its tumors. Int J Cancer. 1984;34(2):197–206. doi: 10.1002/ijc.2910340210. [DOI] [PubMed] [Google Scholar]
- 6.Lumachi F, Basso SM. Serum tumor markers in patients with breast cancer. Expert Rev Anticancer Ther. 2004;4(5):921–931. doi: 10.1586/14737140.4.5.921. [DOI] [PubMed] [Google Scholar]
- 7.Khatcheressian JL, Wolff AC, Smith TJ, Grunfeld E, Muss HB, Vogel VG, Halberg F, Somerfield MR, Davidson NE. American society of clinical oncology 2006 update of the breast cancer follow-up and management guidelines in the adjuvant setting. J Clin Oncol. 2006;24(31):5091–5097. doi: 10.1200/JCO.2006.08.8575. [DOI] [PubMed] [Google Scholar]
- 8.Diamandis EP, Fritsche HA, Lilja H, Chan DW, Schwartz MK. Tumor Markers: Physiology, Pathobiology, Technology, and Clinical Applications. Washington, DC.: AACC Press; 2002. pp. 33–63. [Google Scholar]
- 9.Kulasingam V, Diamandis EP. Proteomics analysis of conditioned media from three breast cancer cell lines: a mine for biomarkers and therapeutic targets. Mol Cell Proteomics. 2007;6(11):1997–2011. doi: 10.1074/mcp.M600465-MCP200. [DOI] [PubMed] [Google Scholar]
- 10.Johanning GL. Modulation of breast cancer cell adhesion by unsaturated fatty acids. Nutrition. 1996;12(11-12):810–816. doi: 10.1016/s0899-9007(96)00244-4. [DOI] [PubMed] [Google Scholar]
- 11.Ofori-Acquah SF, King JA. Activated leukocyte cell adhesion molecule: a new paradox in cancer. Transl Res. 2008;151(3):122–128. doi: 10.1016/j.trsl.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 12.Jezierska A, Olszewski WP, Pietruszkiewicz J, Olszewski W, Matysiak W, Motyl T. Activated Leukocyte Cell Adhesion Molecule (ALCAM) is associated with suppression of breast cancer cells invasion. Med Sci Monit. 2006;12(7):BR245–BR256. [PubMed] [Google Scholar]
- 13.Denzinger T, Diekmann H, Bruns K, Laessing U, Stuermer CA, Przybylski M. Isolation, primary structure characterization and identification of the glycosylation pattern of recombinant goldfish neurolin, a neuronal cell adhesion protein. J Mass Spectrom. 1999;34(4):435–446. doi: 10.1002/(SICI)1096-9888(199904)34:4<435::AID-JMS803>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 14.Swart GW. Activated leukocyte cell adhesion molecule (CD166/ALCAM): developmental and mechanistic aspects of cell clustering and cell migration. Eur J Cell Biol. 2002;81(6):313–321. doi: 10.1078/0171-9335-00256. [DOI] [PubMed] [Google Scholar]
- 15.Bowen MA, Aruffo A. Adhesion molecules, their receptors, and their regulation: analysis of CD6-activated leukocyte cell adhesion molecule (ALCAM/CD166) interactions. Transplant Proc. 1999;31(1-2):795–796. doi: 10.1016/s0041-1345(98)01773-4. [DOI] [PubMed] [Google Scholar]
- 16.van Kempen LC, Nelissen JM, Degen WG, Torensma R, Weidle UH, Bloemers HP, Figdor CG, Swart GW. Molecular basis for the homophilic activated leukocyte cell adhesion molecule (ALCAM)-ALCAM interaction. J Biol Chem. 2001;276(28):25783–25790. doi: 10.1074/jbc.M011272200. [DOI] [PubMed] [Google Scholar]
- 17.Kristiansen G, Pilarsky C, Wissmann C, Kaiser S, Bruemmendorf T, Roepcke S, Dahl E, Hinzmann B, Specht T, Pervan J, Stephan C, Loening S, et al. Expression profiling of microdissected matched prostate cancer samples reveals CD166/MEMD and CD24 as new prognostic markers for patient survival. J Pathol. 2005;205(3):359–376. doi: 10.1002/path.1676. [DOI] [PubMed] [Google Scholar]
- 18.Swart GW, Lunter PC, Kilsdonk JW, Kempen LC. Activated leukocyte cell adhesion molecule (ALCAM/CD166): signaling at the divide of melanoma cell clustering and cell migration? Cancer Metastasis Rev. 2005;24(2):223–236. doi: 10.1007/s10555-005-1573-0. [DOI] [PubMed] [Google Scholar]
- 19.Zimmerman AW, Joosten B, Torensma R, Parnes JR, van Leeuwen FN, Figdor CG. Long-term engagement of CD6 and ALCAM is essential for T-cell proliferation induced by dendritic cells. Blood. 2006;107(8):3212–3220. doi: 10.1182/blood-2005-09-3881. [DOI] [PubMed] [Google Scholar]
- 20.McIntosh MW, Pepe MS. Combining several screening tests: optimality of the risk score. Biometrics. 2002;58(3):657–664. doi: 10.1111/j.0006-341x.2002.00657.x. [DOI] [PubMed] [Google Scholar]
- 21.Zheng Y, Katsaros D, Shan SJ, de la Longrais I, Porpiglia M, Scorilas A, Kim NW, Wolfert RL, Simon I, Li L, Feng Z, Diamandis EP. A multiparametric panel for ovarian cancer diagnosis, prognosis, and response to chemotherapy. Clin Cancer Res. 2007;13(23):6984–6992. doi: 10.1158/1078-0432.CCR-07-1409. [DOI] [PubMed] [Google Scholar]
- 22.Stoll BA. Biological mechanisms in breast cancer invasiveness: relevance to preventive interventions. Eur J Cancer Prev. 2000;9(2):73–79. doi: 10.1097/00008469-200004000-00002. [DOI] [PubMed] [Google Scholar]
- 23.Sommers CL. The role of cadherin-mediated adhesion in breast cancer. J Mammary Gland Biol Neoplasia. 1996;1(2):219–229. doi: 10.1007/BF02013645. [DOI] [PubMed] [Google Scholar]
- 24.Updyke TV, Nicolson GL. Malignant melanoma cell lines selected in vitro for increased homotypic adhesion properties have increased experimental metastatic potential. Clin Exp Metastasis. 1986;4(4):273–284. doi: 10.1007/BF00133592. [DOI] [PubMed] [Google Scholar]
- 25.Benchimol S, Fuks A, Jothy S, Beauchemin N, Shirota K, Stanners CP. Carcinoembryonic antigen, a human tumor marker, functions as an intercellular adhesion molecule. Cell. 1989;57(2):327–334. doi: 10.1016/0092-8674(89)90970-7. [DOI] [PubMed] [Google Scholar]
- 26.Bormer OP. Immunoassays for carcinoembryonic antigen: specificity and interferences. Scand J Clin Lab Invest. 1993;53(1):1–9. doi: 10.3109/00365519309092525. [DOI] [PubMed] [Google Scholar]
- 27.Nap M, Hammarstrom ML, Bormer O, Hammarstrom S, Wagener C, Handt S, Schreyer M, Mach JP, Buchegger F, von Kleist S. Specificity and affinity of monoclonal antibodies against carcinoembryonic antigen. Cancer Res. 1992;52(8):2329–2339. [PubMed] [Google Scholar]
- 28.Soletormos G, Nielsen D, Schioler V, Skovsgaard T, Dombernowsky P. Tumor markers cancer antigen 15.3, carcinoembryonic antigen, and tissue polypeptide antigen for monitoring metastatic breast cancer during first-line chemotherapy and follow-up. Clin Chem. 1996;42(4):564–575. [PubMed] [Google Scholar]
- 29.Hostetter RB, Augustus LB, Mankarious R, Chi KF, Fan D, Toth C, Thomas P, Jessup JM. Carcinoembryonic antigen as a selective enhancer of colorectal cancer metastasis. J Natl Cancer Inst. 1990;82(5):380–385. doi: 10.1093/jnci/82.5.380. [DOI] [PubMed] [Google Scholar]
- 30.Behrens J. The role of cell adhesion molecules in cancer invasion and metastasis. Breast Cancer Res Treat. 1993;24(3):175–184. doi: 10.1007/BF01833258. [DOI] [PubMed] [Google Scholar]
- 31.van Kempen LC, van den Oord JJ, van Muijen GN, Weidle UH, Bloemers HP, Swart GW. Activated leukocyte cell adhesion molecule/CD166, a marker of tumor progression in primary malignant melanoma of the skin. Am J Pathol. 2000;156(3):769–774. doi: 10.1016/S0002-9440(10)64943-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stamey TA, Warrington JA, Caldwell MC, Chen Z, Fan Z, Mahadevappa M, McNeal JE, Nolley R, Zhang Z. Molecular genetic profiling of Gleason grade 4/5 prostate cancers compared to benign prostatic hyperplasia. J Urol. 2001;166(6):2171–2177. [PubMed] [Google Scholar]
- 33.Kristiansen G, Pilarsky C, Wissmann C, Stephan C, Weissbach L, Loy V, Loening S, Dietel M, Rosenthal A. ALCAM/CD166 is up-regulated in low-grade prostate cancer and progressively lost in high-grade lesions. Prostate. 2003;54(1):34–43. doi: 10.1002/pros.10161. [DOI] [PubMed] [Google Scholar]
- 34.Weichert W, Knosel T, Bellach J, Dietel M, Kristiansen G. ALCAM/CD166 is overexpressed in colorectal carcinoma and correlates with shortened patient survival. J Clin Pathol. 2004;57(11):1160–1164. doi: 10.1136/jcp.2004.016238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.King JA, Ofori-Acquah SF, Stevens T, Al Mehdi AB, Fodstad O, Jiang WG. Activated leukocyte cell adhesion molecule in breast cancer: prognostic indicator. Breast Cancer Res. 2004;6(5):R478–R487. doi: 10.1186/bcr815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Davies SR, Dent C, Watkins G, King JA, Mokbel K, Jiang WG. Expression of the cell to cell adhesion molecule, ALCAM, in breast cancer patients and the potential link with skeletal metastasis. Oncol Rep. 2008;19(2):555–561. [PubMed] [Google Scholar]
- 37.Burkhardt M, Mayordomo E, Winzer KJ, Fritzsche F, Gansukh T, Pahl S, Weichert W, Denkert C, Guski H, Dietel M, Kristiansen G. Cytoplasmic overexpression of ALCAM is prognostic of disease progression in breast cancer. J Clin Pathol. 2006;59(4):403–409. doi: 10.1136/jcp.2005.028209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jezierska A, Matysiak W, Motyl T. ALCAM/CD166 protects breast cancer cells against apoptosis and autophagy. Med Sci Monit. 2006;12(8):BR263–BR273. [PubMed] [Google Scholar]
- 39.Ihnen M, Muller V, Wirtz RM, Schroder C, Krenkel S, Witzel I, Lisboa BW, Janicke F, Milde-Langosch K. Predictive impact of activated leukocyte cell adhesion molecule (ALCAM/CD166) in breast cancer. Breast Cancer Res Treat. 2008 doi: 10.1007/s10549-007-9879-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


