Abstract
Microglia are critical nervous system-specific cells influencing brain development, maintenance of the neural environment, response to injury, and repair. They contribute to neuronal proliferation and differentiation, pruning of dying neurons, synaptic remodeling and clearance of debris and aberrant proteins. Colonization of the brain occurs during gestation with an expansion following birth with localization stimulated by programmed neuronal death, synaptic pruning, andaxonal degeneration. Changes inmicroglia phenotype relate to cellular processes including specific neurotransmitter, pattern recognition, or immune-related receptor activation. Upon activation, microglia cells have the capacity to release a number of substances, e.g., cytokines, chemokines, nitric oxide, and reactive oxygen species, which could be detrimental or beneficial to the surrounding cells. With aging, microglia shift their morphology and may display diminished capacity for normal functions related to migration, clearance, and the ability to shift from a pro-inflammatory to an anti-inflammatory state to regulate injury and repair. This shift in microgliapotentially contributes to increased susceptibility and neurodegeneration as a function of age. In the current review, information is provided on the colonization of the brain by microglia, the expression of various pattern recognition receptors to regulate migration and phagocytosis, and the shift in related functions that occur in normal aging.
Keywords: microglia, P2X receptors, aging, development, synapse stripping
1. Introduction
Microglia are resident cells of the brain involved in regulatory processes critical for development, maintenance of the neural environment, response to injury, and subsequent repair. These cells were included in the early mid-nineteenth century description of neuroglia by Virchow (1856). Later, they were classified as a third element in the central nervous system (CNS) morphologically distinct from neurons, astrocytes (Cajal, 1913), and oligodendrocytes (del Rio Hortega, 1932). Microglia sense pathological events in the CNS and serve as brain immune cells to orchestrate innate immune responses. They share phenotypic characteristics and innate immunological functions with other mononuclear phagocytes and express major histocompatibility complex (MHC) antigens, as well as T and B cell markers such as various cluster of differentiation (CD) proteins (Perry et al., 1985; McGeer and McGeer, 1995; Williams et al., 1994). However, they are distinct from other tissue macrophages due to their relative quiescent phenotype and tight regulation by the CNS microenvironment. They often provide the first line of defense against invading microbes and via interactions with neurons can be the first to detect critical changes in neuronal activity and health. In the mature brain, microglia are capable not only of actively monitoring but also controlling the extracellular environment, walling off areas of the CNS from non-CNS tissue, and removing dead or damaged cells. Work by Schmid et al. (2009) suggested that the CNS environment contributes to differentiation of monocytes to a neural specific phenotype, separating microglia from mononuclear phagocytes while maintaining many similar features. This “nervous system specificity” is demonstrated in microglia in their functions associated with synaptic pruning (Bruce-Keller, 1999; Paolicelli et al., 2011; Stevens et al., 2007), phagocytosis of apoptotic neurons and reorganization of neuronal circuitry (Sierra et al., 2010; Tremblay et al., 2010), pruning of axonal collaterals (Gehrmann et al., 1995; Wake et al., 2009), facilitation of axonal sprouting (Nagamoto-Combs et al., 2010), and remyelination of central axons (Olah et al., 2012).
Much of the research on microglia is based upon functions within the adult brain; yet, data from developmental and aging studies suggest that the nature of these functions changes over the lifespan. During development, it is thought that microglia contribute to the formation of the final neural network by stimulating vascularization and assisting in pruning of excess neurons and synapses as well as facilitating cell differentiation. As sentinels in the adult brain, microglia maintain homeostasis and possibly contribute to the neural network by assisting in synaptic remodeling and plasticity (Trapp et al., 2007; Svensson et al., 1993; Moran and Graeber, 2004; Stevens, 2007; Wake, 2009; Perry and O’Connor, 2010), as well as removing excess aberrant proteins and debris accumulating in the brain. With aging, multiple physiological changes occur that are associated with DNA damage, oxidative stress, and telomere shortening (Vijg and Campisi, 2008). Within the brain, this is accompanied by a decrease in synaptic structures (Duan et al., 2003; Hof and Morrison, 2004; Jacobs et al., 1997) and a deficit in synaptic plasticity. With aging, the ability of microglia to express pro-inflammatory cytokines tumor necrosis factor (TNF)α, TNFβ, interleukin (IL)-1α, IL-1β, and IL-6, as well as cytokine receptors (Table 1) is thought to contribute to the mild chronic inflammatory condition that develops. This elevation in pro-inflammatory cytokines is accompanied by a decrease in anti-inflammatory cytokines, such as IL-10 (Ye and Johnson, 2001). These results suggest that aging is associated with an increase in microglia activation and a decreased regulatory status. It has been hypothesized that the active monitoring functions of microglia become compromised due to an onset of cell senescence that hinders the cell’s ability to sense changes in their environment, and thereby limit performance of normal functions. In this case, one might expect that a loss of phagocytic ability of the cells would result in the accumulation of aberrant proteins, such as amyloid beta (Aβ), α synuclein, apolipoprotein E (ApoE), that are known to be associated with neurodegenerative diseases. Senescence, or any mechanism that diminishes the function of microglia, could also have an impact on synaptic plasticity and associated cognitive function. Additionally, with aging, microglia may shift their functional phenotype such that they either express a higher level of proinflammatory factors creating an environment facilitating oxidative stress or thus, be neurotoxic to the neuronal or glial population. Such a shift could also occur in microglia that would inhibit the regulatory aspects or repair capabilities of microglia to downregulate inflammation and promote recovery and tissue remodeling.
Table 1.
Cytokine Receptors on Microglia
| Receptor | Ligand | Expression |
|---|---|---|
| TNFR1 | TNFα | cell-death receptor |
| TNFR2 | TNFα | trophic receptor/2nd cell-death receptor |
| IFNγR | IFNγ | M1 phenotype |
| IFNAR type I IFN | IFNβ | suppression of glutamate and super oxide |
| IL-1R1/IL-1R2 | IL-1α; IL-1β | induction of inflammatory mediators (IL-6) |
| IL-2Rα/β/γ(γc) | IL-2 | augment NO production, M2-like phenotype |
| IL-10R | IL-10 | induction of M2-like deactivation phenotype |
| IL-13R | IL-13 | induction of M2-like phenotype |
| IL-15R | IL-15 | reduced NO production, microglia survival |
| IL-18R (IL-1 related) | IL-18 | attenuation of induced IL-12 |
While characteristic features of microglia structure and function have been identified as they relate to brain development and as they change with aging, there are limited experimental data to fully demonstrate the altered function of microglia cells across the lifespan. However, based upon what we know about the general signaling of the cells for migration and phagocytosis, it could be hypothesized that microglia might change with regard to how they detect changes in the environment and how they respond to those changes, including the severity and duration and nature of the response. The wealth of data on the normal adult brain in response to injury provides a framework for asking critical questions to determine how the cells may function during development and change with aging and the impact of such changes. It is highly likely that a temporal gain or loss of function contributes to defining the nature and function microglia at different stages of the lifecycle. This includes their contribution to brain development and maintaining a healthy neural environment, and possibly the susceptibility of these cells to factors associated with aging. A thorough examination of the existing literature on the physiology of microglia is outside of the current review; rather, the present chapter will focus on data available for microglia during these two ends of the lifestage spectrum. This chapter attempts to set a framework for evaluating multiple aspects of microglia dynamic neural-specific cells as they may apply across the life span.
2. Microglia During Brain Development
Mononuclear phagocytes (macrophages, dendritic cells, monocytes) are hematopoetic cells belonging to the myeloid lineage (van Furth et al., 1979). A major breakthrough in the understanding of the origin of these cells came with the identification of a clonotypic bone marrow precursor by using adoptive transfer monocytes from CX3CR1 green fluorescent protein (GFP) reporter mouse (Varol et al., 2007). These cells are released into the peripheral circulation and transported from the bone marrow to eventually become tissue specific macrophages. The lack of unique expression of cell surface markers between mononuclear phagocytes that infiltrating the brain and activated resident microglia has hindered the ability to discriminate between the two and originally suggested a common origin. This led to the proposal that microglia were like mononuclear phagocytes and thus derived from the bone marrow and seeded to become tissue specific macrophages. This may still be the case for the few circulating blood progenitors penetrating the vascular wall in the neonatal and adult brain or those cells repopulating the mononuclear phagocytes associated with the CNS such as perivascular macrophages. Work by Priller et al. (2001) and others demonstrated GFP-expressing bond marrow cells in the brain several weeks after irradiation and bone marrow transplantation (Eglitis and Mezey, 1997; Massengale et al., 2005). When the head was shielded during irradiation or parabolic mice were examined, a much lower level of engraftment was observed, if at all (Ajami et al., 2011; Ginhoux et al., 2010; Matsumoto and Fujiwara, 1987). Work from Ginhoux et al., (2010) suggested that only approximately 10–20% of donor origin microglia were seen in the parenchyma of bone marrow chimeria mice 10–21 months after transplantation. This low level of engraftment was also observed in a busulfan/CX3CR1GFP+ chimeric mouse (Katzmarski et al., 2013). While parabiotic mice show a lower rate of chimerism, the number of engrafted cells was decreased to only 5% at 1 and 12 months post-parabiosis (Bogunovic et al., 2006). These findings suggested that postnatal microglia are maintained independently of blood-borne hematopoietic progenitors. It is now thought that a large proportion of microglia colonization of the brain occurs prior to birth.
Microglia differentiate from a primitive macrophage population produced by the yolk sac (Alliot et al., 1999; Ginhoux et al., 2010). In colonization of the brain, microglial recruitment and differentiation is suggested to occur in hematopoietic waves during the embryonic and postnatal periods (Chan et al., 2007; Ransohoff and Perry 2009). In using reconstituted sublethally irradiated mice, Ginhoux et al., (2010) were able to identify that only a small proportion (5%) of adult microglia were derived from the transplanted cells with 95% remaining of host origin. Myeloid cells expression the hematopoietic marker CD45 and adult macrophage markers CD11b, F4/80, and CX3CR1 can be detected in the mouse brain starting from E9.5 and by E10.5 could be observed in the cephalic mesenchyme and neuroepithelium (Ginhoux et al., 2010). In the rat, macrophages in the head were observed at E11 infiltrating the neurepithelium at E12 (Sorokin et al., 1992). Using single cell suspensions of mouse brain rudiment, no cells with properties of a microglial progenitor could be detected at E7.5, this increased to a single progenitor per brain rudiment at E8, to three cells at E8.5 followed by a sharp proliferation to reach an average of 1200 cells at E10.5 (Alliot et al., 1999). These cells remained highly proliferative throughout the embryonic period serving to populate the brain parenchyma. In the mouse, approximately 95% of the microglia are generated within the first two post-natal weeks (Alliot et al., 1999). Using a Cre-reporter mouse expressing the runt-related transcription factor 1 (Runx1), Ginhoux et al., (2010) furthered their evaluation of the origin of microglia during development. By fate mapping, reporter expression suggested a yolk sac origin for embryonic microglia and a migration of Runx1+ progenitors through the blood vessels after E8.5 with cells appearing in the brain rudiment at E9.5 (Ginhoux et al., 2010). Additional investigation identified macrophage colony stimulating factor receptor (MCSF-R)-expressing yolk sac precursors present at E8.5–9.5 as the origin for microglia (Schulz et al., 2012). In examining an earlier time in the process, Kierdorf et al (2013) identified cells by flow cytometry expressing cell surface markers characteristic of erythromyeloid progenitors Cd45-/c-kit+. These cells were able to differentiate into Iba-1+/CX3CR1-GFP+ cells and morphologically displayed shorter processes than the more mature microglia. These investigators confirmed the embryonic source of the cells and excluded any contribution from maternal macrophages to the microglia pool.
Colonization of the brain occurs with embryonic vascularization (Ajami et al., 2007) and relies heavily on blood vessels and an active process of blood circulation (Ginhoux et al., 2010). The peri-neural vascular plexus initiates vascularization of the CNS along a caudal-cephalic gradient commencing at the myelencephalon and ascending to the telencephalon (Marin-Padilla, 1985). During this time, cell-cell communication continues between microglia and vascular sprouts allowing for microglia migration towards developing vessels and stimulation of angiogenic activity (Rymo et al., 2011). While the vascular system is a primary avenue for transport, microglia appear in regions of the developing CNS devoid of vascularization (Hurley and Streit, 1995) with brain ventricles or para–meningeal transport serving as alternative routes of entry (review Cuadros and Navascues, 1998). Using immunoelectron microscopy of the rat cerebrum and spinal cord, Lassmann et al. (1991) demonstrated contact between parenchymal microglial process end-feet and the basal lamina of microvessels. This specific class of microglia has been referred to a juxtavascular microglia as distinguished from components of the vascular wall, the perivascular cells (pericytes) and from those microglia within the parenchyma that do not contact blood vessels. Using time-lasped microscopy of brain-slices from immature rodents, Grossmann et al. (2002) demonstrated that juxtavascular microglia are preferentially recruited to blood vessels and migrate along parenchymal surfaces upon stimulation following traumatic injury. Parenchyma microglia tracking along vessels showed a flattened morphology over the vessel surface and extended a leading process parallel to the vessel. A second group of blood vessel-associated microglia remained stationary and apparently anchored to the blood vessel but motile and actively extending processes into the surrounding parenchymal tissue (Grossmann et al., 2002). The heterogeneity of microglia is maintained with regards to brain location and relative distance to the vasculature. In the brain areas receiving the best blood supply, microglia display a more complex process ramification pattern (Rowan and Maxwell, 1981; Vela et al., 1995). Under conditions that decrease capillary flow the normal maturation of microglia to a ramified morphology is delayed (Masuda et al., 2011).
In the human brain, microglia colonization follows a tightly orchestrated process with distinct patterns of microglia migration occurring along radial glia, white matter tracts, as well as the vasculature (Rezaie and Male, 1999). By mid-to-late first trimester, a limited number of amoeboid microglia are present (Andjelkovic et al., 1998; Choi, 1981; Fujimoto et al., 1989) and by 24 weeks of gestation a large accumulation of cells is observed in the germinal matrix (Hutchins et al. 1990). Fetal microglia are located at highly vascularized sites in proximity to the parenchymal wall of radial blood vessels (Rezaie et al., 2005). With further development, microglia align within the subplate region of the telencephalon coinciding with the appearance of neurons, early synaptogenesis, and the establishment of thalamocortical projection fibers (Kostovic and Judas, 2002). As neurons populate and become established in the brain, the necessity for clearance of excess neurons is reduced and with it comes a morphological shift in microglia from a predominant amoeboid morphology normally associated with phagocytic actions. The number of nascent round and amoeboid microglia decreases and an increase is seen in highly ramified cells bearing long, thin, branched processes (Monier et al., 2006). As the cells reach their terminal location, they begin to take on a ramified morphology (Kostovic and Judas, 2002) and by 35 weeks of gestation microglia display a fully developed ramified morphology (Esiri et al., 1991). By birth and during the first few postpartum weeks, microglia disseminate throughout all parts of the brain, occupying defined spatial territories without substantial overlap.
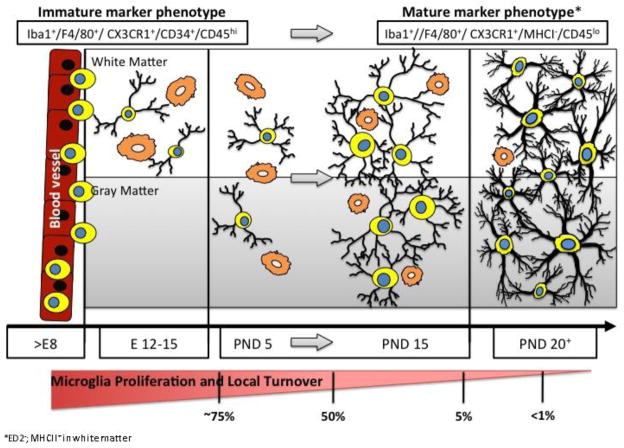
In the mouse, microglia colonization follows a similar pattern to that observed in the human (Fig. 1). Between GD 10–15, when capillary sprouts perforate the CNS, focal degeneration and disintegration of subadjacent glial endfeet stimulate the penetration of endothelial cells and attract monocytes and macrophages from the blood (Marin-Padilla, 1985). Precursors of ramified brain-resident microglia expressing the adult myeloid cell marker, F4/80, are found in the vicinity of CNS capillaries and are characterized by a round or irregular-shaped cell body, with or without pseudopodia (Imamoto and Leblond, 1978). In rats, influx of F4/80+ cells occurs at GD 15–16 with ramified F4/80+ cells appearing in the brain parenchyma by GD 18–19 and becoming more ramified as they fully differentiate (Kaur and Ling, 1991). These cells maintain a highly proliferative state throughout embryonic life (Ginhoux et al., 2010) expressing myeloid cell markers, including cluster of differentiation (CD) 34 and CD45, and protein tyrosine phosphatase receptor type C (PTPRC) (Davoust et al., 2006). In the subcortical white matter, this proliferative state extends into the first week following birth (Hristova et al, 2010).
Figure 1.
Developmental Stages Leading to Establishment of a Mature Population of Brain Microglia. During gestation (E9 – mice; E11- rat) microglial precursors cross the blood vessel wall and begin to take up residence in the brain parenchyma. Microglia progenitor cells (small round cells) line up along the blood vessels. These cells typically possess little cytoplasm and have small or nonexistent appendages (indicated as process-free round cells with distinct nuclei). At early stages of colonization (approx. E12–15), these cells are identifiable in white matter regions (or along vascular/ ventricular margins) possessing an amoeboid/ phagocytic morphology (larger ruffled round cells [orange]). In the gray matter, the cells are somewhat smaller than in the white matter and begin to show processes. During early postnatal stages (~PND5), these highly proliferative mononuclear cells are observed in both white matter and gray matter regions of the brain with showing both amoeboid and process-bearing phenotypes. During a critical period of postnatal microglia development (PND2- PND14), the number of microglia increases dramatically and has been associated with expression of Runx1. During this time, there is an increased ratio of ramified versus amoeboid microglia, with the cells having noticeably more complex process arbors and cytoplasmic material. By ~PND15 are well distributed throughout the brain and can be seen in close proximity to apoptotic neurons (swollen cells [light brown]), facilitating surveillance of the majority of the parenchyma; they exhibit a significantly reduced proliferative capacity; they begin to exhibit more heterogeneity (both within and across brain regions) in terms of their morphology, orientation, and process field organization; and they begin to express a somewhat modified constellation of cell surface markers. These maturation features continue such that by PND20 the adult population of microglia is fairly well established. In the absence of stimulation, these cells are highly ramified with complex process arbors encompassing the entire brain parenchyma integrated around healthy neurons and they possess little to no evidence of proliferation or from the systemic population (Lawson et al., 1992).
During the first postnatal month of life, microglia continue their development with a transition from a prominent phagocytic amoeboid morphology to one of more process bearing non-phagocytic features (Imamoto and Leblond, 1978; Orlowski et al., 2003). This transition is dependent upon the expression of Runt-related transcription factor 1 (Runx1) to inhibit cell proliferation (Zusso et al., 2010). Peak expansion of microglia coincides with increased expression of colony stimulating factor (CSF)-1 receptor (CSF-1R) as a receptor for IL-34 and essential for appropriate brain development, especially in the cortex and olfactory bulb (Erblich et al., 2011). By PND15, the entire parenchyma is filled with ramified microglia within defined territories and no cell overlapping (Lawson et al., 1990; Perry et al., 1985). It was initially thought that during the first 3 weeks of life, only 30% of the cells transform into ramified microglia, while the other 70% degenerate (Imamoto and Leblond, 1978; Wu et al., 1992). Thus, it was thought that the balance between cell proliferation and cell death would serve to regulate the number of cells surviving from the microglial lineage. However, further studies from Dalmau et al. (2003) using double-labeling techniques for microglia and apoptosis, demonstrated that there is a low proportion of microglia undergoing apoptosis during brain development. In the light of a high level of proliferation of microglia, the decrease in microglia density with brain development (Wu et al., 1992), if not due to apoptosis, may simply reflect the dramatic changes of volume expansion in the CNS during development (Dalmau et al., 2003). During these high expansion period in the first two weeks of postnatal life, the maturational progression of microglia can be influenced and thus in turn could alter normal processes of neuronal and glia development. For example, microglia express thyroid hormone (TH) receptors (TRα1 and TRα1) and any shift in thyroid status significantly alters their maturation (Lima et al., 2001). During these first two weeks, hypothyroid animals show a deficit in the density of microglia and a delay in process extension; while, hyperthyroidism accelerates the extension of microglial processes and increases the density of cortical microglia. In addition to altered maturation, microglia can be depleted during this early period. Exposure to ethanol between days 3 and 5 of postnatal life significantly depletes microglia numbers in the cerebellum with a loss of Purkinje cells (Kane et al., 2011).
In the amoeboid state, microglial cells are able to migrate within the brain and serve a critical role in the clearance of excess debris generated during the course of neuronal and glia proliferation (Perry et al., 1985). The arrival of microglia in the brain parenchyma correlates with the presence of apoptotic cells (Rigato et al., 2011; Wakselman et al., 2008). In the postnatal brain this association is considered as a response towards programmed neuronal death or axonal degeneration (Bessis et al., 2007; Mallat et al., 2005; Perry et al., 1985; Rakic and Zecevic, 2000; Schlegelmilch et al., 2011; Wakselman et al., 2008). In addition to a secondary clearance function, an active role for microglia in pruning excess neurons has been suggested. In slice cultures, a direct role for microglia in the cell death of supernumerary Purkinje cells has been demonstrated (Marin-Teva et al., 2004). A similar process has been reported with the death of early postnatal hippocampal neurons (Wakselman et al., 2008).
While clearance of excess cellular debris and aberrant proteins from programmed cell death is critical, the distribution of microglia in the developing mammalian brain suggests a more heterogeneous role (Rezaie et al., 2005). Based upon the body of data, microglia can be considered as critical players in brain development and neural circuitry formation (Erblich et al., 2011; Pont-Lezica et al., 2011). They provide important functions associated with vascularization of the brain, neuronal proliferation, and cell differentiation to a neuronal or astrocyte lineage (Mallat et al., 2005; Ni et al., 2007; Schafer et al., 2010). Examples of these functions are demonstrated in the ability of microglia to facilitate neurogenesis of embryonic cortical cells (Aarum et al., 2003); differentiation of embryonic neural precursor cells to astrocytes (Antony et al., 2011); and differentiation of basal forebrain progenitors into cholinergic neurons (Jonakait et al., 2000). As the neuronal population is established, the demands on microglia shift and the cells take on duties associated with promoting axonal elongation, synaptogenesis, and myelination. Axon pathfinding is facilitated by microglia via modification of the extracellular matrix by thrombospondin (Chamak et al., 1994). Neurotrophic factors such as neuronal growth factor (NGF; Heese et al., 1998), brain derived neurotrophic factor (BDNF; Batchelor et al., 1999; Coull et al., 2005), neurotrophin-3 (NT3), glial derived neurotrophic factor (GDNF) and cytokines with neurotrophic activity (reviewed in Garden and Moller, 2006; Kim and deVellis, 2005) are provided by microglia to the developing brain. Macrophage colony stimulating factor (M-CSF) can promote the proliferation of microglia but it also acts as a neurotrophic factor supporting neuronal survival and neurite outgrowth indirectly through microglia (Michaelson et al., 1996).
The physical association of microglia with synapses implicates microglia with synaptic refinement during development. Process bearing “activated” or “reactive” microglia have been reported in the thalamus, cerebellum, olfactory bulb, and hippocampus during postnatal synaptic remodeling (Fiske and Brunjes, 2000; Perry et al., 1985). The co-localization of neuronal postsynaptic protein within microglia cytoplasm suggests an active process of microglia to engulf and remove dendritic spines during development (Paolicelli et al., 2011). Synapses form as a function of sensory input and with input during the early postnatal days (PND 8–10) an increase in contact of microglia with immature dendritic spines is observed. This leads to a shift in the subsequent loss of these synapses (Tremblay et al., 2010). During postnatal remodeling of the visual cortex, visual experience regulates not only microglia contact but also morphology with cells progressing to a ramified state faster in animals monocular from birth (Rochefort et al., 2002). With synapse maturation or elimination, neurons up-regulate expression of the chemokine, fractalkine (CX3CL1) (Mody et al., 2001), that serves to signal microglia through activation of CX3CR1. Deficits in this receptor (Hughes et al., 2002; Nishiyori et al., 1998) are associated with a delay in synaptic pruning and spine elimination resulting in seizure susceptibility and a reduction in synapse efficiency (Paolicelli et al, 2011). The classical complement cascade has also been implicated in synapse elimination by microglia (Chu et al., 2010; Stevens et al., 2007). Complement receptor 3 (CR3) expression stimulates phagocytosis and spatially associates with complement factor, C1q, at synapses (Gasque et al., 2002). The correlation of complement expression with peak synaptic remodeling in the dorsal lateral geniculate nucleus (Stevens et al., 2007) led to the speculation that complement 3 serves to tag the extranumerary synaptic inputs for CR3-mediated phagocytosis by microglia (Schafer and Stevens, 2010).
Microglia are not only associated with synapse elimination but influence synaptic strength through the release of TNFα (Zhong et al., 2010) and by promoting surface expression of 2-amino-3-(3-hydroxy-5-methyl-isoxazol-4-yl)propanoic acid (AMPA)-type glutamate receptors on hippocampal neurons (Beattie et al., 2002) via β3 integrin expression (Cingolani et al., 2008). Neuronal activity can directly activate microglia (Hung et al., 2010). For example, prolonged inhibition of spontaneous neuronal activity in hippocampal cells stimulates TNFα release, while activity-regulated signals decrease TNFα release (Stellwagen and Malenka, 2006). The authors proposed that this was related to synaptic scaling, where the strength of synapses is modulated in response to changes in network activity. These data suggest s that microglia can detect the functional state of neurons and initiate an appropriate response targeted to the specific neuronal activation pattern. Thus, one can propose that alterations in microglia functioning during synapse formation and maturation of the brain can have significant long-term effects on the final established neural circuitry.
3. Microglia Morphology and Distribution in the Adult Brain
Once established, resident microglia comprise approximately 15–20% of the total number of cells in the brain (Carson et al., 2006b). Slight differences in morphology are seen across brain regions (Harry and Kraft, 2012); however, there is a relative uniform distribution with the exception of a higher relative number of in the olfactory telencephalon, dentate gyrus of the hippocampus, the substantia nigra, and portions of the basal ganglia (Lawson et al., 1990; Mittelbronn et al., 2001; Sharaf et al., 2010). In general, ramified microglia in gray matter show a small amount of perinuclear cytoplasm and a small, dense, and heterochromatic nucleus with processes extending in multiple directions. In white matter, microglial cells align their cytoplasmic extensions in parallel or at right angles to nerve processes. Differences in localization of microglia throughout the brain are suggested to be associated with the microenvironment as well as functional differences and phenotypic heterogeneity, including receptor expression patterns (Olah et al., 2011) and expression of cell surface antigens (Kaur & Ling, 1991). For example, in the corpus callosum, a white matter tract, microglia express a constitutively higher level of the co-stimulatory molecule, CD86 (B7.2) as compared to cells within the cortex (Carson et al., 2007). This expression within the white matter has been proposed to be associated with mechanisms related to nondestructive immunity (Bechmann et al., 2001). Microglia within unique brain regions maintaining a neurogenic capability such as the subgranular zone of the dentate gyrus in the hippocampus, also show a phenotype that allows for phagocytosis at the tips of process extension (Sierra et al., 2010) and exists under higher basal expression levels of CD45 and CD11b (Marshall et al., 2008). This may be linked to the activity of microglia within this region to regulate the cell-death, clearance, and survival of the newly generated neurons in order to ensure an appropriate number of mature dentate granule neurons (McPherson et al., 2011a;b: Sierra et al., 2010). Within brain regions lacking a blood-brain-barrier (BBB) such as the circumventricular organs, microglia display shorter thicker processes. Given their location, this morphology is likely influenced by the presence of serum proteins (Giulian et al., 1995; Fujita et al., 1996; Chamak and Mallat, 1991). Perivascular macrophages represent a separate population of cells located in the basal lamina of brain capillaries and the choroid plexus (Streit et al., 1989; Graeber & Streit, 1990; Guillemin and Brew, 2004; Sedgwick et al., 1991).
4. Phenotype Changes in Microglia
Microglia actively survey surrounding parenchyma via dynamic movement of processes allowing each cell to sample its environment over the course of a few hours (Davalos et al., 2005; Nimmerjahn et al., 2005). Maintaining microglia in such a relatively quiescent state is in part due to specific signals derived from neuronal and astrocyte-derived factors (Cardona et al., 2006; Neumann, 2001; Simard et al., 2006). Healthy neurons accomplish this via secreted and membrane bound signals including, CX3CL1 (Barclay et al., 2002; Hoek et al., 2000; Lyons et al., 2007; Sunnemark et al., 2005; Wright et al., 2003), neurotransmitters (Pocock and Kettenmann, 2007), and neurotrophins. During development, in the normal adult brain, and in the brain following injury, microglia display varied morphologies. The morphological differences in microglia are suggested to represent different functional states. Functional changes in microglia are often accompanied by a morphological transformation to cells with larger somata and shorter, coarser cytoplasmic processes displaying a bushy appearance possibly progressing to a full amoeboid morphology (Hanisch and Kettenmann, 2007; Ransohoff and Perry, 2009). In vivo, the transformation of resident microglia into those with a phagocytic phenotype is strictly regulated and occurs in response to stimuli such as cell death (apoptotic or necrotic), accumulated debris, excess aberrant protein, or the presence of viral or bacterial pathogens. Alternatively, a microglia response can be induced by a variety of input signals and may not necessarily result in a shift to an amoeboid phenotype as has been seen in the morphologies associated with synaptic or neural progenitor cell clearance. Upon the removal and thus absence of the stimulating stimuli or in the presence of the down-regulating signal, activated microglia commonly down-regulate to a ramified phenotype.
Brain macrophages exist in various states of activation within injured tissue and retain the capability to shift their functional phenotype within specific stages of the inflammatory response (Stout et al., 2005; Graeber 2010). In efforts to characterize functional changes and thus activation states, stages of peripheral macrophages have been recently adapted and proposed to describe the various stages that brain macrophages undergo during injury or neurodegeneration (Colton and Wilcock, 2010). Compared to peripheral macrophages or immature dendritic cells, CNS resident microglia do not represent a cellular source to present tissue antigens to T cells located within the draining lymph nodes and thus are relatively ineffective at initiating antigen-driven CD4+ T cell responses (Carson et al., 1998; 2006b). However, expression of MHC class II may serve to re-stimulate T cells in the target organ (Carson et al., 2006b; Siffrin et al., 2007). With presentation, CD4+ T-cells are stimulated toward one of a broad array of phenotypes (Lo et al., 1999; Siffrin et al., 2007). For example, the pro-inflammatory (Th1, Th17) CD4+ T cells promote myeloid cells towards a classical activation state (M1 state) characterized by high expression levels of pro-inflammatory cytokines (IL-1β, IL-12, IL-23, TNFα) as well as inducible nitric oxide synthase (iNOS), CD40, MHC I and MHC II (Goerdt et al., 1999; Colton and Wilcock, 2010; Gordon and Martinez, 2010). In contrast, IL-4- and IL-13+ Th2 CD4+T-cells are essential to promote myeloid cells toward alternative activation states (M2a and M2b) as characterized by expression of macrophage mannose receptor (CD206), Chitinase-3-like-1 (YM1 in rodents), stabilin-1, and arginase I. Under this alternative activation, the cells are functionally associated with enhanced phagocytosis, tissue repair, and parasite elimination. A regulatory activation state (M2c) is promoted by IL-10 and TGFβ-producing Th3 cells and regulatory T cells and characterized by expression of molecules related to tissue repair, reconstruction, and immunosuppression (Colton and Wilcock, 2010; Gordon and Martinez, 2010). Each state may have its own particular phenotype and there are likely transitional phenotypes between wound healing classical and regulatory macrophages (Mosser and Edwards, 2008) and microglia (Colton and Wilcock, 2010; Durafourt et al., 2012). This hypothesis that microglia can display an M1/M2 stage distinction as in peripheral macrophages would account for the heterogeneity of function of microglia (Ajami et al., 2007; Kigerl et al., 2009; Michelucci et al., 2009; Colton and Wilcock, 2010). Microglia are capable of exhibiting a M1 pro-inflammatory phenotype as occurs following activation with lipopolysaccharide (LPS). An M2 anti-inflammatory phenotype can be generated with IL-4, characterized by the release of neurotrophic factors such as insulin like growth factor -1 and anti-inflammatory cytokines such as, IL-10 and expression of YM1 (Perego et al., 2011). Microglia isolated from MS patients undergoing glatiramer acetate therapy displayed type 2 phenotypes, suggestive of a shift from a pro-inflammatory phenotype (Kim et al., 2004). Polarization of microglia has been demonstrated in human adult microglia (Durafourt et al., 2012) following focal cerebral ischemia (Hu et al., 2012) and in a severe relapsing experimental rat model of MS (Mikita we al., 2011). While data are still limited, there are a few studies reporting that aging shifts the sensitivity of activated microglia with diminished anti-inflammatory and M2-promoting effects of IL-4 (Fenn et al., 2012). In the microglia response to the Aβ protein, the brain oligomeric form of Aβ is a stronger inducer of the M1-phenotype as compared to the fibrillar form of the protein Michelucci et al (2009). When the cells are maintained in a cytokine-induced anti-inflammatory environment the level of reactivity is reduced (Michelucci et al., 2009). While the M1/M2 distinction has been considered under injury or disease states, expression of M1 and M2 related factors have been demonstrated in the developing rodent brain (Lenz et al., 2013). Thus, with additional experimental studies, examination of microglia responses and regulatory factors within the framework of an M1/M2 response may lead to a better understanding of the diverse functions of these cells and how they shift during various stages of life. For example, the inability of microglia to follow a normal healthy pattern could likely contribute to neurodegeneration, neuroinflammation, and absence of repair and injury resolution, which could occur under disease conditions or with aging.
5. Receptors for Microglia Activation
The normal functional capacity of microglia to rapidly return an injured tissue back to normal homeostasis is a critical component for maintaining a healthy tissue environment. This response requires migration of microglia to the damaged area and phagocytosis of aberrant material. To accomplish this, microglia express many surface receptors allowing for a direct interaction with the target. A multitude of signals posing potential threat to brain homeostasis are sensed by microglial receptors (Hanisch & Kettenmann, 2007) including the presence of inwardly-rectifying potassium (K+) channels (Kettenmann et al., 1993) other ion channels (Table 2), receptors for adenosine triphosphate (ATP; Langosch et al, 1994; Walz et al., 1993), calcitonin gene-related peptide (Priller et al., 1995), pattern recognition receptors (PRR), and multiple neurotransmitter (Table 3), neurohormone receptors (Table 4) and chemokines (Table 5). Initiation of a microglia response can involve purinergic receptors (P2X, P2Y; Langosch et al., 1994), the scavenger receptor CD36 (Stolzing and Grune, 2004), toll-like receptors (TLRs) (Landreth and Reed-Geaghan, 2009), and chemokine receptors (Cartier et al., 2005). These include the CX3C chemokine fractalkine receptor (CX3CR1) (Cardona et al., 2006; Lee et al., 2010) and CXCR3 as triggered by the neuronal chemokine CCL21 elevated with cell death (de Jong et al., 2005) and regulated by CXCL10 (Dijkstra et al., 2004). Signaling through these receptors can induce changes in membrane potential, intracellular calcium, cellular motility, and cytokine release (for review see, Kettenmann et al. 2011; Pocock and Kettenmann, 2007).
Table 2.
Ion Channels in Microglia
| Channel Type/Subunit | In vivo/In vitro | Expression |
|---|---|---|
| ORAI1/CRAC | rat primary | Activation with ER Ca2+ depletion |
| TRPM7; TRPC6, TRPM2, | primary | Activation for Ca2+ release/IL-6 |
| TRPV1; TRPM4 | primary | Activation for Ca2+ release |
| IK1R, Kir 2.1 | primary/brain slice | Activated microglia |
| IKDR; Kv1.1; Kv1.2; Kv1.3; Kv1.5 | primary/brain slice | Activated microglia |
| Ca2+-dependent K | primary/brain slice | Increased in activated microglia |
| G protein-activated K+ | mouse primary | metabotropic receptor activation |
| TTX-sensitive INA | primary | 20% rat; 95% brain tumors |
| Nav1.1, Nav1.5, Nav1.6 | primary | TTX blocked LPS activation |
| Nav1.6 | EAE mice/MS human | optic nerve/spinal cord |
| Volume-regulated Cl- | mouse primary/cell line | proliferation, phagocytosis |
| CLIC-1 chloride | rat primary/cell line | proliferation and ROS |
| Proton channels | primary/cell lines | respiratory burst, cell volume |
| Aquaporins | rat | LPS activated microglia |
| Connexins | rat, human, mouse | activated microglia TBI |
Source indicates species and type of cell culture or brain slice preparation
Table 3.
Neurotransmitter Receptors in Microglia
| Receptor | Source | Expression |
|---|---|---|
| A1 A2A; A2B; A3 | rat/mouse primary | proliferation, survival, cytokine, trophic effect |
| P2X1, P2X4, P2X7 | rat brain sections | Embryonic P2x1, P2X4; Adult P2X4; P2X7 |
| P2X4 | rat spinal cord | Activated microglia/neuropathic pain |
| P2X7 | primary/line/slice | Activated microglia, cytokine release |
| P2Y2; P2Y6; P2Y12; P2Y13 | Mouse primary | Microglia activation, migration, pain |
| GluR1, GluR2, GluR3, GluR4 | primary/cell line | TNFα, cytoskeleton remodeling, chemotaxis |
| mGluR5a | rat primary | Ca2+ transient |
| mGluR2, mGluR4 | rat primary | TNFα, microglia activation |
| mGluR6, mGluR8 | rat primary | reduced neurotoxicity |
| GABAB(1a); b(1b); B(1c) | rat/mouse primary/slice | increased facial nerve axotomy microglia |
| α7 nACh | rat/mouse primary | nicotine triggered Ca2+ transients |
| α1A, α2A, β1, β2 | rat primary | cytokine release |
| Dopamine (D1, D2) | rat/mouse primary/slice | activated outward K+ currents |
| AMPA/KA | primary | only on proliferating microglia |
Source indicates species and type of cell culture
Table 4.
Neurohormone and Neuromodulator Receptors on Microglia
| Receptor | Source | Expression |
|---|---|---|
| PAF | rat/mouse primary | Ca2+ entry, IL-6 expression |
| Bradykinin (B1; B2) | rat primary | B1 LPS induced; B2 resting |
| Histamine | rat primary | Ca2+ release |
| Endothelin ETB | mouse, human/primary | Ca2+ release |
| Cannabinoid (CB1, CB2) | rat, mouse, human/primary | Activated microglia, cytokine |
| Angiotensin II (AT1, AT2) | rat embryo/primary | AT1 LPS induced; AT2 resting |
| Somatostatin (sst2, sst3, sst4) | rat primary | inhibited proliferation |
| Glucocorticoid | rat primary | inhibited proliferation |
| Mineralcorticoid | rat primary | microglia |
| Opioid (MOR) | rat primary | upregulates P2X4 expression |
| Neuokinin (NK-1) | human embryo/mouse | activation of NF-κB |
| VIP (VPAC1) | rat primary | inhibits LPS-induced activation |
| Trk-B1 | rat primary | BDNF triggered Ca2+ release |
Source indicates species and type of cell culture
Table 5.
Chemokine Receptors on Microglia
| Receptor | Agonists | Expression |
|---|---|---|
| CCR1, CCR2 | CCR2-MCP-1 | Ca2+ transients |
| CCR5 | MIP-1α, RANTES | Ca2+ transients |
| CXCR1, CXCR3, CCR3 | expression increased by TNFα and IFNγ | |
| CCR5 | increase with hypoxia/ischemia in rat forebrain | |
| CCR5 | MIP-1α-induced K+ current human brain | |
| CXCR3 | CCL21T | Activated vol-regulated Cl− channels |
5.1. Distinguishing what to destroy and what to protect
One of the necessary functions of microglia in performing their tasks appropriately and efficiently is the ability to distinguish “friend from foe” (Carson et al., 2006a; 2006b). This function can serve to influence the purpose and outcome of a microglia response and direct outcome effects on other neural cells. In many cases, the ability of the cell to discriminate between self and invader is dependent on the presence or absence of antigen presentation (Medzhitov and Janeway, 2000). In the periphery, the rapid innate and slow adaptive immune response systems are responsible for the “self”-identifier function and the integrated system allows for a specific response based upon the nature of the threat (Lo et al., 1999). In contrast to the periphery, there is limited infiltration of T-cells into the brain and microglia are relatively ineffective at initiating antigen-driven CD4+ T-cell responses (Carson et al., 2006b). There however, is evidence suggesting that microglia are capable of contributing to the antigen driven response in autoimmune diseases such as, multiple sclerosis (Coyle, 2011).
Even in the absence of a strong APC, a productive innate immune response to successfully drive a rapid response to insult is dependent upon a number of factors including expression of pattern recognition receptors (PRRs) (for review, Krishnaswamy et al., 2013). PRRs expressed on microglia include Toll-like receptors (TLRs; TLR 1–9 (Farina et al., 2005); TLR2 (Babcock et al., 2006; Lehnardt et al., 2007), and TLR4 (Lehnardt et al., 2003; Visintin et al., 2001)), receptor for advanced glycation end products (RAGE), and specific scavenger receptors targeted to detect the aberrant expression of phosphatidylserine (PS) on the extracellular surface of dying cells (Ravichandran, 2003). Ligation of PRRs leads to the activation of signal transduction pathways and transcriptional and post-transcriptional molecules of the interferon regulator factor families. These molecules modulate pro-inflammatory target genes encoding cytokines, chemokines, enzymes, and other molecules essential for pathogen elimination (Akira et al., 2006). In comparison, signaling of the triggering receptor expressed on myeloid cells-2 (TREM-2) was found to facilitate debris clearance by microglia in vitro in the absence of an inflammatory response (Takahashi et al., 2005). Based upon studies examining the various TLRs, it was suggested that innate immune response activation in the brain is tailored according to the cell type and environmental signal. For example, TLR2 and TLR4 are capable of sensing damage induced by ischemia and, as such, boost the pro-inflammatory response leading to an increase in infarct size (Caso et al., 2008; Kilic et al., 2008; Lehnardt et al., 2007). TLR2-mediated responses are primarily associated with release of IL-6 and IL-1β (Jack et al., 2005) and thus the outcome would be related to those cytokine-signaling pathways. The presence of activated microglia is required for a TLR4 response in astrocytes and to optimize the TLR2 and TLR3 response (Holm et al., 2012). TLR4 is used by microglia and astrocytes to detect Aβ (Landreth and Reed-Geaghan, 2009) and the release of heat shock protein 60 from dying CNS cells (Lehnardt et al., 2008). In a pro-oxidant and inflammatory environment, RAGE (Neeper et al., 1992; Schmidt et al., 1992) is activated on microglia and responds to the increased production of Aβ, serum amyloid A (SAA), S100 protein, and high-mobility group protein B1 (HMGB1). Increased production of these ligands is observed with cellular dysfunction and inflammation (Schmidt et al., 2009; Srikanth et al., 2011). Upon detection, signal-dependent transcription factors are activated to up-regulate inflammatory response genes and initiate the clearance of the aberrant protein (Reed-Geaghan et al., 2009; Walter et al., 2007). RAGE increases M-CSF release from neurons, activating the cognate receptor, c-fms, on microglia resulting in a prolonged survival of microglia within the injured tissue (Yan et al., 1997). However, RAGE also recognizes PS and induces apoptotic cell clearance (Friggeri et al., 2011). HMGB1 is an intracellular protein that activated p53 and NF-κB transcription factors and through ERK-mediated signaling pathway, inhibits the phagocytosis of apoptotic cells (Friggeri et al., 2010).
Degenerated neurons also release several signaling molecules, including nucleotides, cytokines, and chemokines, to recruit microglia (Biber et al., 2007). Signaling between microglia and degenerating neurons is conducted by a number of different mechanisms with one of the strongest signals being adenosine triphosphate (ATP). ATP release can mediate purinergic signaling to promote microglia process extension and phagocytosis. Microglia recognize the release of purine from dying cells by purinergic receptors. The physiological effects of purines and pyrimidines are mediated through purinoceptors classified as metabotropic P1 adenosine receptors, metabotropic P2Y purinoceptors, and ionotropic P2X purinoceptors (Fredholm et al., 2007; North, 2002; Surprenant and North, 2009). Receptor expression is abundant in glia with microglia expressing P2X4, P2X7, P2Y2, P2Y4, P2Y6, P2Y12, and P2Y14 receptors (Verkhratsky et al., 2009) and purine metabolism-related enzymes are expressed by microglia in the developing brain (Dalmau et al., 1998). In the case of neuropathic pain, one such signaling molecule is the chemokine, CCL2 (monocyte chemoattractant protein 1, MCP-1). Its cognate receptor, CCR2, is expressed on microglia (Zhang et al., 2007) and upon activation, promotes cell surface expression of P2X4R (Toyomitsu et al., 2012) as a critical modulator for the pain response (Trang et al., 2009; Tsuda et al., 2003; 2009; Ulmann et al., 2008). Also critical for the up-regulation of P2X4R are chemokines released from neurons e.g., CCL21 (Biber et al., 2011), interferon gamma (Tsuda et al., 2009), fibronectin (Tsuda et al., 2009), as well as protease tryptase activating proteinase-activated receptor 2 (PAR2) from mast cells (Yuan et al., 2010), and brain derived neurotrophic factor (BDNF) released from microglia (Trang et al., 2009). P2XRs are expressed on rat microglia during early development (Xiang and Burnstock, 2005). P2X7R expressing microglia are diffusely scattered throughout the brain (Yu et al., 2008) and are induced with brain injury and in neurodegenerative disorders including amyotrophic lateral sclerosis (Yiangou et al., 2006), Alzheimer’s disease (Takenouchi et al., 2010), ischemia (Melani et al., 2006) localized injection of Aβ1–42 (McLarnon et al., 2006) or LPS (Choi et al., 2007), and kainite-induced seizures (Rappold et al., 2006). As with other microglia receptors, the outcome of P2X7R activation can be cytotoxic or trophic. Activation of P2X7 receptors promotes release of IL-1α and IL-1β (Bernardino et al., 2008; Ferrari et al., 1997; Mingam et al., 2008), TNFα (Hide et al., 2000), superoxide (Parvathenani et al., 2003), and plasminogen (Inoue et al., 1998). However, a dual role is proposed given that P2X7R activation can protect neurons against glutamate-induced neurotoxicity (Suzuki et al., 2004).
In comparison to the P2X receptors, P2Y receptors can promote down-regulation of a pro-inflammatory response. Activation of P2YR inhibits LPS-induced release of TNFα, IL-1β, IL-6, and IL-12 (Boucsein et al., 2003; Ogata et al, 2003) and activation of P2Y1/P2Y11R increases release of the anti-inflammatory cytokine, IL-10 (Seo et al., 2008). P2Y12Rs are localized on the cell processes and somatic membrane of quiescent microglia. The expression of this receptor contributes to the cell’s ability to migrate, proliferate, and extend processes toward a lesion (Haynes et al., 2006). As with other stimuli of chemotaxis, this is associated with engagement of integrin-β1 signaling (Ohsawa et al., 2010), K+ channel activation (Wu et al., 2007) and ATP signaling (Ohsawa et al., 2007). Migration of microglia mediated by the P2Y12 receptor is dependent upon activation of phosphoinositide 3-kinase (PI3K) and Akt pathways (Irino et al., 2008). Microglia express subclasses of the P1 adenosine receptors (A1, A2A, A2B, and A3) (Dare et al., 2007; Fredholm et al., 2001; Hammarberg et al., 2003). It has been suggested that a co-stimulation of purinergic and adenosine receptors is required for cell migration with the ATP/adenosine balance regulated by expression of the prototype nucleoside triphosphate diphosphohydrolase, CD39 (Draheim et al., 1999).
While ATP serves as the more potent signal, microglia chemotaxis can be mediated by other receptors including α-amino-3-hydroxy-5-methylisoxazole-4-propanoic acid (AMPA), serotonin (Krabbe et al., 2012), histamine (Ferreira et al., 2012), dopamine and epinephrine (Farber et al., 2005), bradykinin (Ifuku et al., 2007), metabotropic glutamate receptors (mGluR) (Liu et al., 2009; McMullan et al., 2012), cannabinoid receptors (McHugh, 2012), platelet-activating factor receptors (Aihara et al., 2000), as well as, expression of CD39 ectonucleotidase (Draheim et al., 1999). Noda et al (2000) reported the presence of AMPA/kainite (KA) type receptors on microglia but on only a subpopulation of the cells. While KA-induced inward currents could be induced in only 20% of quiescent cells, this increased to 80% when the cells were in a proliferative state (Yamada et al., 2006). This suggests that the influence of KA type receptor activation is directed more toward immature or proliferating microglia rather than process bearing cells. While increased excitatory neurotransmission and purinergic signaling can increase microglial process motility, inhibitory neurotransmissions have been implicated in diminished motility. The inhibitory neurotransmitter, γ–aminobutyric acid (GABA), has been implicated in the decrease of microglia process motility in the brain (Fontainhas et al., 2011; Nimmerjahn et al., 2005) and retinal explants (Fontainhas et al., 2011).
6. Microglia in the Aged Brain
It has been proposed that microglia change as a function of aging and that such change may reflect a diminished capability for the cells to perform their normal function (Streit, 2006; Streit and Xue, 2012). To maintain homeostasis cells are required to rapidly respond in a manner that will allow for clearance of aberrant proteins (ApoE, α-synuclein, Aβ42), invading pathogens, damaged tissue debris, synaptic stripping and remodeling. The activation of microglia cells and pro-inflammatory cytokine signaling representing an activation of the innate immune system can serve in a neuroprotective manner with responding and clearing invading pathogens and local debris or protein (for reviews: Aravalli et al., 2007; Glezer et al., 2006; 2007; Lehnardt, 2010; Olson and Miller, 2004; Rodgers and Miller, 2012). Such actions ensure appropriate signaling to extend these clearance processes and transition the response to one of resolution and repair, providing trophic support with the release of various growth factors. Any deficit in the ability of microglia to perform these functions, whether due to a decrease in cell number or diminished function, would have significant impact on the state of health of the brain.
When the overall distribution of microglia was examined in the mouse brain, cells were found less evenly distributed in the aged cortex as compared to the young adult (Tremblay et al., 2012). Dendritic arbors are smaller, less symmetrical and more elongated rather than circular in shape (Sierra et al., 2007; Tremblay et al., 2012). In examining the evolution of structural differences in cortical microglia from rats aged 3 to 30 months, Vaughan and Peters (1974) observed changes in cell shape and an increase in cell number Similar to the morphology in the brain, live cell imaging of retinal microglia in situ demonstrated that aged microglia are smaller with less ramifications, have slower process motility, and as a response to ATP they become less dynamic and ramified. Upon a laser injury, aged microglia show slower migration response and remain at the injury site longer, as compared to young microglia suggesting not only a deficit in the initial response but also in the down-regulatory signaling for resolution (Damani et al., 2011). With aging, microglia are less restricted from the outer retina and accumulate in the subretinal space (Xu et al., 2008). This shift in position brings microglia into contact with photoreceptors and retinal pigmented epithelial cells (Ma et al., 2012). In the retina, a para-inflammatory state exists with aging (Xu et al., 2009). This is a tissue adaptive response to noxious stress or system malfunction and exists under conditions that are intermediate between basal and inflammatory states. A dysregulation of this intermediate state has been linked to the immune dysfunction associated with age-related macular degeneration (Chen et al., 2011). With normal aging, microglia aggregate in the outer retina and acquire intracellular autofluorescent lipofuscin deposits (Xu et al., 2008). Examination of A2E accumulation as representative of ocular lipofuscin, increased microglia activation, lowered expression of chemokine receptors, suppressed chemotaxis and increased complement activation (Ma et al., 2013). The accumulation of lipofuscin in microglia has also been described in the brains of rats aged between 3 and 30 months (Vaughan and Peters, 1974).
In both rat and human brains observations of altered microglia morphology have been observed and associated a more reactive/activated phenotype as a function of aging (Vaughan and Peters, 1974; Samorajski, 1976; Schuitemaker et al., 2010). Markers normally present in activated microglia are also elevated in aged microglia including MHC II antigens, CD11b, CD14, and pattern recognition receptors (Finch et al., 2002; Frank et al., 2006; Letiembre et al., 2007; Ogura et al., 1994; Perry et al., 1993). Basal pro-inflammation cytokine levels are elevated with aging (Sheng et al., 1998; Ye and Johnson, 1999); yet, data on anti-inflammatory cytokines such as IL-10 are less than clear (Ye and Johnson, 2001; Sierra et al., 2007). In addition to a possible shift in pro-inflammatory proteins with aging, changes in ligands and their receptors important for ensuring normal microglia function are changed. Signaling between CX3CL1 and its receptor CX3CR1 is critical for microglia migration in the adult brain yet in the aged brain expression levels of each are diminished in the aged brain (Bachstetter et al., 2011; Wynne et al., 2010). This shift is evident under basal conditions and upon induction by LPS (Wynne et al., 2010). Not only are these receptors which contribute to an activation of microglia diminished but levels of CD200 are also decreased (Frank et al., 2006) suggesting of loss of the regulatory ability of neurons to maintain microglia in a quiescent state.
While it was initially thought the changes in cell morphology observed in the aged brain represented cells in a more activated state, additional research has found that upon morphological changes in cytoplasmic structure with aging were not characteristic of activation (Sheng et al., 1998; Miller and Streit, 2007) but rather reflective of dystrophy and senescence (Streit et al., 2008). Microglial cells showed cytoplasmic inclusions, de-ramification of processes, and membrane blebbing with aging. The dysmorphic characteristics of aged microglia suggested that, rather than the cells being maintained in an over-activated state, they displayed decreased ability to mount a normal response to injury (Sheng et al., 1998). Age-related changes in cytokine production and dysmorphic microglia morphology (Perry et al., 1993; Sheffield and Berman, 1998) have been proposed to underlie microglia contributions to neurodegenerative diseases. Recent work demonstrated that a systemic challenge with LPS to aged mice elevated the level of cytokine synthesis as compared to young animals (Dilger and Johnson, 2008; Henry et al., 2009). It was considered that such an exaggerated response could represent either a “primed” state with over-production of pro-inflammatory cytokines or a diminished induction of anti-inflammatory factors. The phenomenon “priming” represents a phenotypic shift of microglial cells toward a more sensitized state. This primed microglia will then respond to a secondary “triggering” stimulus more rapidly and to a greater degree than would be expected if non-primed.
Further examination by Streit and colleagues (2008) suggested that microglial senescence is triggered by an intracellular oxidative stress response to enhanced intracellular accumulation of iron. Alternatively, the senescence may be related to a decrease in replicative ability as a result of a lifetime of activation/replication resulting in telomere attrition, DNA damage, and chromatin perturbation (Flanary and Streit, 2004). Microglia exhibit telomere shortening and decreased telomerase activity with aging (Flanary and Streit, 2004) and in Alzheimer s disease brains (Flanary et al., 2007) supporting the hypothesis of microglial replicative senescence in normal and pathological aging. Thus, age-related changes observed in receptor expression such as a downregulation of CX3CR1 (Wynne et al., 2010), diminished motility, and speed of an acute response to injury (Damani et al., 2011) may be the result of cell senescence. While a classic outcome of cell senescence is a diminished proliferative ability, it is likely that a more general blunting of functional activities occurs. This could involve a decrease in other properties of microglia such as production of neurotrophic factors, phagocytosis, and protein clearance. How senescence is induced is still uncertain. It may be the result of intracellular oxidative stress as suggested by Streit (2008). Or related to the increased demand for clearance of such proteins as Aβ (Streit et al., 2004), or existing in a prolonged activated state.
It has been hypothesized that changes in the aging microglia drive pathogenic progression of diseases or injury through a diminution of neuroprotective functions, increase in neurotoxicity, and dysregulation of responses to signals and perturbations (Flanary et al., 2007; Lopes et al., 2008; Luo et al., 2010). Evidence of microglia dysregulation in response to perturbation is accumulating from studies models of infection/inflammatory challenge (Norden and Godbout, 2012; Sierra et al., 2007; Sparkman et al., 2005), stroke (Buchanan et al., 2008; Rosczyk et al., 2008), and trauma (Sandhir et al., 2008). In each case the response tended to be larger and sustained for a longer period of time. However, age-related neurodegeneration might not only be due to a loss of neuroprotective properties, but also to the actual loss of microglia (Streit et al., 2008). Work by Baker et al. (2011) demonstrated that lifetime removal of cyclin-dependent kinase inhibitor 2A (p16Ink4a)-positive senescent cells in the adipose tissue, skeletal muscle, and eye resulted in a delayed onset of age-related pathologies. Furthermore, they demonstrated that late-life clearance of senescent cells attenuated the progression of already established age-related disorders. While this work was not conducted in the nervous system, one could envision that a deficit for any organ system to clear dysfunctional cells would result in an adverse and potentially “disease-related” environment.
One of the more prominent features of microglia is their normal phagocytic capability to clear invading pathogens, aberrant proteins, and debris from the CNS. Thus, the loss of efficiency in this function would likely result in a more toxic environment for the neural cells. Cytoplasmic projections from microglia surround Aβ fibrillary aggregates and microglia cultured from aged mice showed a diminished ability to internalize Aβ peptide which would translate to a lack of amyloid elimination by parenchymal microglia. Recent work demonstrated an age related shift in CD47 (Rh-related antigen, integrin-associated signal transducer)-dependent ability of isolated microglia to phagocytize Aβ fibrils. In this case, microglia isolated from brain at birth or at 2 months of age could effectively phagocytize Aβ fibrils; however, this ability was lost when cells were isolated from 6-month-old animals (Floden and Combs, 2011). When cells from each age group, including 12–17 month old animals, were examined for expression of putative Aβ interacting proteins, only CD36 was shown to decrease as a function of age suggesting that adult cells remained able to interact with Aβ fibrils, just not to phagocytize it. This effect was not due to a generalized loss of phagocytic capability as no age related difference was observed in clearance of bacteria, suggesting a specific alteration with regards to Aβ and possibly other aberrant protein. Whether this loss of function reflects actions in vivo or occurs as the result of culturing techniques is not clear, however, when isolated microglia were allowed to adhere to AD or control brain sections the cells obtained at birth showed a greater ability to decrease plaque load as compared to microglia obtained from 6–8 month old brains. While interesting and supportive of a more effective clearance process with the younger microglia, the impact of a loss of cell viability in the older cultures could not allow for a direct comparison of function. In the older cultures, more microglia adhered to Aβ immunoreactive plaques in the AD brain as compared to that seen in with younger microglia. This data suggests concern for other specific loss of functions of microglia that may occur not only with maturation of the brain but also with increased aging. One hypothesis is that interactions with Aβ peptide or other inflammatory stimuli in the diseased brain drives the cells to an early and maintained alternative activation state that shifts the ability of the cells to respond in a normal manner (Floden and Combs, 2011). In the apolipoprotein E (ApoE) transgenic mice as a model of risk factor for AD, an age-related and genotype-related deficit was observed in the ability of the animals to mount a host-resistance response to injury (Harry et al., 2000). Until the age of 8 months, constitutive mRNA levels of pro-inflammatory cytokines were not altered between mice expressing various allelic forms of the human ApoE gene (ApoE2, ApoE3, ApoE4, or ApoE null). With hippocampal injury and activation of resident microglia, no differences were observed at 2 months of age; however, by 8 months of age the ApoE4 allele mice showed a reduced ability to mount a host-response and pro-inflammatory cytokine response. In this model, microglia did not yet display morphological dystrophic phenotypes and microglia activation upon injury was diminished.
7. Conclusions
While characteristic features of microglia structure and function have been identified as they relate to brain development and as they change with aging, there are limited experimental data to fully demonstrate the functions or alterations thereof in microglia cells across the lifespan. However, based upon what is known about the general signaling of the cells and their different functions, it could be assumed that microglia cells change with regard to how they detect changes in the environment, how they respond to those changes, their capability for motility and phagocytosis, the severity and duration of the a response, and the nature of the response. Modifications to resident immune cells of the brain can manifest as alterations in the intrinsic function of the cells or in interactions with ongoing cellular processes. The nature of these alterations can be dependent upon life-stage events (e.g., development, senescence), presence/absence of antigen, vascular cell contribution, leakage of the blood-brain-barrier to serum proteins and other factors. The outcome is also dependent upon the contribution of T-cells and the cellular source of the brain macrophages, i.e., whether the response is driven by resident microglia or infiltrating macrophages. Our knowledge of the function of microglia is expanding; yet, we are still limited with regards to biochemical and molecular changes that occur during development and in the aged brain (for review, Kettenmann et al., 2011). While only critical concepts and limited specific features associated with microglia are presented in the current review, we believe that they set the framework for readers to begin to integrate new information as it becomes available. After having been categorized as brain macrophages, mounting data suggests that not only are microglia heterogeneous with regards to normal morphology and reaction to injury, but that this heterogeneity likely crosses over to the multiple roles that microglia play throughout the lifespan. Gaining a better understanding of their capability and regulatory processes will allow for a more accurate picture of their functions and thus, allow for targeted intervention strategies for approaching brain injury and neurodevelopmental and neurodegenerative disorders.
Acknowledgments
This research was supported by the National Toxicology Program Division, National Institute of Environmental Health Sciences, National Institutes of Health, Department of Health and Human Services #1Z01ES101623 and ES021164. The views expressed in this article are those of the author and they do not necessarily represent the views or policies of the National Toxicology Program.
Abbreviations
- ATP
Adenosine triphosphate
- AD
Alzheimer’s disease
- Aβ
amyloid beta
- BBB
blood brain barrier
- Cl-
chloride
- CD
cluster of differentiation
- CR3
complement receptor 3
- C1q
complement 1q
- CNS
central nervous system
- CX3CL1
fractalkine or neurotactin
- CX3CR1
fractalkine receptor
- GD
gestational day
- IFN
interferon
- MHC
major histocompatibility complex
- MAPK
mitogen-activated protein kinase
- PND
postnatal day
- IL
Interleukin
- LPS
lipopolysaccharide
- NO
nitric oxide
- PRR
pattern recognition receptors
- PI3K
phosphoinositide 3-kinase
- K+
potassium
- P2
purinergic receptors
- TREM-2
triggering receptor expressed on myeloid cells-2
- TLR
toll-like receptors
- TGFβ
transforming growth factor beta
- TNF
tumor necrosis factor
Footnotes
Conflict of Interest Statement
The author declares that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aarum J, Sandberg K, Haeberlein SL, Persson MA. Migration and differentiation of neural precursor cells can be directed by microglia. Proceedings of the National Academy of Sciences USA. 2003;100:15983–15988. doi: 10.1073/pnas.2237050100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aihara M, Ishii S, Kume K, Shimizu T. Interaction between neurone and microglia mediated by platelet-activating factor. Genes to Cells. 2000;5:397–406. doi: 10.1046/j.1365-2443.2000.00333.x. [DOI] [PubMed] [Google Scholar]
- Ajami B, Bennett JL, Krieger C, Tetzlaff W, Rossi FM. Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nature Neuroscience. 2007;10:1538–1543. doi: 10.1038/nn2014. [DOI] [PubMed] [Google Scholar]
- Ajami B, Bennett JL, Krieger C, McNagny KM, Rossi FM. Infiltrating monocytes trigger EAE progression, but do not contribute to the resident microglia pool. Nature Neuroscience. 2011;14:1142–1149. doi: 10.1038/nn.2887. [DOI] [PubMed] [Google Scholar]
- Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Alliot F, Godin I, Pessac B. Microglia derive from progenitors, originating from the yolk sac, and which proliferate in the brain. Brain Research Developmental Brain Research. 1999;117:145–152. doi: 10.1016/s0165-3806(99)00113-3. [DOI] [PubMed] [Google Scholar]
- Andjelkovic AV, Nikolic B, Pachter JS, Zecevic N. Macrophages/microglial cells in human central nervous system during development: an immunohistochemical study. Brain Research. 1998;814:13–25. doi: 10.1016/s0006-8993(98)00830-0. [DOI] [PubMed] [Google Scholar]
- Antony JM, Paquin A, Nutt SL, Kaplan DR, Miller FD. Endogenous microglia regulate development of embryonic cortical precursor cells. Journal of Neuroscience Research. 2011;89:286–298. doi: 10.1002/jnr.22533. [DOI] [PubMed] [Google Scholar]
- Aravalli RN, Peterson PK, Lokensgard JR. Toll-like receptors in defense and damage of the central nervous system. Journal of Neuroimmune Pharmacology, 2007. 2007;2:297–312. doi: 10.1007/s11481-007-9071-5. [DOI] [PubMed] [Google Scholar]
- Babcock AA, Wirenfeldt M, Holm T, Nielsen HH, Dissing-Olesen L, Toft-Hansen H, et al. Toll-like receptor 2 signaling in response to brain injury: an innate bridge to neuroinflammation. Journal of Neuroscience. 2006;26:12826–12837. doi: 10.1523/JNEUROSCI.4937-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachstetter AD, Morganti JM, Jernberg J, Schlunk A, Mitchell SH, Brewster KW, et al. Fractalkine and CX 3 CR1 regulate hippocampal neurogenesis in adult and aged rats. Neurobiology of Aging. 2011;32:2030–2044. doi: 10.1016/j.neurobiolaging.2009.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker DJ, Wijshake T, Tchkonia T, LeBrasseur NK, Childs BG, van de Sluis, et al. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature. 2011;479:232–236. doi: 10.1038/nature10600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barclay AN, Wright GJ, Brooke G, Brown MH. CD200 and membrane protein interactions in the control of myeloid cells. Trends in Immunology. 2002;23:285–290. doi: 10.1016/s1471-4906(02)02223-8. [DOI] [PubMed] [Google Scholar]
- Batchelor PE, Liberatore GT, Wong JY, Porritt MJ, Frerichs F, Donnan GA, Howells DW. Activated macrophages and microglia induce dopaminergic sprouting in the injured striatum and express brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor. Journal of Neuroscience. 1999;19:1708–1716. doi: 10.1523/JNEUROSCI.19-05-01708.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beattie EC, Stellwagen D, Morishita W, Bresnahan JC, Ha BK, Von Zastrow M, et al. Control of synaptic strength by glial TNFalpha. Science. 2002;295:2282–2285. doi: 10.1126/science.1067859. [DOI] [PubMed] [Google Scholar]
- Bechmann I, Peter S, Beyer M, Gimsa U, Nitsch R. Presence of B7–2 (CD86) and lack of B7–1 (CD(80) on myelin phagocytosing MHC-II-positive rat microglia is associated with nondestructive immunity in vivo. FASEB Journal. 2001;15:1086–1088. doi: 10.1096/fj.00-0563fje. [DOI] [PubMed] [Google Scholar]
- Bernardino L, Balosso S, Ravizza T, Marchi N, Ku G, Randle JC, et al. Inflammatory events in hippocampal slice cultures prime neuronal susceptibility to excitotoxic injury: a crucial role of P2X7 receptor-mediated IL-1beta release. Journal of Neurochemistry. 2008;106:271–280. doi: 10.1111/j.1471-4159.2008.05387.x. [DOI] [PubMed] [Google Scholar]
- Beschorner R, Simon P, Schauer N, Mittelbronn M, Schluesener HJ, Trautmann K, Dietz K, Meyermann R. Reactive astrocytes and activated microglial cells express EAAT1, but not EAAT2, reflecting a neuroprotective potential following ischaemia. Histopathology. 2007;50:897–910. doi: 10.1111/j.1365-2559.2007.02703.x. [DOI] [PubMed] [Google Scholar]
- Bessis A, Bechade C, Bernard D, Roumier A. Microglial control of neuronal death and synaptic properties. Glia. 2007;55:233–238. doi: 10.1002/glia.20459. [DOI] [PubMed] [Google Scholar]
- Biber K, Neumann H, Inoue K, Boddeke HW. Neuronal ‘On’ and ‘Off’ signals control microglia. Trends in Neurosciences. 2007;30:596–602. doi: 10.1016/j.tins.2007.08.007. [DOI] [PubMed] [Google Scholar]
- Biber K, Tsuda M, Tozaki-Saitoh H, Tsukamoto K, Toyomitsu E, Masuda T, et al. Neuronal CCL21 up-regulates microglia P2X4 expression and initiates neuropathic pain development. EMBO Journal. 2011;30:1864–1873. doi: 10.1038/emboj.2011.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucsein C, Zacharias R, Farber K, Pavlovic S, Hanisch UK, Kettenmann H. Purinergic receptors on microglial cells: functional expression in acute brain slices and modulation of microglial activation in vitro. The European Journal of Neuroscience. 2003;17:2267–2276. doi: 10.1046/j.1460-9568.2003.02663.x. [DOI] [PubMed] [Google Scholar]
- Bruce-Keller AJ. Microglial-neuronal interactions in synaptic damage and recovery. Journal of Neuroscience Research. 1999;58:191–201. doi: 10.1002/(sici)1097-4547(19991001)58:1<191::aid-jnr17>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Buchanan JB, Sparkman NL, Chen J, Johnson RW. Cognitive and neuroinflammatory consequences of mild repeated stress are exacerbated in aged mice. Psychoneuroendocrinology. 2008;33:755–765. doi: 10.1016/j.psyneuen.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogunovic M, Ginhoux F, Wagers A, Loubeau M, Isola LM, Lubrano L, et al. Identification of a radio-resistant and cycling dermal dendritic cell population in mice and men. Journal of Experimental Medicine. 2006;203:2627–2638. doi: 10.1084/jem.20060667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cajal R. Contribucion al conocimiento de la meuroglia del cerebro humano. Trab Lab Investigative Biology. 1913:11. [Google Scholar]
- Cardona AE, Pioro EP, Sasse ME, Kostenko V, Cardona SM, Dijkstra IM. Control of microglial neurotoxicity by the fractalkine receptor. Nature Neuroscience. 2006;9:917–924. doi: 10.1038/nn1715. [DOI] [PubMed] [Google Scholar]
- Carson MJ, Thrash JC, Walter B. The cellular response in neuroinflammation: The role of leukocytes, microglia and astrocytes in neuronal death and survival. Clinical Neuroscience Research. 2006a;6:237–245. doi: 10.1016/j.cnr.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson MJ, Doose JM, Melchior B, Schmid CD, Ploix CC. CNS immune privilege: hiding in plain sight. Immunology Review. 2006b;213:48–65. doi: 10.1111/j.1600-065X.2006.00441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson MJ, Reilly CR, Sutcliffe JG, Lo D. Mature microglia resemble immature antigen-presenting cells. Glia. 1998;22:72–85. doi: 10.1002/(sici)1098-1136(199801)22:1<72::aid-glia7>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Carson MJ, Bilousova TV, Puntambekar SS, Melchior B, Doose JM, Ethell IM. A rose by any other name? The potential consequences of microglial heterogeneity during CNS health and disease. Neurotherapeutics. 2007;4:571–579. doi: 10.1016/j.nurt.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartier L, Hartley O, Dubois-Dauphin M, Krause KH. Chemokine receptors in the central nervous system: role in brain inflammation and neurodegenerative diseases. Brain Research Reviews. 2005;48:16–42. doi: 10.1016/j.brainresrev.2004.07.021. [DOI] [PubMed] [Google Scholar]
- Caso JR, Pradillo JM, Hurtado O, Leza JC, Moro MA, Lizasoain I. Toll-like receptor 4 is involved in subacute stress-induced neuroinflammation and in the worsening of experimental stroke. Stroke. 2008;39:1314–1320. doi: 10.1161/STROKEAHA.107.498212. [DOI] [PubMed] [Google Scholar]
- Chamak B, Mallat M. Fibronectin and laminin regulate the in vitro differentiation of microglial cells. Neuroscience. 1991;45:513–527. doi: 10.1016/0306-4522(91)90267-r. [DOI] [PubMed] [Google Scholar]
- Chan WY, Kohsaka S, Rezaie P. Brain Research Brain Research Review. 2007;53:344–354. doi: 10.1016/j.brainresrev.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Chen M, Forrester JV, Xu H. Dysregulation in retinal para-inflammation and age-related retinal degeneration in CCL2 or CCR2 deficient mice. PLoS One. 2011;6(8):e22818. doi: 10.1371/journal.pone.0022818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi BH. Hematogenous cells in the central nervous system of developing human embryos and fetuses. Journal of Comparative Neurology. 1981;196:683–694. doi: 10.1002/cne.901960412. [DOI] [PubMed] [Google Scholar]
- Choi HB, Ryu JK, Kim SU, McLarnon JG. Modulation of the purinergic P2X7 receptor attenuates lipopolysaccharide-mediated microglial activation and neuronal damage in inflamed brain. Journal of Neuroscience. 2007;27:4957–4968. doi: 10.1523/JNEUROSCI.5417-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu Y, Jin X, Parada I, Pesic A, Stevens B, Barres B, Prince DA. Enhanced synaptic connectivity and epilepsy in C1q knockout mice. Proceedings of the National Academy of Sciences USA. 2010;107:7975–7980. doi: 10.1073/pnas.0913449107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cingolani LA, Thalhammer A, Yu LM, Catalano M, Ramos T, Colicos MA, Goda Y. Activity-dependent regulation of synaptic AMPA receptor composition and abundance by beta3 integrins. Neuron. 2008;58:749–762. doi: 10.1016/j.neuron.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colton CA, Wilcock DM. Assessing activation states in microglia. CNS & Neurological Disorders Drug Targets. 2010;9:174–191. doi: 10.2174/187152710791012053. [DOI] [PubMed] [Google Scholar]
- Coull JA, Beggs S, Boudreau D, Boivin D, Tsuda M, Inoue K, et al. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature. 2005;438:1017–1021. doi: 10.1038/nature04223. [DOI] [PubMed] [Google Scholar]
- Coyle PK. Dissecting the immune component of neurologic disorders: a grand challenge for the 21st century. Frontiers in Neurology. 2011;2:37. doi: 10.3389/fneur.2011.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuadros MA, Navascues J. The origin and differentiation of microglial cells during development. Progress in Neurobiology. 1998;56:173–189. doi: 10.1016/s0301-0082(98)00035-5. [DOI] [PubMed] [Google Scholar]
- Dalmau I, Vela JM, Gonzalez B, Castellano B. Expression of purine metabolism-related enzymes by microglial cells in the developing rat brain. The Journal of Comparative Neurology. 1998;398:333–346. doi: 10.1002/(sici)1096-9861(19980831)398:3<333::aid-cne3>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Dalmau I, Finsen B, Tonder N, Zimmer J, Gonzalez B, Castellano B. Development of microglia in the prenatal rat hippocampus. The Journal of Comparative Neurology. 1997;377:70–84. [PubMed] [Google Scholar]
- Dalmau I, Vela JM, Gonzalez B, Finsen B, Castellano B. Dynamics of microglia in the developing rat brain. The Journal of Comprative Neurology. 2003;458:144–157. doi: 10.1002/cne.10572. [DOI] [PubMed] [Google Scholar]
- Damani MR, Zhao L, Fontainhas AM, Amaral J, Fariss RN, Wong WT. Age-related alterations in the dynamic behavior of microglia. Aging Cell. 2011;10:263–276. doi: 10.1111/j.1474-9726.2010.00660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dare E, Schulte G, Karovic O, Hammarberg C, Fredholm BB. Modulation of glial cell functions by adenosine receptors. Physiology & Behavior. 2007;92:15–20. doi: 10.1016/j.physbeh.2007.05.031. [DOI] [PubMed] [Google Scholar]
- Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, et al. ATP mediates rapid microglial response to local brain injury in vivo. Nature Neuroscience. 2005;8:752–758. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- Davoust N, Vuaillat C, Cavillon G, Domenget C, Hatterer E, Bernard A, et al. Bone marrow CD34+/B220+ progenitors target the inflamed brain and display in vitro differentiation potential toward microglia. FASEB Journal. 2006;20:2081–2092. doi: 10.1096/fj.05-5593com. [DOI] [PubMed] [Google Scholar]
- de Jong EK, Dijkstra IM, Hensens M, et al. Vesicle mediated transport and release of CCL21 in endangered neurons: a possible explanation for microglia activation remote from a primary lesion. Journal of Neuroscience. 2005;25:7548–7557. doi: 10.1523/JNEUROSCI.1019-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Rio Hortega P. Microglia. In: Penfield W, editor. Cytology and cellular pathology of the nervous system. New York: Hoeber; 1932. pp. 481–584. [Google Scholar]
- Dijkstra IM, Hulshof S, van der Valk P, Boddeke HWGM, Biber K. Cutting edge: activity of human adult microglia in response to CC chemokine ligand 21. Journal of Immunology. 2004;172:2744–2747. doi: 10.4049/jimmunol.172.5.2744. [DOI] [PubMed] [Google Scholar]
- Dilger RN, Johnson RW. Aging, microglial cell priming, and the discordant central inflammatory response to signals from the peripheral immune system. Journal of Leukocyte Biology. 2008;84:932–939. doi: 10.1189/jlb.0208108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draheim HJ, Prinz M, Weber JR, Weiser T, Kettenmann H, Hanisch UK. Induction of potassium channels in mouse brain microglia: cells acquire responsiveness to pneumococcal cell wall components during late development. Neuroscience. 1999;89:1379–1390. doi: 10.1016/s0306-4522(98)00407-2. [DOI] [PubMed] [Google Scholar]
- Duan H, Wearne SL, Rocher AB, Macedo A, Morrison JH, Hof PR. Age-related dendritic and spine changes in corticocortically projecting neurons in macaque monkeys. Cerebellar Cortex. 2003;13:950–961. doi: 10.1093/cercor/13.9.950. [DOI] [PubMed] [Google Scholar]
- Durafourt BA, Moore CS, Zammit DA, Johnson TA, Zaguia F, Guiot MC, et al. Comparison of polarization properties of human adult microglia and blood-derived macrophages. Glia. 2012;60:717–727. doi: 10.1002/glia.22298. [DOI] [PubMed] [Google Scholar]
- Eglitis MA, Mezey E. Hematopoietic cells differentiate into both microglia and macroglia in the brains of adult mice. Proceedings of the National Academy of Sciences USA. 1997;94:4080–4085. doi: 10.1073/pnas.94.8.4080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erblich B, Zhu L, Etgen AM, Dobrenis K, Pollard JW. Absence of colony stimulation factor-1 receptor results in loss of microglia, disrupted brain development and olfactory deficits. PloS One. 2011;6:e26317. doi: 10.1371/journal.pone.0026317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esiri MM, al Izzi MS, Reading MC. Macrophages, microglial cells, and HLA-DR antigens in fetal and infant brain. Journal of Clinical Pathology. 1991;44:102–106. doi: 10.1136/jcp.44.2.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farber K, Pannasch U, Kettenmann H. Dopamine and noradrenaline control distinct functions in rodent microglial cells. Molecular and Cellular Neurosciences. 2005;29:128–138. doi: 10.1016/j.mcn.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Farina C, Krumbholz M, Giese T, Hartmann G, Aloisi F, Meinl E. Preferential expression and function of Toll-like receptor 3 in human astrocytes. Journal of Neuroimmunology. 2005;159:12–19. doi: 10.1016/j.jneuroim.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Fenn AM, Henry CJ, Huang Y, Dugan A, Godbout JP. Lipopolysaccharide-induced interleukin (IL)-4 receptor-α expression and corresponding sensitivity to the M2 promoting effects of IL-4 are impaired in microglia of aged mice. Brain Behavior Immunity. 2012;26:766–777. doi: 10.1016/j.bbi.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari D, Chiozzi P, Falzoni S, Hanau S, Di Virgilio F. Purinergic modulation of interleukin-1 beta release from microglial cells stimulated with bacterial endotoxin. The Journal of Experimental Medicine. 1997;185:579–582. doi: 10.1084/jem.185.3.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira R, Santos T, Goncalves J, Baltazar G, Ferreira L, Agasse F, Bernardino L. Histamine modulates microglia function. Journal of Neuroinflammation. 2012;9:90. doi: 10.1186/1742-2094-9-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch CE, Morgan TE, Rozovsky I, et al. Microglia and aging in the brain. In: Streit WJ, editor. Microglia in the regenerating and degenerating CNS. New York: Spriner Verlag; 2002. pp. 275–305. [Google Scholar]
- Fiske BK, Brunjes PC. Microglial activation in the developing rat olfactory bulb. Neuroscience. 2000;96:807–815. doi: 10.1016/s0306-4522(99)00601-6. [DOI] [PubMed] [Google Scholar]
- Flanary BE, Sammons NW, Nguyen C, Walker D, Streit WJ. Evidence that aging and amyloid promote microglial cell senescence. Rejuvenation Research. 2007;10:61–74. doi: 10.1089/rej.2006.9096. [DOI] [PubMed] [Google Scholar]
- Flanary BE, Streit WJ. Progressive telomere shortening occurs in cultured rat microglia, but not astrocytes. Glia. 2004;45:75–88. doi: 10.1002/glia.10301. [DOI] [PubMed] [Google Scholar]
- Floden AM, Combs CK. Microglia demonstrate age-dependent interaction with amyloid-beta fibrils. Journal of Alzheimer’s disease : JAD. 2011;25:279–293. doi: 10.3233/JAD-2011-101014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontainhas AM, Wang M, Liang KJ, Chen S, Mettu P, Damani M, Fariss RN, Li W, Wong WT. Microglial morphology and dynamic behavior is regulated by ionotropic glutamatergic and GABAergic neurotransmission. PLoS One. 2011 Jan 25;6(1):e15973. doi: 10.1371/journal.pone.0015973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MG, Barrientos RM, Biedenkapp JC, Rudy JW, Watkins LR, Maier SF. mRNA up-regulation of MHC II and pivotal pro-inflammatory genes in normal brain aging. Neurobiology of Aging. 2006;27:717–722. doi: 10.1016/j.neurobiolaging.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, APIJ, Jacobson KA, Klotz KN, Linden J. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacological Reviews. 2001;53:527–552. [PMC free article] [PubMed] [Google Scholar]
- Fredholm BB, Chern Y, Franco R, Sitkovsky M. Aspects of the general biology of adenosine A2A signaling. Progress in neurobiology. 2007;83:263–276. doi: 10.1016/j.pneurobio.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Friggeri A, Yang Y, Banerjee S, Park YJ, Liu G, Abraham E. HMGB1 inhibits macrophage activity in efferocytosis through binding to the αvβ3-integrin. American Journal of Physiology. 2010;299:C1267–C1276. doi: 10.1152/ajpcell.00152.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friggeri A, Banerjee S, Biswas S, et al. Participation of the receptor for advanced glycation end products in efferocytosis. Journal of Immunology. 2011;186:6191–6198. doi: 10.4049/jimmunol.1004134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto E, Miki A, Mizoguti H. Histochemical study of the differentiation of microglial cells in the developing human cerebral hemispheres. Journal of Anatomy. 1989;166:253–264. [PMC free article] [PubMed] [Google Scholar]
- Fujita H, Tanaka J, Toku K, Tateishi N, Suzuki Y, Matsuda S, Sakanaka M, Maeda N. Effects of GM-CSF and ordinary supplements on the ramification of microglia in culture: a morphometrical study. Glia. 1996;18:269–281. doi: 10.1002/(sici)1098-1136(199612)18:4<269::aid-glia2>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Garden GA, Moller T. Microglia biology in health and disease. Journal of Neuroimmune Pharmacology. 2006;1:127–137. doi: 10.1007/s11481-006-9015-5. [DOI] [PubMed] [Google Scholar]
- Gasque P, Neal JW, Singhrao SK, McGreal EP, Dean YD, Van BJ, Morgan BP. Roles of the complement system in human neurodegenerative disorders: pro-inflammatory and tissue remodeling activities. Molecular Neurobiology. 2002;25:1–17. doi: 10.1385/mn:25:1:001. [DOI] [PubMed] [Google Scholar]
- Gehrmann J, Matsumoto Y, Kreutzberg GW. Microglia: intrinsic immuneffector cell of the brain. Brain Research Brain Research Reviews. 1995;20:269–287. doi: 10.1016/0165-0173(94)00015-h. [DOI] [PubMed] [Google Scholar]
- Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330:841–845. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giulian D, Li J, Bartel S, Broker J, Li X, Kirkpatrick JB. Cell surface morphology identifies microglia as a distinct class of mononuclear phagocyte. The Journal of neuroscience Neuroscience. 1995;15:7712–7726. doi: 10.1523/JNEUROSCI.15-11-07712.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glezer I, Lapointe A, Rivest S. Innate immunity triggers oligodendrocyte progenitor reactivity and confines damages to brain injuries. FASEB Journal. 2006;20:750–752. doi: 10.1096/fj.05-5234fje. [DOI] [PubMed] [Google Scholar]
- Glezer I, Simard AR, Rivest S. Neuroprotective role of the innate immune system by microglia. Neuroscience. 2007;147:867–883. doi: 10.1016/j.neuroscience.2007.02.055. [DOI] [PubMed] [Google Scholar]
- Goerdt S, Politz O, Schledzewski K, Birk R, Gratchev A, Guillot P, et al. Alternative versus classical activation of macrophages. Pathobiology. 1999;67:222–226. doi: 10.1159/000028096. [DOI] [PubMed] [Google Scholar]
- Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32:593–604. doi: 10.1016/j.immuni.2010.05.007. [DOI] [PubMed] [Google Scholar]
- Graeber MB. Changing face of microglia. Science. 2010;330:783–788. doi: 10.1126/science.1190929. [DOI] [PubMed] [Google Scholar]
- Graeber MB, Streit WJ. Perivascular microglia defined. Trends in Neurosciences. 1990;13:366. doi: 10.1016/0166-2236(90)90020-B. [DOI] [PubMed] [Google Scholar]
- Grossmann R, Stence N, Carr J, Fuller L, Waite M, Dailey ME. Justavascular microglia migrate along brain microvessels following activation during early postnatal development. Glia. 2002;37:229–240. [PubMed] [Google Scholar]
- Guillemin GJ, Brew BJ. Microglia, macrophages, perivascular macrophages, and pericytes: a review of function and identification. Journal Leukocyte Biology. 2004;75:388–397. doi: 10.1189/jlb.0303114. [DOI] [PubMed] [Google Scholar]
- Hammarberg C, Schulte G, Fredholm BB. Evidence for functional adenosine A3 receptors in microglia cells. Journal of Neurochemistry. 2003;86:1051–1054. doi: 10.1046/j.1471-4159.2003.01919.x. [DOI] [PubMed] [Google Scholar]
- Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nature Neuroscience. 2007;10:1387–1394. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- Harry GJ, Kraft AD. Microglia in the developing brain: a potential target with lifetime effects. Neurotoxicology. 2012;33:191–206. doi: 10.1016/j.neuro.2012.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harry GJ, Lefebvre d’Hellencourt C, Bruccoleri A, Schmechel D. Age-dependent cytokine responses: trimethyltin hippocampal injury in wild-type, APOE knockout, and APOE4 mice. Brain, Behavior, and Immunity. 2000;14:288–304. doi: 10.1006/brbi.2000.0606. [DOI] [PubMed] [Google Scholar]
- Haynes SE, Hollopeter G, Yang G, Kurpius D, Dailey ME, Gan WB, et al. The P2Y12 receptor regulates microglial activation by extracellular nucleotides. Nature Neuroscience. 2006;9:1512–1519. doi: 10.1038/nn1805. [DOI] [PubMed] [Google Scholar]
- Heese K, Fiebich BL, Bauer J, Otten U. NF-kappaB modulates lipopolysaccharide-induced microglial nerve growth factor expression. Glia. 1998;22:401–407. doi: 10.1002/(sici)1098-1136(199804)22:4<401::aid-glia9>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Henry CJ, Huang Y, Wynne AM, Godbout JP. Peripheral lipopolysaccharide (LPS) challenge promotes microglial hyperactivity in aged mice that is associated with exaggerated induction of both pro-inflammatory IL-1beta and anti-inflammatory IL-10 cytokines. Brain Behavior and Immunity. 2009;23:309–317. doi: 10.1016/j.bbi.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hide I, Tanaka M, Inoue A, Nakajima K, Kohsaka S, Inoue K, Nakata Y. Extracellular ATP triggers tumor necrosis factor-alpha release from rat microglia. Journal of Neurochemistry. 2000;75:965–972. doi: 10.1046/j.1471-4159.2000.0750965.x. [DOI] [PubMed] [Google Scholar]
- Hoek RM, Ruuls SR, Murphy CA, Wright GJ, Goddard R, Zurawski SM, et al. Down-regulation of the macrophage lineage through interaction with OX2 (CD200) Science. 2000;290:1768–1771. doi: 10.1126/science.290.5497.1768. [DOI] [PubMed] [Google Scholar]
- Hof PR, Morrison JH. The aging brain: morphomolecular senescence of cortical circuits. Trends in Neuroscience. 2004;27:607–613. doi: 10.1016/j.tins.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Holm TH, Draeby S, Owens T. Microglia are required for astroglial toll-like receptor 4 response and for optimal TLR2 and TLR3 response. Glia. 2012;60:630–638. doi: 10.1002/glia.22296. [DOI] [PubMed] [Google Scholar]
- Hristova M, Cuthill D, Zbarsky V, Acosta-Saltos A, Wallace A, Blight K, et al. Activation and deactivation of periventricular white matter phagocytes during postnatal mouse development. Glia. 2010;58:11–28. doi: 10.1002/glia.20896. [DOI] [PubMed] [Google Scholar]
- Hu X, Li P, Guo Y, Wang H, Leak RK, Chen S, Gao Y, Chen J. Microglia/macrophage polarization dynamics reveal novel mechanism of injury expansion after focal cerebral ischemia. Stroke. 2012;43:3063–3070. doi: 10.1161/STROKEAHA.112.659656. [DOI] [PubMed] [Google Scholar]
- Hughes PM, Botham MS, Frentzel S, Mir A, Perry VH. Expression of fractalkine (CX3CL1) and its receptor, CX3CR1, during acute and chronic inflammation in the rodent CNS. Glia. 2002;37:314–327. [PubMed] [Google Scholar]
- Hung J, Chansard M, Ousman SS, Nguyen MD, Colicos MA. Activation of microglia by neuronal activity: results from a new in vitro paradigm based on neuronal-silicon interfacing technology. Brain Behavior and Immunity. 2010;24:31–40. doi: 10.1016/j.bbi.2009.06.150. [DOI] [PubMed] [Google Scholar]
- Hurley SD, Streit WJ. Griffonia simplicifolia II lectin labels a population of radial glial cells in the embryonic rat brain. Developmental Neuroscience. 1995;17:324–334. doi: 10.1159/000111302. [DOI] [PubMed] [Google Scholar]
- Hutchins KD, Dickson DW, Rashbaum WK, Lyman WD. Localization of morphologically distinct microglial populations in the developing human fetal brain: implications for ontogeny. Brain Research Developmental Brain Research. 1990;55:95–102. doi: 10.1016/0165-3806(90)90109-c. [DOI] [PubMed] [Google Scholar]
- Ifuku M, Farber K, Okuno Y, Yamakawa Y, Miyamoto T, Nolte C, et al. Bradykinin-induced microglial migration mediated by B1-bradykinin receptors depends on Ca2+ influx via reverse-mode activity of the Na+/Ca2+ exchanger. Journal of Neuroscience. 2007;27:13065–13073. doi: 10.1523/JNEUROSCI.3467-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamoto K, Leblond CP. Radioautographic investigation of gliogenesis in the corpus callosum of young rats. II. Origin of microglial cells. Journal of Comparative Neurology. 1978;180:139–163. doi: 10.1002/cne.901800109. [DOI] [PubMed] [Google Scholar]
- Inoue K, Nakajima K, Morimoto T, Kikuchi Y, Koizumi S, Illes P, Kohsaka S. ATP stimulation of Ca2+-dependent plasminogen release from cultured microglia. British Journal of Pharmacology. 1998;123:1304–1310. doi: 10.1038/sj.bjp.0701732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irino Y, Nakamura Y, Inoue K, Kohsaka S, Ohsawa K. Akt activation is involved in P2Y12 receptor-mediated chemotaxis of microglia. Journal of Neuroscience Research. 2008;86:1511–1519. doi: 10.1002/jnr.21610. [DOI] [PubMed] [Google Scholar]
- Jack CS, Arbour N, Manusow J, Montgrain V, Blain M, McCrea E, et al. TLR signaling tailors innate immune responses in human microglia and astrocytes. Journal of Immunology. 2005;175:4320–4330. doi: 10.4049/jimmunol.175.7.4320. [DOI] [PubMed] [Google Scholar]
- Jacobs B, Driscoll L, Schall M. Life-span dendritic and spine changes in areas 10 and 18 of human cortex: a quantitative Golgi study. Journal of Comparative Neurology. 1997;386:661–680. [PubMed] [Google Scholar]
- Jonakait GM, Wen Y, Wan Y, Ni L. Macrophage cell-conditioned medium promotes cholinergic differentiation of undifferentiated progenitors and synergizes with nerve growth factor action in the developing basal forebrain. Experimental Neurology. 2000;161:285–296. doi: 10.1006/exnr.1999.7255. [DOI] [PubMed] [Google Scholar]
- Kane CJ, Phelan KD, Han L, Smith RR, Xie J, Douglas JC, et al. Protection of neurons and microglia against ethanol in a mouse model of fetal alcohol spectrum disorders by peroxisomes proliferator-activated receptor-γ agonists. Brain Behavior and Immunity. 2011;25:S137–145. doi: 10.1016/j.bbi.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur C, Ling EA. Study of the transformation of amoeboid microglial cells into microglia labelled with the isolectin Griffonia simplicifolia in postnatal rats. Acta Anat (Basel) 1991;142:118–125. doi: 10.1159/000147175. [DOI] [PubMed] [Google Scholar]
- Kettenmann H, Banati R, Walz W. Electrophysiological behavior of microglia. Glia. 1993;7:93–101. doi: 10.1002/glia.440070115. [DOI] [PubMed] [Google Scholar]
- Kettenmann H, Hanisch U-K, Noda M, Verkhratsky A. Physiology of Microglia. Physiology Review. 2011;91:461–553. doi: 10.1152/physrev.00011.2010. [DOI] [PubMed] [Google Scholar]
- Kierdorf K, Erny E, Goldmann T, Sander V, Schulz C, Perdiguero EG, et al. Microglia emerge from erythromyeloid precursors via Pu.1- and Irf8-dependent pathways. Nature Neuroscience. 2013;16:273–279. doi: 10.1038/nn.3318. [DOI] [PubMed] [Google Scholar]
- Kigerl KA, Gensel JC, Ankeny DP, Alexander JK, Donnelly DJ, Popovich PG. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. Journal of Neuroscience. 2009;29:13435–13444. doi: 10.1523/JNEUROSCI.3257-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilic U, Kilic E, Matter CM, Bassetti CL, Hermann DM. TLR-4 deficiency protects against focal cerebral ischemia and axotomy-induced neurodegeneration. Neurobiology of Disease. 2008;31:33–40. doi: 10.1016/j.nbd.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Kim SU, de Vellis J. Microglia in health and disease. Journal of Neuroscience Research. 2005;81:302–313. doi: 10.1002/jnr.20562. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Ifergan I, Antel JP, Seguin R, Duddy M, Lapierre Y, et al. Type 2 monocyte and microglia differentiation mediated by glatiramer acetate therapy in patients with multiple sclerosis. Journal of Immunology. 2004;172:7144–7153. doi: 10.4049/jimmunol.172.11.7144. [DOI] [PubMed] [Google Scholar]
- Kostovic I, Judas M. Correlation between the sequential ingrowth of afferents and transient patterns of cortical lamination in preterm infants. Anatomical Record. 2002;267:1–6. doi: 10.1002/ar.10069. [DOI] [PubMed] [Google Scholar]
- Krabbe G, Matyash V, Pannasch U, Mamer L, Boddeke HW, Kettenmann H. Activation of serotonin receptors promotes microglial injury-induced motility but attenuates phagocytic activity. Brain, Behavior, and Immunity. 2012;26:419–428. doi: 10.1016/j.bbi.2011.12.002. [DOI] [PubMed] [Google Scholar]
- Krishnaswamy JK, Chu T, Eisenbarth SC. Beyond pattern recognition: NOD-like receptors in dendritic cells. Trends in Immunology. 2013 doi: 10.1016/j.it.2012.12.003. http://dx.doi.org/10.1016/j.it.2012.12.003. [DOI] [PMC free article] [PubMed]
- Landreth GE, Reed-Geaghan EG. Toll-like receptors in Alzheimer’s disease. Current topics in Microbiology and Immunology. 2009;336:137–153. doi: 10.1007/978-3-642-00549-7_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langosch JM, Gebicke-Haerter PJ, Norenberg W, Illes P. Characterization and transduction mechanisms of purinoceptors in activated rat microglia. British Journal of Pharmacology. 1994;113:29–34. doi: 10.1111/j.1476-5381.1994.tb16169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson LJ, Perry VH, Dri P, Gordon S. Heterogeneity in the distribution and morphology of microglia in the normal adult mouse brain. Neuroscience. 1990;39:151–170. doi: 10.1016/0306-4522(90)90229-w. [DOI] [PubMed] [Google Scholar]
- Lawson LJ, Perry VH, Gordon S. Turnover of resident microglia in the normal adult mouse brain. Neuroscience. 1992;48:405–415. doi: 10.1016/0306-4522(92)90500-2. [DOI] [PubMed] [Google Scholar]
- Lee S, Varvel NH, Konerth ME, et al. CX3CR1 deficiency alters microglial activation and reduces β-amyloid deposition in two Alzheimer’s disease mouse models. American Journal of Pathology. 2010;177:2549–2562. doi: 10.2353/ajpath.2010.100265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehnardt S. Innate immunity and neuroinflammation in the CNS: the role of microglia in Toll-like receptor-mediated neuronal injury. Glia, 2010. 2010;58:253–63. doi: 10.1002/glia.20928. [DOI] [PubMed] [Google Scholar]
- Lehnardt S, Lehmann S, Kaul D, Tschimmel K, Hoffmann O, Cho S, et al. Toll-like receptor 2 mediates CNS injury in focal cerebral ischemia. Journal of neuroimmunology. 2007;190:28–33. doi: 10.1016/j.jneuroim.2007.07.023. [DOI] [PubMed] [Google Scholar]
- Lehnardt S, Massillon L, Follett P, Jensen FE, Ratan R, Rosenberg PA, et al. Activation of innate immunity in the CNS triggers neurodegeneration through a Toll-like receptor 4-dependent pathway. Proceedings of the National Academy of Sciences USA. 2003;100:8514–8519. doi: 10.1073/pnas.1432609100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehnardt S, Schott E, Trimbuch T, Laubisch D, Krueger C, Wulczyn G, et al. A vicious cycle involving release of heat shock protein 60 from injured cells and activation of toll-like receptor 4 mediates neurodegeneration in the CNS. Journal of Neuroscience. 2008;28:2320–2331. doi: 10.1523/JNEUROSCI.4760-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz KM, Nugent BM, Haliyur R, McCarthy MM. Microglia are essential to masculinization of brain and behavior. Journal of Neuroscience. 2013;33:2761–2772. doi: 10.1523/JNEUROSCI.1268-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letiembre M, Hao W, Liu Y, Walter S, Mihaljevic I, Rivest S, et al. Innate immune receptor expression in normal brain aging. Neuroscience. 2007;146:248–254. doi: 10.1016/j.neuroscience.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Lima FR, Gervais A, Colin C, Izembart M, Neto VM, Mallat M. Regulation of microglial development: a novel role for thyroid hormone. Journal of Neuroscience. 2001;21:2028–2038. doi: 10.1523/JNEUROSCI.21-06-02028.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu GJ, Nagarajah R, Banati RB, Bennett MR. Glutamate induces directed chemotaxis of microglia. The European Journal of Neuroscience. 2009;29:1108–1118. doi: 10.1111/j.1460-9568.2009.06659.x. [DOI] [PubMed] [Google Scholar]
- Lo D, Feng L, Li L, Carson MJ, Crowley M, Pauza M, et al. Integrating innate and adaptive immunity in the whole animal. Immunological Reviews. 1999;169:225–239. doi: 10.1111/j.1600-065x.1999.tb01318.x. [DOI] [PubMed] [Google Scholar]
- Lopes KO, Sparks DL, Streit WJ. Microglial dystrophy in the aged and Alzheimer’s disease brain is associated with ferritin immunoreactivity. Glia. 2008;56:1048–1060. doi: 10.1002/glia.20678. [DOI] [PubMed] [Google Scholar]
- Luo XG, Ding JQ, Chen SD. Microglia in the aging brain: relevance to neurodegeneration. Molecular Neurodegeneration. 2010;5:12. doi: 10.1186/1750-1326-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons A, Downer EJ, Crotty S, Nolan YM, Mills KH, Lynch MA. CD200 ligand receptor interaction modulates microglial activation in vivo and in vitro: a role for IL-4. Journal of Neuroscience. 2007;27:8309–8313. doi: 10.1523/JNEUROSCI.1781-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma W, Zhao L, Wong WT. Microglia in the outer retina and their relevance to pathogenesis of age-related macular degeneration. Advances Experimental Medical Biology. 2012;723:37–42. doi: 10.1007/978-1-4614-0631-0_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma W, Coon S, Zhao L, Fariss RN, Wong WT. A2E accumulation influences retinal microglial activation and complement regulation. Neurobiology of Aging. 2013;34:943–960. doi: 10.1016/j.neurobiolaging.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallat M, Marin-Teva JL, Cheret C. Phagocytosis in the developing CNS: more than clearing the corpses. Current opinion in neurobiology. 2005;15:101–107. doi: 10.1016/j.conb.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Marin-Padilla M. Early vascularization of the embryonic cerebral cortex: Golgi and electron microscopic studies. J Comp Neurol. 1985;241:237–249. doi: 10.1002/cne.902410210. [DOI] [PubMed] [Google Scholar]
- Marin-Teva JL, Dusart I, Colin C, Gervais A, van Rooijen N, Mallat M. Microglia promote the death of developing Purkinje cells. Neuron. 2004;41:535–547. doi: 10.1016/s0896-6273(04)00069-8. [DOI] [PubMed] [Google Scholar]
- Marshall GP, 2nd, Demir M, Steindler DA, Laywell ED. Subventricular zone microglia possess a unique capacity for massive in vitro expansion. Glia. 2008;56:1799–1808. doi: 10.1002/glia.20730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massengale M, Wagers AJ, Vogel H, Weissman IL. Hematopoietic cells maintain hematopoietic fates upon entering the brain. Journal of Experimental Medicine. 2005;201:1579–1589. doi: 10.1084/jem.20050030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda T, Croom D, Hida H, Kirov SA. Capillary blood flow around microglial somata determines dynamics of microglial processes in ischemic conditions. Glia. 2011;59:1744–1753. doi: 10.1002/glia.21220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto Y, Fujiwara M. Absence of donor-type major histocompatibility complex class I antigen-bearing microglia in the rat central nervous system of radiation bone marrow chimeras. Journal Neuroimmunology. 1987;17:71–82. doi: 10.1016/0165-5728(87)90032-4. [DOI] [PubMed] [Google Scholar]
- McGeer PL, McGeer EG. The inflammatory response system of brain: implications for therapy of Alzheimer and other neurodegenerative diseases. Brain Research Brain Research Review. 1995;21:195–218. doi: 10.1016/0165-0173(95)00011-9. [DOI] [PubMed] [Google Scholar]
- Medzhitov R, Janeway CA., Jr How does the immune system distinguish self from nonself? Seminars in Immunology. 2000;12:185–188. doi: 10.1006/smim.2000.0230. [DOI] [PubMed] [Google Scholar]
- McHugh D. GPR18 in Microglia: implications for the CNS and endocannabinoid system signalling. British Journal of Pharmacology. 2012 doi: 10.1111/j.1476-5381.2012.02019.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLarnon JG, Ryu JK, Walker DG, Choi HB. Upregulated expression of purinergic P2X(7) receptor in Alzheimer disease and amyloid-beta peptide-treated microglia and in peptide-injected rat hippocampus. Journal of Neuropathology and Experimental Neurology. 2006;65:1090–1097. doi: 10.1097/01.jnen.0000240470.97295.d3. [DOI] [PubMed] [Google Scholar]
- McMullan SM, Phanavanh B, Li GG, Barger SW. Metabotropic glutamate receptors inhibit microglial glutamate release. ASN Neuro. 2012;4(5):art:e00094. doi: 10.1042/AN20120044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherson CA, Aoyama M, Harry GJ. Interleukin (IL)-1 and IL-6 regulation of neural progenitor cell proliferation with hippocampal injury: differential regulatory pathways in the subgranular zone (SGZ) of the adolescent and mature mouse brain. Brain, Behavior, and Immunity. 2011;25:850–862. doi: 10.1016/j.bbi.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherson CA, Kraft AD, Harry GJ. Injury-induced neurogenesis: consideration of resident microglia as supportive of neural progenitor cells. Neurotoxicity Research. 2011;19:341–352. doi: 10.1007/s12640-010-9199-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melani A, Amadio S, Gianfriddo M, Vannucchi MG, Volonte C, Bernardi G, et al. P2X7 receptor modulation on microglial cells and reduction of brain infarct caused by middle cerebral artery occlusion in rat. Journal of Cerebral Blood Flow and Metabolism. 2006;26:974–982. doi: 10.1038/sj.jcbfm.9600250. [DOI] [PubMed] [Google Scholar]
- Michaelson MD, Bieri PL, Mehler MF, Xu H, Arezzo JC, Pollard JW, et al. CSF-1 deficiency in mice results in abnormal brain development. Development. 1996;122:2661–2672. doi: 10.1242/dev.122.9.2661. [DOI] [PubMed] [Google Scholar]
- Michelucci A, Heurtaux T, Grandbarbe L, Morga E, Heuschling P. Characterization of the microglial phenotype under specific pro-inflammatory and anti-inflammatory conditions: Effects of oligomeric and fibrillar amyloid-beta. Journal of Neuroimmunology. 2009;210:3–12. doi: 10.1016/j.jneuroim.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Mikita J, Dubourdieu-Cassagno N, Deloire MS, Vekris A, Biran M, Raffard G, et al. Altered M1/M2 activation patterns of monocytes in severe relapsing experimental rat model of multiple sclerosis. Amelioration of clinical status by M2 activated monocyte administration. Multiple Sclerosis. 2011;17:2–15. doi: 10.1177/1352458510379243. [DOI] [PubMed] [Google Scholar]
- Miller KR, Streit WJ. The effects of aging, injury and disease on microglial function: a case for cellular senescence. Neuron Glia Biology. 2007;3:245–253. doi: 10.1017/S1740925X08000136. [DOI] [PubMed] [Google Scholar]
- Mingam R, De Smedt V, Amedee T, Bluthe RM, Kelley KW, Dantzer R, et al. In vitro and in vivo evidence for a role of the P2X7 receptor in the release of IL-1 beta in the murine brain. Brain, Behavior, and Immunity. 2008;22:234–244. doi: 10.1016/j.bbi.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittelbronn M, Dietz K, Schluesener HJ, Meyermann R. Local distribution of microglia in the normal adult human central nervous system differs by up to one order of magnitude. Acta Neuropathology. 2001;101:249–255. doi: 10.1007/s004010000284. [DOI] [PubMed] [Google Scholar]
- Mody M, Cao Y, Cui Z, Tay KY, Shyong A, Shimizu E, Pham K, Schultz P, Welsh D, Tsien JZ. Genome-wide gene expression profiles of the developing mouse hippocampus. Proceedings of the National Academy of Science of the USA. 2001;98:8862–8867. doi: 10.1073/pnas.141244998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monier A, Evrard P, Gressens P, Verney C. Distribution and differentiation of microglia in the human encephalon during the first two trimesters of gestation. Journal of Comparative Neurology. 2006;499:565–582. doi: 10.1002/cne.21123. [DOI] [PubMed] [Google Scholar]
- Moran LB, Graeber MB. The facial nerve axotomy model. Brain Research Brain Research Reviews. 2004;44:154–178. doi: 10.1016/j.brainresrev.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nature reviews Immunology. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagamoto-Combs K, Morecraft RJ, Darling WG, Combs CK. Long-term gliosis and molecular changes in the cervical spinal cord of the rhesus monkey after traumatic brain injury. J Neurotrauma. 2010;27:565–585. doi: 10.1089/neu.2009.0966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neeper M, Schmidt AM, Brett J, Yan SD, Wang F, Pan YC, et al. Cloning and expression of a cell surface receptor for advanced glycosylation end products of proteins. Journal of Biological Chemistry. 1992;267:14998–15004. [PubMed] [Google Scholar]
- Neumann H. Control of glial immune function by neurons. Glia. 2001;36:191–199. doi: 10.1002/glia.1108. [DOI] [PubMed] [Google Scholar]
- Neumann H, Kotter MR, Franklin RJM. Debris clearance by microglia: an essential link between degeneration and regeneration. Brain. 2009;132:288–295. doi: 10.1093/brain/awn109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni L, Acevedo G, Muralidharan B, Padala N, To J, Jonakait GM. Toll-like receptor ligands and CD154 stimulate microglia to produce a factor(s) that promotes excess cholinergic differentiation in the developing rat basal forebrain: implications for neurodevelopmental disorders. Pediatric Research. 2007;61:15–20. doi: 10.1203/01.pdr.0000249981.70618.18. [DOI] [PubMed] [Google Scholar]
- Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- Nishiyori A, Minami M, Ohtani Y, Takami S, Yamamoto J, Kawaguchi N, et al. Localization of fractalkine and CX3CR1 mRNAs in rat brain: does fractalkine play a role in signaling from neuron to microglia? FEBS Letters. 1998;429:167–172. doi: 10.1016/s0014-5793(98)00583-3. [DOI] [PubMed] [Google Scholar]
- Noda M, Nakanishi H, Nabekura J, Akaike N. AMPA-kainate subtypes of glutamate receptor in rat cerebral microglia. Journal of Neuroscience. 2000;20:251–258. doi: 10.1523/JNEUROSCI.20-01-00251.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norden DM, Godbout JP. Microglia of the aged brain: primed to be activated and resistant to regulation. Neuropathology and Applied Neurobiology. 2012;39:19–34. doi: 10.1111/j.1365-2990.2012.01306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North RA. Molecular physiology of P2X receptors. Physiological Reviews. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- Ogata T, Chuai M, Morino T, Yamamoto H, Nakamura Y, Schubert P. Adenosine triphosphate inhibits cytokine release from lipopolysaccharide-activated microglia via P2y receptors. Brain Research. 2003;981:174–183. doi: 10.1016/s0006-8993(03)03028-2. [DOI] [PubMed] [Google Scholar]
- Ogura K, Ogawa M, Yoshida M. Effects of ageing on microglia in the normal rat brain: immunohistochemical observations. Neuroreport. 1994;5:1224–1226. doi: 10.1097/00001756-199406020-00016. [DOI] [PubMed] [Google Scholar]
- Ohsawa K, Irino Y, Nakamura Y, Akazawa C, Inoue K, Kohsaka S. Involvement of P2X4 and P2Y12 receptors in ATP-induced microglial chemotaxis. Glia. 2007;55:604–616. doi: 10.1002/glia.20489. [DOI] [PubMed] [Google Scholar]
- Ohsawa K, Irino Y, Sanagi T, Nakamura Y, Suzuki E, Inoue K, et al. P2Y12 receptor-mediated integrin-beta1 activation regulates microglial process extension induced by ATP. Glia. 2010;58:790–801. doi: 10.1002/glia.20963. [DOI] [PubMed] [Google Scholar]
- Olah M, Biber K, Vinet J, Boddeke HW. Microglia phenotype diversity. CNS & Neurological Disorders Drug Targets. 2011;10:108–118. doi: 10.2174/187152711794488575. [DOI] [PubMed] [Google Scholar]
- Olah M, Amor S, Brouwer N, Vinet J, Eggen B, Biber K, et al. Identification of a microglia phenotype supportive of remyelination. Glia. 2012;60:306–321. doi: 10.1002/glia.21266. [DOI] [PubMed] [Google Scholar]
- Olson JK, Miller SD. Microglia initiate central nervous system innate and adaptive immune responses through multiple TLRs. Journal of Immunology. 2004;173:3916–3924. doi: 10.4049/jimmunol.173.6.3916. [DOI] [PubMed] [Google Scholar]
- Orlowski D, Soltys Z, Janeczko K. Morphological development of microglia in the postnatal rat brain. A quantitative study. International Journal of Developmental Neuroscience. 2003;21:445–450. doi: 10.1016/j.ijdevneu.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, et al. Synaptic pruning by microglia is necessary for normal brain development. Science. 2011;333:1456–1458. doi: 10.1126/science.1202529. [DOI] [PubMed] [Google Scholar]
- Parvathenani LK, Tertyshnikova S, Greco CR, Roberts SB, Robertson B, Posmantur R. P2X7 mediates superoxide production in primary microglia and is up-regulated in a transgenic mouse model of Alzheimer’s disease. Journal of Biological Chemistry. 2003;278:13309–13317. doi: 10.1074/jbc.M209478200. [DOI] [PubMed] [Google Scholar]
- Perego C, Fumagalli S, De Simoni MG. Temporal pattern of expression and colocalization of microglia/macrophage phenotype markers following brain ischemic injury in mice. Journal of Neuroinflammation. 2011;8:174. doi: 10.1186/1742-2094-8-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry VH, Hume DA, Gordon S. Immunohistochemical localization of macrophages and microglia in the adult and developing mouse brain. Neuroscience. 1985;15:313–326. doi: 10.1016/0306-4522(85)90215-5. [DOI] [PubMed] [Google Scholar]
- Perry VH, Matyszak MK, Fearn S. Altered antigen expression of microglia in the aged rodent CNS. Glia. 1993;7:60–67. doi: 10.1002/glia.440070111. [DOI] [PubMed] [Google Scholar]
- Perry VH, O’Connor V. The role of microglia in synaptic stripping and synaptic degeneration: a revised perspective. ASN Neuro. 2010;2:e00047. doi: 10.1042/AN20100024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pocock JM, Kettenmann H. Neurotransmitter receptors on microglia. Trends in Neurosciences. 2007;30:527–535. doi: 10.1016/j.tins.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Pont-Lezica L, Bechade C, Belarif-Cantaut Y, Pascual O, Bessis A. Physiological roles of microglia during development. Journal of Neurochemistry. 2011;119:901–908. doi: 10.1111/j.1471-4159.2011.07504.x. [DOI] [PubMed] [Google Scholar]
- Priller J, Haas CA, Reddington M, Kreutzberg GW. Calcitonin gene-related peptide and ATP induce immediate early gene expression in cultured rat microglial cells. Glia. 1995;15:447–457. doi: 10.1002/glia.440150408. [DOI] [PubMed] [Google Scholar]
- Priller J, Flugel A, Wehner T, Boentert M, Haas CA, et al. Targeting gene-modified hematopoietic cells to the central nervous system: use of green fluorescent protein uncovers microglial engraftment. Nature Medicine. 2001;7:1356–1361. doi: 10.1038/nm1201-1356. [DOI] [PubMed] [Google Scholar]
- Rakic S, Zecevic N. Programmed cell death in the developing human telencephalon. The European journal of neuroscience. 2000;12:2721–2734. doi: 10.1046/j.1460-9568.2000.00153.x. [DOI] [PubMed] [Google Scholar]
- Ransohoff RM, Perry VH. Microglial physiology: unique stimuli, specialized responses. Annual Review of Immunology. 2009;27:119–145. doi: 10.1146/annurev.immunol.021908.132528. [DOI] [PubMed] [Google Scholar]
- Rappold PM, Lynd-Balta E, Joseph SA. P2X7 receptor immunoreactive profile confined to resting and activated microglia in the epileptic brain. Brain Research. 2006;1089:171–178. doi: 10.1016/j.brainres.2006.03.040. [DOI] [PubMed] [Google Scholar]
- Ravichandran KS. “Recruitment signals” from apoptotic cells: invitation to a quiet meal. Cell. 2003;113:817–820. doi: 10.1016/s0092-8674(03)00471-9. [DOI] [PubMed] [Google Scholar]
- Reed-Geaghan EG, Savage JC, Hise AG, Landreth GE. CD14 and toll-like receptors 2 and 4 are required for fibrillar A{beta}-stimulated microglial activation. Journal of Neuroscience. 2009;29:11982–11992. doi: 10.1523/JNEUROSCI.3158-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezaie P, Dean A, Male D, Ulfig N. Microglia in the cerebral wall of the human telencephalon at second trimester. Cerebral cortex. 2005;15:938–949. doi: 10.1093/cercor/bhh194. [DOI] [PubMed] [Google Scholar]
- Rezaie P, Male D. Colonisation of the developing human brain and spinal cord by microglia: a review. Microscopy Research and Technique. 1999;45:359–382. doi: 10.1002/(SICI)1097-0029(19990615)45:6<359::AID-JEMT4>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Rigato C, Buckinx R, Le-Corronc H, Rigo JM, Legendre P. Pattern of invasion of the embryonic mouse spinal cord by microglial cells at the time of the onset of functional neuronal networks. Glia. 2011;59:675–695. doi: 10.1002/glia.21140. [DOI] [PubMed] [Google Scholar]
- Rochefort N, Quenech’du N, Watroba L, Mallat M, Giaume C, Milleret C. Microglia and astrocytes may participate in the shaping of visual callosal projections during postnatal development. Journal of Physiology Paris. 2002;96:183–92. doi: 10.1016/s0928-4257(02)00005-0. [DOI] [PubMed] [Google Scholar]
- Rodgers JM, Miller SD. Cytokine control of inflammation and repair in the pathology of multiple sclerosis. Yale Journal of Biology and Medicine. 2012;85:447–468. [PMC free article] [PubMed] [Google Scholar]
- Rosczyk HA, Sparkman NL, Johnson RW. Neuroinflammation and cognitive function in aged mice following minor surgery. Experimental Gerontology. 2008;43:840–846. doi: 10.1016/j.exger.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowan RA, Maxwell DS. Patterns of vascular sprouting in the postnatal development of the cerebral cortex of the rat. American Journal of Anatomy. 1981;160:247–255. doi: 10.1002/aja.1001600303. [DOI] [PubMed] [Google Scholar]
- Rymo SF, Gerhardt H, Wolfhagen Sand F, Lang R, Uv A, Betsholtz C. A two-way communication between microglial cells and angiogenic sprouts regulates angiogenesis in aortic ring cultures. PloS One. 2011;6:e15846. doi: 10.1371/journal.pone.0015846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samorajski T. How the human brain responds to aging. Journal of the American Geriatric Society. 1976;24:4–11. doi: 10.1111/j.1532-5415.1976.tb03246.x. [DOI] [PubMed] [Google Scholar]
- Sandhir R, Onyszchuk G, Berman NE. Exacerbated glial response in the aged mouse hippocampus following controlled cortical impact injury. Experimental Neurology. 2008;213:372–380. doi: 10.1016/j.expneurol.2008.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer DP, Stevens B. Synapse elimination during development and disease: immune molecules take centre stage. Biochemical Society Transactions. 2010;38:476–481. doi: 10.1042/BST0380476. [DOI] [PubMed] [Google Scholar]
- Schlegelmilch T, Henke K, Peri F. Microglia in the developing brain: from immunity to behaviour. Current Opinion in Neurobiology. 2011;21:5–10. doi: 10.1016/j.conb.2010.08.004. [DOI] [PubMed] [Google Scholar]
- Schmid CD, Melchior B, Masek K, Puntambekar SS, Danielson PE, Lo DD, et al. Differential gene expression in LPS/IFNgamma activated microglia and macrophages: in vitro versus in vivo. Journal of Neurochemistry. 2009;109(Suppl 1):117–125. doi: 10.1111/j.1471-4159.2009.05984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid CD, Sautkulis LN, Danielson PE, Cooper J, Hasel KW, Hilbush BS, et al. Heterogeneous expression of the triggering receptor expressed on myeloid cells-2 on adult murine microglia. Journal of Neurochemistry. 2002;83:1309–1320. doi: 10.1046/j.1471-4159.2002.01243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt AM, Sahagan B, Nelson RB, Selmer J, Rothlein R, Bell JM. The role of RAGE in amyloid-beta peptide-mediated pathology in Alzheimer’s disease. Current Opinion in Investigational Drugs. 2009;10:672–680. [PubMed] [Google Scholar]
- Schmidt AM, Vianna M, Gerlach M, Brett J, Ryan J, Kao J, et al. Isolation and characterization of two binding proteins for advanced glycosylation end products from bovine lung which are present on the endothelial cell surface. Journal of Biological Chemistry. 1992;267:14987–14997. [PubMed] [Google Scholar]
- Schuitemaker A, Van Der Doef TF, Boellaard R, Van Der Flier WM, Yaqub M, Windhorst AD, et al. Microglial activation in healthy aging. Neurobiology of Aging. 2010;33:1067–1072. doi: 10.1016/j.neurobiolaging.2010.09.016. [DOI] [PubMed] [Google Scholar]
- Schulz C, Gomez-Perdiguero E, Chorro L, Szabo-Rogers H, Cagnard N, Kierdorf K, et al. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science. 2012;336(6077):86–90. doi: 10.1126/science.1219179. [DOI] [PubMed] [Google Scholar]
- Sedgwick JD, Schwender S, Imrich H, Dorries R, Butcher GW, Meulen W. Isolation and direct characterization of resident microglial cells from the normal and inflamed central nervous system. Proceedings of the National Academy of Sciences USA. 1991;88:I7438–7442. doi: 10.1073/pnas.88.16.7438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo DR, Kim SY, Kim KY, Lee HG, Moon JH, Lee JS, et al. Cross talk between P2 purinergic receptors modulates extracellular ATP-mediated interleukin-10 production in rat microglial cells. Experimental & Molecular Medicine. 2008;40:19–26. doi: 10.3858/emm.2008.40.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharaf A, Kriegistein K, Spittau B. Distribution of microglia in the postnatal murine nigrostraital system. Cell Tissue Research. 2010 Dec 19; doi: 10.1007/s00441-012-1537-y. E pub. [DOI] [PubMed] [Google Scholar]
- Sheffield LG, Berman NE. Microglial expression of MHC class II increases in normal aging of nonhuman primates. Neurobiology of Aging. 1998;19:47–55. doi: 10.1016/s0197-4580(97)00168-1. [DOI] [PubMed] [Google Scholar]
- Sheng JG, Mrak RE, Griffin WS. Enlarged and phagocytic, but not primed, interleukin-1 alpha-immunoreactive microglia increase with age in normal human brain. Acta Neuropathologica. 1998;95:229–234. doi: 10.1007/s004010050792. [DOI] [PubMed] [Google Scholar]
- Sierra A, Gottfried-Blackmore AC, Mcewen BS, Bulloch K. Microglia derived from aging mice exhibit an altered inflammatory profile. Glia. 2007;55:412–424. doi: 10.1002/glia.20468. [DOI] [PubMed] [Google Scholar]
- Sierra A, Encinas JM, Deudero JJ, Chancey JH, Enikolopov G, Overstreet-Wadiche LS, et al. Microglia shape adult hippocampal neurogenesis through apoptosis-coupled phagocytosis. Cell Stem Cell. 2010;7:483–495. doi: 10.1016/j.stem.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siffrin V, Brandt AU, Herz J, Zipp F. New insights into adaptive immunity in chronic neuroinflammation. Advances in Immunology. 2007;96:1–40. doi: 10.1016/S0065-2776(07)96001-0. [DOI] [PubMed] [Google Scholar]
- Simard AR, Soulet D, Gowing G, Julien JP, Rivest S. Bone marrow-derived microglia play a critical role in restricting senile plaque formation in Alzheimer’s disease. Neuron. 2006;49:489–502. doi: 10.1016/j.neuron.2006.01.022. [DOI] [PubMed] [Google Scholar]
- Sorokin SP, Hoyt RR, Jr, Blunt DG, McNelly NA. Macrophage development: II. Early ontogeny of macrophage populations in brain, liver, and lungs of rat embryos as revealed by a lectin marker. Anatomical Record. 1992;232:527–550. doi: 10.1002/ar.1092320410. [DOI] [PubMed] [Google Scholar]
- Sparkman NL, Martin LA, Calvert WS, Boehm GW. Effects of intraperitoneal lipopolysaccharide on Morris maze performance in year-old and 2-month-old female C57BL/6J mice. Behavioral Brain Research. 2005;159:145–151. doi: 10.1016/j.bbr.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Srikanth V, Maczurek A, Phan T, Steele M, Westcott B, Juskiw D, et al. Advanced glycation endproducts and their receptor RAGE in Alzheimer’s disease. Neurobiology of Aging. 2011;32:763–777. doi: 10.1016/j.neurobiolaging.2009.04.016. [DOI] [PubMed] [Google Scholar]
- Stellwagen D, Malenka RC. Synaptic scaling mediated by glial TNF-alpha. Nature. 2006;440:1054–1059. doi: 10.1038/nature04671. [DOI] [PubMed] [Google Scholar]
- Stevens B, Allen NJ, Vazquez LE, Howell GR, Christopherson KS, Nouri N, et al. The classical complement cascade mediates CNS synapse elimination. Cell. 2007;131:1164–1178. doi: 10.1016/j.cell.2007.10.036. [DOI] [PubMed] [Google Scholar]
- Stolzing A, Grune T. Neuronal apoptotic bodies: phagocytosis and degradation by primary microglial cells. The FASEB Journal. 2004;18:743–745. doi: 10.1096/fj.03-0374fje. [DOI] [PubMed] [Google Scholar]
- Stout RD, Jiang C, Matta B, Tietzel I, Watkins SK, Suttles J. Macrophages sequentially change their functional phenotype in response to changes in microenvironmental influences. Journal of Immunology. 2005;175:342–349. doi: 10.4049/jimmunol.175.1.342. [DOI] [PubMed] [Google Scholar]
- Streit WJ, Sammons NW, Kuhns AJ, Sparks DL. Dystrophic microglia in the aging human brain. Glia. 2004;45:208–212. doi: 10.1002/glia.10319. [DOI] [PubMed] [Google Scholar]
- Streit WJ, Xue QS. Alzheimer’s disease, neuroprotection, and CNS immunosenescence. Frontiers in Pharmacology. 2012;3:138. doi: 10.3389/fphar.2012.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit WJ, Xue QS. The brain’s aging immune system. Aging and Disease. 2010;1:254–261. [PMC free article] [PubMed] [Google Scholar]
- Streit WJ, Braak H, Xue QS, Bechmann I. Dystrophic (senescent) rather than activated microglial cells are associated with tau pathology and likely precede neurodegeneration in Alzheimer’s disease. Acta Neuropathologica. 2009;118:475–485. doi: 10.1007/s00401-009-0556-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit WJ, Graeber MB, Kreutzberg GW. Expression of Ia antigen on perivascular and microglial cells after sublethal and lethal motor neuron injury. Experimental Neurology. 1989;105:115–126. doi: 10.1016/0014-4886(89)90111-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit WJ, Miller KR, Lopes KO, Njie E. Microglial degeneration in the aging brain--bad news for neurons? Frontiers in Bioscience. 2008;13:3423–3438. doi: 10.2741/2937. [DOI] [PubMed] [Google Scholar]
- Sunnemark D, Eltayeb S, Nilsson M, Wallstrom E, Lassmann H, Olsson T, et al. CX3CL1 (freactalkine) and CX3CR1 expression in myelin oligodendrocyte glycoprotein-induced experimental autoimmune encephalomyelitis: kinetics and cellular origin. Journal Neuroinflammation. 2005;2:17. doi: 10.1186/1742-2094-2-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surprenant A, North RA. Signaling at purinergic P2X receptors. Annual Review of Physiology. 2009;71:333–359. doi: 10.1146/annurev.physiol.70.113006.100630. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Hide I, Ido K, Kohsaka S, Inoue K, Nakata Y. Production and release of neuroprotective tumor necrosis factor by P2X7 receptor-activated microglia. Journal of Neuroscience. 2004;24:1–7. doi: 10.1523/JNEUROSCI.3792-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson M, Aldskogius H. Synaptic density of axotomized hypoglossal motorneurons following pharmacological blockade of the microglial cell proliferation. Experimental Neurology. 1993;120:123–131. doi: 10.1006/exnr.1993.1046. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Rochford CD, Neumann H. Clearance of apoptotic neurons without inflammation by microglial triggering receptor expressed on myeloid cells-2. Journal of Experimental Medicine. 2005;201:647–657. doi: 10.1084/jem.20041611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takenouchi T, Sekiyama K, Sekigawa A, Fujita M, Waragai M, Sugama S, et al. P2X7 receptor signaling pathway as a therapeutic target for neurodegenerative diseases. Archivum Immunologiae et Therapiae Experimentalis. 2010;58:91–96. doi: 10.1007/s00005-010-0069-y. [DOI] [PubMed] [Google Scholar]
- Toyomitsu E, Tsuda M, Yamashita T, Tozaki-Saitoh H, Tanaka Y, Inoue K. CCL2 promotes P2X4 receptor trafficking to the cell surface of microglia. Purinergic Signalling. 2012;8:301–310. doi: 10.1007/s11302-011-9288-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trang T, Beggs S, Wan X, Salter MW. P2X4-receptor-mediated synthesis and release of brain-derived neurotrophic factor in microglia is dependent on calcium and p38-mitogen-activated protein kinase activation. Journal of Neuroscience. 2009;29:3518–3528. doi: 10.1523/JNEUROSCI.5714-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapp BD, Wujek JR, Criste GA, Jalabi W, Yin X, Kidd GJ, et al. Evidence for synaptic stripping by cortical microglia. Glia. 2007;55:360–368. doi: 10.1002/glia.20462. [DOI] [PubMed] [Google Scholar]
- Tremblay ME, Lowery RL, Majewska AK. Microglial interactions with synapses are modulated by visual experience. PLoS biology. 2010;8:e1000527. doi: 10.1371/journal.pbio.1000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay ME, Zettel ML, Ison JR, Allen PD, Majewska AK. Effects of aging and sensory loss on glial cells in mouse visual and auditory cortices. Glia. 2012;60:541–558. doi: 10.1002/glia.22287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda M, Shigemoto-Mogami Y, Koizumi S, Mizokoshi A, Kohsaka S, Salter MW, et al. P2X4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature. 2003;424:778–783. doi: 10.1038/nature01786. [DOI] [PubMed] [Google Scholar]
- Tsuda M, Toyomitsu E, Kometani M, Tozaki-Saitoh H, Inoue K. Mechanisms underlying fibronectin-induced up-regulation of P2X4R expression in microglia: distinct roles of PI3K-Akt and MEK-ERK signalling pathways. Journal of Cellular and Molecular Medicine. 2009;13:3251–3259. doi: 10.1111/j.1582-4934.2009.00719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmann L, Hatcher JP, Hughes JP, Chaumont S, Green PJ, Conquet F, et al. Up-regulation of P2X4 receptors in spinal microglia after peripheral nerve injury mediates BDNF release and neuropathic pain. Journal of Neuroscience. 2008;28:11263–11268. doi: 10.1523/JNEUROSCI.2308-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Furth R, Raeburn JA, van Zwet TL. Characteristics of human mononuclear phagocytes. Blood. 1979;54:485–500. [PubMed] [Google Scholar]
- Varol C, Landsman L, Fogg DK, Greenshtein L, Gildor B, Margalit R. Monocytes give rise to mucosal, but not splenic, conventional dendritic cells. Journal of Experimental Medicine. 2007;204:171–180. doi: 10.1084/jem.20061011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan DW, Peters A. Neuroglial cells in the cerebral cortex of rats from young adulthood to old age: an electron microscope study. Journal of Neurocytology. 1974;3:405–429. doi: 10.1007/BF01098730. [DOI] [PubMed] [Google Scholar]
- Vela JM, Dalmau I, González B, Castellano B. Morphology and distribution of microglial cells in the young and adult mouse cerebellum. Journal of Comparative Neurology. 1995;361:602–616. doi: 10.1002/cne.903610405. [DOI] [PubMed] [Google Scholar]
- Verkhratsky A, Krishtal OA, Burnstock G. Purinoceptors on neuroglia. Molecular Neurobiology. 2009;39:190–208. doi: 10.1007/s12035-009-8063-2. [DOI] [PubMed] [Google Scholar]
- Vijg J, Campisi J. Puzzles, promises and a cure for ageing. Nature. 2008;454:1065–1071. doi: 10.1038/nature07216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virchow R. Gesammelte Abhandlungen zyr wissenschaftlischen Medizin. Frankfurt: Verlag von Meidinger Sohn & Comp; 1856. [Google Scholar]
- Visintin A, Mazzoni A, Spitzer JH, Wyllie DH, Dower SK, Segal DM. Regulation of Toll-like receptors in human monocytes and dendritic cells. Journal of Immunology. 2001;166:249–255. doi: 10.4049/jimmunol.166.1.249. [DOI] [PubMed] [Google Scholar]
- Wake H, Moorhouse AJ, Jinno S, Kohsaka S, Nabekura J. Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. Journal of Neuroscience. 2009;29:3974–3980. doi: 10.1523/JNEUROSCI.4363-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakselman S, Bechade C, Roumier A, Bernard D, Triller A, Bessis A. Developmental neuronal death in hippocampus requires the microglial CD11b integrin and DAP12 immunoreceptor. Journal of Neuroscience. 2008;28:8138–8143. doi: 10.1523/JNEUROSCI.1006-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter S, Letiembre M, Liu Y, Heine H, Penke B, Hao W, et al. Role of the toll-like receptor 4 in neuroinflammation in Alzheimer’s disease. Cellular Physiology and Biochemistry. 2007;20:947–956. doi: 10.1159/000110455. [DOI] [PubMed] [Google Scholar]
- Walz W, Ilschner S, Ohlemeyer C, Banati R, Kettenmann H. Extracellular ATP activates a cation conductance and a K+ conductance in cultured microglial cells from mouse brain. Journal of Neuroscience. 1993;13:4403–4411. doi: 10.1523/JNEUROSCI.13-10-04403.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams K, Ulvestad E, Antel J. Immune regulatory and effector propereties of human adult microglia studies in vitro and in situ. Advances in Neuroimmunology. 1994;4:273–281. doi: 10.1016/s0960-5428(06)80267-6. [DOI] [PubMed] [Google Scholar]
- Wright GJ, Cherwinski H, Foster-Cuevas M, Brooke G, Puklavec MJ, Bigler M, et al. Characterization of the CD200 receptor family in mice and humans and their interactions with CD200. Journal of Immunology. 2003;171:3034–3046. doi: 10.4049/jimmunol.171.6.3034. [DOI] [PubMed] [Google Scholar]
- Wu LJ, Vadakkan KI, Zhuo M. ATP-induced chemotaxis of microglial processes requires P2Y receptor-activated initiation of outward potassium currents. Glia. 2007;55:810–821. doi: 10.1002/glia.20500. [DOI] [PubMed] [Google Scholar]
- Wu CH, Wen CY, Shieh JY, Ling EA. A quantitative and morpho- metric study of the transformation of ameboid microglia into ramified microglia in the developing corpus callosum in rats. Journal of Anatomy. 1992;181:4423–4430. [PMC free article] [PubMed] [Google Scholar]
- Wynne AM, Henry CJ, Huang Y, Cleland A, Godbout JP. Protracted downregulation of CX3CR1 on microglia of aged mice after lipopolysaccharide challenge. Brain Behavior and Immunity. 2010;24:1190–1201. doi: 10.1016/j.bbi.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang Z, Burnstock G. Expression of P2X receptors on rat microglial cells during early development. Glia. 2005;52:119–126. doi: 10.1002/glia.20227. [DOI] [PubMed] [Google Scholar]
- Xu H, Chen M, Manivannan A, Lois N, Forrester JV. Age-dependent accumulation of lipofuscin in perivascular and subretinal microglia in experimental mice. Aging Cell. 2008;7:58–68. doi: 10.1111/j.1474-9726.2007.00351.x. [DOI] [PubMed] [Google Scholar]
- Yamada J, Sawada M, Nakanishi H. Cell cycle-dependent regulation of kainite-induced inward currents in microglia. Biochemical and Biophysical Research Communications. 2006;349:913–919. doi: 10.1016/j.bbrc.2006.08.126. [DOI] [PubMed] [Google Scholar]
- Yan SD, Zhu H, Fu J, et al. Amyloid-β peptide-receptor for advanced glycation endproduct interaction elicits neuronal expression of macrophage-colony stimulating factor: a proinflammatory pathway in Alzheimer disease. Proceedings of the National Academy of Sciences USA. 1997;94:5296–5301. doi: 10.1073/pnas.94.10.5296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye SM, Johnson RW. Increased interleukin-6 expression by microglia from brain of aged mice. Journal of Neuroimmunology. 1999;93:139–148. doi: 10.1016/s0165-5728(98)00217-3. [DOI] [PubMed] [Google Scholar]
- Ye SM, Johnson RW. An age-related decline in interleukin-10 may contribute to the increased expression of interleukin-6 in brain of aged mice. Neuroimmunomodulation. 2001;9:183–192. doi: 10.1159/000049025. [DOI] [PubMed] [Google Scholar]
- Yiangou Y, Facer P, Durrenberger P, Chessell IP, Naylor A, Bountra C, et al. COX-2, CB2 and P2X7-immunoreactivities are increased in activated microglial cells/macrophages of multiple sclerosis and amyotrophic lateral sclerosis spinal cord. BMC Neurology. 2006;6:12. doi: 10.1186/1471-2377-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Ugawa S, Ueda T, Ishida Y, Inoue K, Kyaw Nyunt A, et al. Cellular localization of P2X7 receptor mRNA in the rat brain. Brain Research. 2008;1194:45–55. doi: 10.1016/j.brainres.2007.11.064. [DOI] [PubMed] [Google Scholar]
- Yuan H, Zhu X, Zhou S, Chen Q, Ma X, He X, et al. Role of mast cell activation in inducing microglial cells to release neurotrophin. Journal of Neuroscience Research. 2010;88:1348–1354. doi: 10.1002/jnr.22304. [DOI] [PubMed] [Google Scholar]
- Zhang J, Shi XQ, Echeverry S, Mogil JS, De Koninck Y, Rivest S. Expression of CCR2 in both resident and bone marrow-derived microglia plays a critical role in neuropathic pain. Journal of Neuroscience. 2007;27:12396–12406. doi: 10.1523/JNEUROSCI.3016-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Y, Zhou LJ, Ren WJ, Xin WJ, Li YY, Zhang T, et al. The direction of synaptic plasticity mediated by C-fibers in spinal dorsal horn is decided by Src-family kinases in microglia: the role of tumor necrosis factor-alpha. Brain, Behavior, and Immunity. 2010;24:874–880. doi: 10.1016/j.bbi.2010.01.007. [DOI] [PubMed] [Google Scholar]
- Zusso M, Methot L, Lo R, Greenhalgh AD, David S, Stifani S. Regulation of postnatal forebrain amoeboid microglial cell proliferation and development by the transcription factor Runx1. Journal of Neuroscience. 2012;32:11285–11298. doi: 10.1523/JNEUROSCI.6182-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]