Abstract
Qidong City, China, has had high liver cancer incidence from endemic hepatitis B virus (HBV) infection and dietary exposure to aflatoxin. Based on etiologic studies, we began interventions in 1980 to reduce dietary aflatoxin and initiate neonatal HBV vaccination. We studied trends in liver cancer incidence rates in the 1.1 million inhabitants of Qidong and examined trends in aflatoxin exposure, staple food consumption, HBV infection markers and annual income. Aflatoxin exposure declined greatly in association with economic reform, increased earnings and educational programs to shift staple food consumption in the total population from moldy corn to fresh rice. A controlled neonatal HBV vaccination trial began in 1983 and ended in November, 1990, when vaccination was expanded to all newborns. Liver cancer incidence fell dramatically in young adults. Compared with 1980–83, the age-specific liver cancer incidence rates in 2005–08 significantly decreased 14-fold at ages 20–24, 9-fold at ages 25–29, 4-fold at ages 30–34, 1.5-fold at ages 35–39, 1.2-fold at ages 40–44 and 1.4-fold at ages 45–49, but increased at older ages. The 14-fold reduction at ages 20–24 might reflect the combined effects of reduced aflatoxin exposure and partial neonatal HBV vaccination. Decrease incidence in age groups >25 years could mainly be attributable to rapid aflatoxin reduction. Compared with 1980–83, liver cancer incidence in 1990–93 significantly decreased 3.4-fold at ages 20–24, and 1.9-fold at ages 25–29 when the first vaccinees were <11 years old.
Introduction
Liver cancer is one of the leading causes of cancer deaths worldwide (1). Qidong City is an area of China with high liver cancer incidence as determined by a well-established population-based cancer registry (2). Two major risk factors identified in Qidong were high prevalence of hepatitis B virus (HBV) infection (3,4) and appreciable dietary aflatoxin exposure (5,6). HBV is a potent liver cancer carcinogen (7,8). HBV infection greatly sensitizes hepatocytes to the mutagenic effects of aflatoxin (9), and aflatoxin exposure tripled the risk of hepatocellular carcinoma (HCC) in a cohort of men with chronic hepatitis B (CHB) in Qidong (6,10). These two risk factors act synergistically in causing HCC in China (6,10,11). Characteristic high frequency 249ser-p53 mutations were first identified in HCC specimens in Qidong, an endemic area of aflatoxin exposure and HBV infection (12), but not found in aflatoxin-induced HCC in monkeys (13). These mutations were found only in HCC specimens from patients with both HBV infection and aflatoxin exposure, serving as a genetic marker of their joint effect in carcinogenesis (6).
Measurements of aflatoxin B1 (AFB1) by thin layer semiquantitative chromatography showed extensive contamination of corn, the major staple food in the population of Qidong in the 1970s. The AFB1-producing Aspergillus flavus fungus was very prevalent in corn, but 11-fold less prevalent in rice and 14.5-fold less prevalent in wheat in Qidong. A sensitive and accurate dosimetric system was developed to measure AFB1 and its main soluble metabolite, aflatoxin M1 (AFM1) in individual urine samples (14–17). Rapid economic development allowed for expanded consumption of commercial rice in the 1980s. Studies of individual aflatoxin exposure and other accumulating scientific evidence, together with economic development, prompted the Municipal Government of Qidong to promote expanded access to commercial rice, which was tested to ensure that AFB1 levels were <5 parts per billion (p.p.b.), as the staple food beginning in 1988.
Neonatal HBV vaccination began in a large controlled clinical trial on 1 September 1983 in Qidong (18,19). This internationally collaborative study was initiated as a World Health Organization (WHO) Demonstration Project to implement its Technical Report Series 691, which claimed that ‘unique opportunity exists for the first time to control a major human cancer by vaccination’ (7,8).
Multifaceted preventive interventions based on multiple etiologic factors might be the most promising approach to control HCC and also to eliminate the underlying CHB in endemic areas (20). Such interventions may also reduce morbidity and mortality from cirrhosis. We began such interventions in Qidong in 1980. Although the full effects of neonatal HBV vaccination will not be apparent until a large portion of the adult population has received neonatal vaccination, substantial reductions in dietary exposure to aflatoxin might have a more immediate impact on HCC incidence in the endemic areas. We, therefore, undertook a detailed analysis of age-specific liver cancer incidence data to identify the age groups showing decreased liver cancer incidence, and when the reduction began to occur. These data provide critical information on the possible effects of diet changes and vaccination on liver cancer incidence in Qidong. Such information has important public health implications for other endemic areas in China and worldwide.
Materials and methods
Study populations and cancer registry
Qidong City has a relatively stable population of about 1.1 million; 11 000–13 000 children were born each year in the 1980s. About 90% of the population of Qidong City lives in rural areas. Corn and cotton were the main agricultural products in Qidong City before the early 1980s. We sometimes refer to Qidong City as Qidong.
Population-based age-specific liver cancer incidence data from 1980 to 2008 were collected by the Cancer Registry in the Qidong Liver Cancer Institute, Qidong City, which began collecting data in 1973. Data from this registry have been used by the International Agency for Research on Cancer (21).
Criteria for liver cancer diagnosis
We diagnosed liver cancer on the basis of progressive enlargement of a firm liver and the presence of one or more space-occupying lesions on ultrasonography, together with either serum alpha-fetoprotein (AFP) >300ng/ml or death within months or both. Since 1986, referring hospitals have also provided computerized axial tomography images. In >80% of these cases, confirmatory evidence was provided by elevated AFP >300ng/ml. Death usually occurred after about 8 months, providing additional confirmatory information. Practically, every case that went to surgery was found to have liver cancer pathologically diagnosed as HCC. The criteria of progressive enlargement of a firm liver and one or more space-occupying lesions on ultrasonography, together with elevated AFP or early death have not changed over the course of the study.
Identification of aflatoxin-producing Aspergillus flavus, average dietary AFB1 intake and individual aflatoxin exposure measurements
We obtained samples of corn (n = 474), rice (n = 115) and wheat (n = 97) from rural families in Qidong in the 1970s. These samples were tested to detect the presence of A.flavus. We tested for AFB1 from detected strains of A.flavus by using thin layer chromatography on extracts of rice media on which detected strains were cultured.
Corn was the main staple food in rural areas of Qidong rural before 1980. During 1973–80, a total of 2272 stored dietary corn samples (ranging from n = 83 to n = 522 samples each year) were collected from households in different rural towns in Qidong for detection of AFB1. AFB1 in samples was semiquantified by thin layer chromatography, using pure AFB1 (Makor Chemicals, Jerusalem, Israel) as standard. The lower limit of detection was 5 p.p.b., equivalent to 5 µg/kg. Estimated average AFB1 exposure per person per year was calculated as: [the annual amount (kg) of corn consumption per capita] × (the fraction of corn samples that were positive for AFB1) × [the mean level of AFB1 (µg/kg) in positive samples in the relevant year]. The AFB1 in all corn samples from 1973 to 1980 was measured by the same group of scientists using the same AFB1 standard purchased from Makor Chemicals, Jerusalem, Israel.
To measure an individual’s daily aflatoxin exposure, we had developed sensitive and reproducible techniques for detecting AFB1 and AFM1 (in urine). AFM1 in a 24h urine sample was immunoconcentrated by monoclonal antibody coated small affinity columns and quantified by high-performance liquid chromatography. The AFB1 exposure on the day tested was estimated by multiplying the measured urinary AFM1 output (ng/l) by the conversion ratio 50 that was determined by our previous experiments (14–17).
Follow-up of a cohort of HBV surface antigen-positive men for AFB1 exposure and incident HCC
A cohort of 145 men with CHB was recruited from two randomly selected towns using population-based sampling in 1987–88. Following informed consent and approval from the Review Board in the Qidong Liver Cancer Institute, the cohort was studied and followed for 13 years to 2000. Hepatitis B virus surface antigen (HBsAg) was measured at recruitment with a radioimmunoassay (RIA) kit from the Beijing Biological Products Institute, Beijing.
In 1988, mean AFM1 was measured from 8 to 10 urine samples collected monthly from each individual and stored frozen at –20ºC. The pooled samples from each man were immunoconcentrated and AFM1 was measured by high-performance liquid chromatography. In this way, we obtained average daily AFM1 output and average daily aflatoxin exposure (by multiplying by 50) for each cohort member in 1988 (10,16,17). In 2000, after 13 years of follow-up, 36 of the 78 men with detectable AFM1, in 1988, had died. Of the 78 – 36 = 42 survivors, 8 left Qidong for work elsewhere and were not accessible, whereas 21 of 34 accessible survivors provided urine samples in 2000 (2 urine samples spaced 4 days apart). Pooled samples from each man were retested for AFM1. The 34 accessible survivors answered questions about their daily staple food consumption.
Data on consumption of corn, rice and wheat in Qidong
The Qidong Food Bureau kindly provided data on annual availability of corn, defined as Qidong production less exports, for the Qidong population from 1973 to 1981, and also provided data on computer media on annual consumption of commercial corn, rice and wheat in the Qidong population for the years 1988 through 2010. We converted these data to consumption per capita by dividing the availability by the yearly population size as shown in Table I for 1973–80 and in Figure 2 for 1973–81 and 1988 through 2010, respectively.
Table I.
Estimated mean intake of dietary corn and AFB1 in Qidong residents in 1973–80
| Year | Mean intake of corn (kg/person/year)a | Number of samples tested for AFB1 | AFB1 positive rate in corn samples (%) | Mean AFB1 in positive samples (p.p.b.)b | Mean intake of AFB1 (mg/year/person)c |
|---|---|---|---|---|---|
| 1973 | 101 | 350 | 32 | 55 | 1.8 |
| 1974 | 82 | 522 | 26 | 29 | 0.6 |
| 1975 | 98 | 422 | 28 | 22 | 0.6 |
| 1976 | 124 | 359 | 50 | 24 | 1.5 |
| 1977 | 106 | 269 | 64 | 48 | 3.3 |
| 1978 | 89 | 161 | 40 | 11 | 0.4 |
| 1979 | 97 | 106 | 33 | 19 | 0.6 |
| 1980 | 65 | 83 | 99 | 37 | 2.4 |
| Mean | 95 | 1.4 |
aMean intake of corn was calculated by dividing the total corn consumption (total corn production minus the amount exported from Qidong) by the population size of Qidong in each year.
bIn the AFB1 positive corn samples, AFB1 level varied from 5 p.p.b. (equivalent to 5 µg AFB1/kg of corn) to >250 p.p.b., as assayed by semiquantitative thin layer chromatography. All the 2272 corn samples from 1973 to 1980 were assayed by same group of scientists using same AFB1 standard.
cMean AFB1 intake in milligram per year per person is obtained by multiplying columns 2, 4 and 5 and dividing by 100 000.
Fig. 2.
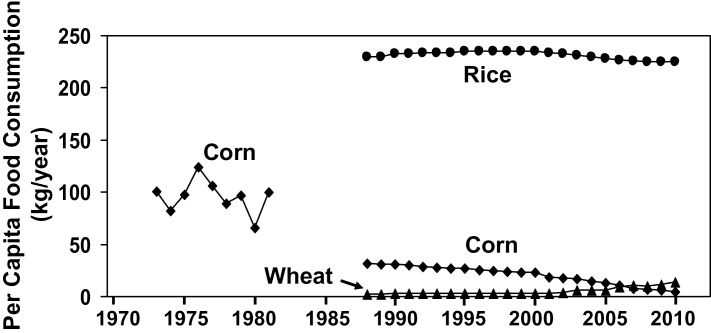
Per capita annual consumption of corn in Qidong from 1973 to 1981 and per capita annual consumption of corn, rice and wheat in Qidong from 1988 to 2010. Corn was the main staple food during 1973–81, when consumption varied from 65 to 124kg/person/year. By 1988, rice was the main staple food, and rice consumption varied from 225 to 235kg/person/year from 1988 to 2010.
Additional information on diet came from a survey of staple food consumption in 2010 in six towns of the Wang Bao and Yun Yang districts of Qidong. This survey covered 36 420 households with 86 555 people in Wang Bao and 31 500 households with 77 226 people in Yun Yang. The total number of people surveyed was 163 781, which was about 15% of the total population of Qidong City.
HBV neonatal vaccination in Qidong and estimation of HBsAg prevalence
HBV neonatal vaccination began on 1 September 1983 as part of a large controlled clinical trial (18,19), promoted by World Health Organization to demonstrate the efficacy of neonatal HBV vaccination to control liver cancer. There were 1831, 9889 and 15 189 newborns vaccinated in 1983–84, 1985–86 and 1987–88, respectively. These numbers of vaccinees and the year vaccinated were useful data for this article. Note that all these vaccinees were <11 years old in 1990–93, but were 17–25 years old in 2005–08. In early November 1990 when the Chinese vaccine began to be commercially available in Qidong, our controlled neonatal HBV vaccination trial was ended. Under the Health Bureau’s administration, HBV vaccination continued without interruption to cover all newborns in Qidong clinics with the same standardized HBV vaccination regimen used in the trial until 2002. The National Expanded Program of Immunization of China assumed responsibility for free universal vaccination of neonates since February 2002 (19).
Following informed consent and Institutional Review Board approval, sera from 2390 men and 4284 women aged 30–69 years from two rural towns, Donghai and Dafeng, were measured to estimate age-specific HBsAg prevalence in rural Qidong adults in 1992. None of these adults had been vaccinated against HBV as neonates because neonatal vaccination first began in 1983. Sensitive RIA kits from Beijing Biological Products Institute were used. Earlier prevalence surveys in 1976 used a reverse passive hemagglutination assay, also from Beijing Biological Products Institute (4).
Evaluation of HCC prevalence in primary liver cancer in Qidong
Among 2606 cases of liver cancer clinically diagnosed in various Qidong hospitals from 1972 to 1976, 259 cases (9.9%) had available pathological diagnoses. Classification of subtypes of liver cancer was performed by senior pathologists using hematoxylin- and eosin-stained slides.
Statistical methods
Population-based age-specific incidence rates for liver cancer in successive time periods were calculated as incidence per 100 000 person-years based on data from the Cancer Registrar of Qidong City in the Qidong Liver Cancer Institute. Confidence intervals (CIs) for Figure 3 and Table II were calculated based on Poisson theory. In particular, the variance of the log relative risk was estimated as the sum of the reciprocals of numbers of cancers. Confidence limits on the log relative risk were based on normal theory and inverted to produce 95% confidence intervals on the relative risk. Poisson regression with allowance for overdispersion was used to compute rate ratios and to test hypotheses on age-specific rates and for comparing rate ratios for older ages with rate ratios for younger age groups (Supplementary Information, available at Carcinogenesis Online). Statistical tests were two sided with significance level 0.05.
Fig. 3.
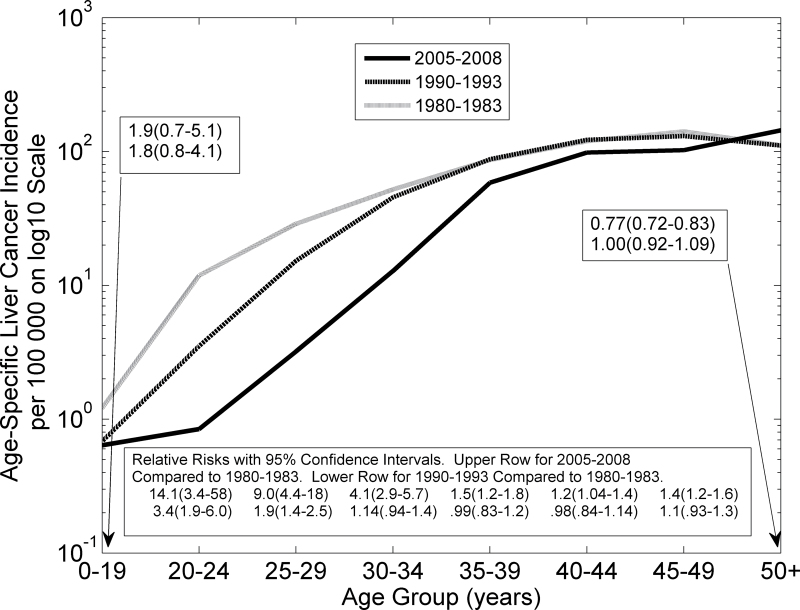
Age-specific incidence rates of liver cancer in Qidong on semi-logarithmic scale. The bold solid curve describes aggregated data from 2005 to 2008, the bold dashed curve aggregated data from 1990 to 1993, and the grey hatched curve aggregated data from 1980 to 1983. Age-specific relative risks with confidence intervals are shown. CIs from the Poisson distribution are based on the assumption that the logarithm of the relative risk is normally distributed with variance estimated by the sum of the reciprocals of the age-specific numbers of incident cases in the two time periods.
Table II.
Age-specific incidence rates for liver cancer and relative risks comparing 1980–83 versus 1990–93 and 1980–83 versus 2005–08 in Qidong City
| Age range (years) | Liver cancer incidence (per 100 000) | Relative risk | 95% CI | P a | Relative risk | 95% CI | P a | ||
|---|---|---|---|---|---|---|---|---|---|
| 1980–83 | 1990–93 | 2005–08 | 1980–1983 versus 1990–1993 | 1980–1983 versus 2005–2008 | |||||
| 0–19 | 1.21 | 0.68 | 0.63 | 1.8 | (0.8–4.1) | 0.1731 | 1.9 | (0.7–5.1) | 0.2019 |
| 20–24 | 11.85 | 3.52 | 0.84 | 3.4 | (1.9–6.0) | <0.0001 | 14.1 | (3.4–58) | 0.0002 |
| 25–29 | 28.82 | 15.26 | 3.20 | 1.9 | (1.4–2.5) | <0.0001 | 9.0 | (4.4–18) | <0.0001 |
| 30–34 | 52.05 | 45.45 | 12.81 | 1.14 | (0.94–1.4) | 0.1814 | 4.1 | (2.9–5.7) | <0.0001 |
| 35–39 | 86.51 | 87.54 | 58.53 | 0.99 | (0.83–1.2) | 0.8886 | 1.5 | (1.2–1.8) | <0.0001 |
| 40–44 | 119.55 | 122.06 | 98.15 | 0.98 | (0.84–1.14) | 0.7862 | 1.2 | (1.04–1.4) | 0.0096 |
| 45–49 | 141.05 | 130.54 | 102.27 | 1.1 | (0.93–1.3) | 0.3265 | 1.4 | (1.2–1.6) | <0.0001 |
| ≥50 | 111.24 | 110.88 | 143.73 | 1.00 | (0.92–1.09) | 0.9419 | 0.77 | (0.72–0.83) | <0.0001 |
aTwo-sided P-values based on the squared log relative risk divided by the estimated variance of the log relative risk. Relative risks in bold type correspond to P < 0.05.
Results
Reduction in aflatoxin exposure from 1970s to 2010
Corn was the main staple food produced and consumed by residents in Qidong before 1982. Aflatoxin-producing Aspergillus flavus was detected in 29.1% (138 of 474) of corn samples, but only 2.6% (3 of 115) of rice samples and 2.1% (2 of 97) of wheat samples in Qidong in the 1970s. The prevalence of aflatoxin-producing A.flavus strains was 11 times higher in corn than in rice and 14.5 times higher than in wheat. Corn was the predominant source of aflatoxin exposure in Qidong residents, especially in rural poor families.
From 1973 to 1980, 2272 corn samples were tested. The percentage of samples with AFB1 >5 p.p.b. rate varied from 26 to 99%, and the mean level of AFB1 in positive samples varied from 11 to 55 p.p.b. in different years (Table I). The estimated AFB1 intake from dietary corn in each year from 1973 to 1980 ranged from 0.4 to 3.3mg/person/year, with a mean exposure of 1.4mg/person/year (Table I). Ten years’ exposure at this average rate would be 14mg, which is comparable with the estimated cumulative exposure to the time of HCC diagnosis in seven men with chronic HBV hepatitis, which ranged from 7 to 29mg [Table 2 in ref. (6)].
By 1983–84, some data suggested that the intake of AFB1 had decreased. Eleven of 40 (27%) rural residents of Qidong had detectable AFM1 (>4ng/l) in urine samples. Among the 11 with detectable AFM1, 2 had estimated intakes of 10–12.5 p.p.b. of AFB1 per day (equivalent to 3.7–4.6mg/person/year), and the other 9 had estimated intakes of about 1.25 p.p.b. of AFB1 per day (0.46mg/person/year). The mean AFB1 intake in these 40 rural residents was 0.3mg/person/year, which was 4.7-fold less than the average AFB1 intake of 1.4mg/person/year from 1973 to 1980 (Table I).
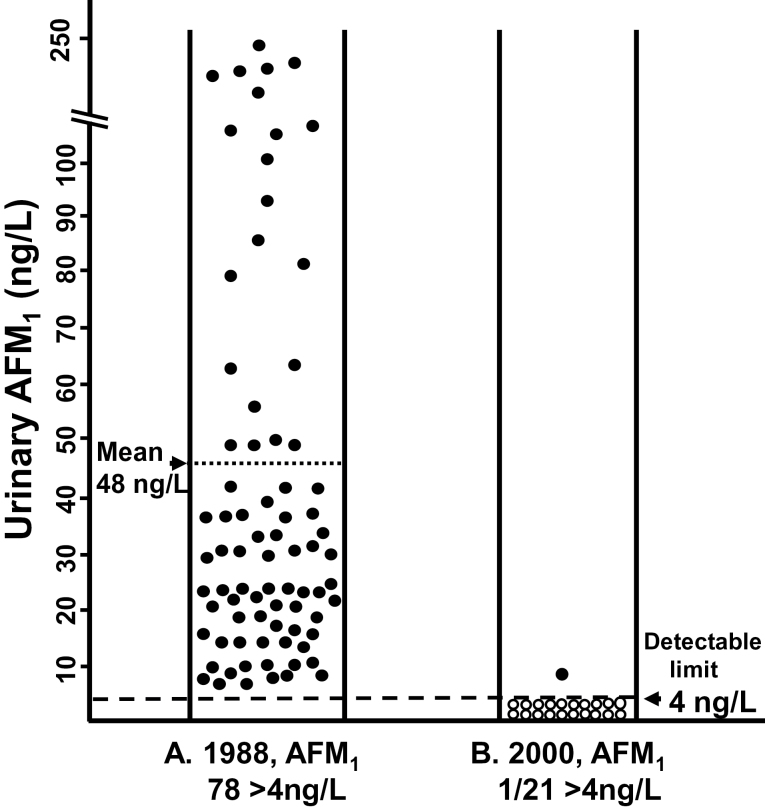
In 1987–88, a cohort of 145 HBsAg-positive men was recruited, and in 1988 they provided 8–10 repeated monthly urine samples. Seventy-eight (54%) of these 145 men had AFM1 levels >4ng/l in pooled urine, with the elevated levels ranging from 5.7ng/l to 243ng/l and with a mean level of 48ng/l (Figure 1, panel A). In 2000, after 13 years of followed-up, 36 (46%) of these 78 HBsAg-positive men with detectable AFM1, in 1988, had died, including 24 from HCC and 7 from end-stage cirrhosis. Eight men left Qidong to work elsewhere. The remaining 34 men were interviewed about diet in 2000, and all reported eating rice as their main staple food in recent years. Twenty-one (62%) of the 34 survivors in Qidong provided two 24h urine samples 4 days apart in 2000. All these 21 men had detectable (>4ng/l) urinary AFM1 in 1988, but only one of them (5%) excreted detectable levels in 2000, and his level was only 9ng/l (Figure 1, panel B). In each of these 21 men, AFM1 levels decreased between 1988 and 2000, and the decreases were statistically significant (P < 10–6 by two-sided sign test).
Fig. 1.
Urinary AFM1 concentrations in a cohort of 145 men with CHB. These men were recruited in 1987–88 and followed to 2000. (A) In 1988, 78 (54%) of the 145 men had urinary AFM1 levels >4ng/l (‘detectable’ levels); these detectable levels ranged from 5.7 to 243ng/l with mean 48ng/l (left panel). (B) In 2000, of the 78 men with detectable AFM1 in 1988, there were 34 accessible survivors, of whom 21 provided 2 urine samples spaced 4 days apart. During the periods of urine collection, they all ate rice as their usual main staple food. Twenty of these men had non-detectable AFM1 levels, and one had a level of 9ng/l (right panel).
Change in staple food consumption in Qidong
Annual per capita corn consumption ranged from 65 to 124kg/person/year in the 1970s and dropped to much lower levels from 1988 to 2010, when it was only 5kg/person/year (Figure 2). Rice became the dominant staple food by 1988, when data first became available, and rice consumption varied in a tight range from 225 to 235kg/person/year from 1988 to 2010 (Figure 2). Wheat consumption has grown since 1988, but remains small. Thus, corn was displaced by rice between 1982 and 1988, and by wheat to a much smaller extent.
This shift from corn to rice was consistent with the data from our survey of 165 781 people in the districts of Wang Bao and Yun Yang in 2010. All of these rural people ate rice in three meals per day as their standard staple food. Ten percent or fewer ate some wheat in the morning meal. The very limited corn consumption included food for domestic animals. The average annual per capita income in rural Qidong increased from 224–324 yuan in 1980–83 to 6069–8376 yuan in 2005–08, which was sufficient to allow people in Qidong to buy rice as their main staple food.
Reduction of age-specific liver cancer incidence in Qidong City
The age-specific incidence of liver cancer in Qidong and relative risks with 95% CIs are shown in Figure 3 (semi-log plot) and Table II. Age-specific liver cancer incidence was higher for each age group (except those aged ≥50 years) in 1980–83 than in 2005–08. Relative risks comparing 1980–83 with 2005–08 (grey hatched line and bold solid line) were 14.1, 9.0 and 4.1 for age groups 20–24, 25–29 and 30–34 years, respectively (Figure 3 and Table II). They diminished toward 1.0 for older age groups. The relative risk for those aged ≥50 years was 0.77 (0.72–0.83). The relative risks for each of the three age groups 20–24, 25–29 and 30–34 years were larger than that of the geometric mean of the relative risks for the age groups 35–39, 40–44 and 45–49 years, with respective P-values 0.003, <0.0001 and <0.0001 (Supplementary Information, available at Carcinogenesis Online). Thus, by 2005–08, after 28 years of preventive measures, there was a dramatic decrease in liver cancer incidence rates in young adults, especially in those in the age range 20–34 years. Although the relative reductions were smaller for those aged 35–49 years, the absolute reductions were comparable in the older and younger age groups because incident rates are higher in the older groups.
Relative risks (with 95% CIs) comparing 1980–83 with 1990–93 (grey hatched line and bold dashed line in Figure 3) were 3.4 (1.9–6.0) and 1.9 (1.4–2.5), respectively, for age groups 20–24 and 25–29 years (see also Table II). These decreases were highly statistically significant (P < 0.0001) after only 10 years of preventive effort. Rates of liver cancer incidence in 1990–93 were significantly higher than in 2005–08 for each age group >24 years, except for those aged 50 and over (Supplementary Information, available at Carcinogenesis Online).
Effect of HBV vaccination on liver cancer incidence
From September 1983 to June 1984, 1831 neonates were first vaccinated against HBV infection in the pilot phase of the controlled trial in Qidong. The oldest of these neonates would be 10 years old in 1993. Therefore, vaccination could have no effect on the 1990–93 incidence rates in those >19 years old. The 1831 neonates vaccinated in 1983–84 would be 24–25 years old in 2008. The number of these vaccinees at age 25 years in 2008 was 378, only 0.59% of the 64 001 people aged 25–29 years in 2005–08. And none of the vaccinees was older than age 25 years in 2005–08. Thus, vaccination could have had no effect on the incidence rates at ages >25 years in 2005–08, and negligible effect at ages >24 years. However, vaccination could have had an appreciable effect on those aged 20–24 in 2005–08 because {(1831×7/8 + 9889×7/8 + 15 186×3/8)/57 414) × 100} = 27.8% of the people aged 20–24 years had been vaccinated. The dramatic 14.1-fold reduction in liver cancer incidence at ages 20–24 years in 2005–08 cannot be entirely explained by vaccination of 27.8% of the population, which could at most reduce liver cancer incidence 1/(1 – 0.278)=1.38-fold. The reduction in liver cancer incidence probably also reflects the benefits of abatement of exposure to aflatoxin over two decades.
In summary, vaccination did not contribute to any of the decreases in liver cancer incidence observed in 1990–93 or to any of the decreases in 2005–08 among those aged >25 years. Although vaccination may have had an effect in 2005–08 among those aged 20–24 years, it can explain only a fraction of the dramatic reduction in liver cancer incidence in that age group.
Relative stability of HBsAg prevalence in non-vaccinated adults
In 1976, a general survey of HBsAg prevalence in adults aged 30–69 years in Qidong yielded prevalence estimates based on the reverse passive hemagglutination assay of 19.1% (1195 of 6270) in men and 16.2% (1365 of 8422) in women (4). In 1984, a survey of HBsAg prevalence in women aged 20–30 years who had just given birth in Qidong yielded a prevalence of 14.2% (167 of 1180) by RIA (18). In 1992, we measured serum HBsAg in adults aged 30–69 years in two rural Qidong towns (Donghai and Dafeng) using RIA. The estimated HBsAg prevalence was 16.6% (397 of 2390) in men and 11.4% (488 of 4284) in women. None of these adults had received neonatal vaccination, which began in 1983. These data indicate that the prevalence of HBV infection may have decreased slightly in non-vaccinated adults from 1976 to 1992, but still remained well >10%.
Prevalence of hepatocellular carcinoma in liver cancer cases
Pathological subclassifications were obtained for liver cancer specimens from 259 adults with clinically diagnosed liver cancer, which accounted for 9.9% of all such cases diagnosed from 1972 to 1976. The pathological diagnoses identified 249 hepatocellular carcinoma (96.1%), 7 mixed HCC and cholangiocarcinoma (2.7%) and 3 cholangiocarcinoma (1.2%). Thus, HCC and the mixed HCC–cholangiocarcinoma cancers constituted 98.8% of adult liver cancer in Qidong.
Discussion
Liver cancer is the fifth most frequently diagnosed cancer but the second most frequent cause of cancer death in men, and is the seventh most commonly diagnosed cancer and the sixth leading cause of cancer death in women worldwide (1). An estimated 748 300 new liver cancer cases and 695 900 liver cancer deaths occurred worldwide in 2008, and half of these cases and deaths were estimated to occur in China (1,22). It was estimated that 78% of liver cancer worldwide is attributable to infection with HBV or hepatitis C virus (23). Among primary liver cancers, HCC is the predominant histological type, accounting for 70–90% of the total liver cancer burden worldwide (24,25). Age-adjusted liver cancer incidence rates in Qidong in 2007 were 76.4/105 for men and 28.1/105 for women, and are among the highest in the world. HCC plus mixed HCC–cholangiocarcinoma accounted for 98.8% of liver cancers in Qidong. Thus, the reduction of liver cancer incidence in young and middle-aged adults following etiological interventions in Qidong predominantly reflects a reduction of HCC incidence.
A report in 2006 identified a declining trend in liver cancer incidence at ages 15–34 years in Qidong, and an increase in those over age 75 (2). In the present report, we compared the age-specific liver cancer incidence rates in 2005–08 and in 1990–93 with rates in 1980–83. In 1990–93, after only about 10 years of preventive interventions, liver cancer incidence was reduced significantly (3.4- and 1.9-fold) compared with 1980–83 in those aged 20–24 and 25–29 years, respectively. None of the people aged 20–29 years in 1990–93 had received neonatal HBV vaccination, which did not begin until 1983. The decreases in liver cancer incidence rates became greater and extended to additional age groups by 2005–08. In fact, by 2005–08, the liver cancer incidence rate decreased 14.1-fold at ages 20–24, 9.0-fold at ages 25–29, 4.1-fold at ages 30–34 and 1.5-fold at ages 35–39. Because there were no vaccinees aged >25 years in 2005–08, we presume that the rapid and dramatic decrease of liver cancer incidence in those aged 25–39 years is mainly attributable to rapid reduction of aflatoxin exposure. The 14.1-fold reduction in incidence at ages 20–24 years is probably due to the combined effects of reduced aflatoxin exposure and HBV vaccination in 27.8% of these young adults. If vaccination were completely effective, it would account for a relative risk of 1/(1 – 0.278) = 1.385, instead of 14.1. The remaining protective effect, 14.1/1.385 = 10.2 must be from some non-vaccine-related factor, such as reduced aflatoxin exposure that began before 1988 (Figure 2).
We anticipate further decreases in liver cancer incidence in Qidong as an increasing proportion of the population becomes vaccinated. Eliminating chronic HBV infection should remove its direct carcinogenic effect as well as its synergistic risk with aflatoxin and other cofactors (5,6,10,20). Investigators in Taiwan (26) reported that vaccination reduced HCC incidence by about 3-fold in children and adolescents aged 6–19 years.
Although HBV neonatal vaccination is one of the most effective measures to reduce liver cancer incidence, as of 2006, only 27% of infants worldwide received the first dose (one of three) within 24h of birth (1). Studies conducted before adults had been vaccinated against HBV as neonates reported decreases in liver cancer incidence in Shanghai of 22% from 1972–74 to 1993–94 (27), and decreases of 17% from 1981 to 2000 in Tianjin, China (28). There was evidence of slightly decreased incidence in urban, but not rural areas in China from 2002 to 2010 (29). However, these decreases are much smaller than those we found in young adults in Qidong, where an active multifaceted program for liver cancer prevention has been pursued.
In contrast, liver cancer incidence rates are increasing in some parts of the world that have low incidence rates, including the USA and Western Europe (1). In the USA, age-adjusted HCC incidence rates tripled between 1975 and 2005 (25). From 2000 to 2005, marked increases occurred among Hispanic, black and white middle-aged men in the USA (25). It was suggested that such increases were related to increased hepatitis C virus infection, and that obesity and diabetes may have contributed (30).
The principal strengths of this study are good surveillance for liver cancer incidence; good information on the timing and extent of neonatal HBV vaccination; annual data on population food consumption and individual survey data on food consumption; data on aflatoxin contamination in corn, rice and wheat and on average individual aflatoxin exposure; and longitudinal follow-up of a cohort of men with chronic hepatitis for aflatoxin in urine and for diet changes. We are taking advantage of a unique historical opportunity to study effects following introduction of HBV vaccination and concomitant changes in population food consumption. A cooperative multidisciplinary team effort and official support were required to obtain this wide variety of relevant data. A limitation of the study is that we are using aggregated data on liver cancer incidence rates and there is a gap (1982–87) in data on population food consumption. An ideal study might produce longitudinal follow-up data on a very large cohort. Each individual would have serial data on food consumption, aflatoxin exposure measured by urinary aflatoxin, vaccination status, hepatitis status and time to liver cancer incidence, if any. However, such a study was not feasible economically, and, if instituted now, would not be able to address key issues in this article concerning the timing of HBV vaccination and diet change. Other limitations include limited numbers of samples for estimating aflatoxin exposure.
In summary, we observed a dramatic reduction in liver cancer incidence in young and middle-aged adults in Qidong that was not the result of HBV vaccination alone and that suggests an important preventive role of reduction in aflatoxin exposure. Appreciable absolute risk reductions extended to those aged 35–49 years, but the relative reductions were greatest in those aged 20–34 years. Although one can anticipate that neonatal HBV vaccination will produce great benefits in future decades as more members of the adult population become protected against HBV hepatitis, a more immediate preventive effect in our study appears to have resulted from a rapid reduction in dietary aflatoxin exposure in this population with endemic HBV hepatitis. This result has important implications for liver cancer prevention in other endemic areas of China and worldwide. Aflatoxin abatement may reduce liver cancer substantially, even before these populations are fully protected against HBV. We would recommend a multifaceted approach, based on etiologic studies to identify endemic areas where cofactors, such as aflatoxin, act synergistically with carcinogenic viruses to cause liver cancer (5,20).
Supplementary material
Supplementary Information can be found at http://carcin.oxfordjournals.org/
Funding
Ministry of Science and Technology, China (36112②, 75610237, 859140310, 2001BA703B06, 2006BA102A03); Intramural Research Program of the National Cancer Institute, National Institutes of Health, USA.
Conflict of Interest Statement: None declared.
Supplementary Material
Glossary
Abbreviations:
- AFB1
aflatoxin B1
- AFM1
aflatoxin M1
- AFP
serum alpha-fetoprotein
- CHB
chronic hepatitis B
- CI
confidence interval
- HCC
hepatocellular carcinoma
- HBsAg
hepatitis B virus surface antigen
- HBV
hepatitis B virus
- p.p.b.
parts per billion
- RIA
radioimmunoassay.
References
- 1. Jemal A., et al. (2011). Global cancer statistics. CA. Cancer J. Clin., 61, 69–90 [DOI] [PubMed] [Google Scholar]
- 2. Chen J.G., et al. (2006). Trends in the incidence of cancer in Qidong, China, 1978–2002. Int. J. Cancer., 119, 1447–1454 [DOI] [PubMed] [Google Scholar]
- 3. Xia Q.J., aka Hsia C.C., et al. (1984). Liver cell cancer in the young and adolescent and its relation to hepatitis B virus (HBV). Chin. J. Oncol., 6, 413–416 [PubMed] [Google Scholar]
- 4. Lu J., et al. (1983). Matched prospective study of primary hepatocellular carcinoma in chronic carriers of HBsAg. Chin. J. Oncol., 5, 406–408 [PubMed] [Google Scholar]
- 5. Sun T., et al. (1984). Carcinogenesis and prevention strategy of liver cancer in areas of prevalence. In Mak T.W. et al. (eds) Cellular and Molecular Biology of Neoplasia., Alan R. Liss Inc. New York, USA: pp. 39–44 [DOI] [PubMed] [Google Scholar]
- 6. Ming L., et al. (2002). Dominant role of hepatitis B virus and cofactor role of aflatoxin in hepatocarcinogenesis in Qidong, China. Hepatology., 36, 1214–1220 [DOI] [PubMed] [Google Scholar]
- 7. World Health Organization (1983). Prevention of liver cancer. Technical Report Series 691., World Health Organization; Geneva: [PubMed] [Google Scholar]
- 8. Zuckerman A., et al. (1983). Prevention of primary liver cancer: report on a meeting of W.H.O. Scientific Group. Lancet., 321, 463–465 [PubMed] [Google Scholar]
- 9. Fujimoto Y., et al. (1994). Alterations of tumor-suppressor genes and allelic losses in human hepatocellular carcinomas in China. Cancer Res., 54, 281–285 [PubMed] [Google Scholar]
- 10. Sun Z.T., et al. (1999). Increased risk of hepatocellular carcinoma in male hepatitis B surface antigen carriers with chronic hepatitis who have detectable urinary aflatoxin metabolite M1. Hepatology., 30, 379–383 [DOI] [PubMed] [Google Scholar]
- 11. Ross R.K., et al. (1992). Urinary aflatoxin biomarkers and risk of hepatocellular carcinoma. Lancet., 339, 943–946 [DOI] [PubMed] [Google Scholar]
- 12. Hsu I.C., et al. (1991). Mutational hotspots in the p53 gene in human hepatocellular carcinomas. Nature., 350, 427–428 [DOI] [PubMed] [Google Scholar]
- 13. Fujimoto Y., et al. (1992). Low frequency of p53 gene mutation in tumors induced by aflatoxin B1 in nonhuman primates. Cancer Res., 52, 1044–1046 [PubMed] [Google Scholar]
- 14. Sun Z.T., et al. (1983). Monoclonal antibody against aflatoxin B1 and its potential applications. Chin. J. Oncol., 5, 401–405 [PubMed] [Google Scholar]
- 15. Sun Z.T., et al. (1983). Prospect of using monoclonal antibodies against aflatoxins in studying human liver carcinogenesis. Acta Acad. Med. Sinicae., 5, 344–345 [Google Scholar]
- 16. Wu S., et al. (1984). Study on urinary excretion of aflatoxin M1 in Beijing and Qidong people. Chin. J. Oncol., 6, 163–167 [PubMed] [Google Scholar]
- 17. Sun T., et al. (1986). Measurement of individual aflatoxin exposure among people having different risk to primary hepatocellular carcinomaIn Hayashi Y. et al. (eds) Diet, Nutrition, and Cancer: Proceedings of the 16th International Symposium of the Princess Takamatsu Cancer Research Fund., Japan Scientific Societies Press; Tokyo, Japan: pp. 225–235 [PubMed] [Google Scholar]
- 18. Sun T., et al. (1986). A pilot study on universal immunization of newborn infants in an area of hepatitis B virus and primary hepatocellular carcinoma. In Mak T.W., Sun T.-T. (eds). Cancer Perspective for Control: Proceedings of an International Symposium Held in Beijing, China, August 18–21, 1985., . Alan R. Liss Inc; New York: pp. 83–90 [Google Scholar]
- 19. Sun Z.T., et al. (2002). Prevention and control of hepatitis B in China. J. Med. Virol., 67, 447–450 [DOI] [PubMed] [Google Scholar]
- 20. Sun T., et al. (1986). Strategies and current trends of etiological prevention of liver cancer. In Harris C.C. (ed). Biochemical and Molecular Epidemiology of Cancer., Alan R. Liss Inc; New York, USA: pp. 283–292 [Google Scholar]
- 21. Parkin D.M., et al. (2002). Cancer incidence in five continents, VIII. IARC Sci. Publ. No. 155., IARC; Lyon: pp. 212–231 [Google Scholar]
- 22. Ferlay J., et al. (2010). GLOBOCAN 2008, Cancer Incidence and Mortality Worldwide: IARC CancerBase No.10., International Agency for Research on Cancer; Year; Lyon, France: http://globocan.iarc.fr (17 August 2010, date last accessed) [Google Scholar]
- 23. Perz J.F., et al. (2006). The contribution of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J. Hepatology., 45, 529–538 [DOI] [PubMed] [Google Scholar]
- 24. Okuda K., et al. (2002). Cholangiocarcinoma: recent progress. Part 1: epidemiology and etiology. J. Gastroenterol. Hepatol., 17, 1049–1055 [DOI] [PubMed] [Google Scholar]
- 25. Altekruse S.F., et al. (2009). Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J. Clin. Oncol., 27, 1485–1491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chang M.H., et al. (2009). Decreased incidence of hepatocellular carcinoma in hepatitis B vaccinees: a 20-year follow-up study. J. Natl. Cancer Inst., 101, 1348–1355 [DOI] [PubMed] [Google Scholar]
- 27. Jin F., et al. (1999). Cancer incidence trends in urban Shanghai, 1972–1994: an update. Int J. Cancer., 83, 435–440 [DOI] [PubMed] [Google Scholar]
- 28. Hao X.S., et al. (2003). Twenty-year trends of primary liver cancer incidence rates in an urban Chinese population. Eur. J. Cancer Prev., 12, 273–279 [DOI] [PubMed] [Google Scholar]
- 29. Guo P., et al. (2012). Trends in cancer mortality in China: an update. Ann. Oncol., 23, 2755–2762 [DOI] [PubMed] [Google Scholar]
- 30. El-Serag H.B. (2007). Epidemiology of hepatocellular carcinoma in USA. Hepatol. Res., 37 (suppl. 2), S88–S94 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.