SUMMARY
Extrinsic cues activate intrinsic signaling mechanisms to pattern neuronal shape and connectivity. We showed previously that three cytoplasmic Ser/Thr kinases, LKB1, SAD-A and SAD-B, control early axon-dendrite polarization in forebrain neurons. Here we assess their role in other neuronal types. We found that all three kinases are dispensable for axon formation outside of the cortex, but that SAD kinases are required for formation of central axonal arbors by subsets of sensory neurons. The requirement for SAD kinases is most prominent in NT-3 dependent neurons. SAD kinases transduce NT-3 signals in two ways through distinct pathways. First, sustained NT-3/TrkC signaling increases SAD protein levels. Second, short duration NT-3/TrkC signals transiently activate SADs by inducing dephosphorylation of C-terminal domains, thereby allowing activating phosphorylation of the kinase domain. We propose that SAD kinases integrate long- and short duration signals from extrinsic cues to sculpt axon arbors within the CNS.
INTRODUCTION
One prominent aspect of neuronal morphogenesis is the series of steps by which axons become progressively more specialized. Initially, one of several short neurites becomes an axon; the others become dendrites (Barnes and Polleux, 2009). Next, the axon elongates, often over long distances (O’Donnell et al., 2009). Once in the target region, the axon branches to form arbors that allow it to synapse onto numerous postsynaptic cells (Schmidt and Rathjen, 2010; Gibson and Ma, 2011). The branches then selectively synapse on appropriate synaptic partners, and form nerve terminals specialized for neurotransmitter release (Jin and Garner, 2008). Later still, terminal arbors are sculpted or rearranged leading to the definitive pattern of connectivity (Luo and O’Leary, 2005). Extrinsic factors in the environment through which the axon grows regulate each of these steps. For many of the steps, guidance and patterning molecules have been identified (Kolodkin and Tessier-Lavigne, 2011), but less is known about the intracellular pathways that respond to and integrate these cues.
We, and others, previously showed that a set of three Ser/Thr kinases, LKB1, SAD-A and SAD-B, control polarization and axon specification in forebrain neurons (Kishi et al., 2005; Barnes et al. 2007; Shelly et al., 2007). LKB1 is a multifunctional kinase that regulates cellular energy homeostasis, polarity and cell proliferation by phosphorylating and activating kinases of the AMPK subfamily, of which SAD-A and SAD-B (also known as Brsk2 and Brsk1, respectively) are members (Alessi et al., 2006). SAD kinases are selectively expressed in the mammalian nervous system and are orthologs of C. elegans Sad-1, a regulator of vesicle clustering at active zones (Kishi et al., 2005; Inoue et al., 2006; Crump et al. 2001). Deletion of LKB1 or both SAD-A and SAD-B causes a loss of polarity in cortical and hippocampal neurons (Kishi et al., 2005; Barnes et al., 2007; Shelly et al., 2007).
Here, we asked whether LKB1 and SAD kinases regulate axonal development in other neurons. Surprisingly, LKB1 and SAD kinases are not required for early stages of axon formation in the spinal cord or brainstem. However, SADs but not LKB1 are required in several types of sensory neurons for a late phase of central axon specialization: the branching of axons in their terminal fields. The requirement for SAD kinases is dramatic in subtypes such as type Ia proprioceptive sensory neurons (IaPSNs) that require peripheral signaling from the neurotrophic factor NT-3, itself known to induce central axon growth (Oakley et al., 1997; Wright et al., 1997; Patel et al., 2003). In fact, NT-3 and its receptor TrkC act in part through SADs and control SAD activity in two distinct ways –they regulate protein levels in response to sustained NT3 signaling, and they regulate kinase activation in response to short term fluctuations. Thus, SAD kinases integrate long- and short-duration signals to sculpt the terminal arbors of sensory neurons.
RESULTS
LKB1 and SAD kinases are dispensable for axon formation by many types of neurons
LKB1, SAD-A and SAD-B are required for neuronal polarization and axon specification in the forebrain (Kishi et al., 2005; Barnes et al. 2007; Shelly et al., 2007). To begin this study, we asked whether these kinases play similar roles in subtelencephalic neurons. For analysis of SAD kinases, we used null SAD-A; SAD-B double mutants, denoted SAD-A/B−/−, which die shortly after birth due to respiratory insufficiency (Kishi et al., 2005). In contrast, LKB1 null mutants fail to develop past E9.5, before most neurons form (Jishage et al., 2002); we therefore used a conditional LKB1 mutant in combination with a Nestin-cre line that acts in all neural progenitors (Tronche et al. 1999; LKB1fl/fl; Nestin-cre, denoted LKB1Nestin-cre). LKB1Nestin-cre mice survived to birth and exhibited cortical defects similar to those demonstrated previously when LKB1 was deleted selectively from cortical progenitors (Barnes et al., 2007): the cortical wall was thinned, apoptosis was prevalent in the cortical plate, segregation of the axonal marker Tau-1 to axons was defective, and axon tracts in the cortical intermediate zone were markedly reduced (Figure S1).
In contrast to cortex, axonal tracts in all other parts of the nervous system examined were present and apparently normal in neonatal SAD-A/B−/− and LKB1Nestin-cre mice. They included the spinal trigeminal tract, axon bundles within the brainstem trigeminal complex (BSTC), ascending tracts in the spinal cord (spinocerebellar, spinothalamic and dorsal funiculus), the optic nerve, and motor and sensory nerves in the periphery (Figure 1 and data not shown). Motor axons in SAD-A/B−/− and LKB1Nestin-cre mice formed neuromuscular junctions on muscle fibers (data not shown), and sensory axons formed specialized endings on peripheral targets (see below). In addition, we deleted SAD-A/B and LKB1 from retinal progenitors using retina-specific cre lines, and found that photoreceptors, retinal bipolar cells and retinal ganglion cells all polarize normally in their absence (M. Samuel, P.E. Voinescu, B.N.L. and J.R.S. submitted). Thus, many types of neurons can form axons in the absence of LKB1 and SAD-A/B kinases.
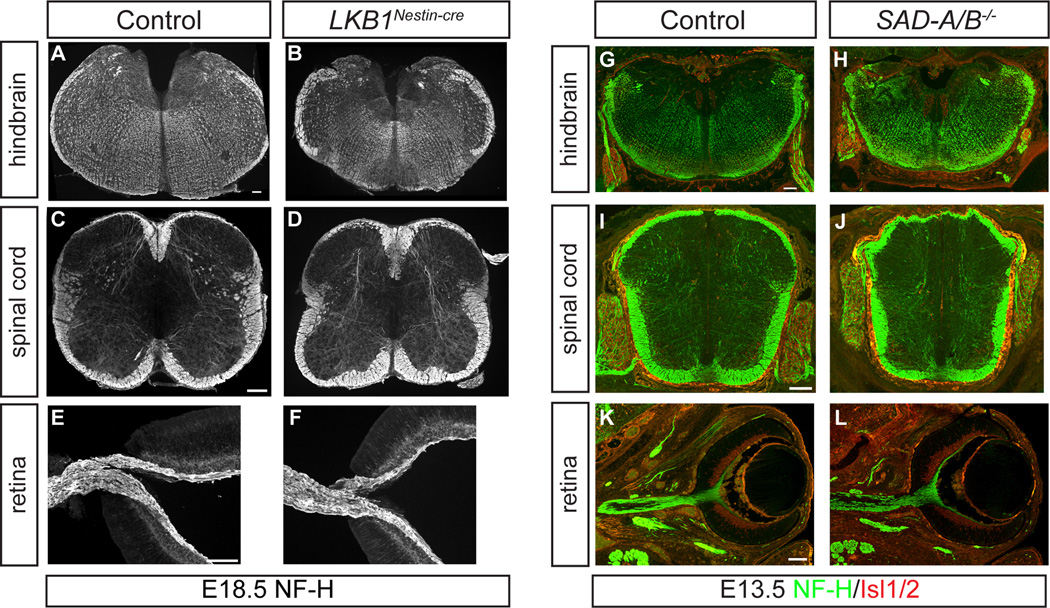
Figure 1. LKB1 and SAD kinases are dispensable for axon formation by subcortical neurons.
(A–F) Neurofilament immunohistochemistry of hindbrain (A,B), spinal cord (C,D) and retina (E,F) from E18.5 control and LKB1Nestin-cre animals reveals that LKB1 deletion in neural progenitors does not affect axon formation throughout the neuraxis. (G–L) Immunohistochemistry for NF-H and Isl1/2 in E13.5 control (G,I,K) or SAD-A/B−/− (H,J,L) hindbrain (G,H), spinal cord (I,J) and retina (K,L) shows that SADs are also dispensable for axon formation in these regions. Scale bars, 100 µm. See also Figure S1.
Proprioceptive sensory neurons require SAD kinases but not LKB1 to form terminal arbors
Immunohistochemical analysis using an antibody that recognizes both SAD-A and SAD-B proteins showed high levels of immunoreactivity in axon tracts of the spinal cord and in peripheral nerves at E13.5–E15.5 (Figure 2A–E). In the peripheral nervous system, SADs were localized in intramuscular axons as well as in sensory axons innervating the mystacial pad. Suitable antibodies for LKB1 localization are not available, but in situ hybridization has shown this kinase to be broadly expressed in the developing nervous system (Barnes et al., 2007). Thus, LKB1 and SADs are expressed in post-mitotic neurons throughout the peripheral and central nervous system after neuronal polarization and axon outgrowth have occurred.
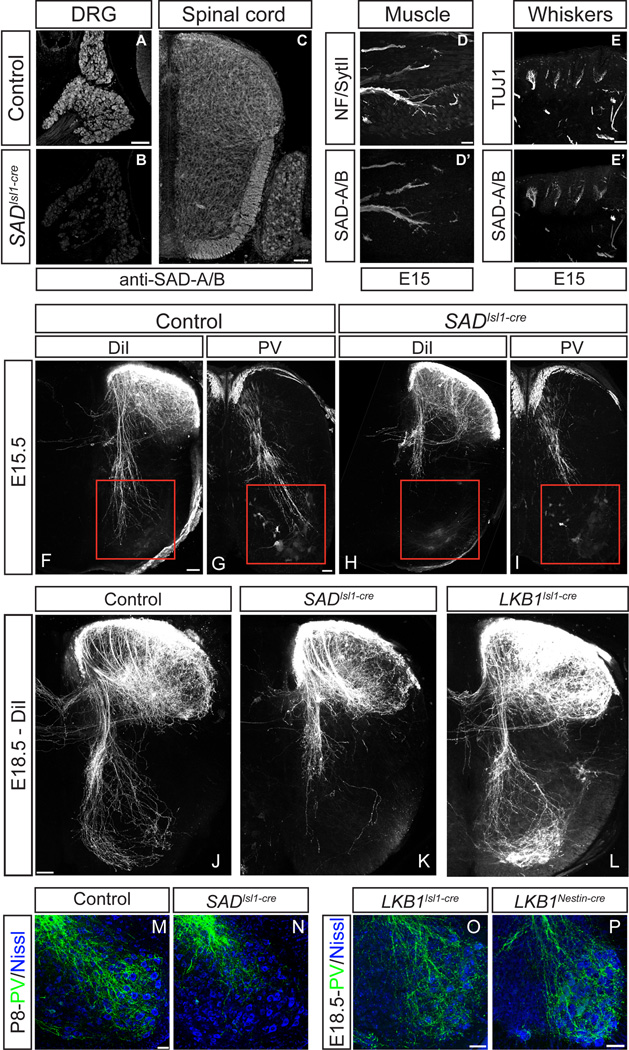
Figure 2. Ia proprioceptive sensory neurons require SADs, but not LKB1, for central axon projections.
(A–E) Immunohistochemistry reveals SAD-A/B in DRG at P0 (A), absent in SADIsl1-cre mutants (B); in spinal cord at E13.5 (C); in intramuscular axons (D; co-labeled with antibodies to neurofilaments and SytII, D’) and in whisker pad at E15.5 (E; co-labeled with TUJ1, E’). (F–I) E15.5 transverse spinal cord sections from animals labeled with DiI (F,H) or anti-Parvalbumin (G,I) show loss of ventral horn projections in SADIsl1-cre mutants compared to controls. (J–P) Ventral horn projections remain truncated in SADIsl1-cre mutants at E18.5 (J,K) and P8 (M,N), whereas deletion of LKB1 has no effect (L,O,P). Scale bars, 50 µm. See also Figure S2.
These patterns of expression raise the possibility that LKB1 and SAD kinases regulate later developmental steps in neurons that do not use them for polarization and axon specification. To test this idea, we deleted LKB1 and SAD kinases from specific neuronal types postmitotically, bypassing early effects of these genes and the perinatal lethality associated with their pan-neuronal deletion. To manipulate SADs, we constructed a conditional allele of SAD-A that, when crossed to Cre recombinase expressing lines, results in a protein null (Figure S2A,B). The conditional SAD-A line was crossed with the SAD-B null allele to create double mutants. We manipulated SAD and LKB1 function in sensory and motor neurons using the Isl1-cre line (Srinivas et al., 2001), which is expressed in DRG and trigeminal sensory neurons, dI3 spinal interneurons and most motor neurons (Figure S2C–E) and effectively deletes SAD and LKB1 kinases from sensory neurons (Figure 2A,B and Figure S2F).
SADIsl1-cre mutants were born at Mendelian ratios, but few animals survived longer than 24 hours after birth. Mutants were hypokinetic and typically had little milk in their stomachs when control littermates had large milk spots (Figure S2F,G). SAD-A−/−, SAD-B−/−, SAD-Afl/fl; SAD-B−/− and SAD-Afl/+; SAD-B−/−; Isl1Cre/+ animals were all viable and fertile, and exhibited no obvious defects. We first examined the role of SADs in the development of axonal projections into the spinal cord by labeling with the tracer DiI. Labeled sensory axons in mutants and controls entered the cord normally at the dorsal root entry zone, bifurcated, and ran many segments rostrally and caudally (data not shown). However, the projections of Type Ia proprioceptive sensory neurons (IaPSNs) into the ventral horn were dramatically disrupted in SADIsl1-cre mice. At E15.5, when this population of axons reaches the ventral spinal cord, SAD mutant axons had arrested their growth in the medial spinal cord adjacent to the central canal (Figure 2F,H). Labeling with antibodies to parvalbumin, a marker of IaPSN axons in spinal cord, confirmed the failure of these axons to reach the ventral horn (Figure 2G,I). The defect was a failure rather than a delay of extension because it persisted through fetal development (Figure 2J,K) and even until P8 in the small number of SADIsl1-cre mice that survived postnatally (Figure 2M,N). A similar phenotype was observed along the entire rostral-caudal extent of the spinal cord (Figure S2H–M). Thus, SAD kinases are not required for growth of sensory axons in the spinal cord, but are required for IaPSNs to form terminal arbors.
In contrast, deletion of LKB1 using Isl1-cre or Nestin-cre had no effect on the IaPSN projections to the ventral horn (Figure 2J,L,O,P). LKB1Isl1-cre mutants survived postnatally and had no apparent sensory or motor deficit, but had to be euthanized at 4–6 weeks of age due to massive gastrointestinal tumors resulting from LKB1 deletion in stomach and duodenum (Bardeesy et al., 2002 and B.N.L., unpublished observations). Thus, in contrast to their dependence on LKB1 for activation in cortex, SAD kinases do not require LKB1 to promote axonal arborization in the spinal cord.
SAD kinases are required for central projections of NT-3 dependent sensory neurons
We next asked whether defects in SADIsl1-cre spinal cord were confined to IaPSNs. Close examination of DiI-labeled spinal cords revealed defects in a second population of sensory neurons. In thoracic segments, a set of axons projects across the midline at the lamina III/IV level. These are central processes of DRG neurons that innervate Merkel cells in mechanoreceptors at the dorsal and ventral midline of the trunk; they terminate in the medial and lateral dorsal horn, respectively (Smith, 1986). Unilateral labeling of the T12 DRG in control animals at P0 labeled the two populations, but few axons crossed the midline in SADIsl1-cre mutants and in only rare instances did axons reach their proper contralateral target (Figure 3A,B).
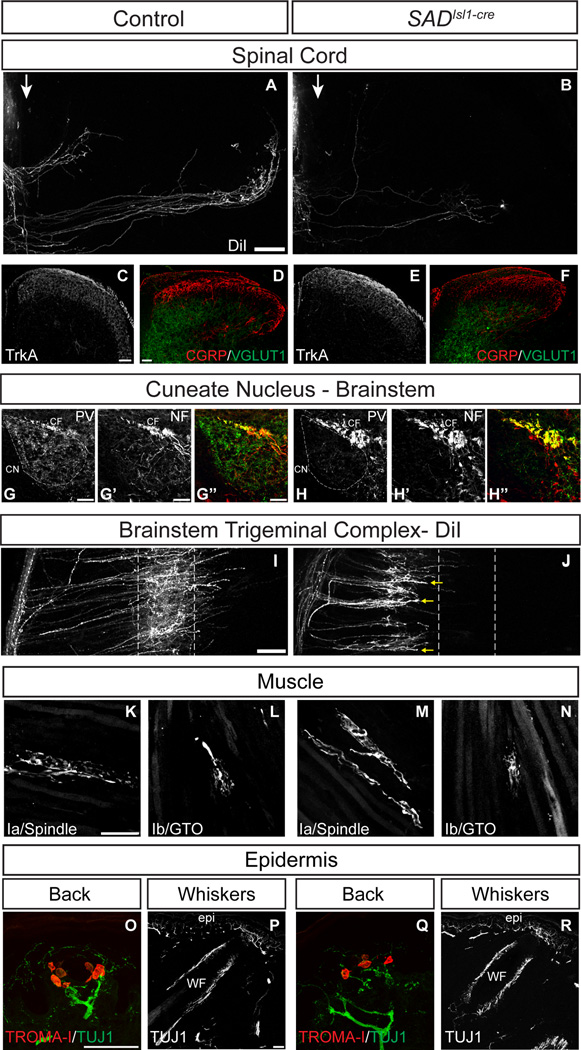
Figure 3. SAD kinases regulate central, but not peripheral axon differentiation in NT-3 dependent sensory neurons.
(A,B) Unilateral labeling of T12 DRG shows loss of midline crossing projections both medially and laterally in the dorsal horn of SADIsl1-cre mutants. Arrows indicates midline. (C–F) Immunohistochemistry of dorsal horn from lumbar spinal cord of P0 (C,E) or P8 (D,F) animals. Loss of SAD kinases in sensory neurons does not effect central projection of axons labeled with TrkA (C,E), CGRP (D,F, red) or VGLUT1 (D,F, green). (G–H’’) Immunohistochemistry using antibodies to PV and NF-H (G’,H’) shows that SAD-deficient axons are prominent in the cuneate fascicle (CF), but fail to grow into the cuneate nucleus (outlined, CN). (I,J) Transganglionic DiI labeling of the B2 whisker follicle shows defects in central axon projections of whisker afferents. DiI labeled axons on the left are in the spinal trigeminal tract adjacent to the SpVc, collaterals branch off and grow into the SpVc and form dense arbors. SADIsl1-cre mutant axons fail to elaborate axon arbors that enter into the correct target (outlined); growth cones tip many of the labeled axons (arrows). (K–R) Parvalbumin immunohistochemistry of E17.5 triceps muscle showing that annulospiral endings of IaPSNs (K,M) and flame-shaped Golgi tendon organs of IbPSNs (L,N) differentiate normally in SADIsl1-cre animals. (O,Q) Immunohistochemistry for TUJ1 (axons) and TROMA-I (Merkel cells) of P0 skin at the dorsal midline shows normal formation of Merkel cell-neurite complexes in SADIsl1-cre mutants. (P,R) TUJ1 immunohistochemistry of P0 mystacial pad shows that whisker follicles (wf) and epidermis (epi) are innervated normally in the absence of SADs. Scale bars, 50 µm. See also Figure S3.
Dense labeling of the dorsal horn with DiI prevented analysis of axonal subpopulations within this region. We therefore labeled control and SADIsl1-cre spinal cord with antibodies to TrkA, which labels terminals of nociceptive sensory axons in laminae I/IIi/IIo; to CGRP, which labels a nociceptor subset in lamina IIi; and to VGLUT1, which labels terminals of cutaneous mechanoreceptors in laminae III/IV (Patel et al., 2000; Chung et al., 1988; Brumovsky et al., 2007). All three sets of neurons targeted correct laminae in SADIsl1-cre mutants (Figure 3C–F). Thus, loss of SAD-A/B affects only some types of sensory axons.
Do SADs also regulate terminal axon arborization of sensory neurons in the brain? We tested this possibility in two ways. First, we examined the ascending axonal projections of cervical IaPSNs, which grow into the brainstem in the cuneate fascicle and then branch and form arbors in the cuneate nucleus (Weinberg et al., 1990). In control animals, numerous parvalbumin-positive proprioceptor axons were present in the cuneate fascicle and nucleus as reported previously (Figure 3G–G’’ and Solback and Celio, 1990). Proprioceptor axons were also abundant in the cuneate fascicle of SADIsl1-cre mutants, but their numbers were dramatically reduced in the cuneate nucleus (Figure 3H–H’’). Thus, in brainstem as in spinal cord, IaPSNs axons grow to the vicinity of their target, but fail to form terminal branches. Second, we used DiI to label central projections of trigeminal sensory neurons that innervate whiskers. These axons grow to the brainstem where they arborize in nuclei of the brainstem trigeminal complex (BSTC) (Erzurumlu et al., 2010). Axons labeled from a single whisker in controls arborize in circumscribed and stereotyped positions within the BSTC (Figures 3I and S3A). Axons labeled in whiskers of SADIsl1-cre mice grew through the spinal trigeminal tract in normal numbers, but had sparse arbors that failed to reach the correct target region in the BSTC and did not branch extensively (Figures 3J and S3B). Neurofilament staining showed no difference in overall structure between mutant and control BSTC (Figure S3C,D). Cumulatively, these data suggest that SAD kinases are required in subsets of sensory neurons for terminal axon arbor formation throughout the CNS.
In SADIsl1-cre mice, SAD kinases are deleted from motor neurons and some populations of interneurons as well as from sensory neurons (Figure S2E). Several observations indicate, however, that loss of SAD kinases from sensory neurons rather than from other cell types accounts for the defects described above. First, although Isl1 is expressed in spinal dI3 interneurons, which may help guide IaPSNs to the spinal cord (Ding et al., 2005), these interneurons were present and migrated to proper positions in SADIsl1-cre mutants (Figure S3E,F). Second, we removed SAD kinases from motor neurons using ChAT-cre, which is active before IaPSN axons reach the ventral horn (Philippidou et al., 2012). SADChAT-cre mutant IaPSN axons grew normally to the ventral horn (Figure S3G,H). Third, Isl1-cre was not expressed in the brainstem targets of whisker afferents or IaPSNs as late as P6 (Figure S3I–J’). Arborization defects in the brainstem are therefore not complicated by deletion of SADs from intrinsic neuronal types.
These results suggest that SAD kinases act cell autonomously in several classes of sensory neuron to regulate formation of central axonal arbors. NT-3 is expressed in the peripheral targets of all classes of SAD-dependent sensory neurons identified (Haeberle et al., 2004; Schechterson and Bothwell, 1992; Farinas et al., 1996; Patapoutian et al., 1999), and both IaPSNs and mechanoreceptive neurons innervating Merkel cells are lost in NT-3 mutants (Ernfors et al., 1994; Farinas et al., 1994; Airaksinen et al., 1996). Moreover, defects in IaPSN central projections described above for SADIsl1-cre mice are similar to those reported previously for NT-3;Bax double mutants in which IaPSNs are spared from apoptosis (Patel et al., 2003). We therefore asked whether SADs interact with the NT-3 signaling pathway.
The neurotrophic factor NT-3 acts through SAD kinases to pattern central projections
Loss of SAD kinases could affect NT-3 signaling in any of three ways. First, SADs might be required for peripheral axons of NT-3-dependent sensory neurons to reach sources of NT-3. Second, SADs might be required for retrograde signaling by NT-3. Third, SADs might mediate effects of NT-3 on axonal arborization. We tested these alternatives in turn.
We examined peripheral projections of sensory neurons innervating muscle (proprioceptors), Merkel cells, and whisker follicles. Parvalbumin-positive proprioceptive axons grew into forelimb and hindlimb muscles of SADIsl1-cre mutants in a manner indistinguishable from controls; within muscles, the IaPSN axons formed characteristic vesicle-rich (synaptotagmin-positive) annulospiral endings on intrafusal muscle fibers of forelimb and hindlimb muscles (Figures 3K,M and S3K–L’’). Golgi tendon organs were also innervated normally in SADIsl1-cre animals (Figure 3L,N). Similarly, in both control and SADIsl1-cre mutants, trunk sensory axons formed normal disc shaped endings on Merkel cells in the epidermis (Figure 3O,Q) and axons in the deep vibrissal nerve innervated whisker follicles (Figure 3P,R). In addition, PV+ DRG neurons acquired a pseudounipolar morphology by E15.5 (Figure S3M,N), a cellular feature that occurs upon peripheral innervation (Matsuda and Uehara, 1984). Thus, defects in central projections of SAD-deficient sensory neurons do not result from failure of peripheral processes to reach sources of neurotrophic factors.
We then asked whether SADs are required in IaPSNs for retrograde signaling by NT-3 through its receptor, TrkC. Expression of TrkC was not affected by the loss of SAD kinases (Figure S4A–B’’). When apoptosis is blocked in the absence of NT-3/TrkC signaling, the size of parvalbumin-positive neurons and levels of the transcription factor ER81 are reduced (Patel et al., 2003). None of these defects were observed in SADIsl1-cre mice (Figure 4A,E and Figure S4C–H) indicating that SAD kinases are not required for retrograde NT-3 signaling or for the acquisition of morphological or molecular characteristics induced by NT-3.
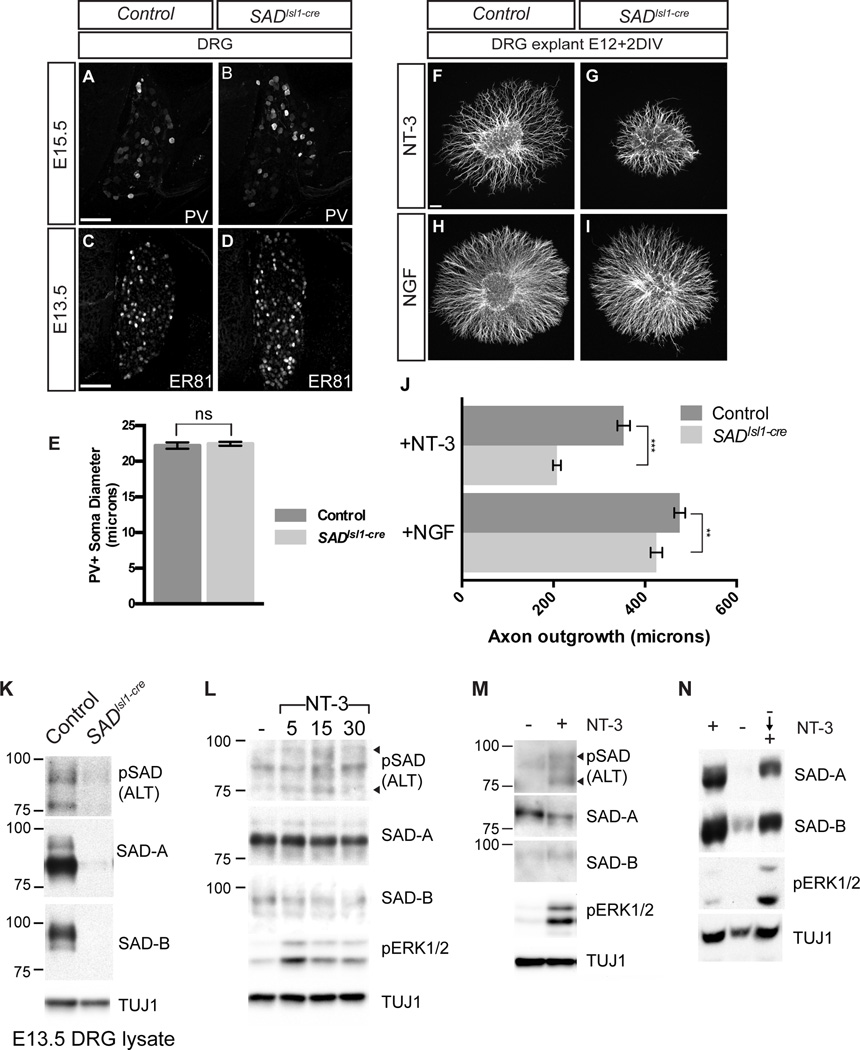
Figure 4. SAD kinases act downstream of NT-3 to induce axon outgrowth.
(A–E) Sections of lumbar DRG stained for PV at E15.5 (A,B) or for ER81 at E13.5 (C,D) and quantification of PV+ soma size (E, mean ± SEM, n≥60 cells per genotype). SADs are not required for transducing signals from peripheral NT-3 sources. (F–J) Axon outgrowth of DRG explants from control (F,H) and SADIsl1-cre mutants (G,I) grown for 2DIV with the indicated neurotrophin shows that SADs are required for NT-3 induced growth in vitro. Scale bars in A, C and F, 100 µm. Quantification (J) shows large decreases in NT-3-induced axon outgrowth and modest effects on NGF-induced growth (mean ± SEM from three experiments; NT-3: n ≥40 ganglia per genotype, p<.0001; NGF: n ≥36 ganglia per genotype; p=.005, unpaired t-test). (K) Immunoblot from control and SADIsl1-cre E13.5 lumbar DRGs shows loss of SAD pALT reactivity in double KO SADIsl1-cre animals. TUJ1 is a loading control. (L) E13.5 + 2DIV DRG cultures grown in NT-3 were starved of NT-3 for 5 hours, then NT-3 was added for the indicated times before cells lysis. (M) Growth of Bax−/− DRG neurons in the absence of neurotrophin for 3 days, followed by stimulation with NT-3 for 15 minutes induces SAD ALT phosphorylation. (N) DRG neurons were grown in the presence of NT-3 for 2DIV then were either grown with NT-3 (+) or without NT-3 for 18 hours (−), or deprived of NT-3 for 18 hours followed by growth with NT-3 for 24 hours (- -> +). See also Figure S4.
To ask whether SADs mediate effects of NT-3 on IaPSNs, we cultured DRG explants from control and SADIsl1-cre animals in the presence of NT-3 and measured axon outgrowth. Under these conditions, only NT-3 dependent neurons survive (Hory-Lee et al. 1993). Outgrowth of axons from these neurons was decreased by nearly half in SADIsl1-cre mutant ganglia relative to controls (Figure 4F,G,J). We also cultured DRG explants in the presence of NGF; under these conditions, IaPSNs die but NGF-dependent neurons survive. Loss of SADs had only a modest effect (12%) on axon outgrowth in these explants (Figure 4H,I,J). These findings indicate that SAD kinases are selectively required for axon growth in response to NT-3.
Does NT-3 signal, in part, by activating SADs? To test this possibility, we cultured wild-type neurons in the presence of NT-3 to promote selective survival of NT-3-dependent neurons, then immunoblotted lysates with antibodies to SAD-A, SAD-B, and to the phosphorylated form of a critical activation loop threonine (ALT) present in both SAD-A and SAD-B (SAD-A T175; SAD-B T187); SAD kinases are catalytically active only when phosphorylated at the ALT site (Lizcano et al., 2004; Barnes et al., 2007). Specificity of the antibodies was demonstrated by absence of immunoreactivity from SADIsl1-cre DRG lysates (Figure 4K). SAD-A and SAD-B were readily detectable in DRGs maintained in NT-3, but their ALT phosphorylated forms were at low levels. When NT-3 was withdrawn for 4–5 hours, SAD protein levels did not change detectably. Re-addition of NT-3 at this time led to significant SAD phosphorylation that was detectable within 5 minutes and peaked by 15 minutes before declining by 30 minutes (Figure 4L). We also tested neurons from Bax−/− mice, which do not require neurotrophins for survival, to confirm the effect of NT-3 on SAD activation. NT-3 stimulation of Bax−/− neurons that had been grown in the absence of neurotrophin for 3 days led to rapid SAD ALT phosphorylation (Figure 4M). Thus, NT-3 can activate SADs.
To assess longer-term effects of NT-3 on SAD, we withdrew the neurotrophin for 16–18 hours in the presence of a caspase inhibitor to prevent apoptosis. In this case, levels of both SAD-A and SAD-B declined dramatically. Re-addition of NT-3 to starved neurons led to recovery of protein levels over the following 24 hours (Figure 4N). Together these results show that NT-3 regulates SAD activity in two distinct ways: total SAD protein levels over long durations and SAD activation with rapid kinetics. We therefore examined the mechanisms underlying long and short term SAD regulation by NT-3.
NT-3 regulates SAD levels post-translationally through the Raf/MEK/ERK pathway
NT-3 might affect SAD protein levels by regulating transcription, mRNA processing and stability, translation, or protein stability. To distinguish among these alternatives, we first measured SAD mRNA levels using quantitative RT-PCR. Levels of SAD mRNAs did not differ significantly between NT-3 treated and starved cultures (Figure 5A). Thus, NT-3 regulates SAD levels at steps following RNA processing. To assess NT-3-dependent translational control, we generated a GFP-tagged SAD-A open reading frame lacking 5’ and 3’ UTRs, in which most translational control elements are found. GFP::SAD-A was introduced to dissociated DRGs using a lentiviral vector. Withdrawal of NT-3 signaling led to similar reductions in endogenous SAD-A and GFP::SAD-A; levels of control proteins were unaffected over this period (Figure 5B). We conclude that NT-3 regulation of SAD protein levels occurs post-translationally.
Figure 5. NT-3 post-translationally regulates SAD protein expression via the Raf/MEK/ERK pathway.
(A) qRT-PCR of SAD-A and SAD-B mRNA shows that SAD mRNA levels are the same in NT-3 starved and NT-3 treated cultures (mean ± SEM, three experiments; 1.0 equals levels in NT-3 treated samples). (B) DRG neurons infected with lentiviral vectors expressing GFP-tagged versions of wild type (WT) or D-box mutant (DBM) SAD-A were grown with NT-3 for 2DIV followed by additional NT-3 treatment (+) or starvation (−) for 18 hours. Quantification of GFP immunoblot signals of (−) relative to (+) NT-3 (mean ± SEM, N=3, p=.025) is shown below representative blot. (C) DRG neurons cultured for 2 DIV with NT-3 were starved for 18 hours (−), followed by NT-3 re-addition for 24 hours in the presence of vehicle, MEK1/2 inhibitor (PD-325901, 10 µM), PI3K inhibitor (LY294002, 50 µM) or mTORC1 inhibitor (rapamycin, 100 nM). (D) DRG neurons were grown for 2DIV in either NT-3 and were treated or starved as in (B) or were treated with NT-3 and MEK1/2 inhibitor. (E) DRG neurons infected with B-RAF V600E-expressing lentivirus were cultured as in (B). (F) Bax-deficient DRG neurons not treated with neurotrophic factors were infected as in (D) and were lysed at 3DIV. All samples were analyzed by immunoblotting with the indicated antibodies. (G) Summary of pathway regulating SAD protein levels.
We examined the SAD protein sequences to determine whether they contain motifs that regulate protein stability. Within the C-terminal domains of SAD-A and SAD-B are highly conserved consensus D box motifs (RxxLxxxxN) that target proteins for ubiquitination by the Anaphase Promoting Complex/Cyclosome (APC/C) E3 ubiquitin ligase (Puram and Bonni, 2011; Li et al., 2012). Multiple subunits of the APC/C are expressed in E13.5 DRGs (data not shown). We expressed a GFP-tagged SAD-A mutant in which the D box was mutated (RxxLxxxxN to AxxAxxxxN) to eliminate binding to APC/C (GFP::SAD-ADBM). While GFP::SAD-AWT was sensitive to NT-3 deprivation, GFP::SAD-ADBM was significantly stabilized (Figure 5B). These data are consistent with a model in which NT-3 controls SAD protein levels by stabilization.
We next examined the pathway that leads from NT-3 to SAD protein stabilization. Three canonical signaling pathways are induced by Trk activation: Raf/MEK/ERK, PI3K/Akt and PLCγ (Reichardt, 2006). We added inhibitors of these pathways along with NT-3 following a period of deprivation. Inhibiting MEK1/2 with PD325901 completely blocked SAD protein increase. LY294002, a PI3K inhibitor, had a modest effect on SAD protein recovery, but long-term treatment with this compound also inhibited ERK1/2 phosphorylation complicating interpretation (Figure 5C). Due to instability of the available PLCγ inhibitors, we were unable to perform long-term pharmacological inhibition of this pathway. We also tested rapamycin, an inhibitor of mTOR, because a recent study reported mTOR-dependent regulation of SAD translation (Choi et al., 2007). Rapamycin had only a slight effect on the increase in SAD protein levels stimulated by NT-3. In addition, blocking MEK1/2 kinases with the specific inhibitor PD-325901 in the presence of NT-3 led to a decline in SAD levels similar to those seen after NT-3 deprivation; as expected, ERK1/2 phosphorylation was also suppressed (Figure 5D).
As a further test of the idea that NT-3 regulates SAD protein levels through the Raf/MEK/ERK pathway, we used lentiviral vectors to express either GFP or constitutively active B-Raf V600E in dissociated DRG neurons. IaPSNs deficient in the B- and C-Raf MAP3Ks, the most upstream components of the MAPK pathway, arrest their growth in the medial spinal cord (Zhong et al., 2007), a phenotype similar to that of SADIsl1-cre mutants. Consistent with this observation, B-Raf V600E increased ERK1/2 phosphorylation in DRG neurons relative to GFP expressing controls, and prevented the decline of SAD protein levels caused by loss of NT-3 signaling (Figure 5E). Constitutive MAPK activation using B-Raf V600E also increased SAD-A/B protein levels in BAX−/− DRG neurons in the absence of neurotrophic factors (Figure 5F). We conclude that sustained NT-3/TrkC signaling via the MAPK pathway is the major mechanism that maintains high SAD-A and –B protein levels in IaPSNs (Figure 5G). Moreover, the effects of Raf MAP3Ks on axonal arborization of IaPSNs (Zhong et al., 2007) may be mediated by SAD kinases.
NT-3 regulates SAD kinase activation by inactivating a C-terminal inhibitory domain
How does NT-3 lead to rapid phosphorylation of the ALT site on SAD kinases and thereby enable their catalytic activity? In light of the surprising finding that LKB1 is not required for SAD-dependent axon branching in vivo (Figure 2), we sought other kinases that might be able to respond to NT-3 and in turn activate SADs. To this end, we used HeLa cells, which do not express LKB1 or SADs. When SADs are transfected into HeLa cells, the ALT remains unphosphorylated and the enzymes exhibit no catalytic activity (Barnes et al., 2007). When LKB1 is expressed in HeLa cells, it binds to its co-factors STRAD and MO25 and phosphorylates SADs on the ALT leading to activation (Lizcano et al., 2004; Barnes et al., 2007). We co-expressed SAD and other potential activating kinases in HeLa cells and assayed SAD ALT phosphorylation. The phenotypic similarities between mutants for Raf kinases SADs noted above suggested Rafs as potential SAD ALT kinases, but expression of a constitutively active Raf kinase (B-RAF V600E) did not induce SAD ALT phosphorylation (Figure S5A). Of several other kinases tested, only TAK1/MAP3K7, together with its co-activator TAB1 (Shibuya et al., 1996), robustly phosphorylated SAD-A and SAD-B at the ALT; it was also active in assays using purified proteins (Figure S5B,C). Moreover, TAK1 is expressed in E13.5 DRG neurons (Figure S5D and Jadrich et al., 2003). However, inactivation of TAK1 using a floxed conditional allele with Isl1-cre and Nestin-cre had no effect on central axon projections of IaPSNs (Figure S5E,F and data not shown). We combined TAK1 and LKB1 conditional alleles to examine whether these two kinases might act as redundant activators of SAD kinases, but observed no defects in IaPSN projections in (LKB1; TAK1)Isl1-cre double mutants (Figure S5G). Thus, neither TAK1 nor LKB1 is required for central axon arbor formation of IaPSNs.
We cannot rule out the possibility that NT-3 signals through ALT kinases that we did not test. However, an alternative mechanism was suggested by biochemical studies of SAD proteins. Immunoblotting revealed highly abundant ~85 kDa and much less abundant 76 kDa forms of SAD-A in brain and sensory ganglia, both absent from SAD-A−/− tissue (Figure 6A). The active (pALT-positive) SAD-A migrated at 76 kDa (Figure 6A). SAD-B migrated more heterogeneously in SDS-PAGE than SAD-A, complicating analysis. We therefore focused on SAD-A, asking whether the 76 and 85 kDa species were generated by distinct mRNAs or by a posttranslational mechanism. We expressed SAD-A with or without LKB1 in HeLa cells and analyzed them by immunoblotting. SAD-A migrated as a doublet of 85 and 76 kDa in both cases, but in the presence of LKB1, only the 76 kDa form of SAD-A was ALT phosphorylated (Figure 6B). We then co-expressed TrkC with SAD-A in the absence of LKB1. Within 15 minutes of adding NT-3 to TrkC expressing HeLa cells, SAD-A protein was largely converted from the 85kDa to the 76kDa form, even though it remained completely dephosphorylated at the ALT site and therefore catalytically inactive (Figure 6C and data not shown). Together, these results suggest that NT-3 can lead to a post-translational modification of SAD-A that renders it activatable by ALT kinases.
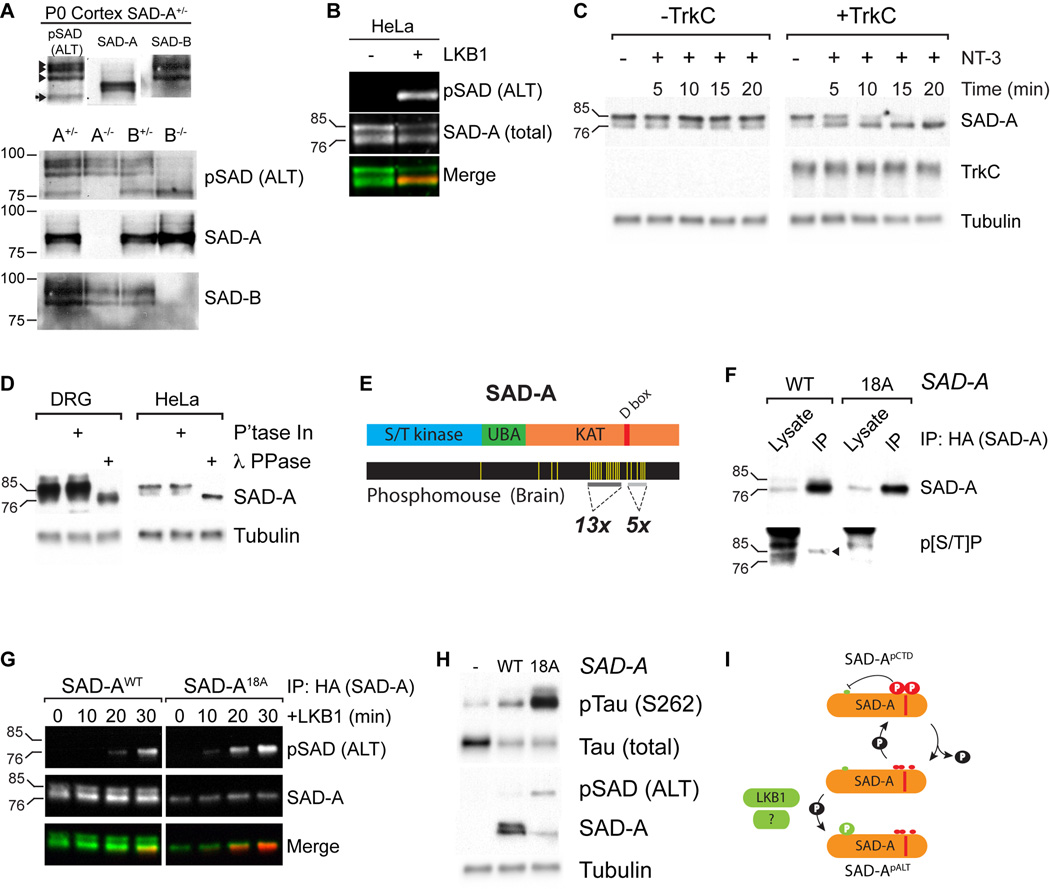
Figure 6. Inhibitory phosphorylation in SAD C-terminal domains blocks kinase activation.
(A) Immunoblots from P0 cortices of animals of the indicated SAD genotype shows that SADs migrate heterogeneously in SDS-PAGE and that the predominant SAD-A pALT species migrates at 76 kDa. (B) Two color immunoblot of lysates from HeLa cells transfected with LKB1 and SAD-A shows that SAD-A is present in 85 and 76 kDa forms, but only the 76 kDa form is ALT phosphorylated. (C) Serum starved HeLa cells transfected with SAD-A without or with TrkC were treated with 50 ng/ml NT-3 for the indicated times followed by cell lysis and immunoblotting. SAD-A shifts from a predominantly 85 kDa form to the 76 kDa form upon NT-3 treatment only in TrkC+ cells. (D) Treatment of lysates of DRG cultures or HeLa cell transfectants with λ protein phosphatase shows that phosphorylation accounts for the difference in molecular weight. (E) Domain structure of SAD-A and Phosphomouse display of identified sites of phosphorylation in mouse brain (yellow lines). (F) Analysis of lysates and anti-HA (SAD-A) immunoprecipitates from HeLa cells expressing WT or 18A SAD-A shows that WT, but not 18A is reactive with anti-p[S/T]P. The anti-p[S/T]P signal in lysate lanes is from non-SAD-A species. (G) Immunoprecipitation of SAD-A followed by kinase assay with purified LKB1/STRAD/MO25. Only dephospho-CTD SAD-A is ALT phosphorylated. (H) Co-expression of Tau and SAD-A WT or 18A in 293T cells followed by immunoblotting. SAD-A18A shows increased kinase activity towards Tau (S262) and increased pALT reactivity. (I) Model for how SAD-A CTD phosphorylation inhibits ALT phosphorylation and activity. See also Figures S5 and S6.
To identify the difference between the 76 and 85 kDa forms of SAD-A, we treated extracts of DRGs and transfected HeLa cells with enzymes that remove putative modifications. Treatment with lambda protein phosphatase led to quantitative conversion of the 85kDa form of SAD-A protein to the 76kDa form (Figure 6D), indicating that SADs are phosphorylated at sites that control their activation state. We then examined a phosphoproteomic database of mouse tissues (Phosphomouse; Huttlin et al., 2010) to identify potential sites of SAD phosphorylation. In mouse brain, SAD-A is phosphorylated on 18 sites in its C-terminal domain (CTD): 16 are proline-directed, p[S/T]P, and of these, 12 are present in a striking proline-rich repeated sequence motif (PXXp[S/T]P) (Figure 6E and Figure S6A). To determine whether these residues are phosphorylated, we expressed a SAD-A mutant in which all 18 S/T residues were mutated to non-phosphorylatable alanine (SAD-A18A). Immunoprecipitation of SAD-A followed by immunoblotting with an antibody that is specific for phosphorylated Ser and Thr residues followed by Pro (p[S/T]P) demonstrated that only the 85 kDa form of wild-type SAD-A was phosphorylated at S/TP motifs (Figure 6F). The SAD-A18A mutant, in contrast, migrated exclusively at 76 kDa (see lysate lanes) and was non-reactive with the p[S/T]P antibody. Thus, some or all of these 18 residues are phosphorylated in SAD-A, and this phosphorylation is a major contributor to migration differences in SDS-PAGE.
We performed two experiments to test the idea that SAD CTD phosphorylation negatively affects the ability of upstream kinases to phosphorylate the ALT site and thereby activate SAD kinase. First we immunoprecipitated SAD-AWT and SAD-A18A from HeLa cells, in which the ALT site remains unphosphorylated (see above), then added exogenous, purified LKB1 and ATP. SAD-AWT was present in both phospho-CTD (85 kDa) and dephospho-CTD (76 kDa) forms. Exogenous LKB1 phosphorylated only the 76 kDa form. SAD-A18A was present in only the 76 kDa form, and this was significantly phosphorylated by LKB1 (Fig. 6G). Second, we expressed either SAD-AWT or SAD-A18A, along with tau (a known SAD substrate, Kishi et al., 2005; Barnes et al., 2007) in 293T cells, which have high levels of LKB1. SAD-A18A accumulated to several fold lower levels than SAD-AWT after transfection (see Discussion), but exhibited dramatically higher levels of SAD pALT phosphorylation and tau kinase activity (Figure 6H). Thus, phosphorylation of the SAD CTD precludes SAD kinase activation (Figure 6I). The fact that SADs are predominantly in the phospho-CTD form in neurons suggests that they are largely inactive under basal conditions.
Regulatory mechanisms controlling SAD-A CTD phosphorylation
To assess mechanisms that regulate phosphorylation of the CTD, we sought CTD kinase(s). Because phosphorylation sites in the CTD are adjacent to proline residues, we treated SAD-expressing HeLa cells with inhibitors of three groups of proline-directed kinases known to play roles in neural development: MEK1/2, GSK3β and cyclin dependent kinases (CDKs) (Newbern et al., 2011; Hur and Zhou, 2010; Su and Tsai, 2011). Only the CDK inhibitor, roscovitine, led to net dephosphorylation of SAD-A as indicated by a shift towards the 76 kDa form (Figure 7A and data not shown). We also treated cultured DRG neurons with roscovitine and found a similar although less pronounced shift in SAD-A mobility (Figure 7A). These results suggest that CDKs are physiological regulators of CTD phosphorylation.
Figure 7. Multiple pathways regulate the phosphorylation state of the SAD-A CTD.
(A) Treatment of SAD-AWT expressing HeLa cells or DRG neurons with 10 µM Roscovitine for 30 minutes causes SAD-A to shift mobility to the dephospho-CTD form. (B) Co-expression of SAD-A (WT or 18A) with CDK5 (WT or Kinase dead: D145N) and p35 in 293T cells followed by immunoblotting. Active CDK5 causes CTD phosphorylation, mobility shift and loss of pALT reactivity of SAD-AWT; SAD-A18A is not affected. (C) Immunoprecipitation for SAD-A followed by immunoblotting shows that NT-3 treatment of TrkC/SAD-A expressing HeLa cells causes dephosphorylation of CTD [S/T]P sites. (D) Treatment of TrkC/SAD-A expressing HeLa cells with MEK1/2 or PLCγ inhibitors partially blocks SAD-A CTD dephosphorylation in response to NT-3. (E) Inhibition of NT-3 induced SAD ALT phosphorylation in DRG neurons by blocking of PLCγ (U73122), but not MEK1/2 (U0126). Ca2+ ionophore A23187 induces SAD ALT phosphorylation independently of NT-3. (F) Treatment of serum starved HeLa cells with calcium ionophore A23187 (5 µM) for 5 minutes induces SAD-A CTD dephosphorylation independently of MEK1/2 activity. (G) Model for NT-3 dependent regulation of SADs. See also Figure S6.
To test this idea directly, we co-expressed either wild type or catalytically inactive CDK5 with the p35 co-activator and SAD-AWT or SAD-A18A. Expression of active but not inactive CDK5 caused SAD-AWT protein to migrate exclusively at 85 kDa, while migration of SAD-A18A was only slightly affected (Figure 7B). Moreover, whereas expression of active CDK5 completely eliminated ALT phosphorylation of SAD-AWT, ALT phosphorylation of SAD-A18A was largely resistant to CDK5-mediated inhibition (Figure 7B). The fact that the SAD-A18A mutant is not completely refractory to the inhibitory effects of CDK5 suggests that there may be other residues involved in mediating SAD-A inhibition. Thus, CDK5 can phosphorylate SAD-A in the CTD, preventing activating phosphorylation at the ALT. These results reveal a novel mechanism in which activation of SAD kinase by canonical activation loop phosphorylation is inhibited by phosphorylation of distal sites in the CTD.
To ask which phosphorylation sites in the SAD-A CTDs are important for inhibition of SAD activation, we divided them into two groups and mutated each separately: the 13 sites in the PXX[S/T]P motifs N-terminal to the D-box (aa 428–468, mutant called SAD-A13A) or the 5 sites (4 of which are [S/T]P) C-terminal to the D-box (aa 490–513, mutant called SAD-A5A; Figure S6A), We expressed the mutants in 293T cells with CDK5 and examined the effects on SAD ALT phosphorylation. CDK5 activation suppressed SAD ALT phosphorylation of both the SAD-A13A and SAD-A5A mutants (Figure S6B). We conclude that no single phosphorylation event in the CTD regulates SAD activity, but rather phosphorylation of residues throughout the SAD CTD is sufficient to block SAD ALT phosphorylation.
What signaling pathway does NT-3 use to regulate phosphorylation of the SAD CTD? We analyzed SAD-A immunoprecipitates from untreated and NT-3 treated cells using the anti-p[S/T]P antibody. Consistent with results presented above (Figure 6C), SAD-A protein from untreated cells was strongly phosphorylated at [S/T]P sites, whereas SAD-A protein from NT-3 treated cells lacked [S/T]P phosphorylation (Figure 7C). Thus, NT-3/TrkC signaling induces net SAD-A CTD dephosphorylation. Inhibitors of MEK1/2 or PLCγ decreased NT-3 dependent SAD-A CTD dephosphorylation in TrkC+ HeLa cells (Figure 7D), indicating that both of these pathways are capable of regulating SAD-A CTD dephsophorylation in response to NT-3. In DRG neurons, inhibition of PLCγ blocked SAD ALT phosphorylation, but inhibition of MEK1/2 did not (Figure 7E), suggesting that neural regulation of CTD phosphorylation is mediated primarily by the PLCγ pathway. PLCγ activation generates diacylglycerol (PKC agonist) and IP3, which leads to release of Ca2+ from intracellular stores. Indeed, elevating intracellular Ca2+ levels using A23187 was sufficient to induce complete SAD-A CTD dephosphorylation in HeLa cells (Figure 7F and Figure S6C) and induced SAD ALT phosphorylation in DRG neurons (Figure 7E). Thus, NT-3 induces SAD ALT phosphorylation in neurons largely through the PLCγ/Ca2+ pathway.
Role of CTD phosphorylation in SAD-dependent axon branching
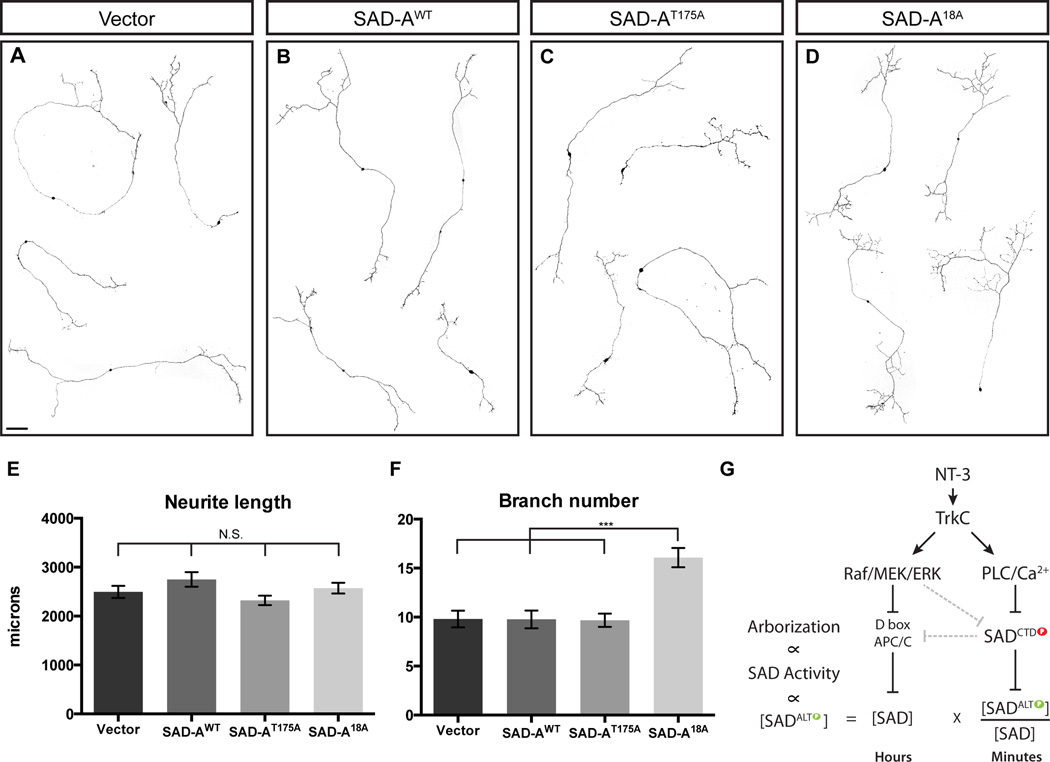
Finally, we tested whether eliminating the inhibitory effects of SAD-A CTD phosphorylation could affect axonal development in neurons. We cultured sensory neurons at low density in 5 ng/ml NT-3, which leads to modest levels of axon branching (Lentz et al. 1999). Expression of wild-type (SAD-AWT) or catalytically inactive (SAD-AT175A) kinase affected neither total axon outgrowth nor the number of branches relative to vector control (Figure 8E,F), consistent with the observation that most SAD-A in neurons is in a CTD-phosphorylated, inactive form (see above). In contrast, expression of SAD-A18A led to significant increase in branching with no effect on total outgrowth (Figure 8E,F). We conclude that augmenting SAD-A activation by preventing inhibitory phosphorylation is sufficient to increase axon branching.
Figure 8. Increasing SAD activation induces DRG axon branching in vitro.
(A–D) Representative images of neurons expressing empty vector control or the indicated SAD-A cDNA constructs. Scale bar equals 100 µm. (E,F) Quantification of neurite length and branch points in cultured neurons. Differences among means in (E) did not reach statistical significance (p>.01 using one way ANOVA with Tukey’s multiple comparisons test; graph shows mean ± SEM; Vector: n=76; WT: n=87; T175A: n=101; 18A: n=93) whereas the difference in branch numbers (F) between SAD-A18A and all others were highly significant (mean ± SEM, p<.0001, One way ANOVA with Tukey’s multiple comparisons test). (G) Model for how NT-3 signaling controls SAD kinase activity by regulating SAD levels and the fraction of SAD that is ALT phosphorylated.
DISCUSSION
We have found that SAD-A and SAD-B kinases, previously implicated in axon specification and polarization of forebrain neurons (Kishi et al., 2005; Barnes et al., 2007) are also required for formation of terminal axonal arbors of sensory neurons, demonstrating that SAD kinases regulate multiple aspects of axonal morphogenesis. We also show that neurotrophin signals regulate SAD kinase activity over multiple time scales (summarized in Figure 8G), suggesting mechanisms by which extrinsic factors could converge on SAD kinases to sculpt axonal morphology.
NT-3-dependent neurons require SAD kinases to form central axonal arbors
Surprisingly, although SAD kinases are required for polarization of forebrain neurons (Kishi et al., 2005; Barnes et al., 2007), they are dispensable for polarization of all subtelencephalic populations tested. In contrast, SAD kinases are required for a late stage of axonal development: the formation of central axonal arbors by subsets of sensory neurons in spinal cord and brainstem. The effect is a highly specific one: SADs are dispensable not only for polarization of these neurons but also for growth of their peripheral axons, initial extension of a central process, bifurcation at the dorsal root entry zone, and collateral formation in the spinal cord and brain. Instead, SADs are required only after axons have reached their target areas, and form highly branched terminal arbors to contact postsynaptic cells.
The requirement for SADs is also highly specific in another respect. Whereas several NT-3-dependent subsets of sensory neurons require SADs for axonal arborization, other subsets, including those that require the related neurotrophin, NGF, are largely SAD-independent. What might account for the differential requirements for SADs? Several lines of evidence suggest that cellular responses to distinct neurotrophins vary widely and are specific to the Trk receptor expressed. For instance, treatment of DRG neurons with NGF, BDNF or NT-3 leads to distinct axon morphologies in culture (Lentz et al., 1999; Ozdinler et al., 2004). More dramatically, substituting TrkC for TrkA in DRG neurons changes the molecular and anatomical properties of cutaneous sensory neurons to those of proprioceptors (Moqrich et al., 2004). SADs may be a component of a TrkC-specific signaling pathway. Alternatively, other signal transduction components may play redundant or compensatory roles in NT-3-independent neurons. The observations that outgrowth from NGF-dependent, TrkA-expressing neurons is slightly decreased in SAD mutants (Figure 4C), and that NGF can stimulate SAD-A ALT phosphorylation (data not shown) support this possibility.
SAD kinases mediate effects of NT-3 on axon branching
Why is axonal branching by NT-3-dependent neurons perturbed in SAD mutants? Knowing that peripheral depots of NT-3 are required for branching, we asked whether SADs might be required for sensory axons to reach these depots or for NT-3 signaling to reach the nucleus and alter gene expression. In fact, SADs were dispensable for both of these developmental steps. In the absence of NT-3, substantial IaPSN axon growth occurs in vivo, but the terminal phase of arbor formation in the spinal cord does not occur (Patel et al., 2003), much as we observe in SADIsl1-cre mutants. We propose that SAD kinases act as effectors of NT-3 signals during axon growth and arbor formation in the CNS, but are not required for NT-3 independent growth modes. Multiple lines of evidence support this hypothesis: NT-3-dependent outgrowth in culture is dramatically attenuated in SAD kinase mutant neurons, NT-3 stimulates SAD activity, and increased SAD activity enhances axonal branching.
We then asked how NT-3 signals to SADs, and found that it does so by two distinct mechanisms that act over different durations but to a common end. Application of NT-3 to sensory neurons increases SAD protein levels over a period of hours and the fraction of SAD that is phosphorylated at a critical activation site (ALT) within minutes. Moreover, as discussed below, distinct molecular pathways link NT-3 to these two effects (summarized in Figure 8G). We propose that this combination of mechanisms allows SAD kinases to integrate short- and long-term signals from distinct sources to provide fine control of arbor formation. For example, peripheral sources of NT-3 might provide tonic increase in SAD levels that enables branching during an appropriate developmental window, whereas NT-3 from sources within the ventral horn, such as motor neurons (Schechterson and Bothwell, 1992; Wright et al., 1997; Genç et al., 2004; Usui et al., 2012) could regulate SAD activity with fine temporal and spatial precision, to precisely sculpt the arbors. Alternatively, other factors in the target region that either promote or inhibit axon growth and branch formation (Wang et al., 1999; Krylova et al., 2000; Messersmith et al., 1995; Ma and Gibson, 2011) might affect SAD activity, allowing the kinases to integrate multiple signals.
Regulation of SAD activation by inhibitory CTD phosphorylation
We used sensory neurons and heterologous cells to map the pathways by which NT-3 increases SAD levels and SAD activity. NT-3 activates the receptor tyrosine kinase TrkC, which then stimulates three pathways in which Raf/MEK/ERK, PLCγ/Ca2+, and PI3K, are key intermediates (Reichardt, 2006). TrkC activation enhances the stability of SADs predominantly through the Raf/MEK/ERK pathway, engagement of which may prevent ubiquitination of SADs by the APC/C complex, which targets them for proteasomal degradation (Puram and Bonni, 2011; Li et al., 2012). In contrast, TrkC activation of the PLCγ/Ca2+ is predominantly responsible for enhancing SAD ALT phosphorylation and thus its catalytic activity.
Kinases in the AMPK family, including SADs, are catalytically active only when phosphorylated at the ALT site (Lizcano et al., 2004). The best studied and seemingly most important ALT kinase is LKB1, which is required for activation of AMPK in many tissues and of SADs in cortex; indeed, cortical phenotypes of SAD-A/B and LKB1 mutants are nearly indistinguishable (Barnes et al., 2007). It was therefore surprising that deletion of LKB1 had no detectable effect on branching of sensory neurons. Instead, we found a unique regulatory mechanism: NT-3 controls ALT phosphorylation indirectly by regulating phosphorylation of the CTD. The CTD is unusual in bearing a large number of closely spaced serine or threonine sites, phosphorylation of which inhibits activating phosphorylation in the catalytic domain. NT-3 signaling controls SAD kinase activation, in part, through regulating the phosphorylation state of the SAD CTD, possibly by activating phosphatases, inhibiting CTD kinases or a combination of the two.
CDK5 is one relevant inhibitor of SAD kinase activity. Evidence from C. elegans is consistent with this hypothesis: Sad-1 gain of function in worms causes vesicle mislocalization to dendrites that is similar to loss of function mutations in Cdk-5 or the related CDK, PCTAIRE1 (Crump et al., 2001; Ou et al., 2010). Mammalian CDK5 plays a large number of roles in neural development (Su and Tsai, 2011), and it will be of interest to determine whether some CDK5 functions may be mediated by SAD regulation and whether other neurally-expressed CDKs (e.g. PCTAIRE1) also contribute to SAD inhibition. An added complexity is that SAD-A has been reported capable of phosphorylating PCTAIRE1 (Chen et al. 2012).
Our studies leave open the identity of the SAD ALT kinase important for sensory axon branching. Possible candidates are members of the STE20 family of kinases (including TAK1/MAP3K7) that can biochemically activate AMPK family members (Figure S5; Timm et al., 2003; Momcilovic et al., 2006). CAMKKβ was also reported to be a SAD ALT kinase (Fujimoto et al., 2008), but we, and others, have not observed such an activity (Bright et al., 2008; B.N.L. unpublished observations). We favor the idea that multiple, redundant kinases perform SAD ALT phosphorylation in DRG neurons, but their ability to access the SAD ALT is dependent upon SAD CTD dephosphorylation. In this scenario, ALT phosphorylation is regulated not by limited availability of an ALT kinase but by CTD-dependent accessibility of the ALT site.
In addition to regulating the activation state of SAD kinases, phosphorylation of the CTD may also play a role in stabilizing the protein: removing the 18 sites of CTD phosphorylation around the D box consistently decreased protein levels in cell lines and in neurons (Figure 6 and data not shown). Phosphorylation of the SAD CTD around the D-box could stabilize the protein by inhibiting interaction with the APC/C, a mechanism similar to that described for the control of securin ubiqutination during anaphase (Holt et al., 2008). Dephosphorylation of the CTD in response to NT-3 could then result in targeting of SADs for degradation, thus extinguishing signals from SADs in the activated, dephospho-CTD form (Figure 8G).
SAD kinases as multifunctional regulators of axonal development in vivo
In the telencephalon, LKB1 and SADs control early axon-dendrite polarization and axon formation (Kishi et al., 2005; Barnes et al., 2007; Shelly et al., 2007). Here, we have shown that SADs affect axonal arborization in some sensory neurons. Initial studies of the C. elegans SAD ortholog, SAD-1, demonstrated a role for this kinase in presynaptic differentiation (Crump et al., 2001) and we found recently that SADs are required for maturation of multiple synaptic types in mouse central and peripheral nervous systems (B.N.L. and J.R.S., in preparation). Finally, Inoue et al. (2006) have reported that SADs modulate presynaptic function in adults. Together, these studies establish SAD kinases as essential regulators of multiple phases of axonal development and function.
A major outstanding question is how SAD kinases activate programs that influence multiple processes of axonal development. While we cannot rule out possible kinase-independent roles of SAD kinases, at least in C. elegans, kinase activity is essential for SAD function in vivo (Crump et al., 2001; Kim et al., 2008), suggesting that substrate phosphorylation is the key functional output. However, only a few SAD substrates have been identified so far, including the microtubule-associated proteins Tau, the cell cycle regulators Wee1 and Cdc25, gamma-tubulin and the nerve terminal component RIM (Barnes et al., 2007; Inoue et al., 2006; Lu et al., 2004; Muller et al., 2010; Alvarado-Kristensson, 2009). Determining how SAD kinases shape neuronal form and connectivity will require examination of how these kinases process upstream signals in distinct developmental contexts and how downstream phosphorylation modifies the activity of yet to be identified substrates.
EXPERIMENTAL PROCEDURES
A full description of experimental procedures, mouse strains and reagents used in this study is presented in Supplementary Experimental Procedures.
Animals
Animals were used in accordance with NIH guidelines and protocols approved by Institutional Animal Use and Care Committee at Harvard University. The SAD-A conditional line was generated by standard methods.
Histology
Immunohistochemistry and DiI labeling was performed on fixed tissue using reagents and methods presented in detail in Supplementary Experimental Procedures.
Cell culture
Culture and transfection of HeLa and 293T cells was performed as described (Barnes et al., 2007). Explant and dissociated cultures of embryonic dorsal root ganglia neurons were prepared using methods similar to those described in Lentz et al. (1999). The Amaxa mouse neuron nucleofector kit and Amaxa Series 2 Nucleofector (Lonza) was used to introduce plasmid DNA into dissociated DRG cells.
Biochemical analysis
Cell and tissue lysis, SDS-PAGE and immunoblotting were performed essentially as described in Barnes et al. (2007). Immunoprecipitations from non-denaturing lysates were performed using anti-HA agarose (Roche). Kinase assays using substrate proteins isolated by immunoprecipitation or purified from bacteria were performed as described (Kim et al., 2008).
Supplementary Material
HIGHLIGHTS.
LKB1 and SAD kinases are required for axon specification only in forebrain.
Subsets of sensory neurons require SADs, but not LKB1, to form central axon arbors.
SAD kinases transduce NT-3 signals to promote terminal arbor formation.
NT-3 controls SAD protein levels and activation state by two distinct mechanisms.
ACKNOWLEDGEMENTS
We thank Marian Carlson, Susan Dymecki, Tom Jessell, Louis Reichardt, Michael Schneider, William Sellers, Li-Huei Tsai, Sander van den Heuvel and Hans Widlund for mouse strains, antibodies and plasmids used in this study; Debbie Pelusi for excellent technical assistance; Arjun Krishnaswamy and members of the Sanes lab for helpful suggestions. This work was supported by grants from the NIH (R01 NS029169 and R21 NS076880). B.N.L. was supported by a fellowship from the Damon Runyon Cancer Research Foundation (DRG-1914-06).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Airaksinen MS, Koltzenburg M, Lewin GR, Masu Y, Helbig C, Wolf E, Brem G, Toyka KV, Thoenen H, Meyer M. Specific subtypes of cutaneous mechanoreceptors require neurotrophin-3 following peripheral target innervation. Neuron. 1996;16:287–295. doi: 10.1016/s0896-6273(00)80047-1. [DOI] [PubMed] [Google Scholar]
- Albers KM, Perrone TN, Goodness TP, Jones ME, Green MA, Davis BM. Cutaneous overexpression of NT-3 increases sensory and sympathetic neuron number and enhances touch dome and hair follicle innervation. J Cell Biol. 1996;134:487–497. doi: 10.1083/jcb.134.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alessi DR, Sakamoto K, Bayascas JR. LKB1-dependent signaling pathways. Annu Rev Biochem. 2006;75:137–163. doi: 10.1146/annurev.biochem.75.103004.142702. [DOI] [PubMed] [Google Scholar]
- Alvarado-Kristensson M, Rodríguez MJ, Silió V, Valpuesta JM, Carrera AC. SADB phosphorylation of gamma-tubulin regulates centrosome duplication. Nat Cell Biol. 2009;11:1081–1092. doi: 10.1038/ncb1921. [DOI] [PubMed] [Google Scholar]
- Arber S, Ladle DR, Lin JH, Frank E, Jessell TM. ETS gene Er81 controls the formation of functional connections between group Ia sensory afferents and motor neurons. Cell. 2000;101:485–498. doi: 10.1016/s0092-8674(00)80859-4. [DOI] [PubMed] [Google Scholar]
- Bardeesy N, Sinha M, Hezel AF, Signoretti S, Hathaway NA, Sharpless NE, Loda M, Carrasco DR, DePinho RA. Loss of the Lkb1 tumour suppressor provokes intestinal polyposis but resistance to transformation. Nature. 2002;419:162–167. doi: 10.1038/nature01045. [DOI] [PubMed] [Google Scholar]
- Barnes AP, Lilley BN, Pan YA, Plummer LJ, Powell AW, Raines AN, Sanes JR, Polleux F. LKB1 and SAD kinases define a pathway required for the polarization of cortical neurons. Cell. 2007;129:549–563. doi: 10.1016/j.cell.2007.03.025. [DOI] [PubMed] [Google Scholar]
- Barnes AP, Polleux F. Establishment of axon-dendrite polarity in developing neurons. Annu Rev Neurosci. 2009;32:347–381. doi: 10.1146/annurev.neuro.31.060407.125536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bright NJ, Carling D, Thornton C. Investigating the regulation of brain-specific kinases 1 and 2 by phosphorylation. J Biol Chem. 2008;283:14946–14954. doi: 10.1074/jbc.M710381200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H-H, Hippenmeyer S, Arber S, Frank E. Development of the monosynaptic stretch reflex circuit. Current Opinion in Neurobiology. 2003;13:96–102. doi: 10.1016/s0959-4388(03)00006-0. [DOI] [PubMed] [Google Scholar]
- Chen X-Y, Gu X-T, Saiyin H, Wan B, Zhang Y-J, Li J, Wang Y-L, Gao R, Wang Y-F, Dong W-P, et al. Brain-selective kinase 2 (BRSK2) phosphorylation on PCTAIRE1 negatively regulates glucose-stimulated insulin secretion in pancreatic β-cells. The Journal of Biological Chemistry. 2012;287:30368–30375. doi: 10.1074/jbc.M112.375618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y-J, Di Nardo A, Kramvis I, Meikle L, Kwiatkowski DJ, Sahin M, He X. Tuberous sclerosis complex proteins control axon formation. Genes & Development. 2008;22:2485–2495. doi: 10.1101/gad.1685008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crump JG, Zhen M, Jin Y, Bargmann CI. The SAD-1 kinase regulates presynaptic vesicle clustering and axon termination. Neuron. 2001;29:115–129. doi: 10.1016/s0896-6273(01)00184-2. [DOI] [PubMed] [Google Scholar]
- Ding Y-Q, Kim J-Y, Xu Y-S, Rao Y, Chen Z-F. Ventral migration of early-born neurons requires Dcc and is essential for the projections of primary afferents in the spinal cord. Development (Cambridge, England) 2005;132:2047–2056. doi: 10.1242/dev.01798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernfors P, Lee KF, Kucera J, Jaenisch R. Lack of neurotrophin-3 leads to deficiencies in the peripheral nervous system and loss of limb proprioceptive afferents. Cell. 1994;77:503–512. doi: 10.1016/0092-8674(94)90213-5. [DOI] [PubMed] [Google Scholar]
- Erzurumlu RS, Murakami Y, Rijli FM. Mapping the face in the somatosensory brainstem. Nat Rev Neurosci. 2010;11:252–263. doi: 10.1038/nrn2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fariñas I, Jones KR, Backus C, Wang XY, Reichardt LF. Severe sensory and sympathetic deficits in mice lacking neurotrophin-3. Nature. 1994;369:658–661. doi: 10.1038/369658a0. [DOI] [PubMed] [Google Scholar]
- Fariñas I, Yoshida CK, Backus C, Reichardt LF. Lack of neurotrophin-3 results in death of spinal sensory neurons and premature differentiation of their precursors. Neuron. 1996;17:1065–1078. doi: 10.1016/s0896-6273(00)80240-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto T, Yurimoto S, Hatano N, Nozaki N, Sueyoshi N, Kameshita I, Mizutani A, Mikoshiba K, Kobayashi R, Tokumitsu H. Activation of SAD kinase by Ca2+/calmodulin-dependent protein kinase kinase. Biochemistry. 2008;47:4151–4159. doi: 10.1021/bi702528r. [DOI] [PubMed] [Google Scholar]
- Genç B, Ozdinler PH, Mendoza AE, Erzurumlu RS. A chemoattractant role for NT-3 in proprioceptive axon guidance. Plos Biol. 2004;2:e403. doi: 10.1371/journal.pbio.0020403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson DA, Ma L. Developmental regulation of axon branching in the vertebrate nervous system. Development (Cambridge, England) 2011;138:183–195. doi: 10.1242/dev.046441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt LJ, Krutchinsky AN, Morgan DO. Positive feedback sharpens the anaphase switch. Nature. 2008;454:353–357. doi: 10.1038/nature07050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hory-Lee F, Russell M, Lindsay RM, Frank E. Neurotrophin 3 supports the survival of developing muscle sensory neurons in culture. Proc Natl Acad Sci USA. 1993;90:2613–2617. doi: 10.1073/pnas.90.7.2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hur E-M, Zhou F-Q. GSK3 signalling in neural development. Nat Rev Neurosci. 2010;11:539–551. doi: 10.1038/nrn2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttlin EL, Jedrychowski MP, Elias JE, Goswami T, Rad R, Beausoleil SA, Villén J, Haas W, Sowa ME, Gygi SP. A tissue-specific atlas of mouse protein phosphorylation and expression. Cell. 2010;143:1174–1189. doi: 10.1016/j.cell.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue E, Mochida S, Takagi H, Higa S, Deguchi-Tawarada M, Takao-Rikitsu E, Inoue M, Yao I, Takeuchi K, Kitajima I, et al. SAD: a presynaptic kinase associated with synaptic vesicles and the active zone cytomatrix that regulates neurotransmitter release. Neuron. 2006;50:261–275. doi: 10.1016/j.neuron.2006.03.018. [DOI] [PubMed] [Google Scholar]
- Jadrich JL, O'connor MB, Coucouvanis E. Expression of TAK1, a mediator of TGF-beta and BMP signaling, during mouse embryonic development. Gene Expr Patterns. 2003;3:131–134. doi: 10.1016/s1567-133x(03)00012-7. [DOI] [PubMed] [Google Scholar]
- Jin Y, Garner CC. Molecular mechanisms of presynaptic differentiation. Annual Review of Cell and Developmental Biology. 2008;24:237–262. doi: 10.1146/annurev.cellbio.23.090506.123417. [DOI] [PubMed] [Google Scholar]
- Jishage K-i, Nezu J-i, Kawase Y, Iwata T, Watanabe M, Miyoshi A, Ose A, Habu K, Kake T, Kamada N, et al. Role of Lkb1, the causative gene of Peutz-Jegher's syndrome, in embryogenesis and polyposis. Proc Natl Acad Sci USA. 2002;99:8903–8908. doi: 10.1073/pnas.122254599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JSM, Lilley BN, Zhang C, Shokat KM, Sanes JR, Zhen M. A chemical-genetic strategy reveals distinct temporal requirements for SAD-1 kinase in neuronal polarization and synapse formation. Neural development. 2008;3:23. doi: 10.1186/1749-8104-3-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishi M, Pan YA, Crump JG, Sanes JR. Mammalian SAD kinases are required for neuronal polarization. Science. 2005;307:929–932. doi: 10.1126/science.1107403. [DOI] [PubMed] [Google Scholar]
- Klein R, Silos-Santiago I, Smeyne RJ, Lira SA, Brambilla R, Bryant S, Zhang L, Snider WD, Barbacid M. Disruption of the neurotrophin-3 receptor gene trkC eliminates la muscle afferents and results in abnormal movements. Nature. 1994;368:249–251. doi: 10.1038/368249a0. [DOI] [PubMed] [Google Scholar]
- Kolodkin AL, Tessier-Lavigne M. Mechanisms and molecules of neuronal wiring: a primer. Cold Spring Harbor Perspectives in Biology. 2011;3 doi: 10.1101/cshperspect.a001727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krylova O, Herreros J, Cleverley KE, Ehler E, Henriquez JP, Hughes SM, Salinas PC. WNT-3, expressed by motoneurons, regulates terminal arborization of neurotrophin-3-responsive spinal sensory neurons. Neuron. 2002;35:1043–1056. doi: 10.1016/s0896-6273(02)00860-7. [DOI] [PubMed] [Google Scholar]
- Lentz SI, Knudson CM, Korsmeyer SJ, Snider WD. Neurotrophins support the development of diverse sensory axon morphologies. J Neurosci. 1999;19:1038–1048. doi: 10.1523/JNEUROSCI.19-03-01038.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Wan B, Zhou J, Wang Y, Luo T, Gu X, Chen F, Yu L. APC/C(Cdh1) targets brain-specific kinase 2 (BRSK2) for degradation via the ubiquitin-proteasome pathway. PLoS ONE. 2012;7:e45932. doi: 10.1371/journal.pone.0045932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lizcano JM, Göransson O, Toth R, Deak M, Morrice NA, Boudeau J, Hawley SA, Udd L, Mäkelä TP, Hardie DG, et al. LKB1 is a master kinase that activates 13 kinases of the AMPK subfamily, including MARK/PAR-1. EMBO J. 2004;23:833–843. doi: 10.1038/sj.emboj.7600110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu R, Niida H, Nakanishi M. Human SAD1 kinase is involved in UV-induced DNA damage checkpoint function. J Biol Chem. 2004;279:31164–31170. doi: 10.1074/jbc.M404728200. [DOI] [PubMed] [Google Scholar]
- Luo L, O'Leary DDM. Axon retraction and degeneration in development and disease. Annu Rev Neurosci. 2005;28:127–156. doi: 10.1146/annurev.neuro.28.061604.135632. [DOI] [PubMed] [Google Scholar]
- Matsuda S, Uehara Y. Prenatal development of the rat dorsal root ganglia. A scanning electron-microscopic study. Cell Tissue Res. 1984;235:13–18. doi: 10.1007/BF00213717. [DOI] [PubMed] [Google Scholar]
- Momcilovic M, Hong S-P, Carlson M. Mammalian TAK1 activates Snf1 protein kinase in yeast and phosphorylates AMP-activated protein kinase in vitro. J Biol Chem. 2006;281:25336–25343. doi: 10.1074/jbc.M604399200. [DOI] [PubMed] [Google Scholar]
- Moqrich A, Earley TJ, Watson J, Andahazy M, Backus C, Martin-Zanca D, Wright DE, Reichardt LF, Patapoutian A. Expressing TrkC from the TrkA locus causes a subset of dorsal root ganglia neurons to switch fate. Nat Neurosci. 2004;7:812–818. doi: 10.1038/nn1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller M, Lutter D, Püschel AW. Persistence of the cell-cycle checkpoint kinase Wee1 in SadA- and SadB-deficient neurons disrupts neuronal polarity. J Cell Sci. 2010;123:286–294. doi: 10.1242/jcs.058230. [DOI] [PubMed] [Google Scholar]
- Newbern JM, Li X, Shoemaker SE, Zhou J, Zhong J, Wu Y, Bonder D, Hollenback S, Coppola G, Geschwind DH, et al. Specific Functions for ERK/MAPK Signaling during PNS Development. Neuron. 2011;69:91–105. doi: 10.1016/j.neuron.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell M, Chance RK, Bashaw GJ. Axon growth and guidance: receptor regulation and signal transduction. Annu Rev Neurosci. 2009;32:383–412. doi: 10.1146/annurev.neuro.051508.135614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley RA, Lefcort FB, Clary DO, Reichardt LF, Prevette D, Oppenheim RW, Frank E. Neurotrophin-3 promotes the differentiation of muscle spindle afferents in the absence of peripheral targets. J Neurosci. 1997;17:4262–4274. doi: 10.1523/JNEUROSCI.17-11-04262.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou C-Y, Poon VY, Maeder CI, Watanabe S, Lehrman EK, Fu AKY, Park M, Fu W-Y, Jorgensen EM, Ip NY, et al. Two cyclin-dependent kinase pathways are essential for polarized trafficking of presynaptic components. Cell. 2010;141:846–858. doi: 10.1016/j.cell.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozdinler PH, Ulupinar E, Erzurumlu RS. Local neurotrophin effects on central trigeminal axon growth patterns. Brain Res Dev Brain Res. 2004;151:55–66. doi: 10.1016/j.devbrainres.2004.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patapoutian A, Backus C, Kispert A, Reichardt LF. Regulation of neurotrophin-3 expression by epithelial-mesenchymal interactions: the role of Wnt factors. Science. 1999;283:1180–1183. doi: 10.1126/science.283.5405.1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel TD, Jackman A, Rice FL, Kucera J, Snider WD. Development of sensory neurons in the absence of NGF/TrkA signaling in vivo. Neuron. 2000;25:345–357. doi: 10.1016/s0896-6273(00)80899-5. [DOI] [PubMed] [Google Scholar]
- Patel TD, Kramer I, Kucera J, Niederkofler V, Jessell TM, Arber S, Snider WD. Peripheral NT3 signaling is required for ETS protein expression and central patterning of proprioceptive sensory afferents. Neuron. 2003;38:403–416. doi: 10.1016/s0896-6273(03)00261-7. [DOI] [PubMed] [Google Scholar]
- Philippidou P, Walsh CM, Aubin J, Jeannotte L, Dasen JS. Sustained Hox5 gene activity is required for respiratory motor neuron development. Nature Neuroscience. 2012;15:1636–1644. doi: 10.1038/nn.3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puram SV, Bonni A. Novel functions for the anaphase-promoting complex in neurobiology. Seminars in cell & developmental biology. 2011;22:586–594. doi: 10.1016/j.semcdb.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichardt LF. Neurotrophin-regulated signalling pathways. Philos Trans R Soc Lond, B, Biol Sci. 2006;361:1545–1564. doi: 10.1098/rstb.2006.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schecterson LC, Bothwell M. Novel roles for neurotrophins are suggested by BDNF and NT-3 mRNA expression in developing neurons. Neuron. 1992;9:449–463. doi: 10.1016/0896-6273(92)90183-e. [DOI] [PubMed] [Google Scholar]
- Schmidt H, Rathjen FG. Signalling mechanisms regulating axonal branching in vivo. Bioessays. 2010;32:977–985. doi: 10.1002/bies.201000054. [DOI] [PubMed] [Google Scholar]
- Shelly M, Cancedda L, Heilshorn S, Sumbre G, Poo M-M. LKB1/STRAD promotes axon initiation during neuronal polarization. Cell. 2007;129:565–577. doi: 10.1016/j.cell.2007.04.012. [DOI] [PubMed] [Google Scholar]
- Shibuya H, Yamaguchi K, Shirakabe K, Tonegawa A, Gotoh Y, Ueno N, Irie K, Nishida E, Matsumoto K. TAB1: an activator of the TAK1 MAPKKK in TGF-beta signal transduction. Science. 1996;272:1179–1182. doi: 10.1126/science.272.5265.1179. [DOI] [PubMed] [Google Scholar]
- Smith CL. Sensory neurons supplying touch domes near the body midlines project bilaterally in the thoracic spinal cord of rats. J Comp Neurol. 1986;245:541–552. doi: 10.1002/cne.902450409. [DOI] [PubMed] [Google Scholar]
- Solbach S, Celio MR. Ontogeny of the calcium binding protein parvalbumin in the rat nervous system. Anat Embryol. 1991;184:103–124. doi: 10.1007/BF00942742. [DOI] [PubMed] [Google Scholar]
- Srinivas S, Watanabe T, Lin CS, William CM, Tanabe Y, Jessell TM, Costantini F. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su SC, Tsai L-H. Cyclin-dependent kinases in brain development and disease. Annual Review of Cell and Developmental Biology. 2011;27:465–491. doi: 10.1146/annurev-cellbio-092910-154023. [DOI] [PubMed] [Google Scholar]
- Timm T, Li X-Y, Biernat J, Jiao J, Mandelkow E, Vandekerckhove J, Mandelkow EM. MARKK, a Ste20-like kinase, activates the polarity-inducing kinase MARK/PAR-1. EMBO J. 2003;22:5090–5101. doi: 10.1093/emboj/cdg447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronche F, Kellendonk C, Kretz O, Gass P, Anlag K, Orban PC, Bock R, Klein R, Schütz G. Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nat Genet. 1999;23:99–103. doi: 10.1038/12703. [DOI] [PubMed] [Google Scholar]
- Usui N, Watanabe K, Ono K, Tomita K, Tamamaki N, Ikenaka K, Takebayashi H. Role of motoneuron-derived neurotrophin 3 in survival and axonal projection of sensory neurons during neural circuit formation. Development (Cambridge, England) 2012;132:1125–1132. doi: 10.1242/dev.069997. [DOI] [PubMed] [Google Scholar]
- Wang KH, Brose K, Arnott D, Kidd T, Goodman CS, Henzel W, Tessier-Lavigne M. Biochemical purification of a mammalian slit protein as a positive regulator of sensory axon elongation and branching. Cell. 1999;96:771–784. doi: 10.1016/s0092-8674(00)80588-7. [DOI] [PubMed] [Google Scholar]
- Weinberg RJ, Pierce JP, Rustioni A. Single fiber studies of ascending input to the cuneate nucleus of cats: I. Morphometry of primary afferent fibers. J Comp Neurol. 1990;300:113–133. doi: 10.1002/cne.903000108. [DOI] [PubMed] [Google Scholar]
- Wright DE, Zhou L, Kucera J, Snider WD. Introduction of a neurotrophin-3 transgene into muscle selectively rescues proprioceptive neurons in mice lacking endogenous neurotrophin-3. Neuron. 1997;19:503–517. doi: 10.1016/s0896-6273(00)80367-0. [DOI] [PubMed] [Google Scholar]
- Zhong J, Li X, McNamee C, Chen AP, Baccarini M, Snider WD. Raf kinase signaling functions in sensory neuron differentiation and axon growth in vivo. Nat Neurosci. 2007;10:598–607. doi: 10.1038/nn1898. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.