Abstract
Interleukin-1 (IL-1) is an important mediator of innate immunity, but can also promote inflammatory tissue damage. During chronic infections, such as tuberculosis, the beneficial antimicrobial role of IL-1 must be balanced with the need to prevent immunopathology. By exogenously controlling the replication of Mycobacterium tuberculosis in vivo, we obviated the requirement for antimicrobial immunity and discovered that both IL-1 production and infection-induced immunopathology were suppressed by lymphocyte-derived interferon-γ (IFN-γ). This effect was mediated by nitric oxide (NO), which we found to specifically inhibit the assembly of the NLRP3 inflammasome via thiol nitrosylation. These data suggest that the NO produced as a result of adaptive immunity is indispensable in modulating the destructive innate inflammatory responses that are elicited during persistent infections.
Inflammation is a tightly regulated process, and multiple signals must be integrated by the innate immune system to determine if the reaction will be initiated. This coordination is particularly apparent in the pathway controlling the proinflammatory cytokine, interleukin-1β (IL-1β). Transcription of the pro-IL-1β gene is induced through the ligation of pattern recognition receptors (e.g. toll-like receptors, TLR) that recognize conserved microbial structures. Subsequent processing of the precursor peptide into its active form is mediated by multi-protein complexes termed, ‘inflammasomes’1. These heterooligomeric structures consist of the IL-1β processing enzyme, caspase-1, the adapter protein, ASC, and a sensor protein of the NLR (NOD-like receptor) or ALR (AIM2-like receptor) families. Depending on the associated NLR or ALR, inflammasome assembly and proteolytic activation of caspase-1 can be initiated by either recognition of microbial patterns or through the perception of host cell injury. Thus, IL-1β production is controlled through the coordination of signals derived from both the presence of microbial colonization and the damage induced by the pathogen2.
The resulting inflammatory reaction is characterized by intravascular coagulation, tissue edema, and neutrophil infiltration. IL-1 can both initiate and propagate this response by regulating leukocyte adhesion to the vascular endothelium and by inducing the production of secondary chemokines that direct neutrophil chemotaxis to the site of tissue damage3. The coordinated action of these responses is generally capable of eradicating the eliciting infection and IL-1 is critically required for resistance to many bacterial and fungal pathogens 4, 5. However, this resistance comes at the cost of significant damage to the host tissue, as activated phagocytes release hydrolytic enzymes and reactive oxygen radicals that are equally toxic to host and microbial cells6. Thus, when the innate immune response is capable of eliminating a pathogen, the resulting self-limiting inflammation is clearly beneficial. However, in the context of persistent infections, it is unclear how the potentially destructive aspects of the IL-1-initiated inflammatory response are controlled to preserve tissue integrity.
A classic example of this paradox is tuberculosis (TB), a chronic infection caused by Mycobacterium tuberculosis (Mtb). Mycobacterial cells are the basis for our most effective immune adjuvants, and are among the most immunostimulatory particles known7, 8. Not surprisingly, pulmonary infection with this pathogen initially elicits a robust induction of IL-1 and neutrophil influx9 in the mouse, but this response is essentially ineffective in controlling the infection10. Bacterial replication only begins to slow with the onset of adaptive immunity. At this point, interferon-γ (IFN-γ) produced by newly recruited lymphocytes acts on the parasitized macrophages to trigger expression of antimicrobial effectors including the inducible isoform of nitric oxide synthase (iNOS). The resulting nitric oxide (NO) inhibits bacterial growth11, but even this adaptive immune response does not significantly reduce the number of viable bacteria. In both murine and nonhuman primate models of TB, a relatively large bacterial burden is maintained for months or even years before disease becomes apparent12, 13. The ability of the host to sustain the persistent presence of this immunostimulatory pathogen in its lungs suggests that regulatory circuits controlling inflammation are likely to be important for maintaining tissue integrity during this asymptomatic period.
Active TB disease eventually afflicts approximately ten percent of infected individuals, resulting in millions of deaths each year. This disease is generally thought to occur as a result of increased Mtb replication in these individuals. However, bacterial burden is not strictly correlated with disease progression in different inbred mouse strains14, and several hallmarks of severe TB disease suggest that insufficiently controlled innate inflammation plays a significant role in pathogenesis. For example, neutrophil accumulation is evident in active TB lesions of both humans15 and mice16. Furthermore, both the IL-1-processing inflammasome complex and the neutrophil have been shown to contribute to the pathology caused by the related pathogen, Mycobacterium marinum17. Therefore, the ability of an individual to modulate tissue-damaging inflammatory responses may be a distinct determinant of TB disease progression, independent of bacterial burden.
The presence of IFN-γ-producing T cells is inversely correlated with pathology in Mtb-infected animals18, and this cytokine has been found to possess anti-inflammatory properties in both infection19 and autoimmune-induced disease models20. These observations indicated that the adaptive immune response might be responsible for suppressing innate inflammatory pathways during persistent Mtb infection. To specifically characterize these putative immunoregulatory roles, we rendered antimicrobial functions unnecessary by deliberately controlling the replication of Mtb in vivo. We found that the same immune axis critical for restricting bacterial replication, IFN-γ and NO, is also necessary to suppress the continual production of IL-1β by the NLRP3 inflammasome, to inhibit persistent neutrophil recruitment, and to prevent progressive tissue damage. These findings indicate that NO plays an unanticipated dual role in promoting resistance to Mtb, and that both its antimicrobial activity and the immunoregulatory function we describe are required to survive this chronic infection.
RESULTS
Adaptive immunity influences IL-1 production
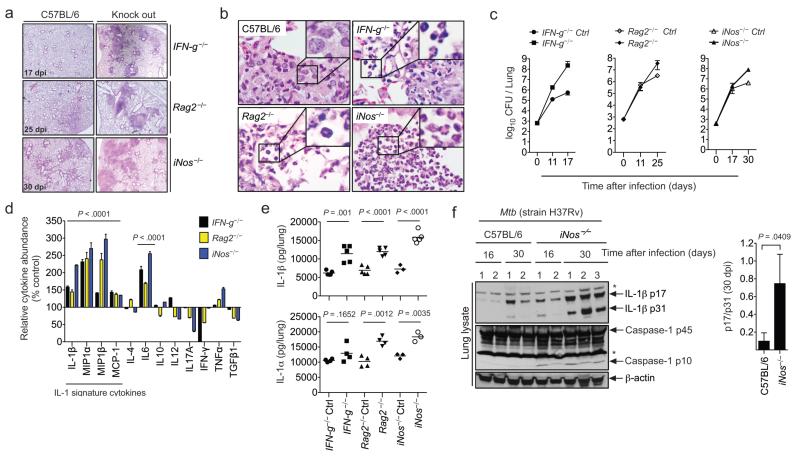
To investigate the potential role of adaptive immunity in regulating innate inflammatory pathways, we examined the mechanisms driving TB pathogenesis in mice lacking mature T and B lymphocytes (Rag2−/−), IFN-γ, (IFN-g−/−) or iNOS (iNos−/−). Following Mtb infection, each of these mutant mouse strains rapidly developed a qualitatively different disease than immunocompetent animals (C57BL/6). The disease was characterized by extensive tissue necrosis, neutrophil infiltration, and relatively high bacterial burdens (Fig. 1a-c). This severe pathology was associated with a distinct cytokine signature (Fig1. d-e dominated by IL-1α, IL-1β, and several monocyte/granulocyte chemo-attractants known to be induced by IL-1, e.g. CCL2 (MCP-1), CCL3 (MIP1α, CCL4 (MIP1β). While lung homogenates of iNOS-deficient animals were found to contain relatively high levels of the immature 31kDa precursor of IL-1β, the accumulation of the mature active form of the cytokine (p17) was even more marked. The ratio of mature/immature cytokine was increased sevenfold in iNOS-deficient animals (Fig. 1f), suggesting that IL-1β processing was enhanced. Consistent with the apparent increase in IL-1β maturation, the active form of caspase-1 (assessed by the p10 fragment) was only detectable in the lungs of iNos−/− mice.
Figure 1. Lymphocytes, IFN-γ, and iNOS modulate TB immunopathology.
a. Paired groups of mutant and wild type C57BL/6 mice were infected with Mtb, and lung histopathology was assessed by hematoxylin/eosin staining on the indicated day post-infection (dpi). b. Lesions in C57BL/6 mice were histocytic, whereas the inflammatory lesions of immunocompromised mice were pyogranulomatous and contained a larger number of neutrophils (insets). c. Bacterial burdens in the lungs of the indicated mice were determined by plating organ homogenates. CFU: colony forming unit. d. Relative cytokine levels were determined by ELISA in lung lysates generated at the same time points as in panel “a”. Data are presented as the % abundance of cytokine in knockout relative to wild type animals. Statistics are provided for cytokines that were significantly increased in all three mutant strains. P values were calculated by two-way ANOVA. e. IL-1β concentrations were determined by ELISA in the lung lysates shown in panel “d”. P values were determined by student’s t-test. Data are representative of two independent experiments. f. Immunoblot analysis of pro-IL-1β (p31) and mature-IL-1β p17 in lung lysates. Numbers indicate individual animals. “*” indicates a nonspecific band. Right panel shows densitometry analysis (ratio of processed to unprocessed IL-1β). Throughout, error bars represent standard deviation (sd). Each group contained 3-5 animals.
While Rag2−/−, IFN-g−/−, and iNos−/− animals consistently produced relatively high amounts of mature IL-1β and related chemokines, the levels of many other cytokines characteristic of both innate and adaptive immune responses were either reduced or unchanged (Fig. 1d). This relatively specific alteration in cytokine profile suggested that IFN-γ-dependent NO might serve a specific role in modulating IL-1β responses. However, the immunodeficient mice lacking this important antimicrobial pathway harbored 30-200 fold more bacteria in their lungs at the time of analysis (Fig. 1c), and we could not exclude the possibility that this increased bacterial burden caused the observed differences in cytokine levels and pathology.
NO directly modulates IL-1 responses and immunopathology
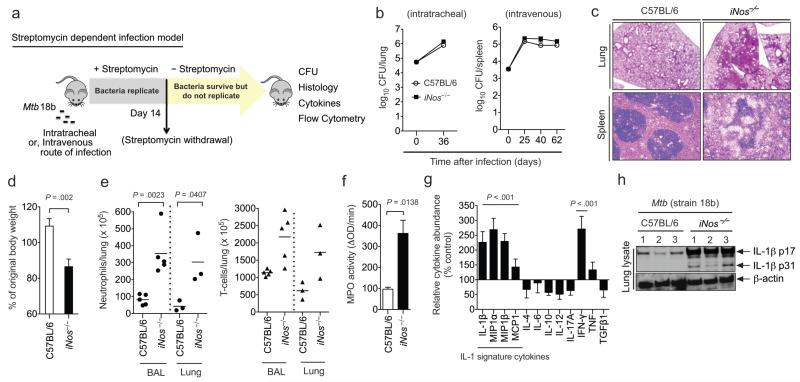
To more directly determine if NO regulated IL-1β and inflammation, we developed an infection model that employs a 16S rRNA mutant of Mtb (strain 18b), which only replicates in the presence of streptomycin 21, 22. The ability to exogenously control bacterial replication allowed the maintenance of bacterial numbers at the same level in both wild type and iNos−/− animals throughout the infection. To colonize the lungs with a physiologically relevant burden of these relatively slow-growing bacteria, mice were inoculated either intratracheally or intravenously. After infection, streptomycin was administered for fourteen days before replication was arrested by drug withdrawal (Fig. 2a). Twenty-two days later, all mice harbored comparable numbers of Mtb in their lungs and spleens (Fig.2b). However, the disease observed in these two mouse strains was profoundly different, and the iNos−/− animals infected by the intratracheal route became moribund by day 36. Increased local inflammatory pathology was apparent in iNos−/− animals regardless of the route of infection, as the tissue architecture of both the lungs and spleens was disrupted by leukocyte infiltration. These mice also suffered from significant wasting, consistent with an increased systemic inflammatory response (Fig. 2c, d).
Figure 2. Nitric oxide’s anti-inflammatory activities are independent of its anti-mycobacterial function.
a. The streptomycin-dependent Mtb (strain 18b) infection model. Mice were infected with Mtb 18b and administered streptomycin for two weeks. At the indicated time points after streptomycin withdrawal, disease progression was assessed in lungs (for intratracheal) or spleens (for intravenous). b. Organs of C57BL/6 and iNos−/− mice harbored similar numbers of viable Mtb throughout the infection. c. Representative histopathology of lungs (day 36) and spleen (day 40) of the indicated mouse strains. d. Body weight of intratracheally-infected mice at day 36, relative to day 0. Mean ± sd of two independent experiments, P value was determined by student’s t-test. e. Neutrophil and T cell counts in bronchoalveolar lavage (BAL) and dissociated lung tissue (Lung) were quantified by flow cytometry. P values were determined by two-way ANOVA. f. Myeloperoxidase activity per milligram of protein in lung lysates was quantified. P value was determined by student’s t-test. g. Relative cytokine levels in lung homogenates were determined by ELISA (presented as in Fig. 1d). P values were determined by two-way ANOVA. h. IL-1β in lung lysates was assessed by immunoblot. Data are representative of 2 independent experiments. All bar graphs depict the mean ± sd of 3-5 mice.
The cellular composition and cytokine profile of lesions in iNos−/− animals were distinct from their wild type counterparts and consistent with an IL-1-mediated inflammatory disease. Using flow cytometry, we found that the airways and dissociated lung tissue of the iNos−/− mice contained increased numbers of neutrophils (CD11b+ Gr1hi), while T cell infiltration (CD3+) increased to a lesser degree. Increased neutrophil recruitment was confirmed by quantifying myeloperoxidase (MPO) activity in lung homogenates (Fig. 2e,f). This granulocytic infiltration was associated with an IL-1β-dominated cytokine signature similar to that observed in animals infected with wild type bacteria, and the active proteolytic fragment of IL-1β was only found in lung lysates of iNos−/− mice (Fig. 2g and h). In contrast to IL-1β and the related chemokines, the production of a distinct proinflammatory mediator, IL-6, was correlated with bacterial burden and not NO production per se (Fig. 1d and 2g). Together, these data suggested that NO modulates immunopathology independent of its role in restricting Mtb growth, and that IFN-γ-stimulated NO plays a specific role in regulating the expression and maturation of IL-1β.
Exacerbated pathology depends on IL-1 and ASC
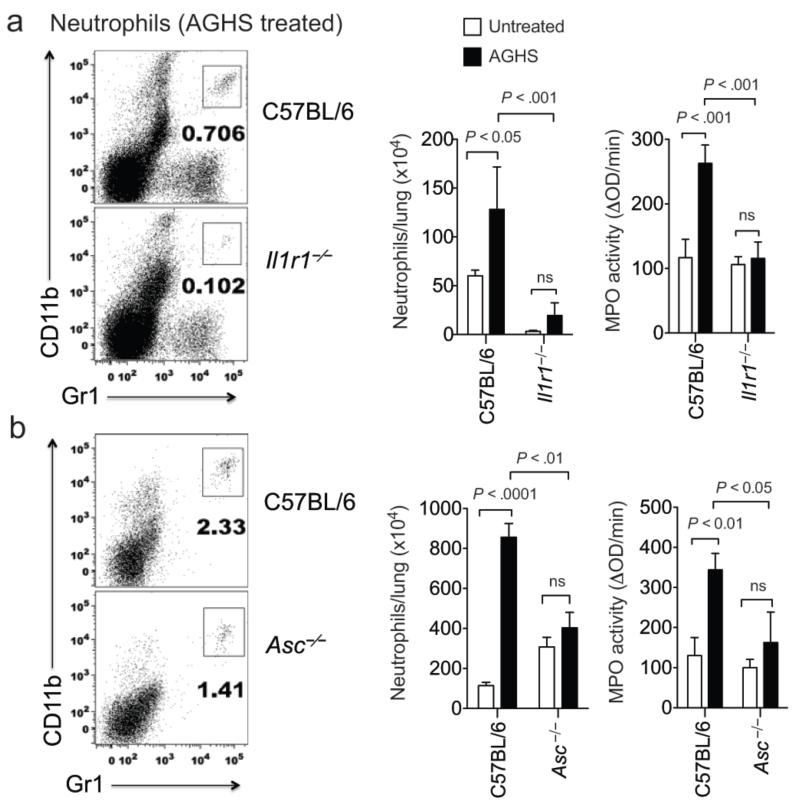
The coincident increase in both IL-1β and neutrophil infiltration in iNOS-deficient mice suggested that this cytokine might play a causal role in the observed immunopathology. To test this hypothesis, we infected wild type and IL-1 receptor-deficient mice (Il1r1−/−) with Mtb and quantified the influx of neutrophils into the lung following treatment with the iNOS inhibitor, aminoguanidine hemisulfate (AGHS). To ensure that differential bacterial growth did not influence inflammation, the streptomycin-dependent infection model was used. Similar to our observations in iNos−/− mice, AGHS treatment increased the recruitment of neutrophils into the lung, as assessed by both flow cytometry and MPO assay. This increase was abrogated in Il1r1−/− mice, demonstrating that the inflammatory response seen in iNos−/− mice depended upon IL-1 signaling (Fig. 3a), and not upon related cytokines such as IL-18.
Figure. 3. IL-1R1 and ASC promote the enhanced neutrophil recruitment observed upon iNOS inhibition.
a. C57BL/6 and Il1r1−/− mice were infected intratracheally with Mtb 18b and treated with or without aminoguanidine hemisulfate (AGHS) throughout the infection. Seven weeks after streptomycin withdrawal (62 days post-infection), neutrophils (CD11b+, Gr1hi cells) were quantified in the BAL by flow cytometry and in the lung lysates by assaying myeloperoxidase activity per milligram of total protein. Representative flow cytometry plots of AGHS treated mice are shown. b. Asc−/− and C57BL/6 control mice were infected with Mtb 18b. Four weeks after streptomycin withdrawal, neutrophil numbers and myeloperoxidase activity in the dissociated lung tissues are shown as in panel “a”. Bars represent the mean ± sd of 4-6 mice, P values were calculated by two-way ANOVA; “ns” indicates P > 0.05.
IL-1β maturation appeared to be altered by NO (Fig. 1f), but the protease(s) responsible for this event in vivo remained unclear. Caspase-1-containing inflammasome complexes are absolutely required for the maturation of IL-1β in Mtb-infected macrophages in vitro23, 24. However, the cell types producing IL-1β in vivo might be distinct25 and inflammasome-independent mechanisms appear to dominate in immunocompetent mice chronically infected with Mtb26. To determine if the inflammasome was required for the increased IL-1β activity we observed upon iNOS inhibition, we assessed the role of this complex in Mtb-induced inflammation. Consistent with previous reports, we could discern no significant effect of ASC deletion in mice infected with streptomycin-dependent Mtb. However, the increased neutrophil recruitment observed upon AGHS treatment was reversed in Asc−/− animals (Fig. 3b). This phenotype was very similar to that seen in Il1r1−/− mice. Thus, the immunopathology observed following iNOS inhibition was caused by inflammasome-dependent IL-1.
IFN-γ suppresses IL-1β in Mtb-infected macrophages
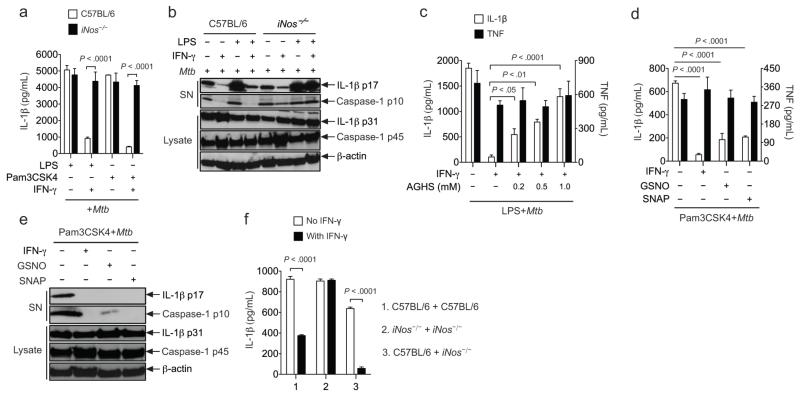
We investigated the mechanisms by which IFN-γ and NO regulated IL-1β in Mtb-infected macrophages. Consistent with previous reports23, 24, IL-1β production in response to Mtb infection depended on the NLRP3-ASC-caspase-1 complex and is likely secondary to membrane damage mediated by the bacterial ESX1 secretion system (Supplementary Fig. 1a,b). As predicted by our in vivo observations, the pretreatment of Mtb-infected macrophages with IFN-γ specifically inhibited IL-1β release, but had no effect on the production of an unrelated cytokine, TNF. This suppression required signaling through the IFN-γ receptor 1, was independent of IFN-γ produced by the macrophages (Fig. 4a,b), and required IFN-γ stimulation prior to infection (Supplementary Fig. 1c). IFN-γ pretreatment did not affect bacterial uptake or reduce the viability of the macrophages (Supplementary Fig. 2a,b), suggesting a direct effect on IL-1β production.
Figure 4. IFN-γ activation of macrophages inhibits IL-1β processing.
a. 24 hours before Mtb infection (multiplicity of infection, MOI, =10), bone marrow derived macrophages (BMDMs) were primed with/without LPS and stimulated with/without IFN-γ. IL-1β and TNF were measured in the supernatants at the indicated time points post-infection. b. The indicated genotypes of BMDMs were treated as shown, and the IL-1β concentration in the supernatant was measured 24 hours later by ELISA. c. At 24 hours post infection, the amount of pro-IL-1β (p31) in cell lysates and mature-IL-1β (p17) in supernatants (SN) were assessed by immunoblotting. Cell lysate samples were normalized for total protein content. d. BMDMs were primed with the indicated TLR stimulant (LPS: TLR4, Pam3CSK4: TLR2, poly (I:C): TLR3) and infected as in panel “a”. IL-1β and IL-1α levels in the supernatant were measured 24 hours later by ELISA. Throughout the figure, bars represent mean ± sd of at least 3 replicates. P values were calculated by two-way ANOVA. Data are representative of three (b) to six (a, c-d) independent experiments.
By a number of criteria, IFN-γ appeared to suppress the inflammasome-dependent maturation of IL-1 and not the expression of the cytokine’s proform. By immunoblot, IFN-γ did not affect the abundance of pro-IL-1β in cell lysates, but specifically inhibited the processing and release of the mature cytokine into the supernatant (Fig. 4c). Furthermore, suppression by IFN-γ was equally apparent whether pro-IL1β expression was induced by Mtb alone or by prestimulation with either the TLR2 ligand, Pam3CSK4, the TLR4 ligand, lipopolysaccharide (LPS), or the TLR3 ligand, poly (I:C) (Fig. 4a, d). Finally, release of, IL-1α, which is regulated by inflammasome-dependent caspase-1 signaling 27, was similarly suppressed by IFN-γ (Fig. 4d), further implying that IFN-γ modulates inflammasome activity.
IFN-γ specifically inhibits NLRP3 inflammasome activity
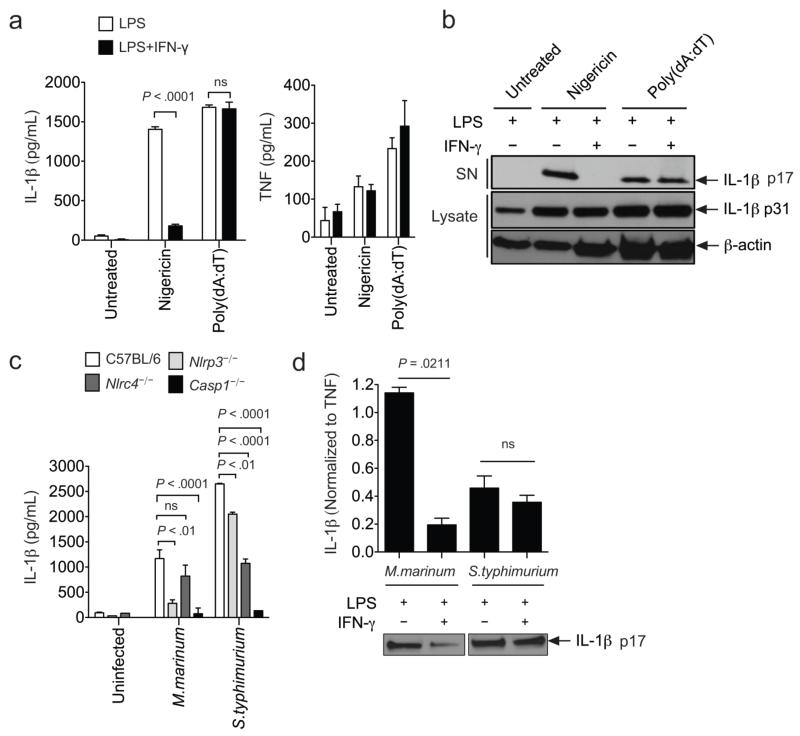
By treating macrophages with defined inflammasome activators, we found that the suppressive effect of IFN-γ was specific to the NLRP3 inflammasome. NLRP3-dependent IL-1β processing and release in response to the pore-forming toxin nigericin28 was inhibited by IFN-γ pretreatment (Fig. 5a,b). In contrast, IFN-γ had no effect on IL-1β production in response to B-form dsDNA, poly (dA:dT), which depends on the distinct AIM2-containing inflammasome29. As we observed in Mtb-infected macrophages, IFN-γ specifically suppressed IL-1β processing and did not affect TNF secretion induced upon LPS priming.
Figure 5. IFN-γ specifically inhibits NLRP3 inflammasome activation.
a and b. BMDMs primed with LPS±IFN-γ were treated with 10μM Nigericin for 1hr to stimulate the NLRP3 inflammasome or transfected with poly(dA:dT) to stimulate the AIM2 inflammasome. Cytokines were measured by ELISA (a) or immunoblotting (b). P values were calculated by two-way ANOVA. c. Different intracellular pathogens trigger a distinct combination NLRP3 and NLRC4 inflammasomes. BMDMs of the indicated genotypes were infected with M. marinum (MOI=10) or S. typhimurium (MOI=25), and the IL-1β found in the supernatants after 6hrs (S.typhimurium) and 24hrs (M.marinum) was measured by ELISA. P values were calculated by student’s t-test (two tailed). d. LPS-primed BMDMs were treated with or without IFN-γ before infection with M.marinum and S.typhimurium. IL-1β and TNF were measured in the culture supernatants as in panel “c”. To determine statistical significance, the IL-1β levels were first normalized to the amount of TNF produced, and then compared using a student’s t-test (two tailed). “ns” indicates P > 0.05. The same supernatant samples were probed by western blotting for the presence of mature IL-1β (p17). Throughout the figure, bars represent the mean ± sd of at least 3 replicates. Data are representative of two (a-c) to four (d) independent experiments.
Bacteria with distinct cellular structures or pathogenic strategies trigger different inflammasome complexes. Those that disrupt cellular membranes generally act on the NLRP3 complex, and those that express flagellin (or similar proteins) have the capacity to trigger the NLRC4 inflammasome30. To determine if IFN-γ suppression was NLRP3-specific in the context of these more complex stimulants, we infected macrophages with pathogens that engage different inflammasomes. Consistent with previous studies31, NLRP3 was the predominant NLR required for M. marinum-induced IL-1β production, and NLRC4 dominated the response to Salmonella typhimurium32 (Fig. 5c). Following IFN-γ treatment, only the NLRP3-dependent IL-1β production produced by M. marinum infection was efficiently suppressed (Fig. 5d). Thus, even in the context of genuine bacterial infection, IFN-γ specifically suppressed NLRP3-dependent responses.
IFN-γ stimulated NO suppresses IL-1β maturation
Our in vivo observations suggested that IFN-γ suppression of IL-1β processing might rely on NO production. Indeed, genetic deletion of iNos restored IL-1β processing and release in IFN-γ-treated macrophages infected with Mtb (Fig. 6a,b). Chemical inhibition of iNOS enzymatic activity with aminoguanidine33 also abrogated the ability of IFN-γ to suppress IL-1β secretion (Fig. 6c), implicating NO as the mediator of this suppression. Similarly, the direct addition of NO donors, such as S-nitroso-N-acetyl-DL-penicillamine (SNAP) and S-nitrosoglutathione (GSNO), inhibited the processing and release of IL-1β as efficiently as IFN-γ (Fig. 6d,e). These donors produced a modestly lower total amount of nitrite as IFN-γ-treated macrophages over the time course of the experiment (Supplementary Fig. 3a, b) and did not alter the secretion of TNF, indicating that nontoxic doses were applied. Consistent with the freely diffusible nature of NO, wild type macrophages had the ability to suppress IL-1β production from co-cultured iNOS deficient cells in trans (Fig. 6f). We conclude that NO is necessary for IFN-γ to suppress IL-1β and sufficient to mediate this effect.
Figure 6. Nitric oxide is necessary for IFN-γ-mediated suppression of IL-1β processing.
a and b. BMDMs were primed with LPS or Pam3CSK4 ± IFN-γ and infected with Mtb (MOI=10). 24 hours later, cell lysates and supernatants were analyzed by ELISA for IL-1β (a) or immunoblotting for IL-1β and caspase-1 (b). Pro-forms in lysates and mature products in supernatants are indicated. c. Pam3CSK4-primed BMDMs were treated with aminoguanidine hemisulfate (AGHS) before Mtb infection. Cytokines were measured in culture supernatants after 24 hrs. d and e. Macrophages were primed with Pam3CSK4 and treated with IFN-γ or NO donors, S-nitrosoglutathione (GSNO) or S-nitroso-N-acetyl-DL-penicillamine (SNAP), before Mtb infection. Secretion of IL-1β and TNF was measured by ELISA. IL-1β and caspase-1 processing in cell lysates and supernatants was assessed by immunoblotting. f. BMDMs from C57BL/6 and iNos−/− mice were co-cultured in the indicated combinations (1:1 ratio), primed with Pam3CSK4 ± IFN-γ, and infected with Mtb. 24 hours later, IL-1β secretion was measured by ELISA. One-way or two-way ANOVA was applied to calculate P values, as appropriate. “ns” indicates P > 0.05. Bars represent the mean ± sd of at least 3 replicates. Data are representative of two (c-f) to five (a and b) independent experiments.
NO inhibits the assembly of the NLRP3 inflammasome
NO has the ability to regulate gene expression by inducing cGMP synthesis34, suggesting that this compound might act by altering the abundance of inflammasome components. However, IFN-γ did not appreciably alter the expression levels of the relevant inflammasome proteins, NLRP3, ASC, caspase-1, and pro-IL-1β, in cell lysates (data not shown). Furthermore, the cell membrane permeable cGMP analog 8-bromo-cGMP (8-Br-cGMP) had no effect on IL-1β release when added to macrophage cultures before and during Mtb infection (Supplementary Fig. 4).
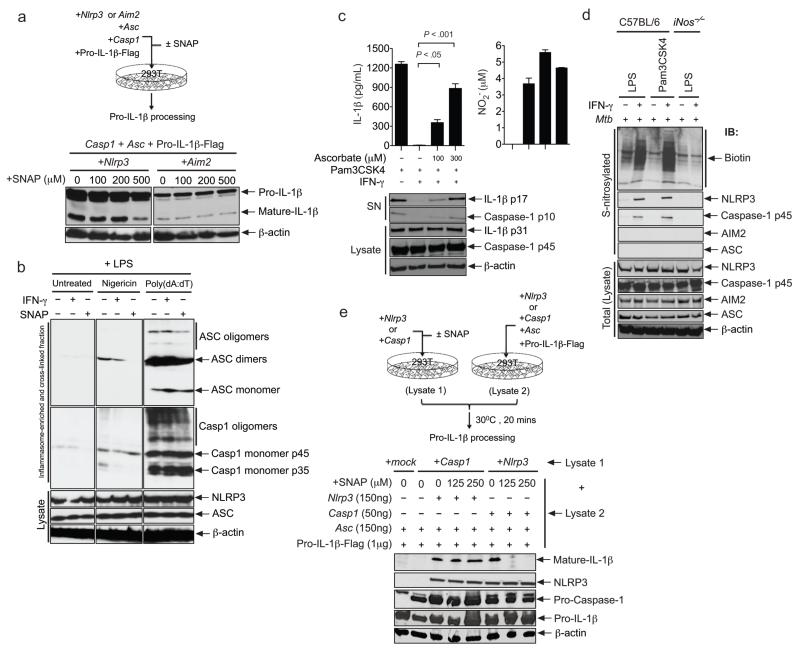
These observations suggested that that NO might act posttranslationally to regulate inflammasome function. To investigate this possibility, we reconstituted the relevant protein complexes in 293T cells. Co-expression of NLRP3 (or AIM2), ASC, caspase-1, and pro-IL-1β in this heterologous system resulted in spontaneous processing of both caspase-1 and IL-1β. The addition of an NO donor (SNAP) inhibited the NLRP3-dependent maturation of IL-1β in these transfected cells, but had no effect on the AIM2 inflammasome, implying that this compound might directly modulate the assembly or activity of the NLRP3 inflammasome (Fig. 7a).
Figure 7. IFN-γ-induced nitric oxide posttranslationally inhibits the assembly and activation of the NLRP3 inflammasome via thiol nitrosylation.
a. Inflammasomes were reconstituted in 293T cells by transfection. Cells were treated ± SNAP, and IL-1β processing was assessed by immunoblotting. b. BMDMs were primed with LPS ± IFN-γ or LPS ± SNAP, and stimulated with nigericin or poly (dA:dT). ASC oligomerization was assayed by cross-linking inflammasome components, followed by immunoblotting. Predicted mono- and multi-meric forms are indicated. “Lysate” indicates unfractionated lysates. c. BMDMs were treated with dehydroascorbate concomitantly with Pam3CSK4 ± IFN-γ. NO production and IL-1β release were assessed at 16 hours (upper panels). Pro- and mature forms of IL-1β and caspase-1 were assessed by immunoblotting (lower panel). Data are representative of at least three independent experiments. P values were determined by student’s t-test. d. BMDMs were subjected to the indicated treatments. 16 hours later, S-nitrosylated cysteines were biotinylated, and the corresponding proteins were purified using streptavidin. Total S-nitrosylated species or the indicated proteins were detected in the biotinylated or total lysate fractions by immunoblotting. e. Independent 293T cultures were transfected with NLRP3 or caspase-1 expression plasmids, treated ± SNAP, and lysed after 16 hours (lysate 1). Separate cultures were transfected to produce the other three components of the complex (lysate 2). Lysates 1 and 2 were mixed and IL-1β processing was assessed by immunoblotting.
To determine if NO altered inflammasome assembly, we evaluated the oligomerization of ASC that occurs during the formation of this complex35. Intact inflammasomes were isolated from stimulated macrophages by differential centrifugation, cross-linked, and detected by immunoblotting. The addition of either IFN-γ or SNAP to these cells inhibited the formation of the ASC and caspase-1 oligomers that are induced by the NLRP3 stimulant nigericin. This inhibition of assembly correlated with a lack of caspase-1 maturation into the processed 35kDa form (Fig. 7b). In contrast, IFN-γ and NO had little effect on the AIM2-dependent oligomerization of ASC and caspase-1 in response to poly (dA:dT), and the subsequent maturation of caspase-1. Thus, in both macrophages and transfected 293T cells NO specifically inhibited the assembly and activity of the NLRP3 inflammasome complex.
NO regulates inflammasome activity via S-nitrosylation
In addition to its gene regulatory roles, NO also has the ability to directly alter protein function through the chemical modification of cysteine residues36. To determine if the observed NO-dependent regulation of inflammasome activity was due to S-nitrosylation, we chemically reversed these modifications with ascorbate. This mild reductant abrogates the nitrosylation of thiols without affecting the ability to form disulfide bonds37. Ascorbate treatment had no effect on the production of NO by macrophages, but reversed the IFN-γ-mediated inhibition of IL-1β release in a dose-dependent manner (Fig. 7c). The processing of both IL-1β and caspase-1 were fully restored by this treatment indicating that the IFN-γ and NO-mediated suppression of inflammasome activity relied on S-nitrosylation.
The posttranslational effect of NO on inflammasome assembly and activity implied that this protein complex might be directly modified by S-nitrosylation. Using the ‘biotin-switch’ method38 we labeled S-nitrosocysteines in macrophage lysates with biotin and isolated the associated proteins with streptavidin. Upon IFN-γ stimulation of Mtb-infected macrophages, we detected iNOS-dependent nitrosylation of a variety of cellular proteins (Fig. 7d). This nitrosylated protein fraction contained NLRP3 and caspase-1, but not AIM2 or ASC.
The S-nitrosylation of NLRP3 and caspase-1 suggested that the modification of one or both of these proteins might be responsible for the inhibition of IL-1β processing. Indeed, nitrosylation of the caspase-1 active site thiol has been previously proposed to regulate the proteolytic activity of this enzyme39. To identify the relevant cellular target(s) of NO, we developed a reconstituted IL-1β processing system in which NLRP3 or caspase-1 could be individually expressed and chemically modified in 293T cells. When these cell lysates (containing either NLRP3 or caspase-1) were incubated with lysates from cells transfected with the remaining inflammasome components, IL-1β processing was only observed if NLRP3, ASC, caspase-1 and pro-IL-1β were simultaneously present. This ability to reconstitute a functional inflammasome from separately produced components afforded the opportunity to assess the specific effects of nitrosylation on either NLRP3 or caspase-1. SNAP treatment of cells expressing the NLRP3 protein efficiently inhibited the IL-1β processing activity of mixed lysates, whereas treatment of caspase-1 expressing cells had no effect on IL-1β processing at any non-cytotoxic concentration (Fig. 7e). To further investigate the effect of NO on caspase-1 activity, we determined the effect of SNAP treatment on the low level of caspase-1 autoprocessing that is observed upon overexpression of this protein alone in 293T cells. At concentrations that inhibit NLRP3-dependent caspase-1 maturation, this compound had no effect on caspase-1 autoprocessing (Supplementary Fig. 5). Together, these observations indicated that nitrosylation of NLRP3, but not caspase-1, was sufficient to suppress inflammasome activity. We propose that nitric oxide-mediated inflammasome inhibition represents an important mechanism by which the adaptive immune response prevents persistent inflammatory tissue damage during chronic infection. (Supplementary Fig. 6).
DISCUSSION
These observations establish a mechanism whereby IFN-γ-induced NO inhibits NLRP3-dependent IL-1 responses. To specifically investigate the importance of this regulatory effect, we developed a new animal model of TB, which obviates the necessity for antimicrobial immunity. Using this model, we found that this NO-mediated IL-1 suppression promotes resistance to Mtb by modulating the inflammatory pathology caused by this persistent infection.
Together, the posttranslational inhibition of inflammasome assembly, the presence of nitrosylated inflammasome components, and the restoration of IL-1β production by ascorbate indicate that NO modulates inflammasome activity via thiol nitrosylation. Although we detected the S-nitrosylation of both NLRP3 and caspase-1, three lines of evidence indicate that only the former represents a plausible target of regulation. Firstly, the ability of IFN-γ or NO to specifically inhibit the NLRP3 inflammasome and not other caspase-1 containing complexes indicates that caspase-1 is unlikely to be a functional nitrosylation target in this context. Secondly, IFN-γ and NO inhibit ASC oligomerization in nigericin-stimulated macrophages, a process that is independent of caspase-140. Finally, we found that nitrosylation of NLRP3, but not caspase-1, suppressed IL-1β processing by reconstituted inflammasomes. Thus, we conclude that the previously described caspase-1 active site nitrosylation is unlikely to be involved in the process we describe. Instead, the preferential target of S-nitrosylation appears to be the NLRP3 protein itself, although we cannot exclude the contribution of an NLRP3-specific cofactor. The NLRP3 protein is extremely rich in cysteines that are clustered in the leucine-rich repeat region proposed to be the sensing domain of the protein41. Identifying which of these 43 cysteines are functional targets of S-nitrosylation may prove to be an effective way to define the repeats most important for triggering IL-1β responses.
The mechanism by which NO modulates the inflammasome is conceptually similar to the inhibition of IL-1 processing mediated by the superoxide radical. This compound promotes the glutathionylation of caspase-1 at cysteines distal to the active site of the protease 42, which could alter inflammasome assembly in a manner analogous to S-nitrosylation. The ability of both reactive oxygen species (ROS) and NO to modulate IL-1 processing emphasizes the important position of the inflammasome as an integration point for pro- and anti-inflammatory pathways, and highlights the central role of redox signaling in this process. Through the coordination of these signals, unproductive inflammation can be suppressed either by the over-accumulation of superoxide-producing phagocytes or by the emergence of IFN-γ-producing lymphocytes. However, while ROS- and NO-mediated IL-1 inhibition share many similarities, the specificity of NO- toward the NLRP3 inflammasome implies that the outcome of this regulation is distinct. The specificity of NO-mediated inhibition indicates that this compound will only affect a discrete set of inflammatory states that depend on NLRP3. These include chronic bacterial infections in which membrane perturbation is the primary inducer of IL-1, and sterile inflammatory lesions initiated by indigestible particles. We note that NO administration has been shown to ameliorate the inflammatory disease promoted by both chronic pulmonary infection43 and cholesterol crystal deposition44, and our observations suggest a mechanism to explain this therapeutic effect.
The differential importance of the inflammasome in Mtb-infected macrophages versus intact mice has been difficult to reconcile. Previous studies have shown that the NLRP3 inflammasome is essential for IL-1β processing in macrophages, but caspase-1-independent mechanisms appear to dominate during chronic infection in mice. Our observations provide an explanation for this apparent paradox. Only in the absence of iNOS could we unveil the contribution of the inflammasome in Mtb-infected animals. These findings suggest that caspase-1 activating signals are produced during infection, but inflammasome complexes are not formed due to the inhibitory effect of NO.
While we show that IFN-γ and NO inhibit IL-1β processing, the mechanisms by which these molecules modulate inflammation likely extend beyond the simple inhibition of inflammasome activity. IFN-γ has been proposed to play an anti-inflammatory role in Mtb infection through the modulation of TH17 cells or direct effects on neutrophil survival45. In addition we observed that iNOS mutation increased the levels of both pro- and mature-IL-1β in the lungs of Mtb-infected mice, suggesting that NO might also influence pro-IL-1β expression. Regardless, our observation that ASC was required for the excessive inflammation seen in iNOS-deficient animals indicates that the modulation of inflammasome activity plays an important, if not singular, role in mediating the anti-inflammatory effects of IFN-γ and NO.
In the context of Mtb infection, we show that the ability of NO to suppress granulocytic inflammation is distinct from its antibacterial function and that both activities appear to be essential for controlling disease. The immunomodulatory activity of NO emphasizes the coordination that is required to balance the competing need for appropriate antimicrobial responses with the necessity to preserve the delicate pulmonary architecture. IL-1β plays a central role in this balance during Mtb infection, as both an important mediator of anti-mycobacterial immunity46 and a driver of tissue damage. Mtb causes a wide spectrum of disease and multiple pro- and anti-inflammatory pathways, including those independent of IL-1, could contribute to pathology. Indeed, the balance between proinflammatory leukotriene B4 and anti-inflammatory lipoxin A4 (LXA4) may alter the outcome of TB disease by modulating TNF47. As we suggest for IL-1β, it has been proposed that TNF must be maintained at an optimal concentration to prevent either bacterial overgrowth or excess immunopathology. LXA4 has been shown both to inhibit TNF secretion and induce NO production. Our observation that NO inhibits IL-1 production suggests a possible mechanism by which eicosanoids could coordinately regulate both of these proinflammatory cytokines to minimize tissue damage. The emerging importance of anti-inflammatory mediators, such as NO and LXA4, in host resistance to Mtb indicates that immunomodulatory pathways are an important determinant of disease progression that could be manipulated to prevent TB disease. Indeed, supplementation with L-arginine, the substrate for iNOS, has been shown to increase NO production and improve the clinical outcome of HIV-negative TB patients undergoing chemotherapy48. Thus, NO-donors or IL-1 antagonists may represent useful adjuncts to anti-TB therapy, which could ameliorate the inflammatory tissue damage that occurs during lengthy antimycobacterial treatment regimens.
METHODS
Mice
C57BL/6 and Rag2−/− (Model # RAGN12) mice were obtained from Taconic farms. IFN-g−/− (B6.129S7-IFN-gtm1Ts/J, stock#002827) and iNos−/− (B6.129P2-Nos2tm1Lau/J, stock#002609) mice were obtained from the Jackson Laboratory. IFN-gr1−/− mice were a gift from Dr. Susan Swain (University of Massachusetts Medical School, UMMS). All other mutant mice were provided by Dr Kate Fitzgerald. Housing and experimentation was performed in accordance with the guidelines set forth by the department of animal medicine of UMMS and Institutional Animal Care and Use Committee.
Bacteria
Wild type M.tuberculosis (Mtb) was a phthiocerol dimycocerosate-positive strain of H37Rv, and was cultured in 7H9 medium. Strain 18b21, 22, was provided by Dr Antonio Campos-Neto, and was cultured in the same media supplemented with 50 μg/mL of streptomycin sulfate (Sigma, St. Louis, MO). Salmonella enterica serovar typhimurium was provided by Dr. Beth McCormick, UMMS.
Mouse infection
For strain 18b, mycobacteria were suspended in phosphate-buffered saline (PBS)-Tween 80 (0.05%), dissociated by sonication, and delivered intravenously at ~1×106 or intratracheally at ~3×105 CFU per mouse. Mice were administered a daily dose of 2 mg of streptomycin sulfate for two weeks. For wild type H37Rv, iNos−/− mice and their controls were challenged with ~200 CFU using an aerosol generation device (Glas-Col, Terre Haute, IN). Rag2−/−, IFN-g−/− mice and their controls were infected with ~106 CFU of H37Rv via the intravenous route. All the mice were of C57BL/6 background. For aminoguanidine treatment, groups of mice were supplied with water supplemented with 2.5% aminoguanidine and 2.5% glucose 7 days prior to Mtb infection, and this was maintained throughout.
Myeloperoxidase (MPO) assay
After perfusion with PBS, lung tissue was homogenized in 1 ml of MPO buffer (50 mM K2HPO4, pH 5.4, 0.5% hexadecyl trimethyl ammonium bromide (HTAB), 10 mM EDTA). The homogenate was frozen and thawed twice. After centrifugation at 2500g for 15 minutes at 4°C, the supernatant was collected and serially diluted. 25μl of diluted supernatant was added to 25 μl of assay buffer (16.7 mg/ml of o-dianisidine dihydrochloride in 50 mM K2HPO4, pH 5.4) and 200 μl of development solution (30 μl of 31.1% H2O2 per 10 ml of 50 mM K2HPO4, pH 5.4). The change in OD460 was measured between 0 and 25 minutes, and MPO activity was calculated as ΔOD/min per milligram of total protein in the lysates.
Flow Cytometry
Single cell suspensions were prepared from bronchoalveolar lavage and PBS-perfused lung tissue as described previously49. Cells were stained with anti-CD3-PE (clone 145-2C11), anti-CD11b-Pacific Blue (clone M1/70) and anti-Ly6G-PE (RB6-8C5) antibodies, and analyzed with an LSR II flow cytometer.
Macrophage infections
Bone marrow from mouse femurs was differentiated into macrophages for 7 days in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 20% L929 cell conditioned medium, 10% fetal bovine serum, 2 mM L-glutamine and 1 mM sodium pyruvate. BMDMs were infected with Mtb at an MOI of 10 for 4 hours, washed with PBS, and cultured in serum free media for 18 hrs. For Mycobacterium marinum, BMDMs were infected for 4 hours (MOI = 10), washed twice, and incubated for overnight in serum-free medium containing gentamicin (50 μg/ml). For Salmonella enterica serovar typhimurium, BMDMs were infected for 1h, washed, incubated for one hour in medium-containing gentamicin (50 μg/ml), and incubated for an additional 4 hours with culture medium containing gentamicin (10 μg/ml). Cytokines were measured in the supernatants generated during the final incubation.
Cytokine and nitric oxide measurements
Cytokine concentrations were quantified using commercial ELISA kits (BD Opt EIA) or the Multi-analyte ELISArray kit from SA Biosciences (Cat No. MEM-005A). Nitric oxide was measured using the Griess reagent (Sigma, Cat No. G4410). Immunoblotting was conducted using anti-murine caspase-1 p10 (sc-514, Santa Cruz Biotechnology), anti-murine IL-1β (R&D systems, Minneapolis, MN), rabbit polyclonal anti-murine AIM2 (generously provided by Dr E. Alnemri), or anti-murine NLRP3 (Enzo-Life sciences). All samples were normalized for total protein content.
Detection of S-nitrosylated proteins
The biotin switch assay for detecting S-nitrosylated proteins was performed essentially as described38. Biotinylated proteins were purified by streptavidin-agarose (Pierce). Bound proteins were recovered in elution buffer (20 mM HEPES, pH 7.7, 100 mM NaCl, 1 mM EDTA, and 100 mM of 2-mercaptoethanol) and visualized by immunoblotting.
ASC oligomerization assay
ASC oligomerization assay was performed as described with minor modifications35. Briefly, immortalized BMDMs from C57BL/6 mice50 were primed with LPS (200 ng/ml) and IFN-γ (200U/mL) for 3 hrs or treated with NO donor SNAP (500μM) for 30 min before the addition of inflammasome ligands. The cells were stimulated with poly(dA:dT) (1.5 μg/106 cells; for 3 hrs) and nigericin (10 μM; for 30 min). The cytosolic fraction of these cells was centrifuged at 5000 rpm to pellet the inflammasome complexes, which were treated with 2mM of disuccinimidyl suberate (DSS). The cross-linked proteins were separated on 10% SDS-polyacrylamide gels and analyzed for ASC oligomerization by immunoblotting with a rat monoclonal antibody against ASC (kind gift from Dr. Gabriel Nunez, University of Michigan).
Inflammasome reconstitution in 293T cells
The inflammasome reconstitution assay shown in figure 7a was performed as described29 with minor modifications. 2 × 104 293T cells were transfected with human ASC (20 ng), caspase1 (5 ng), guassia-luciferase FLAG-tagged IL-1β (80 ng), AIM2 (20 ng), and NLRP3 (20 ng) expression plasmids. After 2-4 h, the cells were treated with different concentrations of SNAP (125, 250 and 500 μM). Sixteen hours later, cells were lysed and analyzed by immunoblotting with anti-IL-1β (3ZD, National Cancer Institute, NIH) or anti-FLAG M2 antibodies (Sigma).
in vitro inflammasome reconstitution assay
For the experiments described in Figure 7e, independent cultures of 4× 105 293T cells were transfected with the indicated combinations of human ASC (150 ng), caspase1 (50ng), guassia-luciferase FLAG-tagged IL-1β (1γg) and NLRP3 (150ng) expression plasmids. After 4 hrs, the cells were treated with SNAP (125 and 250γM). Sixteen hours later, cells were lysed with cold lysis buffer (25mM Tris.Cl, pH, 7.4, 150mM NaCl, 1mM EDTA, 1% NP-40, 5% glycerol, 1mM nucuproine, 1mM PMSF) and snap frozen in liquid N2. The lysates were mixed in different combinations, incubated at 30°C for 20 minutes to facilitate inflammasome activation, and analyzed by immunoblot with goat anti-mouse IL-1β (R&D systems), anti-mouse caspase-1 antibody (clone 5B10; eBioscience) or anti mouse-NLRP3 (Enzo life sciences).
Supplementary Material
Acknowledgements
We thank Antonio Campos-Neto for providing Mtb strain 18b; Beth McCormick, Kenneth Rock, Rabinarayan Mishra, Amit Kumar Pandey and Jinhee Lee for their scientific insight; and Heather Ducharme for animal husbandry. This work was supported by NIH grants (AI064282 to CMS, HL064884 to HK, and AI083713 to KAF), the Howard Hughes Medical Institute (CMS), and a postdoctoral fellowship (NIH U54 AI057159) from the New England Regional Center of Excellence for Biodefense and Emerging Infectious Diseases to VAKR.
Footnotes
Author Contributions
BBM and CMS conceived the project and designed the experiments. BBM performed most of the experiments. VAKR, GWM performed specific experiments. AJM provided veterinary pathology expertise. BBM and CMS wrote the manuscript. HK, KAF provided scientific insight and critical review of the manuscript. CMS oversaw the project.
REFERENCES
- 1.Lamkanfi M, Dixit VM. Inflammasomes: guardians of cytosolic sanctity. Immunol Rev. 2009;227:95–105. doi: 10.1111/j.1600-065X.2008.00730.x. [DOI] [PubMed] [Google Scholar]
- 2.Vance RE, Isberg RR, Portnoy DA. Patterns of pathogenesis: discrimination of pathogenic and nonpathogenic microbes by the innate immune system. Cell Host Microbe. 2009;6:10–21. doi: 10.1016/j.chom.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519–550. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- 4.Dinarello CA. Role of interleukin-1 in infectious diseases. Immunol Rev. 1992;127:119–146. doi: 10.1111/j.1600-065x.1992.tb01411.x. [DOI] [PubMed] [Google Scholar]
- 5.Hise AG, et al. An essential role for the NLRP3 inflammasome in host defense against the human fungal pathogen Candida albicans. Cell Host Microbe. 2009;5:487–497. doi: 10.1016/j.chom.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nathan C, Ding A. Nonresolving inflammation. Cell. 2010;140:871–882. doi: 10.1016/j.cell.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 7.Pandey AK, et al. NOD2, RIP2 and IRF5 play a critical role in the type I interferon response to Mycobacterium tuberculosis. PLoS Pathog. 2009;5:e1000500. doi: 10.1371/journal.ppat.1000500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coulombe F, et al. Increased NOD2-mediated recognition of N-glycolyl muramyl dipeptide. J Exp Med. 2009;206:1709–1716. doi: 10.1084/jem.20081779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kang DD, Lin Y, Moreno JR, Randall TD, Khader SA. Profiling early lung immune responses in the mouse model of tuberculosis. PLoS One. 6:e16161. doi: 10.1371/journal.pone.0016161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seiler P, et al. Early granuloma formation after aerosol Mycobacterium tuberculosis infection is regulated by neutrophils via CXCR3-signaling chemokines. Eur J Immunol. 2003;33:2676–2686. doi: 10.1002/eji.200323956. [DOI] [PubMed] [Google Scholar]
- 11.MacMicking JD, et al. Identification of nitric oxide synthase as a protective locus against tuberculosis. Proc Natl Acad Sci U S A. 1997;94:5243–5248. doi: 10.1073/pnas.94.10.5243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Capuano SV, 3rd, et al. Experimental Mycobacterium tuberculosis infection of cynomolgus macaques closely resembles the various manifestations of human M. tuberculosis infection. Infect Immun. 2003;71:5831–5844. doi: 10.1128/IAI.71.10.5831-5844.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.North RJ, Jung YJ. Immunity to tuberculosis. Annu Rev Immunol. 2004;22:599–623. doi: 10.1146/annurev.immunol.22.012703.104635. [DOI] [PubMed] [Google Scholar]
- 14.Chackerian AA, Perera TV, Behar SM. Gamma interferon-producing CD4+ T lymphocytes in the lung correlate with resistance to infection with Mycobacterium tuberculosis. Infect Immun. 2001;69:2666–2674. doi: 10.1128/IAI.69.4.2666-2674.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eum SY, et al. Neutrophils are the predominant infected phagocytic cells in the airways of patients with active pulmonary TB. Chest. 2010;137:122–128. doi: 10.1378/chest.09-0903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eruslanov EB, et al. Neutrophil responses to Mycobacterium tuberculosis infection in genetically susceptible and resistant mice. Infect Immun. 2005;73:1744–1753. doi: 10.1128/IAI.73.3.1744-1753.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carlsson F, et al. Host-detrimental role of Esx-1-mediated inflammasome activation in mycobacterial infection. PLoS Pathog. 6:e1000895. doi: 10.1371/journal.ppat.1000895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mogues T, Goodrich ME, Ryan L, LaCourse R, North RJ. The relative importance of T cell subsets in immunity and immunopathology of airborne Mycobacterium tuberculosis infection in mice. J. Exp. Med. 2001;193:271–280. doi: 10.1084/jem.193.3.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nandi B, Behar SM. Regulation of neutrophils by interferon-gamma limits lung inflammation during tuberculosis infection. J Exp Med. 208:2251–2262. doi: 10.1084/jem.20110919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Minguela A, Pastor S, Mi W, Richardson JA, Ward ES. Feedback regulation of murine autoimmunity via dominant anti-inflammatory effects of interferon gamma. J Immunol. 2007;178:134–144. doi: 10.4049/jimmunol.178.1.134. [DOI] [PubMed] [Google Scholar]
- 21.Honore N, Marchal G, Cole ST. Novel mutation in 16S rRNA associated with streptomycin dependence in Mycobacterium tuberculosis. Antimicrob Agents Chemother. 1995;39:769–770. doi: 10.1128/AAC.39.3.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kashino SS, Ovendale P, Izzo A, Campos-Neto A. Unique model of dormant infection for tuberculosis vaccine development. Clin Vaccine Immunol. 2006;13:1014–1021. doi: 10.1128/CVI.00120-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McElvania Tekippe E, et al. Granuloma formation and host defense in chronic Mycobacterium tuberculosis infection requires PYCARD/ASC but not NLRP3 or caspase-1. PLoS One. 2010;5:e12320. doi: 10.1371/journal.pone.0012320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mishra BB, et al. Mycobacterium tuberculosis protein ESAT-6 is a potent activator of the NLRP3/ASC inflammasome. Cell Microbiol. 2010;12:1046–1063. doi: 10.1111/j.1462-5822.2010.01450.x. [DOI] [PubMed] [Google Scholar]
- 25.Mayer-Barber KD, et al. Innate and adaptive interferons suppress IL-1alpha and IL-1 beta production by distinct pulmonary myeloid subsets during Mycobacterium tuberculosis infection. Immunity. 35:1023–1034. doi: 10.1016/j.immuni.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mayer-Barber KD, et al. Caspase-1 independent IL-1beta production is critical for host resistance to Mycobacterium tuberculosis and does not require TLR signaling in vivo. J Immunol. 2010;184:3326–3330. doi: 10.4049/jimmunol.0904189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keller M, Ruegg A, Werner S, Beer HD. Active caspase-1 is a regulator of unconventional protein secretion. Cell. 2008;132:818–831. doi: 10.1016/j.cell.2007.12.040. [DOI] [PubMed] [Google Scholar]
- 28.Pelegrin P, Surprenant A. Pannexin-1 couples to maitotoxin- and nigericin-induced interleukin-1beta release through a dye uptake-independent pathway. J Biol Chem. 2007;282:2386–2394. doi: 10.1074/jbc.M610351200. [DOI] [PubMed] [Google Scholar]
- 29.Hornung V, et al. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature. 2009 doi: 10.1038/nature07725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Franchi L, et al. Cytosolic flagellin requires Ipaf for activation of caspase-1 and interleukin 1beta in salmonella-infected macrophages. Nat Immunol. 2006;7:576–582. doi: 10.1038/ni1346. [DOI] [PubMed] [Google Scholar]
- 31.Koo IC, et al. ESX-1-dependent cytolysis in lysosome secretion and inflammasome activation during mycobacterial infection. Cell Microbiol. 2008;10:1866–1878. doi: 10.1111/j.1462-5822.2008.01177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Broz P, et al. Redundant roles for inflammasome receptors NLRP3 and NLRC4 in host defense against Salmonella. J Exp Med. 207:1745–1755. doi: 10.1084/jem.20100257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chan J, Tanaka K, Carroll D, Flynn J, Bloom BR. Effects of nitric oxide synthase inhibitors on murine infection with Mycobacterium tuberculosis. Infect Immun. 1995;63:736–740. doi: 10.1128/iai.63.2.736-740.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hobbs AJ, Ignarro LJ. Nitric oxide-cyclic GMP signal transduction system. Methods Enzymol. 1996;269:134–148. doi: 10.1016/s0076-6879(96)69016-8. [DOI] [PubMed] [Google Scholar]
- 35.Fernandes-Alnemri T, et al. The pyroptosome: a supramolecular assembly of ASC dimers mediating inflammatory cell death via caspase-1 activation. Cell Death Differ. 2007;14:1590–1604. doi: 10.1038/sj.cdd.4402194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stamler JS, Lamas S, Fang FC. Nitrosylation. the prototypic redox-based signaling mechanism. Cell. 2001;106:675–683. doi: 10.1016/s0092-8674(01)00495-0. [DOI] [PubMed] [Google Scholar]
- 37.Forrester MT, Foster MW, Stamler JS. Assessment and application of the biotin switch technique for examining protein S-nitrosylation under conditions of pharmacologically induced oxidative stress. J. Biol. Chem. 2007;282:13977–13983. doi: 10.1074/jbc.M609684200. [DOI] [PubMed] [Google Scholar]
- 38.Jaffrey SR, Snyder SH. The biotin switch method for the detection of S-nitrosylated proteins. Sci STKE. 2001;2001:l1. doi: 10.1126/stke.2001.86.pl1. [DOI] [PubMed] [Google Scholar]
- 39.Dimmeler S, Haendeler J, Nehls M, Zeiher AM. Suppression of apoptosis by nitric oxide via inhibition of interleukin-1beta-converting enzyme (ICE)-like and cysteine protease protein (CPP)-32-like proteases. J. Exp. Med. 1997;185:601–607. doi: 10.1084/jem.185.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Broz P, von Moltke J, Jones JW, Vance RE, Monack DM. Differential requirement for Caspase-1 autoproteolysis in pathogen-induced cell death and cytokine processing. Cell Host Microbe. 2010;8:471–483. doi: 10.1016/j.chom.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rathinam VA, Vanaja SK, Fitzgerald KA. Regulation of inflammasome signaling. Nat Immunol. 2012;13:333–332. doi: 10.1038/ni.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meissner F, Molawi K, Zychlinsky A. Superoxide dismutase 1 regulates caspase-1 and endotoxic shock. Nat Immunol. 2008;9:866–872. doi: 10.1038/ni.1633. [DOI] [PubMed] [Google Scholar]
- 43.Hopkins N, Gunning Y, O’Croinin DF, Laffey JG, McLoughlin P. Anti-inflammatory effect of augmented nitric oxide production in chronic lung infection. J Pathol. 2006;209:198–205. doi: 10.1002/path.1963. [DOI] [PubMed] [Google Scholar]
- 44.Duewell P, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 464:1357–1361. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nandi B, Behar SM. Regulation of neutrophils by interferon-gamma limits lung inflammation during tuberculosis infection. J. Exp. Med. 2011;208:2251–2262. doi: 10.1084/jem.20110919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fremond CM, et al. IL-1 receptor-mediated signal is an essential component of MyD88-dependent innate response to Mycobacterium tuberculosis infection. J Immunol. 2007;179:1178–1189. doi: 10.4049/jimmunol.179.2.1178. [DOI] [PubMed] [Google Scholar]
- 47.Tobin DM, et al. The lta4h locus modulates susceptibility to mycobacterial infection in zebrafish and humans. Cell. 2010;140:717–730. doi: 10.1016/j.cell.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schon T, et al. Arginine as an adjuvant to chemotherapy improves clinical outcome in active tuberculosis. Eur Respir J. 2003;21:483–488. doi: 10.1183/09031936.03.00090702. [DOI] [PubMed] [Google Scholar]
- 49.Martens GW, et al. Tuberculosis susceptibility of diabetic mice. Am J Respir Cell Mol Biol. 2007;37:518–524. doi: 10.1165/rcmb.2006-0478OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Roberson SM, Walker WS. Immortalization of cloned mouse splenic macrophages with a retrovirus containing the v-raf/mil and v-myc oncogenes. Cell Immunol. 1988;116:341–351. doi: 10.1016/0008-8749(88)90236-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.