Abstract
To determine whether the Fas/Fas ligand (FasL) (CD95/CD178) system contributes to the development of an inflammatory response in vivo, 2.5 μg of bacterial lipopolysaccharide (LPS; endotoxin) per g was administered intranasally to healthy mice (C57BL/6) and mutant mice deficient in either Fas (lpr mice) or FasL (gld mice). Sustained LPS-induced neutrophilic inflammation in the lungs was attenuated in both lpr and gld mice. These observations provide further evidence of a proinflammatory role for the Fas/FasL system in the lungs.
The Fas/Fas ligand (FasL) (CD95/CD178) system is recognized for its importance in the regulation of apoptosis in a wide variety of tissues. As a “death receptor,” Fas is generally considered to exclusively transmit a death signal, resulting in a biologically silent, noninflammatory mechanism for the elimination of cells, especially during the regulation and resolution of immune and inflammatory responses (6, 13). However, accumulating evidence indicates that the Fas/FasL system may also preferentially signal cellular proinflammatory responses associated with tissue injury under certain circumstances (1, 4, 14). Data from both animals and humans implicating the Fas/FasL system as a potential mediator of acute inflammatory lung injury have previously been reported (7-10).
A growing body of literature suggests that a complex interaction exists between bacterial lipopolysaccharide (LPS; endotoxin)-induced inflammatory responses and the Fas/FasL system. Experimental evidence suggests that LPS may induce apoptosis via Fas-associated death domain (FADD)-dependent mechanisms (3). LPS has been reported to initiate caspase activation and apoptosis in endothelial cell lines via a CD14- and FADD-dependent mechanism (3). In another study, mice developed lung endothelial injury, as well as evidence of apoptosis in neutrophils (polymorphonuclear leukocytes [PMN]), macrophages, and cells of the alveolar walls, within 24 h of intratracheal instillation of LPS (5). These changes were attenuated by the administration of a Fas inhibitory antibody, suggesting that the Fas/FasL system participates in apoptotic lung injury induced by intratracheal LPS in mice. In a third study, it was shown that FADD modulates proinflammatory responses in endothelial cells. Specifically, the overexpression of FADD blocked the activation of NF-κB following LPS exposure in an endothelial cell line (2). Furthermore, LPS-induced expression of the cytokines interleukin-6 and KC was enhanced in FADD-deficient murine fibroblasts (2).
The present study was conducted to determine whether the Fas/FasL system plays a role in the development of an inflammatory response to LPS in vivo. The response to intranasal instillation of LPS (2.5 μg/g) was examined in healthy mice (C57BL/6) and mutant mice deficient in either Fas (lpr mice) or FasL (gld mice).
MATERIALS AND METHODS
The animal protocol was approved by the Animal Care Committee of the University of Washington, Seattle. Briefly, male mice weighing 20 to 30 g were anesthetized with inhaled halothane. The mice were either C57BL/6 mice (B&K Universal, Seattle, Wash.) or naturally occurring mutant mice lacking the Fas receptor (lpr mice) or FasL (gld mice) (Jackson Laboratories) on the C57BL/6 background. Each mouse received Escherichia coli O111:B4 LPS (Sigma, St. Louis, Mo.) at a dose of 2.5 μg/g by intranasal instillation as previously described (11). The animals were allowed to recover from anesthesia, returned to their cages, and given free access to water and food. After 6, 24, or 48 h, the animals were euthanized with ketamine and xylazine, their thoraxes were rapidly opened, and the animals were exsanguinated by direct cardiac puncture. The lungs were dissected free, and the tracheas were cannulated in order to perform bronchoalveolar lavage (BAL) or to fix the lungs. BAL was performed by instilling 0.9% NaCl containing 0.6 mM EDTA in two separate 0.5-ml aliquots. The BAL fluid (BALF) was recovered by gentle suction and placed on ice for immediate processing. The lungs were fixed by inflation with 10% neutral buffered formalin at a transpulmonary pressure of 15 cm H2O and embedded in paraffin. An aliquot of BALF was processed immediately for total and differential cell counts. Total cells were counted with a hemacytometer, and differential cell counts were obtained from cytospin preparations stained with modified Wright-Giemsa stain (Diff-Quik; American Scientific Products, McGaw Park, Ill.). The remainder of the lavage fluid was spun at 200 × g for 30 min, and the supernatant was removed aseptically and stored in individual aliquots at −70°C. The total protein concentration in BALF was measured by using the bicinchoninic acid method (Pierce Co., Rockford, Ill.). The cytokines KC and tumor necrosis factor alpha (TNF-α) were measured in BALF by using commercially available immunoassays (R&D Systems, Minneapolis, Minn.). Comparisons among groups were made with factorial analysis of variance and the Tukey post hoc test with Graph-Pad InStat software (San Diego, Calif.). A P value of <0.05 was considered significant.
RESULTS
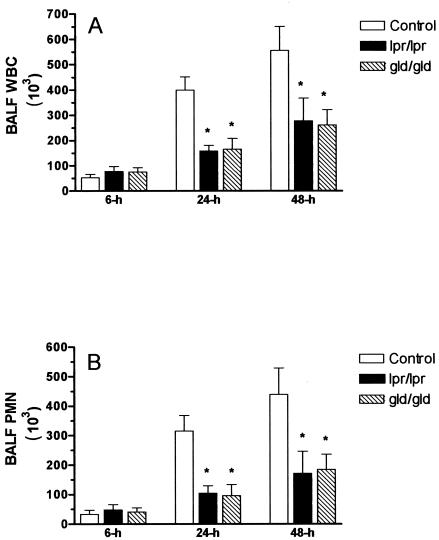
All of the animals survived for the duration of the experiments. Six hours after intranasal instillation of LPS, the numbers of leukocytes in BALF specimens were similar for the three groups of mice (Fig. 1). In contrast, at 24 and 48 h, BALF from the lpr and gld animals contained significantly fewer white blood cells (WBC) than BALF from wild-type C57BL/6 animals (P < 0.05) (Fig. 1A). The difference in numbers of WBC was due to the presence of lower numbers of PMN in the BALF from lpr animals [PMN at 24 h, (104 ± 25) × 103; PMN at 48 h, (171 ± 75) × 103] and gld animals [PMN at 24 h, (96 ± 37) × 103; PMN at 48 h, (184 ± 52) × 103] than in wild-type C57BL/6 animals [PMN at 24 h, (315 ± 53) × 103; at 48 h, (439 ± 90) × 103] (P < 0.05) (Fig. 1B). The numbers of mononuclear cells remained similar in all three groups of animals at all times examined (data not shown).
FIG. 1.
Total numbers of WBC (A) and PMN (B) in BALF from wild-type C57BL/6, Fas-deficient lpr, and FasL-deficient gld mice 6, 24, and 48 h after intranasal administration of E. coli LPS (2.5 μg/g). Asterisk, P < 0.05.
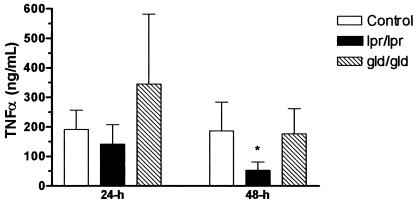
Concentrations of the proinflammatory cytokine TNF-α and the murine chemokine KC were measured in the BALF from animals euthanized 24 and 48 h after the administration of LPS. The concentrations of KC in BALF were not significantly different among the groups of animals at the times examined. In contrast, the concentration of TNF-α was significantly decreased in BALF from lpr mice (53 ± 28 ng/ml [mean ± standard error of the mean]) compared to that in wild-type C57BL/6 mice (186 ± 98 ng/ml) at the 48-h time point following LPS administration (P < 0.05) (Fig. 2).
FIG. 2.
Concentrations of TNF-α in the BALF of wild-type C57BL/6, Fas-deficient lpr, and FasL-deficient gld mice 24 and 48 h after intranasal administration of E. coli LPS (2.5 μg/g). Asterisk, P < 0.05.
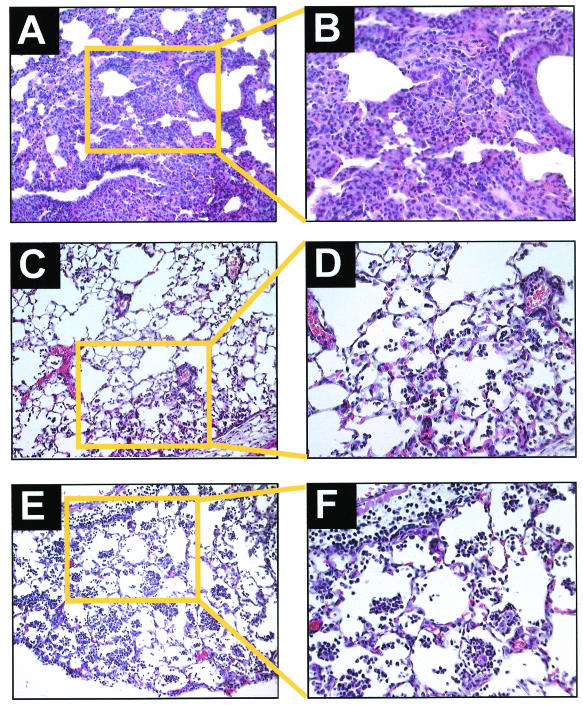
Representative histopathological samples from animals euthanized 24 h after receiving intranasal LPS are shown in Fig. 3. Dense neutrophilic infiltrates and poor expansion were present in lungs from wild-type C57BL/6 mice (Fig. 3A and B). In contrast, only moderate neutrophilic infiltration was present in lungs from lpr (Fig. 3C and D) and gld (Fig. 3E and F) mice.
FIG. 3.
Lung tissue sections stained with hematoxylin-eosin from wild-type C57BL/6 (A and B), Fas-deficient lpr (C and D), and FasL-deficient gld (E and F) mice 24 h after intranasal administration of E. coli LPS (2.5 μg/g). Lungs from C57BL/6 mice show dense neutrophilic alveolar infiltrates and poor inflation (magnifications, ×100 [A] and ×400 [B]). Lungs from lpr (magnifications, ×100 [C] and ×400 [D]) and gld (magnifications, ×100 [E] and ×400 [F]) mice show healthy alveolar walls and moderate neutrophilic infiltrates without intra-alveolar protein deposition.
DISCUSSION
These results demonstrate that the Fas/FasL system mediates, at least in part, sustained pulmonary neutrophilic inflammation in response to LPS administration in mice. The lung neutrophilic inflammatory response was impaired in both Fas-deficient lpr mice and FasL-deficient gld mice at 24 and 48 h following the administration of LPS. Decreased recruitment of PMN to the airspaces resulting from functional impairment of the Fas/FasL system might account for the differences in responses observed in lpr and gld mice compared to that in wild-type C57BL/6 mice. The Fas/FasL system might play a direct role in PMN recruitment to the lung airspaces, as suggested by previous reports of soluble FasL serving as a PMN chemoattractant (11, 15). However, we have been unable to confirm that FasL functions directly as a chemotactic factor for neutrophils (12). Another possibility is that the Fas/FasL system may induce the production of chemotactic cytokines from resident tissue cells, such as macrophages (12). Interestingly, the decreased neutrophilic inflammatory responses observed in lpr and gld mice were not associated with reduced concentrations of the potent murine PMN chemokine, KC, in the BALF from lpr and gld mice compared to that from wild-type C57BL/6 mice, although there were lower concentrations of TNF-α in the lpr mice at the 48-h time point. A third possibility, given previous findings suggesting that the Fas/FasL system may play an important role in the pathogenesis of acute lung injury in both animals and humans (7-10), is that Fas/FasL-mediated disruption of the alveolar-endothelial barrier is required to allow chemotactic cytokines to reach the intraluminal surface for presentation to and recruitment of circulating PMN (5).
In summary, our results demonstrate that the acute inflammatory response to LPS is blunted in the lungs of mice functionally deficient in either Fas or FasL. To our knowledge, these findings represent the first reported evidence indicating that the Fas/FasL system plays a role in LPS-induced inflammation in vivo. Furthermore, these observations provide further evidence of a proinflammatory role for the Fas/FasL system in the lungs.
Acknowledgments
This work was supported in part by the Medical Research Service of the Seattle VAMC and by Public Health Service grants HL70840-01 (G.M.-B.), HL65892 (T.R.M), and HL62995 (W.C.L.) from the National Institutes of Health.
REFERENCES
- 1.Allison, J., H. M. Georgiou, A. Strasser, and D. L. Vaux. 1997. Transgenic expression of CD95 ligand on islet beta cells induces a granulocytic infiltration but does not confer immune privilege upon islet allografts. Proc. Natl. Acad. Sci. USA 94:3943-3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bannerman, D. D., J. C. Tupper, J. D. Kelly, R. K. Winn, and J. M. Harlan. 2002. The Fas-associated death domain protein suppresses activation of NF-κB by LPS and IL-1β. J. Clin. Investig. 109:419-425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choi, K. B., F. Wong, J. M. Harlan, P. M. Chaudhary, L. Hood, and A. Karsan. 1998. Lipopolysaccharide mediates endothelial apoptosis by a FADD-dependent pathway. J. Biol. Chem. 273:20185-20188. [DOI] [PubMed] [Google Scholar]
- 4.Kang, S. M., D. B. Schneider, Z. Lin, D. Hanahan, D. A. Dichek, P. G. Stock, and S. Baekkeskov. 1997. Fas ligand expression in islets of Langerhans does not confer immune privilege and instead targets them for rapid destruction. Nat. Med. 3:738-743. [DOI] [PubMed] [Google Scholar]
- 5.Kitamura, Y., S. Hashimoto, N. Mizuta, A. Kobayashi, K. Kooguchi, I. Fujiwara, and H. Nakajima. 2001. Fas/FasL-dependent apoptosis of alveolar cells after lipopolysaccharide-induced lung injury in mice. Am. J. Respir. Crit. Care Med. 163:762-769. [DOI] [PubMed] [Google Scholar]
- 6.Krammer, P. H. 2000. CD95's deadly mission in the immune system. Nature 407:789-795. [DOI] [PubMed] [Google Scholar]
- 7.Matute-Bello, G., C. W. Frevert, W. C. Liles, M. Nakamura, J. T. Ruzinski, K. Ballman, V. A. Wong, C. Vathanaprida, and T. R. Martin. 2001. Fas/FasL system mediates epithelial injury, but not pulmonary host defenses, in response to inhaled bacteria. Infect. Immun. 69:5768-5776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matute-Bello, G., W. C. Liles, C. W. Frevert, M. Nakamura, K. Ballman, C. Vathanaprida, P. A. Kiener, and T. R. Martin. 2001. Recombinant human Fas-ligand induces alveolar epithelial cell apoptosis and lung injury in rabbits. Am. J. Physiol. 281:L328-L335. [DOI] [PubMed] [Google Scholar]
- 9.Matute-Bello, G., W. C. Liles, K. P. Steinberg, P. A. Kiener, S. Mongovin, E. Y. Chi, M. Jonas, and T. R. Martin. 1999. Soluble Fas ligand induces epithelial cell apoptosis in humans with acute lung injury (ARDS). J. Immunol. 163:2217-2225. [PubMed] [Google Scholar]
- 10.Matute-Bello, G., R. K. Winn, M. Jonas, E. Y. Chi, T. R. Martin, and W. C. Liles. 2001. Fas (CD95) induces alveolar epithelial cell apoptosis in vivo: implications for acute pulmonary inflammation. Am. J. Pathol. 158:153-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ottonello, L., G. Tortolina, M. Amelotti, and F. Dallegri. 1999. Soluble Fas ligand is chemotactic for human neutrophilic polymorphonuclear leukocytes. J. Immunol. 162:3601-3606. [PubMed] [Google Scholar]
- 12.Park, D. R., A. R. Thomsen, C. W. Frevert, U. Pham, S. J. Skerrett, P. A. Kiener, and W. C. Liles. 2003. Fas (CD95) induces proinflammatory cytokine responses by human monocytes and monocyte-derived macrophages. J. Immunol. 170:6209-6216. [DOI] [PubMed] [Google Scholar]
- 13.Savill, J., and V. Fadok. 2000. Corpse clearance defines the meaning of cell death. Nature 407:784-788. [DOI] [PubMed] [Google Scholar]
- 14.Schaub, F. J., D. K. Han, W. C. Liles, L. D. Adams, S. A. Coats, R. K. Ramachandran, R. A. Seifert, S. M. Schwartz, and D. F. Bowen-Pope. 2000. Fas/FADD-mediated activation of a specific program of inflammatory gene expression in vascular smooth muscle cells. Nat. Med. 6:790-796. [DOI] [PubMed] [Google Scholar]
- 15.Seino, K., K. Iwabuchi, N. Kayagaki, R. Miyata, I. Nagaoka, A. Matsuzawa, K. Fukao, H. Yagita, and K. Okamura. 1998. Chemotactic activity of soluble Fas ligand against phagocytes. J. Immunol. 161:4484-4488. [PubMed] [Google Scholar]