Abstract
Objective
To prospectively evaluate relationships among serum cytokine levels, innate immune responsiveness, and mortality in a multicenter cohort of critically ill children with influenza infection.
Design
Prospective, multicenter, observational study.
Setting
Fifteen pediatric ICUs among members of the Pediatric Acute Lung Injury and Sepsis Investigators network.
Patients
Patients ≤18 yrs old admitted to a PICU with community-acquired influenza infection. A control group of outpatient children was also evaluated.
Interventions
ICU patients underwent sampling within 72 hrs of ICU admission for measurement of a panel of 31 serum cytokine levels and quantification of whole blood ex vivo lipopolysaccharide-stimulated tumor necrosis factor-α production capacity using a standardized stimulation protocol. Outpatient control subjects also underwent measurement of tumor necrosis factor-α production capacity.
Measurements and Main Results
Fifty-two patients (44 survivors, eight deaths) were sampled. High levels of serum cytokines (granulocyte macrophage colony-stimulating factor, interleukin-6, interleukin-8, interferon-inducible protein-10, monocyte chemotactic protein-1, and macrophage inflammatory protein-1α) were associated with mortality (p < 0.0016 for each comparison) as was the presence of secondary infection with Staphylococcus aureus (p = 0.007), particularly methicillin-resistant S. aureus (p < 0.0001). Nonsurvivors were immunosuppressed with leukopenia and markedly reduced tumor necrosis factor-α production capacity compared with outpatient control subjects (n = 21, p < 0.0001) and to ICU survivors (p < 0.0001). This association remained after controlling for multiple covariables. A tumor necrosis factor-α response <250 pg/mL was highly predictive of death and longer duration of ICU stay (p < 0.0001). Patients with S. aureus coinfection demonstrated the greatest degree of immunosuppression (p < 0.0001).
Conclusions
High serum levels of cytokines can coexist with marked innate immune suppression in children with critical influenza. Severe, early innate immune suppression is highly associated with both S. aureus coinfection and mortality in this population. Multicenter innate immune function testing is feasible and can identify these high-risk children.
Keywords: cytokine, immunity, immunoparalysis, influenza, pediatric, Staphylococcus aureus
The host response to influenza infection can trigger over-whelming production of pro- and anti-inflammatory mediators in adults (1–4). Although influenza is an important cause of morbidity and mortality in children, remarkably little is known about the innate immune response to severe influenza infection in young hosts. Recent evidence from adult and pediatric populations indicates that a state of severe and prolonged hyporesponsiveness of the innate immune system can occur in the context of critical illness. This suppression is characterized by a marked reduction in the capacity of whole blood to produce the proinflammatory cytokine tumor necrosis factor (TNF)-α on ex vivo stimulation with lipopolysaccharide (LPS) and has been termed immunoparalysis in its most severe form (5, 6). Although a normal subject’s blood should produce robust amounts of TNF-α on ex vivo LPS stimulation, severe reductions in TNF-α production capacity have been associated with the development of secondary infections and death in critically ill adults and children (5–10). Some have suggested that this state of reduced leukocyte proinflammatory cytokine production capacity can coexist with high serum levels of cytokines (11), although this phenomenon is understudied in children.
To date, studies of immunoparalysis have been limited to small single-center investigations (6–10, 12). There are currently no approved therapies to treat innate immune suppression associated with critical illness, although several small studies suggest that it may be reversible with beneficial effects on clinical outcomes (5, 13, 14). How the influenza virus influences the innate immune system is of particular interest given its strong association with secondary bacterial infection and the known inhibitory effects of influenza on leukocyte function in vivo and in vitro (15–17). Pro- and anti-inflammatory therapies have been proposed as adjunctive treatments for influenza infection (18–21), but a lack of prospective immune monitoring data in the pediatric population has made therapeutic decision-making difficult in children. This multicenter, prospective, observational study was designed to test the hypothesis that mortality from influenza in critically ill children and young adults is associated with both hypercytokinemia and innate immune suppression.
MATERIALS AND METHODS
This study was carried out from December 2008 to November 2009 at 15 children’s medical centers across the United States (as part of a larger observational study) selected for their ability to perform LPS stimulation assays. Study sites were recruited from the Pediatric Acute Lung Injury and Sepsis Investigator’s (PALISI) network. This study was approved by the Institutional Review Board at each study site. Informed consent, and subject assent when appropriate, was obtained from the parent or legal guardian of all subjects before study participation.
Patients
Patients ≤18 yrs of age admitted to a PICU with community-acquired influenza infection from December 2008 to November 2009 were eligible to participate. Influenza infection was defined as a positive test for influenza by direct antigen test, direct fluorescent antibody test, polymerase chain reaction test, or viral culture. Additional inclusion criteria included the presence of 1) evidence of lower respiratory tract infection; 2) acute respiratory failure treated with mechanical ventilation regardless of etiology; 3) central nervous system involvement (e.g., coma, seizure); or 4) shock requiring vasopressors. Lower respiratory tract infection was defined as having any of the following: 1) an infiltrate or effusion on chest radiograph and need for supplemental oxygen to maintain systemic oxygen saturations ≥95%; 2) the presence of rales, wheezes, or decreased aeration on chest auscultation; or 3) tachypnea, retractions, or shortness of breath. Shock was defined as receiving >60 mL/kg of resuscitative fluid and/or inotrope or vasopressor support. Exclusion criteria included the presence of isolated upper respiratory tract infection in the absence of other inclusion criteria and/or the presence of nosocomially acquired influenza infection as defined by the onset of influenza-like symptoms and a positive influenza test >48 hrs from hospital admission. After July 7, 2009, we began excluding patients with underlying conditions (respiratory, neurologic, cardiovascular, immunologic, metabolic, and endocrine) predisposing to more severe influenza infection as defined by the Advisory Committee on Immunization Practices (22) and patients who were not severely critically ill (those without marked impairment in mental status or without use of vasopressors or mechanical ventilator support). These steps were taken to enrich our cohort with previously healthy patients who had severe critical illness, a subset of patients that was underrepresented in our initial cohort.
Throughout the entire study period, sites followed the recommendations published by the Centers for Disease Control and Prevention, which called for screening all symptomatic patients admitted to the PICU for influenza and testing all intubated patients for secondary bacterial infection by sending a Gram stain and culture of endotracheal secretions (CD-CHAN-00268-2008-01-30-ADV-N). Patient management was at the discretion of the treating clinicians.
Contemporaneous outpatient control subjects were recruited from the outpatient phlebotomy area at Nationwide Children’s Hospital. Children aged 0–18 yrs presenting for elective phlebotomy were eligible. Children were excluded if they had subjective or objective fever within the past 24 hrs, use of nonsteroidal anti-inflammatory drugs within the past 48 hrs, pre-existing immunosuppression including history of transplantation of any kind, active treatment for malignancy, active treatment of an autoimmune disorder, systemic corticosteroid treatment within the past month, and presence of a known immunosuppressive medication on the patient’s current medication list.
Laboratory Testing
Enrolled influenza subjects included in these analyses underwent blood sampling at a single time point within 72 hrs of ICU admission. Intubated subjects also underwent endotracheal tube sampling for viral coinfection screening using FilmArray multiplex polymerase chain reaction (Idaho Technology, Salt Lake City, UT).
For serum cytokine analyses, whole blood was collected in an anticoagulant-free tube (Becton Dickinson, Franklin Lakes, NJ) and allowed to clot for 30 mins. Tubes were then centrifuged at 1000 × g for 10 mins (within 60 mins of collection), and serum was aliquoted and stored at −80°C and sent to the Cytokine Reference Laboratory at the University of Minnesota where cytokine/chemokine levels were quantified by multiplex assay on the Luminex platform (Luminex, Austin, TX) and Bioplex 2.0 software (Biorad, Hercules, CA). Cytokines and chemokines evaluated with a human-specific 30-plex (Millipore, Billerica, MA) included TNF-α, interferon (IFN)-α2, IFN-γ, eotaxin (CCL11), granulocyte colony stimulating factor, granulocyte-macrophage colony stimulating factor (GM-CSF), growth-related oncogene-α (CXCL1), interleukin (IL)-1α, IL-1β, IL-1 receptor antagonist, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-12 p40, IL-12 p70, IL-13, IL-17, IFN-inducible protein (IP)-10 (CXCL10), monocyte chemotactic protein (MCP)-1 (CCL2), macrophage-derived chemokine (CCL22), macrophage inflammatory protein (MIP)-1α (CCL3), MIP-1β (CCL4), regulated on activation normal T cell expressed and secreted (CCL5), soluble CD 40 ligand (sCD40L), vascular endothelial growth factor, and fractalkine. IFN-β was evaluated as a single plex using a bead set from Panomics (Fremont, CA). All values were interpolated from standard curves of the corresponding human recombinant proteins provided with the multiplex kits.
Quantification of the capacity of subjects’ innate immune system to respond to a challenge was done by measurement of ex vivo LPS-induced TNF-α production capacity. Whole blood was collected in a heparinized tube (Becton Dickinson, Franklin Lakes, NJ) and placed on ice. Within 60 mins of collection, 50 μL of whole blood was added to a tube containing 500 pg/mL LPS (phenol-extracted from Salmonella abortus equi [Sigma, St. Louis, MO]) and incubated for 4 hrs at 37°C. This was done in duplicate for each sample. Tubes were then centrifuged (1000 × g for 5 mins), and supernatants were frozen at −80°C. To assure uniformity of results, LPS stimulation solution was made and shipped monthly by the Immune Surveillance Laboratory at The Research Institute at Nationwide Children’s Hospital. Kits were quality controlled at the site of manufacture such that intrabatch variation in TNF-α stimulation capacity was <10%. Supernatants from each participating study site were shipped quarterly to the Immune Surveillance Laboratory for TNF-α determination. TNF-α was quantified in post-stimulation supernatants using the Immulite automated chemiluminometer (Siemens Healthcare Diagnostics, Deerfield, IL), and average values from duplicate samples were reported.
Although complete blood counts were not part of the study protocol, complete blood counts and differential data obtained within 24 hrs of immune function testing were used to calculate absolute cell counts when available.
Outpatient control subjects also underwent measurement of whole blood ex vivo TNF-α production capacity as well as quantitation of a more limited panel of plasma cytokines (IL-6 [detection threshold: 2 pg/mL] and IL-10 [detection threshold: 5 pg/mL]) using the Immulite automated chemiluminometer. These cytokines were chosen to screen for evidence of systemic inflammation and the counterregulatory anti-inflammatory response in these subjects.
Definitions
Race and ethnicity (Hispanic or non-Hispanic) were defined by the child’s parents. Pediatric Risk of Mortality III acute physiology score (PRISM III) (23) was used to measure severity of illness within the first 24 hrs of admission. Noninvasive ventilation was defined as delivery of continuous positive airway pressure or bilevel positive airway pressure from a mechanical ventilator through a mask. Acute lung injury and acute respiratory distress syndrome (ARDS) were defined as acute onset of hypoxia (Pao2/Fio2 ratio of ≤300 for acute lung injury and ≤200 for ARDS) with bilateral infiltrates on chest radiograph and no evidence of left heart failure. Shock requiring inotropic or vasopressor support was defined as the use of a dopamine infusion >5 μg/kg/min or any epinephrine, norepinephrine, phenylephrine, dobutamine, or milrinone infusion to maintain blood pressure. Viral coinfections were defined as polymerase chain reaction positivity for any virus other than influenza from study airway samples using the FilmArray method. Bacterial secondary infections were defined as the growth of any pathogen from a sterile body site (lower airway, blood, urine) or polymerase chain reaction positivity for known pathogens (e.g., Mycoplasma species) from samples obtained within 48 hrs of ICU admission. Mortality was defined as death during the hospitalization.
Statistical Analyses
The clinical and cytokine markers were evaluated and summarized both quantitatively and graphically through exploratory data analyses. Between-group comparisons were made with the Mann–Whitney U test for continuous variables and Fisher’s exact test or χ2 test for categorical variables. Univariate and multivariable logistic regression models were created to evaluate the relationships among ex vivo TNF-α production capacity, clinical covariables, and mortality. Covariables for the multivariate models included PRISM III score, presence of secondary bacterial infection on admission, presence of Staphylococcus aureus infection on admission, 2009 H1N1 status, presence of at least one underlying condition, and presence of an immunocompromised state at baseline. Identification of these clinical factors to include was based on univariate modeling results as well as all subsets regression. Given the limited numbers of nonsurvivors, multivariable analyses were restricted to two covariates (ex vivo TNF-α production capacity and a clinical factor). To evaluate goodness of fit in the logistic regression models, we looked at the model’s likelihood ratio tests as well as the (pseudo) R2 tests that were specific to the logistic regression setting. Cytokine values (including ex vivo TNF-α production capacity) were log-transformed for regression analyses. A receiver operating characteristic curve with area under the curve analysis was used to further evaluate the relationship between ex vivo LPS-induced TNF-α production capacity and mortality.
The number of ICU-free days was also evaluated using univariate and multivariable generalized linear regression models in a similar approach as that described for the hospital mortality analyses but without the event-based constraints. Finally, time to ICU discharge was evaluated, where the number of days in the ICU until discharge in the first 28 days was used; those patients who were still in the ICU at 28 days were censored at 28 days. Those patients who died before 28 days in the ICU were treated as competing events. Cumulative incidence models were used to assess the time to ICU discharge while controlling for the competing risk of hospital mortality within 28 days.
A p value < 0.05 was considered significant throughout, except for the serum cytokine/chemokine analyses where a p value of < 0.0016 was considered significant after Bonferroni correction for multiple comparisons within the 31-mediator panel (0.05/31). Analyses were done using the Windows version of the statistical program R Version 2.11.1 (The R Foundation for Statistical Computing, Vienna, Austria) and Prism5 (GraphPad, La Jolla, CA) software.
RESULTS
Subjects
Of 197 eligible subjects, 146 (73%) were enrolled. Of these, 53 (36%) underwent sampling within the first 72 hrs of PICU admission. One subject (a survivor) was excluded from analysis as a result of deviation from the specimen collection protocol, leaving 52 (44 survivors [85%], eight nonsurvivors [15%]). In the cohort as a whole, survivors and nonsurvivors were similar in terms of gender, age, ethnicity, race, and presence of comorbidities (Table 1).
TABLE 1.
Subject Demographics and Mortality Analyses for Subjects With Influenza (n = 52)
| Overall (n = 52) |
Survived (n = 44) |
Died (n = 8) | p a | Relative Risk (95% Confidence Interval) |
|
|---|---|---|---|---|---|
| Gender (%) | |||||
| Male | 36 (69) | 31 (70) | 5 (63) | 0.69 | 0.89 (0.50–1.6) |
| Female | 16 (31) | 13 (30) | 3 (37) | ||
|
| |||||
| Age (yrs) | 7.3 (2.4–14) | 7.3 (2.4–14) | 9.7 (1.9–14) | 0.95 | |
|
| |||||
| Age category (%) | |||||
| <1 yr | 8 (15.4) | 6 (13.6) | 2 (25.0) | 0.50 | |
| ≤1 yr to <13 yrs | 29 (55.8) | 26 (59.1) | 3 (37.5) | ||
| ≤13 yrs to 18 yrs | 15 (28.8) | 12 (27.3) | 3 (37.5) | ||
|
| |||||
| Ethnicity (%) | |||||
| Hispanic/Latino | 15 (28.9) | 12 (27.3) | 3 (37.5) | 0.68b | |
| Not Hispanic/Latino | 35 (67.3) | 31 (70.4) | 4 (50.0) | ||
| Unable to determine | 2 (3.8) | 1 (2.3) | 1 (12.5) | ||
|
| |||||
| Race (%) | |||||
| White | 38 (73.1) | 32 (72.7) | 6 (75.0) | 0.23 | |
| Black | 8 (15.4) | 8 (18.2) | 0 (0.0) | ||
| Asian or other | 6 (11.5) | 4 (9.1) | 2 (25.0) | ||
|
| |||||
| Underlying conditions (%) | |||||
| None (previously healthy) | 24 (46.2) | 18 (40.9) | 6 (75.0) | 0.12c | 0.42 (0.12–1.4) |
| One or mored | 28 (53.8) | 26 (59.1) | 2 (25.0) | ||
| Respiratory | 22 (42.3) | 20 (45.5) | 2 (25.0) | ||
| Cardiovascular | 5 (9.6) | 5 (11.4) | 0 (0.0) | ||
| Renal | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Neuromuscular | 11 (21.2) | 10 (22.7) | 1 (12.5) | ||
| Oncologic | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Metabolic | 1 (1.9) | 1 (2.3) | 0 (0.0) | ||
|
| |||||
| Baseline immunocompromise (%) |
2 (3.8) | 2 (4.5) | 0 (0.0) | Not applicable |
|
Data represent median (interquartile range) unless otherwise specified.
Fisher’s exact, χ2, or Mann–Whitney test where appropriate.
Hispanic vs. other.
Previously healthy vs. one or more underlying conditions.
Subcategory percentages may total >100% as a result of the presence of children with multiple comorbidities.
The ICU clinical characteristics of influenza subjects are shown in Table 2. The median time between ICU admission and blood sampling was in the second ICU day for both survivors and nonsurvivors. All nonsurvivors were invasively mechanically ventilated compared with 68% of survivors. Among those receiving invasive mechanical ventilation, high-frequency oscillatory ventilation was used in 21% of patients (eight of 38). Of these, two were survivors and six were nonsurvivors. The exclusive use of noninvasive ventilatory support was rare, accounting for only 5.8% of subjects (all survivors). Extracorporeal membrane oxygenation (ECMO) support was used in six subjects, all nonsurvivors, four of whom were on ECMO at the time of sample collection. Nonsurvivors had higher PRISM III scores, were more likely to have shock (eight of eight) and ARDS (seven of eight), and demonstrated a trend toward longer lengths of ICU stay. Forty-one subjects (79%) had a complete blood count with differential performed within 24 hrs of study sampling (33 survivors, eight nonsurvivors). In this subset, nonsurvivors were significantly more leukopenic with the drop in cell numbers appearing to be consistent across the monocyte, neutrophil, and lymphocyte lineages.
TABLE 2.
ICU Clinical Characteristics and Mortality Analyses (n = 52)
| Overall (n = 52) | Survived (n = 44) | Died (n = 8) | p a | Relative Risk (95% Confidence Interval) |
|
|---|---|---|---|---|---|
| Time to sampling (hrs)b | 31 (15–48) | 31 (15–48) | 38 (24–54) | 0.29 | |
|
| |||||
| Pediatric Risk of Mortality III score |
6 (1–9) | 3 (1–9) | 20 (7–33) | 0.002 | |
|
| |||||
| Length of ICU stay (days) | 8.0 (6.0–20) | 7.2 (5.8–16) | 21 (4.5–35) | 0.05 | |
|
| |||||
| Mechanical ventilation (%) | |||||
| None | 11 (21.2) | 11 (25.0) | 0 (0.0) | 0.09c | 1.5 (1.2–1.8) |
| Noninvasive only | 3 (5.8) | 3 (6.8) | 0 (0.0) | ||
| Invasive | 38 (73.0) | 30 (68.2) | 8 (100) | ||
| Conventional only | 30 (57.6) | 28 (63.5) | 2 (25.0) | ||
| High frequency oscillatory ventilation |
8 (15.4) | 2 (4.5) | 6 (75.0) | ||
|
| |||||
| Acute respiratory distress syndrome (%) |
14 (26.9) | 7 (15.9) | 7 (87.5) | 0.0002 | 19 (2.6–141) |
|
| |||||
| Shock (%) | 20 (38.5) | 12 (27.2) | 8 (100) | 0.0002 | Not applicable |
|
| |||||
| Extracorporeal membrane oxygenation use (%) |
6 (11.5) | 0 (0.0) | 6 (75.0) | < 0.0001 | Not applicable |
|
| |||||
| WBC count (K/mm3)d | 7.5 (4.5–13) | 10 (5.7–16) | 1.0 (0.63–3.4) | < 0.0001 | |
| Absolute monocyte count (cells/mm3) |
421 (188–863) | 493 (299–986) | 57 (27–90) | 0.0001 | |
| Absolute neutrophil count (cells/mm3) |
4480 (1050–9920) | 6200 (3250–11070) | 340 (245–788) | 0.0002 | |
| Absolute lymphocyte count (cells/mm3) |
1465 (604–1940) | 1670 (955–2055) | 364 (192–748) | 0.005 | |
Data represent median (interquartile range) unless otherwise specified.
Fisher’s exact, χ2, or Mann–Whitney test where appropriate.
From the time of ICU admission.
Invasive mechanical ventilation vs. all other.
From subjects with a complete blood count within 24 hrs of cytokine sampling (33 survivors, eight nonsurvivors).
The microbiologic characteristics of influenza subjects are shown in Table 3. Nearly half of subjects (48%) were confirmed or suspected to have had 2009 H1N1 with two-thirds of the remaining subjects positive for seasonal influenza A. Children with 2009 H1N1 were more likely to have had shock (p = 0.02), ARDS (p = 0.005), and S. aureus secondary bacterial infection (p = 0.02). The 2009 H1N1 was more prevalent in nonsurvivors (seven of eight) than survivors (18 of 44) and was significantly associated with mortality (p = 0.02). There was no difference in the use of antiviral medications either during the hospital course or within 48 hrs of symptom onset between survivors and nonsurvivors.
TABLE 3.
Infection Characteristics and Mortality Analyses (n = 52)
| Overall (n = 52) |
Survived (n = 44) |
Died (n = 8) |
p a | Relative Risk (95% Confidence Interval) |
|
|---|---|---|---|---|---|
| Results of influenza testing (%) | |||||
| Influenza A | |||||
| 2009 H1N1 (confirmed) | 20 (38.5) | 15 (34.1) | 5 (62.5) | 0.02b | 2.1 (1.4–3.3) |
| 2009 H1N1 (suspected) | 5 (9.6) | 3 (6.8) | 2 (25.0) | ||
| Other influenza A | 18 (34.6) | 18 (40.9) | 0 (0.0) | ||
| Influenza B | 8 (15.4) | 7 (15.9) | 1 (12.5) | ||
| Influenza not typed | 1 (1.9) | 1 (2.3) | 0 (0.0) | ||
|
| |||||
| Antiviral use (%) | |||||
| During hospital course | 40 (76.9) | 32 (72.7) | 8 (100) | 0.17 | |
| Within 48 hrs of symptoms | 29 (55.8) | 24 (54.5) | 5 (62.5) | 1.00 | |
|
| |||||
| Bacterial coinfection at admission (%) | |||||
| None | 34 (65.4) | 32 (72.7) | 2 (25.0) | 0.015c | 2.8 (1.5–5.1) |
| Staphylococcus aureus | 11 (21.2) | 6 (13.6) | 5 (62.5) | 0.007d | 4.6 (1.8–11) |
| Methicillin-resistant S. aureus | 6 | 1 | 5 | < 0.0001e | 38 (5.3–275) |
| Methicillin-sensitive S. aureus | 5 | 5 | 0 | ||
| Group A streptococcus | 2 (3.8) | 2 (4.6) | 0 (0.0) | ||
| Other bacteria | 5 (9.6) | 4 (9.1) | 1 (12.5) | ||
|
| |||||
| Viral coinfection at time of collection (%) | |||||
| None | 32 (61.5) | 27 (61.4) | 5 (62.5) | 0.70f | 0.69 (0.20–2.4) |
| Respiratory syncytial virus | 1 (1.9) | 1 (2.3) | 0 (0.0) | ||
| Adenovirus | 1 (1.9) | 1 (2.3) | 0 (0.0) | ||
| Parainfluenza | 2 (3.8) | 2 (4.5) | 0 (0.0) | ||
| Herpes simplex virus | 1 (1.9) | 0 (0.0) | 1 (12.5) | ||
| Human metapneumovirus | 2 (3.8) | 2 (4.5) | 0 (0.0) | ||
| Rhinovirus | 8 (15.4) | 7 (15.9) | 1 (12.5) | ||
| Other virus | 3 (5.8) | 3 (6.8) | 0 (0.0) | ||
| No testing performed | 2 (3.8) | 1 (2.3) | 1 (12.5) | ||
Fisher’s exact or χ2 test where appropriate.
2009 H1N1 (confirmed or suspected) vs. all others.
Any bacterial coinfection vs. none.
Staphylococcus aureus coinfection vs. none or other bacteria.
Methicillin-resistant S. aureus coinfection vs. none or other bacteria;
any viral coinfection vs. none or not tested.
Secondary bacterial infections were diagnosed at admission more frequently in nonsurvivors (six of eight) than survivors (12 of 44). The airway was the primary source of secondary bacterial infection in both nonsurvivors (six of six) and survivors (11 of 12) with one survivor demonstrating bacteremia. Having any secondary bacterial infection at admission was significantly associated with mortality (p = 0.015), but that association was stronger if the infection with S. aureus (p = 0.007). Among nonsurvivors with secondary bacterial infections, S. aureus was the most common pathogen (five of six) with one nonsurvivor having pneumonia with Enterobacter cloacae. S. aureus also was the most common isolate among survivors with secondary bacterial infection (six of 12), with group A streptococcus, Haemophilus influenza, Moraxella catarrhalis, Mycoplasma pneumoniae, and Pseudomonas aeruginosa also represented. Notably, all of the S. aureus isolates from nonsurvivors were methicillin-resistant (MRSA), whereas only one of the six staphylococcal isolates from survivors was MRSA. Additional viruses were identified commonly in the respiratory secretions of both survivors (39%) and nonsurvivors (25%), with rhinovirus being the most common among all subjects (eight of 52).
Control Subjects
Twenty-one outpatients were enrolled and sampled at Nationwide Children’s Hospital as control subjects. They included 11 males and ten females with a median age of 11 yrs (range, 8.2–13 yrs) (p = 0.14 vs. all influenza subjects, p = 0.84 vs. influenza nonsurvivors). Influenza testing was not performed on these subjects, but all were afebrile by history and had no influenza-like symptoms at the time of sampling.
Systemic Cytokine/Chemokine Levels
As shown in Table 4, serum cytokine/chemokine profiles were significantly different between survivors and nonsurvivors. Six mediators (GM-CSF, IL-6, IL-8, IP-10, MCP-1, and MIP-1α) were significantly higher in nonsurvivors at an α level < 0.0016, thus meeting the Bonferroni correction for multiple comparisons (see Supplement Digital Content 1, http://links.lww.com/CCM/A519 for results from the full panel of mediators). In contrast, systemic cytokine concentrations were low among outpatient controls with IL-6 and IL-10 levels typically at or near the threshold of detection: IL-6: 2.6 (2.0–5.8) pg/mL and IL-10: 5 (5) pg/mL.
TABLE 4.
Serum Cytokine/Chemokine Data and Mortality Analyses (Mann–Whitney)
| Survivors (n = 44) | Nonsurvivors (n = 8) | ||
|---|---|---|---|
| Cytokine/Chemokine | Median (Interquartile Range) pg/mL |
Median (Interquartile Range) pg/mL |
p |
|
Granulocyte macrophage colony
stimulating factor |
0 (0–0) | 52 (3.9–451) | < 0.0001 |
| IL-1 receptor antagonist | 36 (11–140) | 1661 (113–6933) | 0.002 |
| IL-6 | 16 (4.7–52) | 4426 (260–8877) | < 0.0001 |
| IL-8 | 7.0 (0–34) | 1211 (14–4938) | < 0.0001 |
| IL-10 | 19 (8.8–47) | 451 (66–1559) | 0.03 |
| Interferon-inducible protein-10 | 1905 (620–3956) | 17200 (4132–27270) | 0.004 |
| Monocyte chemotactic protein-1 | 375 (226–700) | 5265 (1852–7124) | < 0.0001 |
| Macrophage inflammatory protein-1 α | 0 (0–0) | 68 (3.3–231) | 0.0008 |
| Tumor necrosis factor-α | 5.3 (3.4–8.5) | 17 (7.6–125) | 0.003 |
IL = interleukin.
Biomarkers with a p value < 0.01 are shown. Those with p < 0.0016 (to allow for multiple comparisons) are displayed in italics. Data from the complete biomarker panel can be found in Supplement Digital Content 1, http://links.lww.com/CCM/A519.
Children with 2009 H1N1 had significantly higher serum levels of IFN-γ, GM-CSF, IL-8, IL-17, MIP-1α, and vascular endothelial growth factor compared with children with other influenza types. The median concentrations of these mediators, however, were typically quite low even in children with 2009 H1N1 (see Supplement Digital Content 2, http://links.lww.com/CCM/A519). Children with S. aureus secondary infection had higher levels of IFN-γ, GM-CSF, IL-8, IL-17, MIP-1α, and vascular endothelial growth factor compared with children with either no secondary infection or infection with a different organism. In these analyses, the median concentrations of mediators were higher in the S. aureus group than those seen in the 2009 H1N1 analyses (see Supplement Digital Content 3, http://links.lww.com/CCM/A519).
Ex Vivo TNF-α Production Capacity
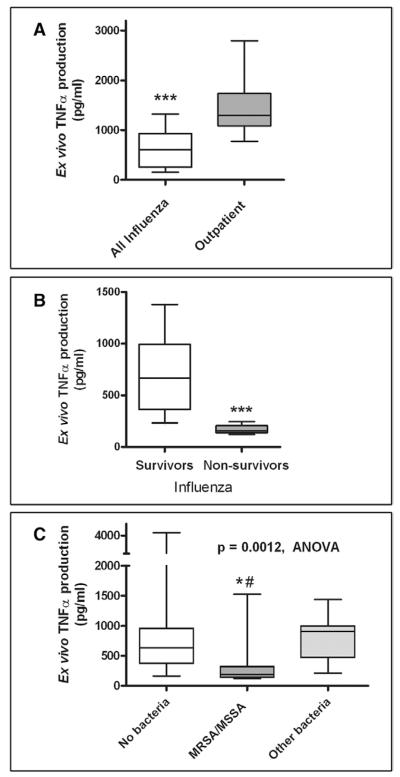
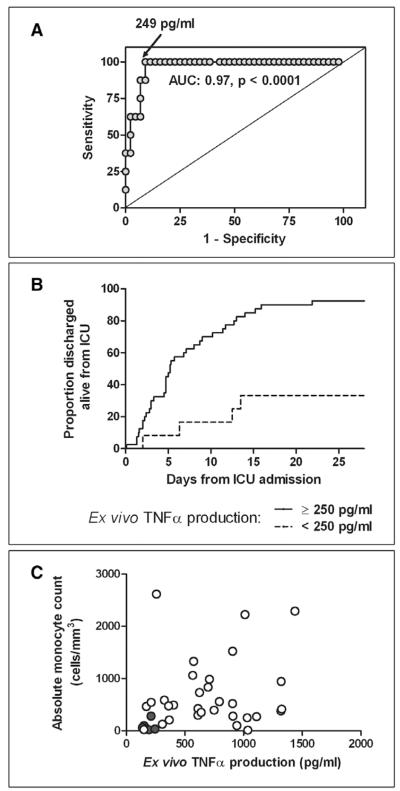
Despite high levels of circulating proinflammatory cytokines, critically ill children with influenza demonstrated lower ex vivo TNF-α production capacity compared with outpatient control subjects (610 [254–934] vs. 1297 [1088–1739] pg/mL, p < 0.0001) (Fig. 1A). Furthermore, influenza nonsurvivors had markedly lower ex vivo TNF-α production capacity compared with survivors (157 [138–206] vs. 667 [364–997] pg/mL, p < 0.0001) (Fig. 1B). Reduced ex vivo TNF-α production capacity was associated with mortality by univariate logistic regression (p = 0.009) and remained significantly associated with mortality after controlling for PRISM III score, presence of secondary bacterial infection at admission, 2009 H1N1 status, presence of underlying conditions, and baseline immunocompromise (Table 5). Receiver operating characteristic curve analysis indicates an excellent ability of the ex vivo TNF-α production capacity to distinguish between survivors and nonsurvivors (area under the curve: 0.97, p < 0.0001) with an optimum discriminatory cutoff of 249 pg/mL (Fig. 2A).
Figure 1.
Ex vivo tumor necrosis factor (TNF)-α production. A, Critically ill children with influenza infection (n = 52) demonstrated lower ex vivo TNF-α production capacity within the first 72 hrs of PICU stay compared with a cohort of children sampled in the outpatient phlebotomy department (n = 21) (p < 0.0001). B, Influenza nonsurvivors (n = 8) had markedly lower ex vivo TNF-α production capacity compared with those who survived (n = 44) (p < 0.0001). C, Subjects presenting with secondary infections resulting from Staphylococcus aureus (n = 11) demonstrated significantly reduced innate immune function compared with those with no bacterial coinfection (n = 34) and those with coinfection with other bacterial species (n = 7) (p = 0.001, analysis of variance [ANOVA]). Boxes represent median and interquartile range. Whiskers represent tenth to 90th percentile. ***p < 0.0001, *p < 0.05 vs. no bacteria, #p < 0.05 vs. other bacteria. MRSA = methicillin-resistant S. aureus; MSSA = methicillin-sensitive S. aureus
TABLE 5.
Logistic Regression Analyses of Ex Vivo Tumor Necrosis Factor-α Production Capacity and Mortality
| Ex Vivo Tumor Necrosis Factor-α Production |
||
|---|---|---|
| p | Pseudo R2 | |
| Univariate | ||
| Ex vivo tumor necrosis factor-α production | 0.009 | 0.70 |
|
| ||
| Bivariate analysis adjusting for: | ||
| Pediatric Risk of Mortality III score | 0.03 | 0.74 |
| Presence of secondary bacterial infection at admission | 0.014 | 0.70 |
| Presence of Staphylococcus aureus infection at admission | 0.014 | 0.70 |
| 2009 H1N1 status | 0.03 | 0.72 |
| Presence of at least one underlying condition | 0.012 | 0.71 |
| Presence of immunocompromised state at baseline | 0.009 | 0.70 |
Figure 2.
Ex vivo tumor necrosis factor (TNF)α production capacity, clinical outcomes, and relationship to absolute monocyte count. A, This assay demonstrated an outstanding capability to discriminate survivors from nonsurvivors within the first 72 hrs of PICU stay with an optimum cutoff point of 249 pg/mL. B, An ex vivo TNF-α production capacity >250 pg/mL was associated with faster time to ICU discharge (p = 0.0006). This remained significant after adjusting for Pediatric Risk of Mortality III score (p = 0.01). C, Lower ex vivo TNF-α production capacity was associated with a lower absolute monocyte count (AMC) (p = 0.002, r2 = 0.23), although nine survivors (open circles) had AMCs as low as nonsurvivors (gray circles). Eight of these survivors had ex vivo TNF-α production capacity >250 pg/mL. AUC = area under the curve.
On analyzing ICU-free days through day 28 (when those who died were given a value of 0), lower ex vivo TNF-α production capacity was associated with fewer ICU-free days by univariate linear regression (p = 0.009). The clinical variable that was most strongly associated with length of ICU stay by linear regression was PRISM III score (p = 0.0005). When performing bivariate regression including PRISM III score, ex vivo TNF-α production capacity remains significantly associated with ICU-free days (p = 0.04). Similarly, by time-to-event analysis, with death as a competing factor, ex vivo TNF-α production is significantly associated with time to ICU discharge (p = 0.005). If ex vivo TNF-α production is dichotomized (<250 pg/mL vs. ≥250 pg/mL), this relationship becomes stronger (p = 0.0006) (Fig. 2B). This remains significant after adjusting for PRISM III score (p = 0.01).
Among all subjects with influenza, children with 2009 H1N1 had lower ex vivo TNF-α production capacity than children with other influenza strains (256 [167–1060] vs. 696 [398–906], p = 0.046). Similarly, children with S. aureus secondary infection had lower TNF-α responses than children with either no bacterial coinfection or coinfection with different bacteria (Fig. 1C). There was no difference in ex vivo TNF-α production capacity between children with and without underlying medical conditions (470 [176–1025] vs. 623 [300–906], p = 0.50).
There was an association between lower absolute monocyte counts (AMC) in children with lower ex vivo TNF-α production capacity (p = 0.002, R2 = 0.23) (Fig. 2C). The highest AMC among nonsurvivors was 282 cells/mm3. There were nine survivors with AMC at or below this level and eight of nine of those had an ex vivo TNF-α production capacity >250 pg/mL. Although both thresholds (AMC ≤288 cells/mm3 and ex vivo TNF-α production capacity <250 pg/mL) demonstrated 100% specificity and positive predictive value for mortality, ex vivo TNF-α production was more sensitive (67% vs. 47%) with better negative predictive value (91% vs. 73%).
DISCUSSION
Our study represents the first multicenter evaluation of the relationships among circulating cytokines/chemokines, innate immune function, and outcomes in children with critical illness resulting from influenza. We were fortunate to have the infrastructure for this study in place at the time of the 2009 H1N1 pandemic, allowing us to enroll patients with seasonal and 2009 H1N1 influenza infections. The serum cytokine/chemokine profiles of nonsurvivors were characterized by high levels of proinflammatory mediators; however, nonsurvivors were also markedly immunosuppressed with leukopenia and severe impairment of ex vivo TNF-α production capacity. In addition, we report an association between S. aureus bacterial coinfection, often fatal, and a state of severe immune suppression, which was not seen in patients coinfected with other bacteria.
Data from the United States (24–28) and abroad (29–31) indicate that children undergoing ICU care in the 2008–2009 influenza season tended to be older than previous seasons, had high likelihoods of having an underlying medical condition, and were likely to be treated with invasive mechanical ventilation. Our cohort was similar in these regards although it was enriched, over time, with children with severe disease (mechanical ventilation, shock) and those without comorbidities. Accordingly, ARDS and shock were highly prevalent in our study. In fact, all of the subjects treated with ECMO had circulatory failure in addition to hypoxemic respiratory failure. This may explain why the ECMO survival rate in this study was lower than that reported in other case series of ECMO use for respiratory failure in patients with 2009 H1N1 (32–34).
Although 2009 H1N1 was the most common influenza strain in our cohort and was associated with mortality, it should be noted that our study represents a convenience sample of children admitted to selected pediatric referral centers. In addition, the enrollment strategy was adjusted midstudy to insure representation of subjects across demographic and illness severity types. There were undoubtedly critically ill children with milder 2009 H1N1 disease who were not included in our study, precluding further comment on 2009 H1N1-specific mortality risk relative to other virus strains. The relationships between S. aureus secondary bacterial infection (particularly with MRSA) and mortality were more highly significant.
The relationships between systemic cytokine levels and outcomes from pediatric influenza to date have been largely limited to survivors. For example, elevated systemic levels of IL-6, IL-12, and IFN-γ were found in a ten-patient group of critically ill children with seasonal influenza (all survivors) compared with noninfected control subjects (15). The multicenter design of our study allowed for the sampling of eight nonsurvivors. Despite a rigorous α level of 0.0016 for our cytokine/chemokine comparisons, we were able to demonstrate statistically significant elevations of serum GM-CSF, IL-6, IL-8, IP-10, MCP-1, and MIP-1α in nonsurvivors. These represent proinflammatory mediators whose primary roles are to mobilize and activate innate immune cells (GM-CSF), activate the acute phase response (IL-6), or serve as chemokines (IL-8, IP-10, MCP-1, and MIP-1α). Mediators that demonstrated a trend toward higher levels in nonsurvivors (p < 0.01) included counterregulatory, anti-inflammatory mediators such as IL-1 receptor antagonist and IL-10. These data are in agreement with the finding of hypercytokinemia in adult influenza nonsurvivors (2).
Adult serum studies and cell culture experiments have suggested that 2009 H1N1 influenza may induce a more robust cytokine response compared with seasonal influenza with elevated levels of mediators such as IL-6, IL-8, IL-10, IP-10, and growth-related oncogene (35, 36). The serum cytokine profiles of children with 2009 H1N1 influenza have been reported in several small case series in recent years although these studies have focused on influenza survivors. Kim et al (37) demonstrated higher serum levels of IL-6 and IL-10 in 26 tachypneic children with H1N1 compared with those without tachypnea, a finding reproduced in a mixed adult–pediatric study including nine children with severe 2009 H1N1 (38). Takano et al (39) showed higher serum levels of IL-1β, IL-2, IL-4, and MCP-1 in ten children with 2009 H1N1 who were mechanically ventilated compared with 11 who were not, whereas Ohta et al (40) found higher levels of IL-5 and IL-6 in 15 children with severe 2009 H1N1 compared with children with uncomplicated disease. Our data are in agreement with these findings with elevations seen in the levels of many of the same mediators in children with 2009 H1N1. These findings may not be specific to 2009 H1N1, however, given that S. aureus secondary infection was so common in our patients with 2009 H1N1 and the cytokine profile in subjects with S. aureus was so similar.
Despite the fact that only two subjects were immunocompromised at baseline, nonsurvivors demonstrated marked impairment of innate immune function. Whole blood from immunocompetent hosts should produce robust amounts of the proinflammatory cytokine TNF-α on ex vivo stimulation with LPS, a finding we observed in our outpatient control population. Activation of the Toll-like receptor pathway is used, in this context, as a readout of innate immune cell responsiveness given the fact that TNF-α is produced quickly by circulating innate immune cells (predominately monocytes) after a LPS challenge. This allows for a 4-hr incubation time, which is favorable for study logistics. This bioassay for innate immune function has been used in a number of single-center studies of adult and pediatric critical illness with lower TNF-α production capacity being associated with mortality (5, 10, 12, 13, 41, 42). This study marks the first time that this assay has been used in a multicenter fashion, highlighting the feasibility of prospective, multicenter functional immune-monitoring studies.
Our critically ill children demonstrated a reduced capacity to produce TNF-α compared with outpatient control subjects, a finding that was expected as part of the compensatory anti-inflammatory response syndrome that often follows a proinflammatory insult. However, the degree of reduction in TNF-α production capacity that we observed in nonsurvivors was severe with a level <250 pg/mL being highly predictive of mortality by receiver operating characteristic curve analysis. This threshold compares favorably with the definition of immunoparalysis (200 pg/mL using this assay) that we and others have used to identify subjects at high risk of death in unrelated patient populations (5, 13). Severe leukopenia was very common in nonsurvivors in our cohort, and this undoubtedly contributed to the development of the immunoparalyzed phenotype that we observed. Indeed, an AMC <288 cells/mm3 was strongly associated with mortality. Twenty percent of survivors, however, demonstrated severely reduced AMC counts, and ex vivo TNF-α production capacity was better able to identify those at risk for death.
The contributors to innate immune suppression associated with critical illness are poorly understood and are thought to include anti-inflammatory cytokine production (9, 42) as well as genetic and epigenetic factors (41, 43). Recent evidence suggests that negative regulation of host innate and adaptive immunity may be important for influenza pathogenesis (3, 15, 44, 45). Treatment of mice with the immunostimulatory cytokine IFN-γ in the early stages of influenza infection has been shown to be protective (18), and intact airway macrophage function is known to be important for limiting lung injury during influenza infection in mice (46). Intriguing evidence suggests that influenza infection may specifically increase susceptibility to S. aureus secondary infection in the lung (16, 17).
Subjects in our cohort with S. aureus infection demonstrated a greater degree of immune suppression than those without secondary bacterial infection or infection with a different organism. S. aureus is known to produce host immune evasion factors including those that dampen the innate and adaptive immune responses (47), but an association between S. aureus infection and the immunoparalyzed phenotype has not been previously reported. Similarly, it is unclear if the increased mortality risk associated with MRSA infection was related to MRSA-specific immunosuppression or to enhanced virulence of the isolates. We view these relationships to be highly deserving of future study.
An important aspect of this study is the demonstration of the feasibility of multicenter, provocative immune function testing. Studies of innate immune function in critical illness to date have relied on single-center reports that largely focus on monocyte HLA-DR expression and/or ex vivo TNF-α production capacity (5, 6, 8, 10, 12, 13, 41, 42, 48). Monocyte HLA-DR measurement is problematic for multicenter studies in which it requires considerable on-site processing of fresh blood specimens, and the measurements can be difficult to standardize across flow cytometers and antibody lots. Provocative testing such as ex vivo LPS-induced cytokine production profiling poses different challenges. There is currently no single industry standard reagent for ex vivo stimulation or protocol for cytokine determination. Accordingly, it is difficult to determine thresholds of immune responsiveness that are associated with clinical outcomes when stimulation protocols differ between investigators.
We elected to measure ex vivo LPS-induced TNF-α production capacity using a protocol that required only basic laboratory equipment that is likely to be available in most tertiary care medical centers. The stimulation procedure was done on-site and required minimal technician time. The LPS stimulation reagents were produced and quality controlled at a single site and shipped monthly to participating centers, ensuring a ready supply of highly standardized, fresh reagents. Lastly, the resulting supernatants were shipped back to a central laboratory where TNF-α was quantified from all supernatants on the same highly automated, good laboratory practices instrument.
The identification of potential treatment thresholds is important because mounting evidence suggests that innate immune suppression associated with critical illness may be reversible. Several small single-center clinical trials have shown that systemic therapy with GM-CSF can improve monocyte HLA-DR expression and/or ex vivo TNF-α production capacity in critically ill adults and children with immunoparalysis with improvement in clinical outcome (5, 42, 49–51). Administration of IFN-γ has shown promise in this population as well (13, 52, 53). In these studies, augmentation of innate immune function occurred without worsening systemic inflammation. Although our nonsurvivors had higher serum levels of GM-CSF than survivors, these levels (median 52 pg/mL) were far below the peak serum concentrations seen in patients undergoing GM-CSF therapy for reconstitution of bone marrow after chemotherapy, which are typically >1000 pg/mL (54). Some authors have advocated for immunosuppressive therapies in the setting of severe influenza, including glucocorticoids or peroxisome proliferator-activated receptor-γ agonists (19, 21). We suggest that patient-specific immune monitoring be incorporated into immunomodulatory trials to aid in treatment assignment. It is possible that immunostimulatory therapies such as GM-CSF targeting both leukopenia and innate immune cell responsiveness may have a role in the treatment of high-risk children with immunoparalysis associated with influenza infection.
Our study has several notable limitations. First, the patient population studied represents a convenience sample, which has been enriched for a higher severity of illness. This has allowed us to investigate the immunobiology of the highest risk subjects in our critically ill population but makes it difficult to compare the epidemiology of our cohort with that of a broader, less ill population. Second, despite the study’s multicenter design, we experienced a small number of deaths in our cohort. A larger study will be needed to more fully explore potentially important confounders including age, race, and socioeconomic status. Still, strong associations between innate immune function and mortality were seen. Also, complete blood count testing was not protocolized for this study, and 25% of survivors did not undergo white blood cell count measurement. Subsequent studies with closely timed complete blood counts and immune function measurements would be informative. It is quite likely that the interplay between innate and adaptive immune function, including relationships among helper, cytotoxic, and regulatory T cells, is important in children with influenza and should be the subject of future study as well. Indeed, mechanisms of immune suppression, including differences in cell populations and expression levels of pattern recognition receptors, were not evaluated in our study and are deserving of investigation. Lastly, this study involved sampling at only one time point. We limited our analyses to samples obtained within the first 72 hrs of ICU admission to identify early predictors of mortality. It is probable that more longitudinal measurements would yield additional information about risk factors for adverse outcomes from critical influenza.
CONCLUSIONS
Despite these limitations, our study demonstrated a strong relationship between mortality and reduced innate immune responsiveness in critically ill patients in a multicenter fashion using highly standardized reagents and measurement techniques. As such, it demonstrates the feasibility of large-scale immune monitoring of the kind that will be necessary to develop and test immunostimulatory therapies in this population. In addition, our data provide evidence that hypercytokinemia and severe innate immune suppression can coexist in children who go on to die with influenza. We also provide evidence that secondary infection with S. aureus may be associated with severe innate immune suppression in this population. We speculate that a goal of reducing systemic cytokine levels associated with severe influenza without addressing concomitant immune hyporesponsiveness may be inappropriate. Rather, research into the mechanisms and reversal of immunoparalysis, perhaps through immunostimulatory therapy in selected children, should be a high priority in future influenza seasons.
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge the input of Nicole Dowling, PhD, and Marta Gwinn, PhD, National Office of Public Health Genomics, Centers for Disease Control and Prevention, on the study design. In addition, we acknowledge the hard work and essential collaboration of the following PALISI PICFlu Study Investigators who made major contributions to this study: Children’s Hospital of Los Angeles, Los Angeles, CA (Barry Markovitz, MD, Jeff Terry, MBA); Children’s Hospital and Research Center, Oakland, CA (Heidi Flori, MD, Julie Simon, RN); Children’s Hospital of Orange County, Orange, CA (Nick Anas, MD, Stephanie Osborne, RN); Denver Children’s Hospital, Denver, CO (Angela Czaja, MD, Sandra B. Lindahl, RN); Children’s Healthcare of Atlanta at Eggleston, Atlanta, GA (Matthew Paden, MD, Rich Toney, RN); Kosair Children’s Hospital, Louisville, KY (Vicki Montgomery, MD, Kara Richardson, RN); Boston Children’s Hospital, Boston, MA (Adrienne Randolph, MD, MSc, Grace Yoon, RN, NNP, Ryan Sullivan, RN, Ana Agan, BA); Children’s Hospital and Clinics of Minnesota, Minneapolis, MN (Stephen Kurachek, MD, Erin Olson, RN, CCRP); Children’s Hospital of Nebraska, Omaha, NE (Edward Truemper, MD, Machelle Zink, BSN, MEd); Nationwide Children’s Hospital, Columbus, OH (Mark W. Hall, MD, Kristin Greathouse, BSN, MS); Penn State Children’s Hospital, Hershey, PA (Neal Thomas, MD, Jill Raymond, RN, MSN, Debra Spear, RN); Children’s Hospital of Philadelphia, Philadelphia, PA (Mark Helfaer, MD, Mary Ann DiLiberto, RN, Jillian Egan, RN); Texas Children’s Hospital, Houston, TX (Laura L. Loftis, MD, Nancy Jaimon, RN, Ursula Kyle, MS, RD/LD); Dell Children’s Medical Center, Austin, TX (Renee A. Higgerson, MD, LeeAnn Christi, RN); and University of Virginia Children’s Medical Center, Charlottesville, VA (Douglas F. Willson, MD, Christine Traul, MD).
Supported, in part, by the Centers for Disease Control and Prevention, Atlanta, GA; America’s Health Insurance Plans Foundation; the Health Respiratory Research Network of the Fonds de Recherche en Santé du Québec; the National Institutes of Health (AI084011); and the Research Institute at Nationwide Children’s Hospital. This work represents the findings of the authors and not necessarily the views of the Centers for Disease Control and Prevention or the National Institutes of Health.
Footnotes
See also p. 364.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (http://journals.lww.com/ccmjournal).
The authors have not disclosed any potential conflicts of interest.
For information regarding this article, Mark.Hall@NationwideChildrens.org
REFERENCES
- 1.Bermejo-Martin JF, Ortiz de Lejarazu R, Pumarola T, et al. Th1 and Th17 hypercytokinemia as early host response signature in severe pandemic influenza. Crit Care. 2009;13:R201. doi: 10.1186/cc8208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.To KK, Hung IF, Li IW, et al. Delayed clearance of viral load and marked cytokine activation in severe cases of pandemic H1N1 2009 influenza virus infection. Clin Infect Dis. 2010;50:850–859. doi: 10.1086/650581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bermejo-Martin JF, Martin-Loeches I, Rello J, et al. Host adaptive immunity deficiency in severe pandemic influenza. Crit Care. 2010;14:R167. doi: 10.1186/cc9259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Itoh Y, Shinya K, Kiso M, et al. In vitro and in vivo characterization of new swine-origin H1N1 influenza viruses. Nature. 2009;460:1021–1025. doi: 10.1038/nature08260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hall MW, Knatz NL, Vetterly C, et al. Immunoparalysis and nosocomial infection in children with multiple organ dysfunction syndrome. Intensive Care Med. 2011;37:525–532. doi: 10.1007/s00134-010-2088-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Volk HD, Reinke P, Krausch D, et al. Monocyte deactivation—Rationale for a new therapeutic strategy in sepsis. Intensive Care Med. 1996;22(Suppl 4):S474–S481. doi: 10.1007/BF01743727. [DOI] [PubMed] [Google Scholar]
- 7.Monneret G, Lepape A, Voirin N, et al. Persisting low monocyte human leukocyte antigen-DR expression predicts mortality in septic shock. Intensive Care Med. 2006;32:1175–1183. doi: 10.1007/s00134-006-0204-8. [DOI] [PubMed] [Google Scholar]
- 8.Ho YP, Sheen IS, Chiu CT, et al. A strong association between down-regulation of HLA-DR expression and the late mortality in patients with severe acute pancreatitis. Am J Gastroenterol. 2006;101:1117–1124. doi: 10.1111/j.1572-0241.2006.00495.x. [DOI] [PubMed] [Google Scholar]
- 9.Hall MW, Gavrilin MA, Knatz NL, et al. Monocyte mRNA phenotype and adverse outcomes from pediatric multiple organ dysfunction syndrome. Pediatr Res. 2007;62:597–603. doi: 10.1203/PDR.0b013e3181559774. [DOI] [PubMed] [Google Scholar]
- 10.Allen ML, Hoschtitzky JA, Peters MJ, et al. Interleukin-10 and its role in clinical immunoparalysis following pediatric cardiac surgery. Crit Care Med. 2006;34:2658–2665. doi: 10.1097/01.CCM.0000240243.28129.36. [DOI] [PubMed] [Google Scholar]
- 11.Bone RC. Immunologic dissonance: A continuing evolution in our understanding of the systemic inflammatory response syndrome (SIRS) and the multiple organ dysfunction syndrome (MODS) Ann Intern Med. 1996;125:680–687. doi: 10.7326/0003-4819-125-8-199610150-00009. [DOI] [PubMed] [Google Scholar]
- 12.Boomer JS, To K, Chang KC, et al. Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA. 2011;306:2594–2605. doi: 10.1001/jama.2011.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Döcke WD, Randow F, Syrbe U, et al. Monocyte deactivation in septic patients: Restoration by IFN-gamma treatment. Nat Med. 1997;3:678–681. doi: 10.1038/nm0697-678. [DOI] [PubMed] [Google Scholar]
- 14.Meisel C, Schefold JC, Pschowski R, et al. Granulocyte-macrophage colony-stimulating factor to reverse sepsis-associated immunosuppression: A double-blind, randomized, placebo-controlled multicenter trial. Am J Respir Crit Care Med. 2009;180:640–648. doi: 10.1164/rccm.200903-0363OC. [DOI] [PubMed] [Google Scholar]
- 15.Heltzer ML, Coffin SE, Maurer K, et al. Immune dysregulation in severe influenza. J Leukoc Biol. 2009;85:1036–1043. doi: 10.1189/jlb.1108710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kudva A, Scheller EV, Robinson KM, et al. Influenza A inhibits Th17-mediated host defense against bacterial pneumonia in mice. J Immunol. 2011;186:1666–1674. doi: 10.4049/jimmunol.1002194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Small CL, Shaler CR, McCormick S, et al. Influenza infection leads to increased susceptibility to subsequent bacterial superinfection by impairing NK cell responses in the lung. J Immunol. 2010;184:2048–2056. doi: 10.4049/jimmunol.0902772. [DOI] [PubMed] [Google Scholar]
- 18.Weiss ID, Wald O, Wald H, et al. IFN-gamma treatment at early stages of influenza virus infection protects mice from death in a NK cell-dependent manner. J Interferon Cytokine Res. 2010;30:439–449. doi: 10.1089/jir.2009.0084. [DOI] [PubMed] [Google Scholar]
- 19.Bassaganya-Riera J, Song R, Roberts PC, et al. PPAR-gamma activation as an anti-inflammatory therapy for respiratory virus infections. Viral Immunol. 2010;23:343–352. doi: 10.1089/vim.2010.0016. [DOI] [PubMed] [Google Scholar]
- 20.Zheng BJ, Chan KW, Lin YP, et al. Delayed antiviral plus immunomodulator treatment still reduces mortality in mice infected by high inoculum of influenza A/H5N1 virus. Proc Natl Acad Sci USA. 2008;105:8091–8096. doi: 10.1073/pnas.0711942105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee KY, Rhim JW, Kang JH. Hyperactive immune cells (T cells) may be responsible for acute lung injury in influenza virus infections: A need for early immune-modulators for severe cases. Med Hypotheses. 2011;76:64–69. doi: 10.1016/j.mehy.2010.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kroger AT, Atkinson WL, Marcuse EK, et al. Advisory Committee on Immunization Practices (ACIP) Centers for Disease Control and Prevention (CDC): General recommendations on immunization: Recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2006;55:1–48. [PubMed] [Google Scholar]
- 23.Pollack MM, Patel KM, Ruttimann UE. PRISM III: An updated Pediatric Risk of Mortality score. Crit Care Med. 1996;24:743–752. doi: 10.1097/00003246-199605000-00004. [DOI] [PubMed] [Google Scholar]
- 24.Randolph AG, Vaughn F, Sullivan R, et al. Pediatric Acute Lung Injury and Sepsis Investigator’s Network and the National Heart, Lung, and Blood Institute ARDS Clinical Trials Network: Critically ill children during the 2009–2010 influenza pandemic in the United States. Pediatrics. 2011;128:e1450–e1458. doi: 10.1542/peds.2011-0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morgan CI, Hobson MJ, Seger B, et al. 2009 pandemic influenza A (H1N1) in critically ill children in Cincinnati, Ohio. Pediatr Crit Care Med. 2012;13:e140–e144. doi: 10.1097/PCC.0b013e318228845f. [DOI] [PubMed] [Google Scholar]
- 26.Cox CM, Blanton L, Dhara R, et al. 2009 Pandemic influenza A (H1N1) deaths among children—United States, 2009–2010. Clin Infect Dis. 2011;52(Suppl 1):S69–S74. doi: 10.1093/cid/ciq011. [DOI] [PubMed] [Google Scholar]
- 27.Tamma PD, Turnbull AE, Milstone AM, et al. Clinical outcomes of seasonal influenza and pandemic influenza A (H1N1) in pediatric inpatients. BMC Pediatr. 2010;10:72. doi: 10.1186/1471-2431-10-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bagdure D, Curtis DJ, Dobyns E, et al. Hospitalized children with 2009 pandemic influenza A (H1N1): Comparison to seasonal influenza and risk factors for admission to the ICU. PLoS One. 2010;5:e15173. doi: 10.1371/journal.pone.0015173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Farias JA, Fernández A, Monteverde E, et al. Critically ill infants and children with influenza A (H1N1) in pediatric intensive care units in Argentina. Intensive Care Med. 2010;36:1015–1022. doi: 10.1007/s00134-010-1853-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jouvet P, Hutchison J, Pinto R, et al. Canadian Critical Care Trials Group H1N1 Collaborative: Critical illness in children with influenza A/pH1N1 2009 infection in Canada. Pediatr Crit Care Med. 2010;11:603–609. doi: 10.1097/PCC.0b013e3181d9c80b. [DOI] [PubMed] [Google Scholar]
- 31.Kendirli T, Demirkol D, Yildizdas D, et al. Critically ill children with pandemic influenza (H1N1) in pediatric intensive care units in Turkey. Pediatr Crit Care Med. 2012;13:e11–e17. doi: 10.1097/PCC.0b013e31820aba37. [DOI] [PubMed] [Google Scholar]
- 32.Freed DH, Henzler D, White CW, et al. Canadian Critical Care Trials Group: Extracorporeal lung support for patients who had severe respiratory failure secondary to influenza A (H1N1) 2009 infection in Canada. Can J Anaesth. 2010;57:240–247. doi: 10.1007/s12630-009-9253-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Turner DA, Rehder KJ, Peterson-Carmichael SL, et al. Extracorporeal membrane oxygenation for severe refractory respiratory failure secondary to 2009 H1N1 influenza A. Respir Care. 2011;56:941–946. doi: 10.4187/respcare.01066. [DOI] [PubMed] [Google Scholar]
- 34.Davies A, Jones D, Bailey M, et al. Australia and New Zealand Extracorporeal Membrane Oxygenation (ANZ ECMO) Influenza Investigators: Extracorporeal membrane oxygenation for 2009 influenza A (H1N1) acute respiratory distress syndrome. JAMA. 2009;302:1888–1895. doi: 10.1001/jama.2009.1535. [DOI] [PubMed] [Google Scholar]
- 35.Lee N, Wong CK, Chan PK, et al. Cytokine response patterns in severe pandemic 2009 H1N1 and seasonal influenza among hospitalized adults. PLoS One. 2011;6:e26050. doi: 10.1371/journal.pone.0026050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Song BM, Kang YM, Kim HS, et al. Induction of inflammatory cytokines and Toll-like receptors in human normal respiratory epithelial cells infected with seasonal H1N1, 2009 pandemic H1N1, seasonal H3N2, and highly pathogenic H5N1 influenza virus. Viral Immunol. 2011;24:179–187. doi: 10.1089/vim.2010.0125. [DOI] [PubMed] [Google Scholar]
- 37.Kim YH, Kim JE, Hyun MC. Cytokine response in pediatric patients with pandemic influenza H1N1 2009 virus infection and pneumonia: Comparison with pediatric pneumonia without H1N1 2009 infection. Pediatr Pulmonol. 2011;46:1233–1239. doi: 10.1002/ppul.21496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yu X, Zhang X, Zhao B, et al. Intensive cytokine induction in pandemic H1N1 influenza virus infection accompanied by robust production of IL-10 and IL-6. PLoS One. 2011;6:e28680. doi: 10.1371/journal.pone.0028680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takano T, Tajiri H, Kashiwagi Y, et al. Cytokine and chemokine response in children with the 2009 pandemic influenza A (H1N1) virus infection. Eur J Clin Microbiol Infect Dis. 2011;30:117–120. doi: 10.1007/s10096-010-1041-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ohta R, Torii Y, Imai M, et al. Serum concentrations of complement anaphylatoxins and proinflammatory mediators in patients with 2009 H1N1 influenza. Microbiol Immunol. 2011;55:191–198. doi: 10.1111/j.1348-0421.2011.00309.x. [DOI] [PubMed] [Google Scholar]
- 41.Cornell TT, Sun L, Hall MW, et al. Clinical implications and molecular mechanisms of immunoparalysis after cardiopulmonary bypass. J Thorac Cardiovasc Surg. 2012;143:1160–1166. e1. doi: 10.1016/j.jtcvs.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fumeaux T, Pugin J. Role of interleukin-10 in the intracellular sequestration of human leukocyte antigen-DR in monocytes during septic shock. Am J Respir Crit Care Med. 2002;166:1475–1482. doi: 10.1164/rccm.200203-217OC. [DOI] [PubMed] [Google Scholar]
- 43.Westendorp RG, Langermans JA, Huizinga TW, et al. Genetic influence on cytokine production in meningococcal disease. Lancet. 1997;349:1912–1913. doi: 10.1016/s0140-6736(05)63910-4. [DOI] [PubMed] [Google Scholar]
- 44.van der Sluijs KF, van Elden LJ, Nijhuis M, et al. IL-10 is an important mediator of the enhanced susceptibility to pneumococcal pneumonia after influenza infection. J Immunol. 2004;172:7603–7609. doi: 10.4049/jimmunol.172.12.7603. [DOI] [PubMed] [Google Scholar]
- 45.Raftogiannis M, Antonopoulou A, Baziaka F, et al. Indication for a role of regulatory T cells for the advent of influenza A (H1N1)-related pneumonia. Clin Exp Immunol. 2010;161:576–583. doi: 10.1111/j.1365-2249.2010.04208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tate MD, Pickett DL, van Rooijen N, et al. Critical role of airway macrophages in modulating disease severity during influenza virus infection of mice. J Virol. 2010;84:7569–7580. doi: 10.1128/JVI.00291-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim HK, Thammavongsa V, Schneewind O, et al. Recurrent infections and immune evasion strategies of Staphylococcus aureus>. Curr Opin Microbiol. 2012;15:92–99. doi: 10.1016/j.mib.2011.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ploder M, Pelinka L, Schmuckenschlager C, et al. Lipopolysaccharide-induced tumor necrosis factor alpha production and not monocyte human leukocyte antigen-DR expression is correlated with survival in septic trauma patients. Shock. 2006;25:129–134. doi: 10.1097/01.shk.0000191379.62897.1d. [DOI] [PubMed] [Google Scholar]
- 49.Nierhaus A, Montag B, Timmler N, et al. Reversal of immunoparalysis by recombinant human granulocyte-macrophage colony-stimulating factor in patients with severe sepsis. Intensive Care Med. 2003;29:646–651. doi: 10.1007/s00134-003-1666-6. [DOI] [PubMed] [Google Scholar]
- 50.Presneill JJ, Harris T, Stewart AG, et al. A randomized phase II trial of granulocyte-macrophage colony-stimulating factor therapy in severe sepsis with respiratory dysfunction. Am J Respir Crit Care Med. 2002;166:138–143. doi: 10.1164/rccm.2009005. [DOI] [PubMed] [Google Scholar]
- 51.Rosenbloom AJ, Linden PK, Dorrance A, et al. Effect of granulocyte-monocyte colony-stimulating factor therapy on leukocyte function and clearance of serious infection in nonneutropenic patients. Chest. 2005;127:2139–2150. doi: 10.1378/chest.127.6.2139. [DOI] [PubMed] [Google Scholar]
- 52.Kox WJ, Bone RC, Krausch D, et al. Interferon gamma-1b in the treatment of compensatory anti-inflammatory response syndrome. A new approach: Proof of principle. Arch Intern Med. 1997;157:389–393. [PubMed] [Google Scholar]
- 53.Nakos G, Malamou-Mitsi VD, Lachana A, et al. Immunoparalysis in patients with severe trauma and the effect of inhaled interferon-gamma. Crit Care Med. 2002;30:1488–1494. doi: 10.1097/00003246-200207000-00015. [DOI] [PubMed] [Google Scholar]
- 54.Liljefors M, Nilsson B, Mellstedt H, et al. Influence of varying doses of granulocyte-macrophage colony-stimulating factor on pharmacokinetics and antibody-dependent cellular cytotoxicity. Cancer Immunol Immunother. 2008;57:379–388. doi: 10.1007/s00262-007-0377-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.