Abstract
Human cytomegalovirus (HCMV) is a common agent of congenital infection and causes severe disease in immunocompromised patients. Current approved therapies focus on inhibiting viral DNA replication. The HCMV kinase pUL97 contributes to multiple stages of viral infection including DNA replication, controlling the cell cycle, and virion maturation. Our studies demonstrate that pUL97 also functions by influencing immediate early (IE) gene expression during the initial stages of infection. Inhibition of kinase activity using the antiviral compound maribavir or deletion of the UL97 gene resulted in decreased expression of viral immediate early genes during infection. Expression of pUL97 was sufficient to transactivate IE1 gene expression from the viral genome, which was dependent on viral kinase activity. We observed that pUL97 associates with histone deacetylase 1 (HDAC1). HDAC1 is a transcriptional corepressor that acts to silence expression of viral genes. We observed that inhibition or deletion of pUL97 kinase resulted in increased HDAC1 and decreased histone H3 lysine 9 acetylation associating with the viral major immediate early (MIE) promoter. IE expression during pUL97 inhibition or deletion was rescued following inhibition of deacetylase activity. HDAC1 associates with chromatin by protein-protein interactions. Expression of active but not inactive pUL97 kinase decreased HDAC1 interaction with the transcriptional repressor protein DAXX. Finally, using mass spectrometry, we found that HDAC1 is uniquely phosphorylated upon expression of pUL97. Our results support the conclusion that HCMV pUL97 kinase regulates viral immediate early gene expression by phosphorylation-mediated disruption of HDAC1 binding to the MIE promoter.
INTRODUCTION
Human cytomegalovirus (HCMV) is a betaherpesvirus that establishes lifelong infections in its hosts. Similar to other human herpesviruses, it is ubiquitous, with the majority of the world's population being seropositive (1). HCMV is an opportunistic pathogen that causes a range of diseases in immunocompromised patients and is an agent of common congenital infection. Current approved treatments include pharmaceutical compounds that are efficacious but demonstrate high toxicity, limiting their use in patients. Additionally, HCMV can develop resistance to the antiviral compounds (2). For these reasons, identifying new treatment options that are both safe and effective is necessary.
The HCMV kinase pUL97 is a serine/threonine-specific kinase that phosphorylates the antiviral nucleoside ganciclovir. This modification is necessary for activating ganciclovir's antiviral activity (3, 4). pUL97 is a tegument protein delivered to infected cells, and newly expressed pUL97 protein begins increasing around 5 hours postinfection (hpi) (5, 6). The kinase exists in multiple isoforms, which have distinct expression patterns within the cell (7, 8). Deletion or inactivation of the kinase results in an ∼6-fold decrease in viral DNA accumulation and up to 100-fold decrease in viral yield (9, 10). pUL97 phosphorylates viral proteins, such as pUL44 and pUL83 (pp65), and host proteins including retinoblastoma protein (pRB), RNA polymerase II, elongation factor delta, lamin A/C, and lamin-associated protein p32 (6, 11–18). Phosphorylation of pRB by pUL97 stimulates cell cycle progression at early times during infection (13, 19, 20). In the absence of pUL97 during infection, aggregates of promyelocytic nuclear body (PML-NB)-associated viral and cellular proteins form in the nucleus (9, 18, 20, 21). The kinase is thought to reduce aggregation by disrupting PML-NBs and phosphorylating viral proteins (18, 21, 22). Furthermore, cellular lamin-associated protein p32 recruits pUL97 to nuclear lamina, promoting lamina disassembly and viral nucleocapsid egress (12, 16). Finally, in a pUL97-deficient infection, cytoplasmic viral assembly compartments do not form correctly and noninfectious viral particles accumulate (23, 24). Several of these activities have resulted in HCMV pUL97 being identified as a viral cyclin-dependent kinase (v-CDK) (12, 13). v-CDK proteins are conserved among herpesviruses and phosphorylate diverse targets (20).
Our previous mass spectrometry screen for proteins that associate with histone deacetylase 1 (HDAC1) during HCMV infection identified peptides corresponding to pUL97 (25). HDACs are enzymes that remove an acetyl group from lysine residues of histone and nonhistone proteins. HDAC1 is a class I HDAC, and protein complexes recruit and regulate class I HDAC-mediated changes in order to control transcriptional repression (reviewed in reference 26). During infection, histones rapidly become associated with the HCMV genome upon entry into the nucleus (27–29). Initially, histone acetylation is low at viral promoters (27–29). As infection progresses, changes in histone acetylation at promoters is associated with changes in transcription of viral genes, beginning with immediate early (IE) promoters, including the major immediate early (MIE) promoter (29). HDAC1 along with the Ets-2 transcription factor has been shown to repress the MIE promoter (30). Furthermore, chemical inhibition of HDACs results in alterations in the histone modification patterns and increases in viral gene expression (28, 30, 31).
Several HCMV proteins function by releasing cell-mediated inhibition of viral gene expression. The viral protein pUL82 (pp71) degrades the PML-NB protein DAXX, and this event contributes toward the initiation of immediate early gene expression (32). DAXX interacts with HDAC1, and together they repress transcription (33). Also, the HCMV pUL123 (IE1) protein inhibits histone deacetylation (34) and, along with pUL122 (IE2), has been shown to affect the repressive actions of HDAC1, 2, and 3 (34–37). Other HCMV proteins interact with HDACs or HDAC-containing complexes, including pUL29/28, which associates with the nucleosome remodeling and deacetylase complex, NuRD, influencing both viral (25) and cellular gene expression (38). In general, regulation of deacetylation is a central event during infection by other herpesviruses. Infection by members of the alphaherpesvirus family such as varicella zoster virus (VZV), herpes simplex virus 1 (HSV-1), and pseudorabies virus (PRV) induces increased phosphorylation of HDAC1 and HDAC2 (39–41). Expression of VZV open reading frame 66 (ORF66), HSV-1 US3, and PRV US3 kinases in cells is sufficient to induce HDAC1 and/or HDAC2 phosphorylation (39–41). In an ORF66 mutant viral infection, treatment with the class I HDAC inhibitor, sodium butyrate, increases immediate early protein expression at late times, plaque sizes, and viral yields (40). Class I HDAC inhibitors also increase the plaquing efficiency of a PRV kinase-deficient virus but not HSV-1 (41).
Within this work, we demonstrate that the HCMV kinase pUL97 can also phosphorylate HDAC1 and influence viral protein expression. Going beyond previous studies, we show that pUL97 kinase activity is necessary and sufficient to transactivate the HCMV MIE promoter in the context of the viral genome and regulate immediate early RNA expression during infection. We observed that the activity occurred during the onset of infection and was maintained in the presence of cycloheximide, suggesting a role for tegument-delivered pUL97. Furthermore, we observed that either deletion of the kinase or inhibition of pUL97 kinase activity decreased acetylation of histone H3 while increasing HDAC1 protein association with the MIE promoter. Our studies provide mechanistic insight into the functional impact of HCMV kinase-mediated phosphorylation of HDAC1.
MATERIALS AND METHODS
Biological reagents.
The wild-type HCMV strain AD169 (ADwt) was obtained from the AD169 bacterial artificial chromosome (BAC) clone (42). A UL97 substitution mutant, ADdel97 (del97), was constructed using BAC recombineering (43, 44). The UL97 gene was replaced by inserting the Escherichia coli galK gene into the UL97 open reading frame of the AD169 wild-type BAC clone (forward primer, 5′-TCGGTGTGGTAGCTAGTGCAGCCTTAGGAACAGGGAAGACTGTCGCCACTCCTGTTGACAATTAATCATCGGCA-3′, and reverse, 5′-AGCCGCATGAGGCAAAAGCCCAGCACGTTACCCAGCGCCGACAGCTCCGATCAGCACTGTCCTGCTCCTT-3′) (Integrated DNA Technologies, Inc., Coralville, IA). AD169 viral stocks were prepared by infecting primary human fibroblasts (HFF) at a low multiplicity of infection (<0.1 PFU/ml). ADdel97 and matched AD169 stocks were prepared by cotransfecting BAC DNA with the pCGN-pUL82HA expression vector. To concentrate viral stocks, culture medium and cleared cell lysate were pelleted through a sorbitol cushion (20% d-sorbitol, 50 mM Tris-HCl [pH 7.2], 1 mM MgCl2) at 55,000 × g for 1 h. Titers were defined as infectious units (IU) per ml and determined by using serial dilutions of viral stocks to infect HFFs in 12-well dishes, fixing cells at 48 hpi, staining with an antibody against pUL123 (IE1), and counting the UL123-positive cells per well. To validate viral titers, we infected HFFs with ADwt, ADwt plus 40 μM maribavir (MBV), or ADdel97 at 0.01 IU/ml, fixed them at 48 hpi, and stained them for pUL123-positive cells.
HFFs, MRC-5 lung fibroblast cells, U-2 OS osteosarcoma cells, and U373 astrocytoma cells were propagated in Dulbecco's modified Eagle medium (DMEM) (Life Technologies, Carlsbad, CA) supplemented with 7% fetal bovine serum (FBS) (Life Technologies) and 1% penicillin-streptomycin (Life Technologies). ADdel97 and matched AD169 viral stocks were propagated in DMEM supplemented with 15% FBS and 1% penicillin-streptomycin following transfection. All infections were completed using cells grown to ∼90% confluence and serum starved (0.5% serum) for 24 to 48 h.
Cells were infected at a multiplicity of infection of 1 IU per cell in DMEM containing 7% FBS and 1% penicillin-streptomycin and exposed to the viral inoculum for 2 h, unless otherwise noted. Compounds were added to the culture medium at the start of infection and reapplied after removing the inoculum. Cells were treated with 40 μM maribavir (Viropharma, Exton, PA), 300 nM trichostatin A (Sigma, St. Louis, MO), 100 μg/ml cycloheximide (MP Biomedicals, Santa Ana, CA), or dimethyl sulfoxide (DMSO) as a vehicle (Sigma). Electroporation of plasmid or BAC DNA was carried out in DMEM at 260 mV for 23 ms in a 4-mm electroporation cuvette. Cells were transfected using Fugene 6 (Roche, Indianapolis, IN) following the manufacturer's protocol. The pCGN-UL82HA, pCGN-UL97HA, and pCGN-UL97K355MHA plasmids were generously provided by Robert Kalejta (University of Wisconsin—Madison) (20). Vector controls were performed using a pCGN vector. The pBJ5-HDAC1Flag plasmid was graciously provided by Edward Seto (45). Point mutations of the pBJ5-HDAC1Flag were created using the Change-IT Multiple Mutation site-directed mutagenesis kit (Affymetrix, Santa Clara, CA). The following primers were used to generate the mutations: T208A, 5′-phospho-AGAGTACTTCCCAGGTGCGGGTGACCTACGGGATATC-3′; T208E, 5′-phospho-AGAGTACTTCCCAGGTGAGGGTGACCTACGGGATATC-3′; and T208D, 5′-phospho-AGAGTACTTCCCAGGTGACGGTGACCTACGGGATATC-3′ with the reverse primer 5′-phospho-CCAATGCTGAGGAGATGACCAAGTACCACAGC-3′. (Underlining identifies the locations of nucleotide substitutions.) E. coli was transformed with the generated plasmids, and transformants were screened by sequencing using an ABI 3500 Genetic Analyzer (ABI, Oyster Bay, NY).
Antibodies used for Western blot (WB), immunoprecipitation (IP), chromatin immunoprecipitation (ChIP), or immunofluorescence analysis (IF) include mouse anti-FLAG M2 (WB, IP, and IF) (Sigma), mouse anti-HDAC1 2E10 (WB and ChIP) (EMD Millipore, Billerica, MA), mouse anti-GAPDH (glyceraldehyde-3-phosphate dehydrogenase) clone 0411 (WB) (Santa Cruz Biotechnology, Santa Cruz, CA), mouse anti-acetyl-histone H3 Lys 9 (ChIP) (EMD Millipore), mouse anti-HA clone HA-7 (WB and IP) (Sigma-Aldrich), and mouse control IgG (Santa Cruz Biotechnology, Santa Cruz, CA). The antibodies against HCMV proteins were mouse anti-pUL123 clone 1B12 (WB and IF), mouse anti-pUL122 clone 3A9 (WB), mouse anti-pUL82 clone 2H10-9 (WB and IF), mouse anti-pUL83 clone 8F5 (WB and IF), mouse anti-pUL99 clone 10B4-29 (WB), and mouse anti-pUL38 clone 3D12 (WB) (46, 47), a gift from T. Shenk (Princeton University), mouse anti-pUL85 (WB), a gift from W. Gibson (The Johns Hopkins University School of Medicine), and mouse anti-pUL44 (Virusys, Taneytown, MD). Secondary antibodies for Western blot analysis were goat anti-mouse horseradish peroxidase (HRP) and donkey anti-rabbit HRP (Jackson ImmunoResearch, West Grove, PA), and for immunofluorescence donkey anti-mouse IgG (H+L) Alexa Fluor 488 (Life Technologies) was used.
Analysis of protein and RNA.
Western blot analysis of steady-state protein levels were performed on cells lysed by sonication in a radioimmunoprecipitation assay (RIPA) lysis buffer (50 mM Tris-Cl [pH 8.0], 150 mM NaCl, 0.5% NP-40). Protein from lysates were resolved by sodium dodecyl sulfate 8 or 10% polyacrylamide gel electrophoresis (SDS-PAGE) and transferred by semidry transfer to Protran nitrocellulose membrane (Whatman Inc., Piscataway, NJ). Blocking was completed in 5% milk in phosphate-buffered saline (PBS) containing 0.1% Tween 20 (PBS-T). Membranes were incubated in primary antibody followed by a secondary antibody conjugated to HRP, both diluted in 5% milk in PBS-T. Antibodies were detected with ECL (GE Healthcare, Piscataway, NJ) and film or FluorChem HD2 (ProteinSimple, Santa Clara, CA) for protein quantification. Immunoprecipitation experiments were initiated by lysing cells using sonication in a RIPA lysis buffer and using Dynabeads Protein G (Life Technologies). Lysates were spun for 2 min at ∼15,000 × g, and supernatants were precleared for 30 min at 4°C with beads. Following two washes with lysis buffer, beads were preconjugated to antibody for 30 min at room temperature and then washed twice with lysis buffer. Samples were incubated with antibody for 3 h at 4°C with rotation. The beads were washed five times with lysis buffer and resuspended in Laemmli sample buffer. The immunoprecipitated samples and lysate controls were evaluated by Western blotting. All buffers used for lysis or washes contained Complete Mini EDTA-free Protease Inhibitor Cocktail (Roche) and PhosStop (Roche).
Cellular and viral RNA was quantified using quantitative reverse transcriptase PCR (qRT-PCR). RNA was collected from samples using TRIzol Reagent (Life Technologies), and cDNA was synthesized using 2 μg of DNase-treated RNA, random hexamers, and SuperScript III reverse transcriptase (Life Technologies). qPCR of cDNA was performed using the following primers: UL123 (5′-GCCTTCCCTAAGACCACCAAT-3′ and 5′-ATTTTCTGGGCATAAGCCATAATC-3′), UL38 (5′-ACGGTGCTATCGTGCTGGAGTATT-3′ and 5′-AAGACCATCACCAGGTCGTCCATA-3′), UL44 (5′-GCCCGATTTCAATATGGAGTTCAG-3′ and 5′-CGGCCGAATTCTCGCTTTC-3′), UL54 (5′-CACCAAAGACACGTCGTT-3′ and 5′-GTCCTTTGCGACCAGAAT-3′), UL97 (5′-GACGCCGTCTAACACGTATACC-3′ and 5′-GGTTCCTACCTTCTCTGTTGCC-3′), and GAPDH (5′-ACCCACTCCTCCACCTTTGAC-3′ and 5′-CTGTTGCTGTAGCCAAATTCGT-3′). Quantification was accomplished by using FastStart Universal SYBR green Master (Roche) and the 7900HT Fast Real-Time PCR system. Relative abundance was determined using a standard curve consisting of 10-fold serial dilutions of one sample.
Cell cycle analysis.
For cell cycle analysis, HFFs were grown to ∼80% confluence in DMEM with 7% FBS. Cells were then serum starved in DMEM with 0.5% FBS and 40 μM MBV or DMSO for 48 h. Subsequently, cells were released from starvation into DMEM with 7% FBS and either MBV or DMSO. After 24 h postrelease, cells were harvested and fixed with 70% ethanol, and DNA content was analyzed by treating cells with Cell Cycle Reagent (EMD Millipore) for 30 min. Cell cycle analysis was performed on a Guava EasyCyte Mini flow cytometer (EMD Millipore). Flow cytometry data were analyzed using FlowJo Analysis Software (FlowJo, Ashland, OR). Flow cytometry data were analyzed and gated identically using FlowJo Analysis Software (FlowJo, Ashland, OR).
Cell doubling was performed from HFFs following release from serum starvation. Cells (7.5 × 104) were plated onto 6-well dishes in DMEM with 7% FBS. Following attachment of cells to the plate, serum starvation was performed in DMEM with 0.5% FBS. After 48 h, cells were released from starvation into DMEM with 7% FBS containing either DMSO or 40 μM MBV. To perform counting, cells were collected by dissociating into 250 μl of 1% trypsin (Life Technologies, Carlsbad, CA) and counted on a hemocytometer (Hausser Scientific, Horsham, PA) using a Nikon Eclipse TS100 inverted microscope set at the 10× objective lens (Nikon Inc., Melville, NY). Each biological sample was counted in technical replicate, and results were averaged.
Analysis of transactivation and viral entry.
For transactivation assays, 2.5 μg ADdel97 BAC DNA was cotransfected with a pCGN expression vector into 1.5 × 105 MRC5 cells using electroporation or U373 cells using Fugene 6. Cells were plated onto 12-well dishes. At 7 days postinfection (dpi) (MRC5 cells) or 48 hpi (U373 cells), cells were fixed using 4% paraformaldehyde, permeabilized using 100% methanol, and blocked using 3% bovine serum albumin (BSA) dissolved in PBS-T. Cells were then incubated for 1 h with mouse anti-pUL123 (1:500 dilution in 3% BSA PBS-T). Cells were washed three times with PBS-T, incubated for 1 h with anti-mouse IgG Alexa 488 (1:1,000 dilution in 3% BSA PBS-T), and then washed three times with PBS-T. Finally, 3% SlowFade Gold Antifade Reagent with DAPI (4′,6-diamidino-2-phenylindole) (Life Technologies) in PBS-T was added to the wells. pUL123-positive cells in each transfection were counted using a Nikon Eclipse TS100 inverted microscope set at the 20× objective lens (Nikon Inc., Melville, NY). Following quantification, cells were prepared for images of pUL123 and hemagglutinin (HA) double-positive cells by incubating cells for 1 h with mouse anti-HA conjugated Zenon Alexa Fluor 568. Conjugation was performed using the Zenon Mouse IgG1 Labeling kit (Life Technologies) following the manufacturer's protocol. To assess entry, HFFs were infected at 1 IU/cell for 1 h, after which time they were fixed and permeabilized as described above. Fixed cells were incubated for 1.5 h with antibody against pUL83 (1:500 dilution in 3% BSA PBS-T). Cells were washed with PBS-T and then incubated with anti-mouse IgG Alexa 488 (1:1,000 dilution in 3% BSA PBS-T) for 1 h. For images containing DAPI, SlowFade with DAPI was added to the cells for 20 min and then washed with PBS-T before imaging using a 40× objective lens (Nikon Inc., Melville, NY).
Chromatin immunoprecipitation assay.
HFFs (5 × 106) were plated onto one 150-mm dish as described in “Biological reagents” above. Cells were fixed and protein-chromatin complexes cross-linked in 1% ChIP grade formaldehyde (Thermo Fisher Scientific, Waltham, MA) for 10 min and neutralized with 125 mM glycine for 5 min. Three washes with ice-cold phosphate-buffered saline were performed before cells were scraped, collected, and lysed in 500 μl SDS lysis buffer (1% sodium dodecyl sulfate, 10 mM EDTA, 50 mM Tris-HCl [pH 8.1]) containing Complete Mini EDTA-free Protease Inhibitor Cocktail (Roche). Lysates were sonicated to ∼1 kbp using a microtip sonicator. Lysates were centrifuged at 18,000 × g at 4°C for 10 min to remove insoluble material. A fraction of supernatant was kept for the input measurement and to test shearing efficiency. The supernatant was precleared with 10 μl Dynabeads Protein G (Life Technologies) without antibody.
The supernatant was diluted with 5 volumes ChIP Dilution buffer (0.01% SDS, 1.1% Triton X-100, 1.2 mM EDTA, 1.67 M Tris-HCl [pH 8.1], 176 mM NaCl). Supernatant was combined with 10 μg of antibody and incubated overnight at 4°C with rotation. Antibody complexes were precipitated with 60 μl Dynabeads Protein G for 2 h with rotation at 4°C. The bound complexes were collected by magnet and washed once with low-salt buffer (0.1% SDS, 1% Triton X-100, 2 mM EDTA, 20 mM Tris-HCl [pH 8.1], 150 mM NaCl), once with high-salt buffer (0.1% SDS, 1% Triton X-100, 2 mM EDTA, 20 mM Tris-HCl [pH 8.1], 500 mM NaCl), once with LiCl wash (250 mM LiCl, 1% IGEPAL-CA630 [octylphenoxypolyethoxyethanol], 1% Na deoxycholate, 1 mM EDTA), and twice with TE buffer (10 mM Tris-HCl [pH 8.1], 1 mM EDTA). Complexes were eluted from beads twice in 250 μl elution buffer (40 mM NaHCO3, 1% SDS, 10 mM dithiothreitol [DTT]) at room temperature for 15 min. The beads were removed, and the eluate was reverse cross-linked using NaCl (final concentration of 4%, wt/vol) at 65°C for 12 h. Eluted proteins were digested in 10 mM EDTA and 40 mM Tris-HCl (pH 6.5) using 40 μg proteinase K (Roche) at 45°C for 1 h. DNA was isolated using the Gel/PCR DNA Fragment Extraction kit (IBI Scientific, Peosta, IA) per manufacturer protocols and eluted using 30 μl Milli-Q filtered H2O (Millipore). Viral and cellular DNA levels were determined using quantitative PCR with sequence specific primers, as described above. PCR primers used were against the major immediate early promoter (MIEP) (5′-CTTACGGGACTTTCCTACTTG-3′ and 5′-CGATCTGACGGTTCACTAA-3′) or beta-actin (5′-CATTGCCGACAGGATGCA-3′ and 5′-GCCGATCCACACGGAGTACT-3′).
HDAC activity assays.
For HDAC activity assays, immunoprecipitation was performed as stated above with the following modifications. Cells were lysed in Triton X-100 lysis buffer (20 mM HEPES-K, 100 mM KOAc, 2 mM MgCl2, 0.1% Tween 20, 1 μM ZnCl2, 1 μM CaCl2, 0.5% Triton X-100, 500 mM NaCl). Following washes, the bead-antibody-protein complex or lysate was subjected to the HDAC Fluor-de-Lys Activity assay according to the manufacturer's protocol (Enzo, Plymouth Meeting, PA). Fluorescence was analyzed on a Synergy2 plate reader (BioTek, Winooski, VT) per HDAC Fluor-de-Lys Activity assay manufacturer recommendations at an excitation wavelength of 485 nm, emission wavelength of 528 nm, and gain of 50. Protein was eluted from beads in Laemmli sample buffer. Lysates and immunoprecipitated sample were analyzed by Western blotting and FluorChem HD2 (Proteinsimple) for protein quantification.
Phosphopeptide enrichment and mass spectrometry.
Mass spectrometry experiments were initiated by transfecting U-2 OS cells using Fugene 6 as described above. Immunoprecipitation was then performed using the protocol outlined in the HDAC Activity assay methods. Following immunoprecipitation, protein was eluted from beads with 0.4 M NH4OH at 25°C for 20 min with agitation. Disulfide bonds were reduced in 10 mM DTT at 25°C for 30 min, and free cysteines were alkylated in 10 mM iodoacetamide at 25°C for 30 min. Protein was digested with 1 μg of Trypsin Gold, mass spectrometry grade (Promega, Madison, WI) at 37°C for 12 to 15 h. Digestion was stopped by trifluoric acid added to a final concentration of 0.1%. The sample was enriched for phosphopeptides using PHOS-select Iron Affinity Gel (Sigma) according to the manufacturer's protocol. Supernatant and washes from the PHOS-select Iron Affinity Gel phosphopeptide enrichment were saved and further enriched for monophosphorylated peptides using Proteo Extract Phosphopeptide Enrichment TiO2 (Calbiochem, Gibbstown, NJ) according to the manufacturer's protocol. The eluates from the PHOS-select Iron Affinity Gel and Calbiochem Proteo Extract Phosphopeptide Enrichment TiO2 enrichments were combined and cleaned using ZipTip C18 (Millipore) according to the manufacturer's protocol.
Mass spectral analysis was performed on an LTQ-Orbitrap Velos mass spectrometer (Thermo Scientific, Waltham, MA, USA) coupled with a NanoAquity UPLC (ultraperformance liquid chromatographer) (Waters Corporation) equipped with an autosampler and interfaced with a nanoelectrospray ion source. Peptide separation took place on a 5-μm C18 nanocolumn (50 μm inner diameter [i.d.]] by 150 mm). The fused silica capillaries (Polymicro Technologies, Phoenix, AZ, USA) for the columns were pulled by a micropipette puller P-2000 (Sutter Instrument Company, CA, USA) and packed with C18 resin using a bomb-loader. The solvents A and B used for chromatographic separation of peptides were 2% acetonitrile in 0.1% formic acid and 98% acetonitrile in 0.1% formic acid, respectively. The peptides injected onto the microcapillary column were resolved at the rate of 200 nl/min, using the following gradient conditions: 0 to 10 min, 2 to 20% B; 120 min, 40% B; 135 min, 60% B; 150 min, 70% B; 158 min, 98%B, which was held for 4 min and then switched to 98% A and held for another 18 min. The ions eluted from the column were electrosprayed at a voltage of 1.75 kV. The ion transfer temperature was kept at 250°C. No auxiliary or sheath gas was used.
Survey full-scan mass spectrometry (MS) spectra (m/z, 300 to 2,000) were recorded in the Orbitrap analyzer at a resolution of 30,000 followed by tandem mass spectrometry (MS/MS) of the 10 most intense peptide ions in the linear ion trap analyzer. Neutral loss-triggered multistage activation for simultaneous fragmentation of neutral loss product and precursor was enabled at m/z of −98, −80, −49, 40, −32.6, and −26.7 relative to the precursor ion, corresponding to a neutral loss of phosphate moiety from +1, +2, and +3 charged ions. The chromatographic and mass spectral functions were controlled using the Xcalibur data system (ThermoFinnigan, Palo Alto, CA, USA). Peptides were identified using the Mascot and SEQUEST search algorithm for combined human cytomegalovirus and human protein database at a global false-discovery rate of 5%. The resulting files were evaluated using Visualize (48).
RESULTS
Inhibition of the HCMV pUL97 kinase disrupts immediate early and early gene expression.
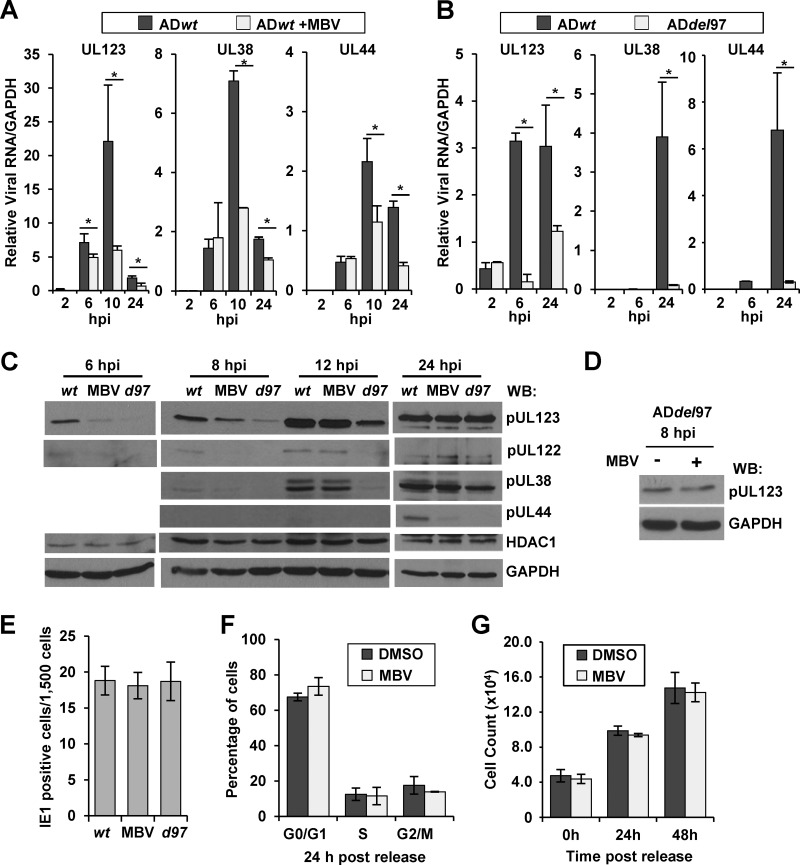
HCMV pUL97 acts at multiple stages during viral replication (49). To determine whether pUL97 could influence viral gene expression, we completed infections in the presence of the pUL97 kinase inhibitor, maribavir (MBV) (50). MBV is in clinical trials and functions as an ATP-competitive UL97 kinase inhibitor (51, 52). For these studies, primary human fibroblasts were infected at a multiplicity of 1 infectious unit (IU) per cell using HCMV strain AD169 (ADwt) in the presence or absence of 40 μM MBV (53). At the indicated times, we determined changes in viral gene expression using quantitative RT-PCR and normalized expression to cellular GAPDH RNA (Fig. 1A). The addition of MBV at the time of infection resulted in a significant decrease in expression of the immediate early gene UL123, with an average decrease of 1.4-fold at 6 hpi, 3.7-fold at 10 hpi, and 2.7-fold at 24 hpi (Fig. 1A). We also evaluated expression of two early HCMV genes, UL38 and UL44. Expression of UL38 decreased 2.5-fold at 10 hpi and 1.7-fold at 24 hpi, while UL44 decreased 2-fold at 10 hpi and 3.5-fold at 24 hpi (Fig. 1A). These data suggest that pUL97 kinase activity does contribute to efficient HCMV gene expression at early times during infection.
Fig 1.
Disruption of HCMV pUL97 kinase activity alters viral gene expression at early times of infection. (A) Human foreskin fibroblasts were infected at 1 IU/cell with wild-type HCMV strain AD169 (ADwt) and treated with either DMSO vehicle or 40 μM maribavir (MBV). Viral RNA was harvested at multiple times and quantified by qRT-PCR relative to cellular GAPDH using sequence-specific primers. Error bars represent standard deviations from the means of two biological replicate experiments, and statistical significance was measured by Student's t test (*, P < 0.05). (B) Fibroblasts were infected using either ADwt or a UL97 galK substitution virus, ADdel97, at 1 IU/cell. Viral RNA was quantified as described above, and the data were evaluated as described above. (C) Western blot analysis using whole-cell lysates from infected fibroblasts treated as described above using the indicated antibodies. d97, ADdel97. (D) Western blot analysis of lysates from ADdel97-infected cells at 8 hpi with DMSO or MBV. (E) Cells were infected with ADwt, ADwt with 40 μM MBV, or ADdel97 at 0.01 IU/cell and fixed at 48 hpi, stained using an antibody against pUL123 and using DAPI for DNA, and evaluated by fluorescence microscopy. The data show the standard deviations and the means of 5 fields from each of 2 biological replicates, quantified at a magnification of ×100. (F) Fibroblasts were serum starved for 48 h and treated with 40 μM MBV or DMSO. Cells were released by the addition of serum with either 40 μM MBV or DMSO and analyzed for DNA content at 24 h using flow cytometry. The data show means and standard deviations from two biological replicates. (G) Fibroblasts (7.5 × 104) were serum starved for 48 h. Cells were released from starvation by adding media containing serum and either DMSO or 40 μM MBV. Cells were dissociated from the plate and counted at the designated time points. The data show the averages of two biological replicates. Error bars are the standard deviations of biological replicates.
To confirm that the observed defect is due to pUL97 and not an unknown off-target effect of the compound, we repeated our studies using a recombinant HCMV mutant that contains the bacterial galK gene in place of the UL97 gene (ADdel97). Although the ADdel97 virus grew slowly, which is consistent with studies by other laboratories (9, 10, 54), we prepared the viral stocks in the absence of complementation. This method of preparation was used to avoid pUL97 being packaged into viral particles. We infected fibroblasts using a multiplicity of 1 IU/cell with either ADdel97 or ADwt. Again via qRT-PCR, we observed significant decreases in UL123 gene expression at 6 and 24 hpi using ADdel97. Furthermore, we quantified significant decreases in both UL38 and UL44 expression at 24 hpi. These data show that HCMV pUL97 contributes to efficient HCMV gene expression at immediate early and early times of infection.
To determine whether pUL97 could also influence steady-state viral protein levels, we completed Western blot analysis on whole-cell lysates following infection using either ADwt, ADwt with MBV, or ADdel97 virus. We observed reduced levels of HCMV pUL123 (IE1) as well as pUL122 (IE2) at 6 and 8 hpi following inhibition of kinase activity or upon removal of the UL97 gene (Fig. 1C). Treatment of the ADdel97 viral infection with MBV did not further reduce pUL123 expression at 8 hpi (Fig. 1D). By 24 hpi, however, we observed wild-type levels of pUL123 and pUL122 protein expression under each condition. We also detected differences in pUL44 expression at 24 hpi (Fig. 1C). For pUL38, we observed a difference in expression between ADwt and both ADwt with MBV and ADdel97 at 8 hpi but only ADdel97 at 24 hpi (Fig. 1C). ADdel97 exhibited larger defects than MBV-mediated inhibition, which is likely due to the multiple roles of pUL97 including virion assembly at late times (9, 10, 16, 21, 23, 54). For all of these studies, we determined the titers of our viral stocks by staining for pUL123-postive cells at 48 hpi. To ensure that the transient defect in viral gene expression was not affecting our ability to accurately determine viral titers, we infected fibroblasts at 1 IU/cell using stocks of ADwt or ADdel97 for which titers had been determined, and we quantified pUL123-positive cells at 48 hpi. The infections were completed using ADwt, ADwt with MBV, or ADdel97. Under these conditions, equivalent numbers of cells were positive between infections (Fig. 1E), indicating that the method is valid. Next, we completed an additional control to determine whether 40 μM MBV could influence cell cycle progression. HCMV pUL97 is a viral cyclin-dependent kinase (v-CDK) (12, 13, 20), and IE expression is sensitive to changes in the cell cycle. For this control, we serum starved subconfluent fibroblasts using 0.5% FBS for 48 h and released cells using 7% FBS with and without 40 μM MBV. At 24 hpi, we evaluated DNA content using flow cytometry as an indicator of the cell cycle phases. As shown in Fig. 1F, 40 μM MBV did not result in observable changes in cellular DNA content compared to the DMSO-treated control cells. We also evaluated cell doubling following release, and the presence of MBV did not alter cell doubling compared to DMSO-treated cells (Fig. 1G). Overall, our studies support the conclusion that HCMV pUL97 contributes to efficient HCMV gene expression beginning at immediate early times of infection.
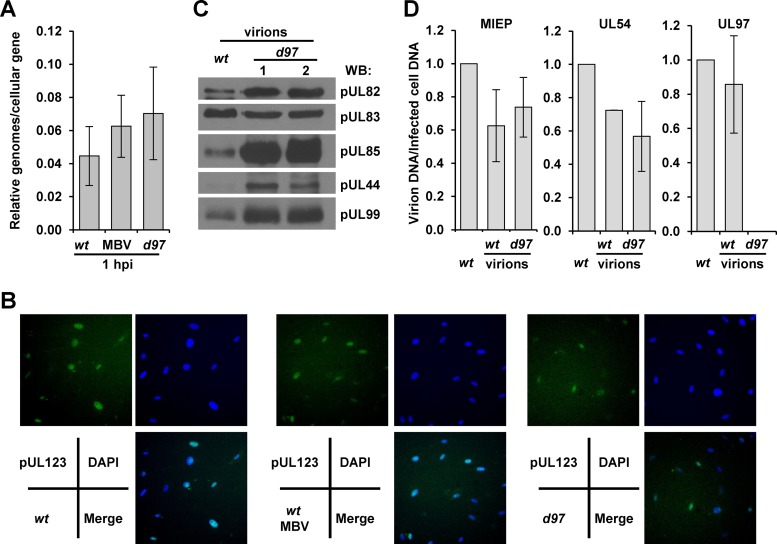
While we suspected that pUL97 was promoting immediate early gene expression, disruption of kinase activity or deletion of the kinase could also influence entry. To control for possible viral entry defects, we infected cells again with 1 IU/cell using ADwt, ADwt with MBV, or ADdel97 virus and quantified viral genomes relative to a cellular gene at 1 hpi (Fig. 2A). We detected similar levels of viral DNA associated with the infected cells, suggesting that the defect was not due to differences in viral entry. To provide additional evidence, we completed immunofluorescence analysis using an antibody against the major HCMV tegument protein, pUL83 (pp65) (55). pUL83 is delivered to the nucleus upon entry and participates in regulating cellular responses to infection (56–59). Again at 1 hpi and 1 IU/cell, we observed similar numbers of cells between infections that were positive for the tegument protein (Fig. 2B). However, for the ADdel97 virus, the signal intensity of pUL83 staining was lower, which is likely due to packaging defects occurring during preparation of the viral stock in the absence of pUL97 (21, 23, 24). To address this possibility, we evaluated the virion protein and DNA composition in equal infectious units between ADwt and ADdel97. Using Western blot analysis, we observed in ADdel97 virions increased levels of pUL82 (pp71), pUL85 (mCP), pUL44, and pUL99 (pp28) but reduced levels of pUL83 compared to equivalent infectious units of ADwt (Fig. 2C). Looking at virion DNA content, we observed similar levels of viral DNA using qPCR and primers to the major immediate early promoter (MIEP) and UL54 sequences between viruses (Fig. 2D). Primers designed against the UL97 gene failed to amplify a signal from ADdel97 virions, confirming the absence of the gene (Fig. 2D). These data demonstrate that deletion of UL97 impacts ADdel97 virion protein levels but not DNA content when comparing equivalent infectious units of ADwt. Since multiple tegument proteins influence IE gene expression, these differences likely contribute to the greater defect observed for ADdel97 compared to MBV-treated wild-type virus.
Fig 2.
Characterization of entry and viral particle content following HCMV kinase disruption. (A) Fibroblasts were exposed to 1 IU/cell using ADwt, ADwt with 40 μM MBV, or ADdel97 virus and washed, and total cellular DNA was harvested at 1 hpi. Viral genomes were quantified using primers to MIEP relative to cellular DNA by qPCR; the data represent standard deviations and means of two biological replicates. (B) Cells were infected as above, fixed at 1 hpi, stained using an antibody against pUL83 and using DAPI for DNA, and evaluated by fluorescence microscopy at a magnification of ×400. (C) Viral particles from ADwt or two independent stocks of ADdel97 prepared from separate BAC transfections were isolated using centrifugation through a sucrose cushion. Equivalent infectious units (IU) of each stock were evaluated using Western blot analysis and the indicated antibodies. (D) Equivalent IUs of ADwt and ADdel97 virions were analyzed for DNA content by qPCR using the indicated primer sets. The data were normalized to DNA content from fibroblasts infected with the equivalent IU of ADwt. The data represent the standard deviations and means of two biological replicates.
Expression of pUL97 is sufficient to enhance immediate early UL123 expression from the viral genome.
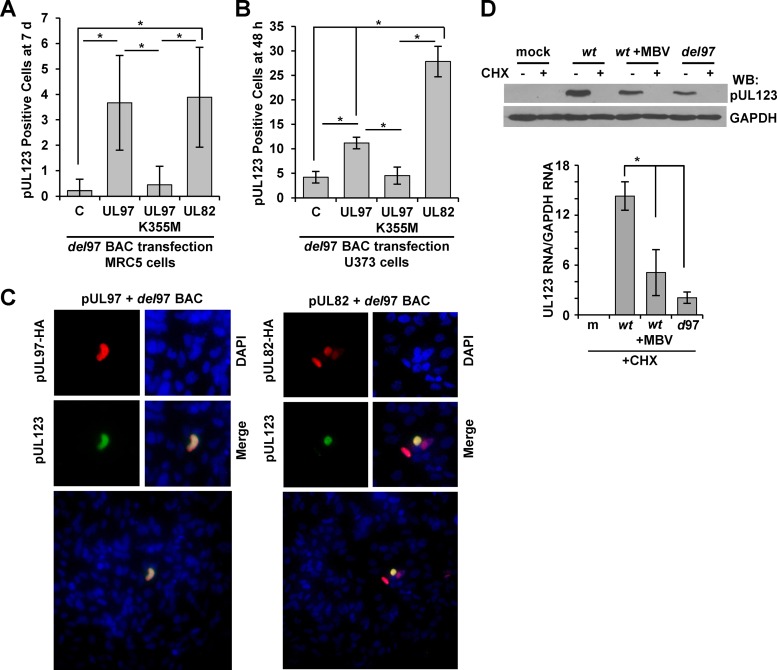
We were next interested in determining whether expression of pUL97 was sufficient to promote HCMV immediate early gene expression. For these studies, we electroporated ADdel97 viral genomic DNA into fibroblasts with equal amounts of either an empty vector, a vector expressing pUL97, or a vector expressing the kinase-inactive version, pUL97K355M (13) (Fig. 3A). As a positive control, we also included an expression vector encoding the transactivating protein pUL82 (32). After 7 days posttransfection, we evaluated the cells for the expression of pUL123 protein from the HCMV genome. Using immunofluorescence analysis and an antibody specific to pUL123, we quantified a significant increase in pUL123-positive cells upon transfection of pUL97 in comparison to either empty vector or kinase-inactive pUL97K355M (Fig. 3A). Furthermore, we observed similar numbers of pUL123-positive cells between pUL97 and pUL82 transfections (Fig. 3A). We repeated the experiments using U373 human astrocytoma cells, which have a higher capacity to express transfected DNA. Cells were transfected using lipid-based transfection with ADdel97 genome along with the different expression vectors, and pUL123-positive cells were identified at 48 h posttransfection. Again, we quantified a significant increase in pUL123-positive cells upon transfection of the pUL97 expression vector in comparison to both empty and pUL97K355M vectors (Fig. 3B). In U373 cells, however, pUL82 promoted higher levels of pUL123-positive cells than pUL97 (Fig. 3B). Representative fluorescent images of pUL123-positive cells, which were later costained using an antibody against the HA epitope in pUL97 and pUL82, are shown in Fig. 3C. Taken together, our data demonstrate that active pUL97 kinase is sufficient to promote increased HCMV pUL123 protein expression from the viral genome.
Fig 3.
Expression of kinase-active pUL97 is sufficient to enhance HCMV pUL123 expression from the viral genome. (A) Primary fibroblasts were electroporated with the ADdel97 BAC DNA and either pCGN, pCGN-UL97HA (UL97), pCGN-UL97K355MHA (UL97K355M), or pCGN-UL82HA (UL82) expression vectors. Cells were fixed at 7 days posttransfection, stained using an antibody against pUL123, and evaluated by immunofluorescence analysis. Error bars represent standard deviations from the means of six biological replicates, and statistical significance (*) was measured using analysis of variance (ANOVA). (B) U373 cells were transfected using lipid transfection and the same DNAs as described above. pUL123-positive cells were quantified at 48 h posttransfection. Error bars represent standard deviations from the means of nine biological replicates, and statistical significance (*) was measured using a one-way ANOVA. (C) Representative immunofluorescence from panel B demonstrating a pUL123-positive cell along with HA-positive cells and DAPI for DNA content. (D) Fibroblasts were pretreated for 1 h with 100 μg/ml cycloheximide (CHX) and then mock infected or infected with ADwt, ADwt with 40 μM MBV, or ADdel97 at 1 IU/cell. CHX-mediated inhibition of protein expression was confirmed by Western blotting of mock-infected or infected lysates harvested at 8 hpi using the indicated antibodies. RNA was collected at 6 hpi, and UL123 RNA was analyzed by qRT-PCR and normalized to cellular GAPDH using sequence-specific primers. Error bars represent standard deviations from the means of 2 biological replicates, and one-way ANOVA was used to measure statistical significance (*).
HCMV pUL97 is a component of virions, and we next investigated whether tegument-delivered pUL97 could influence immediate early gene expression. For these studies, we cultured cells in the presence or absence of cycloheximide starting 1 h prior to infection in order to inhibit viral protein translation (60). Cells were then infected at 1 IU/cell using ADwt, ADwt with MBV, or ADdel97. To ensure that viral protein synthesis was inhibited, we collected whole-cell lysates at 8 hpi and studied pUL123 protein levels using Western blot analysis. Under these conditions, cycloheximide treatment inhibited pUL123 protein synthesis to below detectable levels (Fig. 3D). In the absence of cycloheximide, we detected pUL123 during ADwt infection but reduced levels in MBV-treated ADwt and ADdel97 infections, as expected. To assess the impact of cycloheximide treatment on viral RNA levels, we pretreated cells as described above, infected them at 1 IU/cell, and harvested total RNA at 6 hpi. Using qRT-PCR, we observed significant decreases in UL123 RNA levels in the ADdel97 and MBV-treated ADwt infections compared to those in ADwt infection alone (Fig. 3D). These results indicate that tegument-derived pUL97 is contributing to the activation of UL123 IE gene expression upon infection.
pUL97 interacts with HDAC1 and alters histone H3 acetylation at the HCMV MIE promoter.
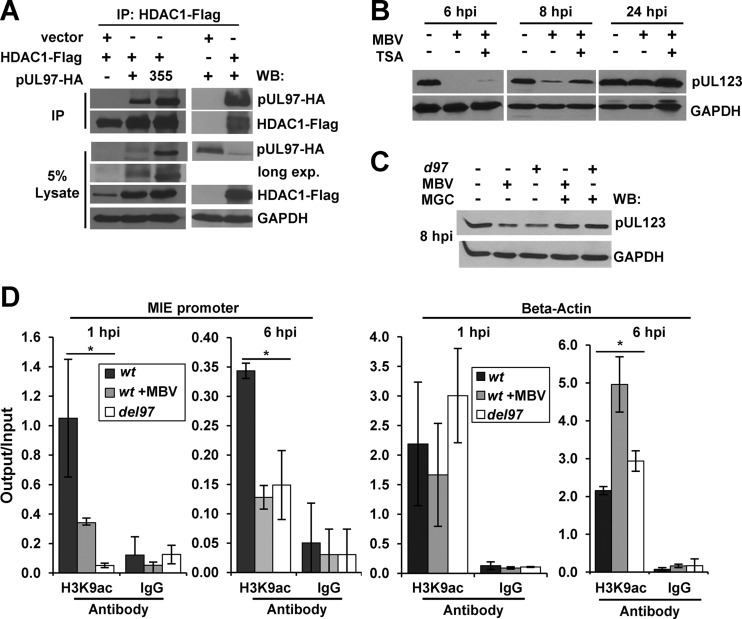
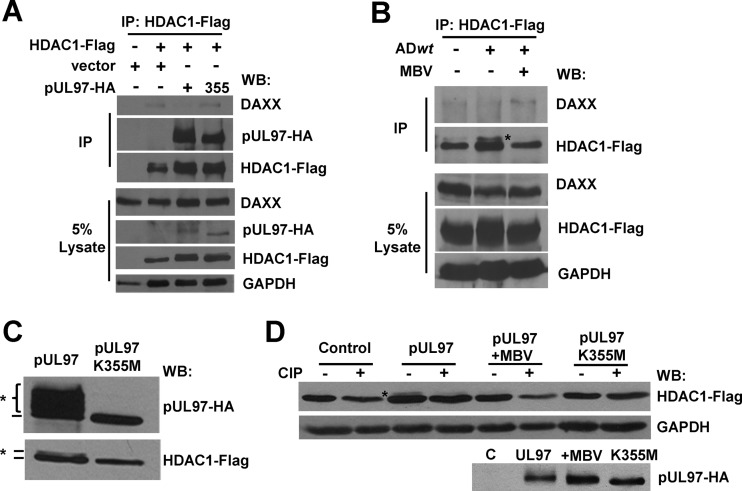
In our previous studies, we observed an association between pUL97 and HDAC1 during HCMV infection using mass spectrometry and HDAC1 as the bait protein (25). We were next interested in investigating the role of HDAC1 in pUL97-mediated regulation of HCMV gene expression. To determine whether the interaction between pUL97 and HDAC1 can also occur in the absence of infection, we transfected U-2 OS osteosarcoma cells with an HDAC1 expression vector containing the FLAG epitope along with vectors for either pUL97 or pUL97K355M containing the HA epitope. We immunoprecipitated HDAC1 and any associated proteins using an antibody against the FLAG epitope and completed Western blot analysis using an antibody against HA. We detected proteins corresponding to the correct molecular weight of pUL97 and pUL97K355M (Fig. 4A). We did not observe these proteins in the absence of either pUL97 expression vectors or HDAC1-FLAG following immunoprecipitation with the FLAG antibody (Fig. 4A). Our data demonstrate that pUL97 as well as inactive-kinase pUL97K355M can associate with HDAC1 and confirm our previous observation (25).
Fig 4.
pUL97 interacts with HDAC1 and alters H3 acetylation at the MIE promoter. (A) U-2 OS cells were transfected with an empty or HDAC1-Flag expression vector along with either empty vector control or pCGN-UL97HA or pCGN-UL97K355M (355) expression vectors. Immunoprecipitation was completed using whole-cell lysates from 48 h posttransfection and an antibody against the FLAG epitope. Western blotting was completed using the indicated antibodies. (B) Fibroblasts were infected at 1 IU/cell with ADwt and treated with DMSO vehicle, 40 μM MBV, or MBV and 300 μM trichostatin A (TSA). Western blot analysis was completed using whole-cell lysates collected at the indicated times using the indicated antibodies. (C) Cells were infected with ADwt (−) or with ADdel97 (d97) (+) at 1 IU/cell and treated with either DMSO, 40 μM MBV, or MBV with 0.8 μM mocetinostat (MGC). Western blot analysis was completed using whole-cell lysate collected at 8 hpi and the indicated antibodies. (D) Cells were infected with ADwt, ADwt with 40 μM MBV, or ADdel97 virus at 1 IU/cell. Chromatin immunoprecipitation was performed at 1 and 6 hpi using an antibody against histone 3 acetylated at lysine 9 (H3K9ac) or an IgG control. Levels of the viral MIE promoter and beta-actin sequence were quantified using qPCR. Data are presented as output relative to input quantity and the standard deviations of the means of two biological replicates with statistical significance (*) calculated using a one-way ANOVA.
We postulated that pUL97 may be acting to increase gene expression through opposing HDAC1-mediated repression of immediate early transcription. To test this possibility, we determined the protein levels of pUL123 during infection under several conditions. Again, infected cells were treated with MBV to inhibit pUL97 kinase activity, resulting in a delay in HCMV pUL123 expression (Fig. 4B). To evaluate the impact of deacetylase activity, we included the pan-HDAC inhibitor, trichostatin A (TSA), which has been previously demonstrated to increase immediate early gene expression (61). When 300 nM TSA was combined with MBV, we observed a partial rescue of pUL123 expression, which was most evident at 8 hpi (Fig. 4B). To confirm these results, we repeated the experiment using the deacetylase inhibitor mocetinostat (MGCD0103), at 0.8 μM, which specifically disrupts HDAC1 and 2 activity (50% inhibitory concentration [IC50] for HDAC1, 0.15 μM; for HDAC2, 0.29 μM; and for HDAC3, 1.66 μM) (62). Under these conditions, the addition of mocetinostat also resulted in a rescue of MBV-mediated or UL97 substitution-mediated disruption of pUL123 expression at 8 hpi (Fig. 4C). Together, these studies provide evidence that pUL97 is opposing the repressive effects of HDAC1 on HCMV IE gene expression.
One way in which HDACs repress transcription is by deacetylating histones associated with promoters (63–67). To determine whether pUL97 could alter the level of histone acetylation at the HCMV MIE promoter, we completed chromatin immunoprecipitation (ChIP). We focused our studies on histone H3 lysine 9 acetyl (H3K9ac), which is a mark of active transcription (68). For these experiments, we infected fibroblasts at a multiplicity of 1 IU/cell using ADwt, ADwt plus MBV, or ADdel97 and completed ChIP at 1 and 6 hpi using an antibody against H3K9ac. Infections involving both ADdel97 and MBV treatment of ADwt virus resulted in decreased MIE promoter sequence associating with H3K9ac at 1 and 6 hpi compared to untreated wild-type virus (Fig. 4D). We detected no differences between each infection using nonspecific IgG (Fig. 4D). As a control, we used primers to the beta-actin gene, and although the data were highly variable, we observed no statistically significant difference in H3K9ac binding at 1 hpi (Fig. 4D). However, we did observe elevated levels of beta-actin sequence in the MBV-treated and ADdel97 samples at 6 hpi (Fig. 4D). Possible explanations include loss of pUL97-mediated changes directly at this gene or indirect effects due to changes in immediate early gene expression. However, we did not observe changes in beta-actin protein levels at this time (Fig. 4B). Overall, our results suggest that disruption of pUL97 is associated with decreased amounts of H3K9ac associated with the HCMV MIE promoter. Furthermore, these data support the hypothesis that pUL97 is disrupting HDAC1-mediated repression.
pUL97 kinase activity alters HDAC1 association with the MIE promoter.
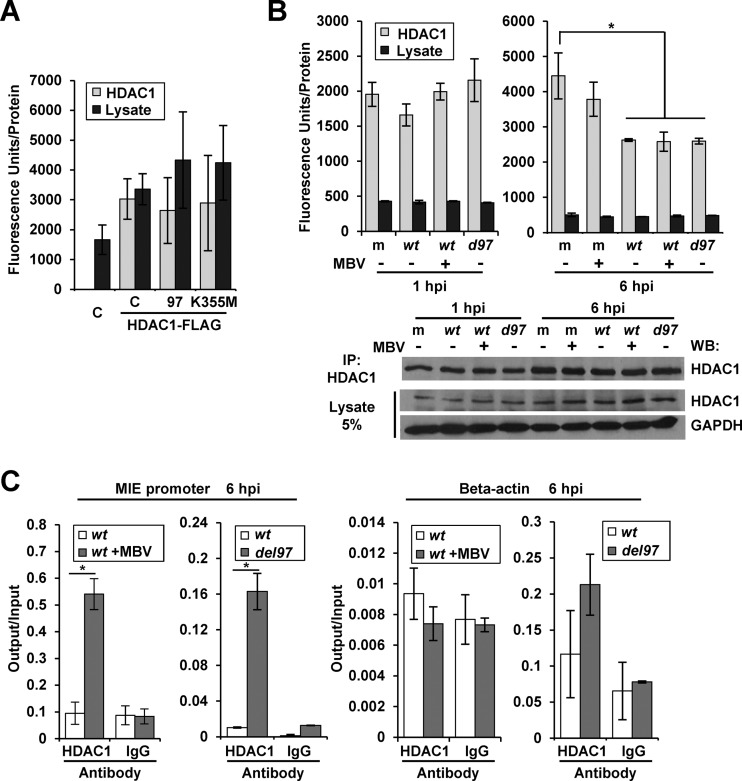
Park et al. (36) have reported that total HDAC2 deacetylase activity decreases early during HCMV infection. We were interested in determining the impact of pUL97 kinase activity on deacetylase activity in the presence and absence of infection. Using U-2 OS cells, we transfected HDAC1 containing the FLAG epitope in the presence of either empty, pUL97, or pUL97K355M expression vectors. HDAC1 was isolated by immunoprecipitation, and the associated deacetylase activity was quantified using a fluorometric HDAC activity assay (Fig. 5A) and normalized to the amount of HDAC1 protein isolated as measured by chemiluminescence from the Western blot (Fig. 6A). Although we detected an elevation in activity following transfection and immunoprecipitation of HDAC1, neither pUL97 nor pUL97K355M appeared to influence deacetylase activity (Fig. 5A). To evaluate activity during infection, we infected fibroblasts using ADwt, ADwt plus MBV, or ADdel97 virus and again immunoprecipitated HDAC1. We quantified deacetylase activity relative to the total amount of HDAC1 isolated as determined by chemiluminescence analysis (Fig. 5B, lower panel). At 1 hpi, we did not observe differences in HDAC1-associated activity between samples (Fig. 5B). At 6 hpi, we observed a significant decrease in activity when comparing infected samples to either the uninfected mock or mock plus MBV samples. However, no difference was observed between ADwt and ADwt plus MBV or ADdel97 infections. These data demonstrate that HDAC1-associated deacetylase activity is decreased at 6 hpi during HCMV infection but this occurs independently of HCMV pUL97.
Fig 5.
Association of HDAC1 with the MIE promoter increases in the absence of kinase activity. (A) To assess deacetylase activity, U-2 OS cells were transfected with an empty vector control (C) or cotransfected with HDAC1-Flag expression vector and empty control (C) or pCGN-UL97HA (97) or pCGN-UL97K355MHA (K355M) expression vectors. Immunoprecipitation was performed with an anti-FLAG antibody and used in a fluorescence-based HDAC activity assay. Activity was normalized to immunoprecipitated HDAC1 levels determined by Western blotting and chemiluminescence detection (Fig. 6A). Activity was also measured in 1% of the lysate and normalized to total protein levels. Error bars represent standard deviations from the means of five biological replicates. (B) Fibroblasts were mock infected (m), infected with ADwt (wt) virus, or infected with ADdel97 (d97) at 1 IU/cell and treated with DMSO vehicle or 40 μM MBV. Immunoprecipitation using an antibody against HDAC1 was performed from lysates collected at 1 or 6 hpi. Deacetylase activity was determined using the isolated proteins with a fluorescence-based HDAC activity assay. Activity was normalized to immunoprecipitated HDAC1 levels as determined by Western blotting and chemiluminescence detection (lower panel). Activity was also measured in 0.5% of the lysate and normalized to total protein levels. Error bars denote standard deviations from the means of two biological replicates and statistical significance by one-way ANOVA (*). (C) Fibroblasts were infected at 1 IU/cell with ADwt (wt) and treated with DMSO or 40 μM MBV at the time of infection or infected with ADwt or ADdel97 (del97) virus. Chromatin immunoprecipitation was performed with an antibody to HDAC1 or IgG control from samples collected at 6 hpi. Viral MIE promoter or the beta-actin sequence was quantified using qPCR. Data are presented as output relative to input DNA, and error bars represent the standard deviations from the means of two biological replicates with statistical significance calculated using Student's t test (*, P < 0.05).
Fig 6.
pUL97-mediated changes in HDAC1 phosphorylation and binding to DAXX. (A) U-2 OS cells were transfected with an empty or HDAC1-Flag expression vector and cotransfected with an empty vector or pUL97HA or pUL97K355M (355) expression vector. Samples were immunoprecipitated with a Flag-specific antibody and analyzed by Western blotting using the indicated antibodies. (B) U373 cells were transfected with an HDAC1-Flag expression vector for 36 h and then mock infected (−) or infected at 3 IU/cell with ADwt in the presence or absence of 40 μM MBV. At 1 hpi, immunoprecipitation was performed with a Flag-specific antibody and analyzed by Western blotting using the indicated antibodies. The asterisk marks a potential modification of HDAC1. (C) U-2 OS cells were transfected with an HDAC1-Flag expression vector along with pUL97-HA or pUL97K355M-HA expression vector. Samples were evaluated by Western blotting using whole-cell lysates and the indicated antibodies. Modifications are marked. (D) Cells were transfected as described above, including an additional sample containing HDAC1 with empty vector or the pUL97-HA expression vector with DMSO vehicle or 40 μM MBV added at 1 h prior to transfection. Lysates were split into two aliquots and were treated with calf intestinal phosphatase (CIP) (+) or were left untreated (−). Proteins were evaluated by Western blotting using the indicated antibodies.
In general, HDAC1 associates with complexes on chromatin but does not directly bind to DNA to mediate transcriptional repression (65). Furthermore, Reeves et al. have demonstrated that HDAC1 associates with the HCMV MIE promoter after 24 hpi in conjunction with IE2 to mediate repression at the cis-repressive sequence (CRS) (37). An alternative hypothesis explaining our observations is that pUL97 alters the association of HDAC1 with the promoter. To evaluate this possibility, we utilized ChIP analysis to quantify changes in endogenous HDAC1 associating with the MIE promoter at 6 hpi. Following the addition of MBV to ADwt infection, we quantified an average increase of 5.7-fold in MIE promoter sequence associating with HDAC1 compared to the untreated infection (Fig. 5C). We repeated the experiment using the ADdel97 virus and quantified a 16-fold increase relative to infection using ADwt (Fig. 5C). This response was not observed for the beta-actin gene (Fig. 5C). These data demonstrate that inhibition of pUL97 activity results in elevated levels of HDAC1 at the MIE promoter. These changes correlate with the observed decrease in H3K9ac levels at the promoter.
HCMV pUL97 kinase-dependent phosphorylation of HDAC1 and reduced DAXX interaction.
HDAC1 is recruited to DNA through interactions with multiple proteins (reviewed in references 26 and 69). With this in mind, we evaluated changes in HDAC1's association with several known binding partners upon pUL97 expression. The interactions of HDAC1 with MTA2, Sin3A, and Rbbp48 were unchanged when pUL97 was coexpressed in U-2 OS cells compared to either empty vector or expression of pUL97K355M (data not shown). HDAC1 has also been demonstrated to interact with the transcriptional repressor DAXX (33). DAXX acts to silence HCMV IE expression in a deacetylase-dependent manner (32, 33). To evaluate the DAXX interaction with HDAC1, we transfected U-2 OS cells with a vector expressing HDAC1-FLAG and either empty or pUL97 or pUL97K355M expression vectors (Fig. 6A). Following immunoprecipitation with an antibody against the FLAG epitope, we detected low levels of DAXX by Western blotting using empty vector or pUL97K355M (Fig. 6A). Although the effect is not robust, we consistently observed a reduction in DAXX associating with HDAC1 upon expression of active pUL97 kinase (Fig. 6A). During infection, steady-state levels of DAXX are decreased at 1 hpi and are substantially decreased by 2 hpi in a pp71-dependent manner (32). We therefore evaluated the influence of pUL97 activity on the interaction between DAXX and HDAC1 at 1 hpi. U373 cells were transfected with a HDAC1-FLAG expression vector and were either mock infected or infected at 3 IU/cell using ADwt virus. Furthermore, cells were treated with DMSO or with 40 μM MBV. Immunoprecipitation with an anti-Flag antibody followed by analysis using Western blot revealed a small but consistent increase in DAXX association with HDAC1 during MBV treatment of infected cells (Fig. 6B) compared to DMSO control. These data show that pUL97 promotes a reduction in the association between HDAC1 and DAXX.
In the above-described experiment, we observed a higher-molecular-weight band for HDAC1 in the infected but not the MBV-treated sample (Fig. 6B), suggesting the possibility that HDAC1 is being modified in a kinase-dependent manner. Furthermore, kinases from alphaherpesviruses have been shown to phosphorylate HDAC1 and/or 2 (40, 41, 70). Therefore, we decided to investigate whether HCMV pUL97 could influence the phosphorylation state of HDAC1. For these experiments, we completed Western blot analysis using whole-cell lysates from U-2 OS cells cotransfected with vectors expressing HDAC1 and either wild-type pUL97 or kinase-inactivated pUL97K355M (Fig. 6C). As a control for kinase activity, we observed substantial autophosphorylation occurring with the wild-type kinase but not K355M when using an antibody against the HA epitope in pUL97 (Fig. 6C). When we used an antibody against the FLAG epitope in HDAC1, we detected a band with increased mobility following transfection with wild-type pUL97 but not with the K355M mutant (Fig. 6C), which suggests that HDAC1 is being modified in the presence of pUL97.
To determine whether the modification is the result of pUL97-mediated phosphorylation, we repeated the experiment in the presence or absence of phosphatase treatment. For these studies, we cotransfected U-2 OS cells with a vector expressing HDAC1 and either wild-type pUL97, pUL97 plus MBV, or pUL97K355M expression vector. We isolated whole-cell lysates and treated one half of the sample with calf intestinal phosphatase. In the untreated sample half expressing both HDAC1 and pUL97, we again detected a modification of HDAC1 by Western blotting (Fig. 6D). Treatment of this sample with phosphatase resulted in a reduction in the band intensity, suggesting that this modification is sensitive to phosphatase treatment (Fig. 6D). We did not observe the higher-mobility species of HDAC1 either in the control transfection, in MBV-treated samples, or when using the inactive kinase (Fig. 6D). Our results show that HCMV pUL97 is promoting the phosphorylation of HDAC1.
Identification of amino acids in HDAC1 being phosphorylated by viral and cellular kinases.
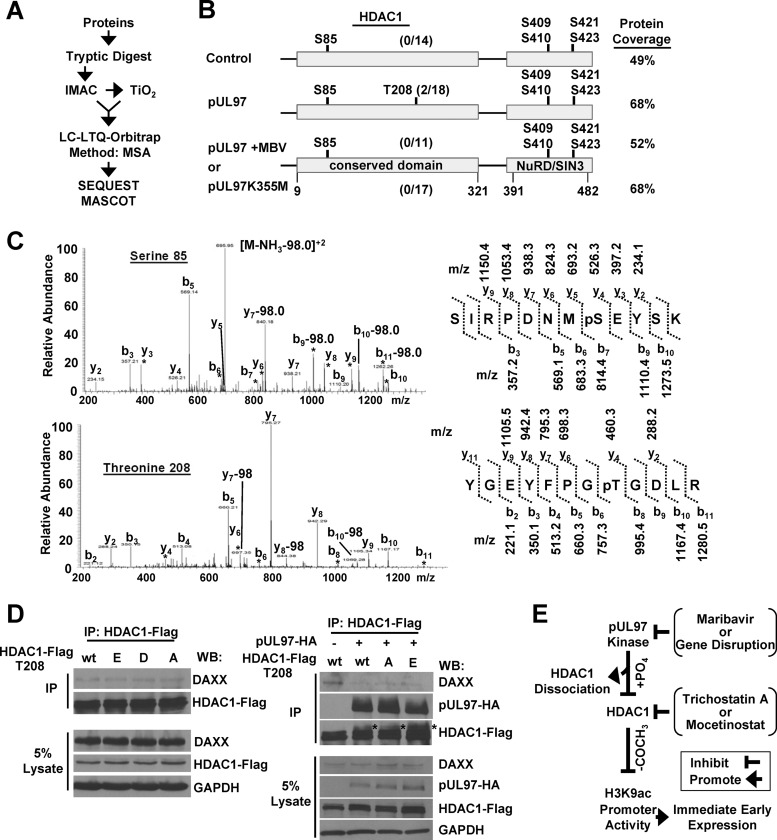
We were next interested in trying to identify the amino acids in HDAC1 that might be differentially phosphorylated by the HCMV kinase. For these studies, we used a phosphopeptide enrichment strategy in-line with a high mass accuracy mass spectrometer (Fig. 7A). The expression vectors for pUL97 and HDAC1 were transfected into U-2 OS cells. As controls, we also transfected HDAC1 with either an empty vector or the pUL97 plus MBV or pUL97K355M expression vector. HDAC1 and associated proteins were isolated by immunoprecipitation followed by in-solution tryptic digestion and phosphopeptide enrichment using immobilized metal ion affinity chromatography (IMAC) and TiO2. Sites of phosphorylation were identified using a high mass accuracy LTQ-Orbitrap mass spectrometer. Under these conditions, we observed peptides to pUL97 associating with HDAC1 under all conditions except when using the empty expression vector, providing additional evidence for the association of pUL97 with HDAC1 (data not shown).
Fig 7.
HDAC1 is uniquely phosphorylated in the presence of kinase-active pUL97. (A) Strategy used to identify phosphopeptides. U-2 OS cells were transfected with an HDAC1-Flag expression vector and cotransfected with an empty vector or pUL97HA, pUL97HA with MBV, or pUL97K355M expression vector. Samples were immunoprecipitated with a Flag-specific antibody, and isolated proteins were digested by trypsin, enriched for phosphopeptides using a combination of IMAC and TiO2 resins, and loaded onto an LTQ-Orbitrap mass spectrometer. Samples were analyzed using multistage activation (MSA), and the resulting spectra were evaluated using Sequest and Mascot algorithms. (B) Sites of phosphorylation identified in HDAC1 from three biological replicate experiments. The overall percentage of the total protein coverage using trypsin is noted. The numbers represent amino acid positions in HDAC1, and the ratio in parentheses represents the number of phosphorylated peptides over the total number of peptides observed. (C) Representative spectra demonstrating b and y ions with a 98-Da mass shift matching the phosphorylation at serine 85 or threonine 208 in HDAC1 as well as identified m/z of the b and y ions. (D) U-2 OS cells were transfected with HDAC1-Flag expressing vectors that were either wild type (T208) or carried an alanine (T208A), glutamic acid (T208E), or aspartic acid (T208D) substitution. Immunoprecipitation was performed with a Flag-specific antibody and analyzed using Western blotting with the indicated antibodies. U-2 OS cells were cotransfected with empty vector or a pUL97HA expressing vector and HDAC1-Flag expressing vectors containing T208, T208A, or T208E. A Flag-specific antibody was used for immunoprecipitation and analyzed by Western blotting with the indicated antibodies. (E) Model showing pUL97 interaction with and phosphorylation of HDAC1 during HCMV infection, resulting in decreased association of HDAC1 with the MIE promoter, increased histone H3-lysine 9 acetylation, and subsequent IE gene expression. Inhibition of kinase activity or gene disruption abrogates these activities, while the addition of HDAC inhibitors compensates for the absence of pUL97 kinase.
Sites of phosphorylation were identified in peptides by the presence of an 80-Da (HPO3−) or 98-Da (H3PO4−) mass shift using the Sequest and Mascot algorithms. The experiments were completed in triplicate, and spectra containing the shift were manually validated. In cells receiving HDAC1 with the empty vector control, we detected 64% of the tryptic peptides representing 49% of the total HDAC1 protein sequence and observed several sites of phosphorylation (Fig. 7B). These included serine 85 (S85), S409 or S410 (S409/S410), S421, and S423 and are the result of cellular kinase activity, since pUL97 was not included in these samples (Fig. 7B). For S409/S410, we were unable to determine which of the two neighboring amino acids was phosphorylated. Phosphorylation at amino acids S409/S410, S421, and S423 has been previously observed by other laboratories (71–73), but S85 represents a newly identified site of phosphorylation. A representative spectrum for S85 is shown in Fig. 7C. The same amino acids were observed to be phosphorylated in samples with pUL97, MBV plus pUL97, and the inactive kinase (Fig. 7B). Unique to only the sample expressing the active pUL97 kinase, we observed phosphorylation of HDAC1 at threonine 208 (T208) (Fig. 7B). Examples of the spectra for this modification are presented in Fig. 7C, and this also represents a newly identified site of phosphorylation in HDAC1. We did observe the unmodified form of this peptide within the control, MBV-treated, and inactive-kinase samples (Fig. 7B). These studies have identified precise amino acids being phosphorylated in HDAC1 in the presence and absence of HCMV kinase activity, and this includes two previously unknown sites of phosphorylation, S85 and T208.
To assess the role of phosphorylation occurring at T208 in modulating the HDAC1-DAXX interaction, we constructed substitution mutants of HDAC1 at T208 using either alanine to prevent phosphorylation (T208A) or a glutamic acid (T208E) or aspartic acid (T208D) to mimic phosphorylation. These expression vectors were cotransfected into U-2 OS cells with either empty vector or the pUL97 kinase. We immunoprecipitated HDAC1 using an antibody against FLAG and evaluated changes in association between HDAC1 and DAXX by Western blotting (Fig. 7D). In the absence of pUL97 expression, changes to HDAC1 T208 did not alter the HDAC1-DAXX interaction. Upon pUL97 expression, we again observed a reduction in the amount of DAXX associating with HDAC1 (Fig. 7D). However, this reduction also occurred when using either the T208A or the T208E HDAC1 mutant (T208D was not tested) (Fig. 7D). Increased pUL97-dependent phosphorylation was observed for all constructs (Fig. 7D). These data confirmed the observations that expression of pUL97 increases phosphorylation of HDAC1 and disrupts the HDAC1-DAXX interaction yet phosphorylation at T208 is not necessary for the changes.
DISCUSSION
The HCMV pUL97 kinase functions at multiple stages of the viral replication cycle, including genome replication (9, 10) and virion egress (9, 12). We have determined that the pUL97 kinase also contributes to HCMV immediate early gene expression by disrupting histone deacetylase 1 (HDAC1)-mediated repression at the viral major immediate early (MIE) promoter. Our studies have resulted in the model presented in Fig. 7E. We observed that inhibiting kinase activity by the antiviral compound maribavir disrupted HCMV UL123 RNA and protein expression (Fig. 1A and C), albeit transiently. We also observed disruption of early gene and protein expression (Fig. 1A and C). The IE defect was rescued following treatment with the broad HDAC inhibitor trichostatin A (Fig. 4B). We validated these observations using a UL97-deficient viral infection (Fig. 1B and C) as well as the HDAC inhibitor mocetinostat at a concentration specific for HDAC1 and 2 inhibition (Fig. 4C). These data indicate that pUL97 is a negative regulator of the cellular transcriptional repressor HDAC1 during infection (Fig. 7E).
In a proteomic screen for HDAC1 binding proteins, we previously observed that pUL97 associates with HDAC1 during HCMV infection (25). Within this report, we have demonstrated that HDAC1 interacts with both the active and inactive versions of the kinase (Fig. 4A). However, in the presence of active pUL97 only, we observed an increased mobility shift in HDAC1 (Fig. 6), which was sensitive to treatment with a phosphatase (Fig. 6D). These findings demonstrate that pUL97 is both interacting with and promoting HDAC1 phosphorylation during infection. Phosphorylation of HDAC1 as well as HDAC2 has been previously demonstrated for members of the alphaherpesvirus family VZV, HSV-1, and PRV and shown to be dependent upon the viral kinase (39–41). In an ORF66 mutant viral infection, treatment with the class I HDAC inhibitor sodium butyrate increases immediate early protein expression at late times, plaque sizes, and viral yields (40, 41). The molecular mechanism behind these changes remains unknown. Interestingly, transient HSV-1 US3 kinase expression enhances expression of a reporter gene driven by the HCMV MIE promoter, which was sensitive to HDAC1 inhibition (70). Our studies demonstrate that HCMV kinase-mediated phosphorylation of HDAC1 and associated changes in viral protein expression are a conserved activity among alpha- and betaherpesviruses.
In general, phosphorylation of HDAC1 has been associated with alterations in both deacetylase activity and protein interactions (71, 73–77). Unique to our studies, we have demonstrated that disruption of pUL97 by either maribavir treatment or gene substitution resulted in increased levels of HDAC1 associating with the HCMV MIE promoter (Fig. 5C). These changes coincided with changes in histone H3K9 acetylation, with the observation of decreased levels of acetylation upon disruption of pUL97 during infection (Fig. 4D). Recent studies by Mounce et al. reported in an accompanying article (78) have demonstrated similar HDAC1-dependent changes involving the gammaherpesvirus MHV-68 Orf36 viral kinase during infection. However, from our studies, we cannot exclude the possibility that disruption of kinase activity is influencing total H3 content at the MIE promoter. Finally, HDAC1-associated deacetylase activity was not altered upon expression of pUL97 in the presence (Fig. 5B) or absence (Fig. 5A) of infection, ruling out the possibility of kinase-mediated disruption of deacetylase activity. Our data support a model (Fig. 7E) in which HCMV kinase-mediated phosphorylation of HDAC1 causes dissociation of HDAC1 from the promoter, resulting in increased levels of H3K9ac and immediate early gene expression.
We identified sites of phosphorylation in HDAC1 in the presence and absence of HCMV kinase activity using a high mass accuracy mass spectrometer. Phosphorylation of HDAC1 has been previously reported from global phosphoproteome screens under diverse conditions at Y221 (79), S393 (80), S406 (81), and S409 (72). Phosphorylation also occurs at S421 and 423 as determined by mass spectrometry using HDAC1 isolated from cells (73), and mutations at S421 or S423 decreased interactions with the transcriptional repressors mSin3A, CoREST, and NuRD components RBBP7 and MTA2 (73). Hyperphosphorylation of HDAC1 can also alter interactions with repressors Sin3A and YY1 (74). In this report, we detected phosphorylation at S409/S410, S421, and S423 (Fig. 7B) as well as at a previously unknown site, S85 (Fig. 7C), by immunoisolation of HDAC1 and high mass accuracy mass spectrometry. These sites of phosphorylation were observed in the presence and absence of active kinase. Upon expression of active pUL97, we detected phosphorylation at an additional unique site, T208 (Fig. 7B), which was not observed in the other samples. In previous studies on alphaherpesvirus kinases, substitution of S406 to an alanine in HDAC1 or the equivalent residue in HDAC2 resulted in loss of viral kinase-mediated phosphorylation (40, 41), implicating this site as the target of phosphorylation. Also, the levels of HDAC2 phosphorylation were influenced by substitutions at additional serine residues (40). We observed peptides containing S406 but did not observe phosphorylation. However, this does not negate S406 as a possible site of phosphorylation. From these studies, we have demonstrated that pUL97 promotes increased HDAC1 phosphorylation and have identified two new unpublished sites of phosphorylation in HDAC1, specifically S85 and, unique to pUL97 expression, T208 (Fig. 7C).
Phosphorylation of HDAC1 affects protein binding partners, and HDAC1 is recruited to DNA through interactions with multiple proteins (reviewed in references 26 and 69). We evaluated the impact of pUL97 expression on several known HDAC1 binding partners including MTA2, Sin3A, Rbbp48, and DAXX. Interestingly, we consistently observed a modest decrease in the interaction between DAXX and HDAC1 upon expression of active kinase in the presence (Fig. 5B) and absence (Fig. 5A) of infection. Cellular DAXX is a component of promyelocytic leukemia protein nuclear bodies (PML-NB), which are involved in antiviral responses and demonstrated to inhibit HCMV IE gene expression (82). DAXX represses viral gene expression in an HDAC-dependent manner, and DAXX has been shown to interact with HDAC1 (32, 33). Our data suggest that pUL97 kinase activity may contribute to relieving PML-NB-mediated silencing by decreasing HDAC1 interaction with DAXX (Fig. 5B). This observation is supported by previous findings that pUL97 and other herpesvirus kinases can influence PML-NBs (18, 20, 22). HDAC1 interacts with diverse transcriptional regulators, and it is likely that pUL97-mediated phosphorylation of HDAC1 is influencing additional interactions. We evaluated the role of HDAC1 T208 in influencing the interaction with DAXX by introducing an alanine or glutamic acid. Under these conditions, pUL97 was still able promote increased phosphorylation of HDAC1 as well as disrupt the interaction with DAXX (Fig. 7D), demonstrating that T208 is not necessary. These results implicate the role of multiple sites of phosphorylation and/or an indirect role of pUL97, which is consistent with studies on VZV US3 and HDAC2 (40).
We have demonstrated the disruption of HCMV immediate early RNA and protein expression following infection using a UL97-deficient virus (Fig. 1B) as well as wild-type virus with a kinase inhibitor (Fig. 1A). Also unique to this report, our data show that pUL97-mediated regulation of MIE promoter is occurring at immediate early times of infection (Fig. 1). We observed kinase-dependent changes beginning at 1 hpi (Fig. 4D), suggesting a role for tegument-delivered pUL97 (5, 6). Supporting this possibility, transient expression of pUL97 but not inactive kinase was sufficient to enhance MIE promoter activity following transfection of UL97-deficient HCMV genomes into cells (Fig. 3A and B). Furthermore, the kinase-dependent defect in IE RNA expression was maintained upon cycloheximide treatment and inhibition of viral protein synthesis (Fig. 3D). These data support the conclusion that tegument-delivered pUL97 contributes to MIE promoter activation.
The transient nature of the regulation by pUL97 implicates compensatory activities by other HCMV proteins. Several other viral tegument proteins have been demonstrated to influence the MIE promoter by modulating cellular factors including pUL82 (pp71) (32), pUL83 (pp65) (83), pUL29/28 (25, 84) and pUL35 (85, 86). Furthermore, both HCMV pUL82 and pUL123 (IE1) disrupt PML-NBs. The HCMV tegument protein pUL82 promotes degradation of DAXX and displacement of ATRX (32, 87), while pUL123 disrupts PML-NB components PML and Sp100 (82, 88, 89). In the absence of pUL97 kinase activity, it is conceivable that a low level of IE1 expression still occurs, eventually resulting in sufficient PML disruption and a robust onset of IE expression. Activation of the MIE promoter is essential for HCMV replication, and it is not surprising to have identified another viral protein involved in its regulation. Our results support the conclusion that HCMV pUL97 regulates viral immediate early gene expression by phosphorylation-mediated disruption HDAC1 binding to the MIE promoter (Fig. 7E).
ACKNOWLEDGMENTS
We thank the following people for generously providing reagents: R. Kalejta for the pCGN-UL97HA, pCGN-UL97K355M-HA, and pGCN-UL82HA expression vectors and T. Shenk and W. Gibson for HCMV antibodies. We are grateful to B. Mounce, V. Tarakanova, and J. Savaryn for thoughtful discussion. B. Halligan and R. Gundry provided helpful insight into phosphopeptide enrichment and data analysis. We also thank B. Hoffman for valuable assistance with instrumentation.
This work is supported by NIH grant R01AI083281 to S.S.T. and NIH Ruth L. Kirschstein NRSA F31AI098402-01 to T.M.B.
Footnotes
Published ahead of print 24 April 2013
REFERENCES
- 1. Mocarski E, Pass TSRF. 2007. Cytomegaloviruses, p 2702–2772 In Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Straus SE. (ed), Fields virology, 5th ed Lippincott Williams & Wilkins, Philadelphia, PA [Google Scholar]
- 2. Hakki M, Chou S. 2011. The biology of cytomegalovirus drug resistance. Curr. Opin. Infect. Dis. 24:605–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Littler E, Stuart AD, Chee MS. 1992. Human cytomegalovirus UL97 open reading frame encodes a protein that phosphorylates the antiviral nucleoside analogue ganciclovir. Nature 358:160–162 [DOI] [PubMed] [Google Scholar]
- 4. Sullivan V, Talarico CL, Stanat SC, Davis M, Coen DM, Biron KK. 1992. A protein kinase homologue controls phosphorylation of ganciclovir in human cytomegalovirus-infected cells. Nature 359:85. [DOI] [PubMed] [Google Scholar]
- 5. van Zeijl M, Fairhurst J, Baum EZ, Sun L, Jones TR. 1997. The human cytomegalovirus UL97 protein is phosphorylated and a component of virions. Virology 231:72–80 [DOI] [PubMed] [Google Scholar]
- 6. Wolf DG, Honigman A, Lazarovits J, Tavor E, Panet A. 1998. Characterization of the human cytomegalovirus UL97 gene product as a virion-associated protein kinase. Arch. Virol. 143:1223–1232 [DOI] [PubMed] [Google Scholar]
- 7. Webel R, Milbradt J, Auerochs S, Schregel V, Held C, Nobauer K, Razzazi-Fazeli E, Jardin C, Wittenberg T, Sticht H, Marschall M. 2011. Two isoforms of the protein kinase pUL97 of human cytomegalovirus are differentially regulated in their nuclear translocation. J. Gen. Virol. 92:638–649 [DOI] [PubMed] [Google Scholar]
- 8. Webel R, Solbak SM, Held C, Milbradt J, Gross A, Eichler J, Wittenberg T, Jardin C, Sticht H, Fossen T, Marschall M. 2012. Nuclear import of isoforms of the cytomegalovirus kinase pUL97 is mediated by differential activity of NLS1 and NLS2 both acting through classical importin-alpha binding. J. Gen. Virol. 93:1756–1768 [DOI] [PubMed] [Google Scholar]
- 9. Krosky PM, Baek MC, Coen DM. 2003. The human cytomegalovirus UL97 protein kinase, an antiviral drug target, is required at the stage of nuclear egress. J. Virol. 77:905–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wolf DG, Courcelle CT, Prichard MN, Mocarski ES. 2001. Distinct and separate roles for herpesvirus-conserved UL97 kinase in cytomegalovirus DNA synthesis and encapsidation. Proc. Natl. Acad. Sci. U. S. A. 98:1895–1900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Baek MC, Krosky PM, Pearson A, Coen DM. 2004. Phosphorylation of the RNA polymerase II carboxyl-terminal domain in human cytomegalovirus-infected cells and in vitro by the viral UL97 protein kinase. Virology 324:184–193 [DOI] [PubMed] [Google Scholar]
- 12. Hamirally S, Kamil JP, Ndassa-Colday YM, Lin AJ, Jahng WJ, Baek MC, Noton S, Silva LA, Simpson-Holley M, Knipe DM, Golan DE, Marto JA, Coen DM. 2009. Viral mimicry of Cdc2/cyclin-dependent kinase 1 mediates disruption of nuclear lamina during human cytomegalovirus nuclear egress. PLoS Pathog. 5:e1000275. 10.1371/journal.ppat.1000275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hume AJ, Finkel JS, Kamil JP, Coen DM, Culbertson MR, Kalejta RF. 2008. Phosphorylation of retinoblastoma protein by viral protein with cyclin-dependent kinase function. Science 320:797–799 [DOI] [PubMed] [Google Scholar]
- 14. Kawaguchi Y, Matsumura T, Roizman B, Hirai K. 1999. Cellular elongation factor 1delta is modified in cells infected with representative alpha-, beta-, or gammaherpesviruses. J. Virol. 73:4456–4460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Krosky PM, Baek MC, Jahng WJ, Barrera I, Harvey RJ, Biron KK, Coen DM, Sethna PB. 2003. The human cytomegalovirus UL44 protein is a substrate for the UL97 protein kinase. J. Virol. 77:7720–7727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Marschall M, Marzi A, aus dem Siepen P, Jochmann R, Kalmer M, Auerochs S, Lischka P, Leis M, Stamminger T. 2005. Cellular p32 recruits cytomegalovirus kinase pUL97 to redistribute the nuclear lamina. J. Biol. Chem. 280:33357–33367 [DOI] [PubMed] [Google Scholar]
- 17. Milbradt J, Webel R, Auerochs S, Sticht H, Marschall M. 2010. Novel mode of phosphorylation-triggered reorganization of the nuclear lamina during nuclear egress of human cytomegalovirus. J. Biol. Chem. 285:13979–13989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Prichard MN, Sztul E, Daily SL, Perry AL, Frederick SL, Gill RB, Hartline CB, Streblow DN, Varnum SM, Smith RD, Kern ER. 2008. Human cytomegalovirus UL97 kinase activity is required for the hyperphosphorylation of retinoblastoma protein and inhibits the formation of nuclear aggresomes. J. Virol. 82:5054–5067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kamil JP, Hume AJ, Jurak I, Munger K, Kalejta RF, Coen DM. 2009. Human papillomavirus 16 E7 inactivator of retinoblastoma family proteins complements human cytomegalovirus lacking UL97 protein kinase. Proc. Natl. Acad. Sci. U. S. A. 106:16823–16828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kuny CV, Chinchilla K, Culbertson MR, Kalejta RF. 2010. Cyclin-dependent kinase-like function is shared by the beta- and gamma- subset of the conserved herpesvirus protein kinases. PLoS Pathog. 6:e1001092. 10.1371/journal.ppat.1001092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Prichard MN, Britt WJ, Daily SL, Hartline CB, Kern ER. 2005. Human cytomegalovirus UL97 kinase is required for the normal intranuclear distribution of pp65 and virion morphogenesis. J. Virol. 79:15494–15502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tower C, Fu L, Gill R, Prichard M, Lesort M, Sztul E. 2011. Human cytomegalovirus UL97 kinase prevents the deposition of mutant protein aggregates in cellular models of Huntington's disease and ataxia. Neurobiol. Dis. 41:11–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Azzeh M, Honigman A, Taraboulos A, Rouvinski A, Wolf DG. 2006. Structural changes in human cytomegalovirus cytoplasmic assembly sites in the absence of UL97 kinase activity. Virology 354:69–79 [DOI] [PubMed] [Google Scholar]
- 24. Goldberg MD, Honigman A, Weinstein J, Chou S, Taraboulos A, Rouvinski A, Shinder V, Wolf DG. 2011. Human cytomegalovirus UL97 kinase and nonkinase functions mediate viral cytoplasmic secondary envelopment. J. Virol. 85:3375–3384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Terhune SS, Moorman NJ, Cristea IM, Savaryn JP, Cuevas-Bennett C, Rout MP, Chait BT, Shenk T. 2010. Human cytomegalovirus UL29/28 protein interacts with components of the NuRD complex which promote accumulation of immediate-early RNA. PLoS Pathog. 6:e1000965. 10.1371/journal.ppat.1000965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cunliffe VT. 2008. Eloquent silence: developmental functions of Class I histone deacetylases. Current Opin. Genet. Dev. 18:404–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cuevas-Bennett C, Shenk T. 2008. Dynamic histone H3 acetylation and methylation at human cytomegalovirus promoters during replication in fibroblasts. J. Virol. 82:9525–9536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Murphy JC, Fischle W, Verdin E, Sinclair JH. 2002. Control of cytomegalovirus lytic gene expression by histone acetylation. EMBO J. 21:1112–1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nitzsche A, Paulus C, Nevels M. 2008. Temporal dynamics of cytomegalovirus chromatin assembly in productively infected human cells. J. Virol. 82:11167–11180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wright E, Bain M, Teague L, Murphy J, Sinclair J. 2005. Ets-2 repressor factor recruits histone deacetylase to silence human cytomegalovirus immediate-early gene expression in non-permissive cells. J. Gen. Virol. 86:535–544 [DOI] [PubMed] [Google Scholar]
- 31. Huang Y, Tang Q, Nguyen M, Dulal K, Wang W, Zhu H. 2011. Histone deacetylase 3, not histone deacetylase 2, interacts with the major immediate early locus of human cytomegalovirus. Virol. J. 8:151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Saffert RT, Kalejta RF. 2006. Inactivating a cellular intrinsic immune defense mediated by Daxx is the mechanism through which the human cytomegalovirus pp71 protein stimulates viral immediate-early gene expression. J. Virol. 80:3863–3871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Li H, Leo C, Zhu J, Wu X, O'Neil J, Park EJ, Chen JD. 2000. Sequestration and inhibition of Daxx-mediated transcriptional repression by PML. Mol. Cell. Biol. 20:1784–1796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tang Q, Maul GG. 2003. Mouse cytomegalovirus immediate-early protein 1 binds with host cell repressors to relieve suppressive effects on viral transcription and replication during lytic infection. J. Virol. 77:1357–1367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nevels M, Paulus C, Shenk T. 2004. Human cytomegalovirus immediate-early 1 protein facilitates viral replication by antagonizing histone deacetylation. Proc. Natl. Acad. Sci. U. S. A. 101:17234–17239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Park JJ, Kim YE, Pham HT, Kim ET, Chung YH, Ahn JH. 2007. Functional interaction of the human cytomegalovirus IE2 protein with histone deacetylase 2 in infected human fibroblasts. J. Gen. Virol. 88:3214–3223 [DOI] [PubMed] [Google Scholar]
- 37. Reeves M, Murphy J, Greaves R, Fairley J, Brehm A, Sinclair J. 2006. Autorepression of the human cytomegalovirus major immediate-early promoter/enhancer at late times of infection is mediated by the recruitment of chromatin remodeling enzymes by IE86. J. Virol. 80:9998–10009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Savaryn JP, Reitsma JM, Bigley TM, Halligan BD, Qian Z, Yu D, Terhune SS. 2013. Human cytomegalovirus pUL29/28 and pUL38 repression of p53-regulated p21CIP1 and caspase 1 promoters during infection. J. Virol. 87:2463–2474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Poon AP, Liang Y, Roizman B. 2003. Herpes simplex virus 1 gene expression is accelerated by inhibitors of histone deacetylases in rabbit skin cells infected with a mutant carrying a cDNA copy of the infected-cell protein no. 0. J. Virol. 77:12671–12678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Walters MS, Erazo A, Kinchington PR, Silverstein S. 2009. Histone deacetylases 1 and 2 are phosphorylated at novel sites during varicella-zoster virus infection. J. Virol. 83:11502–11513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Walters MS, Kinchington PR, Banfield BW, Silverstein S. 2010. Hyperphosphorylation of histone deacetylase 2 by alphaherpesvirus US3 kinases. J. Virol. 84:9666–9676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yu D, Smith GA, Enquist LW, Shenk T. 2002. Construction of a self-excisable bacterial artificial chromosome containing the human cytomegalovirus genome and mutagenesis of the diploid TRL/IRL13 gene. J. Virol. 76:2316–2328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Murphy E, Vanicek J, Robins H, Shenk T, Levine AJ. 2008. Suppression of immediate-early viral gene expression by herpesvirus-coded microRNAs: implications for latency. Proc. Natl. Acad. Sci. U. S. A. 105:5453–5458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Warming S, Costantino N, Court DL, Jenkins NA, Copeland NG. 2005. Simple and highly efficient BAC recombineering using galK selection. Nucleic Acids Res. 33:e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yang WM, Yao YL, Sun JM, Davie JR, Seto E. 1997. Isolation and characterization of cDNAs corresponding to an additional member of the human histone deacetylase gene family. J. Biol. Chem. 272:28001–28007 [DOI] [PubMed] [Google Scholar]
- 46. Terhune S, Torigoi E, Moorman N, Silva M, Qian Z, Shenk T, Yu D. 2007. Human cytomegalovirus UL38 protein blocks apoptosis. J. Virol. 81:3109–3123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhu H, Shen Y, Shenk T. 1995. Human cytomegalovirus IE1 and IE2 proteins block apoptosis. J. Virol. 69:7960–7970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Halligan BD, Greene AS. 2011. Visualize: a free and open source multifunction tool for proteomics data analysis. Proteomics 11:1058–1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Prichard MN. 2009. Function of human cytomegalovirus UL97 kinase in viral infection and its inhibition by maribavir. Rev. Med. Virol. 19:215–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Biron KK, Harvey RJ, Chamberlain SC, Good SS, Smith AA, III, Davis MG, Talarico CL, Miller WH, Ferris R, Dornsife RE, Stanat SC, Drach JC, Townsend LB, Koszalka GW. 2002. Potent and selective inhibition of human cytomegalovirus replication by 1263W94, a benzimidazole L-riboside with a unique mode of action. Antimicrob. Agents Chemother. 46:2365–2372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chou S, Marousek GI. 2008. Accelerated evolution of maribavir resistance in a cytomegalovirus exonuclease domain II mutant. J. Virol. 82:246–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Shannon-Lowe CD, Emery VC. 2010. The effects of maribavir on the autophosphorylation of ganciclovir resistant mutants of the cytomegalovirus UL97 protein. Herpesviridae 1:4. 10.1186/2042-4280-1-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Reitsma JM, Savaryn JP, Faust K, Sato H, Halligan BD, Terhune SS. 2011. Antiviral inhibition targeting the HCMV kinase pUL97 requires pUL27-dependent degradation of Tip60 acetyltransferase and cell-cycle arrest. Cell Host Microbe 9:103–114 [DOI] [PubMed] [Google Scholar]
- 54. Prichard MN, Gao N, Jairath S, Mulamba G, Krosky P, Coen DM, Parker BO, Pari GS. 1999. A recombinant human cytomegalovirus with a large deletion in UL97 has a severe replication deficiency. J. Virol. 73:5663–5670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Nowak B, Gmeiner A, Sarnow P, Levine AJ, Fleckenstein B. 1984. Physical mapping of human cytomegalovirus genes: identification of DNA sequences coding for a virion phosphoprotein of 71 kDa and a viral 65-kDa polypeptide. Virology 134:91–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Gilbert MJ, Riddell SR, Plachter B, Greenberg PD. 1996. Cytomegalovirus selectively blocks antigen processing and presentation of its immediate-early gene product. Nature 383:720–722 [DOI] [PubMed] [Google Scholar]
- 57. McLaughlin-Taylor E, Pande H, Forman SJ, Tanamachi B, Li CR, Zaia JA, Greenberg PD, Riddell SR. 1994. Identification of the major late human cytomegalovirus matrix protein pp65 as a target antigen for CD8+ virus-specific cytotoxic T lymphocytes. J. Med. Virol. 43:103–110 [DOI] [PubMed] [Google Scholar]
- 58. Odeberg J, Plachter B, Branden L, Soderberg-Naucler C. 2003. Human cytomegalovirus protein pp65 mediates accumulation of HLA-DR in lysosomes and destruction of the HLA-DR alpha-chain. Blood 101:4870–4877 [DOI] [PubMed] [Google Scholar]
- 59. Schmolke S, Drescher P, Jahn G, Plachter B. 1995. Nuclear targeting of the tegument protein pp65 (UL83) of human cytomegalovirus: an unusual bipartite nuclear localization signal functions with other portions of the protein to mediate its efficient nuclear transport. J. Virol. 69:1071–1078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Chambers J, Angulo A, Amaratunga D, Guo H, Jiang Y, Wan JS, Bittner A, Frueh K, Jackson MR, Peterson PA, Erlander MG, Ghazal P. 1999. DNA microarrays of the complex human cytomegalovirus genome: profiling kinetic class with drug sensitivity of viral gene expression. J. Virol. 73:5757–5766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Meier JL. 2001. Reactivation of the human cytomegalovirus major immediate-early regulatory region and viral replication in embryonal NTera2 cells: role of trichostatin A, retinoic acid, and deletion of the 21-base-pair repeats and modulator. J. Virol. 75:1581–1593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Beckers T, Burkhardt C, Wieland H, Gimmnich P, Ciossek T, Maier T, Sanders K. 2007. Distinct pharmacological properties of second generation HDAC inhibitors with the benzamide or hydroxamate head group. Int. J. Cancer 121:1138–1148 [DOI] [PubMed] [Google Scholar]
- 63. Alland L, Muhle R, Hou H, Jr, Potes J, Chin L, Schreiber-Agus N, DePinho RA. 1997. Role for N-CoR and histone deacetylase in Sin3-mediated transcriptional repression. Nature 387:49–55 [DOI] [PubMed] [Google Scholar]
- 64. Hassig CA, Fleischer TC, Billin AN, Schreiber SL, Ayer DE. 1997. Histone deacetylase activity is required for full transcriptional repression by mSin3A. Cell 89:341–347 [DOI] [PubMed] [Google Scholar]
- 65. Heinzel T, Lavinsky RM, Mullen TM, Soderstrom M, Laherty CD, Torchia J, Yang WM, Brard G, Ngo SD, Davie JR, Seto E, Eisenman RN, Rose DW, Glass CK, Rosenfeld MG. 1997. A complex containing N-CoR, mSin3 and histone deacetylase mediates transcriptional repression. Nature 387:43–48 [DOI] [PubMed] [Google Scholar]
- 66. Laherty CD, Yang WM, Sun JM, Davie JR, Seto E, Eisenman RN. 1997. Histone deacetylases associated with the mSin3 corepressor mediate mad transcriptional repression. Cell 89:349–356 [DOI] [PubMed] [Google Scholar]
- 67. Yang WM, Inouye C, Zeng Y, Bearss D, Seto E. 1996. Transcriptional repression by YY1 is mediated by interaction with a mammalian homolog of the yeast global regulator RPD3. Proc. Natl. Acad. Sci. U. S. A. 93:12845–12850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Nicolas E, Roumillac C, Trouche D. 2003. Balance between acetylation and methylation of histone H3 lysine 9 on the E2F-responsive dihydrofolate reductase promoter. Mol. Cell. Biol. 23:1614–1622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Sengupta N, Seto E. 2004. Regulation of histone deacetylase activities. J. Cell. Biochem. 93:57–67 [DOI] [PubMed] [Google Scholar]
- 70. Poon AP, Gu H, Roizman B. 2006. ICP0 and the US3 protein kinase of herpes simplex virus 1 independently block histone deacetylation to enable gene expression. Proc. Natl. Acad. Sci. U. S. A. 103:9993–9998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Cai R, Kwon P, Yan-Neale Y, Sambuccetti L, Fischer D, Cohen D. 2001. Mammalian histone deacetylase 1 protein is posttranslationally modified by phosphorylation. Biochem. Biophys. Res. Commun. 283:445–453 [DOI] [PubMed] [Google Scholar]
- 72. Huttlin EL, Jedrychowski MP, Elias JE, Goswami T, Rad R, Beausoleil SA, Villen J, Haas W, Sowa ME, Gygi SP. 2010. A tissue-specific atlas of mouse protein phosphorylation and expression. Cell 143:1174–1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Pflum MK, Tong JK, Lane WS, Schreiber SL. 2001. Histone deacetylase 1 phosphorylation promotes enzymatic activity and complex formation. J. Biol. Chem. 276:47733–47741 [DOI] [PubMed] [Google Scholar]
- 74. Galasinski SC, Resing KA, Goodrich JA, Ahn NG. 2002. Phosphatase inhibition leads to histone deacetylases 1 and 2 phosphorylation and disruption of corepressor interactions. J. Biol. Chem. 277:19618–19626 [DOI] [PubMed] [Google Scholar]
- 75. Karwowska-Desaulniers P, Ketko A, Kamath N, Pflum MK. 2007. Histone deacetylase 1 phosphorylation at S421 and S423 is constitutive in vivo, but dispensable in vitro. Biochem. Biophys. Res. Commun. 361:349–355 [DOI] [PubMed] [Google Scholar]
- 76. Pluemsampant S, Safronova OS, Nakahama K, Morita I. 2008. Protein kinase CK2 is a key activator of histone deacetylase in hypoxia-associated tumors. Int. J. Cancer 122:333–341 [DOI] [PubMed] [Google Scholar]
- 77. Tsai SC, Seto E. 2002. Regulation of histone deacetylase 2 by protein kinase CK2. J. Biol. Chem. 277:31826–31833 [DOI] [PubMed] [Google Scholar]
- 78. Mounce BC, Mboko WP, Bigley TM, Terhune SS, Tarakanova VL. 2013. A conserved gammaherpesvirus protein kinase targets histone deacetylases 1 and 2 to facilitate viral replication in primary macrophages. J. Virol. 87:7314–7325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Rush J, Moritz A, Lee KA, Guo A, Goss VL, Spek EJ, Zhang H, Zha XM, Polakiewicz RD, Comb MJ. 2005. Immunoaffinity profiling of tyrosine phosphorylation in cancer cells. Nat. Biotechnol. 23:94–101 [DOI] [PubMed] [Google Scholar]
- 80. Olsen JV, Blagoev B, Gnad F, Macek B, Kumar C, Mortensen P, Mann M. 2006. Global, in vivo, and site-specific phosphorylation dynamics in signaling networks. Cell 127:635–648 [DOI] [PubMed] [Google Scholar]
- 81. Wang B, Malik R, Nigg EA, Korner R. 2008. Evaluation of the low-specificity protease elastase for large-scale phosphoproteome analysis. Anal. Chem. 80:9526–9533 [DOI] [PubMed] [Google Scholar]
- 82. Ahn JH, Hayward GS. 1997. The major immediate-early proteins IE1 and IE2 of human cytomegalovirus colocalize with and disrupt PML-associated nuclear bodies at very early times in infected permissive cells. J. Virol. 71:4599–4613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Cristea IM, Moorman NJ, Terhune SS, Cuevas CD, O'Keefe ES, Rout MP, Chait BT, Shenk T. 2010. Human cytomegalovirus pUL83 stimulates activity of the viral immediate-early promoter through its interaction with the cellular IFI16 protein. J. Virol. 84:7803–7814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Mitchell DP, Savaryn JP, Moorman NJ, Shenk T, Terhune SS. 2009. Human cytomegalovirus UL28 and UL29 open reading frames encode a spliced mRNA and stimulate accumulation of immediate-early RNAs. J. Virol. 83:10187–10197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Liu Y, Biegalke BJ. 2002. The human cytomegalovirus UL35 gene encodes two proteins with different functions. J. Virol. 76:2460–2468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Schierling K, Buser C, Mertens T, Winkler M. 2005. Human cytomegalovirus tegument protein ppUL35 is important for viral replication and particle formation. J. Virol. 79:3084–3096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Lukashchuk V, McFarlane S, Everett RD, Preston CM. 2008. Human cytomegalovirus protein pp71 displaces the chromatin-associated factor ATRX from nuclear domain 10 at early stages of infection. J. Virol. 82:12543–12554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Ahn JH, Hayward GS. 2000. Disruption of PML-associated nuclear bodies by IE1 correlates with efficient early stages of viral gene expression and DNA replication in human cytomegalovirus infection. Virology 274:39–55 [DOI] [PubMed] [Google Scholar]
- 89. Korioth F, Maul GG, Plachter B, Stamminger T, Frey J. 1996. The nuclear domain 10 (ND10) is disrupted by the human cytomegalovirus gene product IE1. Exp. Cell Res. 229:155–158 [DOI] [PubMed] [Google Scholar]