Summary
A global human immunodeficiency virus-1 (HIV-1) vaccine will have to elicit immune responses capable of providing protection against a tremendous diversity of HIV-1 variants. In this review, we first describe the current state of the HIV-1 vaccine field, outlining the immune responses that are desired in a global HIV-1 vaccine. In particular, we emphasize the likely importance of Env-specific neutralizing and non-neutralizing antibodies for protection against HIV-1 acquisition and the likely importance of effector Gag-specific T lymphocytes for virologic control. We then highlight four strategies for developing a global HIV-1 vaccine. The first approach is to design specific vaccines for each geographic region that include antigens tailor-made to match local circulating HIV-1 strains. The second approach is to design a vaccine that will elicit Env-specific antibodies capable of broadly neutralizing all HIV-1 subtypes. The third approach is to design a vaccine that will elicit cellular immune responses that are focused on highly conserved HIV-1 sequences. The fourth approach is to design a vaccine to elicit highly diverse HIV-1-specific responses. Finally, we emphasize the importance of conducting clinical efficacy trials as the only way to determine which strategies will provide optimal protection against HIV-1 in humans.
Keywords: HIV, vaccines, viral diversity, immune responses
This article is part of a series of reviews covering HIV Immunology appearing in Volume 254 of Immunological Reviews.
Introduction
A global human immunodeficiency virus-1 (HIV-1) vaccine will need to elicit durable, potent, and comprehensive immune responses to provide protection against highly diverse HIV-1 variants 1–2. There is more reason than ever to be hopeful about the development of an effective global HIV-1 vaccine. The RV144 trial conducted in Thailand demonstrated that an HIV-1 vaccine was capable of eliciting modest and transient protection against HIV-1 acquisition 3. A follow-up evaluation of the immune correlates of reduced HIV-1 risk in RV144 has revealed hopeful leads on how to improve vaccine efficacy, particularly in terms of the importance of Env-specific antibodies 4. Meanwhile, recent advances have allowed the discovery and characterization of broadly reactive neutralizing antibodies isolated from HIV-1-infected individuals 5–11. Recent studies of acute HIV-1 infection have also shown that the viral burden at the point of transmission may not be as formidable as first believed 12, involving single transmitter/founder viruses that may be easier to neutralize 13.
Given the vast diversity of HIV-1 worldwide, an effective global HIV-1 vaccine will need to provide protection against a diverse landscape of HIV-1 sequences in multiple demographic populations. Moreover, the development of a global HIV-1 vaccine will require large-scale clinical trials in human subjects. In this review, we first describe the current state of the HIV-1 vaccine field, outlining immune responses that may be desirable in a global HIV-1 vaccine. We then discuss select strategies for addressing the challenge of HIV-1 diversity.
A global vaccine needs a global reach
While there are many challenges in HIV-1 vaccine development, a key hurdle is the tremendous genetic diversity of globally circulating strains of HIV-1 14–19. Because of the ability of HIV-1 to evade immune responses through mutational escape, there is constant viral evolution within populations and individual hosts. The genetic diversity of HIV-1 is attributable in part to the low fidelity of its reverse transcriptase, the large number of replication cycles of the virus, the influence of innate and adaptive immune responses, and the ability for HIV-1 to tolerate this diversity 14.
There are thirteen distinct HIV-1 subtypes and sub-subtypes that are linked geographically or epidemiologically, with within-subtype variation of envelope proteins of 15–20%, and between-subtype variation of up to 35% 14,15. Moreover, there are additional circulating recombinant forms (CRFs) generated from genetic mixing in persons dually infected with different subtypes. HIV-1 also diversifies extensively within each host. For example, Korber et al. 21 have demonstrated that the variability of HIV-1 within one host is comparable to the global variation of influenza A. This genetic diversity makes it difficult to design an HIV-1 vaccine that will be immunologically relevant in the face of such a variety of HIV-1 sequences.
Env-specific antibodies to protect against HIV-1 acquisition
HIV-1 genetic diversity makes it particularly challenging to design a vaccine that can elicit broadly protective antibodies. There is an increasing consensus that antibodies specific to HIV-1 envelope (Env) will likely be required to block acquisition of HIV-1 22–23. Follow-up analyses of RV144 showed that antibodies to variable loops 1 and 2 (V1V2) regions of HIV-1 Env were associated with a reduced risk of HIV-1 acquisition 3,4. A recent genetic analysis provided further support for the importance of V2-specific antibodies by demonstrating that the RV144 vaccine had increased efficacy against viruses that matched the Env immunogen in the V2 location 25. Similarly, a recent study in non-human primates from our group demonstrated that SIV vaccines using adenovirus and poxvirus vectors afforded partial protection against neutralization-resistant SIVmac251 acquisition in rhesus monkeys, and that Env-specific antibodies were associated with decreased SIV infection risk 26. These vaccine-elicited antibodies included antibodies against V2 as well as other epitopes. Our group also demonstrated that Env was required to achieve significant protection against SIVmac251 challenge, and similar correlates have been reported by other laboratories against SIVsmE660 challenges 27–28.
Neutralizing Env-specific antibodies have also been shown to protect against HIV-1 and simian/human immunodeficiency virus (SHIV) acquisition in passive transfer experiments in non-human primates 29–39. For example, passive transfer of the neutralizing monoclonal antibodies 2F5, 2G12, and 4E10 was shown by Mascola, Hessell, and others 31–34 to afford partial protection against intravenous and mucosal SHIV challenge. In addition, Hessell, Burton, and colleagues 35,36 showed that high serum concentrations of the neutralizing monoclonal antibody b12 protected macaques from intravenous SHIV challenge and that low concentrations of b12 protected against low-dose intravaginal SHIV challenge. Monoclonal b12 has also been shown to protect against SHIV infection when given at high-doses intravaginally 37–38. More recently, the potent neutralizing antibody PGT121 was shown to protect against high-dose mucosal SHIV challenge in macaques at serum concentrations significantly lower than needed for protection in prior studies 39.
Non-neutralizing antibodies might also have the potential to afford partial protection against HIV-1 infection 40–41. Non-neutralizing antibodies include various effector functions mediated by the Fc region of the antibody, which triggers the innate immune system to destroy the virus or virus-infected cells. Follow-up analysis of RV144 showed that in participants with low serum immunoglobulin A (IgA) responses, high levels of antibody-dependent cellular cytotoxicity (ADCC) correlated with a reduced risk of HIV-1 infection 42. In addition, Liao and colleagues 43 recently demonstrated that V2-specific antibodies isolated from RV144 vaccines mediated ADCC against HIV-1-infected CD4+ T cells from RV144 subjects with breakthrough infections, and that this activity was dependent on V2 position 169 in breakthrough Envs. Theoretically, increased serum IgA responses might blunt the protective action of non-neutralizing antibodies because the IgA Fc region does not mediate the same effector functions, which might explain why serum IgA levels positively correlated with HIV-1 infection risk in RV144 vaccinees 4. It has also been shown that broadly neutralizing antibodies also rely on the Fc region for part of their protective activity 7.
The above studies suggest that Env-specific antibodies will likely be necessary to protect against HIV-1 acquisition. However, these studies do not directly address the challenge of HIV-1 diversity. For example, the immunogens used in RV144 matched the local Thai circulating strains of subtype B and the circulating recombinant form CRF01_AE 3. It is likely that the Env-specific antibodies elicited in RV144 would afford a lower degree of protection against other subtypes of HIV-1 found elsewhere in the world. However, it remains unclear how a vaccine can elicit antibodies that will recognize the substantial heterogeneity of Env sequences globally. Moreover, HIV-1 has other mechanisms to evade the humoral immune system, including low Env spike density on the virion surface, heavy glycosylation, conformational shielding of highly conserved Env epitopes, and mimicry of Env carbohydrates and proteins of host molecules 9–45.
Cellular immune responses for virologic control
Given the challenges in eliciting broadly protective Env-specific antibodies, it is not likely that any vaccine would achieve 100% sterilizing immunity in all vaccinees; breakthrough HIV-1 infections will likely occur. Thus, it would be beneficial for an HIV-1 vaccine also to elicit immune responses capable of controlling viral replication 46. A wealth of the literature has shown that cellular immune responses can mediate control of viremia in HIV-1-infected humans and SIV-infected rhesus monkeys, including CD8+ T lymphocytes 47–57, NK cells 58, and CD4+ T lymphocytes 59–60. Moreover, vaccine trials in non-human primates have shown that sustained virologic control is achievable after heterologous SIV challenges. For example, our group has shown that adenovirus serotype 26 prime and modified vaccinia Ankara (MVA) boost expressing SIV antigens led to a 2.32 log reduction in mean set point viral load following stringent SIVmac251 challenge, and immune correlates of virologic control included the magnitude and breadth of Gag-specific cellular immune responses 26. Hansen et al. 56 also demonstrated that early profound and durable control of SIV replication were achieved in approximately half of rhesus monkeys immunized with a rhesus cytomegalovirus vector-based vaccine.
Whereas Env-specific antibodies appear necessary to block HIV-1 acquisition, Gag-specific cellular immune responses appear important for virologic control. For example, we have shown that Gag-specific CD8+ T cells correlated with both in vivo and in vitro virologic control following SIV challenge in vaccinated monkeys; no association was seen with Env- or Pol-specific CD8+ T cells 61. This result is consistent with studies demonstrating the association of Gag-specific cellular immune responses with virologic control in HIV-1-infected individuals 62–68 and SIV-infected rhesus monkeys 26–71. In addition to Gag, Vif and Nef may contribute to virologic control in certain settings, such as Mamu-B*08 monkeys 72.
Another critical aspect of cellular immune responses is the location and phenotype of cellular immune responses elicited by vaccination. For example, Fukazawa and colleagues 73 demonstrated that the degree of protection mediated by a live attenuated SIV vaccine strongly correlated with the magnitude and function of SIV-specific, effector T cells in lymph nodes. They also demonstrated that the maintenance of these protective T cells was associated with the persistent replication of vaccine virus in follicular helper T cells.
Despite these observations in non-human primates, virologic control has yet to be achieved in clinical trials of HIV-1 vaccines in human subjects. Neither VAX003/004, the Step study, nor RV144 showed significant impact on viral loads in vaccine recipients who became infected with HIV-1 3,74. However, there was evidence for immune selection pressure on breakthrough HIV-1 sequences in the Step study, suggesting that vaccine-elicited cellular immune responses can exert immunologically relevant biologic effects in humans 76.
Strategies for a global HIV-1 vaccine
The current state of HIV-1 vaccine research suggests that an effective global HIV-1 vaccine will need to elicit Env-specific antibodies to block HIV-1 acquisition and that these humoral immune responses will need to include either neutralizing or non-neutralizing antibodies (Fig. 1). In addition, a global HIV-1 vaccine will need to elicit cellular immune responses to control viral replication for breakthrough HIV-1 infections. These cellular immune responses will most likely need to include Gag-specific CD8+ T cells.
Figure 1.
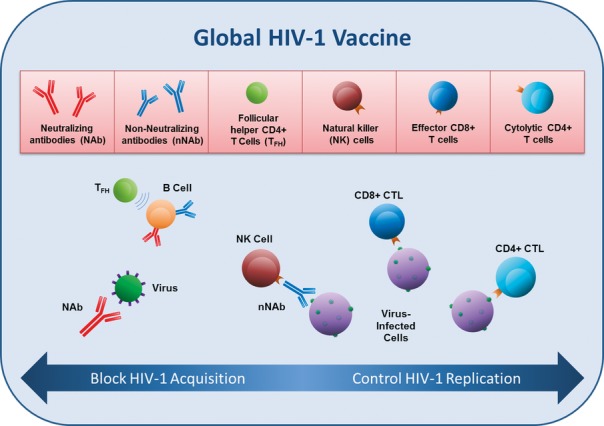
Immune responses targeted by a global HIV–1 vaccine.
It is unclear which HIV-1 antigens to include in a vaccine to address the challenge of global HIV-1 sequence diversity. Currently, there are four major strategies toward selecting antigens for a global HIV-1 vaccine (Table 1). The first approach is to design specific vaccines for each geographic region that include antigens tailor-made to match local circulating HIV-1 strains. The goal of these vaccines is to elicit HIV-1 subtype-specific immune responses that will have a higher likelihood of recognizing local strains. The second approach is to design a vaccine that will elicit Env-specific antibodies capable of broadly neutralizing all HIV-1 subtypes. The third approach is to design a vaccine that will elicit immune responses that are focused on highly conserved HIV-1 sequences. The rationale for this strategy is that these responses will recognize a multitude of different HIV-1 subtypes via a shared epitope target. The fourth strategy is to design a vaccine to elicit highly diverse HIV-1-specific responses. Here the rationale is that the greater the breadth and depth of HIV-1 epitopes recognized by vaccinees, the greater the chance that these immune responses will match the transmitting HIV-1 strain.
Table 1.
Strategies to overcome the challenge of HIV-1 diversity
| Vaccines to elicit regional HIV-1-specific immune responses |
| Subtype AE immunogens for Southeast Asia |
| Subtype C immunogens for South Africa |
| Subtype A immunogens for East Africa |
| Subtype B immunogens for United States and Europe |
| Vaccines to elicit broadly neutralizing antibodies |
| Env monomer immunogens |
| Env trimer immunogens |
| Scaffolded neutralizing antibody epitopes |
| Germline targeted immunogens |
| Vaccines to elicit highly conserved HIV-1-specific cellular immune responses |
| Conserved epitope immunogens |
| Conserved region immunogens |
| Sector immunogens |
| Vaccines to elicit highly diverse HIV-1-specific immune responses |
| Multi-clade immunogens |
| Mosaic immunogens |
Vaccines to elicit regional HIV-1-specific immune responses
One approach to overcoming the challenge of HIV-1 diversity is to design region-specific vaccines to elicit immune responses specific to local circulating HIV-1 strains. Such a region-specific vaccine strategy was adopted in RV144, which used immunogens that matched the local Thai circulating strains of subtype B and the circulating recombinant form CRF01_AE 3. As discussed above, it would not be likely that such a region-specific vaccine would be relevant in other regions with different subtypes. Such vaccines would therefore need to be tailored for specific regions of the world. For example, as a follow-up to RV144, ALVAC vectors and gp120 proteins are being produced with subtype C immunogens for future clinical trials in South Africa 12. In theory, similar vaccines could be developed for subtype B in North America and Europe, subtype A in east Africa, and so on. A limitation of this approach is that it would likely be very difficult to test and license multiple HIV-1 vaccines in different regions of the world. Moreover, many regions such as central Africa have multiple circulating subtypes, sub-subtypes, and CRFs in one area.
Even if it proves difficult to develop multiple region-specific vaccines, there is substantial interest in improving the RV144 vaccine for use in Thailand. As noted above, the ALVAC vector and gp120 protein used in RV144 provided 31% protection against HIV-1 acquisition. While this degree of protection was modest and transient, there is interest in improving the RV144 vaccine regimen for possible licensure in high-risk populations 12. It was noted that vaccine efficacy in RV144 appeared to decline from 60% at 1 year to 31% at 3.5 years, suggesting that increasing the frequency and number of booster doses might improve vaccine efficacy. One approach to building on RV144, therefore, is to use essentially the same vaccine regimen (ALVAC prime/gp120 boost) but expand the immunization schedule significantly; clinical trials in Thailand of such an approach are planned 12.
In addition to changing the RV144 vaccine schedule, there are also efforts to improve upon the ALVAC vector used in RV144. Like other poxviruses, ALVAC is well suited to be a vaccine vector because of its large genome (allowing for the integration of foreign DNA), thermostability, and the fact that genome replication occurs in the cytoplasm 77. It was advanced into Phase III trials in Thailand based on earlier clinical studies that showed that ALVAC vectors expressing subtype B Gag and CRF01_AE Env elicited antibody responses and cellular immune responses 12–78. Nevertheless, alternative poxvirus vectors, such as MVA and NYVAC, are candidates for replacing ALVAC in RV144-like vaccine formulations 12–84. Future studies using NYVAC vectors and gp120 protein boosts are planned for South Africa.
Vaccines to elicit broadly neutralizing antibodies
A prominent but elusive aim of the field is to develop an HIV-1 vaccine that will elicit antibodies that can neutralize all circulating HIV-1 sequences 6–10. Neutralizing antibodies do not develop until late in natural infection and in only 10–30% of HIV-1-infected individuals 9–85. Until recently, the field was limited by relatively few broadly neutralizing monoclonal antibodies and a limited number of epitopic targets 6. However, recent developments in high throughput single-cell BCR-amplification assays have helped revolutionize the field, leading to the isolation and characterization of dozens of new broadly neutralizing monoclonal antibodies 11–90. To date, four highly conserved regions on HIV-1 Env are targeted by broadly neutralizing antibodies, including the CD4+ binding site (CD4bs), a quaternary site on the V1V2 loops, carbohydrates on the outer domain, and the membrane-proximal external region 9. For example, Walker and colleagues 11 described 17 new PGT antibodies that neutralize broadly across subtypes, some of which were 10-fold more potent than the broadly neutralizing antibodies PG9, PG16, and VRC01. In addition, Scheid and colleagues 87 identified a novel class of potent antibodies that mimic CD4 binding entitled ‘highly active agonistic CD4bs antibodies’, which include broadly neutralizing antibodies NIH45-46 and 3BNC117. Huang and colleagues have also described 10E8, a HIV-1 gp41 membrane-proximal external region-specific antibody that neutralized approximately 98 percent of tested viruses, which was non-self-reactive 90.
Despite the discovery of these remarkable broadly neutralizing monoclonal antibodies, it is still unclear how to elicit such antibodies by immunization. In fact, a major gap in the HIV-1 vaccine field is the absence of immunogens capable of eliciting neutralizing antibodies of substantial breadth. Many of the neutralizing monoclonal antibodies arise from extensive somatic mutation of heavy chains after years of chronic viral infection. The current effort to elicit broadly neutralizing antibodies via immunization uses the structure of previously identified neutralizing antibodies as a starting point for immunogen design. For example, several laboratories have used previously identified antibodies, such as the V1V2-directed antibodies PG9, PG16, and CH01-CH04, to screen for binding to gp120 envelope monomers which can then be developed into potential immunogen candidates 10. Other researchers have centered on designing Env immunogens to elicit antibodies that resemble the VRC01 antibody, which binds the highly conserved conformational CD4 binding site 10–44. Protein scaffolds have also been used to express neutralizing epitopes 91,92.
Env proteins might be trimers, monomers, or scaffolded neutralizing antibody epitopes, but all face the same challenge of achieving extensive somatic mutation seen in the broadly reactive neutralizing monoclonal antibodies isolated from chronically infected individuals. One approach to overcome this challenge is to design vaccines that target the germline precursors of the desired antibodies, and that aim to drive appropriate affinity maturation, so-called ‘B-cell-lineage vaccine design’ 45,86. Another novel approach is to use vector-mediated gene transfer to produce broadly neutralizing antibodies directly 95–96.
Vaccines to elicit highly conserved HIV-1-specific cellular immune responses
A third approach to address the challenge of HIV-1 diversity is to design vaccines that will elicit cellular immune responses specific to highly conserved HIV-1 regions. The hypothesis underlying this strategy is that immune responses specific for conserved HIV-1 regions will recognize a multitude of different HIV-1 subtypes as the diverse strains all share a common highly conserved epitope target and that these immune responses will impose a high fitness cost on any HIV-1 escape viral mutants 18–99. An initial emphasis was on selecting natural sequence antigens that may be most conserved among circulating HIV-1 strains. The Step study adopted this approach, using an adenovirus serotype 5 vector to express subtype B Gag, Pol, and Nef sequences that were selected to be phylogenetically close to consensus B sequences 100–101. There is evidence that this vaccine exerted immune selection pressure on breakthrough HIV-1 sequences, but cellular immune breadth was narrow and insufficient to mediate virologic control 76.
Several other HIV-1 vaccine immunogens have been designed with the goal of inducing responses against conserved epitopes, with varying success in preclinical and clinical studies. For example, the HIVA immunogen, first described in 2000, was derived from the p24 and p17 segments of HIV-1 clade A Gag fused to a string of 25 partially overlapping cytotoxic T-lymphocyte (CTL) epitopes 102. HIVA was shown to induce multiple HIV-1-specific CTL epitopes when expressed by DNA and MVA vectors in rhesus monkeys 103 and in a small phase 1 clinical trial in humans 104. However, when the HIVA immunogen was tried in larger clinical trials, the immunogen induced minimal HIV-1-specific T lymphocyte responses 105. Similarly, the EP HIV-1090 immunogen, first described in 2003 as part of a DNA vaccine, was derived from 21 CTL epitopes of HIV-1 that bound multiple HLA types and represented conserved sequences from multiple HIV-1 subtypes 106. When tested in humans, EP HIV-1090 and a later version, EP-1233, were both poorly immunogenic 80–107. These studies suggested that polyepitope immunogens were not optimally processed or presented by human immune systems and were not good candidates for inducing conserved-specific T lymphocyte responses.
Another strategy is to include longer fragments of conserved HIV-1 regions, as is done in the immunogen HIVconsv. When expressed by DNA and viral vector vaccines, HIVconsv has been shown to be immunogenic in preclinical studies 108–109. However, we have shown in non-human primates that at least in certain situations full-length HIV-1 immunogens elicit increased magnitude and breadth of cellular immune responses compared with conserved-region-only HIV-1 immunogens 110. Phase 1 clinical trials of the safety and immunogenicity of HIVconsv are ongoing (NCT01151319 and NCT01024842).
A variation of this approach is to design immunogens based strictly on conserved HIV-1 segments with mutable regions excluded completely 111. In contrast to the sequences in HIVA and EP-1033, the conserved sequences included in these immunogens do not necessarily have to correspond to any known T-cell epitope. Similarly, Dahirel and colleagues proposed designing immunogens based on HIV-1 sequence sectors that exhibit higher order conservation as measured by random matrix theory 62. These are sectors in which multiple mutations are very rare, suggesting they are regions of immunologic vulnerability.
Vaccines to elicit highly diverse HIV-1-specific immune responses
The above vaccine strategies share a common goal to elicit immune responses specific to highly conserved HIV-1 regions, with the hypothesis that these responses will recognize conserved sequences shared by a wide variety of HIV-1 strains. A contrasting strategy is to design vaccines that elicit diverse immune responses specific for a broad array of HIV-1-specific sequences. The hypothesis underlying this strategy is that the greater and more diverse the immune responses, the greater the likelihood that there will be a match to the transmitting HIV-1 strain. Diverse immune responses include T-cell and B-cell specificities that recognize multiple HIV-1 regions (breadth) and also multiple variants of HIV-1 epitopes for each epitopic locus (depth).
Currently, there are two primary approaches for eliciting broad HIV-1-immune responses via vaccination. The first is to design multivalent immunogens that represent multiple different HIV-1 clades 112. For example, the U.S. Military HIV Research Program has developed HIV-1 immunogens based on the predominant HIV-1 subtypes in Kenya, Tanzania, Uganda, and Thailand 113, and a recent Phase I clinical study showed that this vaccine was well tolerated and elicited durable cell-mediated and humoral immune responses 82. Similarly, a phase II clinical trial sponsored by the HIV Vaccines Trial Network (HVTN 505, NCT00865566) tested the DNA prime/Ad5 boost vaccine expressing Env proteins from subtypes A, B, and C developed by the NIH Vaccine Research Center.
The second approach to eliciting broad immune responses is the design of so-called ‘mosaic’ immunogens 114. These immunogens are engineered by in silico analysis of global HIV-1 sequences to provide maximal coverage of viral sequence diversity 115. Several laboratories have shown that mosaic HIV-1 immunogens elicited a greater breadth and depth of HIV-1 cellular immune responses than consensus or natural HIV-1 immunogens in non-human primates, as well as comparable or improved Env-specific binding and neutralizing antibody responses 116–117. Moreover, full-length mosaic HIV-1 immunogens elicited greater immune responses than conserved-region-only HIV-1 immunogens 110. Based on these data, mosaic immunogens are progressing into clinical development, in the context of Ad26 and MVA vectors expressing mosaic HIV-1 Gag, Pol, and Env immunogens, as well as in DNA and NYVAC vectors expressing mosaic HIV-1 Env immunogens.
The vaccines described above are intended to be global vaccine concepts. Vaccine delivery vehicles will also need to be globally relevant. One strategy to avoid the problem of anti-vector immunity is to use plasmids containing HIV-1 DNA sequences, such as VRC-HIVDNA016, a 6-plasmid multiclade HIV-1 DNA vaccine used in HVTN 505 118–119. DNA vaccines can further be improved by electroporation 118. Another strategy to minimize anti-vector immunity is to use lower seroprevalence or non-human viruses as vectors 120–125. For example, the lower seroprevalence and low titer adenoviruses such as adenovirus serotype 26 (Ad26) and Ad35 are now being studied as HIV-1 vaccine vectors 120,121. Preclinical studies have shown that these adenoviruses have significant biologic differences from Ad5, the vector used in the Step study that suggested a possible increased risk of HIV-1 acquisition in the subset of vaccinees with baseline anti-vector antibodies 101–133. Ad26 and Ad35 vectors are therefore planned for further clinical development 134–135.
Conclusions
The tremendous global diversity of HIV-1 poses one of the greatest challenges for the development of an effective global HIV-1 vaccine. Recent research has underscored the importance of Env-specific antibodies for blocking HIV-1 acquisition and CD8+ T lymphocytes for mediating virologic control, yet the optimal strategy for confronting HIV-1 sequence diversity remains unknown. Here we have outlined four key strategies for developing a global HIV-1 vaccine, that is, to design vaccines that elicit (i) region-specific immune responses; (ii) broadly neutralizing antibodies; (iii) highly conserved cellular immune responses; or (iv) highly diverse immune responses. The only way to define which of these strategies will provide optimal protection against HIV-1 in humans will be to test a subset of the most promising vaccine strategies in clinical efficacy trials. By confronting the challenge of HIV-1 sequence diversity, the field can move closer to an effective global HIV-1 vaccine.
Acknowledgments
We acknowledge support from the National Institutes of Health (AI052074 to K. E. S.; AI100663, AI096040, AI095985, AI084794, AI078526, and OD011170 to D. H. B.), the Bill and Melinda Gates Foundation (OPP1040741, OPP1033091), the U.S. Military HIV Research Program (W81XWH-11-2-0174, W81XWH-07-2-0067), and the Ragon Institute of MGH, MIT, and Harvard. The authors have no conflicts of interest to declare.
References
- Walker BD, Ahmed R, Plotkin S. Use both arms to beat HIV. Nat Med. 2011;17:1194–1195. doi: 10.1038/nm.2529. [DOI] [PubMed] [Google Scholar]
- Picker LJ, Hansen SG, Lifson JD. New paradigms for HIV/AIDS vaccine development. Ann Rev Med. 2012;63:95–111. doi: 10.1146/annurev-med-042010-085643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rerks-Ngarm S, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009;361:2209–2220. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- Haynes BF, et al. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N Engl J Med. 2012;366:1275–1286. doi: 10.1056/NEJMoa1113425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonsignori M, et al. HIV-1 antibodies from infection and vaccination: insights for guiding vaccine design. TIM. 2012;20:532–539. doi: 10.1016/j.tim.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatatos L. HIV vaccine design: the neutralizing antibody conundrum. Curr Opin Immunol. 2012;24:316–323. doi: 10.1016/j.coi.2012.04.006. [DOI] [PubMed] [Google Scholar]
- Hessell AJ, et al. Fc receptor but not complement binding is important in antibody protection against HIV. Nature. 2007;449:101–104. doi: 10.1038/nature06106. [DOI] [PubMed] [Google Scholar]
- Burton DR, et al. Broadly neutralizing antibodies present new prospects to counter highly antigenically diverse viruses. Science. 2012;337:183–186. doi: 10.1126/science.1225416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders KO, Rudicell RS, Nabel GJ. The design and evaluation of HIV-1 vaccines. AIDS. 2012;26:1293–1302. doi: 10.1097/QAD.0b013e32835474d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong PD, Mascola JR, Nabel GJ. The changing face of HIV vaccine research. J Int AIDS Soc. 2012;15:17407–17412. doi: 10.7448/IAS.15.2.17407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker LM, et al. Broad neutralization coverage of HIV by multiple highly potent antibodies. Nature. 2011;477:466–470. doi: 10.1038/nature10373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connell RJ, Kim JH, Corey L, Michael NL. Human immunodeficiency virus vaccine trials. Cold Spring Harb Perspect Med. 2012;2:a007351. doi: 10.1101/cshperspect.a007351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar-Gonzalez JF, et al. Genetic identity, biological phenotype, and evolutionary pathways of transmitted/founder viruses in acute and early HIV-1 infection. J Exp Med. 2009;206:1273–1290. doi: 10.1084/jem.20090378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ndung'u T, Weiss RA. On HIV diversity. AIDS. 2012;26:1255–1260. doi: 10.1097/QAD.0b013e32835461b5. [DOI] [PubMed] [Google Scholar]
- Taylor BS, Sobieszcyk ME, McCutchan FE, Hammer SM. The challenge of HIV-1 subtype diversity. N Engl J Med. 2008;358:1590–1602. doi: 10.1056/NEJMra0706737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaschen B, et al. Diversity considerations in HIV-1 vaccine selection. Science. 2002;296:2354–2360. doi: 10.1126/science.1070441. [DOI] [PubMed] [Google Scholar]
- Korber BT, Foley B, Gaschen B, Kuiken C. Epidemiological and immunological implications of the global variability of HIV-1. In: Pantaleo G, Walker BD, editors. Retroviral Immunology: Immune Response and Restoration. New Jersey: Humana Press; 2001. pp. 1–32. [Google Scholar]
- Korber BT, Letvin NL, Haynes BF. T-cell vaccine strategies for human immunodeficiency virus, the virus with a thousand faces. J Virol. 2009;83:8300–8314. doi: 10.1128/JVI.00114-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barouch DH, Korber B. HIV-1 vaccine development after Step. Ann Rev Med. 2009;61:2. doi: 10.1146/annurev.med.042508.093728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemelaar J, et al. Global and regional distributions of HIV-1 genetic subtypes and recombinants in 2004. AIDS. 2006;20:W13–W23. doi: 10.1097/01.aids.0000247564.73009.bc. [DOI] [PubMed] [Google Scholar]
- Korber B, et al. Evolutionary and immunological implications of contemporary HIV-1 variation. Brit Med Bull. 2001;58:19–42. doi: 10.1093/bmb/58.1.19. [DOI] [PubMed] [Google Scholar]
- Mascola JR, Montefiori DC. The role of antibodies in HIV vaccines. Ann Rev Med. 2010;28:413–444. doi: 10.1146/annurev-immunol-030409-101256. [DOI] [PubMed] [Google Scholar]
- Hoxie JA. Toward an antibody-based HIV-1 vaccine. Ann Rev Med. 2010;61:135–152. doi: 10.1146/annurev.med.60.042507.164323. [DOI] [PubMed] [Google Scholar]
- Zolla-Pazner S, et al. Analysis of V2 antibody responses induced in vaccinees in the ALVAC/AIDSVAX HIV-1 vaccine efficacy trial. PLoS ONE. 2013;8:e53629. doi: 10.1371/journal.pone.0053629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolland M, et al. Increased HIV-1 vaccine efficacy against viruses with genetic signatures in Env V2. Nature. 2012;490:417–420. doi: 10.1038/nature11519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barouch DH, et al. Vaccine protection against acquisition of neutralization-resistant SIV challenges in rhesus monkeys. Nature. 2012;482:89–93. doi: 10.1038/nature10766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letvin NL, et al. Immune and genetic correlates of vaccine protection against mucosal infection by SIV in monkeys. Sci Transl Med. 2011;3:81ra36. doi: 10.1126/scitranslmed.3002351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai L, et al. Prevention of infection by a granulocyte-macrophage colony-stimulating factor co-expressing DNA/modified vaccinia Ankara simian immunodeficiency virus vaccine. J Infect Dis. 2011;204:164–173. doi: 10.1093/infdis/jir199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba TW, et al. Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian-human immunodeficiency virus infection. Nat Med. 2000;6:200–206. doi: 10.1038/72309. [DOI] [PubMed] [Google Scholar]
- Shibata R, et al. Neutralizing antibody directed against the HIV-1 envelope glycoprotein can completely block HIV-1/SIV chimeric virus infections of macaque monkeys. Nat Med. 1999;5:204–210. doi: 10.1038/5568. [DOI] [PubMed] [Google Scholar]
- Mascola JR, et al. Protection of macaques against pathogenic simian/human immunodeficiency virus 89.6PD by passive transfer of neutralizing antibodies. J Virol. 1999;73:4009–4018. doi: 10.1128/jvi.73.5.4009-4018.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascola JR, et al. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat Med. 2000;6:207–210. doi: 10.1038/72318. [DOI] [PubMed] [Google Scholar]
- Hessell AJ, et al. Broadly neutralizing human anti-HIV antibody 2G12 is effective in protection against mucosal SHIV challenge even at low serum neutralizing titers. PLoS Pathog. 2009;5:e1000433. doi: 10.1371/journal.ppat.1000433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessell AJ, et al. Broadly neutralizing monoclonal antibodies 2F5 and 4E10 directed against the human immunodeficiency virus type 1 gp41 membrane-proximal external region protect against mucosal challenge by simian-human immunodeficiency virus SHIVBa-L. J Virol. 2010;84:1302–1313. doi: 10.1128/JVI.01272-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parren WHI, et al. Antibody protects macaques against vaginal challenge with a pathogenic R5 simian/human immunodeficiency virus at serum levels giving complete neutralization in vitro. J Virol. 2001;75:8340–8347. doi: 10.1128/JVI.75.17.8340-8347.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessell AJ, et al. Effective, low-titer antibody protection against low-dose repeated mucosal SHIV challenge in macaques. Nat Med. 2009;15:951–954. doi: 10.1038/nm.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton DR, et al. Limited or no protection by weakly or nonneutralizing antibodies against vaginal SHIV challenge of macaques compared with a strongly neutralizing antibody. Proc Natl Acad Sci USA. 2011;108:11181–11186. doi: 10.1073/pnas.1103012108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veazey RS, et al. Prevention of virus transmission to macaque monkeys by vaginally applied monoclonal antibody to HIV-1 gp120. Nat Med. 2003;9:343–346. doi: 10.1038/nm833. [DOI] [PubMed] [Google Scholar]
- Moldt B, et al. Highly potent HIV-specific antibody neutralization in vitro translates into effective protection against mucosal SHIV challenge in vivo. Proc Natl Acad Sci USA. 2012;109:18921–18925. doi: 10.1073/pnas.1214785109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson HL. Non-neutralizing antibodies in prevention of HIV infection. Expert Opin Biol The. 2013;13:197–207. doi: 10.1517/14712598.2012.743527. [DOI] [PubMed] [Google Scholar]
- Hope TJ. To neutralize or not, a key HIV vaccine question. Nat Med. 2011;17:1195–1197. doi: 10.1038/nm.2528. [DOI] [PubMed] [Google Scholar]
- Bonsignori M, et al. Antibody-dependent cellular cytotoxicity-mediating antibodies from an HIV-1 vaccine efficacy trial target multiple epitopes and preferentially use the VH1 gene family. J Virol. 2012;86:11521–11532. doi: 10.1128/JVI.01023-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao HX, et al. Vaccine induction of antibodies against a structurally heterogeneous site of immune pressure within HIV-1 Envelope protein variable regions 1 and 2. Immunity. 2013;12:S1074–S7613. doi: 10.1016/j.immuni.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjelloun F, et al. Role of human immunodeficiency virus type 1 envelope structure in the induction of broadly neutralizing antibodies. J Virol. 2012;86:13152–13163. doi: 10.1128/JVI.01110-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes BF, et al. B-cell-lineage immunogen design in vaccine development with HIV-1 as a case study. Nat Biotechnol. 2012;30:423–433. doi: 10.1038/nbt.2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koup RA, Douek DC. Vaccine design for CD8 T lymphocyte responses. Cold Spring Harb Perspect Med. 2011;1:1–15. doi: 10.1101/cshperspect.a007252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott AB, Koup RA. CD8+ T cells in preventing HIV infection and disease. AIDS. 2012;26:1281–1292. doi: 10.1097/QAD.0b013e328353bcaf. [DOI] [PubMed] [Google Scholar]
- Deeks SG, Walker BD. Human immunodeficiency virus controllers: mechanisms of durable virus control in the absence of antiretroviral therapy. Immunity. 2007;27:406–416. doi: 10.1016/j.immuni.2007.08.010. [DOI] [PubMed] [Google Scholar]
- Blackbourn DJ, et al. Suppression of HIV replication by lymphoid tissue CD8+ cells correlates with the clinical state of HIV-infected individuals. Proc Natl Acad Sci USA. 1996;93:13125–13130. doi: 10.1073/pnas.93.23.13125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frahm N, et al. Control of human immunodeficiency virus replication by cytotoxic T lymphocytes targeting subdominant epitopes. Nature Immunol. 2006;7:173–178. doi: 10.1038/ni1281. [DOI] [PubMed] [Google Scholar]
- Hersperger AR, et al. Qualitative features of the HIV-specific CD8+ T-cell response associated with immunologic control. Curr Opin HIV/AIDS. 2011;6:169–173. doi: 10.1097/COH.0b013e3283454c39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hersperger AR, et al. Perforin expression directly ex vivo by HIV-specific CD8+ T-cells is a correlate of HIV elite control. PLoS Pathogen. 2010;6:e1000917. doi: 10.1371/journal.ppat.1000917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sáez-Cirión A, et al. HIV controllers exhibit potent CD8 T cell capacity to suppress HIV infection ex vivo and peculiar cytotoxic T lymphocyte activation phenotype. Proc Natl Acad Sci USA. 2007;104:6776–6781. doi: 10.1073/pnas.0611244104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streeck H, et al. Human immunodeficiency virus type 1-specific CD8+ T-cell responses during primary infection are major determinants of the viral set point and loss of CD4+ T cells. J Virol. 2009;83:7641–7648. doi: 10.1128/JVI.00182-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene JM, et al. Extralymphoid CD8+ T cells resident in tissue from simian immunodeficiency virus SIVmac239-delta-nef-vaccinated macaques suppress SIVmac239 replication ex vivo. J Virol. 2004;84:3362–3372. doi: 10.1128/JVI.02028-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen SG, et al. Profound early control of highly pathogenic SIV by an effector memory T-cell vaccine. Nature. 2011;473:523–527. doi: 10.1038/nature10003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz JE, et al. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science. 1999;283:857–860. doi: 10.1126/science.283.5403.857. [DOI] [PubMed] [Google Scholar]
- Jost S, Altfeld M. Evasion from NK cell-mediated immune responses by HIV-1. Microbe Infect. 2012;14:904–915. doi: 10.1016/j.micinf.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranasinghe S, et al. HIV-specific CD4 T cell responses to different viral proteins have discordant associations with viral load and clinical outcome. J Virol. 2012;86:277–283. doi: 10.1128/JVI.05577-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soghoian DZ, et al. HIV-specific cytolytic CD4 T cell responses during acute HIV infection predict disease outcome. Sci Transl Med. 2012;4:123ra25. doi: 10.1126/scitranslmed.3003165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson KE, Li H, Walker BD, Michael NL, Barouch DH. Gag-specific cellular immunity determines in vitro viral inhibition and in vivo virologic control following simian immunodeficiency virus challenges of vaccinated rhesus monkeys. J Virol. 2012;86:9583–9589. doi: 10.1128/JVI.00996-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahirel V, et al. Coordinate linkage of HIV evolution reveals regions of immunological vulnerability. Proc Natl Acad Sci USA. 2011;108:11530–11535. doi: 10.1073/pnas.1105315108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards B, et al. Magnitude of functional CD8+ T-cell responses to the Gag protein of human immunodeficiency virus type 1 correlates inversely with viral load in plasma. J Virol. 2002;76:2298–2305. doi: 10.1128/jvi.76.5.2298-2305.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julg B, et al. Enhanced anti-HIV functional activity associated with Gag-specific CD8 T-cell responses. J Virol. 2010;84:5540–5549. doi: 10.1128/JVI.02031-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiepiela P, et al. CD8+ T-cell responses to different HIV proteins have discordant associations with viral load. Nat Med. 2007;13:46–53. doi: 10.1038/nm1520. [DOI] [PubMed] [Google Scholar]
- Streeck H, et al. Recognition of a defined region within p24 Gag by CD8+ T cells during primary human immunodeficiency virus type 1 infection in individuals expressing protective HLA class I alleles. J Virol. 2007;81:7725–7731. doi: 10.1128/JVI.00708-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T, et al. Virus inhibition activity of effector memory CD8+ T cells determines simian immunodeficiency virus load in vaccinated monkeys after vaccine breakthrough infection. J Virol. 2012;86:5877–5884. doi: 10.1128/JVI.00315-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuñiga R, et al. Relative dominance of Gag p24-specific cytotoxic T lymphocytes is associated with human immunodeficiency virus control. J Virol. 2006;80:3122–3125. doi: 10.1128/JVI.80.6.3122-3125.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto N, et al. Broadening of CD8+ cell responses in vaccine-based simian immunodeficiency virus controllers. AIDS. 2010;24:2777–2787. doi: 10.1097/QAD.0b013e3283402206. [DOI] [PubMed] [Google Scholar]
- Kawada M, et al. Gag-specific cytotoxic T-lymphocyte-based control of primary simian immunodeficiency virus replication in a vaccine trial. J Virol. 2008;82:10199–10206. doi: 10.1128/JVI.01103-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, et al. Immune control of an SIV challenge by a T-cell-based vaccine in rhesus monkeys. Nature. 2009;457:87–91. doi: 10.1038/nature07469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudd PA, et al. Vaccine-induced CD8+ T cells control AIDS virus replication. Nature. 2012;491:129–133. doi: 10.1038/nature11443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukazawa Y, et al. Lymph node T cell responses predict the efficacy of live attenuated SIV vaccines. Nat Med. 2012;18:1673–1681. doi: 10.1038/nm.2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitisuttithum P, et al. Randomized, double-blind, placebo-controlled efficacy trial of a bivalent recombinant glycoprotein 120 HIV-1 vaccine among injection drug users in Bangkok, Thailand. J Infect Dis. 2006;194:1661–1671. doi: 10.1086/508748. [DOI] [PubMed] [Google Scholar]
- Flynn NM, et al. Placebo-controlled phase 3 trial of a recombinant glycoprotein 120 vaccine to prevent HIV-1 infection. J Infect Dis. 2005;191:654–665. doi: 10.1086/428404. [DOI] [PubMed] [Google Scholar]
- Rolland M, et al. Genetic impact of vaccination on breakthrough HIV-1 sequences from the STEP trial. Nat Med. 2011;17:366–371. doi: 10.1038/nm.2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantaleo G, Esteban M, Jacobs B, Tartaglia J. Poxvirus vector-based HIV vaccines. Curr Opin HIV/AIDS. 2010;5:391–396. doi: 10.1097/COH.0b013e32833d1e87. [DOI] [PubMed] [Google Scholar]
- Nitayaphan S, et al. Safety and immunogenicity of an HIV subtype B and E prime-boost vaccine combination in HIV-negative adults. J Infect Dis. 2004;190:702–706. doi: 10.1086/422258. [DOI] [PubMed] [Google Scholar]
- Hayes P, et al. Safety and immunogenicity of DNA prime and Modified Vaccinia Ankara virus HIV subtype C vaccine boost in healthy adults. Clin Vacc Immunol. 2013;20:397–408. doi: 10.1128/CVI.00637-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorse GJ, et al. DNA and Modified Vaccinia Virus Ankara vaccines encoding multiple cytotoxic and helper T-lymphocyte epitopes of human immunodeficiency virus type 1 (HIV-1) are safe but weakly immunogenic in HIV-1-uninfected, vaccinia virus-naive adults. Clin Vacc Immunol. 2012;19:649–658. doi: 10.1128/CVI.00038-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia F, et al. Safety and immunogenicity of a modified pox vector-based HIV/AIDS vaccine candidate expressing Env, Gag, Pol and Nef proteins of HIV-1 subtype B (MVA-B) in healthy HIV-1 uninfected volunteers: a phase 1 clinical trial (RISVAC02) Vaccine. 2011;29:8309–8316. doi: 10.1016/j.vaccine.2011.08.098. [DOI] [PubMed] [Google Scholar]
- Currier JR, et al. Phase 1 safety and immunogenicity evaluation of MVA-CMDR, a multigenic, recombinant modified vaccinia Ankara-HIV-1 vaccine candidate. PLoS ONE. 2010;5:e13983. doi: 10.1371/journal.pone.0013983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartaglia J, et al. NYVAC: a highly attenuated strain of vaccinia virus. Virology. 1992;188:217–232. doi: 10.1016/0042-6822(92)90752-b. [DOI] [PubMed] [Google Scholar]
- Harari A, et al. An HIV-1 clade C DNA prime, NYVAC boost vaccine regimen induces reliable, polyfunctional, and long-lasting T cell responses. J Exp Med. 2008;205:63–77. doi: 10.1084/jem.20071331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatatos L, Morris L, Burton DR, Mascola JR. Neutralizing antibodies generated during natural HIV infection: good news for an HIV vaccine? Nat Med. 2009;15:866–870. doi: 10.1038/nm.1949. [DOI] [PubMed] [Google Scholar]
- Scheid JF, et al. Sequence and structural convergence of broad and potent HIV antibodies that mimic CD4 binding. Science. 2011;333:1633–1637. doi: 10.1126/science.1207227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheid JF, et al. A method of identification of HIV gp140 binding memory B cells in human blood. J Immunol Meth. 2009;343:65–67. doi: 10.1016/j.jim.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker LM, et al. Broad and potent neutralizing antibodies from an African donor reveal a new HIV-1 vaccine target. Science. 2009;326:285–289. doi: 10.1126/science.1178746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou T, et al. Structural basis for broad and potent neutralization of HIV-1 by antibody VRC01. Science. 2010;329:811–817. doi: 10.1126/science.1192819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, et al. Broad and potent neutralization of HIV-1 by a gp41-specific human antibody. Nature. 2012;491:406–412. doi: 10.1038/nature11544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correia BE, et al. Computational design of epitope-scaffolds allows induction of antibodies specific for a poorly immunogenic HIV vaccine epitope. Structure. 2010;18:1116–1126. doi: 10.1016/j.str.2010.06.010. [DOI] [PubMed] [Google Scholar]
- Ofek G, et al. Elicitation of structure-specific antibodies by epitope scaffolds. Proc Natl Acad Sci USA. 2010;107:17880–17887. doi: 10.1073/pnas.1004728107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolla-Pazner S, et al. Cross-clade HIV-1 neutralizing antibodies induced with V3-scaffold protein immunogens following priming with gp120 DNA. J Virol. 2011;85:9887–9898. doi: 10.1128/JVI.05086-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malherbe DC, et al. Sequential immunization with a subtype B HIV-1 envelope quasispecies partially mimics the in vivo development of neutralizing antibodies. J Virol. 2011;85:5262–5274. doi: 10.1128/JVI.02419-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PR, et al. Vector-mediated gene transfer engenders long-lived neutralizing activity and protection against SIV infection in monkeys. Nat Med. 2009;15:901–906. doi: 10.1038/nm.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balazs AB, et al. Antibody-based protection against HIV infection by vectored immunoprophylaxis. Nature. 2012;481:81–84. doi: 10.1038/nature10660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Létourneau S, et al. Design and pre-clinical evaluation of a universal HIV-1 vaccine. PLoS ONE. 2007;2:e984. doi: 10.1371/journal.pone.0000984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumme ZL, et al. Human leukocyte antigen-specific polymorphisms in HIV-1 Gag and their association with viral load in chronic untreated infection. AIDS. 2008;22:1277–1286. doi: 10.1097/QAD.0b013e3283021a8c. [DOI] [PubMed] [Google Scholar]
- Wang YE, et al. Protective HLA class I alleles that restrict acute-phase CD8+ T-cell responses are associated with viral escape mutations located in highly conserved regions of human immunodeficiency virus type 1. J Virol. 2009;83:1845–1855. doi: 10.1128/JVI.01061-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priddy FH, et al. Safety and immunogenicity of a replication-incompetent adenovirus type 5 HIV-1 clade B gag/pol/nef vaccine in healthy adults. Clin Infect Dis. 2008;46:1769–1781. doi: 10.1086/587993. [DOI] [PubMed] [Google Scholar]
- Buchbinder SP, et al. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomized, placebo-controlled, test-of-concept trial. Lancet. 2008;372:1881–1893. doi: 10.1016/S0140-6736(08)61591-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanke T, McMichael AJ. Design and construction of an experimental HIV-1 vaccine for a year-2000 clinical trial in Kenya. Nat Med. 2000;6:951–955. doi: 10.1038/79626. [DOI] [PubMed] [Google Scholar]
- Wee EG, et al. A DNA/MVA-based candidate human immunodeficiency virus vaccine for Kenya induced multi-specific T cell responses in rhesus macaques. J Gen Virol. 2002;83:75–80. doi: 10.1099/0022-1317-83-1-75. [DOI] [PubMed] [Google Scholar]
- Mwau M, et al. A human immunodeficiency virus 1 (HIV-1) clade A vaccine in clinical trials:stimulation of HIV-specific T-cell responses by DNA and recombinant modified vaccinia virus Ankara (MVA) vaccines in humans. J Gen Virol. 2004;85:911–919. doi: 10.1099/vir.0.19701-0. [DOI] [PubMed] [Google Scholar]
- Jaoko W, et al. Safety and immunogenicity of recombinant low dosage HIV-1 A vaccine candidates vectored by plasmid pTHr DNA or modified vaccinia virus Ankara (MVA) in humans in East Africa. Virology. 2008;26:2788–2795. doi: 10.1016/j.vaccine.2008.02.071. [DOI] [PubMed] [Google Scholar]
- Wilson CC, et al. Development of a DNA vaccine designed to induce cytotoxic T lymphocyte responses to multiple conserved epitopes in HIV-1. J Immunol. 2003;171:5611–5623. doi: 10.4049/jimmunol.171.10.5611. [DOI] [PubMed] [Google Scholar]
- Gorse GJ, et al. Safety and immunogenicity of cytotoxic T-lymphocyte poly-epitope, DNA plasmid (EP HIV-1090) vaccine in healthy, human immunodeficiency virus type 1 (HIV-1)-uninfected adults. Vaccine. 2008;26:215–223. doi: 10.1016/j.vaccine.2007.10.061. [DOI] [PubMed] [Google Scholar]
- Rosario M, et al. Prime-boost regimens with adjuvanted synthetic long peptides elicit T cells and antibodies to conserved regions of HIV-1 in macaques. AIDS. 2012;26:275–284. doi: 10.1097/QAD.0b013e32834ed9b2. [DOI] [PubMed] [Google Scholar]
- Rosario M, et al. Long peptides induce polyfunctional T cells against conserved regions of HIV-1 with superior breadth to single-gene vaccines in macaques. Eur J Immunol. 2010;40:1973–1984. doi: 10.1002/eji.201040344. [DOI] [PubMed] [Google Scholar]
- Stephenson KE, et al. Full-length HIV-1 immunogens induce greater magnitude and comparable breadth of T lymphocyte responses to conserved HIV-1 regions compared with conserved-region-only HIV-1 immunogens in rhesus monkeys. J Virol. 2012;86:11434–11440. doi: 10.1128/JVI.01779-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolland M, Nickle DC, Mullins JI. HIV-1 group M conserved elements vaccine. PLoS Pathog. 2007;3:e157. doi: 10.1371/journal.ppat.0030157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaman MS, et al. Multiclade human immunodeficiency virus type 1 envelope immunogens elicit broad cellular and humoral immunity in rhesus monkeys. J Virol. 2005;79:2956–2963. doi: 10.1128/JVI.79.5.2956-2963.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earl PL, et al. Design and evaluation of multi-gene, multi-clade HIV-1 MVA vaccines. Vaccine. 2009;27:5885–5895. doi: 10.1016/j.vaccine.2009.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurmond J, et al. Web-based design and evaluation of T-cell vaccine candidates. Bioinformatics. 2008;24:1639–1640. doi: 10.1093/bioinformatics/btn251. [DOI] [PubMed] [Google Scholar]
- Fischer W, et al. Polyvalent vaccines for optimal coverage of potential T-cell epitopes in global HIV-1 variants. Nat Med. 2007;13:100–106. doi: 10.1038/nm1461. [DOI] [PubMed] [Google Scholar]
- Barouch DH, et al. Mosaic HIV-1 vaccines expand the breadth and depth of cellular immune responses in rhesus monkeys. Nat Med. 2010;16:319–323. doi: 10.1038/nm.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santra S, et al. Mosaic vaccines elicit CD8+ T lymphocyte responses that confer enhanced immune coverage of diverse HIV strains in monkeys. Nat Med. 2010;16:324–328. doi: 10.1038/nm.2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu MA. DNA vaccines: an historical perspective and view to the future. Immunol Rev. 2011;239:62–84. doi: 10.1111/j.1600-065X.2010.00980.x. [DOI] [PubMed] [Google Scholar]
- Churchyard GJ, et al. A phase IIA randomized clinical trial of a multiclade HIV-1 DNA prime followed by a multiclade rAd5 HIV-1 vaccine boost in healthy adults (HVTN204) PLoS ONE. 2011;6:e21225. doi: 10.1371/journal.pone.0021225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barouch DH, et al. International seroepidemiology of adenovirus serotypes 5, 26, 35, and 48 in pediatric and adult populations. Vaccine. 2011;29:5203–5209. doi: 10.1016/j.vaccine.2011.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barouch DH. Novel adenovirus vector-based vaccines for HIV-1. Curr Opin HIV/AIDS. 2010;5:386–390. doi: 10.1097/COH.0b013e32833cfe4c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes E, et al. Novel adenovirus-based vaccines induce broad and sustained T cell responses to HCV in man. Sci Transl Med. 2012;4:115ra1. doi: 10.1126/scitranslmed.3003155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hara GA, et al. Clinical assessment of a recombinant simian adenovirus ChAd63: a potent new vaccine vector. J Infect Dis. 2012;205:772–781. doi: 10.1093/infdis/jir850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colloca S, et al. Vaccine vectors derived from a large collection of simian adenoviruses induce potent cellular immunity across multiple species. Sci Transl Med. 2012;4:115ra2. doi: 10.1126/scitranslmed.3002925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farina SF, et al. Replication-defective vector based on a chimpanzee adenovirus. J Virol. 2001;75:11603–11613. doi: 10.1128/JVI.75.23.11603-11613.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mast T, et al. International epidemiology of human pre-existing adenovirus (Ad) type-5, type-6, type-26 and type-36 neutralizing antibodies: correlates of high Ad5 titers and implications for potential HIV vaccine trials. Vaccine. 2010;28:950–957. doi: 10.1016/j.vaccine.2009.10.145. [DOI] [PubMed] [Google Scholar]
- Duerr A, et al. Extended follow-up confirms early vaccine-enhanced risk of HIV acquisition and demonstrates waning effect over time among participants in a randomized trial of recombinant adenovirus HIV vaccine (Step Study) J Infect Dis. 2012;206:258–266. doi: 10.1093/infdis/jis342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, et al. Magnitude and phenotype of cellular immune responses elicited by recombinant adenovirus vectors and heterologous prime-boost regimens in rhesus monkeys. J Virol. 2008;82:4844–4852. doi: 10.1128/JVI.02616-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan WG, et al. Comparative analysis of simian immunodeficiency virus gag-specific effector and memory CD8+ T cells induced by different adenovirus vectors. J Virol. 2013;87:1359–1372. doi: 10.1128/JVI.02055-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penaloza-Macmaster P, et al. Alternative serotype adenovirus vaccine vectors elicit memory T cells with enhanced anamnestic capacity compared to Ad5 vectors. J Virol. 2013;87:1373–1384. doi: 10.1128/JVI.02058-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, et al. Adenovirus serotype 26 utilizes CD46 as a primary cellular receptor and only transiently activates T lymphocytes following vaccination of rhesus monkeys. J Virol. 2012;86:10862–10865. doi: 10.1128/JVI.00928-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teigler JE, Iampietro MJ, Barouch DH. Vaccination with adenovirus serotypes 35, 26, and 48 elicits higher levels of innate cytokine responses than adenovirus serotype 5 in rhesus monkeys. J Virol. 2012;86:9590–9598. doi: 10.1128/JVI.00740-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson KE, Hural J, Buchbinder SP, Sinangil F, Barouch DH. Preexisting adenovirus seropositivity is not associated with increased HIV-1 acquisition in three HIV-1 vaccine efficacy trials. J Infect Dis. 2012;205:1806–1810. doi: 10.1093/infdis/jis285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baden LR, et al. First-in-human evaluation of the safety and immunogenicity of a recombinant adenovirus serotype 26 HIV-1 Env vaccine (IPCAVD 001) J Infect Dis. 2013;207:240–247. doi: 10.1093/infdis/jis670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barouch DH, et al. Characterization of humoral and cellular immune responses elicited by a recombinant adenovirus serotype 26 HIV-1 Env vaccine in healthy adults (IPCAVD 001) J Infect Dis. 2013;207:248–256. doi: 10.1093/infdis/jis671. [DOI] [PMC free article] [PubMed] [Google Scholar]


