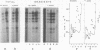Abstract
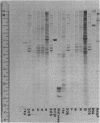
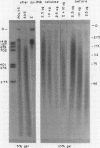
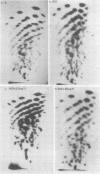
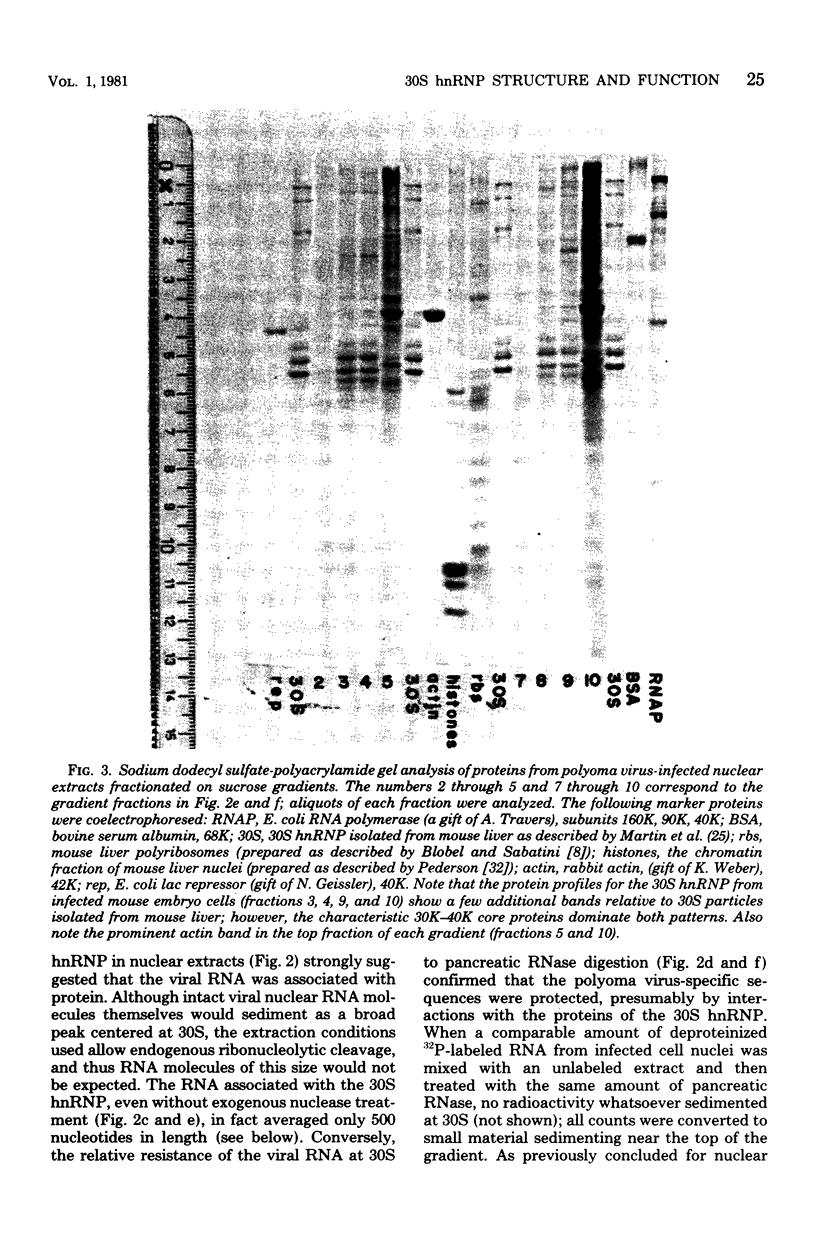
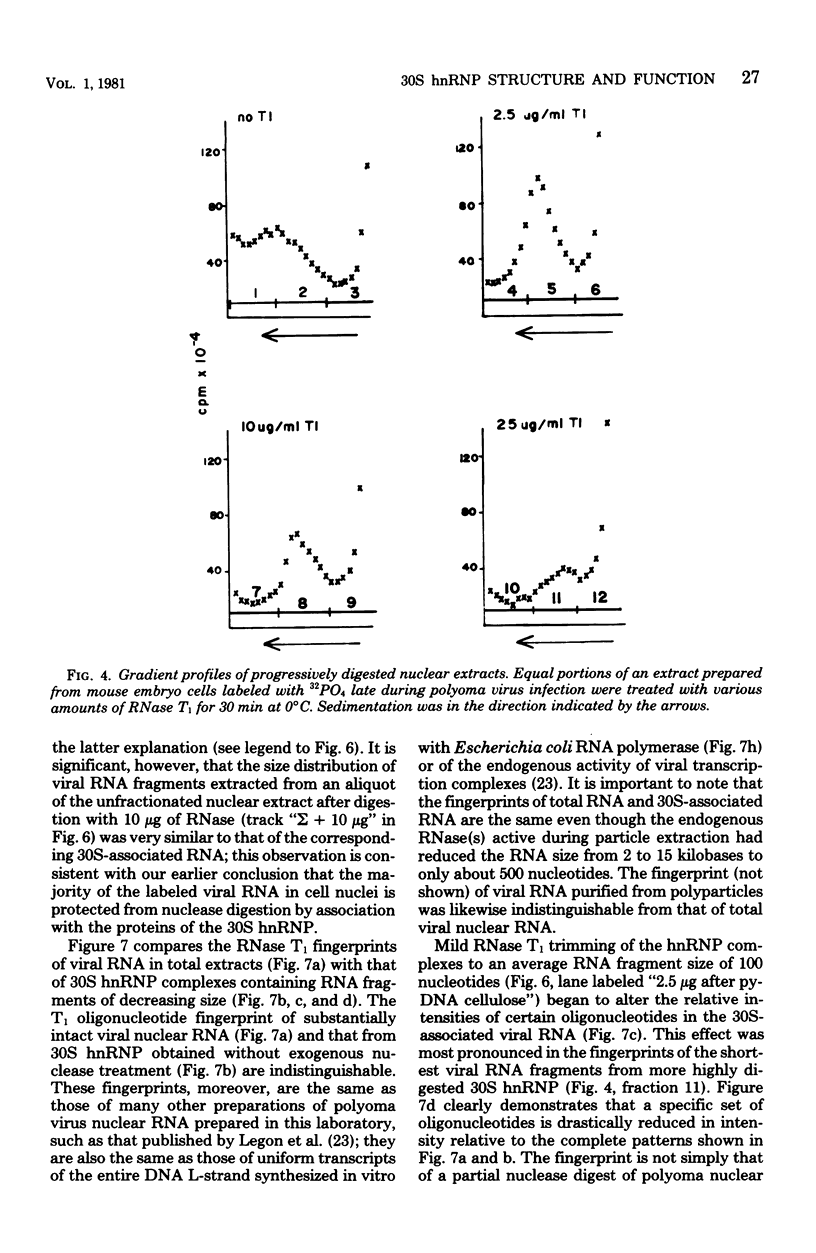
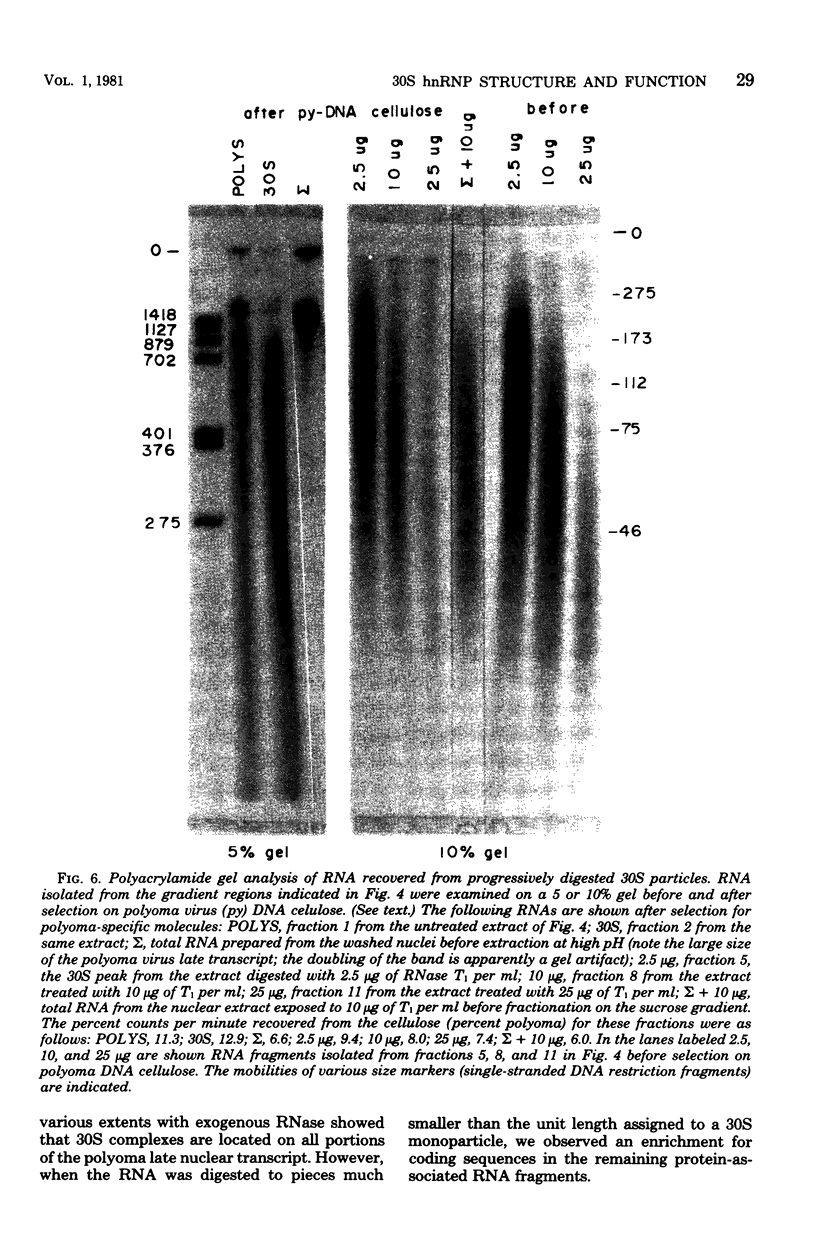
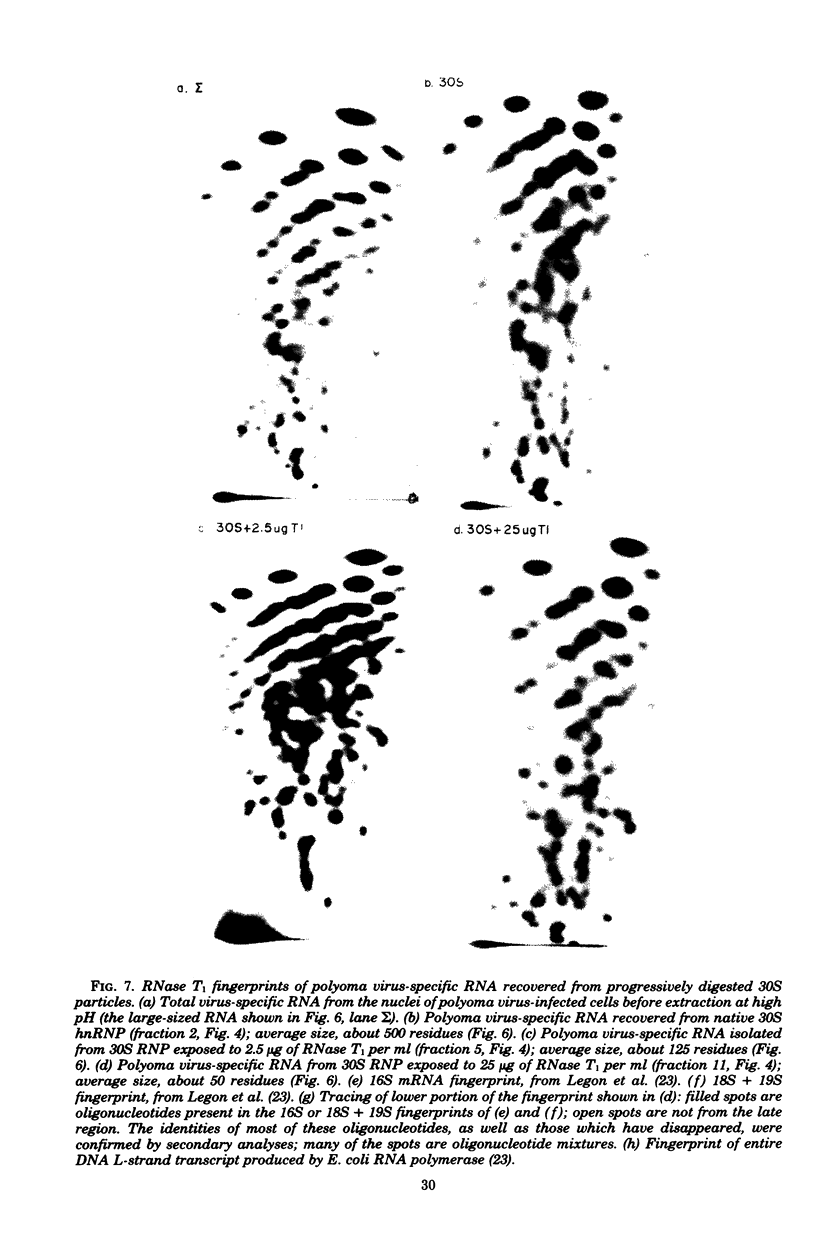
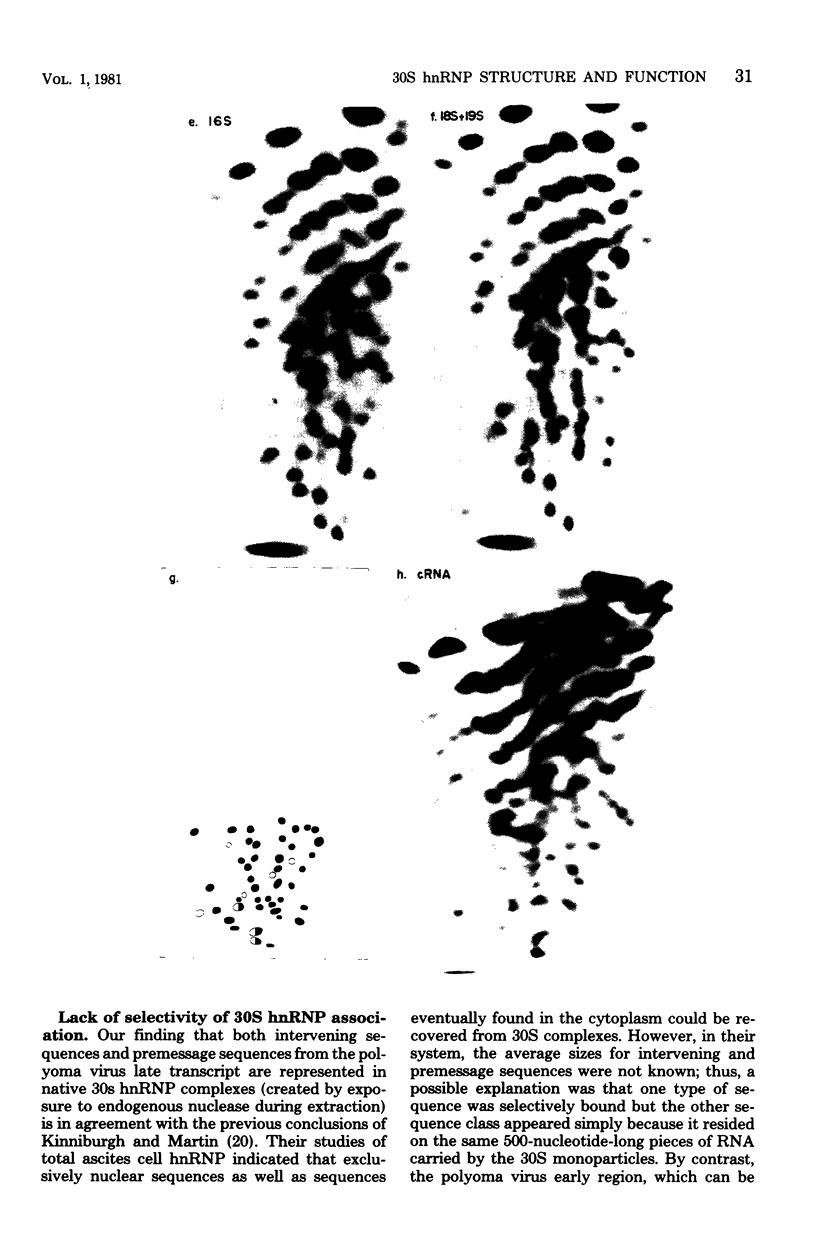
Heterogeneous nuclear ribonucleic acid (hnRNA) molecules in eucaryotic cell nuclei associate with a well-defined group of abundant, highly conserved proteins to form heterogeneous nuclear ribonucleoproteins (hnRNP). The exact manner in which these 30S complexes assemble on nuclear transcripts, however, has not been well documented. To determine whether any site selectivity in the formation of hnRNP can be detected (e.g., preferential recognition of intervening sequences or of premessage regions), we investigated the distribution of 30S hnRNP on a particular nuclear RNA, the polyoma virus late transcript. Hybridization studies showed not only that the majority of polyoma late nuclear RNA sequences can be isolated in the form of 30S complexes, but that the RNP were located equally on intervening sequences and premessage portions of the transcript. The latter conclusion was confirmed by ribonuclease T1 oligonucleotide fingerprint analysis of polyoma virus-specific RNA recovered from native 30S complexes. However, fingerprint analysis of the small segments of viral RNA in the 30S fraction that survived extensive ribonuclease treatment revealed that oligonucleotides corresponding to intervening sequences were preferentially lost. We discuss these findings in relation to the structure of 30S hnRNP and their function in RNA biogenesis.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acheson N. H., Buetti E., Scherrer K., Weil R. Transcription of the polyoma virus genome: synthesis and cleavage of giant late polyoma-specific RNA. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2231–2235. doi: 10.1073/pnas.68.9.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acheson N. H. Polyoma virus giant RNAs contain tandem repeats of the nucleotide sequence of the entire viral genome. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4754–4758. doi: 10.1073/pnas.75.10.4754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aloni Y. Biogenesis and characterization of SV40 and polyoma RNAs in productively infected cells. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):165–178. doi: 10.1101/sqb.1974.039.01.023. [DOI] [PubMed] [Google Scholar]
- Beyer A. L., Christensen M. E., Walker B. W., LeStourgeon W. M. Identification and characterization of the packaging proteins of core 40S hnRNP particles. Cell. 1977 May;11(1):127–138. doi: 10.1016/0092-8674(77)90323-3. [DOI] [PubMed] [Google Scholar]
- Beyer A. L., Miller O. L., Jr, McKnight S. L. Ribonucleoprotein structure in nascent hnRNA is nonrandom and sequence-dependent. Cell. 1980 May;20(1):75–84. doi: 10.1016/0092-8674(80)90236-6. [DOI] [PubMed] [Google Scholar]
- Birg F., Favaloro J., Kamen R. Analysis of polyoma virus nuclear RNA by mini-blot hybridization. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3138–3142. doi: 10.1073/pnas.74.8.3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G., Sabatini D. Dissociation of mammalian polyribosomes into subunits by puromycin. Proc Natl Acad Sci U S A. 1971 Feb;68(2):390–394. doi: 10.1073/pnas.68.2.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvet J. P., Pederson T. Nucleoprotein organization of inverted repeat DNA transcripts in heterogeneous nuclear RNA-ribonucleoprotein particles from HeLa cells. J Mol Biol. 1978 Jul 5;122(3):361–378. doi: 10.1016/0022-2836(78)90195-x. [DOI] [PubMed] [Google Scholar]
- Favaloro J., Treisman R., Kamen R. Transcription maps of polyoma virus-specific RNA: analysis by two-dimensional nuclease S1 gel mapping. Methods Enzymol. 1980;65(1):718–749. doi: 10.1016/s0076-6879(80)65070-8. [DOI] [PubMed] [Google Scholar]
- Flavell A. J., Cowie A., Legon S., Kamen R. Multiple 5' terminal cap structures in late polyoma virus RNA. Cell. 1979 Feb;16(2):357–371. doi: 10.1016/0092-8674(79)90012-6. [DOI] [PubMed] [Google Scholar]
- Flavell A. J., Kamen R. Strand-specific transcription of polyoma virus DNA late during productive infection. J Mol Biol. 1977 Sep 15;115(2):237–242. doi: 10.1016/0022-2836(77)90099-7. [DOI] [PubMed] [Google Scholar]
- Friedmann T., Esty A., LaPorte P., Deininger P. The nucleotide sequence and genome organization of the polyoma early region: extensive nucleotide and amino acid homology with SV40. Cell. 1979 Jul;17(3):715–724. doi: 10.1016/0092-8674(79)90278-2. [DOI] [PubMed] [Google Scholar]
- Griffin B. E., Fried M., Cowie A. Polyoma DNA: a physical map. Proc Natl Acad Sci U S A. 1974 May;71(5):2077–2081. doi: 10.1073/pnas.71.5.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay E., Bambara R., Padmanabhan R., Wu R. DNA sequence analysis: a general, simple and rapid method for sequencing large oligodeoxyribonucleotide fragments by mapping. Nucleic Acids Res. 1974 Mar;1(3):331–353. doi: 10.1093/nar/1.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamen R., Favaloro J., Parker J. Topography of the three late mRNA's of polyoma virus which encode the virion proteins. J Virol. 1980 Feb;33(2):637–651. doi: 10.1128/jvi.33.2.637-651.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karn J., Vidali G., Boffa L. C., Allfrey V. G. Characterization of the non-histone nuclear proteins associated with rapidly labeled heterogeneous nuclear RNA. J Biol Chem. 1977 Oct 25;252(20):7307–7322. [PubMed] [Google Scholar]
- Kinniburgh A. J., Martin T. E. Detection of mRNA sequences in nuclear 30S ribonucleoprotein subcomplexes. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2725–2729. doi: 10.1073/pnas.73.8.2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lamb M. M., Daneholt B. Characterization of active transcription units in Balbiani rings of Chironomus tentans. Cell. 1979 Aug;17(4):835–848. doi: 10.1016/0092-8674(79)90324-6. [DOI] [PubMed] [Google Scholar]
- Legon S., Flavell A. J., Cowie A., Kamen R. Amplification in the leader sequence of late polyoma virus mRNAs. Cell. 1979 Feb;16(2):373–388. doi: 10.1016/0092-8674(79)90013-8. [DOI] [PubMed] [Google Scholar]
- Lukanidin E. M., Zalmanzon E. S., Komaromi L., Samarina O. P., Georgiev G. P. Structure and function of informofers. Nat New Biol. 1972 Aug 16;238(85):193–197. doi: 10.1038/newbio238193a0. [DOI] [PubMed] [Google Scholar]
- Martin T., Billings P., Levey A., Ozarslan S., Quinlan T., Swift H., Urbas L. Some properties of RNA:protein complexes from the nucleus of eukaryotic cells. Cold Spring Harb Symp Quant Biol. 1974;38:921–932. doi: 10.1101/sqb.1974.038.01.094. [DOI] [PubMed] [Google Scholar]
- Martin T., Billings P., Pullman J., Stevens B., Kinniburgh A. Substructure of nuclear ribonucleoprotein complexes. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 2):899–909. doi: 10.1101/sqb.1978.042.01.091. [DOI] [PubMed] [Google Scholar]
- Martin T., Jones R., Billings P. HnRNP core proteins: synthesis, turnover and intracellular distribution. Mol Biol Rep. 1979 May 31;5(1-2):37–42. doi: 10.1007/BF00777486. [DOI] [PubMed] [Google Scholar]
- Maundrell K., Scherrer K. Characterization of pre-messenger-RNA-containing nuclear ribonucleoprotein particles from avian erythroblasts. Eur J Biochem. 1979 Sep;99(2):225–238. doi: 10.1111/j.1432-1033.1979.tb13249.x. [DOI] [PubMed] [Google Scholar]
- McKnight S. L., Miller O. L., Jr Post-replicative nonribosomal transcription units in D. melanogaster embryos. Cell. 1979 Jul;17(3):551–563. doi: 10.1016/0092-8674(79)90263-0. [DOI] [PubMed] [Google Scholar]
- Pagoulatos G. N., Yaniv M. Proteins bound to heterogeneous nuclear RNA of simian-virus-40-infected cells. Eur J Biochem. 1978 Nov 2;91(1):1–10. doi: 10.1111/j.1432-1033.1978.tb20930.x. [DOI] [PubMed] [Google Scholar]
- Patel N. T., Kurosky A., Holoubek V. Isolation and characterization of the predominant protein in nuclear ribonucleoprotein particles from rat liver. Biochim Biophys Acta. 1978 Mar 28;533(1):282–286. doi: 10.1016/0005-2795(78)90573-1. [DOI] [PubMed] [Google Scholar]
- Pederson T. Gene activation in eukaryotes: are nuclear acidic proteins the cause or the effect? Proc Natl Acad Sci U S A. 1974 Mar;71(3):617–621. doi: 10.1073/pnas.71.3.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pederson T. Proteins associated with heterogeneous nuclear RNA in eukaryotic cells. J Mol Biol. 1974 Feb 25;83(2):163–183. doi: 10.1016/0022-2836(74)90386-6. [DOI] [PubMed] [Google Scholar]
- Quinlan T. J., Billings P. B., Martin T. E. Nuclear ribonucleoprotein complexes containing polyadenylate from mouse ascites cells. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2632–2636. doi: 10.1073/pnas.71.7.2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan T. J., Kinniburgh A. J., Martin T. E. Properties of a nuclear polyadenylate-protein complex from mouse ascites cells. J Biol Chem. 1977 Feb 25;252(4):1156–1161. [PubMed] [Google Scholar]
- Samarina O. P., Lukanidin E. M., Molnar J., Georgiev G. P. Structural organization of nuclear complexes containing DNA-like RNA. J Mol Biol. 1968 Apr 14;33(1):251–263. doi: 10.1016/0022-2836(68)90292-1. [DOI] [PubMed] [Google Scholar]
- Sekeris C. E., Niessing J. Evidence for the existence of a structural RNA component in the nuclear ribonucleoprotein particles containing heterogeneous RNA. Biochem Biophys Res Commun. 1975 Feb 3;62(3):642–650. doi: 10.1016/0006-291x(75)90447-7. [DOI] [PubMed] [Google Scholar]
- Soeda E., Arrand J. R., Smolar N., Walsh J. E., Griffin B. E. Coding potential and regulatory signals of the polyoma virus genome. Nature. 1980 Jan 31;283(5746):445–453. doi: 10.1038/283445a0. [DOI] [PubMed] [Google Scholar]
- Steitz J. A. Prokaryotic ribosome binding sites. Methods Enzymol. 1979;60:311–321. doi: 10.1016/s0076-6879(79)60029-0. [DOI] [PubMed] [Google Scholar]
- Stevenin J., Gallinaro-Matringe H., Gattoni R., Jacob M. Complexity of the structure of particles containing heterogeneous nuclear RNA as demonstrated by ribonuclease treatment. Eur J Biochem. 1977 Apr 15;74(3):589–602. doi: 10.1111/j.1432-1033.1977.tb11428.x. [DOI] [PubMed] [Google Scholar]
- Zuckermann M., Manor H., Parker J., Kamen R. Electron microscopic demonstration of the presence of amplified sequences at the 5'-ends of the polyoma virus late mRNAs. Nucleic Acids Res. 1980 Apr 11;8(7):1505–1519. doi: 10.1093/nar/8.7.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]