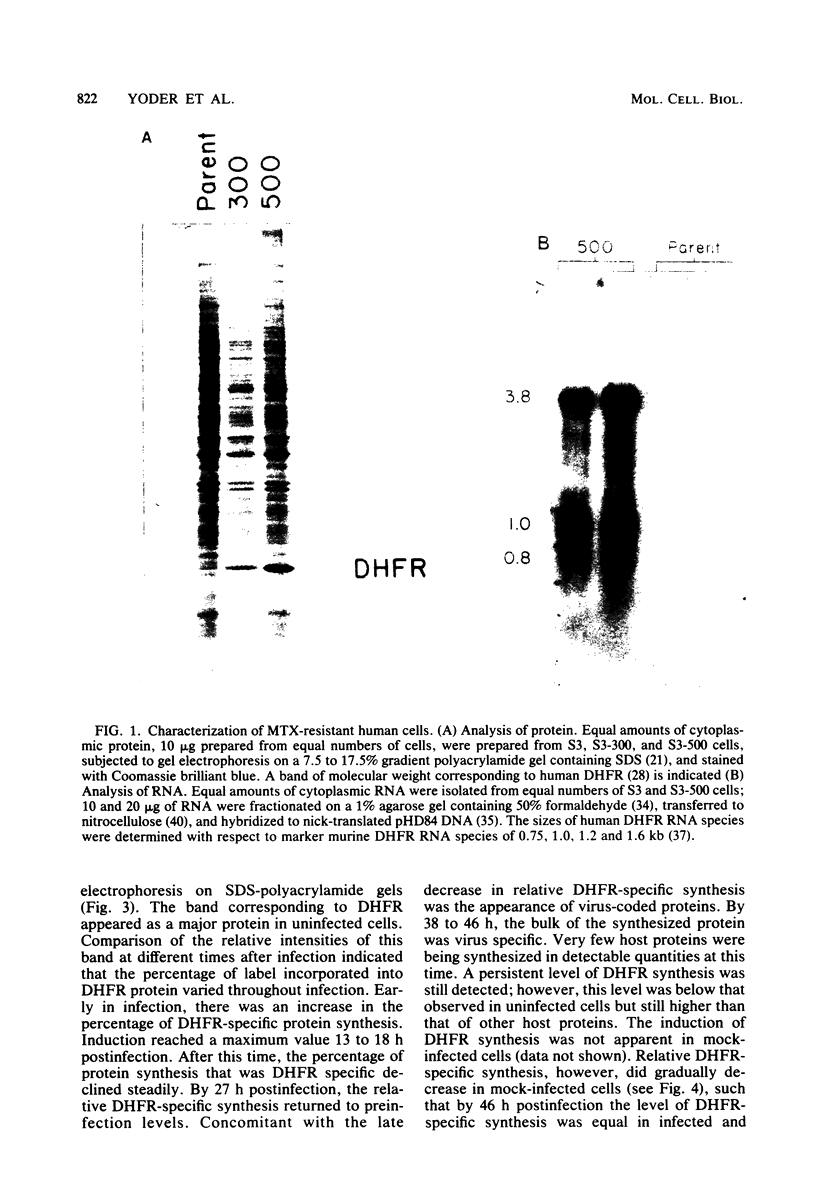
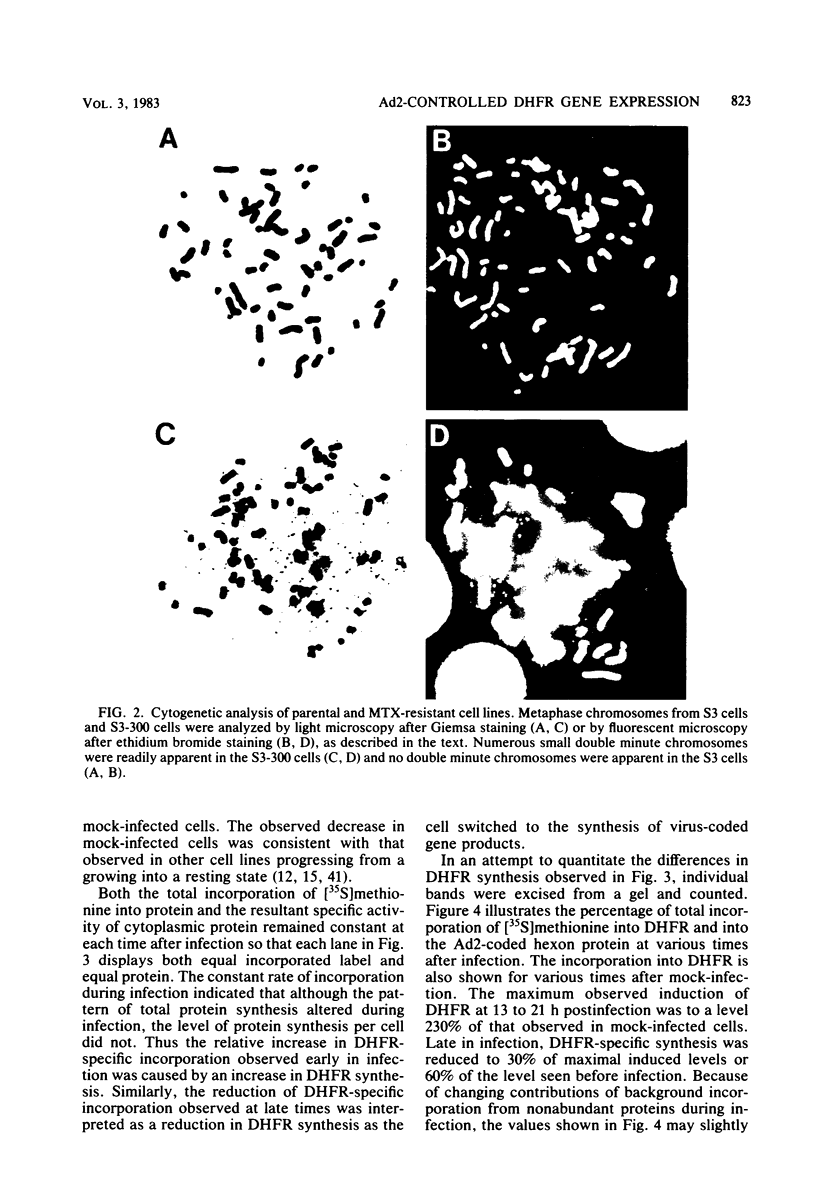
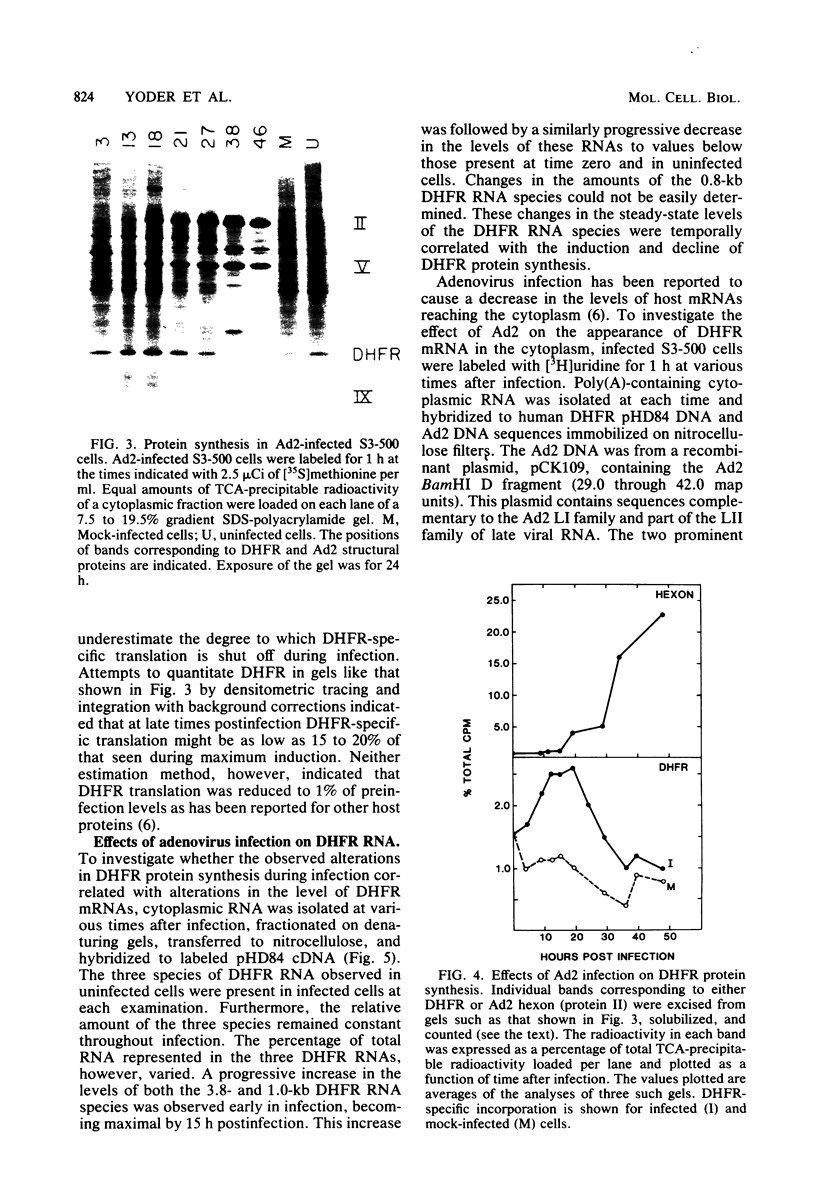
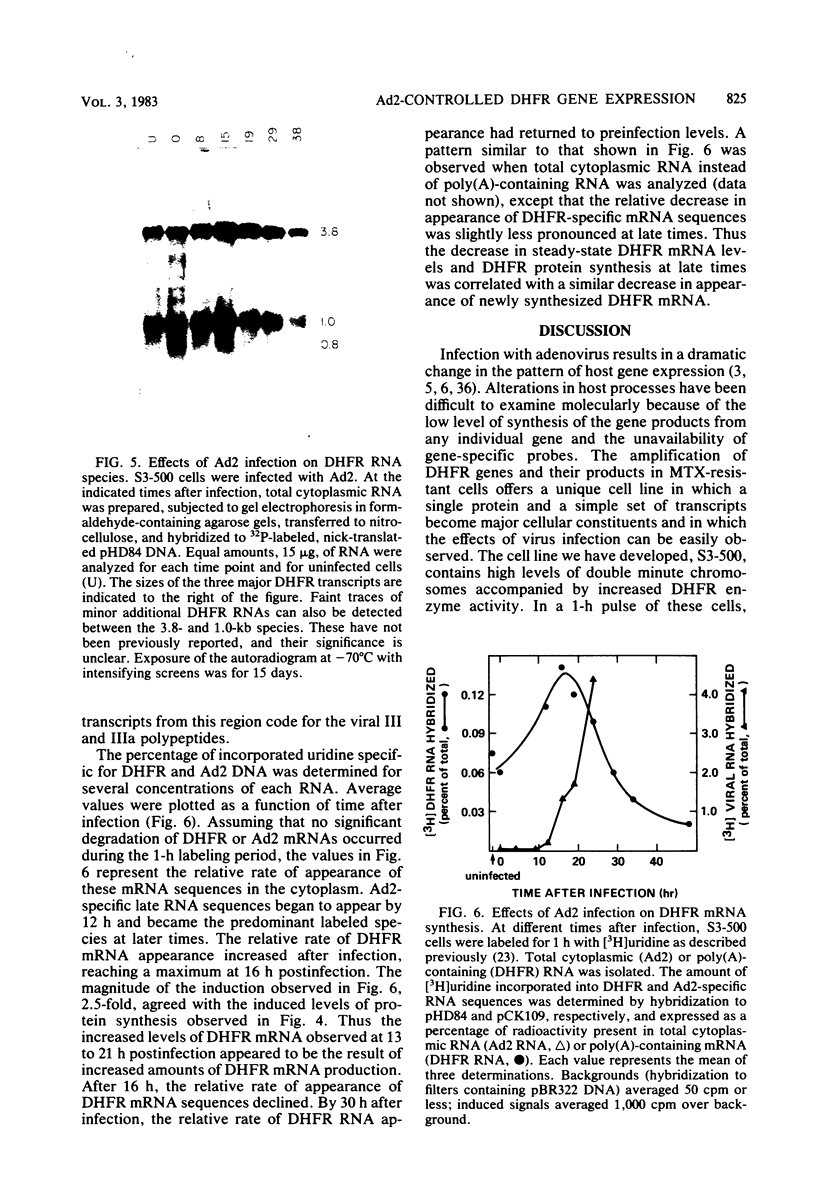
Abstract
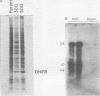
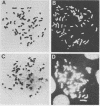
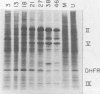
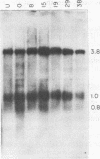
Infection of human cells by adenovirus results in multiple alterations of host gene expression. To examine the effects of viral infection on the expression of a single gene, a line of human cells was developed which is resistant to growth in methotrexate and which contains amplified RNA and protein specific for dihydrofolate reductase (DHFR). Cytogenetic evidence indicated the presence of amplified DNA. Adenovirus infection of these cells caused an induction and subsequent decline in the synthesis of DHFR protein. The maximum DHFR induction occurred 16 to 19 h after infection and reached a level 2.5-fold greater than that observed in uninfected cells. Induction of DHFR protein synthesis was accompanied by concomitant increases in the level of steady-state DHFR-specific cytoplasmic RNA. The relative rate of DHFR mRNA production (i.e., the appearance of DHFR-specific mRNA sequences in the cytoplasm) also increased 2.5-fold during induction. Later in infection, the relative rate of DHFR protein synthesis declined, reaching a level below that observed in uninfected cells. This decline was accompanied by a similar decline in the steady-state levels of DHFR RNA and in the relative rate of synthesis of DHFR mRNA. These data suggest that adenovirus infection controls DHFR gene expression by increasing and subsequently decreasing the relative rate at which DHFR-specific mRNA sequences appear in the cytoplasm and enter the pool of mRNA available for translation.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alt F. W., Kellems R. E., Bertino J. R., Schimke R. T. Selective multiplication of dihydrofolate reductase genes in methotrexate-resistant variants of cultured murine cells. J Biol Chem. 1978 Mar 10;253(5):1357–1370. [PubMed] [Google Scholar]
- Anderson C. W., Baum P. R., Gesteland R. F. Processing of adenovirus 2-induced proteins. J Virol. 1973 Aug;12(2):241–252. doi: 10.1128/jvi.12.2.241-252.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bello L. J., Ginsberg H. S. Inhibition of host protein synthesis in type 5 adenovirus-infected cells. J Virol. 1967 Oct;1(5):843–850. doi: 10.1128/jvi.1.5.843-850.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltz G. A., Flint S. J. Inhibition of HeLa cell protein synthesis during adenovirus infection. Restriction of cellular messenger RNA sequences to the nucleus. J Mol Biol. 1979 Jun 25;131(2):353–373. doi: 10.1016/0022-2836(79)90081-0. [DOI] [PubMed] [Google Scholar]
- Berget S. M., Flint S. J., Williams J. F., Sharp P. A. Adenovirus transcription. IV. Synthesis of viral-specific RNA in human cells infected with temperature-sensitive mutants of adenovirus 5. J Virol. 1976 Sep;19(3):879–889. doi: 10.1128/jvi.19.3.879-889.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk A. J., Lee F., Harrison T., Williams J., Sharp P. A. Pre-early adenovirus 5 gene product regulates synthesis of early viral messenger RNAs. Cell. 1979 Aug;17(4):935–944. doi: 10.1016/0092-8674(79)90333-7. [DOI] [PubMed] [Google Scholar]
- Biedler J. L., Albrecht A. M., Hutchison D. J., Spengler B. A. Drug response, dihydrofolate reductase, and cytogenetics of amethopterin-resistant Chinese hamster cells in vitro. Cancer Res. 1972 Jan;32(1):153–161. [PubMed] [Google Scholar]
- Chang A. C., Nunberg J. H., Kaufman R. J., Erlich H. A., Schimke R. T., Cohen S. N. Phenotypic expression in E. coli of a DNA sequence coding for mouse dihydrofolate reductase. Nature. 1978 Oct 19;275(5681):617–624. doi: 10.1038/275617a0. [DOI] [PubMed] [Google Scholar]
- Chow L. T., Lewis J. B., Broker T. R. RNA transcription and splicing at early and intermediate times after adenovirus-2 infection. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):401–414. doi: 10.1101/sqb.1980.044.01.044. [DOI] [PubMed] [Google Scholar]
- Gudewicz T. M., Morhenn V. B., Kellems R. E. The effect of polyoma virus, serum factors, and dibutyryl cyclic AMP on dihydrofolate reductase synthesis, and the entry of quiescent cells into S phase. J Cell Physiol. 1981 Jul;108(1):1–8. doi: 10.1002/jcp.1041080102. [DOI] [PubMed] [Google Scholar]
- Hendrickson S. L., Wu J. S., Johnson L. F. Cell cycle regulation of dihydrofolate reductase mRNA metabolism in mouse fibroblasts. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5140–5144. doi: 10.1073/pnas.77.9.5140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman R. C., Penman S. Multiple decay rates of heterogeneous nuclear RNA in HeLa cells. Biochemistry. 1977 Jul 26;16(15):3460–3465. doi: 10.1021/bi00634a026. [DOI] [PubMed] [Google Scholar]
- Johnson L. F., Fuhrman C. L., Wiedemann L. M. Regulation of dihydrofolate reductase gene expression in mouse fibroblasts during the transition from the resting to growing state. J Cell Physiol. 1978 Dec;97(3 Pt 2 Suppl 1):397–306. doi: 10.1002/jcp.1040970314. [DOI] [PubMed] [Google Scholar]
- Jones N., Shenk T. An adenovirus type 5 early gene function regulates expression of other early viral genes. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3665–3669. doi: 10.1073/pnas.76.8.3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katze M. G., Persson H., Philipson L. Control of adenovirus early gene expression: posttranscriptional control mediated by both viral and cellular gene products. Mol Cell Biol. 1981 Sep;1(9):807–813. doi: 10.1128/mcb.1.9.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman R. J., Brown P. C., Schimke R. T. Amplified dihydrofolate reductase genes in unstably methotrexate-resistant cells are associated with double minute chromosomes. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5669–5673. doi: 10.1073/pnas.76.11.5669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellems R. E., Morhenn V. B., Pfendt E. A., Alt F. W., Schimke R. T. Polyoma virus and cyclic AMP-mediated control of dihydrofolate reductase mRNA abundance in methotrexate-resistant mouse fibroblasts. J Biol Chem. 1979 Jan 25;254(2):309–318. [PubMed] [Google Scholar]
- Kit S., Dubbs D. R., DeTorres R. A., Melnick J. L. Enhanced thymidine kinase activity following infection of green monkey kidney cells by simian adenoviruses, simian papovavirus SV40, and an adenovirus-SV40 "hybrid". Virology. 1965 Nov;27(3):453–457. doi: 10.1016/0042-6822(65)90133-9. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Ledinko N. Stimulation of DNA synthesis and thymidine kinase activity in human embryonic kidney cells infected by Adenovirus 2 or 12. Cancer Res. 1967 Aug;27(8):1459–1469. [PubMed] [Google Scholar]
- Leys E. J., Kellems R. E. Control of dihydrofolate reductase messenger ribonucleic acid production. Mol Cell Biol. 1981 Nov;1(11):961–971. doi: 10.1128/mcb.1.11.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg U., Persson T., Philipson L. Isolation and characterization of adenovirus messenger ribonucleic acid in productive infection. J Virol. 1972 Nov;10(5):909–919. doi: 10.1128/jvi.10.5.909-919.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN E. M., WORK T. S. Studies on protein and nucleic acid metabolism in virus-infected mammalian cells. IV. The localization of metabolic changes within subcellular fractions of Krebs II mouse-ascites-tumour cells infected with encephalomyocarditis virus. Biochem J. 1961 Dec;81:514–520. doi: 10.1042/bj0810514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters J., Keeley B., Gay H., Attardi G. Variable content of double minute chromosomes is not correlated with degree of phenotype instability in methotrexate-resistant human cell lines. Mol Cell Biol. 1982 May;2(5):498–507. doi: 10.1128/mcb.2.5.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melera P. W., Lewis J. A., Biedler J. L., Hession C. Antifolate-resistant Chinese hamster cells. Evidence for dihydrofolate reductase gene amplification among independently derived sublines overproducing different dihydrofolate reductases. J Biol Chem. 1980 Jul 25;255(14):7024–7028. [PubMed] [Google Scholar]
- Morandi C., Attardi G. Isolation and characterization of dihydrofolic acid reductase from methotrexate-sensitive and -resistant human cell lines. J Biol Chem. 1981 Oct 10;256(19):10169–10175. [PubMed] [Google Scholar]
- Morandi C., Masters J. N., Mottes M., Attardi G. Multiple forms of human dihydrofolate reductase messenger RNA. Cloning and expression in Escherichia coli of their DNA coding sequence. J Mol Biol. 1982 Apr 15;156(3):583–607. doi: 10.1016/0022-2836(82)90268-6. [DOI] [PubMed] [Google Scholar]
- Nevins J. R. Mechanism of activation of early viral transcription by the adenovirus E1A gene product. Cell. 1981 Oct;26(2 Pt 2):213–220. doi: 10.1016/0092-8674(81)90304-4. [DOI] [PubMed] [Google Scholar]
- Nunberg J. H., Kaufman R. J., Schimke R. T., Urlaub G., Chasin L. A. Amplified dihydrofolate reductase genes are localized to a homogeneously staining region of a single chromosome in a methotrexate-resistant Chinese hamster ovary cell line. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5553–5556. doi: 10.1073/pnas.75.11.5553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postel E. H., Levine A. J. Studies on the regulation of deoxypyrimidine kinases in normal, SV40-transformed and SV40- and adenovirus-infected mouse cells in culture. Virology. 1975 Feb;63(2):404–420. doi: 10.1016/0042-6822(75)90313-x. [DOI] [PubMed] [Google Scholar]
- Rave N., Crkvenjakov R., Boedtker H. Identification of procollagen mRNAs transferred to diazobenzyloxymethyl paper from formaldehyde agarose gels. Nucleic Acids Res. 1979 Aug 10;6(11):3559–3567. doi: 10.1093/nar/6.11.3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Russell W. C., Skehel J. J. The polypeptides of adenovirus-infected cells. J Gen Virol. 1972 Apr;15(1):45–57. doi: 10.1099/0022-1317-15-1-45. [DOI] [PubMed] [Google Scholar]
- Setzer D. R., McGrogan M., Nunberg J. H., Schimke R. T. Size heterogeneity in the 3' end of dihydrofolate reductase messenger RNAs in mouse cells. Cell. 1980 Nov;22(2 Pt 2):361–370. doi: 10.1016/0092-8674(80)90346-3. [DOI] [PubMed] [Google Scholar]
- Takahashi M., Ueda S., Ogino T. Enhancement of the thymidine kinase activity of human embryonic kidney cells and newborn hamster kidney cells by infection with human adenovirus types 5 and 12. Virology. 1966 Dec;30(4):742–743. doi: 10.1016/0042-6822(66)90181-4. [DOI] [PubMed] [Google Scholar]
- Tal J., Craig E. A., Raskas H. J. Sequence relationships between adenovirus 2 early RNA and viral RNA size classes synthesized at 18 hours after infection. J Virol. 1975 Jan;15(1):137–144. doi: 10.1128/jvi.15.1.137-144.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiedemann L. M., Johnson L. F. Regulation of dihydrofolate reductase synthesis in an overproducing 3T6 cell line during transition from resting to growing state. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2818–2822. doi: 10.1073/pnas.76.6.2818. [DOI] [PMC free article] [PubMed] [Google Scholar]