Abstract
In the event of a radiological accident or terrorist attack, whole- or partial-body exposure can injure the lungs. To simulate such an incident, we used a single fraction of total-body irradiation (TBI) or whole-thoracic irradiation to induce pneumonitis or pulmonary fibrosis, respectively, in a rat model. The superoxide dismutase and catalase mimetic EUK-207 was given by subcutaneous injection (20 mg/kg/day, 5 days per week, once daily) starting at 7 days after irradiation and stopping before pneumonitis developed. After TBI, morbidity and the increase in breathing rates associated with pneumonitis were significantly improved in rats treated with EUK-207 compared to rats receiving irradiation alone. At 42 days after TBI (the peak of pneumonitis) changes in vascular end points including pulmonary hemodynamics ex vivo and relative arterial density in lungs were also mitigated by EUK-207. At 7 months after whole-thoracic irradiation, EUK-207 reduced synthesis of collagen as assessed by the Sircol collagen assay and Masson’s trichrome staining. Our results demonstrate promise for EUK-207 as a mitigator of radiation pneumonitis and fibrosis. We also demonstrate for the first time mitigation of multiple vascular injuries in the irradiated lung in vivo by EUK-207.
INTRODUCTION
In the event of a radiological accident or terrorist attack, survivors of gastrointestinal and bone marrow syndromes may face the risk of delayed and potentially lethal injuries to the lungs. Radiation-induced lung injury is characterized by acute pneumonitis and chronic fibrosis (1). Acute pneumonitis develops within the first 2 to 3 months after irradiation, while chronic pulmonary fibrosis manifests months or even years later (2). Pneumonitis can be lethal and causes vascular injuries to the lungs (3, 4) including narrowing of arterioles and capillaries (5). Substantial data support the pathophysiological importance of oxidative stress generated after radiation that initiates a cascade of chronic inflammatory reactions (6). It remains controversial whether radiation pneumonitis induces pulmonary fibrosis (7), but pharmacologic interventions that address both injuries will serve as ideal mitigators.
Mitigation refers to therapies that are started after irradiation but before there is overt evidence of clinical disease (8). Despite the fact that the pathogenesis of radiation lung injury is incompletely understood, many studies have described promising mitigators, such as angiotensin converting enzyme (ACE) inhibitors (3, 9), genistein (10), manganese porphyrins (11–14) and salen Mn complexes (15).
The salen Mn complex EUK-207 (Fig. 1) has superoxide dismutase (SOD) and catalase activities. SODs dismutate superoxide to form hydrogen peroxide (H2O2) (16), and catalase (17) decomposes H2O2 into water and oxygen. EUK-207 and other salen Mn complexes, such as the older compound EUK-189, have been reported to mitigate radiation injury in vivo in multiple tissues including the lung (15, 18, 19). EUK-207 and EUK-189 also inhibit apoptosis induced by ionizing radiation in capillary endothelial cell cultures (20). EUK-207, a cyclized salen Mn complex, has greater stability than EUK-189 and attains much higher plasma levels when given subcutaneously to rats (19). But, like EUK-189, EUK-207 lacks oral bioavailability and has to be administered by injection or infusion (21). The need for these routes of administration suggests that shorter drug schedules will be more practical and desirable than more chronic delivery.
FIG. 1.
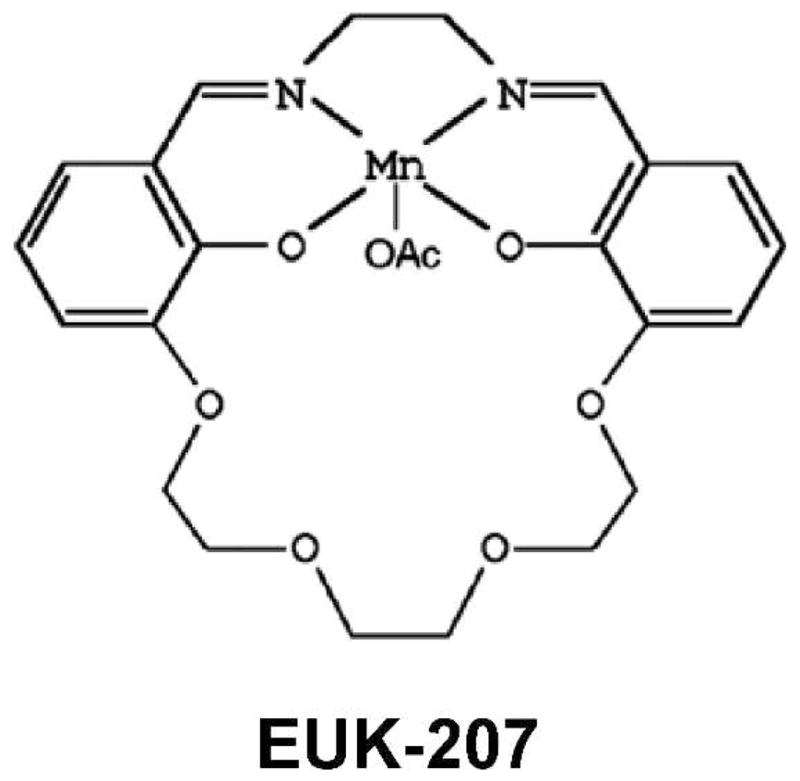
Chemical structure of EUK-207.
Recently, Mahmood et al. (15) reported that EUK-207 mitigated pulmonary fibrosis in Sprague Dawley rats when given subcutaneously (by implanted infusion pumps) starting within 1 h after thoracic irradiation and continuing for 98 days. Our goal is to mitigate radiation-induced lung injury after total-body irradiation (TBI) as well as after whole-thoracic irradiation, both of which are scenarios that are likely to occur in a radiological terrorism event or nuclear accident. We are also interested in identifying shorter durations of EUK-207 therapy required for mitigation and determining whether EUK-207 has an impact on the vascular impairments known to be associated with radiation-induced lung injury.
There are relatively few studies of the development of pneumonitis in rats after a single dose of TBI. Most studies have used partial- (11–14) or whole-thoracic irradiation to assess mitigation (9, 18). Molteni et al. (3) described fractionated TBI in a rat model and demonstrated reduction of lung injury by ACE inhibitors started 10 days before radiation. In addition, Mahmood et al. (22) reported that genistein mitigated lung injuries caused by combined irradiation of 4 Gy TBI plus an additional 8 Gy to the whole thorax of rats.
We used a rat model of a single dose of TBI-induced pneumonitis to mimic a nuclear event. Higher doses of radiation than those tolerated by the gastrointestinal system (12 Gy) are needed to cause pulmonary fibrosis in rats in an experimentally feasible time frame (23). In addition, we have found that after 11 Gy TBI, radiation nephropathy occurs before we can detect pulmonary fibrosis. Therefore, we could not use TBI to study pulmonary fibrosis in the rat and instead used 13 Gy whole-thoracic irradiation to induce fibrosis for our study. This type of strategy is relevant to a sudden exposure after a radiological event because, if an agent mitigates pneumonitis without mitigating pulmonary fibrosis, we may be buying time, but may not be preventing fatal injuries. Nephropathy may be an issue if pneumonitis is prevented, but there are other promising approaches for mitigating that radiation injury [e.g., ACE inhibitors (24)]. Fatal gastrointestinal injury is not of significant concern for mitigation of lung injury after TBI. If the dose is high enough to cause gastrointestinal lethality, no one will live long enough to develop pneumonitis. We have also used a relatively short drug schedule in this study, we started EUK-207 at 7 days after radiation and stopped it before pneumonitis (which peaks at 42 days). Our results demonstrate mitigation by EUK-207 of acute pneumonitis and late lung fibrosis. Furthermore, our data indicate, for the first time that EUK-207 mitigates multiple pulmonary vascular impairments that result from irradiation. As a first step we used only female rats to demonstrate efficacy, but future studies will need to be extended to males, younger and older rats, other rat strains and eventually to other species.
MATERIALS AND METHODS
Animal Care
The study was approved by the Institutional Animal Care and Use Committee (IACUC) at the Medical College of Wisconsin and Zablocki Veterans Affairs Medical Center. Female rats (WAG/ RijCMCR) were housed in a moderate security barrier. Based upon directives from the IACUC of the Medical College of Wisconsin, rats were considered morbid and were euthanized if they met specified veterinarian’s criteria. These included at least 3 of the following: (1) greater than 20% loss in body weight; (2) inactivity on 2 consecutive days, defined as no movement unless actively stimulated; (3) lack of grooming that became worse after 24 h; (4) breathing rates of less than 60 or greater than 250 breaths per minute; and (5) hunched posture, death pose on 2 consecutive days.
Injury Models
We used two radiation injury models: TBI to study radiation pneumonitis (19) and whole-thoracic irradiation to study radiation fibrosis (25). Unanesthetized 8- to 10-week-old rats weighing approximately 140 g were immobilized in a plastic jig and irradiated with a 320-kVp orthovoltage system X rays, with a half-value layer (HVL) of 1.4 mm Cu.
For TBI, as described previously (24), rats were given a single dose of 11 Gy (posterior to anterior) at a dose rate of 1.73 Gy/min. The head was shielded to protect the mouth to facilitate food intake. All irradiated rats received a syngeneic bone marrow transplant (BMT) a few hours after irradiation (26). The irradiated rats and their age-matched controls were randomized for follow up to 42 or 110 days after irradiation. Rats were sacrificed for invasive assays at 42 days after TBI to study vascular and structural end points as this time coincided with the peak of pneumonitis. The experiment was terminated at 110 days by which time surviving rats had recovered from major morbidity due to pneumonitis.
For whole-thoracic irradiation, as described previously (25), rats were treated with a single dose of 13 Gy to the whole thorax at a dose rate of 1.43 Gy/min. The radiation dose was delivered by two equally-weighted lateral beams to improve uniformity. The whole lung, heart and a small amount of liver were in the field. The irradiated rats (N = 61) and their age-matched controls (N = 14) were kept for 7 months (~ 210 days), and were then sacrificed to evaluate pulmonary fibrosis. The lungs were harvested to measure collagen and were sectioned for histopathology.
Administration of Drug
EUK-207 (custom synthesized by Dalton Chemical Laboratories, Toronto) was injected subcutaneously in rats randomized from the irradiated groups. A total of 14 rats given 11 Gy TBI followed by BMT and 20 rats given 13 Gy to the whole thorax, were injected with EUK-207 at 20 mg/kg/day (rats were weighed before injection) once per day for 5 days a week, starting at 7 days after irradiation. Injections were stopped at 35 days after TBI or 30 days after radiation to the thorax.
Breathing Rate Assay
The breathing rates and body weights were measured as described previously (27) in randomly-selected rats from TBI groups from 4 to 12 weeks after irradiation. Rats were restrained in a Plexiglas jig and placed in a transparent, airtight box connected to a differential pressure transducer. The mean breathing rate in each rat was calculated from a minimum of four steady regions of recording lasting at least 15 s each. The breathing rate was expressed as breaths per minute.
Pulmonary Vascular Resistance, Total Lung Angiotensin-Converting Enzyme (ACE) Activity and Terminal Arteriole Count
Pulmonary vascular resistance, total lung angiotensin-converting enzyme (ACE) activity and terminal arteriole count were measured as described previously (28). Briefly, the lungs and heart were harvested en bloc and perfused with 5% bovine serum albumin in physiological saline solution (Equitech-Bio, Kerrville, TX). The pulmonary artery pressure was measured at 30, 20, 15, 10 and 5 ml/min. The flow was then stopped and the closing pressure was recorded. The flow rates were normalized by the rat body weight and the pressure versus flow data were fit to a simple pulmonary vascular distensibility model (29) to determine pulmonary vascular resistance at a flow rate of 100 ml/ min/kg.
For assessment of ACE activity, the ACE substrate N-[3-(2-furyl)-acryloyl]-L-phenyl-alanylglyclglycine (FAPGG) (Sigma-Aldrich, Milwaukee, WI) was perfused through the lung, and the lung ACE-binding activity was measured as the percentage of conversion of FAPGG to FAP in the effluent. FAP was measured spectrophotometrically, and ACE activity was presented in relative activity units (19).
To compare arterial densities, the perfusate in the arteries was replaced with 1-bromo-perfluoro-octane for X-ray contrast. The airway pressure was maintained at 6 mm Hg. The intravascular pressure was set to 30 mm Hg after being conditioned by cycling the pressure from 0 to 35 mm Hg several times. High-magnification microfocal angiographic images of isolated lungs were acquired. Applying a global threshold and a seeded region growing algorithm, the 3D isotropic CT data was used to determine the number of terminal vessels in the arterial tree, expressed as the “terminal arteriole count”.
Sircol Collagen Assay
Sircol collagen assay was employed to study fibrosis. The assay was performed as described previously with minor modification (Biocolor Ltd., Carrickfergus, Northern Ireland) (25). The assay measured the acid and pepsin soluble collagen content in rat lungs. The inferior lobe of the right lung was excised, gently wiped, cut into two and weighed. The top half was used for protein assay. The lower part was homogenized in 1 ml pepsin reagent (Accurate Chemical & Scientific Corp., Westbury, NY) and was used for estimation of collagen as previously described (25). The concentration of collagen in test samples was compared to the linear region of a standard provided by the manufacturer. Collagen content in test samples was normalized to protein content.
Histology
After perfusion and imaging, the inflated lungs from TBI-treated rats were fixed in 10% neutral buffered formalin (Fisher Scientific, Pittsburg, PA), and then embedded in paraffin. Whole mount sections were cut (4 μm), processed and stained with hematoxylin and eosin (H&E) (Richard Allan, Kalamazoo, MI). For rats given whole-thoracic irradiation, the left lung was removed at ~ 210 days and inflated by injecting with 10% neutral buffered formalin. The sections were processed and stained with Masson’s trichrome (Newcomer Supply, Middletown, WI). All histological work was performed by the Children’s Research Institute-Histology Core at the Medical College of Wisconsin.
Quantification of Staining in Lung Sections
Sections stained with H&E were used to monitor vessel wall thickening as an indicator of vascular injury, and the presence of foamy macrophages as a measure of inflammation. All lung sections were reviewed independently by two investigators who were blinded to the treatment groups. Five fields from corresponding areas that did not include large airways and vessels were selected in each section. In each field, the thickness of the vessel walls was scored semi-quantitatively on a three-point scale from 0–2 as shown in Table 1, panel a. All foamy macrophages in the same field were counted and given scores from 0 to 3 as shown in Table 1, panel b. Mean vessel thickening and foamy macrophage scores were calculated for each rat.
TABLE 1.
Description of Scales used for Histological Scoring
| Score | Stain | Description |
|---|---|---|
| a: Vessel wall thickening | H&E | |
| 0 | normal vessel | |
| 1 | thickened vessel wall with visible lumen | |
| 2 | thickened vessel wall with occluded lumen | |
| b: Foamy macrophage | H&E | |
| 0 | no macrophages | |
| 1 | 1–3 macrophages/field | |
| 2 | 3–5 macrophages/field | |
| 3 | >5 macrophages/field | |
| c: Frequency of fibrotic foci (25) | Masson’s trichrome | |
| 0 | no blue staining in alveolar walls | |
| 1 | <25% of alveoli with blue staining in walls | |
| 2 | 25–50% of alveoli with blue staining in walls | |
| 3 | 50–75% of alveoli with blue staining in walls | |
| 4 | >75% of alveoli with blue staining in walls | |
| d: Extent of fibrosis (25) | Masson’s trichrome | |
| 1 | <25% area of alveolar walls are blue | |
| 2 | 25–50% area of alveolar walls are blue | |
| 3 | 50–75% area of alveolar walls are blue | |
| 4 | >75% area of alveolar walls are blue |
To quantify collagen staining by Masson’s trichrome, 4 corresponding fields in each lung section that did not include large airways and the adjacent vessels were selected as described previously (25). A numeric score based on the percentage of alveoli with blue color in the walls was given for each field as shown in Table 1, panel c. This score estimated the frequency of fibrotic (blue) foci in the section. A second score was assigned to estimate the extent of fibrosis in the collagen stained alveoli as shown in Table 1, panel d. The sum of the 2 scores was taken as a composite trichrome blue score for fibrosis in each field, and the average of the four fields was calculated for each animal. Blue color associated with collagen present in the vasculature or airways were not considered for scoring.
Statistical Analysis
The morbidity/mortality of rats following treatments was evaluated by a Kaplan-Meier survival plot and was expressed as percentage morbidity. The significance was analyzed by the Peto-Peto Wilcoxon test. Results for body weight changes, breathing rate, terminal arteriole count, ACE activity, Sircol collagen assay, vessel wall thickness and trichrome staining were compared by one-way analysis of variance (ANOVA). All pairwise multiple comparisons were carried out by the Holm-Sidak test to determine significance. Box-Cox transformations were performed before ANOVA for measurements of pulmonary vascular resistance and macrophage counts since the variance was not constant: the standard deviation increased with the mean and the hypothesis of equal variance was rejected (Fligner test P =0.0003 and P =0.02, respectively). The transformation suggested by the Box-Cox analysis was the inverse for pulmonary vascular resistance and logarithm for macrophage counts. Graph values are expressed as mean ±95% confidence intervals.
A second statistical test was conducted for the breathing rate assays and weight changes to account for differential attrition. Based on a Box-Cox analysis, the breathing intervals (inverse of breathing rate) were calculated as minutes/breath and weight measurements were not modified. A mixed-effects model, with random rat effect and fixed measurement week, and treatment group effects and their interaction were fitted (30). This approach assumed that breathing for animals that were lost to morbidity followed the same general trajectory as for those that survived. The comparisons of interest were estimated based on the model, and were adjusted for multiple testing by the single-step method that was based on multivariate normal distribution (31).
RESULTS
EUK-207 Reduces Morbidity After TBI
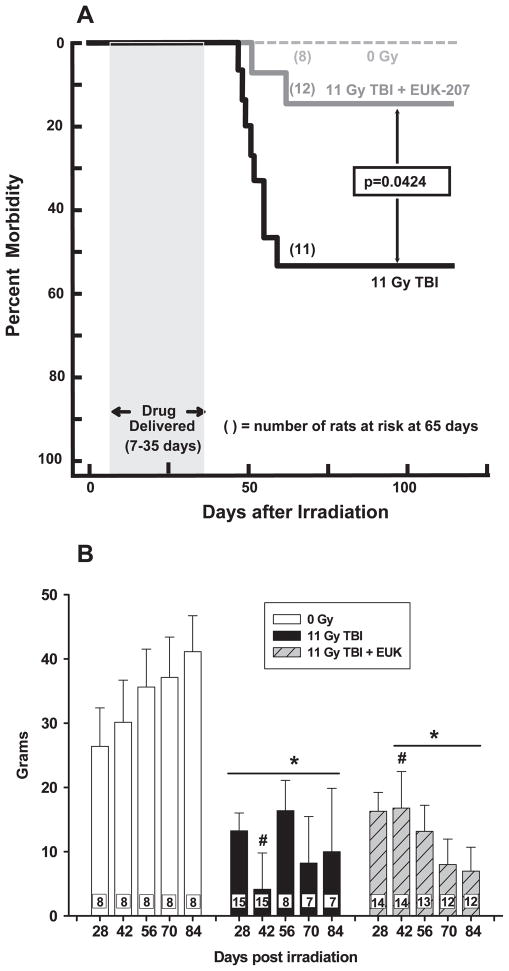
Bone marrow transplant (BMT) was combined with TBI in all cases to minimize death due to bone marrow failure, which occurs before pneumonitis. Over 50% of rats treated with 11 Gy TBI + BMT were morbid by 110 days (Fig. 2A). Rats treated with a short schedule of EUK-207 completed before the onset of pneumonitis (as represented by the gray bar in Fig. 2A) had less morbidity (Fig. 2A).
FIG. 2.
Panel A: Kaplan-Meier plot of morbidity after 11 Gy TBI followed by a bone marrow transplant. EUK-207 was given by subcutaneous injection from 7 to 35 days after irradiation once daily, 5 days per week, at a dose of 20 mg/kg/day. Numbers in parentheses indicate the number of rats at risk at 65 days after irradiation. We started with 8 rats in the unirradiated group: 20 with 11 Gy TBI and 14 with 11 Gy TBI+EUK-207. P = 0.0424 (Peto-Peto-Wilcoxon). Panel B: Body weight change after 11 Gy TBI. Rats were treated with EUK-207 as described in panel A. The increase in body weight in grams, from the day of irradiation to the day marked on the X-axis is presented as mean ± 95% confidence intervals. Irradiated rats had less increase in body weight compared to age-matched normal rats at corresponding times between 28 to 84 days after irradiation. EUK-207 treated rats weighed more than irradiated rats without drug at 42 days. The number in each bar represents the number of rats per group (N): #P < 0.05 vs. each other; *P < 0.05 vs. unirradiated rats at corresponding times.
Nonirradiated rats gained weight steadily over the study (84 days). In contrast, the increase in body weight of irradiated animals was significantly less than age-matched normal animals at corresponding time points from 42 days after irradiation (Fig. 2B). To account for attrition due to pneumonitis, analyses were repeated based on a mixed effects model with random rat effect. The comparisons of interest were estimated and adjusted for multiple testing by the single-step method based on multivariate normal distribution (Table 2). EUK-207 statistically improved body weight increase after 11 Gy TBI over that of irradiated animals without EUK-207 at 42 days (Fig. 2B and Table 2). Overall, the pattern through 84 days with and without EUK-207 after irradiation was similar (Fig. 2B and Table 2), supporting a position that the survival advantage of this treatment was not related to nutritional status alone.
TABLE 2.
Comparison of Weight Changes
| Comparison | Estimatea | SEb | P value | |
|---|---|---|---|---|
| Day 28–day 0 | 11 Gy TBI – 0 Gy | −13.1 | 3.3 | 0.00117 |
| 11 Gy TBI+EUK – 0 Gy | −10.1 | 3.4 | 0.0357 | |
| 11 Gy TBI+EUK – 11 Gy TBI | 3. 1 | 2.8 | 0.95111 | |
| Day 42–day 0 | 11 Gy TBI – 0 Gy | −26 | 3.3 | <0.001 |
| 11 Gy TBI+EUK – 0 Gy | −13.3 | 3.4 | 0.0011 | |
| 11 Gy TBI+EUK - 11 Gy TBI | 12.7 | 2.8 | <0.001 | |
| Day 56–day 0 | 11 Gy TBI – 0 Gy | −19.9 | 3.7 | <0.001 |
| 11 Gy TBI+EUK – 0 Gy | −23 | 3.4 | <0.001 | |
| 11 Gy TBI+EUK – 11 Gy TBI | −3.1 | 3.2 | 0.97862 | |
| Day 70–day 0 | 11 Gy TBI – 0 Gy | −28.7 | 3.7 | <0.001 |
| 11 Gy TBI+EUK – 0 Gy | −29.8 | 3.4 | <0.001 | |
| 11 Gy TBI+EUK – 11 Gy TBI | −1.1 | 3.4 | 1 | |
| Day 84–day 0 | 11 Gy TBI – 0 Gy | −30.9 | 3.7 | <0.001 |
| 11 Gy TBI+EUK – 0 Gy | −34.9 | 3.4 | <0.001 | |
| 11 Gy TBI+EUK – 11 Gy TBI | −4 | 3.4 | 0.91656 |
Estimate: Difference in body weight.
SE: Standard error.
Mitigation of the Radiation-Induced Increase in Breathing Rate by EUK-207
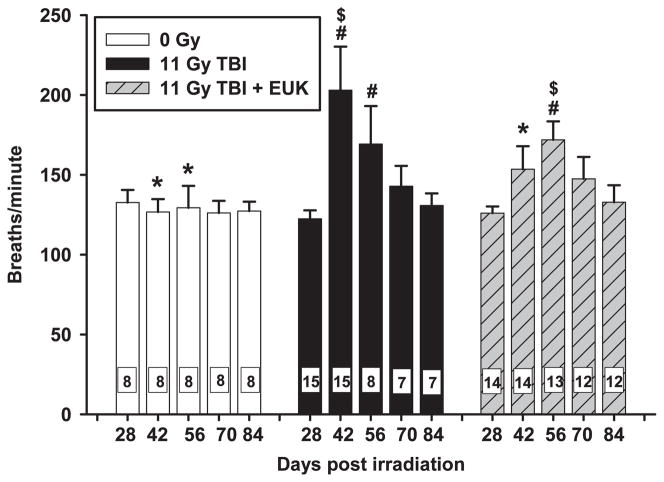
The breathing rates of age-matched control, nonirradiated rats did not change over the course of days 28 through 84 (Fig. 3, open bars). After 11 Gy TBI (Fig. 3, black bars) respiratory rates increased and reached a peak at 42 days. Thereafter, the breathing rates of irradiated animals slowly declined and survivors had statistically identical breathing rates to age-matched normal animals by 70 days. Similar to those of their untreated but irradiated cohorts, breathing rates of irradiated rats treated with EUK-207 (Fig. 3, hatched bars) were significantly increased above those of age-matched normal animals at 56 days, but not at any other times. The peak breathing rate in rats treated with EUK-207 was lower than the peak breathing rate of irradiated rats without drug. In addition to lessened tachypnea with EUK treatment, the peak in the breathing rate after irradiation was shifted to the right by EUK treatment from 42 to 56 days. In contrast, EUK treatment had no effect on time to peak breathing rates or highest values in 13 Gy WTI rats (data not shown).
FIG. 3.
Radiation-induced increase in breathing rate is mitigated by EUK-207. Rats were treated as described in Fig. 2. Breaths per minute (Y-axis) are presented as mean ± 95% confidence intervals. Breathing rate of the irradiated rats without drug (black bars) peaked at 42 days and then decreased over the next few weeks. The peak was attenuated, as well as delayed, to 56 days by EUK-207. The number in each bar represents the number of rats per group (N). #P < 0.05 vs. 0 Gy at corresponding times; *P < 0.05 vs. 11 Gy TBI at corresponding times;, $P < 0.05 between groups (peak 11 Gy TBI (42 days) vs. peak 11 Gy TBI+EUK-207 (56 days)).
To account for attrition due to pneumonitis, analyses were repeated based on a mixed effects model. In the case of respiratory rates, calculations were made using the inverse of the breathing rate (minutes/breath), which is the breathing interval. There was no difference between the breathing intervals of groups at 28, 70 or 84 days (Table 3). However, at 42 days these intervals were different in all three groups (Table 3). At 56 days, age-matched normal rats had a different breathing interval from all irradiated rats, but there was no difference between irradiated rats and those that also received EUK-207 (Table 3). These results are very similar to those shown in Fig. 3.
TABLE 3.
Comparison of Breathing Intervals after Accounting for Attrition
| Days post irradiation | Treatment | Mean breathing intervals | Number of rats survived | Number of rats morbid | Comparison | P value |
|---|---|---|---|---|---|---|
| 28 | Controla | 0.00757 | 8 | 0 | Irradiation vs. control | 0.62 |
| Irradiationb | 0.00821 | 15 | 0 | EUK vs. control | 0.97 | |
| EUKc | 0.00796 | 14 | 0 | EUK vs. irradiation | 0.99 | |
| 42 | Control | 0.00794 | 8 | 0 | Irradiation vs. control | <0.001 |
| Irradiation | 0.0052 | 15 | 0 | EUK vs. control | 0.015 | |
| EUK | 0.00667 | 14 | 0 | EUK vs. irradiation | <0.001 | |
| 56 | Control | 0.00785 | 8 | 0 | Irradiation vs. control | <0.001 |
| Irradiation | 0.00607 | 8 | 7 | EUK vs. control | <0.001 | |
| EUK | 0.00588 | 13 | 1 | EUK vs. irradiation | 1 | |
| 70 | Control | 0.00797 | 8 | 0 | Irradiation vs. control | 0.37 |
| Irradiation | 0.00706 | 7 | 8 | EUK vs. control | 0.089 | |
| EUK | 0.0069 | 12 | 2 | EUK vs. irradiation | 1 | |
| 84 | Control | 0.00788 | 8 | 0 | Irradiation vs. control | 1 |
| Irradiation | 0.00767 | 7 | 8 | EUK vs. control | 0.99 | |
| EUK | 0.00763 | 12 | 2 | EUK vs. irradiation | 1 |
Control, 0 Gy.
Irradiation, 11 Gy TBI.
EUK, 11 Gy + EUK-207.
Mitigation of Vascular and Other Changes in the Lungs at 42 Days by EUK-207
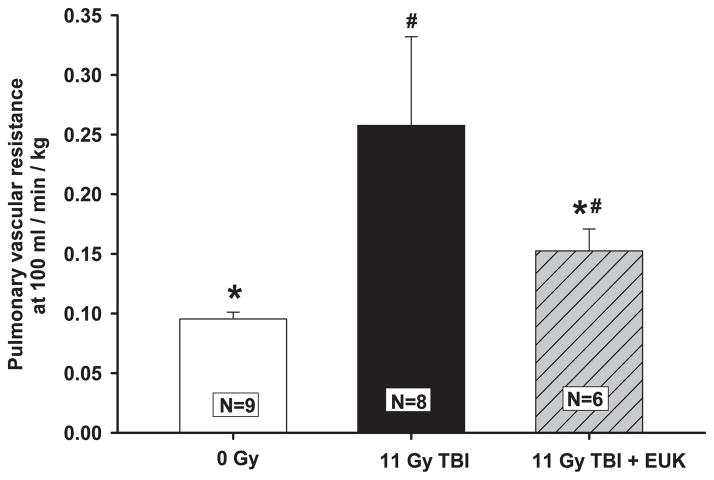
Pulmonary vascular resistance (PVR) measurements in isolated perfused lungs at a flow rate of 100 ml/min/kg at 42 days after TBI are shown in Fig. 4. The PVR was significantly increased after 11 Gy TBI compared to age-matched normal animals, with a relatively wide variance between animals. Irradiated rats treated with EUK-207 had a significantly lower PVR than those receiving 11 Gy TBI alone (Fig. 4), as well as variance among individuals in this group closer to that of nonirradiated hosts.
FIG. 4.
Radiation-induced increase in pulmonary vascular resistance and mitigation by EUK-207. Rats were irradiated and treated with the drug as described in Fig. 2. Pulmonary vascular resistance was measured at 42 days after irradiation. Values are presented as mean ± 95% confidence intervals. N = number of rats per group, #P < 0.05 vs. 0 Gy; *P < 0.05 vs. 11 Gy TBI.
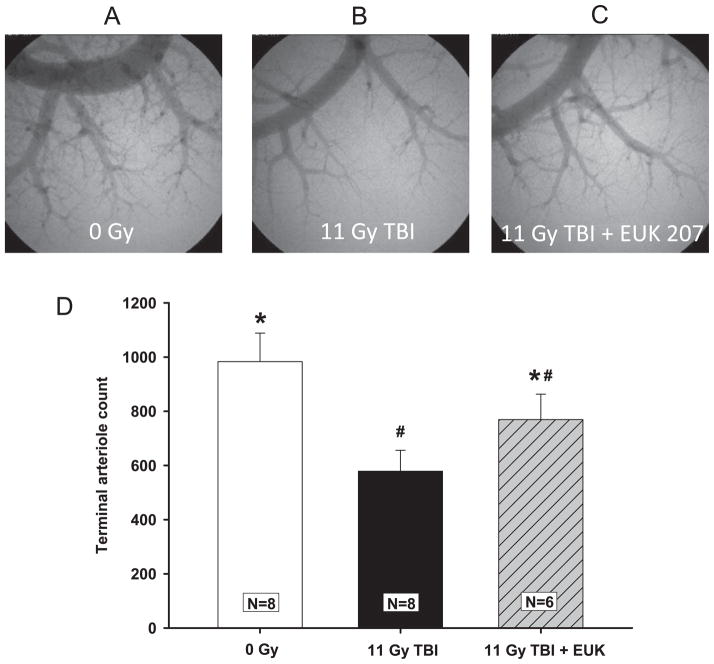
Representative high-magnification planar angiograms of the pulmonary arterial tree from the right lung are shown in Fig. 5A–C. Extensive pulmonary arteriole branching is evident in nonirradiated hosts, and it is clearly diminished in irradiated rats. Terminal arteriole counts (Fig. 5D) performed on isotropic image volumes demonstrated that 11 Gy TBI significantly reduced arterial density, while EUK-207 attenuated this loss.
FIG. 5.
Radiation-induced loss of pulmonary vessels and mitigation by EUK-207. Rats were irradiated and treated with the drug as described in Fig. 2. Panels A, B and C are representative images of the high-power planar angiograms from each group at 42 days after irradiation. Panel D shows the terminal arteriole count presented as mean ± 95% confidence intervals. N =number of rats per group. #P < 0.05 vs. 0 Gy; *P < 0.05 vs. 11 Gy TBI.
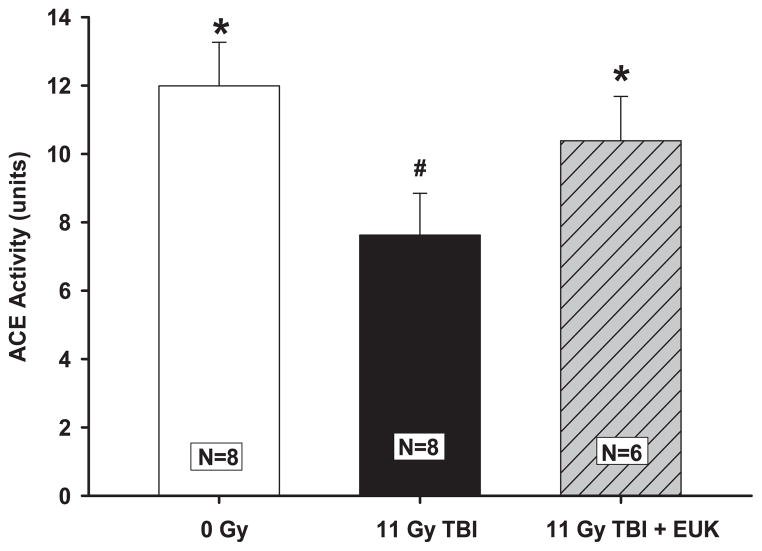
Activity of ACE, an enzyme expressed by vascular endothelial cells, was used to assess endothelial function and vascular surface area in irradiated rats (3, 28, 32). Total lung ACE activity was decreased by roughly one-third after 11 Gy TBI at 42 days. Compared age-matched normal rats or irradiated rats given EUK-207 (Fig. 6). The ACE activity in EUK-207 treated rats was lower, but not different from age-matched normal rats (Fig. 6).
FIG. 6.
Radiation-induced decrease in ACE activity and mitigation by EUK-207. Rats were irradiated and treated with the drug as described in Fig. 2. ACE activity was measured in isolated whole lungs at 42 days after irradiation. Note the decrease in ACE activity with 11 Gy TBI alone compared to age-matched normal rats or irradiated rats given EUK-207. Values are presented as mean ± 95% confidence intervals. N = number of rats per group. #P < 0.05 vs. 0 Gy; *P < 0.05 vs. 11 Gy TBI.
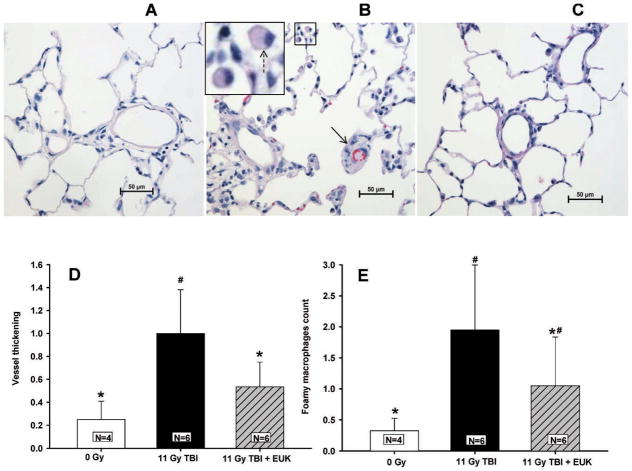
Radiation exposure resulted in two characteristic histological changes in the rat lungs. TBI increased the thickness of arterial walls and increased the number of foamy macrophages per unit area 42 days after irradiation (Fig. 7). EUK-207 reduced both the radiation-associated increases in vessel wall thickening (Fig. 7D) and the numbers of foamy macrophages in the lung (Fig. 7E).
FIG. 7.
Radiation-induced changes in lung structure in H&E stained lung sections. Vessel wall thickening and foamy macrophages were scored in lung sections as described in Materials and Methods. Lungs were fixed at 42 days after irradiation. Representative stained sections are shown as follows: Panel A: age-matched normal rats; panel B: rats treated with 11 Gy TBI alone. The inset is magnified to visualize foamy macrophages (panel C) rats given 11 Gy TBI with EUK-207. Solid arrow marks an occluded vessel; dotted arrow marks a foamy macrophage. Panel D is a graphical representation summarizing vessel wall thickening scores. Panel E summarizes the foamy macrophage scores. Table 1 describes the scales used for scoring. Values are presented as mean ± 95% confidence intervals. N =number of rats per group. #P < 0.05 vs. 0 Gy; *P < 0.05 vs. 11 Gy TBI.
Mitigation of Radiation-Induced Fibrosis at 210 Days by EUK-207
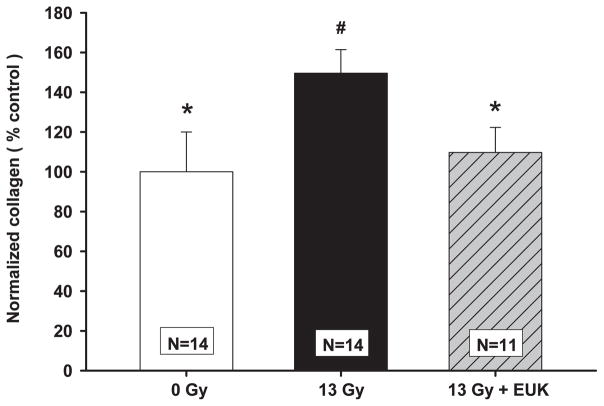
Newly synthesized (pepsin-soluble) lung collagen content was measured at 210 days after 13 Gy whole-thorax irradiation. Irradiated rats had elevated collagen compared to age-matched normal animals (Fig. 8). Pepsin-soluble collagen content of lungs from EUK-207 treated, irradiated rats was not different from that of age-matched normal rats.
FIG. 8.
Radiation-induced increase in pepsin-soluble collagen in lungs and mitigation by EUK-207. Rats were given 13 Gy to the whole thorax. EUK-207 was given by subcutaneous injection from 7 to 30 days (once daily, 5 days per week) after irradiation at a dose of 20 mg/kg/day. Pepsin-soluble collagen was measured at 210 days after irradiation and was normalized by protein content. Values (percentage relative to age-matched normal animals) are presented as mean ± 95% confidence intervals. N = number of rats per group. #P < 0.05 vs. 0 Gy; *P < 0.05 vs. 13 Gy.
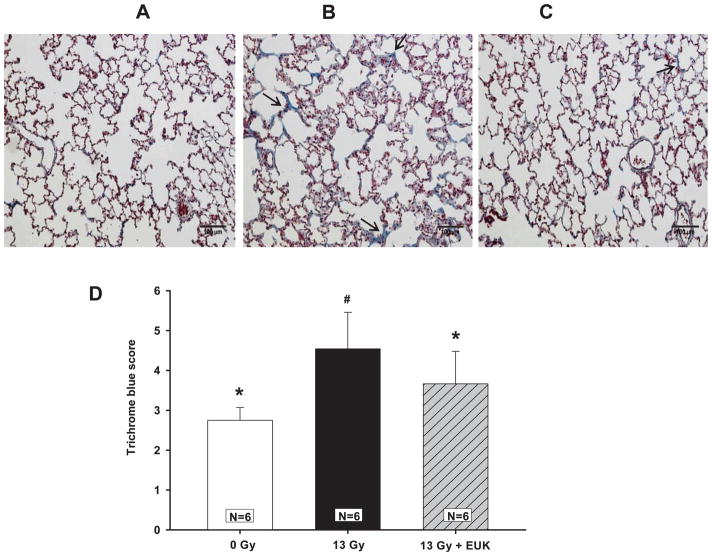
EUK-207 also reduced the increase in collagen in irradiated animals as measured histologically. Blue staining of lung sections with Masson’s trichrome (Fig. 9, arrows) identify collagen foci. The density of blue collagen as measured by our scoring system was increased in irradiated rats over that of age-matched controls. The collagen scores of lungs from rats treated with EUK-207 were not different from those of age-matched normal rats.
FIG. 9.
Radiation-induced increase in fibrotic collagen foci (see arrows) and mitigation by EUK-207 in Masson’s trichrome stained rat lung sections (collagen is stained in blue). Lungs were fixed at 210 days after irradiation. Representative stained sections are shown as follows: Panel A: age-matched normal rats; panel B: rats treated with 13 Gy to the thorax; panel C: rats treated with 13 Gy to the thorax and given EUK-207. Bar =100 μm. Blue color (see arrow) was scored as described in Materials and Methods and Table 1. The results are represented graphically in panel D. Values are presented as mean ± 95% confidence intervals. N = number of rats per group; #P < 0.05 vs. 0 Gy, *P < 0.05 vs. 13 Gy.
DISCUSSION
In this work, we used TBI followed by a BMT to study acute radiation pneumonitis and whole-thorax irradiation to study late pulmonary fibrosis. Single doses of irradiation were used to mimic a radiological terrorism event or an accident. The TBI study showed pneumonitis at 42–70 days after irradiation. After TBI, EUK-207 treatment for 4 weeks attenuated morbidity (Fig. 2A), mitigated the radiation-induced increase in breathing rate (Fig. 3, Table 3), and reduced vascular injuries associated with radiation pneumonitis (Figs. 4–7). In data not shown, rats treated with 13 Gy whole-thoracic irradiation with EUK-207 (n = 20) exhibited less though a statistically insignificant difference in morbidity during pneumonitis over rats receiving whole-thorax irradiation alone (n = 41). However, morbidity was greater (66%) versus only 55% in rats given TBI, with deaths in both groups limited to the period between 40 and 80 days. Pneumonitis was also more severe after 13 Gy to the thorax based on peak breathing rates (~230 ± 11 breaths/minute (mean ± SE, n = 25) after 13 Gy to the thorax versus 200 ± 12 breaths/minute (n =15) after 11 Gy TBI. It is therefore possible that a higher dose of EUK-207 may be needed to provide significant benefit through pneumonitis after 13 Gy WTI. Our results demonstrate that EUK-207 can be started as late as 7 days after irradiation and stopped before pneumonitis and still mitigate radiation pneumonitis as well as the onset of pulmonary fibrosis. A window of 7 days after irradiation would be appropriate to simulate the interval after a mass casualty event between radiation exposure and biodosimetry, to determine the need for therapeutic intervention and the ability to deliver therapy to a large population. The drug was stopped at 35 days after TBI or 30 days after whole-thorax irradiation because we wanted to limit the duration of drug treatment. This time was chosen because it precedes the onset of pneumonitis, when administration of an antioxidant agent may hinder effective control of infection.
Radiation-induced pneumonitis usually becomes apparent after approximately 2–4 months and depending on the severity and extent, can be lethal (33). Clinical signs and symptoms include dyspnea, cough, low-grade fever, and chest discomfort (34). Histological changes such as inflammation and edema of the interstitium and air spaces were reported both in humans and in experimental animals (35, 36). Structural and functional vascular injuries accompanying pneumonitis include narrowing of arterioles and capillaries, development of vascular congestion and increased capillary permeability (5, 37). To our knowledge, this study is the first to show mitigation of lethality due to radiation pneumonitis after a single dose of TBI. Most studies on mitigation of radiation lung injury in animal models have used whole- or partial-thoracic irradiation (9, 18). Following TBI, the gastrointestinal and hematopoietic syndromes could cause death before the onset of pneumonitis. The BMT is given after TBI to prevent hematological toxicity, but lethal doses to the gastrointestinal tract must be avoided to observe pneumonitis at a later time (26). Molteni et al. described a rat fractionated TBI model in which there was no mortality (3). Mahmood et al. reported that genistein had mitigating effects on radiation-induced lung injuries in rats irradiated with either 12 Gy to the lung, or with 8 Gy to the lung and 4 Gy to the whole torso (22), doses that spare the bone marrow and gastrointestinal syndromes. Single-dose TBI (11 Gy) with BMT in our rat model did not induce pulmonary fibrosis before rats were lost to radiation nephropathy starting around 120 days (data not shown). Therefore, we tested the ability of EUK-207 to mitigate the onset of pulmonary fibrosis using a model of 13 Gy whole-thoracic irradiation which shielded the hematopoietic and gastrointestinal systems.
In previous studies, a single injection of salen Mn complexes given prior to irradiation demonstrated enhancement of 30 day survival (38). In that study, it was shown that EUK-189 at 70 mg/kg administered subcutaneously 24 h prior to irradiation had a dose reduction factor of 1.15 in mice after whole-body 60Co irradiation. However, when given after irradiation, a single injection of EUK-189 had limited mitigating effect in rodents, especially in the lung (18). In a rat model of whole- or partial-lung 60Co irradiation (10–20.5 Gy), Langan et al. also showed that 3 injections of EUK-189 after irradiation were effective at reducing micronucleus formation in lung fibroblasts, but did not protect against morbidity at 2–3 months after irradiation (18). Continuous administration of EUK-207 via implanted subcutaneous infusion pumps from 1 h until 14 weeks after irradiation is reported to mitigate lung injuries in rats (15). Brown et al. showed that continuing antioxidant diet supplementation starting 24 h after irradiation is effective at mitigating lethality (39). Furthermore, they reported that a delay of 24 h after radiation of continuous antioxidant diet supplementation is more effective than intervention initiated immediately after the exposure. They hypothesized that this delay in administration of antioxidant diet allows for efficient repair of the radiation injury as well as survival of bone marrow cells (39). In the current study, EUK-207 was administered by subcutaneous daily injections (5 days a week) starting 7 days after irradiation and stopping before the onset of pneumonitis. This schedule simulates the delay anticipated to be inherent to initiating therapy after a mass-casualty event. Furthermore, a short schedule stopping before the onset of pneumonitis could limit potential drug side effects as well as the inconvenience of continuous injections of EUK-207.
We have previously reported mitigation of pulmonary ACE activity by EUK-207 delivered by subcutaneous pumps at 1.8 mg/m2/day, alone or in combination with 100 mg/m2/day of the ACE inhibitor, captopril (19). In that model, survival was not improved after 11 Gy TBI. In the current study, however, daily injection of a higher dose of EUK-207 without captopril reduced morbidity due to radiation pneumonitis by 11 Gy TBI (Fig. 2).
In our model, EUK-207 injected subcutaneously at 20 mg/kg/day to female rats weighing approximately 140 grams statistically improved body weights after 11 Gy TBI at only one time after irradiation, and this increase was very modest (Fig. 2B and Table 2). We do not believe that the beneficial effects of EUK-207 on survival, breathing rates, or fibrosis in our model are attributable solely to small differences in weight of treated and untreated groups. Because of previous reports of no change in body weight with EUK-207 in unirradiated mice (40), and because weights were overall so similar in our irradiated rats with and without EUK-207, EUK-207 treatment without radiation was not assessed in this study.
The peak breathing rate after 11 Gy TBI was attenuated and delayed by EUK-207 (Fig. 3). A delay in symptoms of pneumonitis by EUK-207 could give victims of a larger window of time for treatment. Interestingly in this study, we found an increase in the breathing rate in the irradiated animals treated with EUK-207, while Mahmood et al. (15) did not observe an increase in breathing rate in rats given EUK-207 after thoracic irradiation when the drug was continued for up to 14 weeks. This could be due to a number of reasons: (1) a difference in the dose of irradiation [10 (15) vs. 11 Gy] as the lower dose did not induce lethality in rats; (2) a difference in the irradiation, that is whole thorax (15) vs. TBI (this study); (3) a difference in strain [Sprague Dawley (15) vs. WAG/Rij (this study)]; and (4) continuing drug treatment after the onset of pneumonitis (15) vs. a 4 week treatment (this study).
Vascular damage after irradiation has been studied by many investigators (4, 41–43). We demonstrated impairments in several pulmonary vascular parameters after irradiation at a dose of 10 Gy limited to the thorax (27, 28). In addition, we and others demonstrated that ACE inhibitors mitigate pulmonary vascular injuries (3, 9, 44). In the current study, we showed that 11 Gy TBI, [as with 10 Gy to the thorax only (28)] increased the pulmonary vascular resistance (PVR), and reduced the terminal arteriole count and ACE activity in isolated and perfused lungs (Figs. 4–6). Structural changes in pulmonary blood vessels, such as wall thickening, stenosis and lumen occlusion, could increase the PVR. Histological scoring of vessel wall thickness and lumen size confirmed such thickening in our model (Fig. 7). Another factor contributing to an increase in PVR could be a decrease in vascular density. Faulkner et al. (45) found by histological examination that there was a marked reduction in blood vessels in the rat lung after 30 Gy of 60Co to the right lung. Using high-magnification microfocal angiographic images of the lungs (Fig. 5), we observed a reduction in microvessel density after 11 Gy TBI, which was partially mitigated by EUK-207 (Fig. 5).
ACE activity is an indicator of vascular surface area in the lung (3, 32), and this was protected by EUK-207. ACE activity is also an index of the functional status of the endothelium. We observed a radiation-induced decrease in lung ACE activity that was mitigated by EUK-207 therapy (Fig. 6). While the exact mechanism by which EUK-207 prevents vascular loss, including loss of detectable ACE in our model is not known, its ability to attenuate radiation-induced apoptosis in capillary endothelial cell cultures (20) may be relevant. In addition to a direct protective effect on microvascular endothelium, EUK-207 may contribute to a microenvironment that favors both prevention of, and recovery from, radiation injury to vascular and other lung cells through modulating oxidative post-translational modifications and reactive oxygen species-dependent gene expression.
Among studies of the effects of salen Mn complexes on radiation-induced lung injuries in animal models, our study is the first to test mitigation of vascular end points. This mitigation by EUK-207, a SOD-catalase mimetic, supports the hypothesis that radiation-induced oxidative stress up-regulates multiple pathways pertinent to vascular disease (46). Our data are also consistent with previous findings showing that synthetic SOD/catalase mimetics including EUK-207, mitigate radiation injury to microvascular endothelial cells in culture (20). Taken together, these observations indicate the need for further investigation of the effect of EUK-207 treatment on the time-dependent induction of apoptosis in vascular endothelial cells in vivo, as well as the potential vascular actions of EUK-207 in mitigating radiation injury to other well perfused organs, such as the kidney, gastrointestinal and cardiovascular systems. In this regard, it has been shown that EUK-207, but not EUK-189, mitigates radiation injury in the kidney even though both compounds show similar effectiveness in certain other animal models (19, 40, 47). Since EUK-207 achieves and maintains higher plasma levels than does EUK-189 in rats, it has been suggested that it might act at the level of the vasculature in the renal injury model (19). However, this hypothesis remains to be tested.
Radiation-induced pulmonary fibrosis has also been correlated with oxidant stress (6, 11, 18). Our data show that treatment with the antioxidant EUK-207 in the first month after irradiation to the thorax was able to protect against increased collagen synthesis at 210 days (Figs. 8 and 9). Our results provide indirect evidence that radiation-induced oxidant stress plays an important role in the formation of lung fibrosis. They also suggest that late pulmonary fibrosis is developed from damage incurred in the early stages of injury. Improvement with EUK-207 treatment is noted in the context of modest but insignificant changes in morbidity or mortality associated with pneumonitis in the 13 Gy whole-thoracic irradiation model, for example, lack of EUK-207-associated difference in breathing rates and survival. Given the steep dose response of the lung to radiation, as evidenced by trends to increased morbidity and tachypnea in 13 Gy whole-thoracic irradiation relative to 11 Gy TBI, it is possible that the pneumonitis injury with whole-thoracic irradiation was so severe that a more potent intervention would be needed to improve outcomes through this phase. However, survivors of pneumonitis with 13 Gy and EUK-207 benefited from the standpoint of less fibrosis. Any hypothesis regarding EUK-207 protection from early oxidant stress leading to less fibrosis remains to be tested, and should take into account the severity of pneumonitis indices and requirements to survive this injury to exhibit fibrosis.
Several types of antioxidants have been tested against fibrotic radiation injury to rodent lungs (11–14), since it is believed that pulmonary damage is initiated and sustained by oxidative stress (14, 48, 49). However, except for one (14), these studies represent radiation models relevant to cancer therapy (fractionated radiation to a hemithorax) rather than terrorism or nuclear accidents.
A low-molecular-weight manganese porphyrin-based SOD mimetic (MnTE-2-PyP5+) was reported to decrease collagen deposition when started prior to irradiation (11, 13). The same compound also mitigated radiation lung injury when started 2, 6 and 12 h, but not 24 or 72 h, after irradiation and continued for 14 days (14). The most exciting effect, however, was a reduction in lung fibrosis when the drug was given for 14 days starting at 8 weeks after irradiation. The lungs were assayed at 10 weeks and the experiments were conducted in Fischer rats given a single dose of 28 Gy to the right lung only (14). Longer-term infusion in the same model (with osmotic pumps for 10 weeks starting 1 day after irradiation) also decreased structural damage, collagen deposition, macrophage accumulation and hypoxia at 20 weeks (12). Breathing rates in this model (without any drug intervention) were elevated after 4 weeks and did not recover up to 20 weeks, at which time the experiments were terminated. The lung injury that was induced with a single dose of 28 Gy to one lung does not manifest in a distinct pneumonitis phase separate from fibrosis since the increase in breathing rate does not recover (12) as it does in our TBI or whole-thoracic irradiation models (27). However, the lungs in rats given 28 Gy are fibrotic by 20 weeks [unlike whole-thoracic irradiation with 13 Gy (25)], indicating it is a good model to study fibrosis. Finally, morbidity or lethality were not discussed in these studies (12, 14).
CONCLUSION
Our result show that a short course of EUK-207 starting 7 days after radiation and stopping before pneumonitis mitigates both radiation-induced pneumonitis and pulmonary fibrosis in rats. We used a single high dose of TBI that induces pneumonitis as a model that is relevant to radiological terrorism or nuclear accidents. We also demonstrated mitigation of multiple pulmonary vascular injuries by EUK-207. In addition, we showed mitigation of pneumonitis, and also survival. Using a second model of whole-thoracic irradiation, we demonstrated mitigation of fibrosis with EUK-207. These promising data, along with previous data reported in other organ systems, support the further development of EUK-207 as an agent to prevent multi-organ failure after radiation in the clinic, as well as for use after sudden radiological events.
Acknowledgments
The authors thank Qingping Wu for measuring hemodynamic parameters and vascular counts in isolated perfused lungs, and Jayashree Narayanan for helping with histological analyses and preparing the manuscript. This work was funded by NIH/NAIAD RC-1 AI 81294 and agreement U19 AI67734.
References
- 1.Robbins ME, Brunso-Bechtold JK, Peiffer AM, Tsien CI, Bailey JE, Marks LB. Imaging radiation-induced normal tissue injury. Radiat Res. 2012;177(4):449–66. doi: 10.1667/rr2530.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coggle JE, Lambert BE, Moores SR. Radiation effects in the lung. Environ Health Perspect. 1986;70:261–91. doi: 10.1289/ehp.8670261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Molteni A, Moulder JE, Cohen EF, Ward WF, Fish BL, Taylor JM, et al. Control of radiation-induced pneumopathy and lung fibrosis by angiotensin-converting enzyme inhibitors and an angiotensin II type 1 receptor blocker. Int J Radiat Biol. 2000;76(4):523–32. doi: 10.1080/095530000138538. [DOI] [PubMed] [Google Scholar]
- 4.Roth NM, Sontag MR, Kiani MF. Early effects of ionizing radiation on the microvascular networks in normal tissue. Radiat Res. 1999;151(3):270–7. [PubMed] [Google Scholar]
- 5.Medhora M, Gao F, Jacobs ER, Moulder JE. Radiation damage to the lung: Mitigation by angiotensin-converting enzyme (ACE) inhibitors. Respirology. 2012;17(1):66–71. doi: 10.1111/j.1440-1843.2011.02092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robbins ME, Zhao W. Chronic oxidative stress and radiation-induced late normal tissue injury: a review. Int J Radiat Biol. 2004;80(4):251–9. doi: 10.1080/09553000410001692726. [DOI] [PubMed] [Google Scholar]
- 7.Trott KR, Herrmann T, Kasper M. Target cells in radiation pneumopathy. Int J Radiat Oncol Biol Phys. 2004;58(2):463–9. doi: 10.1016/j.ijrobp.2003.09.045. [DOI] [PubMed] [Google Scholar]
- 8.Moulder JE, Cohen EP. Future strategies for mitigation and treatment of chronic radiation-induced normal tissue injury. Semin Radiat Oncol. 2007;17(2):141–8. doi: 10.1016/j.semradonc.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 9.Ghosh SN, Zhang R, Fish BL, Semenenko VA, Li XA, Moulder JE, et al. Renin-angiotensin system suppression mitigates experimental radiation pneumonitis. Int J Radiat Oncol Biol Phys. 2009;75(5):1528–36. doi: 10.1016/j.ijrobp.2009.07.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calveley VL, Jelveh S, Langan A, Mahmood J, Yeung IW, Van Dyk J, et al. Genistein can mitigate the effect of radiation on rat lung tissue. Radiat Res. 2010;173(5):602–11. doi: 10.1667/RR1896.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vujaskovic Z, Batinic-Haberle I, Rabbani ZN, Feng QF, Kang SK, Spasojevic I, et al. A small molecular weight catalytic metalloporphyrin antioxidant with superoxide dismutase (SOD) mimetic properties protects lungs from radiation-induced injury. Free Radic Biol Med. 2002;33(6):857–63. doi: 10.1016/s0891-5849(02)00980-2. [DOI] [PubMed] [Google Scholar]
- 12.Rabbani ZN, Batinic-Haberle I, Anscher MS, Huang J, Day BJ, Alexander E, et al. Long-term administration of a small molecular weight catalytic metalloporphyrin antioxidant, AEOL 10150, protects lungs from radiation-induced injury. Int J Radiat Oncol Biol Phys. 2007;67(2):573–80. doi: 10.1016/j.ijrobp.2006.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rabbani ZN, Salahuddin FK, Yarmolenko P, Batinic-Haberle I, Thrasher BA, Gauter-Fleckenstein B, et al. Low molecular weight catalytic metalloporphyrin antioxidant AEOL 10150 protects lungs from fractionated radiation. Free Radic Res. 2007;41(11):1273–82. doi: 10.1080/10715760701689550. [DOI] [PubMed] [Google Scholar]
- 14.Gauter-Fleckenstein B, Fleckenstein K, Owzar K, Jiang C, Reboucas JS, Batinic-Haberle I, et al. Early and late administration of MnTE-2-PyP5+ in mitigation and treatment of radiation-induced lung damage. Free Radic Biol Med. 2010;48(8):1034–43. doi: 10.1016/j.freeradbiomed.2010.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mahmood J, Jelveh S, Calveley V, Zaidi A, Doctrow SR, Hill RP. Mitigation of radiation-induced lung injury by genistein and EUK-207. Int J Radiat Biol. 2011;87(8):889–901. doi: 10.3109/09553002.2011.583315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCord JM, Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein) J Biol Chem. 1969;244(22):6049–55. [PubMed] [Google Scholar]
- 17.Loew O. A new enzyme of general occurrence in organismis. Science. 1900;11(279):701–2. doi: 10.1126/science.11.279.701. [DOI] [PubMed] [Google Scholar]
- 18.Langan AR, Khan MA, Yeung IW, Van Dyk J, Hill RP. Partial volume rat lung irradiation: the protective/mitigating effects of Eukarion-189, a superoxide dismutase-catalase mimetic. Radiother Oncol. 2006;79(2):231–8. doi: 10.1016/j.radonc.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 19.Rosenthal RA, Fish B, Hill RP, Huffman KD, Lazarova Z, Mahmood J, et al. Salen Mn complexes mitigate radiation injury in normal tissues. Anticancer Agents Med Chem. 2011;11(4):359–72. doi: 10.2174/187152011795677490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vorotnikova E, Rosenthal RA, Tries M, Doctrow SR, Braunhut SJ. Novel synthetic SOD/catalase mimetics can mitigate capillary endothelial cell apoptosis caused by ionizing radiation. Radiat Res. 2010;173(6):748–59. doi: 10.1667/RR1948.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosenthal RA, Huffman KD, Fisette LW, Damphousse CA, Callaway WB, Malfroy B, et al. Orally available Mn porphyrins with superoxide dismutase and catalase activities. J Biol Inorg Chem. 2009;14(6):979–91. doi: 10.1007/s00775-009-0550-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mahmood J, Jelveh S, Calveley V, Zaidi A, Doctrow SR, Hill RP. Mitigation of lung injury after accidental exposure to radiation. Radiat Res. 2011;176(6):770–80. doi: 10.1667/rr2562.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Williams JP, Brown SL, Georges GE, Hauer-Jensen M, Hill RP, Huser AK, et al. Animal models for medical countermeasures to radiation exposure. Radiat Res. 2010;173(4):557–78. doi: 10.1667/RR1880.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moulder JE, Cohen EP, Fish BL. Captopril and losartan for mitigation of renal injury caused by single-dose total-body irradiation. Radiat Res. 2011;175(1):29–36. doi: 10.1667/RR2400.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kma L, Gao F, Fish BL, Moulder JE, Jacobs ER, Medhora M. Angiotensin converting enzyme inhibitors mitigate collagen synthesis induced by a single dose of radiation to the whole thorax. J Radiat Res (Tokyo) 2012;53(1):10–7. doi: 10.1269/jrr.11035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moulder JE, Fish BL. Late toxicity of total body irradiation with bone marrow transplantation in a rat model. Int J Radiat Oncol Biol Phys. 1989;16(6):1501–9. doi: 10.1016/0360-3016(89)90955-3. [DOI] [PubMed] [Google Scholar]
- 27.Zhang R, Ghosh SN, Zhu D, North PE, Fish BL, Morrow NV, et al. Structural and functional alterations in the rat lung following whole thoracic irradiation with moderate doses: injury and recovery. Int J Radiat Biol. 2008;84(6):487–97. doi: 10.1080/09553000802078396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ghosh SN, Wu Q, Mader M, Fish BL, Moulder JE, Jacobs ER, et al. Vascular injury after whole thoracic x-ray irradiation in the rat. Int J Radiat Oncol Biol Phys. 2009;74(1):192–9. doi: 10.1016/j.ijrobp.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Linehan JH, Haworth ST, Nelin LD, Krenz GS, Dawson CA. A simple distensible vessel model for interpreting pulmonary vascular pressure-flow curves. J Appl Physiol. 1992;73(3):987–94. doi: 10.1152/jappl.1992.73.3.987. [DOI] [PubMed] [Google Scholar]
- 30.Verbeke G, Molenberghs G. Linear mixed models for longitudinal data. New York: Springer-Verlag; 2009. [Google Scholar]
- 31.Hothorn T, Bretz F, Westfall P. Simultaneous inference in general parametric models. Biom J. 2008;50(3):346–63. doi: 10.1002/bimj.200810425. [DOI] [PubMed] [Google Scholar]
- 32.Ward WF, Solliday NH, Molteni A, Port CD. Radiation injury in rat lung. II. Angiotensin-converting enzyme activity. Radiat Res. 1983;96(2):294–300. [PubMed] [Google Scholar]
- 33.Hill RP. Radiation effects on the respiratory system. BJR Suppl. 2005;27:75–81. [Google Scholar]
- 34.Davis SD, Yankelevitz DF, Henschke CI. Radiation effects on the lung: clinical features, pathology, and imaging findings. AJR Am J Roentgenol. 1992;159(6):1157–64. doi: 10.2214/ajr.159.6.1442375. [DOI] [PubMed] [Google Scholar]
- 35.Travis EL. The sequence of histological changes in mouse lungs after single doses of x-rays. Int J Radiat Oncol Biol Phys. 1980;6(3):345–7. doi: 10.1016/0360-3016(80)90145-5. [DOI] [PubMed] [Google Scholar]
- 36.Eldh T, Heinzelmann F, Velalakan A, Budach W, Belka C, Jendrossek V. Radiation-induced changes in breathing frequency and lung histology of C57BL/6J mice are time- and dose-dependent. Strahlenther Onkol. 2012 doi: 10.1007/s00066-011-0046-3. [DOI] [PubMed] [Google Scholar]
- 37.Movsas B, Raffin TA, Epstein AH, Link CJ., Jr Pulmonary radiation injury. Chest. 1997;111(4):1061–76. doi: 10.1378/chest.111.4.1061. [DOI] [PubMed] [Google Scholar]
- 38.Srinivasan V, Doctrow S, Singh VK, Whitnall MH. Evaluation of EUK-189, a synthetic superoxide dismutase/catalase mimetic as a radiation countermeasure. Immunopharmacol Immunotoxicol. 2008;30(2):271–90. doi: 10.1080/08923970801925331. [DOI] [PubMed] [Google Scholar]
- 39.Brown SL, Kolozsvary A, Liu J, Jenrow KA, Ryu S, Kim JH. Antioxidant diet supplementation starting 24 hours after exposure reduces radiation lethality. Radiat Res. 2010;173(4):462–8. doi: 10.1667/RR1716.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Clausen A, Doctrow S, Baudry M. Prevention of cognitive deficits and brain oxidative stress with superoxide dismutase/catalase mimetics in aged mice. Neurobiol Aging. 2010;31(3):425–33. doi: 10.1016/j.neurobiolaging.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cohen EP, Molteni A, Hill P, Fish BL, Ward WF, Moulder JE, et al. Captopril preserves function and ultrastructure in experimental radiation nephropathy. Lab Invest. 1996;75(3):349–60. [PubMed] [Google Scholar]
- 42.Molteni A, Moulder JE, Cohen EP, Fish BL, Taylor JM, Veno PA, et al. Prevention of radiation-induced nephropathy and fibrosis in a model of bone marrow transplant by an angiotensin II receptor blocker. Exp Biol Med. 2001;226(11):1016–23. doi: 10.1177/153537020122601108. [DOI] [PubMed] [Google Scholar]
- 43.Molteni A, Wolfe LF, Ward WF, Ts’ao CH, Molteni LB, Veno P, et al. Effect of an angiotensin II receptor blocker and two angiotensin converting enzyme inhibitors on transforming growth factor-beta (TGF-beta) and alpha-actomyosin (alpha SMA), important mediators of radiation-induced pneumopathy and lung fibrosis. Curr Pharm Des. 2007;13(13):1307–16. doi: 10.2174/138161207780618777. [DOI] [PubMed] [Google Scholar]
- 44.Ward WF, Kim YT, Molteni A, Solliday NH. Radiation-induced pulmonary endothelial dysfunction in rats: modification by an inhibitor of angiotensin converting enzyme. Int J Radiat Oncol Biol Phys. 1988;15(1):135–40. doi: 10.1016/0360-3016(88)90357-4. [DOI] [PubMed] [Google Scholar]
- 45.Faulkner CS, 2nd, Connolly KS. The ultrastructure of 60Co radiation pneumonitis in rats. Lab Invest. 1973;28(5):545–53. [PubMed] [Google Scholar]
- 46.Weintraub NL, Jones WK, Manka D. Understanding radiation-induced vascular disease. J Am Coll Cardiol. 2010;55(12):1237–9. doi: 10.1016/j.jacc.2009.11.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu R, Liu IY, Bi X, Thompson RF, Doctrow SR, Malfroy B, et al. Reversal of age-related learning deficits and brain oxidative stress in mice with superoxide dismutase/catalase mimetics. Proc Natl Acad Sci USA. 2003;100(14):8526–31. doi: 10.1073/pnas.1332809100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fleckenstein K, Zgonjanin L, Chen L, Rabbani Z, Jackson IL, Thrasher B, et al. Temporal onset of hypoxia and oxidative stress after pulmonary irradiation. Int J Radiat Oncol Biol Phys. 2007;68(1):196–204. doi: 10.1016/j.ijrobp.2006.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vujaskovic Z, Anscher MS, Feng QF, Rabbani ZN, Amin K, Samulski TS, et al. Radiation-induced hypoxia may perpetuate late normal tissue injury. Int J Radiat Oncol Biol Phys. 2001;50(4):851–5. doi: 10.1016/s0360-3016(01)01593-0. [DOI] [PubMed] [Google Scholar]