Abstract
cis-Acting elements in the viral genome RNA (vRNA) are essential for the translation, replication, and/or encapsidation of RNA viruses. In this study, a novel conserved cis-acting element was identified in the capsid-coding region of mosquito-borne flavivirus. The downstream of 5′ cyclization sequence (5′CS) pseudoknot (DCS-PK) element has a three-stem pseudoknot structure, as demonstrated by structure prediction and biochemical analysis. Using dengue virus as a model, we show that DCS-PK enhances vRNA replication and that its function depends on its secondary structure and specific primary sequence. Mutagenesis revealed that the highly conserved stem 1 and loop 2, which are involved in potential loop-helix interactions, are crucial for DCS-PK function. A predicted loop 1-stem 3 base triple interaction is important for the structural stability and function of DCS-PK. Moreover, the function of DCS-PK depends on its position relative to the 5′CS, and the presence of DCS-PK facilitates the formation of 5′-3′ RNA complexes. Taken together, our results reveal that the cis-acting element DCS-PK enhances vRNA replication by regulating genome cyclization, and DCS-PK might interplay with other cis-acting elements to form a functional vRNA cyclization domain, thus playing critical roles during the flavivirus life cycle and evolution.
INTRODUCTION
The flavivirus genus contains numerous important agents of human infectious diseases, including dengue virus (DENV), West Nile virus (WNV), Japanese encephalitis virus (JEV), yellow fever virus (YFV), and tick-borne encephalitis virus (TBEV). Human infection by flaviviruses can result in symptoms ranging from mild fever to severe encephalitis and hemorrhagic fever. Due to lack of effective vaccines and specific medicines against many flaviviruses, they pose a significant threat to human health around the world.
Flaviviruses are enveloped RNA viruses with single-stranded, positive-sense genomes. Their 5′ capped viral genome RNA (vRNA) is approximately 10 to 11 kb and contains a single open reading frame (ORF) flanked by 5′ and 3′ untranslated regions (UTRs). The ORF encodes a polyprotein with more than 3,000 residues, which is cotranslationally and/or posttranslationally proteolytically processed into three structural proteins (capsid, pre-membrane/membrane, and envelope) and seven nonstructural proteins (NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5) by viral and cellular proteases. NS3 and NS5 perform essential enzyme activities required for vRNA replication. The C-terminal domain of NS3 has helicase/NTPase (1) and RNA triphosphatase activities (2, 3). The N-terminal domain of NS5 encodes guanylyltransferase (4) and methyltransferase activities (5, 6), whereas the C-terminal domain of NS5 has RNA-dependent RNA polymerase (RdRp) activity. These enzyme activities are involved in vRNA synthesis, 5′ capping, and internal adenosine methylation (7).
The 5′UTR, 3′UTR, and capsid-coding sequence are involved in vRNA replication, translation, and perhaps encapsidation, and many cis-acting elements have been found in these regions. cis-Acting elements at the 5′ end include the 5′-end stem-loop (SLA), which binds viral NS5 RdRp and promotes vRNA replication (8–10); the 5′ upstream of AUG region (UAR); the 5′ downstream of AUG region (DAR); and the 5′ cyclization sequence (CS) (11–14). The last three are required for genome cyclization by base-pairing with corresponding complementary sequences in the 3′UTR. A hairpin in the capsid-coding sequence (cHP) is required for both start codon recognition and vRNA replication (15, 16). One of the critical cis-acting elements at the 3′ end is the 3′-terminal stem-loop (3′SL), which is indispensable for vRNA replication (17–19) and is also the binding site for several cellular factors (20–23). Two conserved elements, DB1 and DB2, which have dumbbell-shaped structures, are also located within the 3′UTR (24). It should be noted that the 5′UAR can fold into a stem-loop (SLB) (8), and partial sequences of 3′UAR and 3′DAR can form a small hairpin (sHP) in the linear conformation of vRNA (25); thus, the balance between the linear and circular vRNA conformations is critical for flavivirus vRNA replication.
Flavivirus cis-acting elements can be classified into elements that are essential for vRNA replication, such as promoters, and elements that are dispensable for vRNA replication but can regulate this process, for example, enhancers. Most known cis-acting elements of flaviviruses are indispensable for vRNA replication. Enhancer-like elements (DB1 and DB2) had only been found in the 3′UTR (24, 26) until recently, when a cis-acting enhancer element, SL6, was discovered in the capsid-coding region of tick-borne flavivirus (27). The results from different groups (28–30) also indicate that the region downstream of the DENV 5′CS regulates vRNA replication, but the mechanism by which this occurs is unknown. In this study, using a combination of bioinformatic, biochemical, and reverse genetic approaches, we identified a novel cis-acting element, the downstream of 5′CS pseudoknot (DCS-PK), in the capsid-coding region, which is conserved in mosquito-borne flaviviruses. DCS-PK enhances vRNA replication by regulating vRNA cyclization, indicating that they might have played a role in the evolution of mosquito-borne flaviviruses.
MATERIALS AND METHODS
Cell culture.
BHK-21 cells were grown in Dulbecco modification of minimal essential medium (DMEM; pH 7.5; Gibco) supplemented with 5% fetal bovine serum (FBS; PAA, Morningside, Queensland, Australia) at 37°C in 5% CO2.
DNA constructs.
The p4-Rluc-Rep replicon was reported previously (31, referred to as DEN-R.luc2A-RP in the original publication). The infectious clone p4 (32) and the host Escherichia coli strain for all of the p4 and replicon constructs, BD1528, were kindly provided by Stephen S. Whitehead. To construct the p4-cHPstop-SP-IRES-Rluc-Rep replicon, the encephalomyocarditis virus (EMCV) internal ribosome entry site (IRES) sequence was fused upstream of the Renilla luciferase (Rluc) gene by overlapping PCR, and the resulting fragment was then fused downstream of the spacer sequence (positions 67 to 707 of the enhanced green fluorescent protein [EGFP] gene ORF). The AscI-XhoI fragment of p4-Rluc-Rep was replaced by an artificial multiple cloning sequence: 5′-GGCGCGCCAAACTTTCCCCTTACGTATTAACATGCATCTTGCGGCCGCGTACAGAGTCTCGAG--3′ (restriction sites for AscI, SnaBI, NotI, and XhoI are underlined from the left to right). The resulting construct was used as a backbone for the assembly of a new replicon. First, a hepatitis D virus ribozyme sequence, followed by a unique AgeI site, was inserted downstream of the 3′ end of DENV cDNA in the backbone construct. The spacer-IRES-Rluc fragment and the AscI-5′UTR-cHPstop-SnaBI fragment, which contains a stop codon in the cHP sequence, were subsequently subcloned into the backbone to obtain p4-cHPstop-SP-IRES-Rluc-Rep.
A cassette cloning strategy was then used to introduce mutations into p4-cHPstop-SP-IRES-Rluc-Rep. The AscI-5′UTR-cHPstop-SnaB I fragment was engineered by overlapping PCR so that its sequence corresponding to position 54 to 90 in the capsid ORF was replaced by the following sequence: 5′-CTCCTCACACACTACGTAAATGGGGAGGAG-3′. The underlined sequence indicates the BseRI restriction site. The resulting construct was named pBseRI-03. Oligonucleotides containing the desired mutations were annealed and cloned into BseRI-treated pBseRI-03 to generate AscI-5′UTR-cHPstop-SnaB I fragments containing different mutations. The corresponding fragments were then subcloned into p4-cHPstop-SP-IRES-Rluc-Rep to obtain replicon mutants.
To introduce mutations into the p4 infectious clone, mutations were introduced into the AscI-NheI-XhoI fragment from p4-Rluc-Rep using a cassette cloning method similar to that described above. The NheI-XhoI fragments were then replaced by the NheI-XhoI fragment from p4. The resulting constructs contained AscI-XhoI fragments from p4 with the desired mutations. The corresponding fragments were subcloned into the p4 plasmid by Eco91I/PauI digestion, and p4 constructs carrying various mutations in DCS-PK were generated. To generate GVD-containing p4 mutants, the SacII-MluI fragment of p4 was substituted with homologous fragments containing GVD mutations. The Eco91I-PauI fragment of the p4-GVD plasmid was substituted with homologous fragments containing mutations. The sequences of the primers and oligonucleotides are available upon request.
RNA structure prediction.
RNA secondary structures were predicted using the mfold online server (33, 34). The pknotsRG (35, 36) and PseudoViewer3 (37, 38) online versions were used to predict and illustrate, respectively, the RNA structures containing pseudoknots.
In vitro transcription.
The RiboMAX large-scale RNA production SP6 kit (Promega) was used for in vitro transcription according to the manufacturer's instructions, with minor changes. For the replicon and infectious RNA assay, linearized DNA was extracted with phenol-chloroform, ethanol precipitated, and dissolved in RNase-free water. Topically, 2 to 3 μg of linearized DNA, 6 μl of 5× SP6 buffer, and 2.5 μl of SP6 enzyme mix were mixed with 6 mM ATP, 6 mM CTP, 6 mM UTP, 2 mM GTP, and 0.83 mM m7GpppG cap analog (Promega) in a 30-μl reaction. The reactions were incubated at 37°C for 3.5 h, and then RQ1 RNase-free DNase I (Promega) was added to the reaction and incubated at 37°C for 20 min to remove the DNA template. For RNA structure probing and RNA binding assays, DNA fragments containing the SP6 promoter and respective viral sequences were amplified by high-fidelity PCR and purified from agarose gels to prepare templates for the transcription of DEN-4 5′ 300-nt RNA, DEN-4 3′UTR RNA, JEV 5′ 320-nt RNA, and WNV 5′ 320-nt RNA. In vitro transcription reactions were topically performed at 37°C for 4.5 h in a 60-μl mixture containing 1 to 2 μg of template DNA, 12 μl of 5× SP6 buffer, 6 μl of SP6 enzyme mix, and 5 mM each of the four ribonucleoside triphosphates, followed by the removal of DNA templates as described above. All in vitro-transcribed RNAs were purified using the RNeasy minikit (Qiagen) according to the RNA cleanup protocol. Purified RNA was quantified by an ND-1000 spectrophotometer (NanoDrop) and stored at −80°C before use.
RNase probing.
Various 5′ end RNAs of flaviviruses were incubated at 85°C for 5 min and slowly cooled to room temperature. The RNAs were then digested by 0.01 U of RNase T1, 0.001 U of RNase V1, or 0.01 ng of RNase A (Ambion). The reactions were performed at 25°C for 15 min in a 10-μl mixture containing 10 mM Tris-HCl (pH 7.0), 100 mM KCl, 10 mM MgCl2, 10 μg of yeast RNA, 1 μg of sample RNA, and the respective RNase. Control reactions without RNase were performed in parallel. Forty microliters of precipitation/inactivation buffer with ethanol was added to stop the reactions. Reactions were incubated at −80°C for 30 min, followed by centrifugation at 15,700 × g and 4°C for 15 min. Pellets were washed with 120 μl of 70% ethanol, air dried, and dissolved in 5 μl of RNase-free water.
NMIA modification.
Various RNAs corresponding to the 5′ end sequences of DENV, JEV, and WNV were modified with N-methylisatoic anhydride (NMIA; Sigma-Aldrich). Experiments were performed as previously described (39). Reactions were incubated at 37°C for 45 min, and then the modified RNAs were purified by using the RNeasy MinElute cleanup kit (Qiagen).
Primer extension.
Purified RNase-treated and NMIA-modified RNA samples were reverse transcribed into cDNA using carboxyfluorescein (FAM)-labeled primers. The primer sequences were as follows: 5′-FAM-GTGGGATGGAAAGGACTC-3′ or 5′-FAM-TTCCCAGAAAAAAGTCC-3′ for DENV, 5′-FAM-TCGGGGCTAATGCTGTAAACT-3′ for JEV, and 5′-FAM-TGCTGTGAACCTGAAGAAC-3′ for WNV. Reverse transcription was performed using Superscript II reverse transcriptase (Invitrogen) in a 20-μl reaction according to the manufacturer's instructions. The FAM-labeled cDNA samples were analyzed by capillary electrophoresis using a 3730xl DNA analyzer (Applied Biosystems). Dideoxy sequencing reactions were performed in parallel. The detection of NMIA reactivity by primer extension is generally known as the SHAPE (selective 2′-hydroxyl acylation analyzed by primer extension) assay (39).
Replicon assay.
A total of 2 × 104 BHK-21 cells were seeded into each well of 48-well plates 1 day prior to transfection and incubated at 37°C in 5% CO2. Transfection was performed with Lipofectamine 2000 reagent (Invitrogen) in triplicate according to the manufacturer's instructions. A total of 250 ng of RNA was transfected into each well, except for p4-Rluc-Rep, of which 500 ng of RNA was transfected. Cell lysates were collected at 6, 24, 48, 72, and/or 96 h after transfection, and Renilla luciferase activity was measured using the Renilla luciferase assay system (Promega) in a GloMax-96 microplate luminometer (Promega).
Electroporation of full-length vRNA.
Portions (5 μg) of each RNA transcript was electroporated into 400 μl of BHK-21 cells at 107 cells/ml in 0.4-cm cuvettes (Bio-Rad) using a Gene Pulser Xcell electroporation system (Bio-Rad) at 270 V and 975 μF. The cells were resuspended in 14 ml of DMEM containing 5% FBS. A total of 0.5 ml of the cell suspension was seeded into each well of 24-well plates, and 0.3 ml of the cell suspension was seeded into each well of 8-well Lab-Tek II chamber slides (Thermo Scientific). Plates and slides were incubated at 37°C in 5% CO2. The medium in the 24-well plates was changed 4 h postelectroporation.
qRT-PCR.
At 4, 24, 48, 72, and 96 h postelectroporation, electroporated BHK-21 cells were washed thoroughly with phosphate-buffered saline (PBS), and then total RNA was isolated using the RNeasy minikit (Qiagen) in triplicate except for the experiment accessing the stability of WT, XT, S1X, S3X, 75.78, and 91.93-GVD RNAs, in which RNA was isolated in duplicate. Quantitative reverse transcription-PCR (qRT-PCR) was performed on a LightCycler 2.0 (Roche) system with the One Step PrimeScript RT-PCR kit (TaKaRa). Probe (5′-FAM-CAGGAGGATTGTGACCATAGAGGCCCA-TAMRA-3′) and primers (forward, 5′-CCCCGGAACAACAGTCACA-3′; reverse, 5′-TGCAGTGGTGGTCCTCAAAG-3′) targeting the NS1 gene were used to detect DENV RNA. Purified full-length RNA transcript was 10-fold serial diluted and used as standards for calculation of RNA copy number.
Immunofluorescence assay (IFA).
Electroporated BHK-21 cells grown in chamber slides were washed with PBS and fixed with cold acetone at 24, 48, 72, and 96 h after electroporation. The fixed cells were incubated with anti-DENV envelope monoclonal antibody (2D73; 20 μg/ml, 40 μl per well) at 37°C for 1 h, followed by three 10-min PBS washes. Goat anti-mouse IgG (1:200 dilution; Alexa Fluor 488-conjugated [Zsbio]) was added, and the slides were incubated at 37°C for 1 h, followed by three 10-min washes with PBS. The cells were then stained with 40 μl of 0.5 μg/ml 4′,6-diamidino-2-phenylindole (DAPI) for 5 min and washed with PBS. The fluorescence images were captured with an Olympus BX51 microscope.
Plaque assay.
Culture supernatants of electroporated BHK-21 cells were collected at 48, 72, and 96 h postelectroporation. The virus titers of the supernatants were determined by plaque assay as described before (40).
RNA binding assay.
A total of 0.3 μg of DEN-4 3′UTR RNA and various amounts (0.24, 0.6, 1.2, or 2.4 μg) of different 5′ 300-nucleotide (nt) RNAs were mixed and diluted to 14-μl reaction mixes, which also contained 5 mM Tris-HCl (pH 8.0) and 0.5 mM EDTA, the 5′:3′ RNA molar ratio in the samples is 1:1, 2.6:1, 5:1, and 10:1, respectively. The samples were then heated at 95°C for 2 min and immediately placed in an ice bath. Next, 6 μl of 3.3× RNA folding buffer (333 mM HEPES [pH 8.0], 20 mM MgCl2, 333 mM NaCl) was added to each sample and mixed well. The RNA samples were incubated at 37°C for 30 min to allow folding into complexes, and then 2.5 μl of gel loading solution (Ambion) was added to each sample. The samples were loaded into 5% native polyacrylamide gels, which had been pre-run in 0.5× Tris-borate-EDTA buffer at 4°C for 30 min, and electrophoresis was conducted at 60 V at 4°C for 4 h. The gels were then stained with SYBR Gold nucleic acid gel stain (Invitrogen) for 20 min and directly visualized with a UV transilluminator. The data were analyzed using the ImageJ package.
RESULTS
Conserved three-stem pseudoknot is located downstream of the 5′CS in mosquito-borne flavivirus.
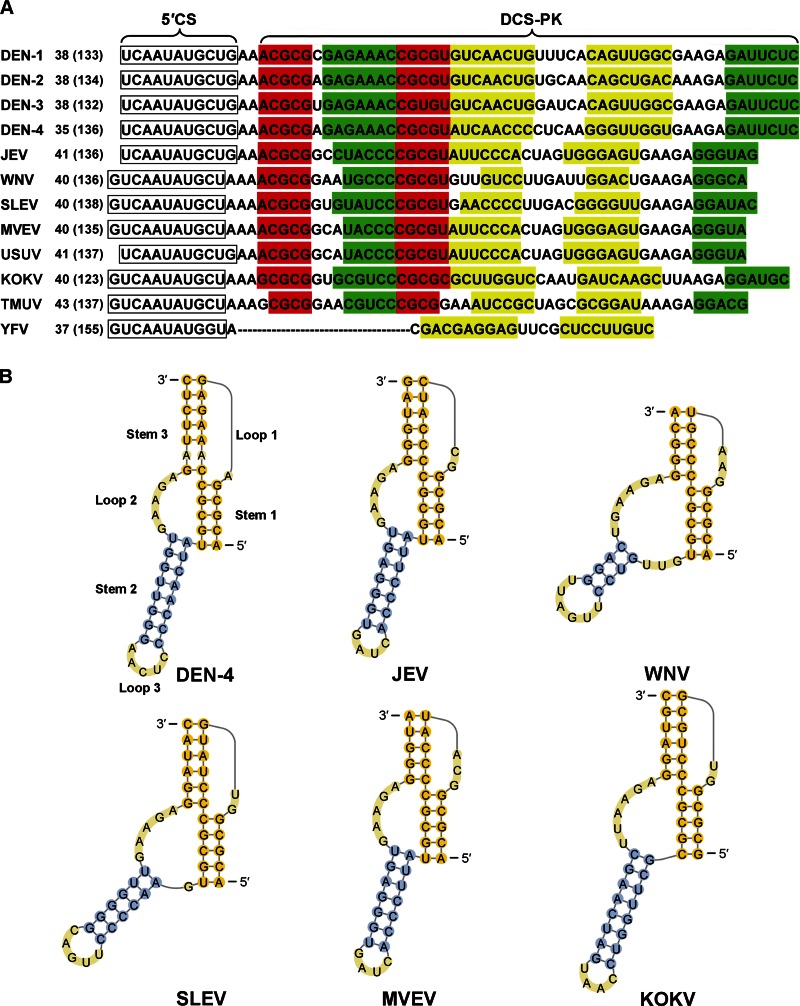
To identify potential cis-acting element(s) downstream of the 5′CS in mosquito-borne flaviviruses, consensus sequences of the capsid-coding region of each flavivirus species were generated and subjected to the mfold web server (33, 34) to obtain structure models. The base-pairing patterns of the two most stable structure models implied that they were possibly coexistence by formation of a pseudoknot (data not shown). Sequence comparison also indicated that the downstream of 5′CS region's base-pairing potential of folding into a pseudoknot is conserved in all mosquito-borne flaviviruses except YFV (Fig. 1A). To address the possibility that the cis-acting element downstream of the 5′CS has a pseudoknot structure, the sequences of downstream of 5′CS regions of different flaviviruses were subjected to pknotsRG (35, 36). As we hypothesized, similar pseudoknot structures were predicted (Fig. 1B), which were designated DCS-PK. The DCS-PK has three stem regions (Fig. 1B), and stem 1 and loop 2 are highly conserved among mosquito-borne flaviviruses (Fig. 1A), whereas the stem 2-loop 3 hairpin, which links the 3′ of stem 1 and 5′ of loop 2, has variable sequences between different virus species. The sequences of loop 1 and stem 3 are also not conserved. These bioinformatic analyses indicate that a conserved RNA pseudoknot may be formed in the capsid-coding region of mosquito-borne flavivirus.
Fig 1.
Structure prediction of DCS-PK among different mosquito-borne flaviviruses. (A) Nucleotide sequences of the downstream of 5′CS region of mosquito-borne flaviviruses. The 5′CSs are boxed, and predicted stem regions of DCS-PK are shown in different colors: red for stem 1, yellow for stem 2, and green for stem 3. The numbers at the beginning of the sequences represent the position of the first shown nucleotide in the capsid ORF, and its position in the viral genome (the latter are shown in parentheses). Abbreviations: DEN-1 to DEN-4, DENV serotypes 1 to 4; SLEV, Saint Louis encephalitis virus; MVEV, Murray Valley encephalitis virus; USUV, Usutu virus; KOKV, Kokobera virus; TMUV, Tembusu virus. The GenBank accession numbers of representative sequences are as follows: DEN-1, EU848545; DEN-2, AY702035; DEN-3, EU081188; DEN-4, AF326573; JEV, M55506; WNV, AY490240; SLEV, EU566860; MVEV, AF161266; USUV, EF206350; KOKV, AY632541; TMUV, JQ289550; YFV, AY640589. (B) DCS-PK structures predicted by pknotRG and drawn with PseudoViewer3. Note that constraints were applied to the stem 1-stem 3 interfaces of several viruses based on sequence comparison shown in panel A and structure probing results shown in Fig. 2.
Structure probing of the DCS-PK structure.
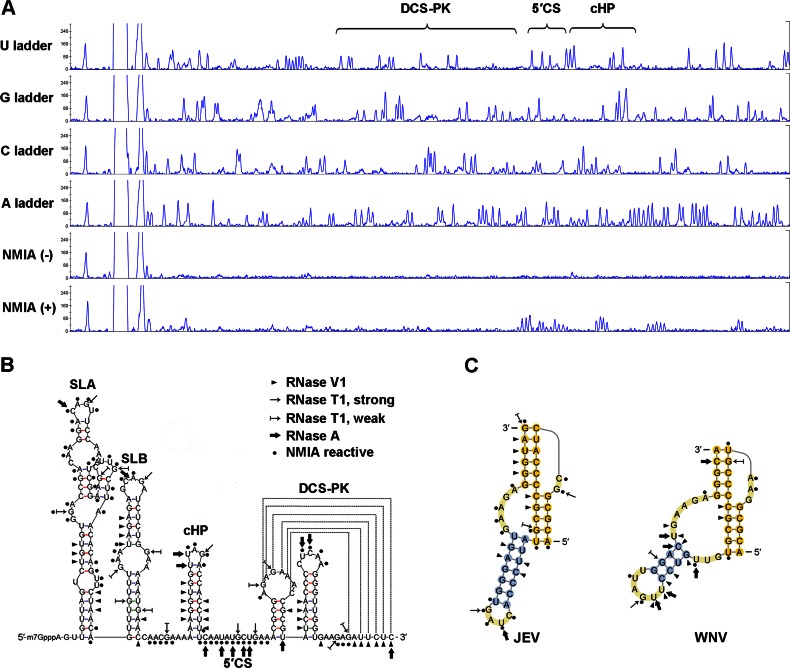
To confirm the physical existence of the predicted DCS-PK structure, RNase V1/T1/A and NMIA were used to probe the 5′ end structure of DENV serotype 4 (DEN-4), WNV, and JEV (Fig. 2 and data not shown). RNase V1, which cleaves double-stranded or base-stacking regions, detected all three DCS-PK stems from DEN-4 and JEV, and stems 1 and 2 from WNV were also RNase V1 sensitive. Single-strand-specific RNase T1/A-sensitive nucleotides were mainly located in loops and junctions. NMIA selectively adds 2′-O adducts to structural flexible nucleotides, and its reactive sites in DCS-PK were mainly located in loop regions and loop-stem interfaces, whereas most nucleotides in the stems showed no SHAPE activity. In summary, the structure probing results support the predicted three-stem pseudoknot structure of DCS-PK.
Fig 2.
Biochemical structure analysis of DCS-PK elements from different flaviviruses. (A) A representative SHAPE experiment for the analysis of the structure of DEN-4 5′ end RNA. Nucleotide ladders were generated by dideoxy-sequencing. The regions corresponding to DCS-PK and several 5′ end RNA elements are indicated. (B) Summary of the RNase probing and NMIA modification results of the DEN-4 5′ RNA containing the DCS-PK element. The major RNA elements of the 5′ end are indicated. The symbols used are explained in the right panel. (C) RNase probing and NMIA modification results of the DCS-PK elements from JEV and WNV. The symbols used are the same in panel B.
DCS-PK is required for efficient vRNA replication and virus propagation.
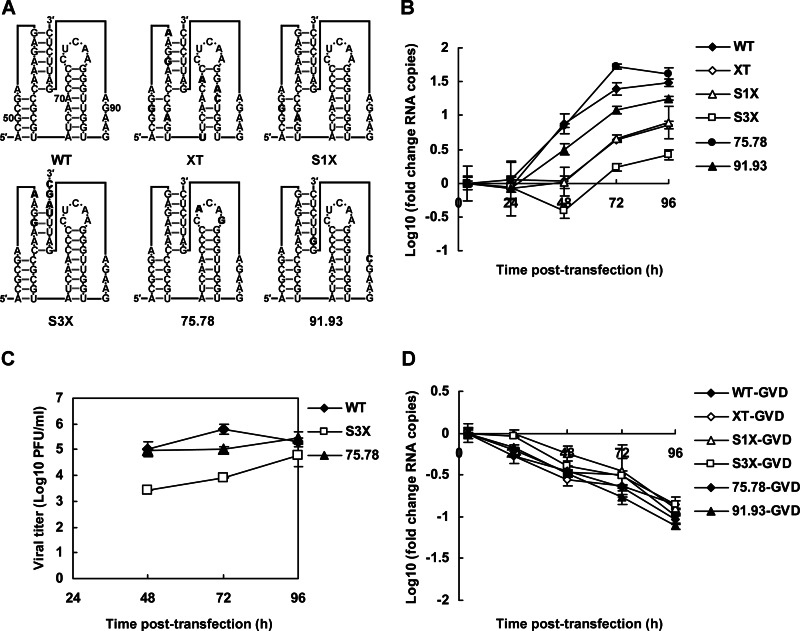
Reverse genetic approaches were used to investigate the role of DCS-PK in virus life cycle. Silent mutations targeting DCS-PK (Fig. 3A) were introduced into the infectious clone p4 of DEN-4 (32). The XT mutant was designed to disrupt the overall secondary structure of DCS-PK. Stem 1 was disrupted in S1X, and S3X included a disrupted stem 3. Mutations were also introduced into the nonconserved loop 3 to generate mutant 75.78, which retained the original secondary structure of DCS-PK. The codon usage frequencies in the above mutants were left unchanged to avoid potential effects on viral translation efficiency. Mutant 91.93, which targeted the bulged A93 of stem 3 and A91 of loop 2 but did not affect DCS-PK secondary interactions, corresponded to a previously reported DEN-2 mutant (28) with reduced vRNA replication. Detection of viral antigens in vRNA-transfected BHK-21 cells by IFA demonstrated that all of the above-mentioned mutant RNAs were infectious. There were fewer DENV-positive cells in XT, 91.93, S1X, and S3X mutant RNA-transfected cells than in wild-type (WT) or 75.78 mutant RNA-transfected cells, demonstrating that the disruption of RNA structure in DCS-PK impairs viral replication (data not shown). To directly assess the effect of DCS-PK on viral genome RNA replication, vRNA levels in transfected cells were determined by qRT-PCR. The vRNA levels in the XT, S1X, and S3X mutant RNA-transfected cells were approximately 1 order of magnitude lower than those of WT and 75.78 RNA-transfected cells at 48 to 72 h after transfection, whereas the latter two exhibited similar vRNA levels at these time points (Fig. 3B). The vRNA level of 91.93 also showed a moderate reduction of ca. 50% at 48 to 72 h, which is in agreement with previous findings (28). The viral loads in the supernatants of the WT-, 75.78-, and S3X-transfected BHK-21 cells were compared. At 48 to 72 h after transfection, the virus titers of S3X were significantly lower than those of WT or mutant 75.78 (Fig. 3C). To exclude the possible impact of mutations on RNA stability, RNAs containing mutations in the conserved GDD motif of NS5 were electroporated into BHK-21 cells. Monitoring of intracellular vRNA levels indicated that the stability of mutant RNAs was similar to that of WT RNA (Fig. 3D). Taken together, the results described above provide strong evidence that the presence of DCS-PK structure enhances vRNA replication and thus facilitates virus propagation. These results also suggest that the function of DCS-PK depends on its secondary structure, as well as some conserved primary sequences.
Fig 3.
Characterization of DEN-4 mutants containing silent mutations in DCS-PK. (A) Demonstration of mutants containing silent mutations in the DCS-PK element. Mutations are indicated in the predicted pseudoknot structure of DCS-PK in boldface. The positions of several nucleotides of DCS-PK in the capsid ORF are indicated in the WT structure. Note that the secondary structures of DCS-PK mutants are only shown to provide a clear illustration and do not represent the actual folding of these mutants. (B) Replication of vRNA mutants in transfected BHK-21 cells. vRNA levels are expressed as fold changes relative to the RNA copies 4 h posttransfection. (C) Growth curves of viruses in the supernatants of vRNA-transfected BHK-21 cells. Virus titers were normalized to transfection efficiency as determined by qRT-PCR at 4 h after transfection. (D) Decay kinetics of various vRNA mutants, which all contained GDD-to-GVD mutations in NS5RdRp. The error bars indicate the standard deviations in all figures.
Secondary structure of DCS-PK is required for its function.
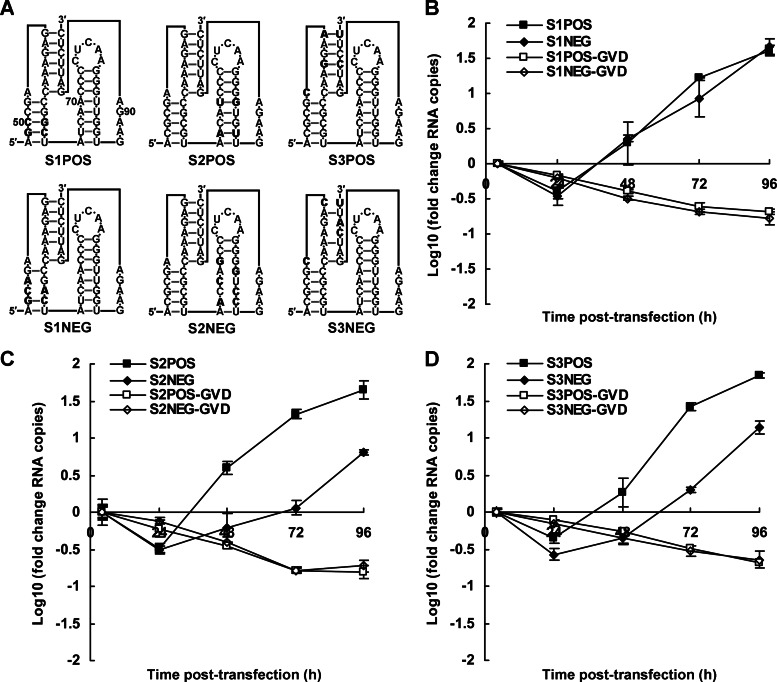
The function of DCS-PK and its dependence on the secondary structure was further investigated with another reverse genetic approach. For each DCS-PK stem region, a pair of mutants was constructed to bypass potential influences of changes in the capsid sequence and/or viral translation on vRNA replication (Fig. 4A). One mutant contained a disrupted stem region (S1NEG, S2NEG, and S3NEG), and the corresponding stem region was restored in the other construct (S1POS, S2POS, and S3POS). The paired mutants encoded the same capsid sequence, which differed from the WT capsid by a few residues. The mutations were carefully chosen to minimize the changes in conserved residues in the capsid sequence, and codon usage frequencies of the paired mutants were remained the same. The vRNA levels of S1POS and S1NEG were similar at all time points after transfection (Fig. 4B). In contrast, at 48 to 96 h after transfection, the vRNA level in the S2POS-transfected cells was significantly higher than that of S2NEG, indicating that S2POS replicated more efficiently (Fig. 4C). Similar results were observed for S3POS and S3NEG (Fig. 4D). The RNA stability was similar between each pair of mutants, as determined by electroporation of NS5 GVD mutant RNAs (Fig. 4B to 4D). IFA detection of viral antigens in vRNA-transfected cells (data not shown) agreed with the results of vRNA replication. Taken together, these results indicate that secondary structure is required for DCS-PK function. For stem 2 and stem 3, maintaining their secondary structures is most likely sufficient for the function of DCS-PK, whereas in the case of stem 1, its highly conserved sequence might be required for DCS-PK function.
Fig 4.
Effects of secondary interactions in DCS-PK on vRNA replication. (A) Mutations targeting various stem regions of DCS-PK. The mutations introduced are indicated in boldface. The nucleotide positions of DCS-PK in the capsid ORF are indicated in the S1POS structure. Note that the secondary structures of DCS-PK mutants are only shown to provide a clear illustration and do not represent the actual folding of these mutants. (B to D) Replication of vRNA mutants containing the disrupted or restored DCS-PK stem 1 (B), stem 2 (C), and stem 3 (D), which were determined by qRT-PCR. Metabolism curves of corresponding GVD-containing RNAs are shown in parallel. The data are expressed as fold changes relative to the RNA level 4 h posttransfection.
The function of stem 2-loop 3 hairpin depends on its secondary structure.
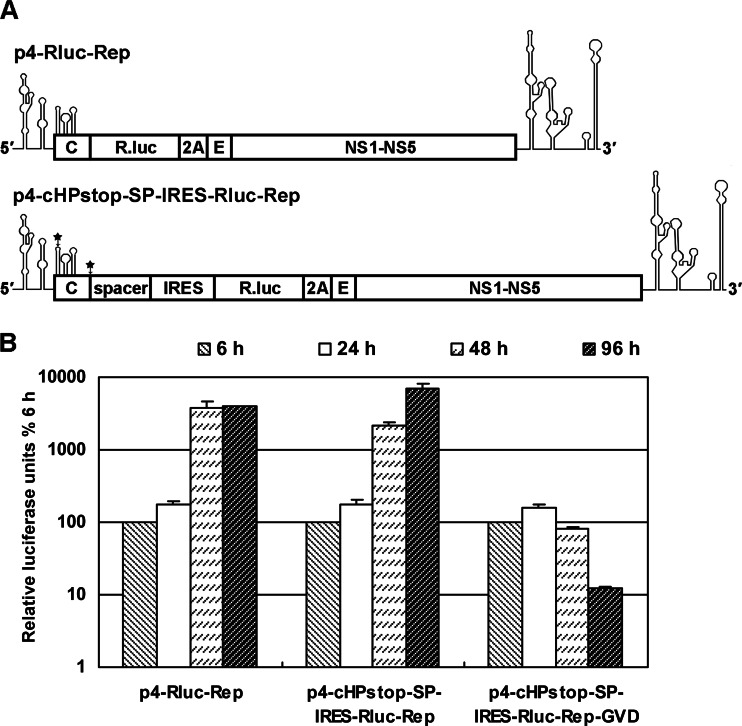
To characterize the structure and function of DCS-PK in detail, a novel DEN-4 replicon was designed (Fig. 5) to bypass the coding role of DCS-PK sequence. In this replicon, the translation of viral protein is under the control of EMCV IRES, and the 0.6-kb spacer sequence separates the 5′UTR-capsid region and IRES to ensure the correct folding of both (Fig. 5A). To abort 5′ cap directed translation of capsid ORF, one stop codon was introduced into the loop sequence of the cHP element (18) without changing its secondary structure. SHAPE analysis showed that the stop codon introduced into cHP did not change the overall secondary structure of DEN-4 5′ end (data not shown). The p4-cHPstop-SP-IRES-RLuc-Rep replicated similarly to p4-Rluc-Rep (Fig. 5B) and was suitable for further mutation experiments.
Fig 5.
Design of replicons uncoupling the coding role of DCS-PK from its function in vRNA replication. (A) Organization of replicons used in the present study. In p4-Rluc-Rep, the Rluc ORF was fused in frame with the capsid ORF, whereas in p4-cHPstop-SP-IRES-Rluc-Rep, the EMCV IRES sequence was located upstream of the Rluc ORF and directed the translation of the Rluc-DENV polypeptide, and a spacer sequence separated the DENV 5′ end and IRES domain to ensure the correct folding of both. The foot-and-mouth disease virus 2A sequence was placed after the Rluc ORF in both replicons to cleave the luciferase peptide with DENV nonstructural proteins. The C terminus of the envelope protein was retained for correct anchoring of DENV polypeptide. The black stars indicate the positions where artificial stop codons were introduced. (B) Replication kinetics of different replicons. Relative luciferase units are expressed as the ratio of light units measured at different time points after transfection to the value at 6 h, which was set to 100%. A replicon containing a mutation in the conserved GDD motif of NS5RdRp (p4-cHPstop-SP-IRES-Rluc-Rep-GVD) is shown as control.
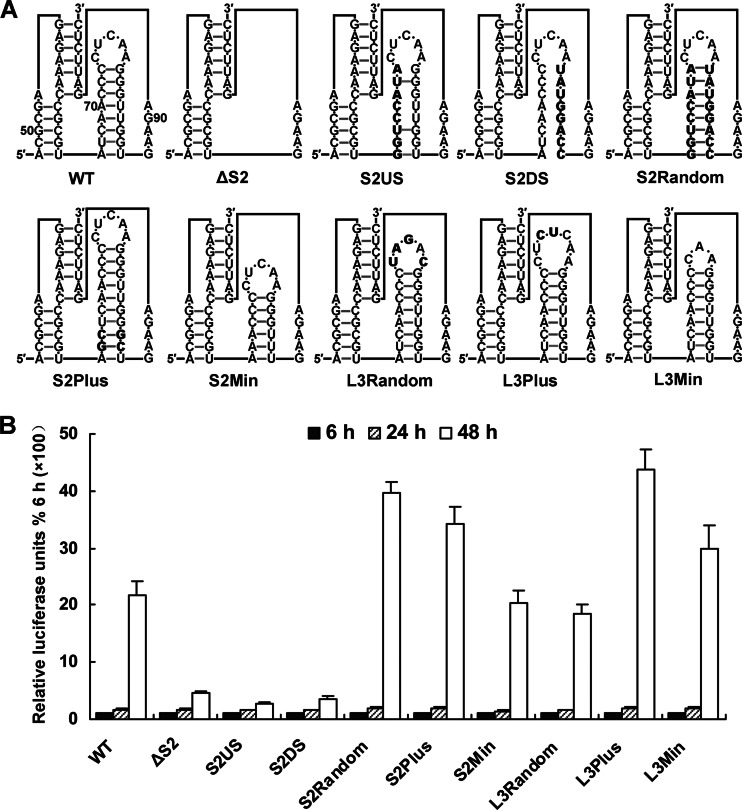
Sequence alignment showed that the primary sequences of the stem 2-loop 3 hairpin are not conserved among flaviviruses, suggesting that the secondary structure is crucial in determining the function of the stem 2-loop 3 hairpin. To verify this hypothesis, a set of mutations (Fig. 6A) were introduced into p4-cHPstop-SP-IRES-RLuc-Rep, and their replication efficiency was monitored by a transient luciferase assay. As shown in Fig. 6B, the role of the stem 2-loop 3 hairpin in vRNA replication was confirmed, as deletion of the entire stem 2-loop 3 hairpin sharply decreased vRNA replication. Randomization of the stem 2 sequence did not impair vRNA replication, whereas randomization of either the upstream or downstream strand sequence, which disrupted stem 2 secondary structure, significantly reduced vRNA replication 48 h posttransfection. Lengthening or shortening stem 2 by 2 bp did not adversely affect vRNA replication. Randomization of 4 of 5 nt in loop 3, lengthening loop 3 by 2 nt, or shortening it by 2 nt also had no harmful effects on vRNA replication. In summary, the above results demonstrate that the function of stem 2-loop 3 hairpin depends on its secondary structure and is independent of primary sequence.
Fig 6.
Mutagenesis of the stem 2-loop 3 hairpin of DCS-PK. (A) Mutations targeting stem 2-loop 3 hairpin are shown. The mutation sites are indicated in bold. The stem 2-loop 3 hairpin was deleted in ΔS2. The S2Random mutant replaced stem 2 with a randomized stem sequence. The S2US and S2DS mutants contained the 5′ and 3′ halves of the randomized stem, respectively. Stem 2 was lengthened in S2Plus, and it was shortened in S2Min. The L3Random, L3Plus, and L3Min included a randomized, lengthened, and shortened loop 3, respectively. This illustration follows the rules outlined in Fig. 3A. (B) Replication of different replicon mutants. Relative luciferase units are expressed as the ratio of light units measured at different time points after transfection to the value at 6 h, which was set to 100%.
Sequence requirements of stem 1-loop 2 for efficient vRNA replication.
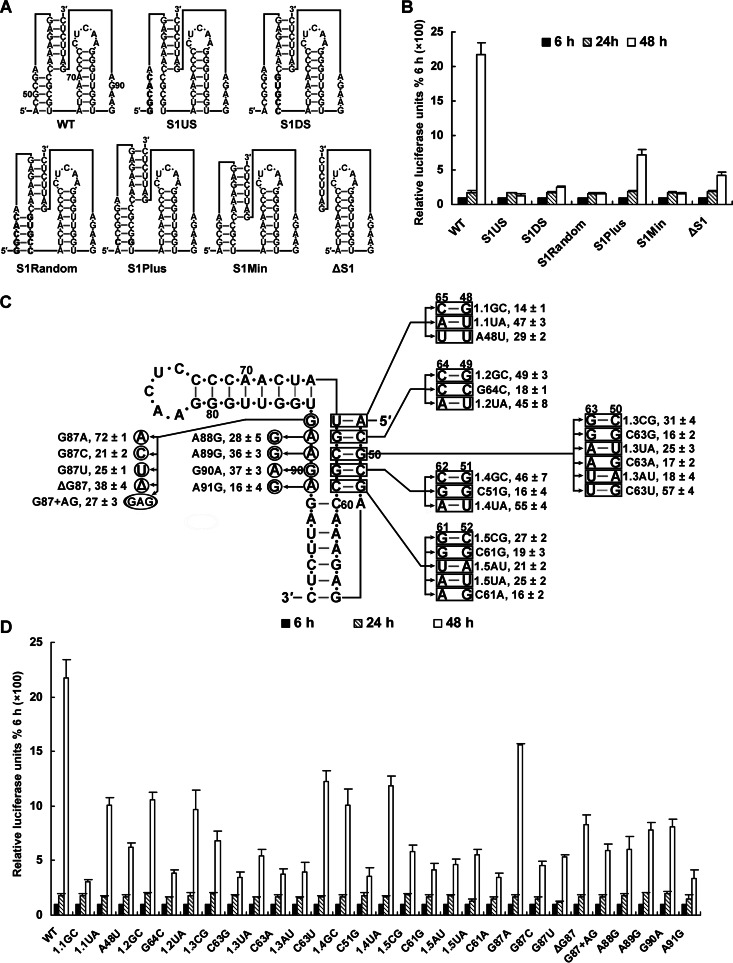
Loop-helix interactions have been found in many H-type pseudoknots (41, 42, 43, 44). Generally, loop 2 crosses the minor groove of stem 1, and loop 1 crosses the major groove of stem 2 (corresponding to stem 3 of DCS-PK), which facilitates the formation of tertiary contacts. Although DCS-PK is not a classical H-type pseudoknot, the conservation of stem 1 and loop 2 sequences (Fig. 1) and the low SHAPE activity of loop 2 (Fig. 2) imply that loop-helix interactions might play roles in its function. To investigate the sequence requirements of stem 1 and loop 2 for vRNA replication, two panels of mutagenesis were performed based on p4-cHPstop-SP-IRES-RLuc-Rep (Fig. 7). First, randomizing, lengthening, or shortening stem 1 and the disruption or deletion of the stem 1 secondary structure significantly reduced vRNA replication (Fig. 7A and B), demonstrating the high sequence dependence on conserved stem 1 for optimal vRNA replication. Next, five base pairs of stem 1 were substituted with different Watson-Crick base pairs (Fig. 7C). Base pair substitutions (1.2GC, 1.3CG, and 1.4GC) significantly enhanced replication compared to single mutations aiming to disrupt the corresponding base pairs (G64C, C63G, and C51G) (Fig. 7D), confirming the importance of secondary interactions in stem 1 for DCS-PK function; however, the replication of all of the base pair-substituted replicons was reduced to some extent compared to the WT replicon. For A48-U65, replacing it with a G-C base pair reduced vRNA replication to ca. 15% relative to the level of the WT replicon 48 h posttransfection (referred to as the percentage of WT below). For G50-C63, the only mutant able to reach 50% of the level of WT was the C63U mutant (G50-C63 Watson-Crick to G50-U63 wobble), whereas mutants containing other base pairs replicated poorly, indicating that G50 and the base-pairing interactions with its partner are absolutely required for DCS-PK function. All of the G52-C61 base pair substitutions reduced vRNA replication to ca. 15 to 30% of the WT. Substitutions of the C49-G64 and C51-G62 pairs had less effect on vRNA replication than substitutions of the other three base pairs. The above results demonstrate that both the base composition and orientation of the base pairs in stem 1 are important for DCS-PK function.
Fig 7.
Mutagenesis of the stem 1-loop 2 region of DCS-PK. (A) Mutations targeting stem 1 are shown in the pseudoknot structure of DCS-PK. The S1Random mutant replaced stem 1 with a randomized stem sequence. The S1US and S1DS mutants contained the 5′ and 3′ halves of the randomized stem, respectively. Stem 1 was lengthened in S1Plus, and it was shortened in S1Min. The ΔS1 mutant contained deletion of the A48 to U65 region in capsid ORF. This illustration follows the rules outlined in Fig. 3A. (B) Replication of replicon mutants shown in panel A. Relative luciferase units are expressed as the percentage of the value 6 h posttransfection, which was set to 100%. (C) Single-base-pair and -nucleotide mutations that were introduced into DCS-PK stem 1-loop 2 region, and their replication efficiency. Nucleotides in rectangles or circles were substituted with the nucleotides indicated by arrows. The “Δ” symbol means deletion. Numbers near the main DCS-PK structure indicate the positions of the corresponding nucleotides in the capsid ORF. The 1.1GC mutant contained the A48-U65 bp substituted with G48-C65, and 1.1UA had a U48-A65 substitution, whereas the A48U mutant, which indicates that A48 was mutated to U, disrupted this base pair. For each base pair, at least three mutants were constructed, and the nomenclature followed the example of the A48-U65 bp. The replication efficiency (mean ± the standard deviation) is listed after the name of the corresponding mutant and is expressed as the ratio of a mutant's relative luciferase units at 48 h after transfection to the value of the WT replicon, which was set to 100. (D) Replication of mutants shown in panel C. Relative luciferase units are expressed as the percentage of the value 6 h posttransfection, which was set to 100%.
Loop 2 of DCS-PK has consensus sequence G/AAAGA. Shortening or lengthening loop 2 significantly reduced vRNA replication (Fig. 7D), indicating the importance of the length of loop 2. All single-base substitutions reduced vRNA replication >2-fold compared to WT, except for G87A, which replicated to ca. 70% of WT. These results agree with sequence alignments, which show that G and A exist at the 5′ first position of DCS-PK loop 2 in different flaviviruses, whereas the other nucleotides are highly conserved (Fig. 1). Thus, the loop 2 nucleotide sequence is critical for optimal vRNA replication. These results indicate that most of the conserved nucleotides in stem 1 and loop 2 are indispensable for optimal vRNA replication. The above results also agree with the common loop-helix interaction feature of classical pseudoknots, suggesting the presence of tertiary interactions between DCS-PK stem 1 and loop 2.
Loop 1-stem 3 interactions are important for the structure and function of DCS-PK.
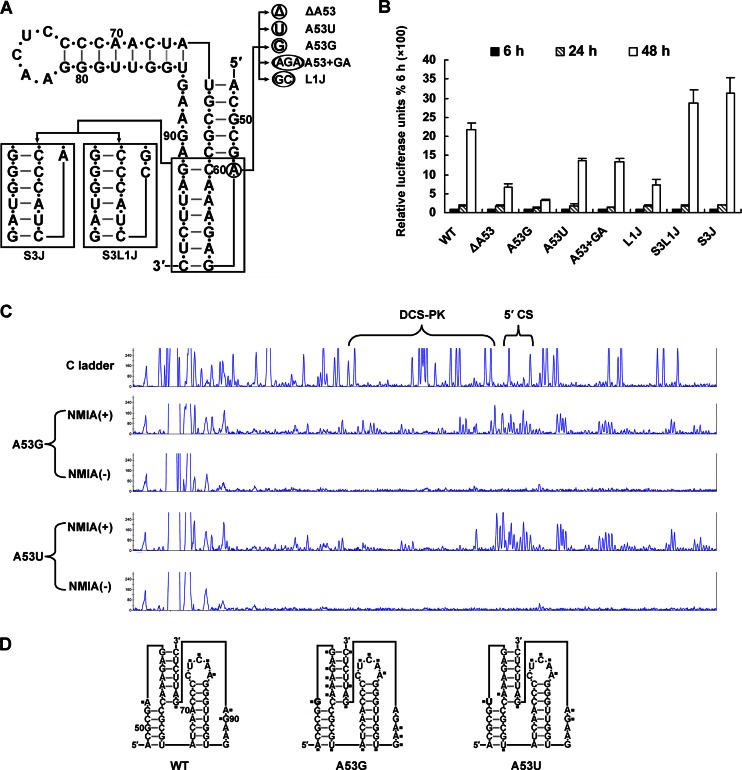
To investigate the role of loop 1 in DCS-PK function, mutagenesis was performed based on the above-mentioned replicon system (Fig. 8A). First, A53G mutation or A53 deletion significantly reduced vRNA replication (Fig. 8B). In contrast, A53U only had little effect on vRNA replication, and the insertion of 2 nt into loop 1 also did not significantly change vRNA replication (Fig. 8B). Thus, the exact length of loop 1 is not required for DCS-PK function, which is different from loop 2. The results of the mutagenesis analysis fit well with the sequence comparison, and loop 1 should contain at least one non-G residue (Fig. 1A).
Fig 8.
Mutagenesis of the loop 1 region of DCS-PK. (A) Mutations introduced into DCS-PK loop 1 region. A53G and A53U replaced A53 with G and U, respectively. The A53+GA mutant increased the length of loop 1 by inserting GA after A53. A53 was deleted in ΔA53. In the L1J mutant, A53 was mutated to GC, which corresponded to the loop 1 sequence of JEV. In S3J, stem 3 was replaced by the homologous stem region from JEV. In S3L1J, the loop 1 and stem 3 sequences were from JEV. (B) The luciferase activity of the replicon mutants shown in panel A 6 to 48 h after transfection. Relative luciferase units are expressed as the percentage of the value 6 h posttransfection, which was set to 100%. (C) Capillary electrophoresis diagrams of a SHAPE experiment with A53U and A53G mutants are shown. ddG dideoxy sequencing is shown as a ladder. The regions corresponding to the DCS-PK and 5′CS are indicated by braces. (D) Comparison of the SHAPE activities of WT, A53G, and A53U DCS-PK. Nucleosides reactive with NMIA are indicated by a black square.
SHAPE analysis was then performed for the loop 1 mutants A53G and A53U (Fig. 8C and D). A53G mutation led to a dramatic increase in the SHAPE activity of stem 3, whereas substituting A53 with U53 did not change the overall SHAPE activity of DCS-PK. Thus, loop 1 is important for the stability of DCS-PK structure. These results suggest that, similar to the loop 1-stem 2 major groove interactions in classical pseudoknots, loop 1 interacts with stem 3 to maintain the structure and function of DCS-PK because the A53G mutation greatly reduced the stability of stem 3 without influencing its secondary interactions. Importantly, substitution of the DCS-PK loop 1 sequence of DEN-4 with the corresponding sequence from JEV (L1J) reduced vRNA replication, whereas replacing loop 1 and stem 3 with homologous regions from JEV (S3L1J) restored vRNA replication. Although the S3J mutant's replication efficiency was similar to WT, these data provide functional evidence that loop 1 interacts with stem 3 (Fig. 8A and B). Taken together, the highly consistent results from SHAPE and mutagenesis demonstrate that the interaction between loop 1 and stem 3 is critical for the structure and function of DCS-PK.
Structural requirement of stem 3 for efficient vRNA replication.
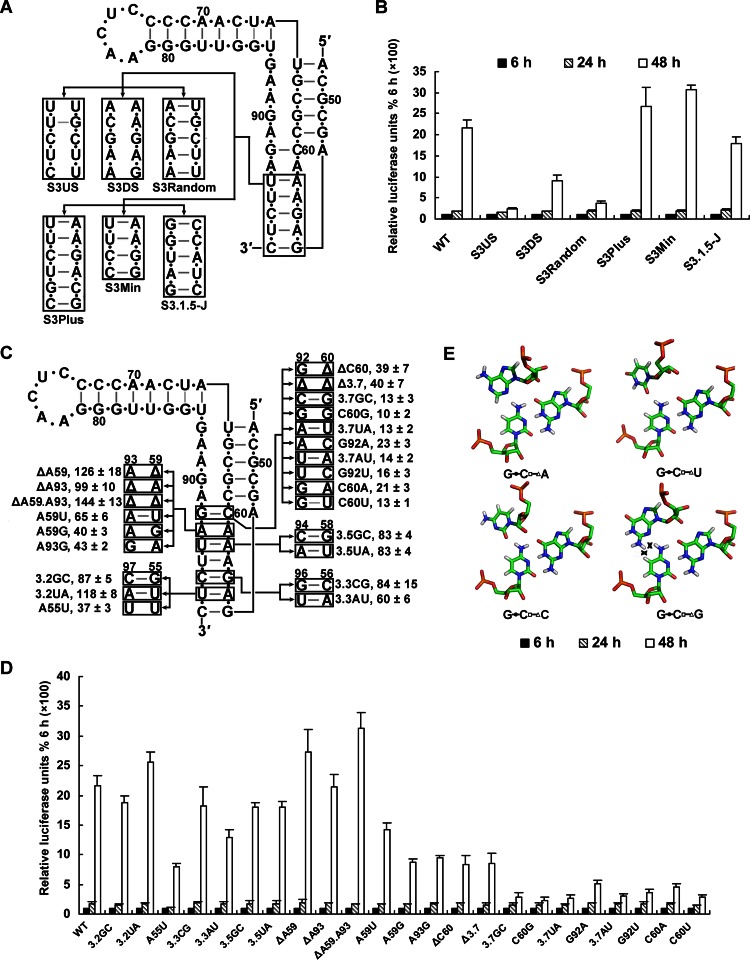
Since the sequence of stem 3 is not conserved among flaviviruses, mutagenesis was performed to investigate the structural requirement of stem 3 for DCS-PK function. As shown in Fig. 9, changing the length of stem 3 by 1 bp or replacing the 5′ helix of stem 3 with the corresponding region from JEV did not affect vRNA replication, but randomization of the 5′ helix of stem 3 or half of it significantly reduced vRNA replication (Fig. 9B). Mutagenesis analysis of bulged A59 and A93, the A55-U97, G56-C96, A58-U94, and C60-G92 bp (Fig. 9C) was performed based on the replicon system. Deletion of A59 or A93 or the combination of both did not affect vRNA replication, and substitution of the A59-A93 bulge with a U-A base pair also had little effect on vRNA replication, suggesting that the bulges in stem 3 are dispensable for DCS-PK function; however, the A59G and A93G mutants showed reduced replication compared to WT (Fig. 9D). Substitution of A55-U97, G56-C96, and A58-U94 with other base pairs did not impair vRNA replication, suggesting that these base pairs are not strictly required for DCS-PK function. In contrast, all of the mutants that deleted, disrupted, or substituted the C60-G92 bp with other base pairs replicated less efficiently than WT (Fig. 9D). These results suggest that the C60-G92 bp is the most likely determinant of stem 3's ability to form tertiary interactions with loop 1 to stabilize the structure of DCS-PK.
Fig 9.
Mutagenesis of the stem 3 region of DCS-PK. (A) Demonstration of mutations targeting the entire stem 3 region of the DCS-PK element. The mutant S3Random had the 5′ helical region of stem 3 replaced by a random double-stranded region, whereas S3US and S3DS served as controls similar to the design of the stem 1 and stem 2 mutants. S3Plus had a lengthened stem 3, and S3Min had a stem 3 that was shortened by 1 bp. The 5′ helical region of stem 3 was replaced by the corresponding region from JEV in the S3.1.5-J mutant. (B) Replication kinetics of the replicon mutants shown in panel A. Relative luciferase units are expressed as the percentage of the value 6 h posttransfection, which was set to 100%. (C) Single-base-pair and -nucleotide mutations that were introduced into DCS-PK stem 3 region. The A59U mutation rendered stem 3 a continuous helix. The ΔA59, ΔA93, and ΔA59.A93 mutants deleted A59, A93, and both A59 and A93, respectively. The A59G and A93G mutants contained the corresponding A substituted with G. The mutant nomenclature and data representation follow the rules described in Fig. 7C. (D) Luciferase activity of the mutant replicon RNAs shown in panel C 6 to 48 h after transfection. Relative luciferase units are expressed as the percentage of the value 6 h after transfection, which was set to 100%. (E) Modeling of the cWW_tHS GCA base triple between loop 1 and stem 3. This illustration follows the Leontis-Westhof nomenclature. The top nucleotides in the shown triples are from loop 1 and the bottom base-pairing nucleotides are from stem 3. Structural files are from the RNA base triples database. Steric clashes are indicated by a cross symbol.
Localization of DCS-PK and its distance relative to the 5′CS.
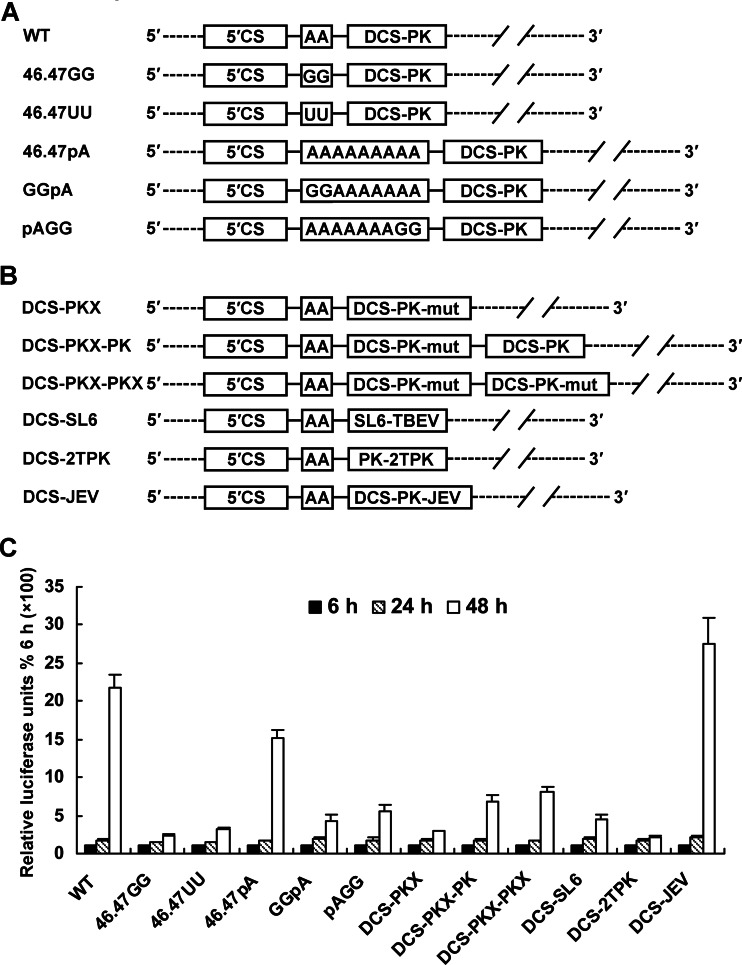
In most flaviviruses, the DCS-PK element is located just 2 to 3 nt downstream of the 5′CS, with two or three adenosine (A) nucleotides as the spacer sequence between them. Mutagenesis using the DEN-4 replicon demonstrated that insertion of more adenosines into the spacer sequence had only a slight effect on vRNA replication, whereas substitutions of adenosines with other nucleotides (G or U) significantly reduced vRNA replication (Fig. 10A and C). Inserting either WT or mutated DCS-PK (DCS-PKX-PK or DCS-PKX-PKX) into the downstream region of the mutated DCS-PK in the original position failed to restore vRNA replication (Fig. 10B and C), confirming that proximity between DCS-PK and 5′CS is required for DCS-PK function. Importantly, the DCS-PK of DEN-4 could be functionally replaced by the JEV DCS-PK but not by the SL6 element of TBEV (27) or the H-type pseudoknot (45) from phage T2 gene 32 mRNA (Fig. 10B and C).
Fig 10.
Interplay of DCS-PK with 5′CS. (A) Illustration of mutations introduced into the spacer region between the 5′CS and DCS-PK. The conserved AA was changed to GG and UU in the 46.47GG and 46.47UU mutants, respectively. Seven additional adenosines were inserted into the 46.47pA spacer sequence. pAGG and GGpA contained AAAAAAAGG and GGAAAAAAA spacer sequences, respectively. (B) The organization of replicon mutants, which contained DCS-PK duplications, and mutants, which contained substitutions of DCS-PK with other RNA elements. DCS-PKX contained a mutated DCS-PK. DCS-PKX-PK contained a mutated DCS-PK followed by a WT DCS-PK, and DCS-PKX-PKX contained two tandem-repeat mutated DCS-PKs. In DCS-SL6, DCS-PK was replaced by SL6 of TBEV. DCS-PK was substituted with pseudoknots from the bacteriophage T2 gene 32 mRNA (PDB 2TPK) in the DCS-2TPK mutant. In DCS-JEV, the DCS-PK of DEN-4 was replaced by the DCS-PK of JEV. (C) Replication of the corresponding replicon mutants. The data are presented as described in Fig. 5 to 9.
DCS-PK modulates vRNA cyclization.
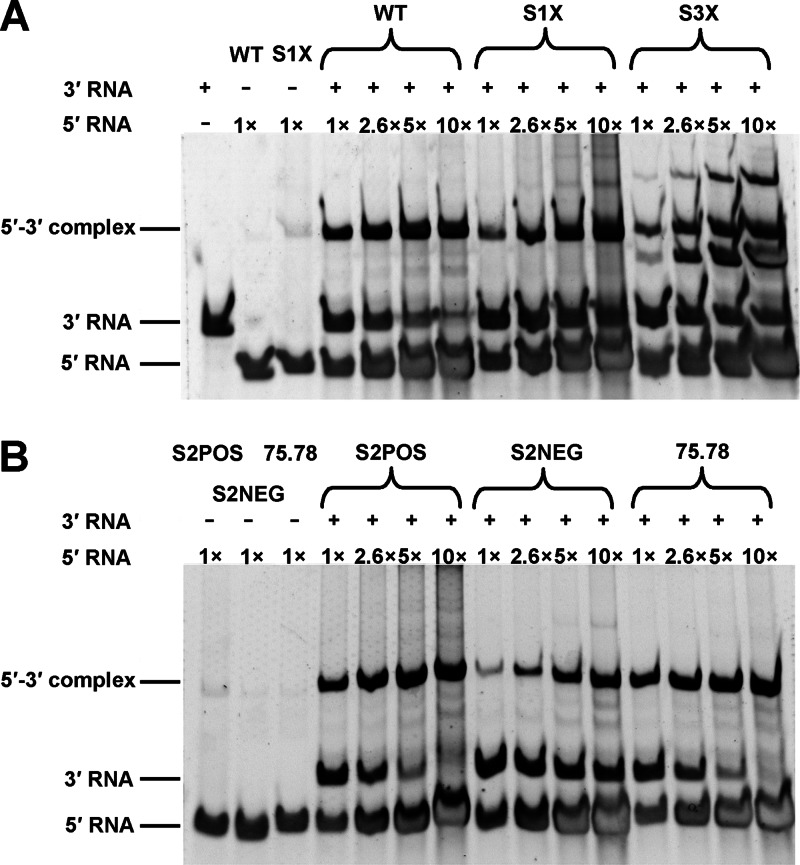
The dependence of DCS-PK function on its proximity to the 5′CS suggests that DCS-PK plays a role in vRNA cyclization. Thus, RNA binding assays were performed to test this hypothesis. The 5′ 300-nt RNAs of WT, S1X, S3X, 75.78, S2POS, and S2NEG were used to bind the 3′UTR RNA (Fig. 11). The percentage of 5′-3′ complexes increased and the percentage of free 3′ RNA decreased as the concentration of 5′ RNA increased. No difference in complex formation was found between 75.78, S2POS, and WT; however, the percentages of 5′-3′ complexes formed by S1X, S3X, and S2NEG 5′ RNA mutants were significantly lower than that formed by WT when the lowest concentration of 5′ RNAs was used (Fig. 11, lane 1×). At a higher 5′ RNA concentration (Fig. 11, lane 10×), the percentage of the remaining free 3′ RNA was apparently higher for S1X, S3X, and S2NEG than for WT 5′ RNA. These results demonstrate that the disrupted DCS-PK structure (Fig. 11, S1X, S3X, and S2NEG) hindered the formation of the 5′-3′ complex and that an intact DCS-PK structure is required for efficient vRNA genome cyclization. The above results agree with the vRNA replication results (Fig. 3 and 4), indicating that DCS-PK enhances vRNA replication by modulating genome cyclization.
Fig 11.
DCS-PK regulates vRNA cyclization. (A) RNA binding assay of 5′ RNA containing WT, S1X, or S3X DCS-PK with 3′ RNA. 5′ RNA and 3′ RNA alone were run in parallel as controls. The 5′ RNA concentration was increased as indicated from 1× to 10×. (B) RNA binding assay of 5′ RNA containing S2POS, S2NEG, or 75.78 DCS-PK with 3′ RNA. The sequences of the DCS-PK mutants are the same as those shown in Fig. 3 and 4.
DISCUSSION
Accumulating evidence from the last few decades has revealed that RNA can fold into complex tertiary structures to achieve various functions (46–48), and many RNA regulation elements with specific tertiary structures have been identified, even in mRNAs, such as frameshifting pseudoknots (49) and riboswitches (50, 51). In the case of flaviviruses, several cis-acting elements have been identified in both the 5′ and 3′UTRs. The discovery of DCS-PK in mosquito-borne flaviviruses further increases our knowledge about the cis-acting RNA elements of flavivirus. The DCS-PK element is located in the capsid-coding region of DENV and Culex-borne flaviviruses, but not YFV. This finding agrees with a phylogenetics study (52) that indicated that the YFV group diverged early and evolved independently, whereas DENV and Culex-borne flaviviruses are closer in evolution. The recently identified tick-borne flavivirus SL6 element (27) shares homology with stem 1 of DCS-PK; however, these two elements are likely to modulate vRNA replication by distinct mechanisms. First, no structural homology was observed between SL6 and DCS-PK; second, the organization of the 5′ ends were quite different between the tick-borne and mosquito-borne flaviviruses. It is likely that the mosquito-borne and tick-borne flaviviruses acquired enhancer elements independently after their separation in evolution and that the enhancers perform their functions by distinct mechanisms, most likely due to the differences in the vector species. However, because the mechanism by which SL6 modulates the replication of tick-borne flavivirus is unclear, further studies are needed to unveil the mechanistic difference between SL6 and DCS-PK.
In the present study, DCS-PK was demonstrated to enhance vRNA replication using biochemical and reverse genetic assays. A recent report (29) suggested that the DENV CCR1 element, which is homologous with the stem 1 hairpin of DCS-PK, serves as a vRNA packaging signal but not a replication enhancer. Our results provide strong evidence that DCS-PK plays a direct role in the vRNA synthesis phase of virus life cycle. Although whether DCS-PK is also involved in vRNA encapsidation was not examined in the present study, it will be interesting to investigate the many functions of DCS-PK by various approaches.
Mutagenesis analysis based on a newly designed DEN-4 replicon indicated the following: (i) the overall secondary structure of the stem 2-loop 3 hairpin is needed for vRNA replication, which is consistent with sequence comparison. (ii) The highly conserved stem 1 and loop 2 sequences are required for DCS-PK function, which is consistent with stem 1-loop 2 interactions in many pseudoknot tertiary structures. The G52-C61 bp, which is located at the 3′ end of stem 1, is the most mutation-sensitive site in stem 1 (Fig. 7), suggesting that more tertiary interactions exist around this site. One possibility is the coaxial stacking with C60-G92 in stem 3. G50 is absolutely required for vRNA replication, suggesting that it directly participates in tertiary interactions. (iii) Tertiary interactions between loop 1 and stem 3 are important for DCS-PK function. Compared to loop 2-stem 1, the loop 1-stem 3 interaction involves fewer nucleotides, which renders the loop 1 sequence more flexible, for example, its tolerance to insertions. The majority of stem 3 bp are sequence independent, although a few mutants that do not change the secondary structure of stem 3 do impair vRNA replication. A possible explanation for this is that most of the nucleotides of stem 3 are not directly involved in tertiary interactions; however, mutations such as A93G and A59G possibly hinder the formation of correct tertiary interactions by steric effects, thus impairing DCS-PK function. The C60-G92 bp in stem 3 is strictly required for vRNA replication, suggesting that a base triple interaction exists between C60-G92 and loop 1. We attempted to model this base triple interaction based on the following facts: (i) loop 1 interacts with the major groove face of stem 3 and forms base triple(s) as in other pseudoknots. (ii) The third base in this base triple interaction can be A, C, or U but not G. Six base triple families whose third nucleotide interacts with the major groove face of a Watson-Crick base pair were identified via the RNA base triples database (http://rna.bgsu.edu/triples/triples.php) (53). Among them, the cWW_tHS GCA (Leontis-Westhof nomenclature [54]) base triple (Fig. 9E) fully satisfies the above requirements and explains the loop 1-stem 3 interactions well. In this model, the A nucleotide in loop 1 can be substituted with C or U, but not G, due to the steric effects between the 2-exocyclic amino group of G and the 4-exocyclic amino group/5-hydrogen of C.
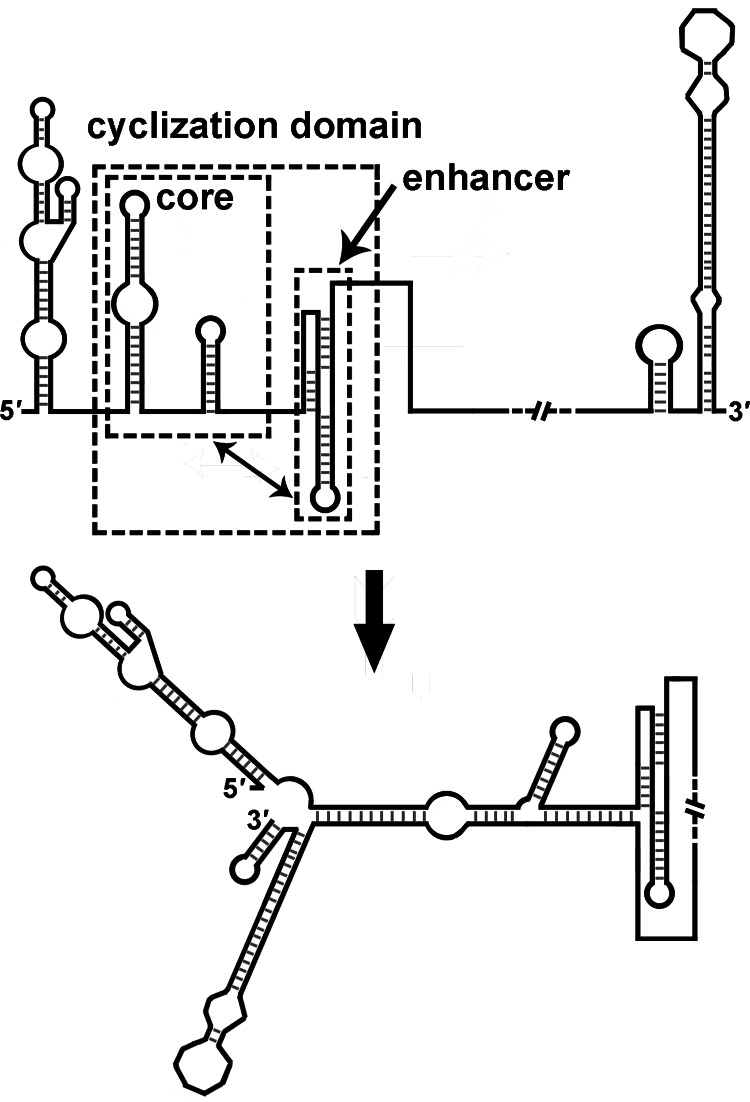
It has been proposed that the initiation of flavivirus negative-strand RNA synthesis involves three phases: (i) the vRNA switches to the circular conformation, which is facilitated by 5′-3′UAR, DAR, and CS hybridization, to bring the 5′ and 3′ ends close and cause the bottom of 3′SL to melt; (ii) the viral RdRp binds to the SLA element at the 5′ end; and (iii) the viral RdRp is translocated to the 3′ end and initiates negative-strand RNA synthesis (8). Friebe and Harris (13) reported that the 5′UAR, 5′DAR, cHP, and 5′CS must act together to achieve their functions, and the sequence downstream of the 5′CS affects vRNA cyclization (30). In this report, we further demonstrated that the DCS-PK element regulates vRNA cyclization, since the disruption of DCS-PK structure hindered the ability of 5′ RNA to bind 3′ RNA, whereas the restoration of DCS-PK structure also recovered the formation of 5′-3′ complex (Fig. 11). We also showed that the function of DCS-PK in vRNA replication depends on interplay with the 5′CS. Based on these results, we propose that the DCS-PK, together with the 5′UAR, 5′DAR, cHP, and 5′CS, form a functional domain that modulates the conformational change of vRNA ends (Fig. 12). The 5′UAR, 5′DAR, cHP, and 5′CS constitute the core of this cyclization domain, whereas DCS-PK helps the core fold into a specific conformation that favors genome cyclization by potential tertiary interactions. More efficient genome cyclization improves the overall efficiency of the vRNA replication process. Further investigation of the structure and function of this cyclization domain will facilitate the in-depth understanding of flavivirus replication and provide new targets for the design of anti-flavivirus drugs.
Fig 12.
Model of flavivirus genome cyclization. The terminal structures are shown in linear (top) and circular (bottom) conformations. The largest region (shown in a dashed-lined box) indicates the proposed cyclization domain. The inner-left boxed region demonstrates the cyclization core, which is composed of the 5′UAR, 5′DAR, cHP, and 5′CS. The inner-right boxed region shows the DCS-PK element. The double-headed arrow indicates the potential tertiary interactions between the enhancer and the cyclization core.
ACKNOWLEDGMENTS
This study was supported by the National Natural Science Foundation of China (31270196, 31000083, and 30972613), National 973 project of China (2012CB518904), and the Open Research Fund Program of the State Key Laboratory of Virology of China (no. 2012005).
We thank Stephen S. Whitehead (National Institute of Allergy and Infectious Diseases, National Institutes of Health) for the p4 infectious clone and Xiao-Yan Che (Zhujiang Hospital, Southern Medical University, China) for 2D73 monoclonal antibody.
Footnotes
Published ahead of print 10 April 2013
REFERENCES
- 1. Li H, Clum S, You S, Ebner KE, Padmanabhan R. 1999. The serine protease and RNA-stimulated nucleoside triphosphatase and RNA helicase functional domains of dengue virus type 2 NS3 converge within a region of 20 amino acids. J. Virol. 73:3108–3116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bartelma G, Padmanabhan R. 2002. Expression, purification, and characterization of the RNA 5′-triphosphatase activity of dengue virus type 2 nonstructural protein 3. Virology 299:122–132 [DOI] [PubMed] [Google Scholar]
- 3. Benarroch D, Selisko B, Locatelli GA, Maga G, Romette JL, Canard B. 2004. The RNA helicase, nucleotide 5′-triphosphatase, and RNA 5′-triphosphatase activities of dengue virus protein NS3 are Mg2+-dependent and require a functional Walker B motif in the helicase catalytic core. Virology 328:208–218 [DOI] [PubMed] [Google Scholar]
- 4. Issur M, Geiss BJ, Bougie I, Picard-Jean F, Despins S, Mayette J, Hobdey SE, Bisaillon M. 2009. The flavivirus NS5 protein is a true RNA guanylyltransferase that catalyzes a two-step reaction to form the RNA cap structure. RNA 15:2340–2350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Egloff MP, Benarroch D, Selisko B, Romette JL, Canard B. 2002. An RNA cap (nucleoside-2′-O)-methyltransferase in the flavivirus RNA polymerase NS5: crystal structure and functional characterization. EMBO J. 21:2757–2768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Geiss BJ, Thompson AA, Andrews AJ, Sons RL, Gari HH, Keenan SM, Peersen OB. 2009. Analysis of flavivirus NS5 methyltransferase cap binding. J. Mol. Biol. 385:1643–1654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dong H, Chang DC, Hua MH, Lim SP, Chionh YH, Hia F, Lee YH, Kukkaro P, Lok SM, Dedon PC, Shi PY. 2012. 2′-O methylation of internal adenosine by flavivirus NS5 methyltransferase. PLoS Pathog. 8:e1002642 doi: 10.1371/journal.ppat.1002642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Filomatori CV, Lodeiro MF, Alvarez DE, Samsa MM, Pietrasanta L, Gamarnik AV. 2006. A 5′ RNA element promotes dengue virus RNA synthesis on a circular genome. Genes Dev. 20:2238–2249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lodeiro MF, Filomatori CV, Gamarnik AV. 2009. Structural and functional studies of the promoter element for dengue virus RNA replication. J. Virol. 83:993–1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dong H, Zhang B, Shi PY. 2008. Terminal structures of West Nile virus genomic RNA and their interactions with viral NS5 protein. Virology 381:123–135 [DOI] [PubMed] [Google Scholar]
- 11. Alvarez DE, Lodeiro MF, Ludueña SJ, Pietrasanta LI, Gamarnik AV. 2005. Long-range RNA-RNA interactions circularize the dengue virus genome. J. Virol. 79:6631–6643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Friebe P, Shi PY, Harris E. 2011. The 5′ and 3′ downstream AUG region elements are required for mosquito-borne flavivirus RNA replication. J. Virol. 85:1900–1905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Friebe P, Harris E. 2010. Interplay of RNA elements in the dengue virus 5′ and 3′ ends required for viral RNA replication. J. Virol. 84:6103–6118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Khromykh AA, Meka H, Guyatt KJ, Westaway EG. 2001. Essential role of cyclization sequences in flavivirus RNA replication. J. Virol. 75:6719–6728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Clyde K, Harris E. 2006. RNA secondary structure in the coding region of dengue virus type 2 directs translation start codon selection and is required for viral replication. J. Virol. 80:2170–2182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Clyde K, Barrera J, Harris E. 2008. The capsid-coding region hairpin element (cHP) is a critical determinant of dengue virus and West Nile virus RNA synthesis. Virology 379:314–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tilgner M, Shi PY. 2004. Structure and function of the 3′ terminal six nucleotides of the West Nile virus genome in viral replication. J. Virol. 78:8159–8171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Elghonemy S, Davis WG, Brinton MA. 2005. The majority of the nucleotides in the top loop of the genomic 3′ terminal stem-loop structure are cis-acting in a West Nile virus infectious clone. Virology 331:238–246 [DOI] [PubMed] [Google Scholar]
- 19. Zeng L, Falgout B, Markoff L. 1998. Identification of specific nucleotide sequences within the conserved 3′-SL in the dengue type 2 virus genome required for replication. J. Virol. 72:7510–7522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yocupicio-Monroy M, Padmanabhan R, Medina F, del Angel RM. 2007. Mosquito La protein binds to the 3′ untranslated region of the positive and negative polarity dengue virus RNAs and relocates to the cytoplasm of infected cells. Virology 357:29–40 [DOI] [PubMed] [Google Scholar]
- 21. Vashist S, Anantpadma M, Sharma H, Vrati S. 2009. La protein binds the predicted loop structures in the 3′ non-coding region of Japanese encephalitis virus genome: role in virus replication. J. Gen. Virol. 90:1343–1352 [DOI] [PubMed] [Google Scholar]
- 22. Davis WG, Blackwell JL, Shi PY, Brinton MA. 2007. Interaction between the cellular protein eEF1A and the 3′-terminal stem-loop of West Nile virus genomic RNA facilitates viral minus-strand RNA synthesis. J. Virol. 81:10172–10187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gomila RC, Martin GW, Gehrke L. 2011. NF90 binds the dengue virus RNA 3′ terminus and is a positive regulator of dengue virus replication. PLoS One 6:e16687 doi: 10.1371/journal.pone.0016687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Olsthoorn RC, Bol JF. 2001. Sequence comparison and secondary structure analysis of the 3′ noncoding region of flavivirus genomes reveals multiple pseudoknots. RNA 7:1370–1377 [PMC free article] [PubMed] [Google Scholar]
- 25. Villordo SM, Alvarez DE, Gamarnik AV. 2010. A balance between circular and linear forms of the dengue virus genome is crucial for viral replication. RNA 16:2325–2335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Alvarez DE, De Lella Ezcurra AL, Fucito S, Gamarnik AV. 2005. Role of RNA structures present at the 3′UTR of dengue virus on translation, RNA synthesis, and viral replication. Virology 339:200–212 [DOI] [PubMed] [Google Scholar]
- 27. Tuplin A, Evans DJ, Buckley A, Jones IM, Gould EA, Gritsun TS. 2011. Replication enhancer elements within the open reading frame of tick-borne encephalitis virus and their evolution within the Flavivirus genus. Nucleic Acids Res. 39:7034–7048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pu SY, Wu RH, Yang CC, Jao TM, Tsai MH, Wang JC, Lin HM, Chao YS, Yueh A. 2011. Successful propagation of flavivirus infectious cDNAs by a novel method to reduce the cryptic bacterial promoter activity of virus genomes. J. Virol. 85:2927–2941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Groat-Carmona AM, Orozco S, Friebe P, Payne A, Kramer L, Harris E. 2012. A novel coding-region RNA element modulates infectious dengue virus particle production in both mammalian and mosquito cells and regulates viral replication in Aedes aegypti mosquitoes. Virology 432:511–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Friebe P, Peña J, Pohl MO, Harris E. 2011. Composition of the sequence downstream of the dengue virus 5′ cyclization sequence (dCS) affects viral RNA replication. Virology 422:346–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yu XD, Qin CF, Jiang T, Chen SP, Han JF, Deng YQ, Liu R, Zhao H, Li XF, Zhu WY, Yu M, Qin ED. 2008. Construction of sub-genomic replicon of dengue virus type 4 with insertion of Renilla luciferase reporter gene. Bull. Acad. Military Med. Sci. 32:101–105 (In Chinese.) [Google Scholar]
- 32. Durbin AP, Karron RA, Sun W, Vaughn DW, Reynolds MJ, Perreault JR, Thumar B, Men R, Lai CJ, Elkins WR, Chanock RM, Murphy BR, Whitehead SS. 2001. Attenuation and immunogenicity in humans of a live dengue virus type-4 vaccine candidate with a 30 nucleotide deletion in its 3′-untranslated region. Am. J. Trop. Med. Hyg. 65:405–413 [DOI] [PubMed] [Google Scholar]
- 33. Zuker M. 2003. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 31:3406–3415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mathews DH, Sabina J, Zuker M, Turner DH. 1999. Expanded sequence dependence of thermodynamic parameters improves prediction of RNA secondary structure. J. Mol. Biol. 288:911–940 [DOI] [PubMed] [Google Scholar]
- 35. Reeder J, Giegerich R. 2004. Design, implementation and evaluation of a practical pseudoknot folding algorithm based on thermodynamics. BMC Bioinform. 5:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Reeder J, Steffen P, Giegerich R. 2007. pknotsRG: RNA pseudoknot folding including near-optimal structures and sliding windows. Nucleic Acids Res. 35:W320–W324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Byun Y, Han K. 2009. PseudoViewer3: generating planar drawings of large-scale RNA structures with pseudoknots. Bioinformatics 25:1435–1437 [DOI] [PubMed] [Google Scholar]
- 38. Byun Y, Han K. 2006. PseudoViewer: web application and web service for visualizing RNA pseudoknots and secondary structures. Nucleic Acids Res. 34:W416–W422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wilkinson KA, Merino EJ, Weeks KM. 2006. Selective 2′-hydroxyl acylation analyzed by primer extension (SHAPE): quantitative RNA structure analysis at single nucleotide resolution. Nat. Protoc. 1:1610–1616 [DOI] [PubMed] [Google Scholar]
- 40. Deng YQ, Dai JX, Ji GH, Jiang T, Wang HJ, Yang HO, Tan WL, Liu R, Yu M, Ge BX, Zhu QY, Qin ED, Guo YJ, Qin CF. 2011. A broadly flavivirus cross-neutralizing monoclonal antibody that recognizes a novel epitope within the fusion loop of E protein. PLoS One 6:e16059 doi: 10.1371/journal.pone.0016059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Batey RT, Rambo RP, Doudna JA. 1999. Tertiary motifs in RNA structure and folding. Angew. Chem. Int. Ed. Engl. 38:2326–2343 [DOI] [PubMed] [Google Scholar]
- 42. Michiels PJ, Versleijen AA, Verlaan PW, Pleij CW, Hilbers CW, Heus HA. 2001. Solution structure of the pseudoknot of SRV-1 RNA, involved in ribosomal frameshifting. J. Mol. Biol. 310:1109–1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kolk MH, van der Graaf M, Wijmenga SS, Pleij CW, Heus HA, Hilbers CW. 1998. NMR structure of a classical pseudoknot: interplay of single- and double-stranded RNA. Science 280:434–438 [DOI] [PubMed] [Google Scholar]
- 44. Su L, Chen L, Egli M, Berger JM, Rich A. 1999. Minor groove RNA triplex in the crystal structure of a ribosomal frameshifting viral pseudoknot. Nat. Struct. Biol. 6:285–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Holland JA, Hansen MR, Du Z, Hoffman DW. 1999. An examination of coaxial stacking of helical stems in a pseudoknot motif: the gene 32 messenger RNA pseudoknot of bacteriophage T2. RNA 5:257–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Holbrook SR. 2008. Structural principles from large RNAs. Annu. Rev. Biophys. 37:445–464 [DOI] [PubMed] [Google Scholar]
- 47. Ferré-D'Amaré AR, Doudna JA. 2000. Crystallization and structure determination of a hepatitis delta virus ribozyme: use of the RNA-binding protein U1A as a crystallization module. J. Mol. Biol. 295:541–556 [DOI] [PubMed] [Google Scholar]
- 48. Toor N, Keating KS, Taylor SD, Pyle AM. 2008. Crystal structure of a self-spliced group II intron. Science 320:77–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Giedroc DP, Cornish PV. 2009. Frameshifting RNA pseudoknots: structure and mechanism. Virus Res. 139:193–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hollands K, Proshkin S, Sklyarova S, Epshtein V, Mironov A, Nudler E, Groisman EA. 2012. Riboswitch control of Rho-dependent transcription termination. Proc. Natl. Acad. Sci. U. S. A. 109:5376–5381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Garst AD, Edwards AL, Batey RT. 2011. Riboswitches: structures and mechanisms. Cold Spring Harbor Perspect. Biol. 3(6):pii:a003533 doi: 10.1101/cshperspect.a003533.rev [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Grard G, Moureau G, Charrel RN, Holmes EC, Gould EA, de Lamballerie X. 2010. Genomics and evolution of Aedes-borne flaviviruses. J. Gen. Virol. 91(Pt 1):87–94 [DOI] [PubMed] [Google Scholar]
- 53. Abu Almakarem AS, Petrov AI, Stombaugh J, Zirbel CL, Leontis NB. 2012. Comprehensive survey and geometric classification of base triples in RNA structures. Nucleic Acids Res. 40:1407–1423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Leontis NB, Westhof E. 2001. Geometric nomenclature and classification of RNA base pairs. RNA 7:499–512 [DOI] [PMC free article] [PubMed] [Google Scholar]