Abstract
Background
Disparity between the sexes in incidence, disease aggressiveness, and prognosis has been observed in various cancers. Although some investigators have reported that male sex is associated with a worse prognosis in thyroid cancer of follicular cell origin, this finding has not been widely studied and is controversial. Thus, using a large population-based database, we sought to better characterize the role of sex in disease aggressiveness and outcome.
Methods
Data of patients with non-medullary thyroid cancer from the Surveillance, Epidemiology, and End Results 17 Registries Database (1988–2007) were used to compare the extent of disease and to analyze the effect of age, sex, race/ethnicity, presence of extrathyroidal extension, lymph node and distant metastases, disease stage, surgical treatment, radiotherapy, tumor size, and histological subtypes on disease-specific survival (DSS) by univariate and multivariate analyses.
Results
We identified 61,523 patients with thyroid cancer (female:male=3.5:1). The median follow-up was 54 months. At diagnosis, 61.2% of men were ≥45 years, compared with 49.7% of women (p<0.01). Men had significantly more aggressive histological subtypes of differentiated thyroid cancer and undifferentiated thyroid cancer, regardless of age group (p<0.01). Moreover, men had significantly more advanced disease at presentation: larger primary tumor size (p<0.01), higher rates of extrathyroidal extension (p<0.01), regional lymph node metastasis (p<0.01), and distant metastasis (p<0.01). DSS stratified by pathology was significantly worse in men (227.5 and 234.3 months in men and women, respectively, p<0.01), despite more total thyroidectomies being performed in men (p<0.01). On multivariate analysis, independent prognostic factors for DSS included age ≥45 years, aggressive histopathology, advanced tumor staging, tumor >4 cm, extrathyroidal extension, lymph node and distant metastasis, less than subtotal thyroidectomy, and post-operative radiation. Sex was not an independent prognostic factor for DSS.
Conclusion
Men with thyroid cancer are more likely to present with more advanced disease, aggressive histological subtypes, and older age. However, sex is not an independent prognostic factor for DSS. Thus, men may benefit from more aggressive screening to detect thyroid cancer at an earlier stage.
Introduction
The disparity in incidence, tumor characteristics, and prognosis has been described in several cancers of sex-nonspecific organs (1–3). The disease heterogeneity is likely from multiple factors, such as the effects of endogenous sex hormones on cancer cells (4) or environmental exposure. The top five cancers with the largest ratio of male-to-female incidence rate are Kaposi sarcoma, lip, larynx, mesothelioma, and urinary bladder. There are only five cancers with higher incidence in women: breast; peritoneum, omentum, and mesentery; thyroid; gallbladder; and anorectum (5). Sex disparity in cancers has often been recognized but infrequently becomes a primary focus of research. A better understanding of factors associated with sex disparity in cancers can lead to improved insight in biological behavior of the tumor, which is essential in developing effective strategies for prevention, detection, and treatment.
Thyroid cancer is the most common endocrine malignancy and is one of the fastest growing cancers diagnosed in the United States (6). The estimated incidence of thyroid cancer is >56,000 cases in 2012, and the incidence is more than three times higher in women (7–9). However, the estimated deaths of women with thyroid cancer was only 1.3 times higher than the death rate in men (780 men and 1000 women in 2012) (9). The incidence rates of differentiated thyroid cancer (DTC) of follicular cell origin rise sharply in women during reproductive age, compared with men, then consistently decline, and equalize by 85 years of age (2). Men tend to have more advanced disease diagnosed at an older age and have lower disease-free survival and higher mortality (2,7,8). However, the available data from small retrospective series or population-based studies did not specifically analyze the role of sex in thyroid cancer.
The objective of this study was, using a large population-based cancer registry, to evaluate the role of sex in disease aggressiveness and treatment outcome of patients with thyroid cancer of follicular cells origin to test the hypothesis that there is a thyroid cancer sex disparity. We analyzed the Surveillance, Epidemiology, and End Results (SEER) cancer registry database to assess the difference between sex by demographics, disease presentation, extent of disease, treatments, histopathology, and outcome.
Materials and Methods
Data acquisition
The SEER program of the National Cancer Institute is a U.S. population–based cancer registry that has been collecting information on patient demographics, incidence, prevalence, tumor characteristics, stage at diagnosis, initial treatment, tumor histopathology, and follow-up vital status since 1973. The data used in this study were derived from 17 registries from various geographic locations throughout the United States, representing 28% of the U.S. population. The SEER data contain unidentifiable patient information and, therefore, this study was exempted from a review and approval of the Office of Human Subject Research at the National Institutes of Health. Cases were identified, and detailed information was captured using SEER*Stat software Release 6.6.2 (April 2010; Cancer Statistics Branch, NCI, Bethesda, MD).
Since radioisotope therapy has been specifically coded in a separate category since 1988, we identified all adult patients (≥20 years of age) who were diagnosed with thyroid cancer, excluding medullary thyroid cancer, from 1988 to 2007. The histological subtypes and their International Classification of Disease codes (ICD-O-3) (10) included in the analysis were papillary thyroid carcinoma (PTC) (8050), papillary adenocarcinoma (8260), follicular thyroid carcinoma (FTC) (8330–8332, 8335), insular carcinoma (8337), follicular variant PTC (FVPTC) (8340), papillary microcarcinoma (8341), PTC with oxyphilic cells (8342), encapsulated PTC (8343), and PTC with columnar cells (8344). The histological differentiation grades analyzed were well, moderately, poorly, and undifferentiated thyroid cancer. We categorized tumor histopathology into four groups based on their prognosis: Group 1 included well and moderately differentiated, conventional PTC, FVPTC, FTC, minimally invasive FTC; group 2 included PTC with oxyphilic cells (Hürthle cells); group 3 included poorly differentiated, columnar, insular, tall cell variant PTC; and group 4 included undifferentiated thyroid cancer. The variables analyzed were age (<45 and ≥45 years of age), sex, and race/ethnicity (white, black, and others) according to the standards for the classification of federal data on race and ethnicity of the United States Office of Management and Budget (11), histology (group 1–4), size of the primary cancer in greatest dimension, the presence of extrathyroidal extension, cervical lymph node metastasis, distance metastasis, types of surgery (less than lobectomy; lobectomy; subtotal, near, or total thyroidectomy), radiotherapy (none, radioisotopes, external radiotherapy [XRT], combined radioisotopes and XRT, and other), and disease-specific survival (DSS). All patients had thyroid cancer as the first and only primary cancer.
Statistical analysis
DSS was calculated in months from the date of diagnosis to death caused by the disease. Survival was censored at the date of last follow-up if no disease-specific death occurred. Cochran–Armitage trend test and Pearson chi-square were used to evaluate the trend and to assess the difference among sex by ordinal and nominal categorical variables, respectively. The Mann–Whitney test was used to compare non-parametric variables. The Kaplan–Meier with log-rank test was used to estimate and compare DSS for both sexes, stratified by tumor stage and histological group. Univariate and multivariate Cox proportional hazard regressions were performed to identify variables associated with disease-specific mortality. A two-tailed p-value <0.05 was considered statistically significant. DSS was the primary outcome measure used in this study.
Statistical analysis was performed using SPSS® v16.0 for Windows (SPSS, Inc., Chicago, IL) and the SAS® software package (SAS Institute, Inc., Cary, NC).
Results
We identified 61,523 patients with histologically confirmed thyroid cancer of follicular cell origin reported to SEER from 1988 to 2007. Patient demographics, tumor characteristics, treatments, and outcome are summarized in Table 1. The median follow-up time in this cohort was 54 months. Men had a slightly shorter follow-up time than women (53 and 54 months, respectively, p=0.01). At the time of diagnosis, 61.2% of men were >45 years of age, compared with 49.7% of women (p<0.01). Men had significantly more aggressive histological subtypes of DTC and undifferentiated thyroid cancer, regardless of age group (p<0.01). Moreover, men had significantly more advanced disease at presentation: larger primary tumor size (p<0.01), higher rates of extrathyroidal extension (p<0.01), regional lymph node metastasis (p<0.01), and distant metastasis (p<0.01). Stratified by histology, there was a strong association between men and larger tumor size (p<0.01) and more advanced stage at presentation (p<0.01) in every histopathological group.
Table 1.
Characteristics of Patients with Thyroid Cancer of Follicular Cell Origin as the First and Only Primary Tumors by Sex, SEER, 1988–2007
| |
Sex |
|
|
|
|---|---|---|---|---|
| Patient characteristics | Male [n (%)]a | Female [n (%)]a | Total [n (%)] | p-Value |
| No. of patients (percent of Total) | 13,642 (22.2) | 47,881 (77.8) | 61,523 | |
| Age ≥45 years | 8345 (61.2)a | 23,820 (49.7)a | 32,165 (52.3) | <0.01 |
| Race/ethnicity | 61,086 | <0.01 | ||
| White | 11,633 (85.8) | 39,004 (82.1) | 50,637 (82.9) | |
| Black | 616 (4.5) | 2838 (6) | 3454 (5.7) | |
| Other | 1305 (9.6) | 5690 (12) | 6995 (11.5) | |
| Histopathologyb | 59,757 | <0.01 | ||
| Group 1 | 11,567 (86.8) | 41,776 (90) | 53,343 (86.7) | |
| Group 2 | 491 (3.7) | 1327 (2.9) | 1818 (3) | |
| Group 3 | 995 (7.5) | 2861 (6.2) | 3856 (6.3) | |
| Group 4 | 269 (2) | 471 (1) | 740 (1.2) | |
| Tumor size (cm) | 56,393 | <0.01 | ||
| ≤1 | 3268 (26.6) | 15,566 (35.3) | 18,834 (33.4) | |
| >1–2 | 2967 (24.2) | 13,192 (29.9) | 16,159 (28.7) | |
| >2–4 | 3745 (30.5) | 11,695 (26.5) | 15,440 (27.4) | |
| >4 | 2300 (18.7) | 3660 (8.3) | 5960 (10.6) | |
| Extrathyroidal extension | 2740 (21) | 7092 (15.2) | 9832 (16.4) | <0.01 |
| Lymph node metastasis | 4058 (45.2) | 8709 (28.9) | 12,767 (32.6) | <0.01 |
| Distant metastasis | 516 (4.9) | 861 (2.3) | 1377 (2.9) | <0.01 |
| Surgery | <0.01 | |||
| Less than lobectomy | 113 (1.1) | 361 (1) | 474 (1) | |
| Lobectomy | 1493 (14.3) | 5809 (15.5) | 7312 (15.2) | |
| Subtotal or total thyroidectomy | 8868 (84.7) | 31,332 (83.5) | 40,200 (83.5) | |
| Radiotherapy | <0.01 | |||
| None | 5790 (43.2) | 23,392 (49.6) | 29,182 (48.2) | |
| Radioisotopes | 6753 (50.4) | 21,768 (46.2) | 28,521 (47.1) | |
| XRT | 422 (3.2) | 909 (1.9) | 1331 (2.2) | |
| Combined XRT and radioisotopes | 166 (1.2) | 256 (0.5) | 422 (0.7) | |
| Other | 265 (2) | 794 (1.7) | 1.059 (1.7) | |
| Median follow-up | 53 months | 54 months | 0.012 | |
| Disease-specific mortality | 688 (5) | 1065 (2.2) | 1753 (2.8) | <0.01 |
Percentage of parameter of each sex.
Histopathology: group 1 included well and moderately differentiated, conventional PTC, FVPTC, FTC, minimally invasive FTC; group 2 included PTC with oxyphilic cells (Hürthle cells); group 3 included poorly differentiated, columnar, insular, tall cell variant PTC; and group 4 included undifferentiated thyroid cancer.
SEER, Surveillance, Epidemiology, and End Results; XRT, external radiotherapy; PTC, papillary thyroid carcinoma; FVPTC, follicular variant papillary carcinoma; FTC, follicular thyroid carcinoma.
To evaluate whether the findings of more advanced disease in men was associated with older age, we divided our cohort into four age groups: 20–34, 35–44, 45–64, and >65 years of age and compared the extent of thyroid cancer and the rates of histological subtypes within the same age group. Men had significantly higher rates of larger tumor size, extrathyroidal extension, and lymph node and distant metastases in all age groups. Men in younger age groups (20–34 and 35–44 years of age) also had more subtotal or total thyroidectomy than women. Radiotherapy (radioisotopes, external radiation, or combination of both) was also more frequently used in men in all age groups (Table 2).
Table 2.
A Comparison of Aggressive Clinicopathological Features and Treatments Between Men and Women with Thyroid Cancer of Follicular Cell Origin As First and Only Primary Tumors By Age Group, SEER 1998–2007
| Age groupa | Patient characteristics | Male [n (%)]b | Female [n (%)]b | p-Value |
|---|---|---|---|---|
| Histopathologyc | ||||
| I | Group 2 | 38 (1.7) | 158 (1.4) | <0.01 |
| Group 3 | 140 (6.4) | 607 (5.4) | ||
| Group 4 | 8 (0.4) | 12 (0.1) | ||
| II | Group 2 | 71 (2.3) | 241 (2.0) | <0.01 |
| Group 3 | 182 (6.0) | 723 (5.9) | ||
| Group 4 | 13 (0.4) | 16 (0.1) | ||
| III | Group 2 | 219 (3.8) | 550 (3.2) | <0.01 |
| Group 3 | 425 (7.4) | 961 (5.7) | ||
| Group 4 | 105 (1.8) | 130 (0.8) | ||
| IV | Group 2 | 163 (6.9) | 378 (6.3) | 0.14 |
| Group 3 | 248 (10.5) | 570 (9.6) | ||
| Group 4 | 143 (6.1) | 313 (5.3) | ||
| Tumor size (cm) | ||||
| I | >2–4 | 707 (35.8) | 3449 (32.6) | <0.01 |
| >4 | 349 (17.7) | 895 (8.5) | ||
| II | >2–4 | 945 (33.6) | 2991 (25.7) | <0.01 |
| >4 | 420 (14.9) | 689 (5.9) | ||
| III | >2–4 | 1796 (27.7) | 3838 (23.3) | <0.01 |
| >4 | 966 (17.9) | 1156 (7.0) | ||
| IV | >2–4 | 597 (28.7) | 1417 (26.3) | <0.01 |
| >4 | 565 (27.1) | 920 (17.0) | ||
| Extrathyroidal extension | ||||
| I | 434 (20.1) | 1530 (13.6) | <0.01 | |
| II | 500 (16.7) | 1558 (12.6) | <0.01 | |
| III | 1137 (20.0) | 2511 (14.5) | <0.01 | |
| IV | 669 (30.2) | 1493 (25.8) | <0.01 | |
| Lymph node metastasis | ||||
| I | 924 (59.7) | 3130 (42.0) | <0.01 | |
| II | 988 (48.1) | 2326 (29.5) | <0.01 | |
| III | 1515 (39.0) | 2322 (20.7) | <0.01 | |
| IV | 631 (42.2) | 931 (26.2) | <0.01 | |
| Distant metastasis | ||||
| I | 44 (2.9) | 122 (1.5) | <0.01 | |
| II | 60 (2.6) | 109 (1.1) | <0.01 | |
| III | 204 (4.3) | 301 (2.1) | <0.01 | |
| IV | 208 (11.4) | 329 (7.0) | <0.01 | |
| Subtotal or total thyroidectomy | ||||
| I | 1374 (89.0) | 7145 (86.2) | 0.01 | |
| II | 2035 (87.7) | 8247 (84.5) | <0.01 | |
| III | 3992 (83.6) | 12162 (82.7) | 0.18 | |
| IV | 1467 (79.9) | 3778 (79.7) | 0.98 | |
| Radiotherapy | ||||
| I | Radioisotopes | 1215 (55.8) | 5760 (51.0) | <0.01 |
| XRT | 33 (1.5) | 146 (1.3) | ||
| Combined XRT and radioisotopes | 23 (1.1) | 48 (0.4) | ||
| II | Radioisotopes | 1641 (54.2) | 5932 (48.0) | <0.01 |
| XRT | 47 (1.6) | 157 (1.3) | ||
| Combined XRT and radioisotopes | 20 (0.7) | 56 (0.5) | ||
| III | Radioisotopes | 2914 (50.1) | 7779 (44.7) | <0.01 |
| XRT | 160 (2.7) | 276 (1.6) | ||
| Combined XRT and radioisotopes | 65 (1.1) | 94 (0.5) | ||
| IV | Radioisotopes | 983 (41.4) | 3397 (38.0) | <0.01 |
| XRT | 182 (7.7) | 330 (5.5) | ||
| Combined XRT and radioisotopes | 58 (2.4) | 58 (1.0) |
Age group of patients: I, 20–34 years of age; II, 35–44 years of age; III, 45–64 years of age;IV, >65 years of age.
Percentage of parameter of each sex.
Histopathology: group 1 included well- and moderately differentiated, conventional PTC, FVPTC, FTC, minimally invasive FTC; group 2 included PTC with oxyphilic cells (Hürthle cells); group 3 included poorly differentiated, columnar, insular, tall cell variant PTC; and group 4 included undifferentiated thyroid cancer.
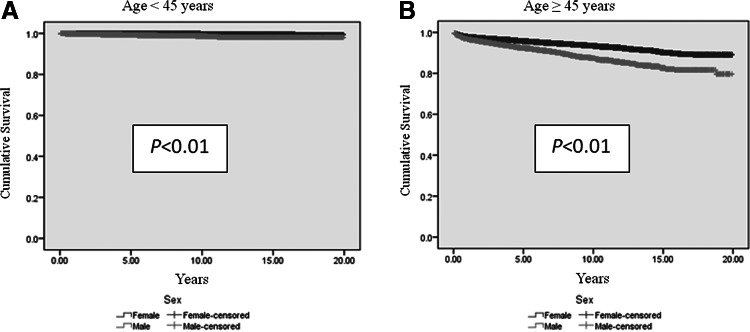
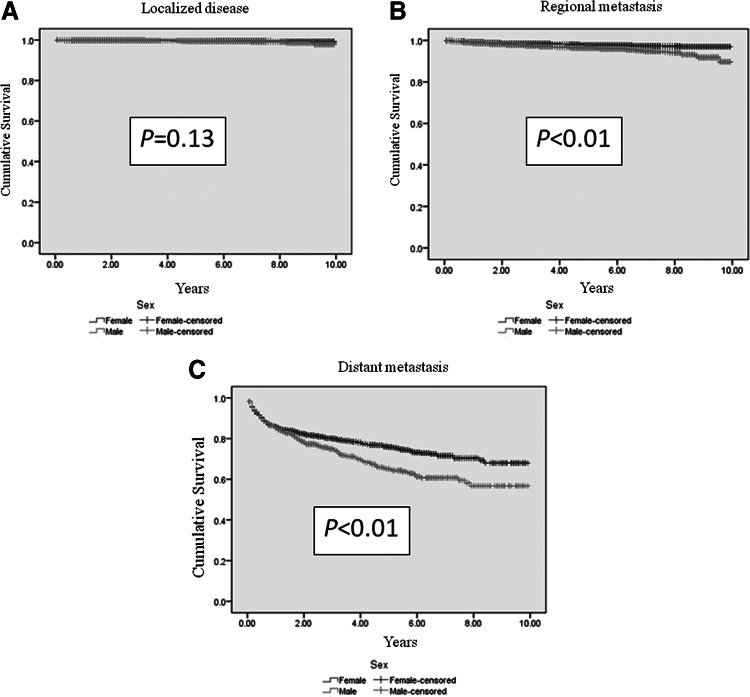
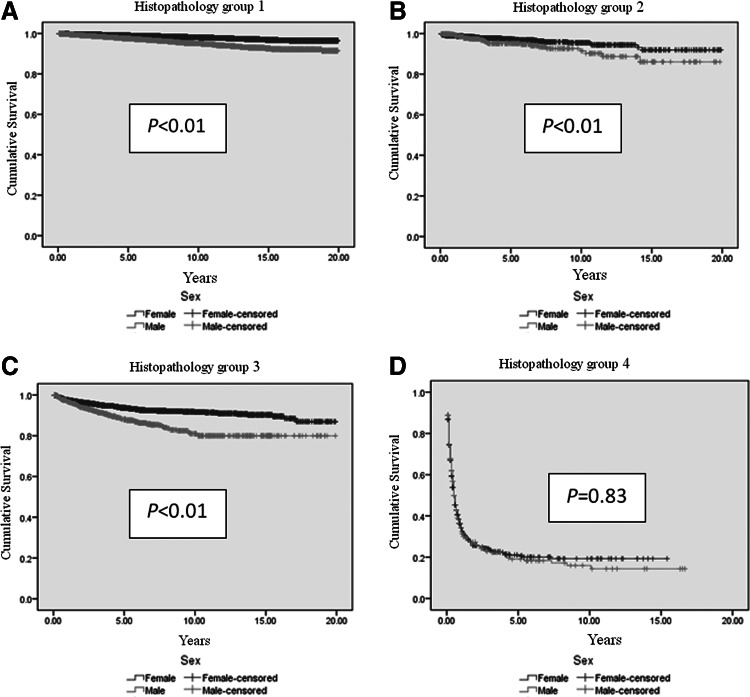
Men had shorter DSS than women, regardless of age (p<0.01; Table 3 and Fig. 1). A shorter DSS was observed in men with regional and distant metastatic cancer (p<0.01; Table 3 and Fig. 2). DSS stratified by histology was significantly worse in men in histopathological groups 1, 2, and 3 (Table 3 and Fig. 3), despite higher rates of total thyroidectomies being performed in men (p<0.01). There was no difference in DSS for patients who had undifferentiated thyroid cancer (group 4). Although univariate analysis showed that sex was a significant prognostic factor associated with DSS (p<0.01), sex was not an independent prognostic factor associated with DSS by multivariate analysis. Independent prognostic factors for disease-specific mortality included age ≥45 years, tumor size ≥4 cm, extrathyroidal extension, lymph node metastasis, distant metastasis, tumor staging, histopathology, and radiation treatment (Table 4).
Table 3.
Comparison of Disease-Specific Survival for Both Sexes by Age, Tumor Stage and Histopathology
| Patient characteristics | Male | Female | Overall | p-Value |
|---|---|---|---|---|
| Age at diagnosis | ||||
| Age <45 years | 19.7±0.03 | 19.9±0.01 | 19.8±0.01 | <0.01 |
| Age ≥45 years | 17.5±0.1 | 18.6±0.05 | 18.3±0.04 | <0.01 |
| Tumor staging | ||||
| Localized | 9.8±0.02 | 9.9±0.01 | 9.9±0.01 | 0.13 |
| Regional | 9.5±0.04 | 9.7±0.02 | 9.6±0.02 | <0.01 |
| Distant | 6.8±0.11 | 7.6±0.12 | 7.3±0.18 | <0.01 |
| Histopathologya | ||||
| Group 1 | 18.9±0.05 | 19.5±0.02 | 19.4±0.02 | <0.01 |
| Group 2 | 18.1±0.35 | 19.0±0.18 | 18.7±0.16 | <0.01 |
| Group 3 | 16.8±0.28 | 18.3±0.13 | 17.9±0.12 | <0.01 |
| Group 4 | 3.4±0.41 | 3.6±0.3 | 3.6±0.26 | 0.83 |
Values are presented as mean±standard deviation. Kaplan–Meier with log-rank test was performed.
Histopathology: group 1 included well- and moderately differentiated, conventional PTC, FVPTC, FTC, minimally invasive FTC; group 2 included PTC with oxyphilic cells (Hürthle cells); group 3 included poorly differentiated, columnar, insular, tall cell variant PTC; and group 4 included undifferentiated thyroid cancer.
FIG. 1.
Estimated disease-specific survival of men with thyroid cancer of follicular cell origin is significantly shorter than women, independent of age. Analyses were performed using Kaplan–Meier with log-rank test. (A) Estimated disease-specific survival of men <45 years is significantly shorter than women (p<0.01). (B) Estimated disease-specific survival of men ≥45 years is significantly shorter than women (p<0.01).
FIG. 2.
Estimated disease-specific survival of men who developed regional (B) (p<0.01) and distant metastasis (C) (p<0.01) from thyroid cancer of follicular cell origin is significantly shorter than women. There is no survival difference between men and women with localized thyroid cancer (A) (p=0.13). Analyses were performed using Kaplan–Meier with log-rank test.
FIG. 3.
Estimated disease-specific survival of men with histopathology group 1 (A) (p<0.01), group 2 (B) (p<0.01), and group 3 (C) (p<0.01) from thyroid cancer of follicular cell in origin is significantly shorter than women. There is no survival difference between men and women with histopathology group 4 (D) (p=0.83). Analyses were performed using Kaplan–Meier with log-rank test. Histopathology: group 1 included well and moderately differentiated, conventional PTC, FVPTC, FTC, minimally invasive FTC; group 2 included PTC with oxyphilic cells (Hürthle cells); group 3 included poorly differentiated, columnar, insular, tall cell variant PTC; and group 4 included undifferentiated thyroid cancer.
Table 4.
Multivariate Cox Proportional Hazard Regression Analysis of Patient Characteristics and Disease-Specific Mortality
| |
|
95% confidence interval for Exp(B) |
|
|
|---|---|---|---|---|
| Patient characteristics | Hazard ratio | Lower | Upper | p-Value |
| Age ≥45 years | 12.2 | 7.3 | 20.4 | <0.01 |
| Race/ethnicity | 0.52 | |||
| Black (reference) | 1.00 | — | — | |
| White | 1.16 | 0.71 | 1.91 | 0.55 |
| Other | 1.35 | 0.77 | 2.36 | 0.30 |
| Male | 0.96 | 0.77 | 1.20 | 0.73 |
| Tumor size (cm) | 1.00 | — | — | <0.01 |
| ≤1 (reference) | — | |||
| >1 to ≤2 | 0.96 | 0.59 | 1.56 | 0.87 |
| >2 to ≤4 | 1.40 | 0.90 | 2.20 | 0.14 |
| >4 | 2.70 | 1.73 | 4.23 | <0.01 |
| Extrathyroidal extension | 1.77 | 1.23 | 2.48 | <0.01 |
| Regional lymph node metastasis | 1.72 | 1.33 | 2.22 | <0.01 |
| Distant metastasis | 1.44 | 1.02 | 2.02 | 0.04 |
| Tumor staging | <0.01 | |||
| Localized (reference) | 1.00 | — | — | — |
| Regional metastasis | 3.76 | 2.25 | 6.29 | <0.01 |
| Distant metastasis | 8.47 | 4.58 | 15.70 | <0.01 |
| Histopathologya | <0.01 | |||
| Group 1 (reference) | 1.00 | — | — | — |
| Group 2 | 2.63 | 1.43 | 4.83 | <0.01 |
| Group 3 | 2.69 | 2.00 | 3.63 | <0.01 |
| Group 4 | 25.16 | 18.07 | 35.04 | <0.01 |
| Subtotal or total thyroidectomy (reference) | 1 | <0.01 | ||
| Lobectomy | 1.42 | 0.98 | 2.06 | <0.01 |
| Less than lobectomy | 2.81 | 1.52 | 5.18 | <0.01 |
| Radiotherapy | <0.01 | |||
| None (reference) | — | — | — | — |
| Radioisotopes | 0.62 | 0.47 | 0.82 | <0.01 |
| XRT | 1.452 | 1.04 | 2.02 | 0.03 |
| Combined XRT and radioisotopes | 1.979 | 1.12 | 3.50 | 0.02 |
| Other | 1.665 | 0.91 | 3.05 | 0.10 |
Sex was not an independent prognostic factor. Significant independent prognostic factors are highlighted in bold.
Histopathology: group 1 included well and moderately differentiated, conventional PTC, FVPTC, FTC, minimally invasive FTC; group 2 included PTC with oxyphilic cells (Hürthle cells); group 3 included poorly differentiated, columnar, insular, tall cell variant PTC; and group 4 included undifferentiated thyroid cancer.
Discussion
While sex disparity in the incidence of thyroid cancer of follicular cell origin has been well-described, comprehensive data analysis of the factors associated with worse outcome in men with thyroid cancer is lacking. We aimed at assessing the difference between sexes by comparing various demographics and clinical data of patients with thyroid cancer of follicular cell origin using a large population-based cancer registry. Our goal was to evaluate the impact of sex, as well as other clinical and pathological variables, on disease-specific mortality. Larger tumors, higher rates of lymph node and distant metastases, and more aggressive tumor histology were observed in men. Although men received more sub- or near- total thyroidectomies and radiotherapy, disease-specific mortality was significantly higher in men. Estimated DSS was lower in men with regional and distant metastasis and all types of histopathology, except in undifferentiated thyroid cancer, which is almost uniformly lethal.
Similar to previously reported studies with relatively smaller cohorts, sex was associated with mortality in univariate analysis (12) but was not an independent prognostic factor in multivariate analysis (12–14). The prognostic significance of sex in univariate analysis is likely due to the association between male sex and more aggressive disease at diagnosis and treatment. Our subgroup analysis showed that advanced thyroid cancer of follicular cell origin was more common in men even at a young age and may support the hypothesis that men inherently have more aggressive tumor behavior rather than a delay in diagnosis. When these confounding factors were controlled for in a multivariate analysis, sex no longer was an independent prognostic factor. In contrast, Gilliland et al. (8) observed that sex was a significant prognostic factor after analyzing relative survival of >15,000 patients with all types of thyroid cancer, including medullary thyroid cancer, using SEER data from 1975 to 1991. Our study focused on thyroid cancer of follicular cell origin in a larger population, and we did not find sex to be an independent prognostic factor. We believe that our study best describes the biological behavior of thyroid cancer of follicular cell origin because (i) only patients with non-medullary thyroid cancer as their only primary cancer were included, (ii) DSS was used in the analyses, and (iii) a subset analysis by histopathological group was performed. Yu et al. (15) focused the analysis only on papillary microcarcinoma from >18,000 patients and described male sex as an independent prognostic factor for shorter overall survival. Although our results suggest that the worse outcome in men is likely due to more advanced stage and higher rates of aggressive histopathology at presentation, consistent with a previous study by Ries et al. (7), the results demonstrated by Yu et al. (15) may suggest a truly aggressive behavior of papillary thyroid cancer in men, even at a small size.
Our study suggests that men are likely to present with more advanced disease and tumors with aggressive histopathology; we did not find a survival difference between men and women when the thyroid cancer was localized to the thyroid gland. This study demonstrated that men with localized disease have an excellent prognosis, similar to women. Our findings do, however, suggest that men with thyroid cancer presented with more advanced disease, and earlier detection or evaluation in men who are symptomatic may decrease the number of patients who present at a later stage and, thus, improve outcome in men with thyroid cancer.
The limitations of the SEER database as compared with other large institutional prospective studies include the lack of some clinical data such as disease recurrence, pathological characteristics that may be associated with poor outcome (e.g., multicentricity), non-uniform treatment, and the absence of centralized pathology review, which may result in the inaccurate diagnosis of uncommon thyroid cancer histopathology. The SEER database does not include information on the presence of chronic lymphocytic thyroiditis on histopathology, which is highly prevalent in women and has been associated with lower rates of capsular invasion and lymph node metastasis (16), as well as longer survival (17) in univariate but not in multivariate analysis. Thus, lymphocytic thyroiditis may be one of several factors associated with superior outcome in women. While there may be bias of treatments toward an aggressive approach in men, such as sub- or near-total thyroidectomy, lymphadenectomy, and radioactive iodine therapy, resulting in higher rates of lymph node and distant metastases found in men, the worse DSS in men, despite these treatments, suggests more aggressive disease at presentation.
In summary, our analyses demonstrate that men present with a more advanced stage of disease, at older age and higher rates of aggressive histopathology. Men have higher thyroid cancer-specific mortality and have a shorter DSS, except when the cancer is localized. However, sex is not an independent prognostic factor for DSS.
Acknowledgment
The research activities performed in this article were supported in part by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health.
Disclosure Statement
All authors have no conflict of interest.
References
- 1.Cerfolio RJ. Bryant AS. Scott E. Sharma M. Robert F. Spencer SA. Garver RI. Women with pathologic stage I, II, and III non-small cell lung cancer have better survival than men. Chest. 2006;130:1796–1802. doi: 10.1378/chest.130.6.1796. [DOI] [PubMed] [Google Scholar]
- 2.Kilfoy BA. Devesa SS. Ward MH. Zhang Y. Rosenberg PS. Holford TR. Anderson WF. Gender is an age-specific effect modifier for papillary cancers of the thyroid gland. Cancer Epidemiol Biomarkers Prev. 2009;18:1092–1100. doi: 10.1158/1055-9965.EPI-08-0976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Naugler WE. Sakurai T. Kim S. Maeda S. Kim K. Elsharkawy AM. Karin M. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science. 2007;317:121–124. doi: 10.1126/science.1140485. [DOI] [PubMed] [Google Scholar]
- 4.Wu MH. Ma WL. Hsu CL. Chen YL. Ou JH. Ryan CK. Hung YC. Yeh S. Chang C. Androgen receptor promotes hepatitis B virus-induced hepatocarcinogenesis through modulation of hepatitis B virus RNA transcription. Sci Transl Med. 2010;2:32ra35. doi: 10.1126/scitranslmed.3001143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cook MB. Dawsey SM. Freedman ND. Inskip PD. Wichner SM. Quraishi SM. Devesa SS. McGlynn KA. Sex disparities in cancer incidence by period and age. Cancer Epidemiol Biomarkers Prev. 2009;18:1174–1182. doi: 10.1158/1055-9965.EPI-08-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hodgson NC. Button J. Solorzano CC. Thyroid cancer: is the incidence still increasing? Ann Surg Oncol. 2004;11:1093–1097. doi: 10.1245/ASO.2004.03.066. [DOI] [PubMed] [Google Scholar]
- 7.Ries LAG, editor; Melbert D, editor; Krapcho M, editor; Mariotto A, editor; Miller BA, editor; Feuer EJ, editor; Clegg L, editor; Horner MJ, editor; Howlader N, editor; Eisner MP, editor; Reichman M, editor; Edwards BK, editor. SEER cancer statistics review, 1975–2004. 2007. http://seer.cancer.gov/csr/1975_2004. [Oct 10;2011 ]. http://seer.cancer.gov/csr/1975_2004
- 8.Gilliland FD. Hunt WC. Morris DM. Key CR. Prognostic factors for thyroid carcinoma. A population-based study of 15,698 cases from the Surveillance, Epidemiology and End Results (SEER) program 1973–1991. Cancer. 1997;79:564–573. doi: 10.1002/(sici)1097-0142(19970201)79:3<564::aid-cncr20>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 9.Siegel R. Naishadham D. Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 10.National Cancer Institute, U.S. National Institutes of Health. Surveillance Epidemiology and End Results: ICD-O-3 Coding Materials. 2012. http://seer.cancer.gov/icd-o-3. [May 21;2012 ]. http://seer.cancer.gov/icd-o-3
- 11.Office of Management and Budget. Revisions to the standards for the classification of federal data on race and ethnicity. 2003. www.whitehouse.gov/omb/fedreg_1997standards. [May 21;2012 ]. www.whitehouse.gov/omb/fedreg_1997standards
- 12.Sebastian SO. Gonzalez JM. Paricio PP. Perez JS. Flores DP. Madrona AP. Romero PR. Tebar FJ. Papillary thyroid carcinoma: prognostic index for survival including the histological variety. Arch Surg. 2000;135:272–277. doi: 10.1001/archsurg.135.3.272. [DOI] [PubMed] [Google Scholar]
- 13.Toniato A. Boschin I. Casara D. Mazzarotto R. Rubello D. Pelizzo M. Papillary thyroid carcinoma: factors influencing recurrence and survival. Ann Surg Oncol. 2008;15:1518–1522. doi: 10.1245/s10434-008-9859-4. [DOI] [PubMed] [Google Scholar]
- 14.Konturek A. Barczynski M. Nowak W. Richter P. Prognostic factors in differentiated thyroid cancer—a 20-year surgical outcome study. Langenbecks Arch Surg. 2012;397:809–815. doi: 10.1007/s00423-011-0899-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu XM. Wan Y. Sippel RS. Chen H. Should all papillary thyroid microcarcinomas be aggressively treated? An analysis of 18,445 cases. Ann Surg. 2011;254:653–660. doi: 10.1097/SLA.0b013e318230036d. [DOI] [PubMed] [Google Scholar]
- 16.Yoon YH. Kim HJ. Lee JW. Kim JM. Koo BS. The clinicopathologic differences in papillary thyroid carcinoma with or without co-existing chronic lymphocytic thyroiditis. Eur Arch Otorhinolaryngol. 2012;269:1013–1017. doi: 10.1007/s00405-011-1732-6. [DOI] [PubMed] [Google Scholar]
- 17.Kebebew E. Treseler PA. Ituarte PH. Clark OH. Coexisting chronic lymphocytic thyroiditis and papillary thyroid cancer revisited. World journal of surgery. 2001;25:632–637. doi: 10.1007/s002680020165. [DOI] [PubMed] [Google Scholar]