Abstract
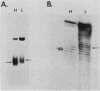
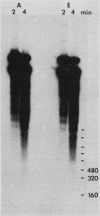
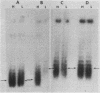
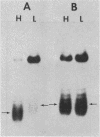
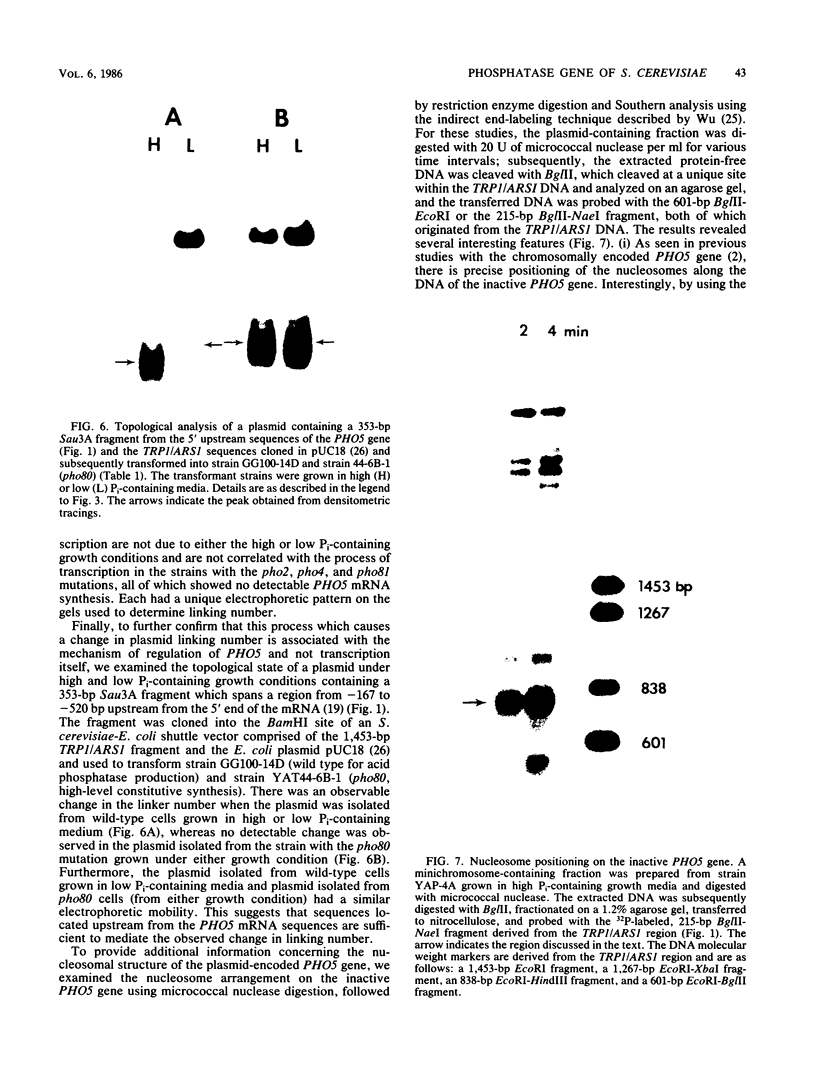
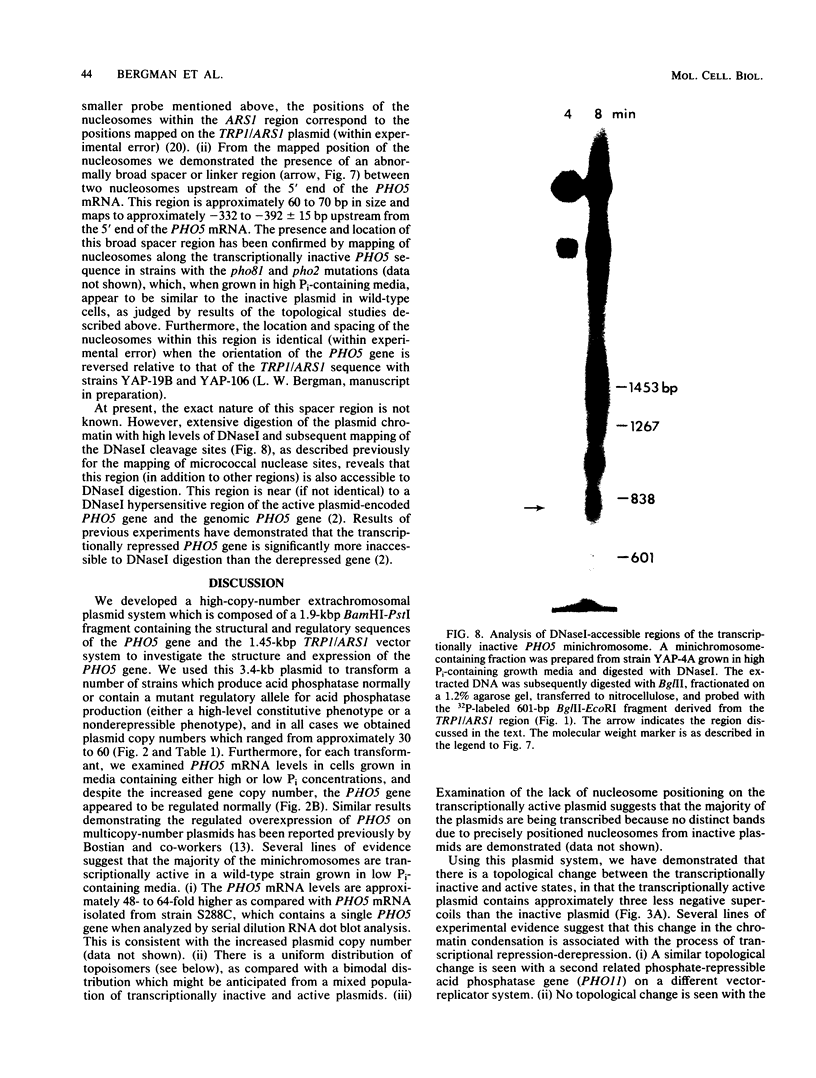
We developed a high-copy-number plasmid system containing the entire structural and regulatory sequences of the phosphate-repressible acid phosphatase (PHO5) gene and the TRP1/ARS1 replicator sequences of the yeast Saccharomyces cerevisiae to investigate the mechanism of repression-derepression of transcription. The resulting plasmid was used to transform either wild-type cells or a number of strains which contain mutations in various trans-acting regulatory loci for the production of acid phosphatase. Results of analysis of mRNA levels isolated from the transformed strains grown under repressed or derepressed conditions suggested that normal transcriptional regulation of the gene persisted, although gene copy number was significantly increased. Analysis of changes in linking number (i.e., the number of negative supercoils) of the plasmid isolated under repressed and derepressed growth conditions revealed that the transcriptionally inactive plasmid contained approximately three more negative supercoils than the transcriptionally active plasmid. This difference in topological state was similarly seen in a plasmid containing a sequence-related acid phosphatase gene (PHO11) under the same regulatory control system, but it was not seen in plasmids isolated from some strains containing mutations which caused either fully constitutive or nonderepressible production of acid phosphatase. Finally, analysis of the nucleosome positioning along the inactive gene sequence revealed that an abnormally broad internucleosomal spacer is present in a region presumed to function in the regulation of transcription by the level of Pi in the growth media.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham J., Feldman J., Nasmyth K. A., Strathern J. N., Klar A. J., Broach J. R., Hicks J. B. Sites required for position-effect regulation of mating-type information in yeast. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 2):989–998. doi: 10.1101/sqb.1983.047.01.113. [DOI] [PubMed] [Google Scholar]
- Bergman L. W., Kramer R. A. Modulation of chromatin structure associated with derepression of the acid phosphatase gene of Saccharomyces cerevisiae. J Biol Chem. 1983 Jun 10;258(11):7223–7227. [PubMed] [Google Scholar]
- Bostian K. A., Lemire J. M., Cannon L. E., Halvorson H. O. In vitro synthesis of repressible yeast acid phosphatase: identification of multiple mRNAs and products. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4504–4508. doi: 10.1073/pnas.77.8.4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brent R., Ptashne M. A bacterial repressor protein or a yeast transcriptional terminator can block upstream activation of a yeast gene. Nature. 1984 Dec 13;312(5995):612–615. doi: 10.1038/312612a0. [DOI] [PubMed] [Google Scholar]
- Chao M. V., Gralla J., Martinson H. G. DNA sequence directs placement of histone cores on restriction fragments during nucleosome formation. Biochemistry. 1979 Mar 20;18(6):1068–1074. doi: 10.1021/bi00573a021. [DOI] [PubMed] [Google Scholar]
- Guarente L. Yeast promoters: positive and negative elements. Cell. 1984 Apr;36(4):799–800. doi: 10.1016/0092-8674(84)90028-x. [DOI] [PubMed] [Google Scholar]
- Kramer R. A., Andersen N. Isolation of yeast genes with mRNA levels controlled by phosphate concentration. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6541–6545. doi: 10.1073/pnas.77.11.6541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohr D. Organization of the GAL1-GAL10 intergenic control region chromatin. Nucleic Acids Res. 1984 Nov 26;12(22):8457–8474. doi: 10.1093/nar/12.22.8457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovett M. A., Guiney D. G., Helinski D. R. Relaxation complexes of plasmids ColE1 and ColE2: unique site of the nick in the open circular DNA of the relaxed complexes. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3854–3857. doi: 10.1073/pnas.71.10.3854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. M., Nasmyth K. A. Role of DNA replication in the repression of silent mating type loci in yeast. Nature. 1984 Nov 15;312(5991):247–251. doi: 10.1038/312247a0. [DOI] [PubMed] [Google Scholar]
- Proffitt J. H. DNase I-hypersensitive sites in the galactose gene cluster of Saccharomyces cerevisiae. Mol Cell Biol. 1985 Jun;5(6):1522–1524. doi: 10.1128/mcb.5.6.1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers D. T., Lemire J. M., Bostian K. A. Acid phosphatase polypeptides in Saccharomyces cerevisiae are encoded by a differentially regulated multigene family. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2157–2161. doi: 10.1073/pnas.79.7.2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin G. M. Three forms of the 5.8-S ribosomal RNA species in Saccharomyces cerevisiae. Eur J Biochem. 1974 Jan 3;41(1):197–202. doi: 10.1111/j.1432-1033.1974.tb03260.x. [DOI] [PubMed] [Google Scholar]
- Shure M., Pulleyblank D. E., Vinograd J. The problems of eukaryotic and prokaryotic DNA packaging and in vivo conformation posed by superhelix density heterogeneity. Nucleic Acids Res. 1977;4(5):1183–1205. doi: 10.1093/nar/4.5.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson R. T., Stafford D. W. Structural features of a phased nucleosome core particle. Proc Natl Acad Sci U S A. 1983 Jan;80(1):51–55. doi: 10.1073/pnas.80.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Struhl K., Stinchcomb D. T., Scherer S., Davis R. W. High-frequency transformation of yeast: autonomous replication of hybrid DNA molecules. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1035–1039. doi: 10.1073/pnas.76.3.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thill G. P., Kramer R. A., Turner K. J., Bostian K. A. Comparative analysis of the 5'-end regions of two repressible acid phosphatase genes in Saccharomyces cerevisiae. Mol Cell Biol. 1983 Apr;3(4):570–579. doi: 10.1128/mcb.3.4.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
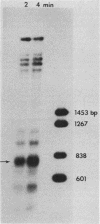
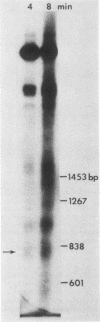
- Thoma F., Bergman L. W., Simpson R. T. Nuclease digestion of circular TRP1ARS1 chromatin reveals positioned nucleosomes separated by nuclease-sensitive regions. J Mol Biol. 1984 Aug 25;177(4):715–733. doi: 10.1016/0022-2836(84)90046-9. [DOI] [PubMed] [Google Scholar]
- Toh-e A., Inouye S., Oshima Y. Structure and function of the PHO82-pho4 locus controlling the synthesis of repressible acid phosphatase of Saccharomyces cerevisiae. J Bacteriol. 1981 Jan;145(1):221–232. doi: 10.1128/jb.145.1.221-232.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschumper G., Carbon J. Sequence of a yeast DNA fragment containing a chromosomal replicator and the TRP1 gene. Gene. 1980 Jul;10(2):157–166. doi: 10.1016/0378-1119(80)90133-x. [DOI] [PubMed] [Google Scholar]
- Weintraub H. Recognition of specific DNA sequences in eukaryotic chromosomes. Nucleic Acids Res. 1980 Oct 24;8(20):4745–4753. doi: 10.1093/nar/8.20.4745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West R. W., Jr, Yocum R. R., Ptashne M. Saccharomyces cerevisiae GAL1-GAL10 divergent promoter region: location and function of the upstream activating sequence UASG. Mol Cell Biol. 1984 Nov;4(11):2467–2478. doi: 10.1128/mcb.4.11.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C. The 5' ends of Drosophila heat shock genes in chromatin are hypersensitive to DNase I. Nature. 1980 Aug 28;286(5776):854–860. doi: 10.1038/286854a0. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]