Background: Cancer cells rely on energy metabolism that requires increased glucose uptake and constitutive NF-κB activity for survival.
Results: Pancreatic cancer cells display elevated O-GlcNAcylation, reduction of which inhibits cell survival and oncogenic NF-κB signaling.
Conclusion: Hyper-O-GlcNAcylation is anti-apoptotic and contributes to NF-κB activation in pancreatic cancer.
Significance: Targeting hyper-O-GlcNAcylation may serve as a novel therapeutic intervention in pancreatic cancer.
Keywords: Apoptosis, NF-kappa B (NF-κB), O-GlcNAc, O-GlcNAcylation, Pancreatic Cancer, Warburg Effect
Abstract
Cancer cell metabolic reprogramming includes a shift in energy production from oxidative phosphorylation to less efficient glycolysis even in the presence of oxygen (Warburg effect) and use of glutamine for increased biosynthetic needs. This necessitates greatly increased glucose and glutamine uptake, both of which enter the hexosamine biosynthetic pathway (HBP). The HBP end product UDP-N-acetylglucosamine (UDP-GlcNAc) is used in enzymatic post-translational modification of many cytosolic and nuclear proteins by O-linked β-N-acetylglucosamine (O-GlcNAc). Here, we observed increased HBP flux and hyper-O-GlcNAcylation in human pancreatic ductal adenocarcinoma (PDAC). PDAC hyper-O-GlcNAcylation was associated with elevation of OGT and reduction of the enzyme that removes O-GlcNAc (OGA). Reducing hyper-O-GlcNAcylation had no effect on non-transformed pancreatic epithelial cell growth, but inhibited PDAC cell proliferation, anchorage-independent growth, orthotopic tumor growth, and triggered apoptosis. PDAC is supported by oncogenic NF-κB transcriptional activity. The NF-κB p65 subunit and upstream kinases IKKα/IKKβ were O-GlcNAcylated in PDAC. Reducing hyper-O-GlcNAcylation decreased PDAC cell p65 activating phosphorylation (S536), nuclear translocation, NF-κB transcriptional activity, and target gene expression. Conversely, mimicking PDAC hyper-O-GlcNAcylation through pharmacological inhibition of OGA suppressed suspension culture-induced apoptosis and increased IKKα and p65 O-GlcNAcylation, accompanied by activation of NF-κB signaling. Finally, reducing p65 O-GlcNAcylation specifically by mutating two p65 O-GlcNAc sites (T322A and T352A) attenuated the induction of PDAC cell anchorage-independent growth. Our data indicate that hyper-O-GlcNAcylation is anti-apoptotic and contributes to NF-κB oncogenic activation in PDAC.
Introduction
Cancer cells preferentially utilize glycolysis instead of oxidative phosphorylation as the main energy source even in the presence of high oxygen tension (Warburg effect) (1). One consequence of cancer cells shifting to the less efficient energy producing glycolysis pathway is a need for greatly increased glucose uptake (2, 3). Cancer cells also consume glutamine at high rates and require high glutamine concentrations to survive (glutamine addiction) (4, 5). A relatively small fraction of glucose (estimated around 2–5%) and glutamine entering cells shunts into the hexosamine biosynthetic pathway (HBP).3 The HBP end product UDP-N-acetylglucosamine (UDP-GlcNAc) is the donor sugar nucleotide used by O-GlcNAc transferase (OGT) in the dynamic enzymatic post-translational modification called O-GlcNAcylation. O-GlcNAc is removed by O-GlcNAcase (OGA). Unlike complex glycosylation that occurs in the secretory pathway, O-GlcNAc is a unique single carbohydrate modification that occurs on serines and threonines of many cytosolic and nuclear proteins. O-GlcNAc in many ways acts analogous to phosphorylation to regulate signaling. O-GlcNAc has complex roles in cellular processes such as cell cycle control, cell stress responses, and transcription (6). Our previous work linked diabetic hyperglycemia to increased glucose flux through the HBP and consequent elevation of O-GlcNAc levels (7). This led us to consider potential novel links between cancer cell metabolism (involving increased glucose and glutamine uptake), elevation of O-GlcNAc, and transformation. While elevated O-GlcNAc (hyper-O-GlcNAcylation) has been observed in several cancer types including breast (8) and prostate (9), potential links between hyper-O-GlcNAcylation and oncogenicity are not clear.
NF-κB is a family of transcription factors with well known roles in immunity and inflammation (10). Abnormal constitutive NF-κB activation has been linked to oncogenic growth/survival of many cancer types (11) including pancreatic ductal adenocarcinoma (PDAC) (12, 13), which accounts for over 90% of pancreatic cancers and is currently the fourth leading cause of cancer deaths in the United States (14). However, oncogenic mutants of NF-κB have not been described, raising questions of how cancer cell constitutive NF-κB activity is regulated. The canonical NF-κB dimer composed of p65 and p50 subunits is sequestered in the cytoplasm by IκBα. In response to stimuli such as TNFα, the IKK complex composed of IKKα, IKKβ and IKKγ phosphorylates IκBα at S32 and S36 and p65 at S536 leading to IκBα proteasome-mediated degradation, release of the NF-κB dimer from sequestration, nuclear translocation of NF-κB, and transcription of target genes including cyclin D1, Vimentin, Bcl-xL, IL-8, VEGF, GLUT3 involved in cell cycle progression, anti-apoptosis, angiogenesis, and glycolysis (10, 15, 16). In rat vascular smooth muscle cells and mesangial cells, hyperglycemia induced O-GlcNAc elevation activates NF-κB, in part through direct modification of p65 (17, 18). However, potential roles for O-GlcNAc in oncogenic NF-κB activation have not been examined.
In the present study, we observed PDAC cell increased HBP flux and hyper-O-GlcNAcylation. Reducing this hyper-O-GlcNAcylation inhibited PDAC cell growth and tumor formation by triggering apoptosis, but did not affect the growth of non-transformed pancreatic epithelial cells. We also found that the NF-κB p65 subunit and upstream activating kinases IKKα/IKKβ were O-GlcNAc modified in PDAC. Reduction of PDAC hyper-O-GlcNAcylation inhibited constitutive NF-κB activity, while elevation of O-GlcNAc activated NF-κB and suppressed apoptosis.
EXPERIMENTAL PROCEDURES
Materials and Reagents
Anti-O-GlcNAc antibody (CTD 110.6) has been described previously (19). The OGT inhibitor Ac-5SGlcNAc and OGA inhibitor NButGT were prepared as described (20, 21). Anti-OGT (DM-17), anti-Flag, and anti-actin were obtained from Sigma. Anti-OGA (14711–1-AP) was from Proteintech (Chicago, IL). Anti-OGT (F-12), Anti-p65, anti-IKKβ, and anti-HDAC were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-IKKα, anti-IKKβ, anti-p65, anti-phospho-p65, and anti-Bcl-xL were obtained from Cell Signaling (Danvers, MA). Human p65 cDNA, a gift from Dr. Jianhua Yang (Baylor College of Medicine) (22), was cloned into p3xFlag-CMV vector (Sigma-Aldrich). T322 and T352 on p65 were mutated to alanines using the QuickChange II Site-directed Mutagenesis Kit (Agilent Technologies). Subsequently, 3×Flag-tagged wild-type (WT) p65 and the T322/352A mutant were cloned into the retroviral vector pBaBe-hygro.
Cell Culture
The immortalized human pancreatic duct epithelial cells (HPDE) established by the transduction of the HPV16-E6E7 genes were kindly provided by Dr. Ming-Sound Tsao (Ontario Cancer Institute, Toronto, Ontario, Canada) (23). These cells were cultured in keratinocyte serum-free (KSF) medium supplied with 5 ng/ml epidermal growth factor (EGF), 50 μg/ml bovine pituitary extract (Invitrogen, Carlsbad, CA), 2.5 g/liter glucose, 2 mm l-glutamine, and 1× antibiotic-antimycotic mixture (Invitrogen). MiaPaCa-2 cells were grown in DMEM containing 4.5 mg/ml d-glucose and 4 mm l-glutamine supplemented with 10% fetal bovine serum (FBS), 2.5% horse serum, 100 units/ml penicillin, and 100 μg/ml streptomycin. BxPC-3 cells were grown in RPMI 1640 supplemented with 10% FBS, 2.5 g/liter glucose, 2 mm l-glutamine, 100 units/ml penicillin, and 100 μg/ml streptomycin. Capan-1 cells were cultured in IMEM supplemented with 20% FBS, 100 units/ml penicillin, and 100 μg/ml streptomycin. Panc-1 and HPAFII cells were grown in DMEM containing 4.5 mg/ml d-glucose and 4 mm l-glutamine supplemented with 10% fetal bovine serum (FBS), 100 units/ml penicillin, and 100 μg/ml streptomycin. All cells were maintained at 37 °C with 5% CO2-humidified atmosphere. BxPC-3 cells stably overexpressing WT p65 or T322/352A p65 were generated after transduction using the pBaBe-hygro retroviral vectors and selection in hygromycin (300 μg/ml) for 7 days.
Cell Proliferation
At 48 h after infection or treatment with Ac-5SGlcNAc or vehicle, cells were seeded into 12-well plates at the concentration of 1.5 × 104/well. Cells were counted approximately every 24 h for 5 consecutive days using a hemocytometer. All measurements were performed in triplicate.
Soft Agar Colony Formation Assay
Growth and survival of cancer cells in soft agar was determined as described (8). See more detail in Supplemental Information.
Measurement of UDP-N-acetylhexosamines
The measurement of nucleotide-linked UDP-N-acetylhexosamines (UDP-HexNAc) composed of UDP-GlcNAc and UDP-N-acetylgalactosamine (UDP-GalNAc), end products of the HBP, was performed as described (24, 25). Briefly, 24 h prior to collection, the media was changed. Cells from a 10 cm plate at 80–90% confluence were pelleted, and 150 μl of 0.3 m perchloric acid (PCA) was added to pellet and sonicated. Soluble fractions were obtained by centrifuging at 10,000 × g for 10 min at 4 °C. PCA was then extracted from the supernatant with 1:4 trioctylamine: 1,1,2-trichloro-trifluoroethane and the aqueous phase was stored at −80 °C until analysis. The acid exacts were analyzed for UDP-HexNAc by high-pressure liquid chromatography (HPLC) using a Whatman Partisil anion exchange column (4.6 × 250 mm) with a concave gradient spanning 50 min from 15 mm H3PO4 (pH 3.8 with NH4OH) to 1 m H3PO4 (pH 4.5 with NH4OH) at a flow rate of 1 ml/min. UDP-HexNAc levels were monitored by UV absorption at 254 nm and quantified by a standard curve method. The level of UDP-HexNAc was normalized to total protein.
Lentiviral shRNA Production and Infection
The lentiviruses expressing shRNAs against OGT were produced as described in Supplemental Information. Forty-eight hours after infection, cells were assayed for proliferation and anchorage-independent growth or selected with puromycin (3 μg/ml) overnight. Lysates were collected 72 h post-transduction for immunoblotting, unless otherwise indicated.
Immunoblotting and Immunoprecipitation
Cells were lysed on ice for 10 min in RIPA buffer (50 mm Tris-HCl, pH 7.4, 1% Igepal CA-630, 0.5% sodium deoxycholate, 150 mm NaCl, 1 mm EDTA, 0.1% SDS) supplemented with protease inhibitor mixture tablet (Roche). Cell lysates were cleared by centrifuging at 16,000 × g for 20 min at 4 °C. The protein concentrations were determined using the BCA Protein Assay Reagent Kit (Pierce Biotechnology). Total cellular proteins were separated on SDS-PAGE. For immunoprecipitation, 3 mg of cell lysate in 1 ml RIPA buffer was incubated with 2 μg of anti-p65 antibody (sc-109) at 4 °C for 1 h. Samples were then added with 30 μl of protein A-agarose and rotated at 4 °C overnight. Agarose beads were washed four times in RIPA buffer. Immunoprecipitates were eluted in 2× SDS sample loading buffer and separated by SDS-PAGE. In some cases, cells were treated with 50 μm NButGT overnight before cell lysis.
Immunofluorescence
BxPC-3 cells were grown on glass coverslips in 6-well culture plates and transiently transfected with either p3XFlag-CMV-OGT (a gift from Dr. Jin Won Cho, Yonsei University) (18) or pLenti4-HA-OGT (a gift from Dr. Lance Wells, the University of Georgia). After 24 h, cells were washed with PBS, fixed for 10 min at room temperature with 4% paraformaldehyde, and permeabilized for 5 min with 0.5% Triton X-100 in PBS, blocked with 3% BSA in PBS with 0.05% Tween-20 (PBST) for an hour, and incubated at 4 °C with anti-Flag (1:500), anti-p65 (1:100), or anti-O-GlcNAc (1:200) antibodies in 3% BSA/PBST overnight, followed by incubation with AlexaFluor 488 goat anti-mouse or AlexaFluor 568 goat anti-rabbit secondary antibody for 1 h in the dark. DNA was stained with 0.5 μg/ml 4′, 6-diamidino-2-phenylindole dihydrochloride (DAPI) in PBS for 5 min (Sigma-Aldrich). Coverslips were mounted with Prolong Antifade Reagent (Molecular Probes). Images were acquired using an Olympus AX70 epi-fluorescence microscope.
Orthotopic Mouse Model
GFP-labeled MiaPaCa-2 cells were generated by infection with pWZL-blast-GFP, a gift from Dr. Robert Weinburg (addgene #12269). Orthotopic transplantation was done on female CB17SCRF mice (7–8 weeks old, Taconic) as previously described (26). Briefly, mice were anesthetized with isoflurane. Abdominal surgical field was prepared by depilation and followed by sterilization with alcohol and povidone-iodine. A small left upper abdominal incision (∼10 mm) was made to expose the peritoneal cavity. The tail of pancreas was located and exposed according to the spleen. One million cells that were infected with scramble, shOGT1, or shOGT2 lentivirus were suspended in 50 μl of cold DMEM and then injected into the tail of pancreas using a 28½ gauge needle. A successful injection of tumor cells was confirmed by visualization of a fluid bleb with minimal fluid leakage. Leakage was prevented by pressing a cotton swab for 1 min against the site of injection. The pancreas was then returned into the peritoneal cavity and the peritoneum and skin were sewed in two layers with 4–0 vicryl. After surgery, buprenorphine was given to mice (0.05–0.1 mg/kg) every 12 h for 4 times. Mice were sacrificed at 8 weeks and tumors in situ were imaged by a dissection fluorescence microscope equipped with a digital camera. Tumors were then harvested and weighed.
Anoikis Assay
Anoikis resistance was induced as previously described (27). See more detail in Supplemental Information.
FITC Annexin V Apoptosis Assay
MiaPaCa-2 cells infected with scramble or shRNAs against OGT were labeled at 96 h post transduction with annexin V-FITC/propidium iodide (BD Bioscience Pharmingen) in accordance with the manufacturer's instructions. The apoptotic fraction was quantified using a Guava PCA-96 flow cytometer (Millipore, Billerica, MA) and Guava CytoSoft 5.3 software (Guava Technologies, Hayward, CA). The same method was used for BxPC-3 cells treated with NButGT.
Statistical Analysis
All the quantitative data are presented as means ± S.D. The statistical significance of differences was determined using Student's two-tailed t test in two groups, and one-way ANOVA in multiple groups. A p value ≤ 0.05 was considered statistically significant.
RESULTS
Hyper-O-GlcNAcylation Occurs in Pancreatic Cancer
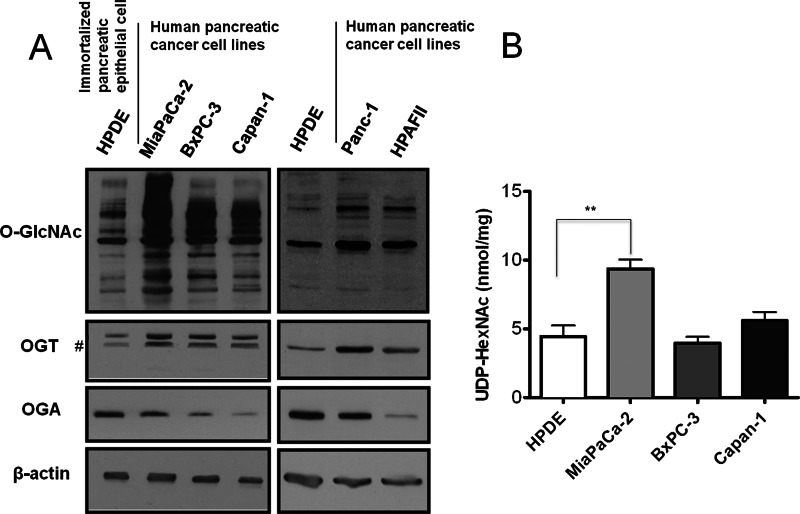
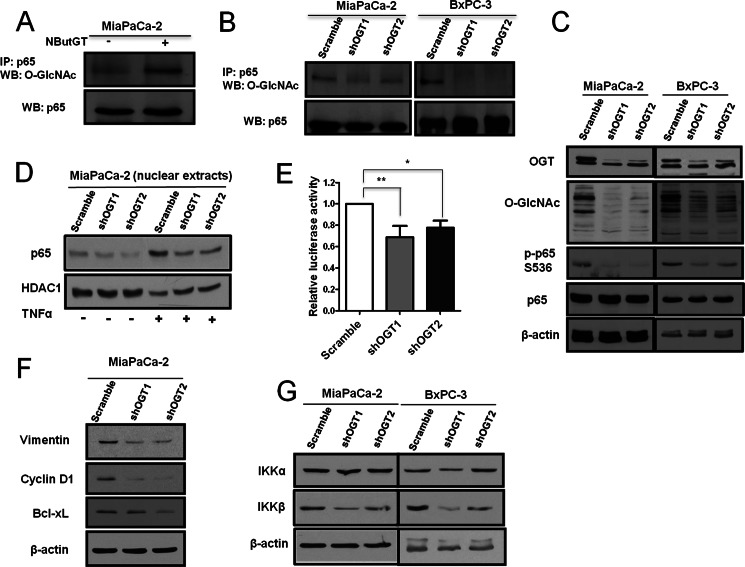
To determine whether O-GlcNAcylation is elevated in pancreatic cancer, we first examined O-GlcNAc modification levels in human pancreatic ductal adenocarcinoma (PDAC) cell lines compared with non-transformed human pancreatic epithelial HPDE cells. Global O-GlcNAcylation was increased in PDAC cells (Fig. 1A). Moreover, OGT levels were elevated in PDAC cells, while levels of OGA were reduced compared with HPDE cells (Fig. 1A). As increased flux through the HBP is known to drive elevation of O-GlcNAc, we examined levels of HBP end products UDP-GlcNAc plus UDP-GalNAc (UDP-HexNAc) in PDAC. The concentration of UDP-HexNAc was almost 2-fold increased in MiaPaCa-2 PDAC cells compared with HPDE cells (Fig. 1B), and correlated with MiaPaCa-2 cells displaying the highest increase in O-GlcNAc levels among PDAC cell lines (Fig. 1A). Finally, we found that PDAC cancer tissue exhibited increased O-GlcNAc staining compared with normal pancreatic ducts (Supplemental Fig. S1). These results indicate that elevated O-GlcNAc modification (hyper-O-GlcNAcylation) occurs in pancreatic cancer.
FIGURE 1.
O-GlcNAc is elevated in human PDAC cells. A, immortalized human pancreatic epithelial HPDE cells and human pancreatic cancer cells MiaPaCa-2, BxPC-3, Capan-1, Panc-1, HPAFII were lysed and analyzed by Western blotting for O-GlcNAc, OGT, and OGA. β-Actin served as a loading control. B, UDP-HexNAc measurement. Sugar nucleotides were extracted from cells and UDP-HexNAc consisting of UDP-GlcNAc and UDP-GalNAc separated by HPLC anion-exchange chromatography were quantified by a standard curve method. The level of UDP-HexNAc was normalized to total protein. Data are the means ± S.D. from at least three independent experiments. **, p < 0.01. #, nonspecific bands.
Reduction of Hyper-O-GlcNAcylation Selectively Inhibits PDAC Cell Proliferation and Anchorage-independent Growth
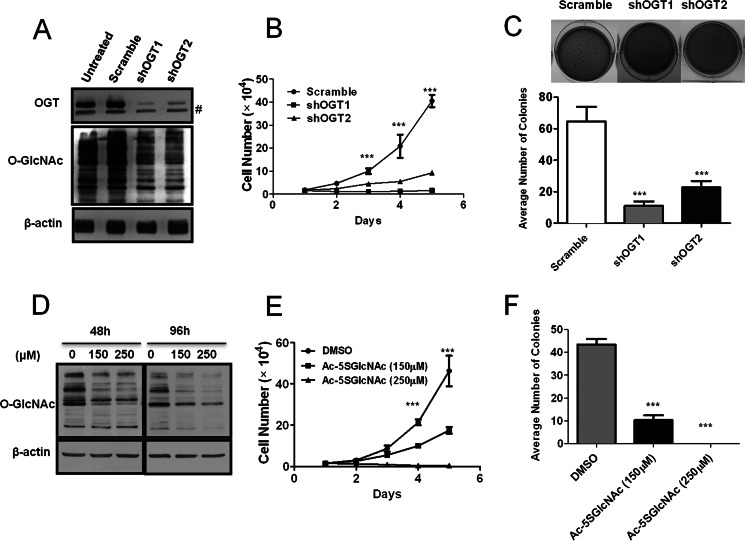
To begin to investigate if hyper-O-GlcNAcylation contributes to PDAC cell-transformed phenotypes, O-GlcNAcylation in MiaPaCa-2 and Panc-1 cells was decreased by OGT silencing (Fig. 2A and supplemental Fig. S2A) and effects on cell proliferation and soft agar growth was determined. OGT knockdown-mediated reduction of hyper-O-GlcNAcylation strongly inhibited PDAC cell proliferation in two-dimensional culture (Fig. 2B and supplemental Fig. S2B), and also inhibited cell proliferation in three-dimensional culture, which more closely mimics the in vivo physiological tumor environment (supplemental Fig. S2D). Suppression of hyper-O-GlcNAcylation also decreased cancer cell anchorage-independent colony growth in soft agar (Fig. 2C and supplemental Fig. S2C). Additionally, in BxPC-3 cells, OGT knockdown reduced hyper-O-GlcNAcylation and blocked anchorage-independent growth induced by overexpression of the p65 subunit of NF-κB (supplemental Fig. S2E). However, OGT silencing to the same extent in non-transformed HPDE cells did not affect cell proliferation (supplemental Fig. S3, A and B). As these results began to indicate that enzymes which regulate O-GlcNAc levels may be novel PDAC therapeutic targets, we examined the effects of a recently developed OGT inhibitor (Ac-5SGlcNAc) (21) on PDAC cells. Ac-5SGlcNAc suppressed MiaPaCa-2 hyper-O-GlcNAcylation (Fig. 2D), and inhibited cell proliferation (Fig. 2E) and soft agar colony formation (Fig. 2F) in a dose-dependent manner. Thus, hyper-O-GlcNAcylation contributes to PDAC cancer cell specific proliferation and anchorage-independent growth.
FIGURE 2.
Reducing hyper-O-GlcNAcylation inhibits PDAC cell proliferation and anchorage-independent growth in vitro. A, MiaPaCa-2 cells were infected with scramble, shOGT1, or shOGT2 pLKO.1 lentivirus. The expression of OGT and O-GlcNAc was examined by Western blotting. B, MiaPaCa-2 cells infected with lentiviruses as in panel A were seeded into 12-well plates 48 h after infection. Cell number was counted for 5 consecutive days using a hemocytometer. C, MiaPaCa-2 cells were infected with lentiviruses as in panel A and placed into soft agar 48 h after infection. Colonies were stained 14 days later and quantified. Representative images are shown in the inset. D, MiaPaCa-2 cells were treated either with DMSO vehicle control or Ac-5SGlcNAc at a concentration of 150 or 250 μm for 48 h or 96 h and lysed. Immunoblot was performed to detect O-GlcNAc. E, MiaPaCa-2 cells were treated as in panel D for 48 h and seeded into 12-well plates. Cell number was counted for five consecutive days using a hemocytometer. F, MiaPaCa-2 cells were treated as in panel D for 48 h and placed into soft agar assay. Colonies were stained 14 days later and quantified. Data represent mean and S.D. of at least three independent experiments. ***, p < 0.001. #, nonspecific bands.
Reduction of Hyper-O-GlcNAcylation Inhibits PDAC Orthotopic Tumor Growth
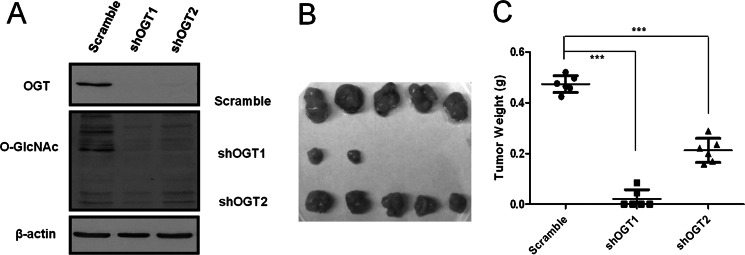
The tumor cell in vivo microenvironment plays critical roles in tumor growth. We examined whether suppression of MiaPaCa-2 cell hyper-O-GlcNAcylation inhibits tumorigenic phenotypes in vivo using orthotopic pancreatic cancer xenografts. GFP expressing MiaPaCa-2 cells infected with shRNA OGT knockdown constructs versus control shRNA were injected into the tail of the pancreas in SCID mice. Eight weeks after implantation, mice were sacrificed and tumors were visualized by GFP signal under a fluorescence dissection microscope (supplemental Fig. S4), harvested and weighed. Suppression of hyper-O-GlcNAcylation by OGT specific knockdown (Fig. 3A) resulted in greatly reduced tumor growth (in some cases, OGT knockdown resulted in no tumor being found) (Fig. 3, B and C). No macroscopic metastasis was observed in liver and adjacent organs. This result indicates that OGT knockdown-mediated suppression of hyper-O-GlcNAcylation in MiaPaCa-2 cells dramatically impairs primary tumor growth.
FIGURE 3.
Reducing hyper-O-GlcNAcylation suppresses PDAC tumor growth in vivo. A, GFP-labeled MiaPaCa-2 cells were infected with scramble, shOGT1, or shOGT2 pLKO.1 lentivirus. The expression of OGT and O-GlcNAc was examined by Western blotting. B, GFP expressing MiaPaCa-2 cells infected with scramble, shOGT1, or shOGT2 lentivirus were orthotopically injected into the pancreas of SCID mice (n = 6 per treatment group). The orthotopic primary tumors were excised 8 weeks after transplantation, and representative tumor masses from each group are shown. C, tumor weight was measured when the orthotopic tumors from each group were removed. Each symbol (dot, square, and triangle) represents the tumor mass from one mouse. The relatively long horizontal lines indicate the mean, and the shorter horizontal lines denote S.D. Statistical analysis was performed by one-way ANOVA and Bonferroni's multiple comparison test. ***, p < 0.001.
Hyper-O-GlcNAcylation is Anti-apoptotic in PDAC Cells
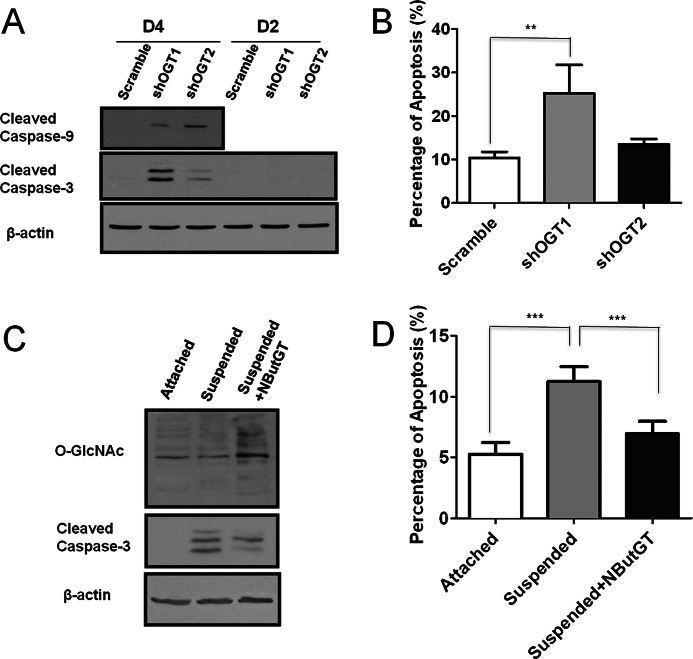
We next investigated whether inhibition of PDAC cell oncogenic phenotypes by reduced hyper-O-GlcNAcylation involved induction of apoptosis. We found that reduction of PDAC hyper-O-GlcNAcylation by OGT knockdown induced caspase-3 and caspase-9 cleavage (Fig. 4A and supplemental Fig. S3C). Consistent with this data, we found significant induction of apoptosis as measured by Annexin V staining (Fig. 4B). The knockdown efficiency of shOGT2 construct was less than that of shOGT1. Correspondingly, while shOGT2 caused a trend toward increased apoptosis, this did not reach statistical significance. Additionally, OGT knockdown to the same extent did not induce caspase-3 activation in non-transformed HPDE cells (supplemental Fig. S3D), consistent with the lack of effect on HPDE cell proliferation. Resistance to suspension-induced cell death (anoikis) is an oncogenic property acquired by cancer cells. Since reduction of PDAC hyper-O-GlcNAcylation induced apoptosis, we reasoned that global elevation of O-GlcNAcylation might provide protection against suspension-induced cell death. To test this, O-GlcNAc levels in BxPC-3 cells were increased by treatment with the OGA inhibitor NButGT for 6 h, and then cultured in suspension for an additional 24 h in the presence of NButGT. We found that pharmacological elevation of O-GlcNAcylation reduced caspase-3 cleavage (Fig. 4C). Consistent with this data, suspension culture-induced apoptosis was significantly decreased in BxPC-3 cells by pharmacological elevation of O-GlcNAcylation (Fig. 4D). These data indicate that hyper-O-GlcNAcylation contributes to PDAC specific cell survival at least in part by inhibition of apoptosis.
FIGURE 4.
PDAC hyper-O-GlcNAcylation is anti-apoptotic. A, MiaPaCa-2 cells were infected with scramble, shOGT1, or shOGT2 pLKO.1 lentivirus and cultured for 2 days or 4 days. Cell lysates were collected and subjected to immunoblotting for cleaved caspases-3 and 9. β-Actin served as a loading control. B, MiaPaCa-2 cells were infected with lentiviruses as in panel A. After 96 h, the percentage of apoptosis was assessed by fluorescence activated cell sorting using Annexin V/PI labeling. C, BxPC-3 cells were pretreated with vehicle or 50 μm NButGT for 6 h and then cultured in suspension conditions with or without NButGT for an additional 24 h. Cell lysates were collected and subjected to immunoblotting for O-GlcNAc and cleaved caspase-3. β-Actin served as a loading control. D, BxPC-3 cells were treated as in panel C. The cells were then labeled with Annexin V/PI labeling, and the percentage of apoptosis was determined by fluorescence-activated cell sorting. Data represent mean and S.D. of at least three independent experiments. **, p < 0.01; ***, p < 0.001.
Reduction of PDAC Hyper-O-GlcNAcylation Down-regulates NF-κB Signaling
We began to address potential mechanisms of how reducing PDAC hyper-O-GlcNAcylation might cause inhibition of PDAC cell survival. NF-κB constitutive activation in many cancers including PDAC (12) contributes to tumorigenic growth (11). Inhibition of NF-κB signaling has been shown to result in PDAC cell apoptosis (13). O-GlcNAc has previously been implicated in regulation of non-transformed mesangial and endothelial cell NF-κB signaling (17, 18). Thus, we considered weather PDAC cell cancer specific O-GlcNAcylation may impinge on oncogenic NF-κB signaling. The p65 subunit of NF-κB immunoprecipitated from PDAC cells was O-GlcNAc modified (Fig. 5A and 6A) and pharmacological elevation of O-GlcNAc by OGA inhibition with NButGT increased p65 O-GlcNAcylation (Fig. 5A), while OGT knockdown in PDAC cells decreased p65 O-GlcNAcylation (Fig. 5B), suggesting dynamic O-GlcNAc cycling on p65 and potential regulation of p65 by PDAC hyper-O-GlcNAcylation. The p65 upstream kinase IKKβ was also O-GlcNAcated in HPDE and PDAC cancer cells (supplemental Fig. S5A), but pharmacological elevation of global O-GlcNAcylation or reduction of hyper-O-GlcNAcylation by OGT knockdown did not alter IKKβ O-GlcNAc levels (supplemental Fig. S5, B and C). We next examined effects of reducing PDAC hyper-O-GlcNAcylation on NF-κB activity. We found that OGT knockdown-mediated reduction of hyper-O-GlcNAcylation in both MiaPAaCa-3 and BxPC-3 cells reduced activating phosphorylation at S536 on p65 (Fig. 5C), which is critical for NF-κB nuclear translocation and activation. Consistent with this data, reduction of MiaPaCa-2 hyper-O-GlcNAcylation decreased basal and TNFα stimulated p65 nuclear localization (Fig. 5D), and resulted in about a 40% decrease in NF-κB luciferase transcriptional activity (Fig. 5E and supplemental Fig. S5D). Reduction of hyper-O-GlcNAcylation in MiaPaCa-2 cells also decreased protein levels of NF-κB transcriptional targets cyclin D1, Vimentin, and Bcl-xL (Fig. 5F). Consistent with this, reduced O-GlcNAcylation in BxPC-3 cells increased expression of E-cadherin (supplemental Fig. S5E), which is indirectly inhibited by NF-κB (28). Finally, expression of IKKβ kinase which acts upstream of p65 (but not IKKα) was decreased upon reduction of hyper-O-GlcNAcylation (Fig. 5G). These results indicate that suppression of hyper-O-GlcNAcylation decreases oncogenic NF-κB signaling in PDAC.
FIGURE 5.
Reducing hyper-O-GlcNAcylation inhibits NF-κB activity. A, MiaPaCa-2 cells were treated with or without NButGT overnight. Cells were lysed and immunoprecipitates of p65 were Western-blotted for p65 and O-GlcNAc. B, MiaPaCa-2 and BxPC-3 cells were infected with scramble, shOGT1, or shOGT2 pLKO.1 lentivirus and treated with NButGT overnight. Immunoprecipitates were Western-blotted to detect p65 and O-GlcNAc. C, MiaPaCa-2 and BxPC-3 cells were infected with scramble shOGT1, or shOGT2 pLKO.1 lentivirus. Cell lysates were subjected to immunoblotting for OGT, O-GlcNAc, phospho-p65 S536, and p65. β-Actin served as a loading control. D, MiaPaCa-2 cells were infected with viruses and then treated or untreated with TNFα (10 ng/ml) for 10 min before nuclear extraction. Nuclear extracts were subjected to immunoblotting for p65. HDAC1 serves as the loading control. E, MiaPaCa-2 cells were infected with lentivirus and then cotransfected with NF-κB luciferase and Renilla luciferase reporter vectors 16 h after infection. The relative luciferase activity was measured 24 h later and normalized with the Renilla activity. F, MiaPaCa-2 cells were infected with scramble, shOGT1, or shOGT2 pLKO.1 lentivirus. Cell lysates were subjected to immunoblotting for Vimentin, cyclin D1, and Bcl-xL. β-Actin served as a loading control. G, MiaPaCa-2 and BxPC-3 cells were infected with scramble, shOGT1, or shOGT2 pLKO.1 lentivirus. Cell lysates were subjected to immunoblotting for IKKα and IKKβ. β-Actin served as a loading control. Error bars indicate S.D. *, p < 0.05; **, p < 0.01.
Elevation of O-GlcNAcylation Activates NF-κB Signaling
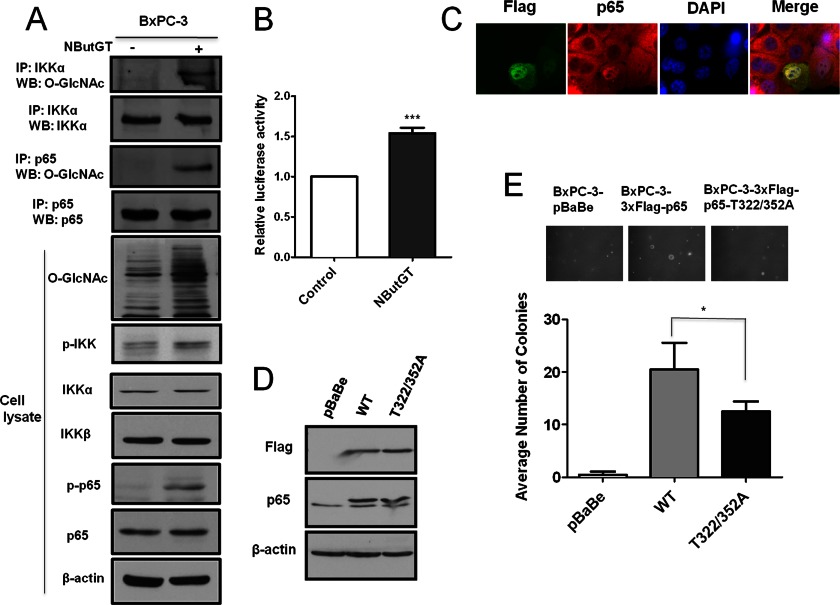
Since reduction of PDAC hyper-O-GlcNAcylation decreased NF-κB signaling, we considered that pharmacological elevation of O-GlcNAc (mimicking cancer cell hyper-O-GlcNAcylation) might promote activation of NF-κB signaling. We found that pharmacological inhibition of OGA with NButGT elevated p65 and IKKα O-GlcNAcylation in BxPC-3 cells accompanied by increased activating phosphorylation of p65 and IKK (Fig. 6A). Consistent with this data, pharmacological elevation of O-GlcNAc increased NF-κB dependent luciferase transcription by over 50% (Fig. 6B). Additionally, cells successfully transfected to overexpress OGT displayed increased NF-κB nuclear localization by immunofluorescent staining (Fig. 6C and supplemental Fig. S6). Therefore, elevation of O-GlcNAcylation up-regulates NF-κB activity in PDAC cells.
FIGURE 6.
Up-regulation of O-GlcNAcylation activates NF-κB. A, BxPC-3 cells were treated with or without 50 μm NButGT overnight. Cells were lysed and IKKα and p65 immunoprecipitates were Western-blotted to detect IKKα, p65, and O-GlcNAc. Whole cell lysates were also subjected to Western blot analysis using indicated antibodies. B, BxPC-3 cells were transfected with NF-κB luciferase reporter pNF-κB-luc WT together with Renilla luciferase reporter plasmid and incubated in the presence of vehicle or 50 μm NButGT for 24 h. The relative luciferase activity was measured and normalized to Renilla activity. C, BxPC-3 cells were transfected with a plasmid expressing Flag-tagged OGT and immunostained with antibodies against Flag (green) and p65 (red) to determine cells overexpressing OGT and the subcellular localization of p65. Nuclei were counterstained with DAPI (blue). D, BxPC-3 cells stably expressing vector, Flag-tagged WT p65 or T322/352A p65 were lysed and subjected to Western blot analysis using indicated antibodies. β-Actin served as a loading control. E, BxPC-3 cells stably expressing vector, Flag-tagged WT p65 or T322/352A p65 were placed into soft agar. Colonies were stained 21 days later and quantified. Representative images are shown in the inset. Error bars indicate S.D. *, p < 0.05.
Site-specific p65 O-GlcNAcylation Contributes to Induction of PDAC Cell Anchorage-independent Growth
As p65 was found to be O-GlcNAcated in PDAC cells, we next examined whether direct O-GlcNAcylation of p65 contributes to NF-κB-dependent transformed phenotypes. Two p65 O-GlcNAc sites have been previously mapped to T322 and 352 (18). We mutated these threonines to alanines (an amino acid that cannot be O-GlcNAc modified) to block p65 specific O-GlcNAcylation at those sites. WT p65 or O-GlcNAc mutant (T322/352A) p65 were over-expressed in BxPC-3 cells (Fig. 6D). Compared with WT p65, the over-expression of O-GlcNAc T322/352A p65 mutant in BxPC-3 cells resulted in fewer and smaller soft agar colonies (Fig. 6E). This result indicates that direct site-specific p65 O-GlcNAcylation facilitates NF-κB activity-dependent PDAC cell growth in soft agar.
DISCUSSION
Symptoms of PDAC usually appear late in disease progression, and thus at time of diagnosis the majority of patients display local infiltration and/or distal metastasis and 80% of PDAC tumors are not resectable (29). Current chemotherapy and radiotherapy are largely ineffective (30). Therefore, more comprehensive understanding of the molecular basis of PDAC is needed to identify potential novel therapeutic targets.
Metabolic reprogramming in cancer, including the Warburg effect, necessitates greatly increased glucose uptake and correlates with increased rates of glycolysis and lactate production and with high expression levels of glucose transporters in cancer cells (2, 3). Indeed, analogues of glucose such as [18F]fluoro-2-deoxy-glucose (FDG) have been widely used diagnostically to image tumors with positron emission tomography (PET) scanning, and to evaluate therapeutic responses in cancer patients (3). In PDAC, MiaPaCa-2 cells have been shown to exhibit a very high rate of lactate production (31). This correlates with high levels of GLUT1 in MiaPaCa-2 cells compared with the PDAC cell lines BxPC-3 and Panc-1 (32, 33). The proliferation of human PDAC cell lines MiaPaCa-2 and Panc-1 is blocked by inhibitors of glycolysis (31, 33). Moreover, MiaPaCa-2 and Panc-1 cells are strictly dependent on glutamine for growth (34). BxPC-3 cells also rely on glutamine for growth even in the presence of high levels of glucose (35). Additionally, oncogenic kras, the predominant mutated oncogene found in PDAC, increases flux of glucose into the HBP via up-regulation of GLUT1, glycolytic enzymes (HK1/2, Gpi1) and GFAT1, the rate-limiting enzyme in the HBP in PDAC cells (36). Consistent with responsiveness of O-GlcNAc levels to HBP flux, we report here for the first time that MiaPaCa-2 metabolism is associated with increased production of HBP end products including UDP-GlcNAc and that this corresponds to hyper-O-GlcNAcylation. These data suggest that general cancer cell metabolic reprogramming resulting in increased uptake of glucose and glutamine could increase flux into the HBP, thereby driving elevation of O-GlcNAc. We also observe that in all PDAC cell lines examined (MiaPaCa-2, BxPC-3, Capan-1, Panc-1, HPAFII) hyper-O-GlcNAcylation is associated with up-regulated OGT levels and down-regulated OGA levels compared with non-transformed pancreatic epithelial HPDE cells. In agreement with our findings in PDAC, we and other groups have now observed elevated O-GlcNAcylation, OGT overexpression, and reduced OGA expression in a variety of human malignancies ranging from nonsolid tumor chronic lymphocytic leukemia (37) to breast (8, 38), prostate (9), lung (39), colorectal (39), and liver (40). These data indicate that hyper-O-GlcNAcylation could be a general hallmark of cancer.
To begin to elucidate potential roles of hyper-O-GlcNAcylation in tumorigenesis, we reduced PDAC hyper-O-GlcNAcylation by OGT knockdown and tested effects on transformed phenotypes. Reducing hyper-O-GlcNAcylation suppressed PDAC cell proliferation, anchorage-independent growth, and orthotopic xenograft tumor growth in mice. Conversely, increasing O-GlcNAc promoted BxPC-3 cell survival in forced suspension culture. However, knockdown of OGT in non-transformed HPDE cells did not affect cell proliferation, indicating cancer cell specific susceptibility to targeting OGT. The extent to which OGT knockdown in HPDE cells decreased O-GlcNAc levels was less than in PDAC cells, perhaps explaining to some extent the cancer specific response to targeting OGT. We also observed that in non-transformed HPDE cells specifically, knock-down of OGT was accompanied by down-regulation of OGA, suggesting a potential feedback mechanism to some extent counterbalance the loss of OGT, perhaps explaining why targeting OGT in HPDE cells decreased O-GlcNAc levels less than in PDAC cells.
The in vitro inhibition of PDAC cell growth by pharmacological inhibition of OGT suggests this may be a potential future therapeutic strategy. However, currently no OGT inhibitor has been shown to be effective in vivo in lowering O-GlcNAc levels, and thus future work will need to involve screening and design of more bioavailable and potent OGT inhibitors.
To elucidate why decreasing hyper-O-GlcNAcylation suppresses tumor transformed phenotypes while not affecting non-transformed cell growth, we considered that PDAC cells might selectively be undergoing apoptosis in response to OGT targeting. Reducing hyper-O-GlcNAcylation in MiaPaCa-2 and Panc-1 cells decreased the expression of the anti-apoptotic protein Bcl-xL and induced pro-apoptotic cleavage of caspases-9, and -3, suggesting that reducing hyper-O-GlcNAcylation triggered the intrinsic apoptotic pathway, which was confirmed by Annexin V/PI staining. Conversely, increasing O-GlcNAc in BxPC-3 cells protected against suspension-induced apoptosis. However, reducing O-GlcNAcylation by knockdown of OGT in non-transformed HPDE cells did not trigger apoptosis. Thus, PDAC hyper-O-GlcNAcylation is essential for cancer cell survival, acting at least in part through anti-apoptotic mechanisms. Given these results, Ogt may act as oncogene, but this has yet to be fully examined.
While we have reported that hyper-O-GlcNAcylation supports transformed phenotypes, the underlying molecular mechanisms through which hyper-O-GlcNAcylation of specific proteins play an essential role in cancer is not understood (8, 9). We have previously reported that knockdown of OGT decreases levels of the oncogenic transcriptional factor FoxM1 in breast and prostate cancer cells, but the mechanism is not known (8, 9). O-GlcNAc modification of Snail in non-metastatic MCF-7 breast cancer cells stabilizes Snail to enhance invasion (41). O-GlcNAc modifies many cytosolic and nuclear proteins, and thus global hyper-O-GlcNAcylation is likely to have influences on transformed phenotypes through a variety of mechanisms. Here, we found that O-GlcNAc directly modified the NF-κB p65 subunit and upstream activating kinases IKKα and β in PDAC cells. Lowering hyper-O-GlcNAcylation decreased IKKβ expression and attenuated p65 activating phosphorylation (S536), nuclear translocation, NF-κB transcriptional activity, and protein levels of NF-κB transcriptional targets cyclin D1, Bcl-xL, and Vimentin, whereas elevating O-GlcNAc increased IKKα and p65 O-GlcNAcylation accompanied by increased IKK and p65 activating phosphorylation, p65 nuclear localization, and NF-κB transcriptional activity. It has been reported that in p53 knockdown cells, glycolysis is up-regulated and IKKβ O-GlcNAc modification at S733 enhances IKKβ kinase activity by preventing an inhibitory phosphorylation event (42). We also observe IKKβ O-GlcNAc modification in PDAC cells, however, global elevation of O-GlcNAc did not lead to increased IKKβ O-GlcNAcylation, but did elevate IKKα and p65 O-GlcNAcylation, suggesting that O-GlcNAc in PDAC cells is dynamically cycling on IKKα and p65, but not on IKKβ. Based on conservation of the IKKβ S733 O-GlcNAc site in IKKα, PDAC O-GlcNAcylation on IKKα might increase its kinase activity. Consistent with this, we observed increased activating IKK phosphorylation, which may contribute to increased PDAC p65 S536 activating phosphorylation. We began to examine p65 site-specific roles for O-GlcNAc in PDAC growth. Compared with wild type p65, a p65 mutant lacking two O-GlcNAc modification sites displayed reduced p65-dependent soft agar colony formation. Thus, direct O-GlcNAcylation of p65 contributes to p65-induced soft agar growth of BxPC-3 cells.
NF-κB is constitutively activated in many cancers, including PDAC (12, 13), however the mechanism underlying constitutive NF-κB activity is not fully understood. Multiple mechanisms have been proposed to explain constitutive activity. Evidence from several studies showed that persistently activated NF-κB activity could be derived from increased IKK activity (13, 43, 44) and from post-translational modification of p65 (e.g. acetylation on K310) (45). Our results suggest that hyper-O-GlcNAcylation could be an explanation for constitutive NF-κB activity in PDAC. Furthermore, constitutive activity of NF-κB has been well documented to support PDAC cell survival, as inhibiting the constitutive activity of NF-κB causes the death of PDAC cells via triggering apoptosis (13, 44, 46). In addition, NF-κB activation by overexpressing p65 in immortalized HaCaT keratinocytes protects against cell death induced by suspension culture (47). Given that reducing hyper-O-GlcNAcylation inhibits oncogenic NF-κB activity and causes the induction of apoptosis and that increasing O-GlcNAc up-regulates NF-κB activity and protects BxPC-3 cells from anoikis, elevated O-GlcNAcylation contributing to pancreatic cancer cell survival likely acts in part through up-regulation of oncogenic NF-κB activity.
In summary, we have shown that metabolic reprogramming and deregulated expression of OGT and OGA in PDAC leads to hyper-O-GlcNAcylation and that hyper-O-GlcNAcylation plays an important role in PDAC cell survival and constitutive NF-κB activity. PDAC cells are selectively susceptible to apoptosis in response to targeting OGT and reduction of O-GlcNAc, while growth of non-transformed pancreatic epithelial cells is not affected. Thus, similar to “oncogene addiction” in many cancers, PDAC cells display an “addiction” to hyper-O-GlcNAcylation. Therefore, suppression of hyper-O-GlcNAcylation by targeting OGT may serve as a novel therapeutic intervention in PDAC.
Supplementary Material
Acknowledgments
We thank Dr. Ben Stanger's laboratory (University of Pennsylvania, Philadelphia, PA) for technical support for the orthotopic mouse model. We also thank Dr. Anil K. Rustgi (University of Pennsylvania, Philadelphia, PA) for providing the pancreatic tissue slides. Dr. Lehua Deng is thanked for the synthesis of compounds.
This work was supported in part by grants from the Natural Sciences and Engineering Research Council of Canada.

This article contains supplemental methods, references, and supplemental Figs. S1–S6.
- HBP
- hexosamine biosynthetic pathway
- GlcNAc
- N-acetylglucosamine
- PDAC
- pancreatic ductal adenocarcinoma
- OGA
- O-GlcNAcase
- HPDE
- human pancreatic duct epithelial cell.
REFERENCES
- 1. Warburg O. (1956) On the origin of cancer cells. Science 123, 309–314 [DOI] [PubMed] [Google Scholar]
- 2. Kroemer G., Pouyssegur J. (2008) Tumor cell metabolism: cancer's Achilles' heel. Cancer Cell 13, 472–482 [DOI] [PubMed] [Google Scholar]
- 3. Vander Heiden M. G., Cantley L. C., Thompson C. B. (2009) Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 324, 1029–1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yuneva M., Zamboni N., Oefner P., Sachidanandam R., Lazebnik Y. (2007) Deficiency in glutamine but not glucose induces MYC-dependent apoptosis in human cells. J. Cell Biol. 178, 93–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. DeBerardinis R. J. (2008) Is cancer a disease of abnormal cellular metabolism? New angles on an old idea. Genet. Med. 10, 767–777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hart G. W., Slawson C., Ramirez-Correa G., Lagerlof O. (2011) Cross talk between O-GlcNAcylation and phosphorylation: roles in signaling, transcription, and chronic disease. Annu. Rev. Biochem. 80, 825–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wells L., Vosseller K., Hart G. W. (2003) A role for N-acetylglucosamine as a nutrient sensor and mediator of insulin resistance. Cell Mol. Life Sci. 60, 222–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Caldwell S. A., Jackson S. R., Shahriari K. S., Lynch T. P., Sethi G., Walker S., Vosseller K., Reginato M. J. (2010) Nutrient sensor O-GlcNAc transferase regulates breast cancer tumorigenesis through targeting of the oncogenic transcription factor FoxM1. Oncogene 29, 2831–2842 [DOI] [PubMed] [Google Scholar]
- 9. Lynch T. P., Ferrer C. M., Jackson S. R., Shahriari K. S., Vosseller K., Reginato M. J. (2012) Critical Role of O-Linked β-N-Acetylglucosamine Transferase in Prostate Cancer Invasion, Angiogenesis, and Metastasis. J. Biol. Chem. 287, 11070–11081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Karin M., Greten F. R. (2005) NF-κB: linking inflammation and immunity to cancer development and progression. Nat. Rev. Immunol. 5, 749–759 [DOI] [PubMed] [Google Scholar]
- 11. Karin M. (2006) Nuclear factor-κB in cancer development and progression. Nature 441, 431–436 [DOI] [PubMed] [Google Scholar]
- 12. Wang W., Abbruzzese J. L., Evans D. B., Larry L., Cleary K. R., Chiao P. J. (1999) The nuclear factor-κB RelA transcription factor is constitutively activated in human pancreatic adenocarcinoma cells. Clin. Cancer Res. 5, 119–127 [PubMed] [Google Scholar]
- 13. Liptay S., Weber C. K., Ludwig L., Wagner M., Adler G., Schmid R. M. (2003) Mitogenic and antiapoptotic role of constitutive NF-κB/Rel activity in pancreatic cancer. Int. J. Cancer 105, 735–746 [DOI] [PubMed] [Google Scholar]
- 14. Siegel R., Naishadham D., Jemal A. (2012) Cancer statistics, 2012. CA Cancer J. Clin. 62, 10–29 [DOI] [PubMed] [Google Scholar]
- 15. Guttridge D. C., Albanese C., Reuther J. Y., Pestell R. G., Baldwin A. S., Jr. (1999) NF-κB controls cell growth and differentiation through transcriptional regulation of cyclin D1. Mol. Cell Biol. 19, 5785–5799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kawauchi K., Araki K., Tobiume K., Tanaka N. (2008) p53 regulates glucose metabolism through an IKK-NF-κB pathway and inhibits cell transformation. Nat. Cell Biol. 10, 611–618 [DOI] [PubMed] [Google Scholar]
- 17. James L. R., Tang D., Ingram A., Ly H., Thai K., Cai L., Scholey J. W. (2002) Flux through the hexosamine pathway is a determinant of nuclear factor κB- dependent promoter activation. Diabetes 51, 1146–1156 [DOI] [PubMed] [Google Scholar]
- 18. Yang W. H., Park S. Y., Nam H. W., Kim do H., Kang J. G., Kang E. S., Kim Y. S., Lee H. C., Kim K. S., Cho J. W. (2008) NFκB activation is associated with its O-GlcNAcylation state under hyperglycemic conditions. Proc. Natl. Acad. Sci. U.S.A. 105, 17345–17350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Comer F. I., Vosseller K., Wells L., Accavitti M. A., Hart G. W. (2001) Characterization of a mouse monoclonal antibody specific for O-linked N-acetylglucosamine. Anal. Biochem. 293, 169–177 [DOI] [PubMed] [Google Scholar]
- 20. Macauley M. S., Whitworth G. E., Debowski A. W., Chin D., Vocadlo D. J. (2005) O-GlcNAcase uses substrate-assisted catalysis: kinetic analysis and development of highly selective mechanism-inspired inhibitors. J. Biol. Chem. 280, 25313–25322 [DOI] [PubMed] [Google Scholar]
- 21. Gloster T. M., Zandberg W. F., Heinonen J. E., Shen D. L., Deng L., Vocadlo D. J. (2011) Hijacking a biosynthetic pathway yields a glycosyltransferase inhibitor within cells. Nature Chemical Biol. 7, 174–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fan Y., Mao R., Zhao Y., Yu Y., Sun W., Song P., Shi Z., Zhang D., Yvon E., Zhang H., Fu S., Yang J. (2009) Tumor necrosis factor-α induces RelA degradation via ubiquitination at lysine 195 to prevent excessive nuclear factor-κB activation. J. Biol. Chem. 284, 29290–29297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Furukawa T., Duguid W. P., Rosenberg L., Viallet J., Galloway D. A., Tsao M. S. (1996) Long-term culture and immortalization of epithelial cells from normal adult human pancreatic ducts transfected by the E6E7 gene of human papilloma virus 16. Am. J. Pathol. 148, 1763–1770 [PMC free article] [PubMed] [Google Scholar]
- 24. Wice B. M., Trugnan G., Pinto M., Rousset M., Chevalier G., Dussaulx E., Lacroix B., Zweibaum A. (1985) The intracellular accumulation of UDP-N-acetylhexosamines is concomitant with the inability of human colon cancer cells to differentiate. J. Biol. Chem. 260, 139–146 [PubMed] [Google Scholar]
- 25. Robinson K. A., Weinstein M. L., Lindenmayer G. E., Buse M. G. (1995) Effects of diabetes and hyperglycemia on the hexosamine synthesis pathway in rat muscle and liver. Diabetes 44, 1438–1446 [DOI] [PubMed] [Google Scholar]
- 26. Rhim A. D., Mirek E. T., Aiello N. M., Maitra A., Bailey J. M., McAllister F., Reichert M., Beatty G. L., Rustgi A. K., Vonderheide R. H., Leach S. D., Stanger B. Z. (2012) EMT and dissemination precede pancreatic tumor formation. Cell 148, 349–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Duxbury M. S., Ito H., Zinner M. J., Ashley S. W., Whang E. E. (2004) CEACAM6 gene silencing impairs anoikis resistance and in vivo metastatic ability of pancreatic adenocarcinoma cells. Oncogene 23, 465–473 [DOI] [PubMed] [Google Scholar]
- 28. Maier H. J., Schmidt-Strassburger U., Huber M. A., Wiedemann E. M., Beug H., Wirth T. (2010) NF-κB promotes epithelial-mesenchymal transition, migration and invasion of pancreatic carcinoma cells. Cancer Lett. 295, 214–228 [DOI] [PubMed] [Google Scholar]
- 29. Varadhachary G. R., Tamm E. P., Abbruzzese J. L., Xiong H. Q., Crane C. H., Wang H., Lee J. E., Pisters P. W., Evans D. B., Wolff R. A. (2006) Borderline resectable pancreatic cancer: definitions, management, and role of preoperative therapy. Ann. Surg. Oncol. 13, 1035–1046 [DOI] [PubMed] [Google Scholar]
- 30. Wolff R. A. (2007) Chemotherapy for pancreatic cancer: from metastatic disease to adjuvant therapy. Cancer J. 13, 175–184 [DOI] [PubMed] [Google Scholar]
- 31. Schneiderhan W., Scheler M., Holzmann K. H., Marx M., Gschwend J. E., Bucholz M., Gress T. M., Seufferlein T., Adler G., Oswald F. (2009) CD147 silencing inhibits lactate transport and reduces malignant potential of pancreatic cancer cells in in vivo and in vitro models. Gut 58, 1391–1398 [DOI] [PubMed] [Google Scholar]
- 32. Ito H., Duxbury M., Zinner M. J., Ashley S. W., Whang E. E. (2004) Glucose transporter-1 gene expression is associated with pancreatic cancer invasiveness and MMP-2 activity. Surgery 136, 548–556 [DOI] [PubMed] [Google Scholar]
- 33. Maher J. C., Savaraj N., Priebe W., Liu H., Lampidis T. J. (2005) Differential sensitivity to 2-deoxy-D-glucose between two pancreatic cell lines correlates with GLUT-1 expression. Pancreas 30, e34–39 [DOI] [PubMed] [Google Scholar]
- 34. Wu M. C., Arimura G. K., Yunis A. A. (1978) Mechanism of sensitivity of cultured pancreatic carcinoma to asparaginase. Int. J. Cancer 22, 728–733 [DOI] [PubMed] [Google Scholar]
- 35. Kaadige M. R., Looper R. E., Kamalanaadhan S., Ayer D. E. (2009) Glutamine-dependent anapleurosis dictates glucose uptake and cell growth by regulating MondoA transcriptional activity. Proc. Natl. Acad. Sci. U.S.A. 106, 14878–14883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ying H., Kimmelman A. C., Lyssiotis C. A., Hua S., Chu G. C., Fletcher-Sananikone E., Locasale J. W., Son J., Zhang H., Coloff J. L., Yan H., Wang W., Chen S., Viale A., Zheng H., Paik J. H., Lim C., Guimaraes A. R., Martin E. S., Chang J., Hezel A. F., Perry S. R., Hu J., Gan B., Xiao Y., Asara J. M., Weissleder R., Wang Y. A., Chin L., Cantley L. C., DePinho R. A. (2012) Oncogenic Kras maintains pancreatic tumors through regulation of anabolic glucose metabolism. Cell 149, 656–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shi Y., Tomic J., Wen F., Shaha S., Bahlo A., Harrison R., Dennis J. W., Williams R., Gross B. J., Walker S., Zuccolo J., Deans J. P., Hart G. W., Spaner D. E. (2010) Aberrant O-GlcNAcylation characterizes chronic lymphocytic leukemia. Leukemia 24, 1588–1598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Krzelak A., Forma E., Bernaciak M., Romanowicz H., Bry M. (2012) Gene expression of O-GlcNAc cycling enzymes in human breast cancers. Clin. Exp. Med. 12, 61–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mi W., Gu Y., Han C., Liu H., Fan Q., Zhang X., Cong Q., Yu W. (2011) O-GlcNAcylation is a novel regulator of lung and colon cancer malignancy. Biochim. Biophys. Acta 1812, 514–519 [DOI] [PubMed] [Google Scholar]
- 40. Zhu Q., Zhou L., Yang Z., Lai M., Xie H., Wu L., Xing C., Zhang F., Zheng S. (2012) O-GlcNAcylation plays a role in tumor recurrence of hepatocellular carcinoma following liver transplantation. Medical Oncology 29, 985–993 [DOI] [PubMed] [Google Scholar]
- 41. Park S. Y., Kim H. S., Kim N. H., Ji S., Cha S. Y., Kang J. G., Ota I., Shimada K., Konishi N., Nam H. W., Hong S. W., Yang W. H., Roth J., Yook J. I., Cho J. W. (2010) Snail1 is stabilized by O-GlcNAc modification in hyperglycaemic condition. EMBO J. 29, 3787–3796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kawauchi K., Araki K., Tobiume K., Tanaka N. (2009) Loss of p53 enhances catalytic activity of IKKβ through O-linked β-N-acetyl glucosamine modification. Proc. Natl. Acad. Sci. U.S.A. 106, 3431–3436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ahmad R., Raina D., Trivedi V., Ren J., Rajabi H., Kharbanda S., Kufe D. (2007) MUC1 oncoprotein activates the IκB kinase β complex and constitutive NF-κB signalling. Nat. Cell Biol. 9, 1419–1427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wilson W., 3rd, Baldwin A. S. (2008) Maintenance of constitutive IκB kinase activity by glycogen synthase kinase-3α/β in pancreatic cancer. Cancer Res. 68, 8156–8163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lee H., Herrmann A., Deng J. H., Kujawski M., Niu G., Li Z., Forman S., Jove R., Pardoll D. M., Yu H. (2009) Persistently activated Stat3 maintains constitutive NF-κB activity in tumors. Cancer Cell 15, 283–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kong R., Sun B., Jiang H., Pan S., Chen H., Wang S., Krissansen G. W., Sun X. (2010) Downregulation of nuclear factor-κB p65 subunit by small interfering RNA synergizes with gemcitabine to inhibit the growth of pancreatic cancer. Cancer Lett. 291, 90–98 [DOI] [PubMed] [Google Scholar]
- 47. Ren Q., Kari C., Quadros M. R., Burd R., McCue P., Dicker A. P., Rodeck U. (2006) Malignant transformation of immortalized HaCaT keratinocytes through deregulated nuclear factor κB signaling. Cancer Res. 66, 5209–5215 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.