Abstract
The ERK MAP kinase signaling cascade plays critical roles in brain development, learning, memory, and cognition. It has recently been appreciated that mutation or deletion of elements within this signaling pathway leads to developmental syndromes in humans that are associated with impaired cognitive function and autism. Here, we review recent studies that provide insight into the biological roles of the ERKs in the brain that may underlie the cognitive deficits seen in these syndromes.
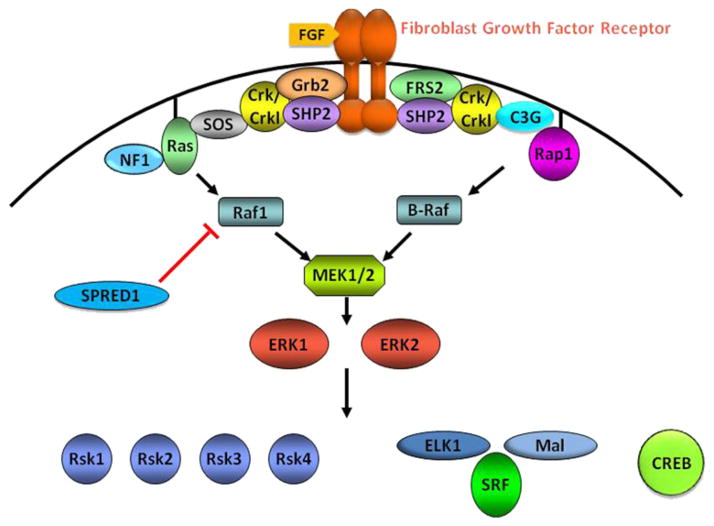
The extracellular signal regulated kinase (ERK) subfamily of mitogen-activated protein (MAP) kinases comprises the central elements of one of the most important and best-studied intracellular signaling pathways (Rubinfeld and Seger, 2005). This pathway mediates the transmission of signals from cell surface receptors to cytoplasmic and nuclear effectors. Indeed, the ERKs are activated in response to extracellular stimuli that target a broad array of receptors (Rubinfeld and Seger, 2005). These receptors are physically and functionally linked to the ERK cascade through a diverse group of molecular adapters that couple them to activation of the GTPases of the Ras family, Ras and Rap1 (Figure 1). Ligand binding results in the conversion of Ras-related GTPases into an active conformation, enabling them to interact with and promote the activation of the raf kinases, which initiate downstream signaling through the ERK cascade. The core elements of the ERK signaling pathway comprise a three-tiered protein kinase cascade including the raf kinases (B-Raf and Raf1) that phosphorylate and activate the MAP kinase kinases, MEK1/2, which in turn activate ERK1/2 (Rubinfeld and Seger, 2005). The ERKs phosphorylate a host of proteins and activate other protein kinases (RSK1–4, MSK1/2, MNK1/2) that are responsible for the regulation of transcription and translation. The ERKs and their immediate targets, the RSKs, are translocated into the nucleus upon activation, allowing the phosphorylation of transcription factors that mediate the direct regulation of gene expression.
Figure 1.
The ERK Signaling Cascade
ERK1/2 share 84% sequence identity but little is known about their isoform-specific actions. Both isoforms are ubiquitously expressed, are coordinately activated, and have identical substrate specificity. The relative expression of ERK2 is greater than that of ERK1, although both are expressed throughout the adult brain. The ERKs are most highly expressed in neurons, compared to all other cell types.
In the nervous system, the ERKs are involved in processes as diverse as the genesis of neural progenitors, learning, and memory. During development the ERKs respond principally to growth factors through the activation of receptor tyrosine kinases. In the mature nervous system, the ERKs are activated in neurons in response to synaptic activity (Davis and Laroche, 2006). Recently, mutations within elements of the ERK signaling cascade have been associated with a number of clinical syndromes that have been collectively termed “neuro-cardio-facial-cutaneous (NCFC) syndromes” (Bentires-Alj et al., 2006). Change in gene copy number of ERK signaling elements has also been described for other clinical phenotypes. New studies have identified individuals with microdeletions encompassing the MAPK1 gene that encodes ERK2 in distal chromosome 22q11 who exhibit cognitive deficits and features of the Di-George syndrome (DGS) spectrum (Shaikh et al., 2007). Further, a deletion of a locus on chromosome 16 that includes the MAPK3 gene encoding ERK1 has been associated with autism and craniofacial anomalies (Kumar et al., 2008; Weiss et al., 2008). These syndromes frequently include CNS developmental abnormalities and functional deficits. Collectively, these findings suggest that genetic alterations involving elements of the ERK signaling cascade are a significant cause of neurodevelopmental and cognitive disorders. A number of recent studies have shed new light on the actions of the ERKs both in neural development and in the mature brain, providing insight into the biological basis of the deficits observed in humans in which signaling through the ERK pathway has been perturbed.
Clinical Syndromes Associated with Altered ERK Signaling
NCFC Syndromes
A group of related syndromes are characterized by cardiac and craniofacial abnormalities and include Costello, Noonan, LEOPARD, and cardio-facio-cutaneous (CFC) syndromes. It has recently been recognized that the mutated genes act within a common genetic pathway regulating ERK activation (Figure 1) (Aoki et al., 2008; Denayer et al., 2008). Importantly, similar phenotypes arise from both gain of function and loss of function of a single allele of elements of the ERK pathway (Table 1). Individuals with mutations associated with these syndromes can have cardiac defects including pulmonic valve stenosis, arrhythmia, and cardiomyopathy. While the cardiac and craniofacial defects in these disorders have been well documented, the CNS manifestations of these disorders have not been extensively studied and less is known about the developmental basis of this part of the phenotype. Noonan syndrome (NS) results from point mutations in genes encoding upstream elements of the ERK cascade, namely SHP2, K-Ras, SOS1, and Raf1 (Gelb and Tartaglia, 2006). This syndrome is phenotypically diverse, and can include developmental delays and behavioral and learning disabilities (Lee et al., 2005). Mental retardation is reported in approximately 25% of patients (Yoshida et al., 2004), but normal cognitive development is reported as well (Tartaglia et al., 2002). Classical features include pulmonic stenosis, short stature, and facial dysmorphia. Approximately half of individuals with NS show gain-of-function mutations in the PTPN11 gene, which encodes SHP2 (Tartaglia et al., 2002). SHP2 plays critical roles in fibroblast growth factor receptor (FGFR) and Trk signaling to the ERKs. Germline mutations in SOS1 have been found in approximately 10% of NS patients, while RAF1 and K-RAS account for about 4%–15% and 1%–2% of mutations, respectively. LEOPARD syndrome (lentigines, EKG abnormalities, ocular hypertelorism, pulmonic stenosis, abnormal genitalia, retardation of growth, and deafness) arises from loss-of-function mutations in PTPN11 and K-RAS that are distinct from those associated with NS, though there is clinical overlap between the two disorders (Bentires-Alj et al., 2006). Two recent reports of gain-of-function mutations in RAF1 were shown to cause Noonan and LEOPARD syndromes, and were associated with increased ERK activation (Pandit et al., 2007; Razzaque et al., 2007). CFC has recently been linked to mutations in K-Ras, B-Raf, and MEK1/2 (Roberts et al., 2006). In CFC syndrome, mental retardation and developmental delay is found in over 80% of cases. These patients exhibit structural CNS anomalies, most prominently reduced cerebral volume and hypoplasia of the frontal lobe and cerebellum. Costello syndrome is associated with activating mutations of H-RAS and has a clinical profile similar to CFC (Rauen, 2007; Roberts et al., 2006). The neurological manifestations of Costello syndrome include frontal lobe atrophy (40% of patients), cerebellar abnormalities (26% of patients), and significant mental retardation (Delrue et al., 2003).
Table 1.
Developmental Disorders with Altered ERK Pathway Signaling
| Syndrome | Mutated Gene | ERK Signaling (Gain/Loss of Function) | Intellectual Impairment |
|---|---|---|---|
| Costello | H-RAS | + | moderate |
| Cardio-facio-cutaneous | K-RAS | + | moderate-severe |
| B-RAF | + and − | moderate-severe | |
| MEK1 | + | moderate-severe | |
| MEK2 | + | moderate-severe | |
| Noonan | PTPN11/ SHP2 | + | variable |
| K-RAS | + | variable | |
| SOS1 | + | variable | |
| LEOPARD | PTPN11/ SHP2 | − | normal-mild |
| RAF1 | + | normal-mild | |
| DiGeorge/22q11 deletion | TBX1−→FGF8 | − | mild-moderate |
| CRKL | − | mild-moderate | |
| Distal 22q11 deletion | ERK2 | − | mild-moderate |
| Neurofibromatosis type 1 | NF1 (mut) | + | normal-moderate |
| NF1 (del) | + | moderate | |
| NF1-like syndrome | SPRED1 | + | mild |
| Autism (del/ dup 16p11.2) | ERK1 | + and − | variable |
| Coffin-Lowry | RSK2 | (−) | severe |
| X-linked mental retardation | RSK4 | (−) | severe |
Neurofibromatosis type 1 (NF1) arises from mutation or deletion of the NF1 gene encoding neurofibromin, a Ras GTPase activating protein that acts as a negative regulator of ERK activation (Ferner, 2007). Over half of individuals with NF1 mutations have intellectual impairment. Individuals with deletions encompassing the NF1 gene exhibit a more severe phenotype and more profound intellectual impairment (Descheemaeker et al., 2004) with craniofacial abnormalities (Leppig et al., 1997). The bases of the impaired neurocognitive functions have been argued to arise from defects in corticogenesis. NF1 is associated with macrocephaly, and murine models with analogous NF1 mutations or deletions exhibit increased proliferation of neural progenitors during embryogenesis that are correlated with ERK activation (Hegedus et al., 2007). However, recent work by Cui et al. (2008) have demonstrated an ongoing requirement for neuronal NF1 and ERK activity in long-term potentiation (LTP), learning, and memory. They reported that loss of NF1 is associated with an enhancement of ERK-dependent GABA release, which contributes to cognitive impairment in murine models of this disorder.
Recently a new syndrome with a similar clinical presentation has been described, termed NF1-like syndrome, which results from loss-of-function mutations in the SPRED1 gene. The SPRED1 gene product normally acts to suppress ERK activation, as does NF1 (Brems et al., 2007). Thus, loss-of-function mutations in NF1 or SPRED1 result in elevation of ERK pathway activation. These individuals are also cognitively impaired and macrocephalic and exhibit facial dysmorphism similar to other NCFC syndromes.
In addition, two genes that are direct targets of the ERK cascade are associated with X-linked mental retardation. Coffin-Lowry syndrome results from mutations at Xp22.2, resulting in loss of function of RSK2 that is associated with severe mental retardation (Yntema et al., 1999). Similarly, deletions at Xq21 that include the RSK4 gene result in a similar phenotype with profound cognitive impairment (Shalin et al., 2006).
DGS (22q11 Deletion syndrome) is the most common (1/3000) microdeletion syndrome in humans. DGS is most frequently associated with loss of a 3 Mb region in chromosome 22q11 (Lindsay, 2001; Scambler, 2000) and has clinical features distinct from the NCFC disease spectrum. DGS is characterized by conotruncal cardiac defects and craniofacial anomalies that may arise from the combinatorial effects of deleting multiple (330) genes. Importantly, two genes (TBX1 and CRKL) that are both deleted in the common 3 Mb deletion of DGS act within a genetic pathway that regulates ERK1/2 signaling in neural crest cells (Frank et al., 2002; Guris et al., 2006; Moon et al., 2006). TBX1 acts to regulate FGF8 expression (Wurdak et al., 2006), and CRKL is an adaptor protein linking the FGFRs (and Trk receptors) to the ERK cascade (Figure 1). The neurological features of DGS have been less intensively studied until recently (Antshel et al., 2005; Schaer et al., 2006). There is a high incidence (45%) of associated psychiatric disease; most commonly, this is bipolar illness and schizophrenia. Imaging studies have revealed that DGS patients exhibit an approximate 10% decrease in overall brain volume (Schaer et al., 2006), with a similar loss of hippocampal volume (Debbane et al., 2006; Deboer et al., 2007), reduction in cortical thickness, decreased cortical gyrification, and cerebellar hypoplasia. Simon and colleagues recently reported hypomorphic fronto-parietal cortical white matter tracts in a group of DGS patients that they associate with nonverbal cognitive impairment (Simon et al., 2008).
The ERK2 gene, MAPK1, is located on chromosome 22, at a position distal to the 3 Mb DGS region. A number of unrelated patients have been identified with deletions of this distal region of chromosome 22q11 that include the MAPK1 gene, but which do not physically overlap the DGS deletion (Ben-Shachar et al., 2008; Rauch et al., 1999; Saitta et al., 1999). Importantly, patients with distal deletions independently exhibit a spectrum of craniofacial abnormalities, cardiac defects, and neurodevelopmental deficits. These findings suggest that haploinsufficiency for different elements of the MAPK cascade can result in similar anatomic and intellectual manifestations, implying sensitivity to dosage of various elements of the ERK signaling cascade.
In early 2008 there were two independent reports linking approximately 1% of all cases of autism with deletions or duplications of a 593 kb region on chromosome 16p11.2. This locus encompasses the MAPK3 gene that encodes ERK1 (Kumar et al., 2008; Weiss et al., 2008). Remarkably, these individuals exhibited dysmorphic facial features and congenital heart defects that were noted to be similar to those observed in DGS (Ballif et al., 2007; Ghebranious et al., 2007). While autism is not a common feature of NCFC syndromes, there are reports of autism in DGS patients and in those with other syndromes.
Developmental Roles of the ERKs
The discovery of the linkage of the ERK pathway to a number of CNS syndromes in humans has served to refocus attention on the underlying actions of these molecules. Remarkably little is known about the biology of the ERKs in vivo, and indeed many assumptions about their roles have been contravened by new data from animals in which the ERKs have been knocked out. Given the importance of these enzymes, it was surprising that genetic inactivation of the ERK1 gene, Mapk3, did not result in an overt phenotype. Knockout of the ERK2 gene, Mapk1, however, resulted in early embryonic lethality, due principally to failed placental/trophoblast development (Aouadi et al., 2006). The recent generation of conditional ERK2 knockouts has provided new insight into the biology of these enzymes. One of the central unanswered questions in understanding the roles of the ERKs in CNS function is to what degree the functional impairments result from abnormalities in development or, alternatively, reflect an ongoing requirement for ERK activity in synaptic transmission, memory formation, and cognitive function.
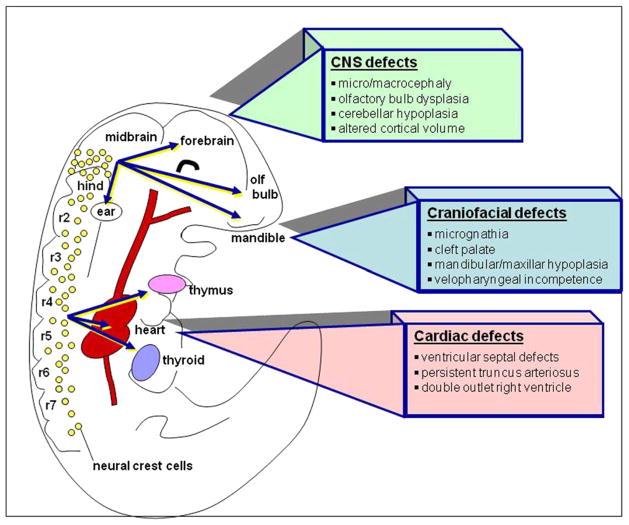
Seminal studies in the mouse (Corson et al., 2003), chick (Lunn et al., 2007), Xenopus (Christen and Slack, 1999), and zebrafish (Shinya et al., 2001) reported the rather surprising finding that activated forms of ERK1/2 were spatially restricted to regions of the embryo that were developing under the influence of FGF, and that ERK activation could be blocked by pharmacological inhibition of FGFR function. Prominent regions that develop in response to FGF action include the branchial arches, migrating neural crest cells, the midbrain/hindbrain boundary, and developing forebrain (Figure 2). These observations appeared counterintuitive, based on a vast literature describing the broad actions of the ERKs in conveying signals from the majority of cell surface receptors. These data provided the first evidence that in vivo these enzymes might direct development in a highly selective manner within circumscribed populations of progenitors. The linkage of ERK activation to FGF signaling has proven to be a key finding in explaining their developmental roles.
Figure 2.
ERK Activity Is Necessary for the Normal Development of Neural Crest and the Central Nervous System
A prominent feature of human syndromes related to mutations in ERK signaling pathways is the coincidence of CNS, cardiac, and craniofacial phenotypes. This coincidence arises from the fact that a subset of neuroepithelial derivatives that are targets of ERK signaling, neural crest cells, contribute not only to development of the nervous system, but to morphogenesis of the heart, cranium, and face (Figure 2).
ERK1/2 in Neural Crest Development
Gail Martin and colleagues demonstrated the essential actions of the FGFs in development through genetic regulation of FGF8 expression within the neural crest and neural tube (Meyers et al., 1998; Storm et al., 2006). Reduction in FGF8 levels resulted in the failure to generate a normal complement of neural crest cells, leading to severe cardiac and craniofacial defects (Meyers et al., 1998). The phenotypic severity was directly related to the levels of FGF8. Importantly, disruption of FGF8 expression in the developing neural tube dramatically affected normal CNS patterning, leading to the failure to develop a cerebellum, olfactory bulb, and a normal cerebral cortex (Chi et al., 2003). These morphogenetic events have subsequently been shown to be reliant upon ERK signaling (Kawauchi et al., 2005). Recently, Newbern and colleagues have reported that genetic inactivation of ERK2 in the developing neural crest results in cardiac abnormalities and craniofacial defects similar to those observed in the FGF8 hypomorphs and analogous to phenotypes observed in humans haploinsufficient for the MAPK1 gene encoding ERK2 (Newbern et al., 2008). Mice in which both ERK isoforms were inactivated in the developing neural crest displayed a more severe phenotype. Significantly, similar phenotypes were observed upon conditional knockout of upstream elements of the ERK cascade, B-Raf/Raf1 and MEK1/2, as well as knockout of the transcription factor SRF, which is a direct downstream target of ERK signaling (Newbern et al., 2008). These data provide clear evidence that signaling through the ERK cascade is essential for normal neural crest development.
ERK1/2 in Cortical Development
The actions of the ERKs in the developing brain have only recently been examined and have revealed a critical role for ERK2 in cortical development. The first study in which ERK2 was conditionally knocked out of the developing brain has provided new insight into the biological actions of this enzyme. Inactivation of ERK2 within cortical neural progenitor cells early in the neurogenic period results in a thinner, but normally organized, cortex. The ERK2-deficient mutant cortex contains fewer neurons but many more astrocytes (Samuels et al., 2008). The neuronal loss was the result of selective suppression of division intermediate progenitor cells. The intermediate progenitor cells are a recently recognized subclass of neural progenitors positioned within the subventricular zone that undergo symmetric terminal divisions to generate neurons that then populate all layers of the developing cortex (Pontious et al., 2008). Previous work had documented a clear requirement for ERK activity in FGF-stimulated cortical progenitor differentiation and neurogenesis (Menard et al., 2002). The cortical phenotypes in the ERK2 conditional knockout mice are consistent with the action of the ERKs in conveying FGF signals. FGFs play critical roles in cortical development and there is good evidence demonstrating ERK activity as a primary effector of growth factor action. Reduction of FGF8 expression suppresses neural progenitor proliferation and increases cell death, resulting in abnormal cortical structure and patterning (Meyers et al., 1998; Storm et al., 2006). Similarly, FGF2 null animals exhibit impaired neural progenitor proliferation, resulting in a 40% reduction in cortical neurons in the mature frontal and parietal cortex (Zheng et al., 2004). Analogous phenotypes are observed upon inactivation of FGFRs (Shin et al., 2004). Conversely, exogenous application of FGF2 (Vaccarino et al., 1999) or expression of a constitutively active FGFR3 (Inglis-Broadgate et al., 2005) resulted in increased numbers of neurons and cortical volume due to stimulation of neural progenitor proliferation as a direct result of enhanced ERK signaling (Thomson et al., 2007).
Compelling evidence supporting a role for the ERKs in cortical neural progenitor proliferation and differentiation has come from analyzing the effects of mutations of the scaffolding molecules FRS2 and SHP2 (Figure 1). FRS2 is an essential adaptor protein linking both the FGFRs and Trks to activation of the ERKs. This linkage is achieved through receptor-dependent phosphorylation of FRS2 that allows the formation of a signaling complex with SHP2 that is necessary for ERK pathway activation (Hadari et al., 2001). Mice that express mutant forms of FRS2 exhibit smaller brains, with an approximate 30% reduction in cortical thickness and fewer neurons (Yamamoto et al., 2005). This effect was shown to be a consequence of dramatically reduced levels of ERK activation, specifically resulting in reduced numbers of proliferating intermediate progenitor cells in the subventricular zone. These cells have recently entered the spotlight as they are believed to underlie the evolutionary expansion in size and the increased surface area and gyrification of the cortex (Pontious et al., 2008). The mutant FRS2 phenotype is very similar to that observed in mice in which ERK2 was conditionally inactivated during cortical neurogenesis (Samuels et al., 2008). Significantly, Freda Miller’s lab has recently shown that deletion or inactivation of the adaptor molecule SHP2, which is mutated in Noonan and LEOPARD syndromes, in cortical progenitors was found to inhibit neurogenesis and promote precocious astrocyte generation through ERK-dependent mechanisms (Gauthier et al., 2007). Conversely, SHP2 gain-of-function mutations led to generation of supernumerary neurons and inhibition of gliogenesis. Conditional inactivation of B-Raf in neural progenitors resulted in reduced cortical thickness in the postnatal brain (Zhong et al., 2007) and hypomyelination (Galabova-Kovacs et al., 2008) phenotypes, which were both associated with reduced ERK activation.
ERKs in Cellular Survival
It has been widely accepted that the ERKs play essential roles in neuronal survival, owing to seminal studies by Greenberg and colleagues (Xia et al., 1995) that were subsequently verified by many other reports using in vitro models. Recent in vivo studies examining the effect of genetic inactivation of the ERKs or their upstream regulators have shown conflicting data. Conditional inactivation of ERK2 in the developing cortex did not result in apoptotic neuronal death (Samuels et al., 2008), nor was cell death observed in mice in which both ERK isoforms were inactivated (I.S.S. and G.E.L., unpublished data). These findings are consistent with studies of cortical development by Miller and colleagues in which MEK (Paquin et al., 2005) and SHP2 (Gauthier et al., 2007) are inactivated in cortical progenitor cells in vivo. The loss of either protein results in reduced ERK activity within these cells and causes them to remain undifferentiated until gliogenic stimuli induce them to differentiate into astrocytes. The resultant cortices are composed of reduced numbers of neurons due to altered fate of the progenitors, thereby defining a role for the ERKs in neuronal differentiation and cell fate determination rather than survival of these cells (Gauthier et al., 2007; Paquin et al., 2005). Similarly, conditional knockout of Raf1 and B-Raf resulted in the loss of ERK signaling leading to reduced neurogenesis (Zhong et al., 2007) and dysmyelination (Galabova-Kovacs et al., 2008), secondary to impaired progenitor proliferation, but not apoptosis. Consistent with these findings, inactivation of both ERK isoforms in B-lymphocyte lineages was associated with reduced proliferation, but not apoptosis (Yasuda et al., 2008). Thus far, studies in vivo have not supported an obligatory role for the ERKs in cellular survival.
ERKs in Memory and Learning
The ERKs are potently activated by synaptic activity, and they are essential for synaptic plasticity related to learning and memory formation in mammals and invertebrates (Davis and Laroche, 2006). Activation of neurotransmitter receptors results in the initiation of both calcium-dependent and -independent signaling mechanisms that serve to activate the Ras-related small GTPases (Thomas and Huganir, 2004). Early work by Sweatt and colleagues demonstrated an absolute requirement for ERK activity in induction of LTP (English and Sweatt, 1996), and subsequent studies have established its necessity for NMDA-dependent and -independent forms of LTP induction and maintenance. The molecular basis of these effects is the subject of intense interest and a number of mechanisms have been investigated including ERK-dependent regulation of AMPA receptor insertion into the postsynaptic membrane, physical remodeling and generation of dendritic spines, Kv4.2 potassium channel function, and the local regulation of protein synthesis (Thomas and Huganir, 2004). Significantly, the Svoboda lab recently reported that glutamate stimulation of a single dendritic spine resulted in sustained alteration of the volume of the stimulated spine and subsequent enlargement of neighboring spines that was reliant upon ERK activation, arguing that such mechanisms underlie the development of LTP (Harvey et al., 2008). The ERKs also have newly appreciated roles in memory consolidation. It was recently reported that in the hippocampus there is circadian oscillation of ERK activation that is essential for persistent and stable memory formation (Eckel-Mahan et al., 2008), documenting another unexpected level of complexity in the actions of the ERKs in memory.
New mouse models targeting the ERKs themselves have provided additional direct evidence of their involvement in learning and memory. Previous behavioral analysis of mice treated with inhibitors of ERK activity demonstrated the importance of ERK signaling in a broad range of memory and learning tasks (Davis and Laroche, 2006). Thus, it was quite surprising that analysis of ERK1 knockout mice revealed only a rather subtle behavioral phenotype (Mazzucchelli et al., 2002; Selcher et al., 2001). Genetic inactivation of the ERK1 gene results in hyperactivity (Selcher et al., 2001), and the animals exhibit a generalized behavioral excitement phenotype with altered responses to amphetamine (Engel et al., 2008) and cocaine (Ferguson et al., 2006; Grueter et al., 2006). ERK1 knockout mice have recently been used as models for mania and bipolar disorder (Engel et al., 2008). ERK1 null mice are also reported to have a paradoxical improvement in a striatal-based long-term memory task and facilitation of LTP in the nucleus accumbens (Mazzucchelli et al., 2002) that was argued to result from compensatory actions of ERK2. The recently recognized genetic linkage of ERK1 to autism suggests that a more extensive behavioral analysis of these animals is in order.
The recent development of conditional ERK2 alleles (Samuels et al., 2008) and ERK2 hypomorphic mice (Satoh et al., 2007) has shed new light on the roles of ERK2 in memory and learning. Samuels and colleagues reported that mice in which ERK2 was knocked out in telencephalic radial glial progenitors exhibit profound deficits in associative learning in a fear conditioning assay (Samuels et al., 2008). Satoh et al. generated ERK2 hypomorphic mice that exhibit an approximate 30% reduction in ERK2 levels and an overtly normal brain (Satoh et al., 2007). Strikingly, these mice exhibit impairment in fear conditioning and deficits in two spatial memory tasks. These data suggest that even modest reduction of ERK2 function is sufficient to result in behavioral impairment. The interpretation of the behavioral phenotypes in hypomorphs, or mice in which the ERKs are inactivated during development, raises the question of whether the deficiencies arise from developmental-related structural perturbations of the brain or are reflective of the acute actions of these enzymes in synaptic function and plasticity. Analysis of mice in which ERK2 has been conditionally inactivated at later developmental times will likely provide insight into the relative actions of ERK2, and these studies are currently underway in several laboratories.
Summary
Taken together these findings argue that mammalian development is exquisitely sensitive to perturbations in signaling through the ERK pathway, and either gain of function or loss of function of a single allele of an ERK pathway gene is sufficient to result in abnormal brain development and function. The recognition that the ERKs play very specific roles in CNS development that are linked to FGF signaling events, and are inexplicably restricted to distinct populations of neural progenitors, has elucidated the novel actions of these enzymes in the morphogenesis and plasticity of the brain. Collectively, these studies underscore our primitive understanding of the biology of the ERK signaling pathway in vivo and its unexpected complexity.
Acknowledgments
We would like to thank Drs. Bruce Gelb, Steven Maricich, Karl Herrup, and David Katz for their comments on the manuscript. We apologize to those authors whose papers we were unable to cite due to space restrictions. This work was supported by grants from the NHLBI (S.C.S.), the NSF (G.E.L.), and the NINDS (I.S.S.).
References
- Antshel KM, Kates WR, Roizen N, Fremont W, Shprintzen RJ. 22q11.2 deletion syndrome: genetics, neuroanatomy and cognitive/ behavioral features keywords. Child Neuropsychol. 2005;11:5–19. doi: 10.1080/09297040590911185. [DOI] [PubMed] [Google Scholar]
- Aoki Y, Niihori T, Narumi Y, Kure S, Matsubara Y. The RAS/ MAPK syndromes: novel roles of the RAS pathway in human genetic disorders. Hum Mutat. 2008;29:992–1006. doi: 10.1002/humu.20748. [DOI] [PubMed] [Google Scholar]
- Aouadi M, Binetruy B, Caron L, Le Marchand-Brustel Y, Bost F. Role of MAPKs in development and differentiation: lessons from knockout mice. Biochimie. 2006;88:1091–1098. doi: 10.1016/j.biochi.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Ballif BC, Hornor SA, Jenkins E, Madan-Khetarpal S, Surti U, Jackson KE, Asamoah A, Brock PL, Gowans GC, Conway RL, et al. Discovery of a previously unrecognized microdeletion syndrome of 16p11.2-p12.2. Nat Genet. 2007;39:1071–1073. doi: 10.1038/ng2107. [DOI] [PubMed] [Google Scholar]
- Ben-Shachar S, Ou Z, Shaw CA, Belmont JW, Patel MS, Hummel M, Amato S, Tartaglia N, Berg J, Sutton VR, et al. 22q11.2 distal deletion: a recurrent genomic disorder distinct from DiGeorge syndrome and velocardiofacial syndrome. Am J Hum Genet. 2008;82:214–221. doi: 10.1016/j.ajhg.2007.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentires-Alj M, Kontaridis MI, Neel BG. Stops along the RAS pathway in human genetic disease. Nat Med. 2006;12:283–285. doi: 10.1038/nm0306-283. [DOI] [PubMed] [Google Scholar]
- Brems H, Chmara M, Sahbatou M, Denayer E, Taniguchi K, Kato R, Somers R, Messiaen L, De Schepper S, Fryns JP, et al. Germline loss-of-function mutations in SPRED1 cause a neurofibromatosis 1-like phenotype. Nat Genet. 2007;39:1120–1126. doi: 10.1038/ng2113. [DOI] [PubMed] [Google Scholar]
- Chi CL, Martinez S, Wurst W, Martin GR. The isthmic organizer signal FGF8 is required for cell survival in the prospective midbrain and cerebellum. Development. 2003;130:2633–2644. doi: 10.1242/dev.00487. [DOI] [PubMed] [Google Scholar]
- Christen B, Slack JM. Spatial response to fibroblast growth factor signalling in Xenopus embryos. Development. 1999;126:119–125. doi: 10.1242/dev.126.1.119. [DOI] [PubMed] [Google Scholar]
- Corson LB, Yamanaka Y, Lai KM, Rossant J. Spatial and temporal patterns of ERK signaling during mouse embryogenesis. Development. 2003;130:4527–4537. doi: 10.1242/dev.00669. [DOI] [PubMed] [Google Scholar]
- Cui Y, Costa RM, Murphy GG, Elgersma Y, Zhu Y, Gutmann DH, Parada LF, Mody I, Silva AJ. Neurofibromin regulation of ERK signaling modulates GABA release and learning. Cell. 2008;135:549–560. doi: 10.1016/j.cell.2008.09.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis S, Laroche S. Mitogen-activated protein kinase/extracellular regulated kinase signalling and memory stabilization: a review. Genes Brain Behav. 2006;5(Suppl 2):61–72. doi: 10.1111/j.1601-183X.2006.00230.x. [DOI] [PubMed] [Google Scholar]
- Debbane M, Schaer M, Farhoumand R, Glaser B, Eliez S. Hippocampal volume reduction in 22q11.2 deletion syndrome. Neuropsychologia. 2006;44:2360–2365. doi: 10.1016/j.neuropsychologia.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Deboer T, Wu Z, Lee A, Simon TJ. Hippocampal volume reduction in children with chromosome 22q11.2 deletion syndrome is associated with cognitive impairment. Behav Brain Funct. 2007;3:54. doi: 10.1186/1744-9081-3-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delrue MA, Chateil JF, Arveiler B, Lacombe D. Costello syndrome and neurological abnormalities. Am J Med Genet A. 2003;123:301–305. doi: 10.1002/ajmg.a.20330. [DOI] [PubMed] [Google Scholar]
- Denayer E, de Ravel T, Legius E. Clinical and Molecular Aspects of RAS-related disorders. J Med Genet. 2008;45:695–703. doi: 10.1136/jmg.2007.055772. [DOI] [PubMed] [Google Scholar]
- Descheemaeker MJ, Roelandts K, De Raedt T, Brems H, Fryns JP, Legius E. Intelligence in individuals with a neurofibromatosis type 1 microdeletion. Am J Med Genet A. 2004;131:325–326. doi: 10.1002/ajmg.a.30346. [DOI] [PubMed] [Google Scholar]
- Eckel-Mahan KL, Phan T, Han S, Wang H, Chan GC, Scheiner ZS, Storm DR. Circadian oscillation of hippocampal MAPK activity and cAMP: implications for memory persistence. Nat Neurosci. 2008 doi: 10.1038/nn.2174. in press. Published online August 10, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel SR, Creson TK, Hao Y, Shen Y, Maeng S, Nekrasova T, Landreth GE, Manji HK, Chen G. The extracellular signal-regulated kinase pathway contributes to the control of behavioral excitement. Mol Psychiatry. 2008 doi: 10.1038/sj.mp.4002135. in press. Published online January 29, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English JD, Sweatt JD. Activation of p42 mitogen-activated protein kinase in hippocampal long term potentiation. J Biol Chem. 1996;271:24329–24332. doi: 10.1074/jbc.271.40.24329. [DOI] [PubMed] [Google Scholar]
- Ferguson SM, Fasano S, Yang P, Brambilla R, Robinson TE. Knockout of ERK1 enhances cocaine-evoked immediate early gene expression and behavioral plasticity. Neuropsychopharmacology. 2006;31:2660–2668. doi: 10.1038/sj.npp.1301014. [DOI] [PubMed] [Google Scholar]
- Ferner RE. Neurofibromatosis 1 and neurofibromatosis 2: a twenty first century perspective. Lancet Neurol. 2007;6:340–351. doi: 10.1016/S1474-4422(07)70075-3. [DOI] [PubMed] [Google Scholar]
- Frank DU, Fotheringham LK, Brewer JA, Muglia LJ, Tristani-Firouzi M, Capecchi MR, Moon AM. An Fgf8 mouse mutant phenocopies human 22q11 deletion syndrome. Development. 2002;129:4591–4603. doi: 10.1242/dev.129.19.4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galabova-Kovacs G, Catalanotti F, Matzen D, Reyes GX, Zezula J, Herbst R, Silva A, Walter I, Baccarini M. Essential role of B-Raf in oligodendrocyte maturation and myelination during postnatal central nervous system development. J Cell Biol. 2008;180:947–955. doi: 10.1083/jcb.200709069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier AS, Furstoss O, Araki T, Chan R, Neel BG, Kaplan DR, Miller FD. Control of CNS cell-fate decisions by SHP-2 and its dysregulation in Noonan syndrome. Neuron. 2007;54:245–262. doi: 10.1016/j.neuron.2007.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelb BD, Tartaglia M. Noonan syndrome and related disorders: dysregulated RAS-mitogen activated protein kinase signal transduction. Hum Mol Genet. 2006;15(Suppl 2):R220–226. doi: 10.1093/hmg/ddl197. [DOI] [PubMed] [Google Scholar]
- Ghebranious N, Giampietro PF, Wesbrook FP, Rezkalla SH. A novel microdeletion at 16p11.2 harbors candidate genes for aortic valve development, seizure disorder, and mild mental retardation. Am J Med Genet A. 2007;143:1462–1471. doi: 10.1002/ajmg.a.31837. [DOI] [PubMed] [Google Scholar]
- Grueter BA, Gosnell HB, Olsen CM, Schramm-Sapyta NL, Nekrasova T, Landreth GE, Winder DG. Extracellular-signal regulated kinase 1-dependent metabotropic glutamate receptor 5-induced long-term depression in the bed nucleus of the stria terminalis is disrupted by cocaine administration. J Neurosci. 2006;26:3210–3219. doi: 10.1523/JNEUROSCI.0170-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guris DL, Duester G, Papaioannou VE, Imamoto A. Dose-dependent interaction of Tbx1 and Crkl and locally aberrant RA signaling in a model of del22q11 syndrome. Dev Cell. 2006;10:81–92. doi: 10.1016/j.devcel.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Hadari YR, Gotoh N, Kouhara H, Lax I, Schlessinger J. Critical role for the docking-protein FRS2 alpha in FGF receptor-mediated signal transduction pathways. Proc Natl Acad Sci USA. 2001;98:8578–8583. doi: 10.1073/pnas.161259898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey CD, Yasuda R, Zhong H, Svoboda K. The spread of Ras activity triggered by activation of a single dendritic spine. Science. 2008;321:136–140. doi: 10.1126/science.1159675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegedus B, Dasgupta B, Shin JE, Emnett RJ, Hart-Mahon EK, Elghazi L, Bernal-Mizrachi E, Gutmann DH. Neurofibromatosis-1 regulates neuronal and glial cell differentiation from neuroglial progenitors in vivo by both cAMP- and Ras-dependent mechanisms. Cell Stem Cell. 2007;1:443–457. doi: 10.1016/j.stem.2007.07.008. [DOI] [PubMed] [Google Scholar]
- Inglis-Broadgate SL, Thomson RE, Pellicano F, Tartaglia MA, Pontikis CC, Cooper JD, Iwata T. FGFR3 regulates brain size by controlling progenitor cell proliferation and apoptosis during embryonic development. Dev Biol. 2005;279:73–85. doi: 10.1016/j.ydbio.2004.11.035. [DOI] [PubMed] [Google Scholar]
- Kawauchi S, Shou J, Santos R, Hebert JM, McConnell SK, Mason I, Calof AL. Fgf8 expression defines a morphogenetic center required for olfactory neurogenesis and nasal cavity development in the mouse. Development. 2005;132:5211–5223. doi: 10.1242/dev.02143. [DOI] [PubMed] [Google Scholar]
- Kumar RA, KaraMohamed S, Sudi J, Conrad DF, Brune C, Badner JA, Gilliam TC, Nowak NJ, Cook EH, Jr, Dobyns WB, Christian SL. Recurrent 16p11.2 microdeletions in autism. Hum Mol Genet. 2008;17:628–638. doi: 10.1093/hmg/ddm376. [DOI] [PubMed] [Google Scholar]
- Lee DA, Portnoy S, Hill P, Gillberg C, Patton MA. Psychological profile of children with Noonan syndrome. Dev Med Child Neurol. 2005;47:35–38. doi: 10.1017/s001216220500006x. [DOI] [PubMed] [Google Scholar]
- Leppig KA, Kaplan P, Viskochil D, Weaver M, Ortenberg J, Stephens K. Familial neurofibromatosis 1 microdeletions: cosegregation with distinct facial phenotype and early onset of cutaneous neurofibromata. Am J Med Genet. 1997;73:197–204. doi: 10.1002/(sici)1096-8628(1997)73:2<197::aid-ajmg17>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Lindsay EA. Chromosomal microdeletions: dissecting del22q11 syndrome. Nat Rev Genet. 2001;2:858–868. doi: 10.1038/35098574. [DOI] [PubMed] [Google Scholar]
- Lunn JS, Fishwick KJ, Halley PA, Storey KG. A spatial and temporal map of FGF/Erk1/2 activity and response repertoires in the early chick embryo. Dev Biol. 2007;302:536–552. doi: 10.1016/j.ydbio.2006.10.014. [DOI] [PubMed] [Google Scholar]
- Mazzucchelli C, Vantaggiato C, Ciamei A, Fasano S, Pakhotin P, Krezel W, Welzl H, Wolfer DP, Pages G, Valverde O, et al. Knockout of ERK1 MAP kinase enhances synaptic plasticity in the striatum and facilitates striatal-mediated learning and memory. Neuron. 2002;34:807–820. doi: 10.1016/s0896-6273(02)00716-x. [DOI] [PubMed] [Google Scholar]
- Menard C, Hein P, Paquin A, Savelson A, Yang XM, Lederfein D, Barnabe-Heider F, Mir AA, Sterneck E, Peterson AC, et al. An essential role for a MEK-C/EBP pathway during growth factor-regulated cortical neurogenesis. Neuron. 2002;36:597–610. doi: 10.1016/s0896-6273(02)01026-7. [DOI] [PubMed] [Google Scholar]
- Meyers EN, Lewandoski M, Martin GR. An Fgf8 mutant allelic series generated by Cre- and Flp-mediated recombination. Nat Genet. 1998;18:136–141. doi: 10.1038/ng0298-136. [DOI] [PubMed] [Google Scholar]
- Moon AM, Guris DL, Seo JH, Li L, Hammond J, Talbot A, Imamoto A. Crkl deficiency disrupts Fgf8 signaling in a mouse model of 22q11 deletion syndromes. Dev Cell. 2006;10:71–80. doi: 10.1016/j.devcel.2005.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newbern J, Zhong J, Wickramasinghe S, Li X, Wu Y, Samuels I, Cherosky N, Karlo J, O’Loughlin B, Wikenheiser J, et al. Mouse and human phenotypes indicate a critical conserved role for ERK2 signaling in neural crest development. Proc Natl Acad Sci USA. 2008 doi: 10.1073/pnas.0805239105. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandit B, Sarkozy A, Pennacchio LA, Carta C, Oishi K, Martinelli S, Pogna EA, Schackwitz W, Ustaszewska A, Landstrom A, et al. Gain-of-function RAF1 mutations cause Noonan and LEOPARD syndromes with hypertrophic cardiomyopathy. Nat Genet. 2007;39:1007–1012. doi: 10.1038/ng2073. [DOI] [PubMed] [Google Scholar]
- Paquin A, Barnabe-Heider F, Kageyama R, Miller FD. CCAAT/ enhancer-binding protein phosphorylation biases cortical precursors to generate neurons rather than astrocytes in vivo. J Neurosci. 2005;25:10747–10758. doi: 10.1523/JNEUROSCI.2662-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontious A, Kowalczyk T, Englund C, Hevner RF. Role of intermediate progenitor cells in cerebral cortex development. Dev Neurosci. 2008;30:24–32. doi: 10.1159/000109848. [DOI] [PubMed] [Google Scholar]
- Rauch A, Pfeiffer RA, Leipold G, Singer H, Tigges M, Hofbeck M. A novel 22q11.2 microdeletion in DiGeorge syndrome. Am J Hum Genet. 1999;64:659–666. doi: 10.1086/302235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauen KA. HRAS and the Costello syndrome. Clin Genet. 2007;71:101–108. doi: 10.1111/j.1399-0004.2007.00743.x. [DOI] [PubMed] [Google Scholar]
- Razzaque MA, Nishizawa T, Komoike Y, Yagi H, Furutani M, Amo R, Kamisago M, Momma K, Katayama H, Nakagawa M, et al. Germ-line gain-of-function mutations in RAF1 cause Noonan syndrome. Nat Genet. 2007;39:1013–1017. doi: 10.1038/ng2078. [DOI] [PubMed] [Google Scholar]
- Roberts A, Allanson J, Jadico SK, Kavamura MI, Noonan J, Opitz JM, Young T, Neri G. The cardiofaciocutaneous syndrome. J Med Genet. 2006;43:833–842. doi: 10.1136/jmg.2006.042796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinfeld H, Seger R. The ERK cascade: a prototype of MAPK signaling. Mol Biotechnol. 2005;31:151–174. doi: 10.1385/MB:31:2:151. [DOI] [PubMed] [Google Scholar]
- Saitta SC, McGrath JM, Mensch H, Shaikh TH, Zackai EH, Emanuel BS. A 22q11.2 deletion that excludes UFD1L and CDC45L in a patient with conotruncal and craniofacial defects. Am J Hum Genet. 1999;65:562–566. doi: 10.1086/302514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels IS, Karlo JC, Faruzzi AN, Pickering K, Herrup K, Sweatt JD, Saitta SC, Landreth GE. Deletion of ERK2 mitogen-activated protein kinase identifies its key roles in cortical neurogenesis and cognitive function. J Neurosci. 2008;28:6983–6995. doi: 10.1523/JNEUROSCI.0679-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh Y, Endo S, Ikeda T, Yamada K, Ito M, Kuroki M, Hiramoto T, Imamura O, Kobayashi Y, Watanabe Y, et al. Extracellular signal-regulated kinase 2 (ERK2) knockdown mice show deficits in long-term memory; ERK2 has a specific function in learning and memory. J Neurosci. 2007;27:10765–10776. doi: 10.1523/JNEUROSCI.0117-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scambler PJ. The 22q11 deletion syndromes. Hum Mol Genet. 2000;9:2421–2426. doi: 10.1093/hmg/9.16.2421. [DOI] [PubMed] [Google Scholar]
- Schaer M, Schmitt JE, Glaser B, Lazeyras F, Delavelle J, Eliez S. Abnormal patterns of cortical gyrification in velo-cardio-facial syndrome (deletion 22q11.2): an MRI study. Psychiatry Res. 2006;146:1–11. doi: 10.1016/j.pscychresns.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Selcher JC, Nekrasova T, Paylor R, Landreth GE, Sweatt JD. Mice lacking the ERK1 isoform of MAP kinase are unimpaired in emotional learning. Learn Mem. 2001;8:11–19. doi: 10.1101/lm.37001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaikh TH, O’Connor RJ, Pierpont ME, McGrath J, Hacker AM, Nimmakayalu M, Geiger E, Emanuel BS, Saitta SC. Low copy repeats mediate distal chromosome 22q11.2 deletions: sequence analysis predicts breakpoint mechanisms. Genome Res. 2007;17:482–491. doi: 10.1101/gr.5986507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalin SC, Egli R, Birnbaum SG, Roth TL, Levenson JM, Sweatt JD. Signal transduction mechanisms in memory disorders. Prog Brain Res. 2006;157:25–41. doi: 10.1016/s0079-6123(06)57003-7. [DOI] [PubMed] [Google Scholar]
- Shin DM, Korada S, Raballo R, Shashikant CS, Simeone A, Taylor JR, Vaccarino F. Loss of glutamatergic pyramidal neurons in frontal and temporal cortex resulting from attenuation of FGFR1 signaling is associated with spontaneous hyperactivity in mice. J Neurosci. 2004;24:2247–2258. doi: 10.1523/JNEUROSCI.5285-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinya M, Koshida S, Sawada A, Kuroiwa A, Takeda H. Fgf signalling through MAPK cascade is required for development of the subpallial telencephalon in zebrafish embryos. Development. 2001;128:4153–4164. doi: 10.1242/dev.128.21.4153. [DOI] [PubMed] [Google Scholar]
- Simon TJ, Wu Z, Avants B, Zhang H, Gee JC, Stebbins GT. Atypical cortical connectivity and visuospatial cognitive impairments are related in children with chromosome 22q11.2 deletion syndrome. Behav Brain Funct. 2008;4:25. doi: 10.1186/1744-9081-4-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storm EE, Garel S, Borello U, Hebert JM, Martinez S, McConnell SK, Martin GR, Rubenstein JL. Dose-dependent functions of Fgf8 in regulating telencephalic patterning centers. Development. 2006;133:1831–1844. doi: 10.1242/dev.02324. [DOI] [PubMed] [Google Scholar]
- Tartaglia M, Kalidas K, Shaw A, Song X, Musat DL, van der Burgt I, Brunner HG, Bertola DR, Crosby A, Ion A, et al. PTPN11 mutations in Noonan syndrome: molecular spectrum, genotype-phenotype correlation, and phenotypic heterogeneity. Am J Hum Genet. 2002;70:1555–1563. doi: 10.1086/340847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas GM, Huganir RL. MAPK cascade signalling and synaptic plasticity. Nat Rev Neurosci. 2004;5:173–183. doi: 10.1038/nrn1346. [DOI] [PubMed] [Google Scholar]
- Thomson RE, Pellicano F, Iwata T. Fibroblast growth factor receptor 3 kinase domain mutation increases cortical progenitor proliferation via mitogen-activated protein kinase activation. J Neurochem. 2007;100:1565–1578. doi: 10.1111/j.1471-4159.2006.04285.x. [DOI] [PubMed] [Google Scholar]
- Vaccarino FM, Schwartz ML, Raballo R, Nilsen J, Rhee J, Zhou M, Doetschman T, Coffin JD, Wyland JJ, Hung YT. Changes in cerebral cortex size are governed by fibroblast growth factor during embryogenesis. Nat Neurosci. 1999;2:246–253. doi: 10.1038/6350. [DOI] [PubMed] [Google Scholar]
- Weiss LA, Shen Y, Korn JM, Arking DE, Miller DT, Fossdal R, Saemundsen E, Stefansson H, Ferreira MA, Green T, et al. Association between microdeletion and microduplication at 16p11.2 and autism. N Engl J Med. 2008;358:667–675. doi: 10.1056/NEJMoa075974. [DOI] [PubMed] [Google Scholar]
- Wurdak H, Ittner LM, Sommer L. DiGeorge syndrome and pharyngeal apparatus development. Bioessays. 2006;28:1078–1086. doi: 10.1002/bies.20484. [DOI] [PubMed] [Google Scholar]
- Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- Yamamoto S, Yoshino I, Shimazaki T, Murohashi M, Hevner RF, Lax I, Okano H, Shibuya M, Schlessinger J, Gotoh N. Essential role of Shp2-binding sites on FRS2alpha for corticogenesis and for FGF2-dependent proliferation of neural progenitor cells. Proc Natl Acad Sci USA. 2005;102:15983–15988. doi: 10.1073/pnas.0507961102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda T, Sanjo H, Pages G, Kawano Y, Karasuyama H, Pouyssegur J, Ogata M, Kurosaki T. Erk kinases link pre-B cell receptor signaling to transcriptional events required for early B cell expansion. Immunity. 2008;28:499–508. doi: 10.1016/j.immuni.2008.02.015. [DOI] [PubMed] [Google Scholar]
- Yntema HG, van den Helm B, Kissing J, van Duijnhoven G, Poppelaars F, Chelly J, Moraine C, Fryns JP, Hamel BC, Heilbronner H, et al. A novel ribosomal S6-kinase (RSK4; RPS6KA6) is commonly deleted in patients with complex X-linked mental retardation. Genomics. 1999;62:332–343. doi: 10.1006/geno.1999.6004. [DOI] [PubMed] [Google Scholar]
- Yoshida R, Hasegawa T, Hasegawa Y, Nagai T, Kinoshita E, Tanaka Y, Kanegane H, Ohyama K, Onishi T, Hanew K, et al. Protein-tyrosine phosphatase, nonreceptor type 11 mutation analysis and clinical assessment in 45 patients with Noonan syndrome. J Clin Endocrinol Metab. 2004;89:3359–3364. doi: 10.1210/jc.2003-032091. [DOI] [PubMed] [Google Scholar]
- Zheng W, Nowakowski RS, Vaccarino FM. Fibroblast growth factor 2 is required for maintaining the neural stem cell pool in the mouse brain subventricular zone. Dev Neurosci. 2004;26:181–196. doi: 10.1159/000082136. [DOI] [PubMed] [Google Scholar]
- Zhong J, Li X, McNamee C, Chen AP, Baccarini M, Snider WD. Raf kinase signaling functions in sensory neuron differentiation and axon growth in vivo. Nat Neurosci. 2007;10:598–607. doi: 10.1038/nn1898. [DOI] [PubMed] [Google Scholar]