Summary
The evolutionarily conserved target of rapamycin (TOR) signaling controls growth, metabolism and aging. In the first robust demonstration of pharmacologically-induced life extension in a mammal, longevity was extended in mice treated with rapamycin, an inhibitor of mechanistic TOR (mTOR). However, detrimental metabolic effects of rapamycin treatment were also reported, presenting a paradox of improved survival despite metabolic impairment. How rapamycin extended lifespan in mice with such paradoxical effects was unclear. Here we show that detrimental effects of rapamycin treatment were only observed during the early stages of treatment. As the treatment continued for 20 weeks, these effects were reversed or diminished; the mice had better metabolic profiles, increased oxygen consumption and ketogenesis, and markedly enhanced insulin sensitivity. Thus, prolonged rapamycin treatment led to beneficial metabolic alterations, consistent with life extension previously observed. Our findings provide a likely explanation of the “rapamycin paradox” and support the potential causal importance of these metabolic alterations in longevity.
INTRODUCTION
mTOR is a master regulator of growth and metabolism. It senses upstream inputs of growth factors (such as insulin), nutrients and energy status to regulate downstream events by its complex 1 (mTORC1) and/or its complex 2 (mTORC2) (Wullschleger et al., 2006). Rapamycin inhibits mTORC1, but longer rapamycin treatment also affects mTORC2 (Sarbassov et al., 2006). With various genetic backgrounds, extension of longevity was reported in mice with knockout of S6K1, a target of mTOR (Selman et al., 2009), and in mice fed (Harrison et al., 2009) or injected (Chen et al., 2009; Anisimov et al., 2011) with rapamycin, but rapamycin treatment was repeatedly reported to produce detrimental metabolic changes usually associated with reduced rather than extended longevity including insulin resistance, hyperlipidemia and glucose intolerance (Houde et al., 2010; Chang et al., 2009; Fraenkel et al., 2008). Thus, it remains unclear how rapamycin extends the lifespan of mice. In various studies, the length of rapamycin treatment ranged from two (Houde et al., 2010; Fraenkel et al., 2008) or six weeks (Chen et al., 2009; Chang et al., 2009) to 1.5-2 years (Harrison et al., 2009; Anisimov et al., 2011), when rapamycin led to longer survivorship. We hypothesized that these paradoxical findings may be due to differences in the duration of treatment, and longer rapamycin treatment may change the metabolic parameters controlled by insulin signaling toward a beneficial profile. To test this hypothesis, we compared the effects of injecting adult male mice with rapamycin for 2, 6 or 20 weeks. Indeed, the mice experienced negative effects of rapamycin treatment including insulin resistance with short duration, but insulin signaling changed from an insulin resistant to insulin sensitive state after 20 weeks of rapamycin treatment.
RESULTS
Duration of Rapamycin Treatment Changed Body Features
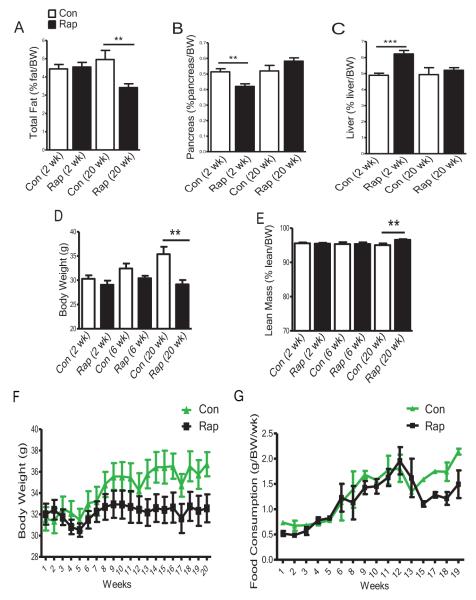
Starting at age of 3 months, the male mice were injected with rapamycin for 2, 6 or 20 weeks following the protocol by Chen et al. (2009), and then sacrificed when rapamycin treatments were completed. With 2 or 6 weeks of rapamycin treatment, adiposity, body weight and food consumption were not altered; however, after 20 weeks of treatment they were reduced dramatically (Figures 1A,S1A,1D,1F and 1G) without significant changes in lean body mass (Figures 1E and S1B). Prolonged rapamycin treatment prevented normal body weight gain (Figure 1D and 1F) mainly due to decreased adiposity (Figure 1A, S1A, 1E, S1B and data not shown). Pancreas mass was reduced after 2 weeks of rapamycin treatment but was restored with 20 weeks of treatment (Figures1B and S1C). Liver mass was increased after 2 weeks of rapamycin treatment, but no longer differed from that of controls after 20 weeks of treatment (Figures 1C and S1D). Thus, body features associated with metabolic syndrome, including smaller pancreas and enlarged liver, appeared in the mice with 2 weeks of rapamycin treatment; but with continued treatment these features returned to normal levels, and adiposity, body weight and food consumption were decreased. The most striking differences between the effects of short versus prolonged rapamycin treatment concern insulin signaling, glucose and lipid homeostasis and metabolism.
Figure 1. Body Characteristics Alter with Duration of Rapamycin Treatment.
(A) Relative total fat mass (absolute value (g)/body weight (BW) (g)) ×100; total fat includes subcutaneous fat from the thighs, a pair of peri-gonadal (visceral) depots, a pair of perinephric depots, and interscapular brown fat. (B) Relative mass of the pancreas. (C) Relative mass of the liver. (D) Body weight. (E) Relative total lean mass (absolute value (g)/body weight (BW) (g)) ×100. Total lean mass is estimated as following: lean mass=body weight-total fat mass. (F) Time course of body weight measured over 20 weeks of rapamycin treatment. (G) Time course of food consumption over 19 weeks of rapamycin treatment. Data are means ± SEM (n=8-16 for control, n=9-16 for rapamycin). P values are calculated between the rapamycin treated and control groups at each time point independently. The error bars represent standard error of the mean (SEM). *P≤ 0.05, **P≤ 0.01, ***P≤ 0.001.
Prolonged Rapamycin Treatment Increased Insulin Sensitivity
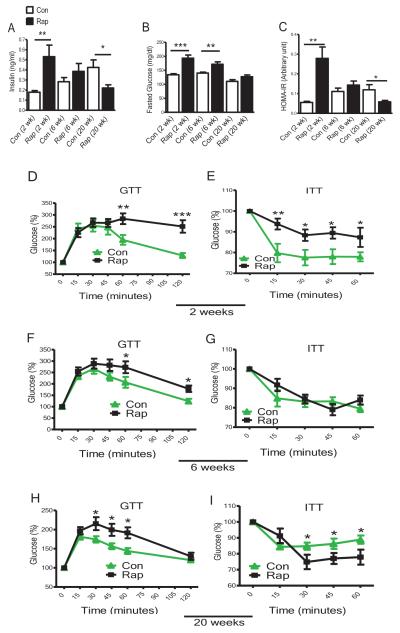
Insulin signaling is important in the control of longevity in both mice and humans, although the specific mechanisms are not completely understood and some findings are controversial (Barzilai et al., 2012). Normally, insulin inhibits hepatic gluconeogenesis and increases lipogenesis. In individuals exhibiting insulin resistance, excess insulin is produced as a compensatory mechanism. When insulin resistance develops, insulin initially loses its ability to inhibit hepatic gluconeogenesis but maintains the ability to enhance lipogenesis. As a result, hyperglycemia and hypertriglyceridemia occur (Matsumoto et al., 2006). mTORC1 is required for insulin-induced stimulation of lipogenesis but not for inhibition of gluconeogenesis (Li et al., 2010), and 2 weeks of rapamycin treatment inhibited lipogenesis and upregulated gluconeogenesis (Houde et al., 2010). Similarly, 2 weeks of rapamycin treatment in our study increased insulin levels 2.5-fold compared with controls (Figure 2A). The mice became glucose intolerant (Figure 2D) and insulin resistant (Figure 2E), with increased glucose (Figure 2B and Table S1) and HOMA-IR (Homeostatic Model for Assessment of Insulin Resistance) levels (Figure 2C). However, with 6 weeks of rapamycin treatment, the mice experienced a transition from insulin resistance toward an improved metabolic state (Figure 2A, 2B, 2C, 2F, 2G and Table S1). Strikingly, with 20 weeks of rapamycin treatment, both insulin levels and insulin sensitivity were altered in a direction opposite to that observed with 2 weeks of treatment: insulin levels decreased (Figure 2A), while insulin sensitivity increased (Figure 2I) drastically compared with the controls. Although glucose intolerance remained, especially at the early stage of GTT (Figure 2H), HOMA-IR values derived from both insulin and glucose levels were significantly reduced (Figure 2C). These findings indicate that with prolonged rapamycin treatment, the mice progressed from being insulin resistant to having improved insulin sensitivity.
Figure 2. Insulin Signaling and Glucose Homeostasis Switch from Insulin Resistant State with Short Rapamycin Treatment to Insulin Sensitive State with Prolonged Rapamycin Treatment.
(A) Plasma insulin levels. (B) Fasted glucose levels. (C) HOMA-IR (Homeostatic model for assessment of insulin resistance): HOMA-IR = (FPI×FPG)/22.5, where FPI is fasted plasma insulin and FPG is fasted plasma glucose. Plasma and fasted glucose values were collected at sacrifice. (D, F & H) Glucose tolerance test (GTT): 16 hrs fasted mice underwent GTT by intraperitoneal (i.p.) injection with 1 g of glucose per kg of BW. (E, G & I) Insulin tolerance test (ITT): mice were injected by i.p. with 1 IU/kg BW of porcine insulin. Data are mean ± SEM (n=8-16 for control, n=9-16 for rapamycin). P values are calculated between the rapamycin treated and control groups at each time point independently. The error bars represent standard error of the mean (SEM).*P≤ 0.05, **P≤ 0.01, ***P≤ 0.001.
Prolonged Rapamycin Treatment Improved Lipid Profile and Metabolism
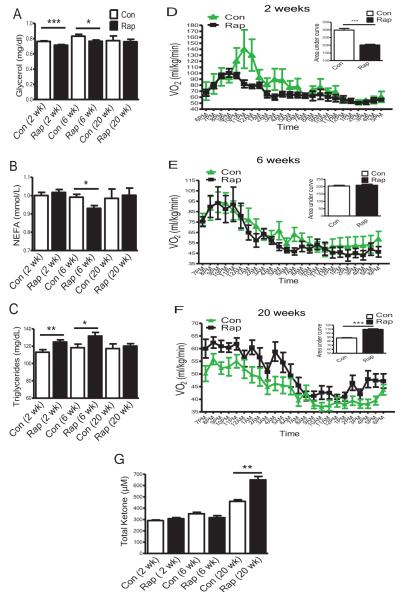
Lipid homeostasis showed similar but more complex responses. Lipolysis breaks down triglycerides into glycerol and free fatty acids (measured as non-esterified fatty acids (NEFA)), and lipogenesis generates triglycerides. Insulin inhibits lipolysis but enhances lipogenesis. Consequently, when insulin levels are high, less glycerol and NEFA, and more triglycerides are generated (Prentki & Madiraju, 2012). In our study, after 2 weeks of rapamycin treatment, the mice had increased insulin levels (Figure 2A); likely explaining lower glycerol (Figure 3A) and higher triglycerides (Figure 3C). After 6 weeks of rapamycin treatment, increase in insulin levels was much less pronounced, but insulin was still higher than in controls (Figure 2A); glycerol and NEFA levels were suppressed (Figures 3A and 3B), while triglycerides remained elevated (Figure 3C). In contrast, after 20 weeks of rapamycin treatment, insulin levels were reduced significantly (Figure 2A) and triglycerides declined to control levels (Figure 3C). Thus, also with regard to lipid homeostasis, the duration of rapamycin treatment determined the direction of the changes. Similar to the findings in human renal transplant patients, who received rapamycin as an immunosuppressant for 12 months (Blum, 2002), hypertriglyceridemia detected after short rapamycin treatment was normalized when the treatment was continued for 20 weeks. Although the mice treated with rapamycin for 20 weeks no longer had hypertriglyceridemia, they could have had elevated levels of free fatty acids, reflecting enhanced lipolysis and reduced lipogenesis. Elevation of free fatty acids is associated with the metabolic syndrome (Prentki & Madiraju, 2012). Interestingly, NEFA levels were not increased by 20 weeks of rapamycin treatment (Figure 3B). What other lipid metabolic changes could have developed with 20 weeks of rapamycin treatment to produce a decrease of free fatty acids? Adipose-specific knockout of Raptor results in mice being lean due to enhanced oxygen consumption (VO2) without affecting respiratory quotient (RQ) (Polak et al., 2008). Could the mice treated with rapamycin for 20 weeks in our study have higher oxygen consumption (VO2) to burn more fatty acids? Similar to the previous report (Cunningham et al., 2007), the mice with 2 weeks of rapamycin treatment had reduced VO2 (Figure 3D), suggesting that they expend less energy. An intermediate state was observed in mice treated with rapamycin for 6 weeks, such that VO2 was not significantly different from the controls (Figure 3E). In contrast, after 20 weeks of rapamycin treatment, the mice had higher VO2 than the controls (Figure 3F), suggesting that they expend more energy, which was consistent with the findings in mice with adipose-specific knockout of Raptor (Polak et al., 2008). Duration of rapamycin treatment did not alter RQ (Figure S2) or spontaneous locomotor activity (data not shown). The present findings indicate that as the length of rapamycin treatment increased from 2 and 6 to 20 weeks, the energy metabolism was switched from lower energy expenditure and reliance on carbohydrates to higher energy expenditure and increased reliance on burning fatty acids. Fatty acid breakdown is also related to ketogenesis. mTOR was reported to control fasting-induced ketogenesis, and this process was linked to modulation of aging (Sengupta, et al., 2010). Could it be possible that with 20 weeks of rapamycin treatment the mice also have enhanced ketogenesis in our study? Indeed, the levels of total ketone bodies were not altered after 2 or 6 weeks of rapamycin treatment, but were significantly increased when the treatment was continued for 20 weeks (Figure 3G).
Figure 3. Lipid Profile, Oxygen Consumption and Total Ketone Body Production Chang Following Different Lengths of Rapamycin Treatment.
(A) Plasma glycerol levels. (B) Plasma NEFA levels. (C) Plasma triglycerides levels. For plasma chemical analysis, mice were fasted for 16 hrs and thusly collected plasma was subjected to the assays. (D, E & F) Oxygen consumption VO2 (ml/kg/min) measured using indirect calorimetry (AccuScan Instruments, Columbus, OH) after 2 weeks (D), 6 weeks (E) or 20 weeks (F) of rapamycin treatment. Data are mean ± SEM (n=8 for control or rapamycin). (G) Plasma total ketone bodies: mice were fasted for 16 hrs, and plasma total ketone bodies were measured using total ketone body kits (Wako, Richmond, VA). Data are mean ± SEM (n=8-16 for control, n=9-16 for rapamycin). The error bars represent standard error of the mean (SEM).*P≤ 0.05, **P≤ 0.01, ***P≤ 0.001.
Both mTORC1 and mTORC2 were Involved in Metabolic Alterations by Duration of Rapamycin Treatment
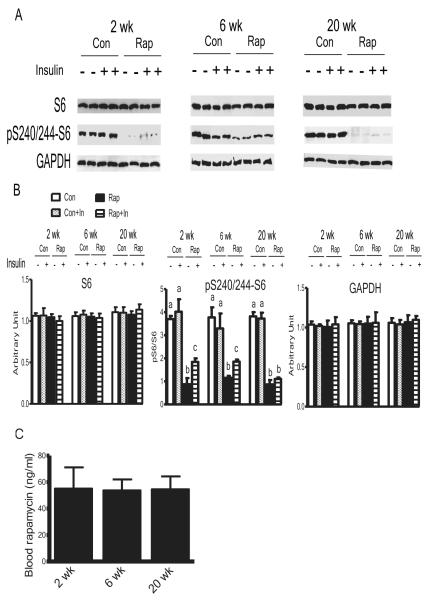
Given those metabolic alterations observed, it is crucial to know if duration of the treatment changed the levels or effectiveness of rapamycin. With the same biochemical readout of rapamycin effectiveness used by Harrison et al., 2009, phosphorylation of ribosomal protein subunit S6 in liver was analyzed. The blood levels of rapamycin (Figure 4C) and inhibition of phosphorylation of S6 with longer rapamycin treatment (Figure 4A and 4B) were similar to those reported by Harrison et al., 2009. However, both the levels of rapamycin and inhibition of pS6 were not altered with duration of the treatments (Figure 4). Although S6 is a remote downstream target of mTORC1, the metabolic switch caused by duration of the treatments was not differentially regulated at the level of pS6. To test whether the regulation was upstream of S6, and whether mTORC2 was also involved; mTOR, Raptor (key component of mTORC1), Rictor (key component of mTORC2), S6K1 and 4EBP1 (direct downstream targets of mTORC1), and AKT (downstream target of mTORC2) were measured. Duration of rapamycin treatment did not change the levels of these proteins. 2 weeks of rapamycin treatment reduced phosphorylation of mTOR, S6K1, 4EBP1, and AKT; 6 weeks of the treatment led to the transition stage; while 20 weeks of rapamycin treatment had reverse effects, compared with shorter rapamycin treatments (Figure S3 and S4). Thus, various measures of insulin signaling and its downstream metabolic effects changed drastically from unfavorable after 2 or 6 weeks of rapamycin treatment toward largely beneficial after 20 weeks of rapamycin treatment. In other words, duration of rapamycin treatment emerges as a key determinant of not only the magnitude but, importantly, also the direction of the responses.
Figure 4. Effects of Different Duration of Rapamycin Treatment on the Blood Levels of Rapamycin and Phosphorylation of S6 in the Liver.
(A) Western blots of S6 and phosphorylation of S6 at S240/244. Western blots are representative of 3 independent experiments. Each treatment includes 6 samples from 6 individual animals. (B) Quantification of the western blots. Data are means ± SEM (n=6). (C) Blood levels of rapamycin. Data are means ± SEM (n=4-6). P values are calculated between the rapamycin treated and control groups at each time point independently. Different letters represent P≤ 0.05. The error bars represent standard error of the mean (SEM).
DISCUSSION
The terms “prolonged” or “chronic” used to describe rapamycin treatment in various studies have been confusing. Several studies used a period of 2 to 6 weeks as prolonged or chronic rapamycin treatment (Houde et al., 2010; Chang et al., 2009; Fraenkel et al., 2008), while much longer periods of exposure (1.5-2 years) showed the ability of rapamycin to extend longevity (Harrison et al., 2009; Anisimov et al., 2011). Although various genetic backgrounds of mice, as well as various diets and methods of rapamycin administration were used in the previous studies (Houde et al., 2010, Lamming et al., 2012), our 2 weeks of rapamycin treatment showed similar detrimental effects of short rapamycin treatment on insulin signaling and related downstream events. Results of 6 weeks of rapamycin treatment generally resembled the effects of 2 weeks of rapamycin treatment, but appeared to represent a transitional state with regard to insulin sensitivity and HOMA-IR. In contrast, 20 weeks of rapamycin treatment had very different and generally “beneficial” effects, which may be a mechanism of extended longevity in mice by rapamycin (Harrison et al., 2009; Anisimov et al., 2011). The present study differed from the previous report by Harrison et al., 2009 in the genetic background of the mice, the source and composition of the diet, and the route of rapamycin administration; however, both studies involved long-term treatment with rapamycin and utilized a diet with a similar fat content (5.0% vs. 4.6%), and both used genetically-heterogeneous mice with partial commonality in the strains from which they were derived.
Rapamycin inhibition of mTOR signaling is primarily due to its actions on mTORC1, although prolonged rapamycin treatment also affects mTORC2. Hepatic mTORC2 was reported to mediate rapamycin-induced insulin resistance; however, short (2 to 4 weeks) rapamycin treatment in mice with hepatic Rictor deletion caused a further pronounced increase of insulin and glucose levels (Lamming et al., 2012). These data indicate that the actions of mTORC1 and mTORC2 may not be fully separate, and instead it may be their balance that determines the final impact on insulin signaling and longevity (Hughes & Kennedy, 2012). Our biochemical data showed that duration of rapamycin treatment affected not only levels of phosphorylation of mTOR, S6K1, 4EBP1, and AKT, but also direction of the responses. Based on our data, we speculated that both mTORC1 and mTORC2 played roles in these differential responses (Figure S3, S4 and data not shown). Unexpectedly, considering the similar blood levels of rapamycin and inhibition of pS6 (Figure 4), prolonged rapamycin treatments in the mice no longer inhibited levels of pmTOR, pS6K1, p4EBP1 or pAKT, while it remained effective in inhibiting pS6 (Figure S3 and S4). The molecular mechanism for the disparate responses of pS6K1 and pS6 remains unclear. However, S6K2 could also play a significant role; as S6K2, not S6K1, is the predominant kinase for S6 phosphorylation (Pende et al., 2004). In addition, phosphorylation of S6K1 at T389 is not the only regulatory mechanism for phosphorylation of S6. Nevertheless, the striking difference in the insulin signaling responses may well underlie the distinct physiological effects, and may potentially explain why prolonged rapamycin treatment is beneficial. The exact mechanisms of the counter-intuitive signaling outcome upon prolonged rapamycin treatment will be investigated in future studies.
In the present study, alterations in insulin sensitivity induced by different durations of rapamycin treatment were closely associated with changes of glucose and lipid homeostasis and metabolism, as well as body composition. After 20 weeks of rapamycin treatment, the mice were lean with enhanced insulin sensitivity (measured by ITT (Figure 2I)), increased oxygen consumption and ketogenesis, and improved serum lipid profile. The mice with prolonged rapamycin treatments showed a certain degree of glucose intolerance, especially at the early stages of GTT (Figure 2H); but those mice were able to clear glucose, albeit at a slower pace due to lower basal levels of insulin and higher insulin sensitivity (Figure 2A and 2I). Most of these phenotypic features are associated with extended longevity in several kinds of mutant mice (Bartke, 2005), and therefore can be viewed as candidate mechanisms of life extension in animals exposed to rapamycin (Harrison et al., 2009; Anisimov et al., 2011). In support of this suggestion, deletion of S6K1 attenuated age-related loss of insulin sensitivity and increased lifespan in mice (Selman et al., 2009). By showing differences between metabolic responses to short versus long-term rapamycin treatment, the present findings provide a likely explanation of the paradox of reported detrimental effects of rapamycin on insulin signaling and its ability to extend longevity.
Experimental Procedures
Mice Maintenance
The animal procedures were approved by the Laboratory Animal Care and Use Committee of Southern Illinois University School of Medicine. Mice were housed under temperature- and light-controlled conditions (22 ± 1°C, 12-h light/12-h dark cycle) with ad libitum access to food (Chow 5001 with 23.4% protein, 5% fat, 5.8% crude fiber) (LabDiet PMI Feeds, Inc., St. Louis, MO). Our breeding colony was developed by mating mice with 129 Ola/ BALB/c background with mice derived from crossing C57BL/6 and C3H strains and thereafter breeding the resulting animals in a closed colony without brother × sister matings. The colony generated thus has a heterogeneous genetic background. Beginning at the same age (3 months), male mice were injected intraperitoneally (i.p.) with 4 mg/kg body weight (BW) rapamycin (LC labs, Woburn, MA) or vehicle every other day following the protocol by Chen et al. (2009), and then sacrificed after 2, 6 or 20 weeks of rapamycin treatment.
Glucose Tolerance Test (GTT) and Insulin Tolerance Test (ITT)
16 hour-fasted mice underwent GTT by i.p. injection with 1 g of glucose per kg of body weight (BW). Blood glucose levels were measured at 0, 15, 30, 45, 60 and 120 minutes using a PRESTO glucometer (AgaMatrix, Salem, NH) for GTT. Non-fasted mice were injected by i.p. with 1 IU porcine insulin (Sigma, St. Louis, MO) per kg of BW. Blood glucose levels were measured at 0, 15, 30 and 60 minutes for ITT. The data for both ITT and GTT are presented as a percentage of baseline glucose.
Indirect Calorimetry
Mice injected for 2, 6 or 20 weeks with rapamycin were subjected to indirect calorimetry (AccuScan Instruments, Columbus, OH). The system uses zirconia, infrared sensors and light beam arrays to monitor oxygen consumption (VO2), carbon dioxide output (VCO2) and spontaneous locomotor activity, inside respiratory chambers in which individual mice were tested. After 24-hour acclimation, mice were monitored in the chambers for 24 hours with ad libitum access to food (Chow 5001) (LabDiet PMI Feeds, Inc., St. Louis, MO) and water. All comparisons are based on animals studied simultaneously in 8 different chambers connected to the same O2, CO2 and light beam sensors. Gas samples were collected and analyzed every 5 minutes per animal, and the data were averaged for each hour.
Assessment of Blood Chemistry
After 2, 6 or 20 weeks of rapamycin treatment, mice were fasted for 16 hrs and sacrificed. Plasma was collected at sacrifice. Following the manufacturer’s protocol, insulin was measured using Mouse Insulin ELISA Kits (Crystal Chem, Downers Grove, IL); total ketone bodies and non-esterified free fatty acids (NEFA) were measured using colorimetric assays from Wako Chemicals (Richmond, VA); glycerol was measured using kits from Sigma (St. Louis, MO) and triglycerides using kits from Pointe Scientific (Canton, MI), respectively. The whole blood samples were used to measure rapamycin levels using HPLC-tandem MS as detailed in Supplemental Information.
Western Blot Analysis
Mice were fasted for 16 hours. Plasma was collected, and then half of the animals from each experimental group were injected with insulin or saline through the liver portal vein to stimulate insulin signaling pathway using a previously described protocol (Bonkowski et al., 2009). 2 minutes after insulin injection, mice were sacrificed and the liver tissues collected. Approximately 500 mg liver samples were homogenized in 0.5 ml ice-cold T-PER tissue protein extraction buffer (Thermo Scientific, Rockford, IL) with protease and phosphatase inhibitors (Sigma, St. Louis, MO). 40 μg of total protein were separated by SDS–polyacrylamide gel electrophoresis using Criterion XT Precast Gel (Bio-Rad, Hercules, CA), and blotted with the antibodies. Antibodies were obtained from Cell Signaling Technology (Beverly, MA) including mTOR, phospho-mTOR (Ser2448), Raptor, phospho-Raptor (Ser792), Rictor, phospho-Rictor (Thr1135), S6K1, phospho-S6K1(Thr389), 4EBP1, phospho-4EBP1(Ser65), AKT, phospho-AKT(Ser473), S6, phospho-S6 (Ser240/244) and GAPDH. Western blots were quantified using Multi Gauge V3.0 software (Fujifilm North America, Edison, NJ).
Statistical Analysis
Data are presented as means ± SEM. The error bars represent standard error of the mean (SEM). Differences between two groups were assessed using the unpaired two-tailed Student’s t-tests. All statistical analyses were conducted using SPSS Statistics 17.0 (SPSS Company, Quarry Bay, Hong Kong).
Supplementary Material
Highlights.
2 weeks of rapamycin treatment in mice had detrimental metabolic effects
6 weeks of rapamycin treatment led to a metabolic transition to the improved state
20 weeks of rapamycin treatment produced reversed and largely beneficial alterations
Acknowledgements
The study was supported by grants from the National Institutes of Health (AG019899, AG038850 and AG031736 to A.B.). We thank Drs. Michal Mastenak and Jianxin Wang for suggestion, Minxiao Yang and Alexander Wang for experimental assistance and the Office of Public Affairs at SIU for artwork.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplemental Information Supplemental information includes 4 figures, 1 table, Supplemental Experimental procedures, and can be found with this article online.
References
- Anisimov VN, Zabezhinski MA, Popovich IG, Piskunova TS, Semenchenko AV, Tyndyk ML, Yurova MN, Rosenfeld SV, Blagosklonny MV. Rapamycin increases lifespan and inhibits spontaneous tumorigenesis in inbred female mice. Cell Cycle. 2011;10:4230–4236. doi: 10.4161/cc.10.24.18486. [DOI] [PubMed] [Google Scholar]
- Bartke A. Role of the growth hormone/insulin-like growth factor system in mammalian aging. Endocrinology. 2005;146:3718–3723. doi: 10.1210/en.2005-0411. [DOI] [PubMed] [Google Scholar]
- Barzilai N, Huffman DM, Muzumdar RH, Bartke A. The critical role of metabolic pathways in aging. Diabetes. 2012;61:1315–1322. doi: 10.2337/db11-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonkowski MS, et al. Disruption of growth hormone receptor prevents calorie restriction from improving insulin action and longevity. PLoS One. 2009;4:e4567. doi: 10.1371/journal.pone.0004567. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Blum CB. Effects of sirolimus on lipids in renal allograft recipients: an analysis using the Framingham risk model. Am. J. Transplant. 2002;2:551–559. doi: 10.1034/j.1600-6143.2002.20610.x. [DOI] [PubMed] [Google Scholar]
- Chang GR, Wu YY, Chiu YS, Chen WY, Liao JW, Hsu HM, Chao TH, Hung SW, Mao FC. Long-term administration of rapamycin reduces adiposity, but impairs glucose tolerance in high-fat diet-fed KK/HlJ mice. Basic Clin. Pharmacol. Toxicol. 2009;105:188–198. doi: 10.1111/j.1742-7843.2009.00427.x. [DOI] [PubMed] [Google Scholar]
- Chen C, Liu Y, Liu Y, Zheng P. mTOR regulation and therapeutic rejuvenation of aging hematopoietic stem cells. Sci. Signal. 2009;2:ra75. doi: 10.1126/scisignal.2000559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham JT, Rodgers JT, Arlow DH, Vazquez F, Mootha VK, Puigserver P. mTOR controls mitochondrial oxidative function through a YY1-PGC-1alpha transcriptional complex. Nature. 2007;450:736–740. doi: 10.1038/nature06322. [DOI] [PubMed] [Google Scholar]
- Fraenkel M, et al. mTOR inhibition by rapamycin prevents beta-cell adaptation to hyperglycemia and exacerbates the metabolic state in type 2 diabetes. Diabetes. 2008;57:945–957. doi: 10.2337/db07-0922. [DOI] [PubMed] [Google Scholar]
- Harrison DE, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houde VP, Brûlé S, Festuccia WT, Blanchard PG, Bellmann K, Deshaies Y, Marette A. Chronic rapamycin treatment causes glucose intolerance and hyperlipidemia by upregulating hepatic gluconeogenesis and impairing lipid deposition in adipose tissue. Diabetes. 2010;59:1338–1348. doi: 10.2337/db09-1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes KJ, Kennedy BK. Rapamycin paradox resolved. Science. 2012;335:1578–1579. doi: 10.1126/science.1221365. [DOI] [PubMed] [Google Scholar]
- Lamming DW, et al. Rapamycin-induced insulin resistance is mediated by mTORC2 loss and uncoupled from longevity. Science. 2012;335:1638–1643. doi: 10.1126/science.1215135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Brown MS, Goldstein JL. Bifurcation of insulin signaling pathway in rat liver: mTORC1 required for stimulation of lipogenesis, but not inhibition of gluconeogenesis. Proc. Natl. Acad. Sci. 2010;107:3441–3446. doi: 10.1073/pnas.0914798107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M, Han S, Kitamura T, Accili D. Dual role of transcription factor FoxO1 in controlling hepatic insulin sensitivity and lipid metabolism. J. Clin. Invest. 2006;116:2464–2472. doi: 10.1172/JCI27047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pende M, Um H, Mieulet V, Sticker M, Goss VL, Mestan J, Mueller M, Fumagalli S, Kozma SC, Thomas G. S6K1(−/−)/S6K2(−/−) mice exhibit perinatal lethality and rapamycin-sensitive 5′-terminal oligopyrimidine mRNA translation and reveal a mitogen-activated protein kinase-dependent S6 kinase pathway. Mol. Cell. Biol. 2004;24:3112–3124. doi: 10.1128/MCB.24.8.3112-3124.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polak P, Cybulski N, Feige JN, Auwerx J, Rüegg MA, Hall MN. Adipose-specific knockout of raptor results in lean mice with enhanced mitochondrial respiration. Cell Metab. 2008;8:399–410. doi: 10.1016/j.cmet.2008.09.003. [DOI] [PubMed] [Google Scholar]
- Prentki M, Madiraju SR. Glycerolipid/free fatty acid cycle and islet β-cell function in health, obesity and diabetes. Mol. Cell. Endocrinol. 2012;353:88–100. doi: 10.1016/j.mce.2011.11.004. [DOI] [PubMed] [Google Scholar]
- Sarbassov DD, Ali SM, Sengupta S, Sheen JH, Hsu PP, Bagley AF, Markhard AL, Sabatini DM. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol. Cell. 2006;22:159–168. doi: 10.1016/j.molcel.2006.03.029. [DOI] [PubMed] [Google Scholar]
- Selman C, et al. Ribosomal protein S6 kinase 1 signaling regulates mammalian life span. Science. 2009;326:140–144. doi: 10.1126/science.1177221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta S, Peterson TR, Laplante M, Oh S, Sabatini DM. mTORC1 controls fasting-induced ketogenesis and its modulation by ageing. Nature. 2010;468:1100–1104. doi: 10.1038/nature09584. [DOI] [PubMed] [Google Scholar]
- Wullschleger S, Loewit R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.