Abstract
We measured the temporal order of replication of EcoRI segments from the murine immunoglobulin heavy-chain constant region (IgCH) gene cluster, including the joining (J) and diversity (D) loci and encompassing approximately 300 kilobases. The relative concentrations of EcoRI segments in bromouracil-labeled DNA that replicated during selected intervals of the S phase in Friend virus-transformed murine erythroleukemia (MEL) cells were measured. From these results, we calculated the nuclear DNA content (C value; the haploid DNA content of a cell in the G1 phase of the cell cycle) at the time each segment replicated during the S phase. We observed that IgCH genes replicate in the following order: alpha, epsilon, gamma 2a, gamma 2b, gamma 1, gamma 3, delta, and mu, followed by the J and D segments. The C value at which each segment replicates increased as a linear function of its distance from C alpha. The average rate of DNA replication in the IgCH gene cluster was determined from these data to be 1.7 to 1.9 kilobases/min, similar to the rate measured for mammalian replicons by autoradiography and electron microscopy (for a review, see H. J. Edenberg and J. A. Huberman, Annu. Rev. Genet. 9:245-284, 1975, and R. G. Martin, Adv. Cancer Res. 34:1-55, 1981). Similar results were obtained with other murine non-B cell lines, including a fibroblast cell line (L60T) and a hepatoma cell line (Hepa 1.6). In contrast, we observed that IgCh segments in a B-cell plasmacytoma (MPC11) and two Abelson murine leukemia virus-transformed pre-B cell lines (22D6 and 300-19O) replicated as early as (300-19P) or earlier than (MPC11 and 22D6) C alpha in MEL cells. Unlike MEL cells, however, all of the IgCH segments in a given B cell line replicated at very similar times during the S phase, so that a temporal directionality in the replication of the IgCH gene cluster was not apparent from these data. These results provide evidence that in murine non-B cells the IgCH, J, and D loci are part of a single replicon.
Full text
PDF

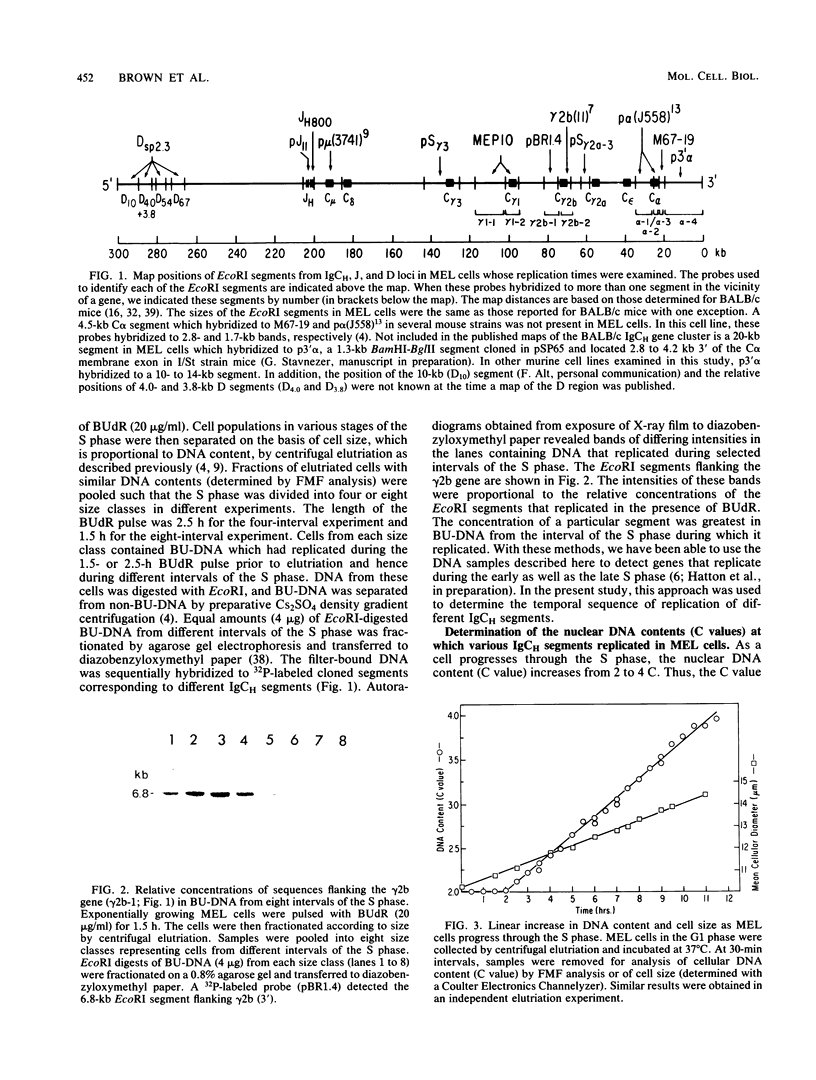
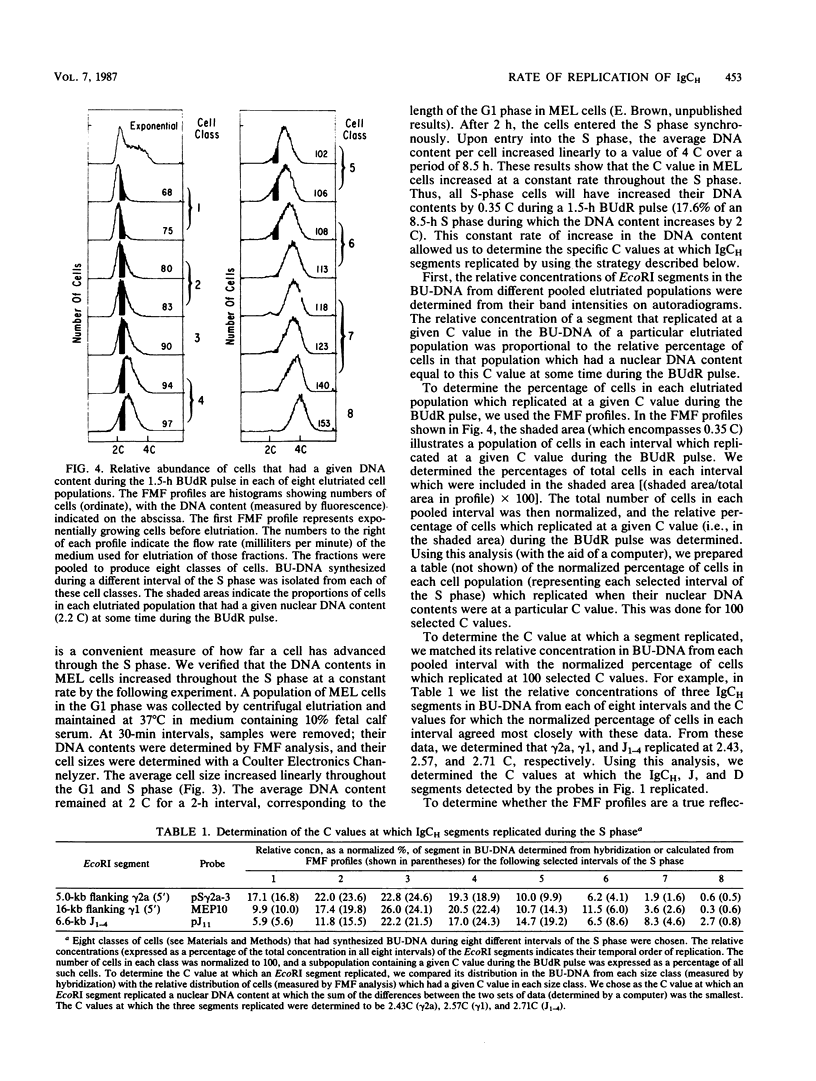
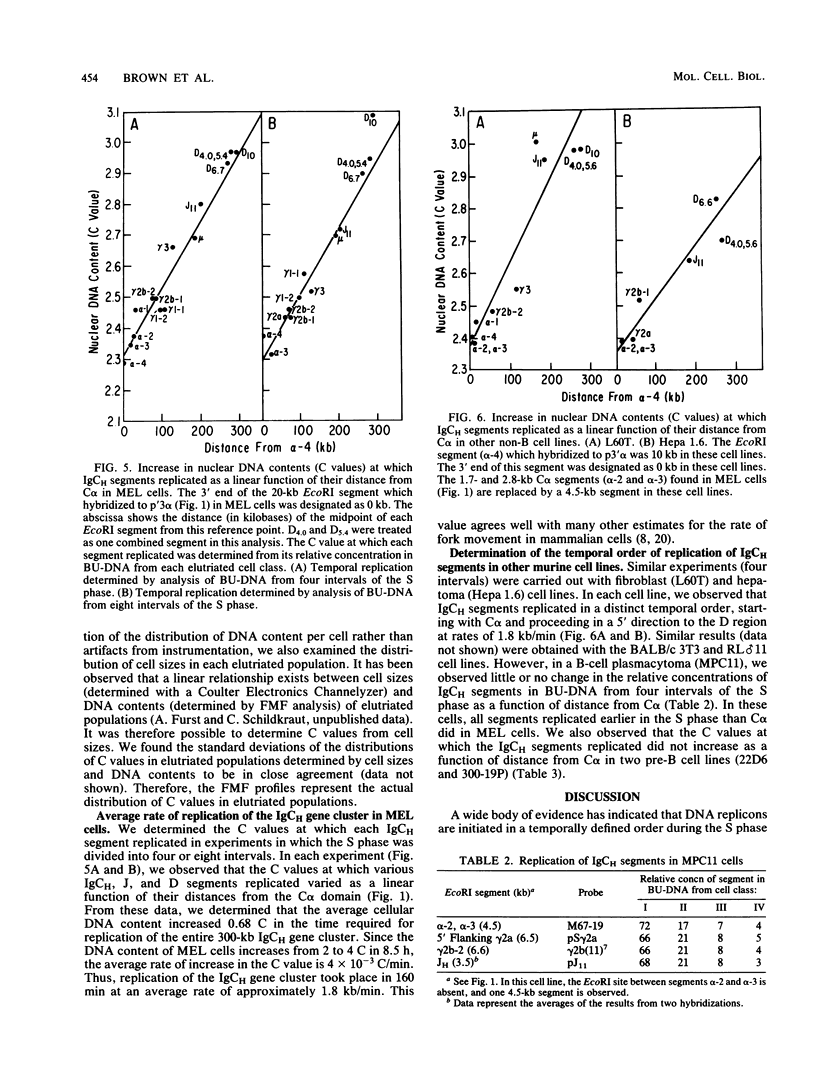


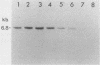
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alt F. W., Baltimore D. Joining of immunoglobulin heavy chain gene segments: implications from a chromosome with evidence of three D-JH fusions. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4118–4122. doi: 10.1073/pnas.79.13.4118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balazs I., Brown E. H., Schildkraut C. L. The temporal order of replication of some DNA cistrons. Cold Spring Harb Symp Quant Biol. 1974;38:239–245. doi: 10.1101/sqb.1974.038.01.027. [DOI] [PubMed] [Google Scholar]
- Blumenthal A. B., Kriegstein H. J., Hogness D. S. The units of DNA replication in Drosophila melanogaster chromosomes. Cold Spring Harb Symp Quant Biol. 1974;38:205–223. doi: 10.1101/sqb.1974.038.01.024. [DOI] [PubMed] [Google Scholar]
- Braunstein J. D., Schulze D., DelGiudice T., Furst A., Schildkraut C. L. The temporal order of replication of murine immunoglobulin heavy chain constant region sequences corresponds to their linear order in the genome. Nucleic Acids Res. 1982 Nov 11;10(21):6887–6902. doi: 10.1093/nar/10.21.6887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown E. H., Schildkraut C. L. Perturbation of growth and differentiation of Friend murine erythroleukemia cells by 5-bromodeoxyuridine incorporation in early S phase. J Cell Physiol. 1979 May;99(2):261–278. doi: 10.1002/jcp.1040990213. [DOI] [PubMed] [Google Scholar]
- Calza R. E., Eckhardt L. A., DelGiudice T., Schildkraut C. L. Changes in gene position are accompanied by a change in time of replication. Cell. 1984 Mar;36(3):689–696. doi: 10.1016/0092-8674(84)90349-0. [DOI] [PubMed] [Google Scholar]
- Chang D. D., Hauswirth W. W., Clayton D. A. Replication priming and transcription initiate from precisely the same site in mouse mitochondrial DNA. EMBO J. 1985 Jun;4(6):1559–1567. doi: 10.1002/j.1460-2075.1985.tb03817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edenberg H. J., Huberman J. A. Eukaryotic chromosome replication. Annu Rev Genet. 1975;9:245–284. doi: 10.1146/annurev.ge.09.120175.001333. [DOI] [PubMed] [Google Scholar]
- Furst A., Brown E. H., Braunstein J. D., Schildkraut C. L. alpha-Globulin sequences are located in a region of early-replicating DNA in murine erythroleukemia cells. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1023–1027. doi: 10.1073/pnas.78.2.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman M. A., Holmquist G. P., Gray M. C., Caston L. A., Nag A. Replication timing of genes and middle repetitive sequences. Science. 1984 May 18;224(4650):686–692. doi: 10.1126/science.6719109. [DOI] [PubMed] [Google Scholar]
- Hay R. T., DePamphilis M. L. Initiation of SV40 DNA replication in vivo: location and structure of 5' ends of DNA synthesized in the ori region. Cell. 1982 Apr;28(4):767–779. doi: 10.1016/0092-8674(82)90056-3. [DOI] [PubMed] [Google Scholar]
- Heintz N. H., Milbrandt J. D., Greisen K. S., Hamlin J. L. Cloning of the initiation region of a mammalian chromosomal replicon. 1983 Mar 31-Apr 6Nature. 302(5907):439–441. doi: 10.1038/302439a0. [DOI] [PubMed] [Google Scholar]
- James C. D., Leffak M. Polarity of DNA replication through the avian alpha-globin locus. Mol Cell Biol. 1986 Apr;6(4):976–984. doi: 10.1128/mcb.6.4.976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KISSANE J. M., ROBINS E. The fluorometric measurement of deoxyribonucleic acid in animal tissues with special reference to the central nervous system. J Biol Chem. 1958 Jul;233(1):184–188. [PubMed] [Google Scholar]
- Kaufmann G., Zannis-Hadjopoulos M., Martin R. G. Cloning of nascent monkey DNA synthesized early in the cell cycle. Mol Cell Biol. 1985 Apr;5(4):721–727. doi: 10.1128/mcb.5.4.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurosawa Y., Tonegawa S. Organization, structure, and assembly of immunoglobulin heavy chain diversity DNA segments. J Exp Med. 1982 Jan 1;155(1):201–218. doi: 10.1084/jem.155.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang R. B., Stanton L. W., Marcu K. B. On immunoglobulin heavy chain gene switching: two gamma 2b genes are rearranged via switch sequences in MPC-11 cells but only one is expressed. Nucleic Acids Res. 1982 Jan 22;10(2):611–630. doi: 10.1093/nar/10.2.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskey R. A., Harland R. M. Replication origins in the eucaryotic chromosome. Cell. 1981 May;24(2):283–284. doi: 10.1016/0092-8674(81)90316-0. [DOI] [PubMed] [Google Scholar]
- Marcu K. B., Banerji J., Penncavage N. A., Lang R., Arnheim N. 5' flanking region of immunoglobulin heavy chain constant region genes displays length heterogeneity in germlines of inbred mouse strains. Cell. 1980 Nov;22(1 Pt 1):187–196. doi: 10.1016/0092-8674(80)90167-1. [DOI] [PubMed] [Google Scholar]
- Martin R. G. The transformation of cell growth and transmogrification of DNA synthesis by simian virus 40. Adv Cancer Res. 1981;34:1–68. doi: 10.1016/s0065-230x(08)60238-9. [DOI] [PubMed] [Google Scholar]
- Montiel J. F., Norbury C. J., Tuite M. F., Dobson M. J., Mills J. S., Kingsman A. J., Kingsman S. M. Characterization of human chromosomal DNA sequences which replicate autonomously in Saccharomyces cerevisiae. Nucleic Acids Res. 1984 Jan 25;12(2):1049–1068. doi: 10.1093/nar/12.2.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierron G., Sauer H. W., Toublan B., Jalouzot R. Physical relationship between replicons and transcription units in Physarum polycephalum. Eur J Cell Biol. 1982 Nov;29(1):104–113. [PubMed] [Google Scholar]
- Reisman D., Yates J., Sugden B. A putative origin of replication of plasmids derived from Epstein-Barr virus is composed of two cis-acting components. Mol Cell Biol. 1985 Aug;5(8):1822–1832. doi: 10.1128/mcb.5.8.1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reth M. G., Alt F. W. Novel immunoglobulin heavy chains are produced from DJH gene segment rearrangements in lymphoid cells. 1984 Nov 29-Dec 5Nature. 312(5993):418–423. doi: 10.1038/312418a0. [DOI] [PubMed] [Google Scholar]
- Reth M. G., Ammirati P., Jackson S., Alt F. W. Regulated progression of a cultured pre-B-cell line to the B-cell stage. 1985 Sep 26-Oct 2Nature. 317(6035):353–355. doi: 10.1038/317353a0. [DOI] [PubMed] [Google Scholar]
- Richardson C. C. Bacteriophage T7: minimal requirements for the replication of a duplex DNA molecule. Cell. 1983 Jun;33(2):315–317. doi: 10.1016/0092-8674(83)90411-7. [DOI] [PubMed] [Google Scholar]
- Roeder W., Maki R., Traunecker A., Tonegawa S. Linkage of the four gamma subclass heavy chain genes. Proc Natl Acad Sci U S A. 1981 Jan;78(1):474–478. doi: 10.1073/pnas.78.1.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth G. E., Blanton H. M., Hager L. J., Zakian V. A. Isolation and characterization of sequences from mouse chromosomal DNA with ARS function in yeasts. Mol Cell Biol. 1983 Nov;3(11):1898–1908. doi: 10.1128/mcb.3.11.1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saffer L. D., Miller O. L., Jr Electron microscopic study of Saccharomyces cerevisiae rDNA chromatin replication. Mol Cell Biol. 1986 Apr;6(4):1148–1157. doi: 10.1128/mcb.6.4.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schibler U., Marcu K. B., Perry R. P. The synthesis and processing of the messenger RNAs specifying heavy and light chain immunoglobulins in MPC-11 cells. Cell. 1978 Dec;15(4):1495–1509. doi: 10.1016/0092-8674(78)90072-7. [DOI] [PubMed] [Google Scholar]
- Seidman M. M., Levine A. J., Weintraub H. The asymmetric segregation of parental nucleosomes during chrosome replication. Cell. 1979 Oct;18(2):439–449. doi: 10.1016/0092-8674(79)90063-1. [DOI] [PubMed] [Google Scholar]
- Shimizu A., Takahashi N., Yaoita Y., Honjo T. Organization of the constant-region gene family of the mouse immunoglobulin heavy chain. Cell. 1982 Mar;28(3):499–506. doi: 10.1016/0092-8674(82)90204-5. [DOI] [PubMed] [Google Scholar]
- Silverstone A., Sun L., Witte O. N., Baltimore D. Biosynthesis of murine terminal deoxynucleotidyltransferase. J Biol Chem. 1980 Jan 25;255(2):791–796. [PubMed] [Google Scholar]
- Smithies O. The control of globin and other eukaryotic genes. J Cell Physiol Suppl. 1982;1:137–143. doi: 10.1002/jcp.1041130421. [DOI] [PubMed] [Google Scholar]
- Taylor J. H., Hozier J. C. Evidence for a four micron replication unit in CHO cells. Chromosoma. 1976 Sep 24;57(4):341–350. doi: 10.1007/BF00332159. [DOI] [PubMed] [Google Scholar]
- Taylor J. H. Increase in DNA replication sites in cells held at the beginning of S phase. Chromosoma. 1977 Jul 18;62(4):291–300. doi: 10.1007/BF00327029. [DOI] [PubMed] [Google Scholar]
- Taylor J. H. Origins of replication and gene regulation. Mol Cell Biochem. 1984;61(2):99–109. doi: 10.1007/BF00222489. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood C., Tonegawa S. Diversity and joining segments of mouse immunoglobulin heavy chain genes are closely linked and in the same orientation: implications for the joining mechanism. Proc Natl Acad Sci U S A. 1983 May;80(10):3030–3034. doi: 10.1073/pnas.80.10.3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates J., Warren N., Reisman D., Sugden B. A cis-acting element from the Epstein-Barr viral genome that permits stable replication of recombinant plasmids in latently infected cells. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3806–3810. doi: 10.1073/pnas.81.12.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurov Y. B., Liapunova N. A. The units of DNA replication in the mammalian chromosomes: evidence for a large size of replication units. Chromosoma. 1977 Apr 19;60(3):253–267. doi: 10.1007/BF00329774. [DOI] [PubMed] [Google Scholar]
- Zannis-Hadjopoulos M., Kaufmann G., Wang S. S., Lechner R. L., Karawya E., Hesse J., Martin R. G. Properties of some monkey DNA sequences obtained by a procedure that enriches for DNA replication origins. Mol Cell Biol. 1985 Jul;5(7):1621–1629. doi: 10.1128/mcb.5.7.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]