Abstract
This article summarizes the molecular and cellular mechanisms that regulate the activity of indoleamine 2,3-dioxygenase (IDO), a potent immune-suppressive enzyme, in dendritic cells (DCs). Specific attention is given to differential up-regulation of IDO in distinct DC subsets, its function in immune homeostasis/autoimmunity, infection and cancer; and the associated immunological outcomes. The review will conclude with a discussion of the poorly defined mechanisms that mediate the long-term maintenance of IDO-expression in response to inflammatory stimuli and how selective modulation of IDO activity may be used in the treatment of disease.
Keywords: Dendritic Cells; Indoleamine 2,3-dioxygenase; Immune tolerance
INTRODUCTION
Dendritic Cells
The dendritic cell (DC) is a cell of the immune system that was first described by Steinman and Cohn (1973). DCs are classified as a highly potent “professional” antigen presenting cell (APC) subset and are 100 to 1000-times more effective than macrophages and B-cells in priming T-cells. DCs perform many functions for the immune system such as: 1) uptake, processing, and presentation of antigens, 2) activation of effector cells such as T-cells and NK-cells, and 3) secretion of cytokines and other immune-modulating molecules to direct the immune response. Due to these characteristics, DCs have been recognized as the primary orchestrators of immune activity. Several outstanding reviews provide extensive information about dendritic cell biology and their immunological functions (Buckwalter and Albert, 2009; Heath and Carbone, 2009; Wallet et al., 2005), therefore this section will be limited to a brief discussion of the highlights.
Although DCs, when first discovered, were primarily thought to mediate immune-activation, it is now appreciated that DCs play an equally important role in immune-tolerance. Indeed, DCs that reside in the mucosa are often bombarded with innocuous foreign antigens and therefore have a predisposition to be tolerogenic (MacDonald et al., 2011). Tolerogenic DCs also play a role in maternal tolerance of the allogeneic fetus (Kammerer et al., 2008; Laskarin et al., 2007).
DCs can promote a tolerogenic environment through many mechanisms. They produce suppressive cytokines such as IL-10, IL-13, or TGFβ, which can have effects on many cells of the immune system. Immune-suppressive DCs also play a critical role in maintaining effector cell quiescence in the tumor microenvironment (Fricke and Gabrilovich, 2006). Tolerogenic DCs can expand immune-suppressive T-regulatory cells (T-regs), which function to inhibit effector cell proliferation and activation. DCs can also express inhibitory molecules such as programmed death ligand receptors (PDL1 and PDL2), CD103, arginase or the immunosuppressive enzyme IDO, which is the focus of this article.
In addition to balancing immune activation and suppression, DCs can qualitatively influence the type of immune response. DCs that produce large amounts of IL-12 can induce very potent TH1 responses and CD8+ T-cell and NK-cell activation, important for fighting viral infection or other intracellular pathogens. Conversely, DCs may produce cytokines such as IL-6 and IL-23, directing the immune activity towards a TH17 response, which has been shown to play an important role in recruitment of neutrophils and macrophages, immune responses against fungal infections such as Candida albicans, and in autoimmune diseases (Dong, 2008). Interestingly, DC production of tryptophan metabolites through IDO can also alter the polarization of T-cells, as well as the direction of the immune response, which is discussed next.
In conclusion, DCs are highly plastic in nature and regulate immune activity by presenting antigen in an immunogenic or tolerogenic fashion via production of numerous immune-activating or immune-inhibitory molecules. Furthermore, DCs can polarize these responses via secretion of different cytokines. The sections below focus on dendritic cell expression of the immunosuppressive enzyme IDO, specifically how it is induced in DCs, which subsets it is induced in, how it is maintained, and the consequences of IDO-expression by DCs in different biological contexts.
Indoelamine 2,3-dioxygenase
Indoelamine 2,3-dioxygenase is an enzyme that catabolizes the essential amino acid tryptophan into the stable metabolite, kynurenine. IDO activity has been found to greatly impact peripheral tolerance and immune regulation. The major mechanisms underlying IDO-mediated immune suppression are outlined below. Further information on the diverse biological activities of IDO and other tryptophan metabolizing enzymes can be found in several excellent reviews (Johnson et al., 2009; Mellor, 2005; Soliman et al., 2010).
The immunosuppressive activity of IDO was first speculated to be solely a function of the physical depletion of tryptophan from the environment, thus “starving” T-cells or other effector cells. Tryptophan starvation can be sensed by cells via activation of GCN2 (general control nonrepressed 2) kinase, which directly binds uncharged tRNAs (Munn et al., 2005). It was found that tryptophan depletion resulted in activation of the GCN2 pathway, the down-regulation of CD3 zeta-chain in CD8+ T-cells (Fallarino et al., 2006) and inhibition of TH17 cell differentiation (Yan et al., 2010). GCN2 also contributes to T-regulatory cell generation in an IDO-high environment (Fallarino et al., 2006).
The second mechanism of immune suppression elicited by IDO is due to the direct effects of the tryptophan metabolites, such as kynurenine on target cells (Frumento et al., 2002; Jasperson et al., 2009; Terness et al., 2002; Zhu, 2010). In addition to kynurenine, other tryptophan metabolites produced by IDO such as 3-hydroxyanthranilic acid and 3-hydroxykynurenine can also induce immunosuppression (Favre et al., 2010; Terness et al., 2002; Yan et al., 2010). In the case of CD4+ T-cells, an environment high in tryptophan metabolites favors expansion of Foxp3+ T-regulatory cells (Fallarino et al., 2006; Favre et al., 2010; Harden et al., 2011). Thus the combined actions of IDO, i.e., direct suppression of effector T-cell activity and concurrent expansion of T-regulatory cells, highlights its pleiotropic functions in immune suppression.
The molecular pathways involved in kynurenine sensing and subsequent development of the immune-suppressive phenotype are not well-understood. Recently, it was discovered that kynurenine can bind to a ligand-activated transcription factor, the aryl-hydrocarbon receptor (AhR) and cause phenotypic changes in immune cells (Mezrich et al., 2010). Specifically, it was found that kynurenine interaction with the AhR in CD4+ T-cells resulted in their polarization to a T-regulatory cell phenotype, thus explaining how this IDO metabolite can directly mediate an immunosuppressive environment (Mezrich et al., 2010).
The above mechanisms are not mutually exclusive, and it is likely that both the physical depletion of tryptophan and the direct action of the metabolites are responsible for the broad immunosuppressive actions of IDO (Fallarino et al., 2006).
Finally, the inhibitory effects of IDO appear to be largely confined to the immune compartment (Habibi et al., 2010; Forouzandeh et al., 2008). In these studies, the resistance of non-hematopoietic cells to IDO was linked to expression of the protein IMPACT (imprinted and ancient), which inhibits GCN2 activation and sensitivity to tryptophan starvation. However, additional unknown mechanism(s) may also be involved in IDO-resistance as forced expression of IMPACT in Jurkat cells (T-cells) rendered them resistant to tryptophan starvation, but did not endow resistance to the tryptophan metabolites (Habibi et al., 2010).
IDO EXPRESSION IN DCs
In the physiological setting, IDO expression by antigen presenting cells is essential to fetal tolerance (Munn et al., 1998; Zhu, 2010); to oral tolerance and maintenance of the immunosuppressive environment in the gut (Matteoli et al., 2010; Onodera et al., 2009); and to suppression of harmful autoimmune activity in general (Grohmann et al., 2003a; Yan et al., 2010). Additionally, IDO-knockout mice experience acute rejection of transplanted MHC-mismatched grafts, and wild-type mice with high tryptophan catabolism experienced longterm survival of the graft (Sucher et al., 2012) demonstrating a critical function for IDO in transplant tolerance.
Expression of this enzyme in other settings however, may not be as beneficial to the individual. For example, IDO activity can be detrimental to mounting an effective immune response against certain pathogens or tumors. In fact, IDO-deficient mice have a tumor-resistant phenotype in a carcinogenesis model of skin papilloma formation (Muller et al., 2008).
IDO Production by Different DC-Subsets
Dendritic cells are a highly heterogeneous cell type, and specialized subsets of DCs allow delegation of different APC functions. Similarly, the ability of DCs to produce IDO does not seem to be equally distributed among the various subsets. CD8α positive DCs express higher amounts of IDO compared to CD8α-negative DCs (Fallarino et al., 2002, 2003). In response to IFNγ, CD8α-positive DCs rapidly express IDO and establish immunological tolerance (Grohmann et al., 2003b). These findings are intriguing in that the CD8α positive DCs have also been identified as the most effective antigen cross-presenters and producers of IL-12 (den Haan et al., 2000; Maldonado-Lopez et al., 1999). CD8α-positive DCs therefore seem to have an inherent dichotomous nature, and may just be the most potent APC, in both tolerogenic and immunogenic settings. As mentioned above, CD8α-negative conventional myeloid DCs, which comprise the majority of the DC found in secondary lymphoid organs, can also express IDO in response to stimuli including IFNγ, CTLA-4-Ig (Fallarino et al., 2003) or ligation with endogenous CTLA-4 on T-regs (Onodera et al., 2009). In this context, whether the CD8α-negative DCs perform a distinct function or simply act as secondary responders that further enhance IDO-mediated tolerogenicity in the peripheral lymphoid tissue is not known.
CD103 expression on DCs has been associated with an immunosuppressive phenotype, and CD103-positive DCs play a central role in the maintenance of gut homeostasis and oral tolerance via production of TGFβ and retinoic acid (Coombes et al., 2007; Sun et al., 2007). However, it was also found in the gut that CD103-positive DCs express higher levels of IDO compared to CD103-negative DCs, and that IDO was very important to the tolerogenic properties of CD103-expressing DCs (Matteoli et al., 2010). Additionally, most of the CD103-positive DCs were also CD8α- and B220-negative, demonstrating again that conventional, myeloid DCs (cDCs) can express IDO.
Plasmacytoid DCs (pDC) have also been described as having high capacity to produce IDO (Daissormont et al., 2011; Lu et al., 2012). Classically known as a critical source of type 1 interferons, pDCs modulate the immune response primarily via cytokines. Sharma et al. (2009) demonstrated that IDO+ pDC could control CD4+ T-cell plasticity. However, pDCs have been described as rather poor at antigen uptake, presentation, and priming compared to cDCs (Reizis et al., 2011) and therefore their expression of IDO, and subsequent production of kynurenine may modulate the immune response at effector sites, rather than modulating T-cell dynamics during priming (where cDC-derived IDO may play a larger role). Indeed, pDCs in the actual physical lesions and plaques of atherosclerosis produce IDO, and depletion of these IDO expressing pDCs resulted in increased T-cell proliferation and pronounced inflammation at the site of the lesion (Daissormont et al., 2011).
Recently, a very specific DC subset has been coined as the true IDO competent DC subset (Baban et al., 2009; Johnson et al., 2010). Phenotypically, these DCs are identified as being CD19+, B220−, CD11c+ and are positive for the B-cell lineage transcription factor Pax5 (Johnson et al., 2010). Additionally, CD19+ DCs coexpress CD8α, which might account for others’ observations that theCD8α expressing DCs are the most potent expressers of IDO. Whereas CD19+ DCs appear to be the primary splenic DC subset that up-regulate IDO in response to CTLA-4 ligation and TLR9 signaling (Baban et al., 2005; Mellor et al., 2005), whether this property extends to all IDO-inducers is not clear. Therefore, not all potential IDO-eliciting agents may work on just any DC subset, but rather specific DC subsets may respond to discrete sets of stimuli for induction of tryptophan catabolism.
Immune Suppressive Agonists of IDO
A number of molecules that induce immune suppression or tolerance have been shown to mediate their activity via IDO. For example, ligation of B7 molecules on the DC with CTLA-4, a co-inhibitory molecule expressed on T-regulatory cells, can induce IDO expression in DCs (Fallarino et al., 2003; Mellor et al., 2004). Administration of CTLA-4-Ig to mice prone to spontaneous abortions enhanced IDO expression with concomitant induction of T-regulatory cells resulting in improved pregnancy outcome (Li et al., 2009). Interactions between PD-1 on T-cells with programmed death-ligands on the DC can also promote the up-regulation of IDO (Baban et al., 2005, 2011; Grohmann et al., 2002). Additionally, the immune suppressive cytokine TGFβ can elicit and maintain IDO expression in plasmacytoid DCs, as well as in conventional CD8α negative and CD8α positive splenic DCs (Belladonna et al., 2008, 2009; Chen, 2011; Pallotta et al., 2011).
STAT-3 is a well known negative regulator of immunogenic DC function, and activated, acetylated STAT-3 was found to induce expression of IDO in bone marrow derived DCs (BMDCs) (Sun et al., 2009). Others demonstrated that a classical immune-suppressive transcription factor, Foxp3, which is normally only expressed by regulatory T-cells, can also confer tolerogenicity when ectopically expressed in DCs (Lipscomb et al., 2010; Munn, 2010). Specifically, Foxp3 expression in DCs induced IDO, resulting in a loss of T-cell priming ability (Lipscomb et al., 2010; Munn, 2010). The true biological significance of these experiments remains to be determined, but the data point to a novel mechanism of creating tolerogenic DC in vitro. A related transcription factor, Foxo3, was shown to be up-regulated in tumor-associated dendritic cells and correlated with their tolerogenic phenotype (Watkins et al., 2011). Silencing of Foxo3 in tumor-associated DCs resulted in decreased expression of several tolerogenic mediators including IDO, confirming the link between Foxo3 and the tolerogenic phenotype (Watkins et al., 2011).
In regards to tolerance induction in the female reproductive tract, an undefined soluble tolerogenic mediator produced by human uterine epithelial cells was able to condition DCs to express IDO, and resulted in decreased T-cell priming abilities (Ochiel et al., 2010). One of the earliest signaling molecules produced by an embryo is human chorionic gonadotropin (hCG), which was also found to induce IDO expression in DCs (Tsampalas et al., 2010; Wan et al., 2008). Interestingly, some tumors can also produce hCG, which may be a mechanism to induce tryptophan catabolism and facilitate tumor-immune escape (Tsampalas et al., 2010).
“Two-Signal” Requirement for IDO-induction
In addition to the qualitative effects mediated by the signaling molecules, the quantity of the signal can affect IDO expression. TLR ligands, which are often used as adjuvants in various experimental settings, can also induce the expression of IDO. Indeed, systemic high-dose administration of the TLR9 agonist CpG, induced IDO expression in DCs, whereas lower concentrations did not (Baban et al., 2011). However, the majority of data point to a “co-stimulation” or “two-signal” requirement for IDO-induction, rather than a simple signaling threshold requirement for inducing its expression in DCs.
IFNγ alone can induce up-regulation of IDO message in DCs (Hwu et al., 2000; Lu et al., 2012); however, addition of a second stimulus, such as CD40L or LPS, results in significantly higher amounts of IDO-induction and tryptophan degradation (Favre et al., 2010; Hwu et al., 2000; Jurgens et al., 2009). Therefore, similar to the requirement for MHC-TCR interaction and co-stimulation in T-cell activation, DCs may also require “two signals” to express physiologically relevant levels of IDO.
Several other studies have demonstrated a role for T-cells and T-cell derived mediators in eliciting IDO from DCs (Hwu et al., 2000; Lu et al., 2012). Although high-dose CpG can induce IDO expression in CD19+ DCs (through activation of the TLR9), this signal alone was not sufficient in RAG1-KO mice, suggesting a necessary role for additional T-cell interactions with DCs (Baban et al., 2011). To this end, 4-1BB/4-1BB-ligand triggered IDO expression in DCs was found to require T-cell production of IFNγ (Choi et al., 2011). Similarly, after allogeneic bone marrow transplant, IFNγ secretion from donor T-cells was essential for induction of IDO and subsequent protection from graft versus host disease (Jasperson et al., 2009). Finally, IFNγ receptor KO mice succumbed to GVHD very rapidly, due to the failure of IDO expression in APCs (Jasperson et al., 2009).
Certain IDO signaling molecules, such as CD28 on T-cells and B7-molecules on DC are present in steady-state conditions, yet these DCs do not express IDO, consistent with a “multiple signaling” requirement for true induction of tryptophan degradation. Further support for this hypothesis is provided by the observation that DC interactions with T-cells can recondition the dendritic cell to switch from an immunogenic to a regulatory phenotype. For example, Grohmann et al. (2003b) found that an immunogenic DC could become tolerogenic (express IDO) after ligation with CTLA-4. It is thus likely that in this setting immunogenic activation constitutes “signal one,” and ligation of CTLA-4 provides “signal two” for induction of tryptophan catabolism. Conversely though, tolerogenic IDO+ DC can become immunogenic after CD40 ligation (Grohmann et al., 2003b).
The latter observation is puzzling considering that CD40L can act synergistically with IFNγ to induce IDO expression in DCs (Hwu et al., 2000). These somewhat contradictory findings raise the possibility that different subsets of DCs may have differential requirements for IDO induction. Specifically, DCs that express IDO in naïve mice during steady-state conditions (Grohmann et al., 2003b) may have different requirements for their induction and maintenance of IDO (or conversion to an immunogenic profile) compared to DCs, which express IDO in response to pro-inflammatory stimuli, such as IFNγ and CD40L.
IDO-eliciting “signal two” may also be supplied by DC in an autonomous manner. Both autocrine IFNα (Manlapat et al., 2007) or TGFβ production (Belladonna et al., 2008), elicited after a “first” IDO signal, were shown to be required for acquisition of a tolerogenic phenotype in CD19+ and CD8α+ DCs, respectively. Moreover, autocrine TNFα production after infection of DCs with Chlamydia pneumoniae was found to be necessary for post-infection up-regulation of IDO in DC (Njau et al., 2009). These observations of autocrine signaling might explain the reports of IDO induction by a single inducer. Furthermore, the previously observed signaling threshold requirement for IDO induction may not just pertain directly to the quantity of the “single IDO-inducer” that is required, but rather may represent the necessity for a certain level of “signal one” to elicit an autocrine “signal two.”
Support for the “two signal” requirement for IDO-expression has also been obtained in human samples (Favre et al., 2010; Hwu et al., 2000; Njau et al., 2009). For example, human monocytes expressed IDO in lepromatous leprosy due to the synergistic actions of IFNγ and the bacterium (likely through pattern recognition receptor signaling) (de Souze et al., 2011). Human-monocyte derived DCs also express IDO and tryptophan metabolizing activity after exposure to IFNγ, however stimulation with IFNγ and a second agonist significantly enhanced IDO levels (Favre et al., 2010).
Not all potential IDO signals can work together as “signal one” and “signal two” to ultimately elicit a tolerogenic phenotype. For example, whereas CD40L and LPS can work synergistically with IFNγ to up-regulate IDO expression (Hwu et al., 2000), CD40L and LPS together are rather weak inducers of IDO. This suggests that a potent “signal one” (such as IFNγ) is required before “signal two” can impart full tryptophan metabolizing ability. Additionally, during an immune response both the “immunogenic IDO agonists” such as B7 molecules and pro-inflammatory cytokines, as well as the “tolerogenic IDO agonists” such as CTLA-4 and PD-1 are up-regulated, thus allowing heightened potential for DCs to receive both “signal one” and “signal two” for the induction of IDO.
In contrast, certain primary signals may actually program a distinct phenotype within the DC to actively inhibit the IDO-eliciting effects of a “signal two”. Orabona et al. (2004) demonstrated that CD28 ligation of DCs resulted in autocrine production of IFNγ, but failed to induce IDO. Further analysis demonstrated that this was due to co-induction of autocrine IL-6 and p38 mitogen-activated protein kinase that inhibited the induction of IDO. Therefore, the order and timing of distinct “signal ones” and “signal twos” may play an important role in the final elicitation of tryptophan catabolism in DCs. Collectively, these data support the idea that IDO is an important homeostatic regulatory molecule that is up-regulated in response to multiple yet specific signals, and is not elicited unless a bona fide immune response is induced.
Positive Feedback Loops – Autocrine Kynurenine
Basal levels of tryptophan degradation in particular dendritic cell subsets may also influence the ability to respond to IDO agonists, either by direct actions of the metabolites or sensing of tryptophan depletion. Manlapat et al. found that in regards to the CD19+DC cell subset, pharmacological inhibition of basal IDO levels before stimulation by B7 ligation resulted in loss of autocrine IFNα, which can feed-forward to elicit more IDO (Manlapat et al., 2007). Conversely, basal tryptophan catabolism did not affect autocrine IFNα induction after TLR9-ligation and the subsequent IDO up-regulation, again demonstrating differential requirements and ordering of “signal one” and “signal two.” Plasmacytoid DCs from GCN2-KO mice (which can not sense tryptophan depletion), fail to up-regulate IDO in response to PMA (Muller et al., 2008). Therefore, DC sensing of tryptophan degradation may function as one of the “signals,” and may play a role in the maintenance of IDO.
The aryl-hydrocarbon receptor, a transcription factor that has been extensively studied in the toxicology field, is well known for binding to the environmental toxin 2,3,7,8-tetrachlorodibenzo-p-dixoin (TCDD/dioxin) and mediating an array of suppressive effects on the immune system (Stockinger et al., 2011; Veldhoen and Duarte, 2010). AhR activation in dendritic cells supports a tolerogenic DC phenotype which promotes T-regulatory cell expansion (Quintana et al., 2010). Dioxin activation of the AhR in DCs induces their expression of IDO, providing one mechanism of the immunosuppressive ability of dioxin (Mezrich et al., 2010; Simones and Shelpherd, 2011). Recently, the IDO metabolite kynurenine was found to be an endogenous ligand for the AhR (Mezrich et al., 2010). Thus, since the AhR is important for IDO induction (Nguyen et al., 2010), and is activated by kynurenine, this mechanism may in part explain how basal levels of tryptophan metabolites are directly sensed by DCs, identifying the kynurenine-AhR axis as an autocrine signal necessary for IDO induction.
Recently, our laboratory has found that IFNγ can directly induce the up-regulation of AhR expression (in addition to IDO mRNA) in splenic DCs in vitro, and in lymph node DCs in vivo as determined by quantitative RT-PCR, after IL-12 and GM-CSF microsphere immunotherapy (Harden JL and Egilmez NK, unpublished observations). This finding, in conjunction with the above findings that kynurenine promotes IDO via AhR, provides an explanation as to how IDO expression can be maintained even after IFNγ signaling subsides. Specifically, the data are consistent with the notion that the initial “spark” provided by the IFNγ-IDO-kynurenine axis results in continuous AhR signaling by autocrine kynurenine, which maintains IDO activity even in the absence of further IFNγ signaling.
PHYSIOLOGICAL CONSEQUENCES OF IDO EXPRESSION IN DCs
IDO as a Component of Homeostatic Immune Regulation
In numerous settings, DCs express IDO as part of a “feedback” process to limit chronic or hyper-activation of the immune system. For example, Dendritic cells that produce IDO can suppress effector T-cell proliferation (Harden et al., 2011; Hwu et al., 2000; Terness et al., 2002), induce down-regulation of the CD3ζ-chain in T-cells, and promote expansion of Foxp3+ regulatory T-cells (Fallarino et al., 2006; Harden et al., 2011). Expression of IDO can also skew CD4+ T-helper cell phenotype from one of TH1 or TH17, to that of a Foxp3+ T-regulatory cell (Baban et al., 2009; Liu et al., 2010; Matteoli et al., 2010; Sharma et al., 2009).
Many molecules that induce IDO expression in DCs have also been found to be involved in the initiation of the immune response. To this end, Muller et al. (2008) demonstrated that plasmacytoid DCs (pDCs) failed to have IDO mediated immunosuppressive abilities if the DCs came from IFNα receptor KO, IFNγ receptor KO, or MyD88 KO mice.
Reagents often used to mature DCs, and in fact, just the process of DC maturation itself often leads to IDO-up-regulation. Indeed, mature DCs express higher levels of IDO and catabolize tryptophan more effectively compared to immature DCs (Chung et al., 2009). For example, human DC matured in vitro by IL-1γ, TNFα, IL-6, and Prostaglandin E2 (PGE2) up-regulated expression of IDO resulting in enhanced tryptophan degradation (Wobser et al., 2007). Dendritic cells matured with LPS or CpG also increased expression of the aryl hydrocarbon receptor (AhR) (Nguyen et al., 2010), which as discussed later in this review, may be critical to the induction and maintenance of IDO in DCs. Additionally, B7 family co-stimulatory molecules (CD80 and CD86), which are up-regulated on mature DCs, can transduce signals that elicit IDO (Fallarino et al., 2003; Grohmann et al., 2003b; Mellor et al., 2004; Nair et al., 2011). Consistent with these findings, DC that lack costimulatory molecules were found to be inferior in attenuating established autoimmune disease compared to costimulatory-sufficient DCs (O’Sullivan et al., 2011).
IFNγ is a crucial cytokine for TH1 responses, including responses to intracellular pathogens and cancer. Conversely, IFNγ can induce IDO in DCs thus limiting the extent of the immune response (Favre et al., 2010; Gu et al., 2010; Harden et al., 2011; Jurgens et al., 2009). Our laboratory has shown that a single, intra-tumoral injection of slow-release IL-12 and GM-CSF microspheres results in the induction of potent anti-tumor immunity (Egilmez et al., 2007; Gu et al., 2010; Kilinc et al., 2006, 2009a, 2009b; Nair et al., 2006). The beneficial effects elicited after IL-12 and GM-CSF treatment were found to be critically dependent upon host production of IFNγ (Harden et al., 2011; Kilinc et al., 2006 2009a, 2009b). However, in this model, IFNγ also induced the expression of IDO in DC, which in turn promoted the expansion of T-regulatory cells resulting in premature termination of effector cell activity (Gu et al., 2010; Harden et al., 2011). Post-treatment IDO expression in myeloid DCs was independent of tumor-produced factors and was likely part of homeostatic immune regulation.
Another cytokine, IL-32, known to be elevated in inflammatory conditions, was recently found to induce IDO in human DCs (Smith et al., 2011). Therefore, pro-inflammatory cytokines, such as IFNγ and IL-32 that are critical to the development of effector responses, may have dichotomous properties, driving not only the initial effector reponse but also the subsequent regulatory activity. In instances of acute infection, such a mechanism would be beneficial by allowing the resolution of inflammation. However, in the tumor setting, where a more prolonged and sustained anti-tumor response is desired, IDO may short-circuit the effectiveness of immunotherapies.
T-cell co-stimulatory molecules can also elicit DC production of IDO. For example, binding of CD28 (Nair et al., 2011) and 4-1BB (Choi et al., 2011) to their ligands on DCs (B7 and 4-1BBL, respectively) resulted in the up-regulation of IDO. However, the functional involvement of T-cell accessory molecules in IDO activity can be complex. For example, two molecules with opposing functions in T-cell physiology, i.e., CD28 and CTLA-4, can both induce IDO expression in DC via interactions with B7-molecules. Separately, in a model of murine cardiac allograft rejection, administration of a CD28 blocking antibody, promoted overall allograft survival, yet resulted in decreased IDO (Zhang et al., 2011). In contrast, the administration of a CTLA-4 blocking antibody resulted in complete allograph rejection and a severe reduction in the IDO levels (Zhang et al., 2011). In this study the CD28 and CTLA-4 blocking reagents were used in combination with a CD154 (CD40L) blocking antibody and/or calcineurin inhibitors, and the effect of blocking CD28 or CTLA-4 in the absence of CD40L on IDO expression was not determined making interpretation difficult.
The exact nature of the mechanisms underlying the seemingly contradictory observations above, that is both immunogenic and tolerogenic agonists can induce IDO expression in DC; and that an immunogenic agonist can also mediate dichotomous effects (i.e. eliciting both DC activation as well as the subsequent tolerogenic phenotype), are yet to be defined. The answer may lie within the timing of the immunogenic versus IDO+ tolerogenic phenotypes, as elicited by the agonist. Quantitative analysis of the transcriptional kinetics of proinflammatory cytokines and IDO in splenic DCs stimulated in vitro with IFNγ showed that up-regulation of immunogenic cytokine mRNAs (such as TNFα) preceded IDO (occurring within 2 hours post-stimulation versus 5 hours after stimulation, respectively; Harden JL and Egilmez NK, unpublished observations). It might also be possible that a particular DC subset becomes immunogenic whereas another DC subset becomes tolerogenic in response to the agonist or its downstream effectors. The plasticity of different DC subsets is still not fully understood, however certain DC subsets are described to produce higher amounts of IDO compared to others as discussed above. Finally, the “two-signal” requirement may also play a role in the switch from an immunogenic to a tolerogenic phenotype.
IDO in Infectious Disease
IDO expression by DCs can modulate immunity to infection. Hoshi et al. (2010) found that IDO−/− mice, or WT mice treated with 1-MT, had a greater survival rate in response to murine retrovirus infection along with increased production of type-1 interferons suggesting a detrimental role for IDO in retroviral infection. In other infectious settings however, a robust immune response can have deleterious effects on the host, such as during influenza infection. Mice infected with influenza virus experienced improved survival in a setting where IDO expression was induced by 4-1BB triggering (Choi et al., 2011).
On the other hand, in parallel experiments by the same group, 4-1BB-mediated elicitation of IDO resulted in increased disease incidence and increased disease severity in mice infected with herpes simplex virus 1 (HSV-1). The tryptophan catabolism elicited in these two different disease models resulted in the same immunosuppressive effects, i.e., loss of CD4+ T-cells, and apoptosis of newly activated effector T-cells and effector-memory T-cells. The contrasting outcomes observed in influenza versus retroviral/HSV-1 infection are likely a reflection of the important role of IDO in maintaining the fine balance between over and under-activation of effector responses in acute infection.
In chronic viral infections, such as HIV, prolonged activation of IDO+ pDC can result in the inhibition of antiviral T-cell responses (Boasso et al., 2011). In the gut of HIV-infected individuals, IDO expression by DCs also results in induction of regulatory T-cells and a subsequent detrimental shift in the TH17/T-reg ratio (Favre et al., 2010). HIV infected individuals also have increased levels of the pro-inflammatory cytokine IL-32, which strongly correlated with increased levels of IDO, permitting both viral replication and major susceptibility to secondary infections (Smith et al., 2011). Enhanced tryptophan catabolism has also been associated with poor prognosis in HIV patients (Favre et al., 2010). These findings correspond to the fact that mortality from HIV is due to excessive immune suppression (in part mediated by IDO), rather than over-activity of the immune system or direct action of the virus on the host.
Several investigators have reported that IDO induction after bacterial infection could be beneficial to the host, resulting in tryptophan depletion and starvation of the bacteria (Muller et al., 2009; Njau et al., 2009). Based on such observations, it has been proposed that IDO may have been originally acquired by mammalian cells as a defense mechanism to starve pathogens of tryptophan, but was subsequently incorporated into the homeostatic immune regulatory processes of the host. Therefore, the role of IDO in bacterial infections is complex.
For example, Mycobacteriumleprae was found to increase IDO expression in human monocytes; and skin biopsies of leprosy patients demonstrated that IDO levels were highest in those patients with the more severe lepromatous disease, compared to less severe tuberculoid and reversal reaction patients (de Souze Sales et al., 2011). Conversely, Rhodococcus equi infection in IDO−/− mice compared to WT mice demonstrated no difference in bacterial growth; however, IDO−/− mice experienced pronounced inflammation and increased immune pathology (Heller et al., 2010). Recently, it has been described that IDO is antimicrobial solely via depletion of tryptophan, whereas immune cells can be influenced by both the loss of tryptophan, as well as the metabolites (Muller et al., 2009).
Additionally, bacteria require significantly higher amounts of tryptophan for growth compared to T-cells (Muller et al., 2009). Therefore, IDO expression may initially limit bacterial growth but could subsequently affect host immunity when tryptophan depletion reaches a critical threshold. In this context, the proper balance between IDO-mediated antimicrobial function versus inhibition of host immunity may determine the ultimate outcome for the host.
IDO in Cancer
In the context of the tumor microenvironment, expression of IDO by DCs can inhibit an effective anti-tumor response in both murine tumor models (Huang et al., 2011; Muller et al., 2008; Munn et al., 2004) and patients (Kuales et al., 2011; Lee et al., 2011; Wobser et al., 2007). Dendritic cells from melanoma patients show expression of IDO mRNA (Wobser et al., 2007). Human studies involving squamous cell carcinoma and melanoma patients also demonstrated a correlation between tumor burden and IDO expression by DCs (Kuales et al., 2011; Lee et al., 2011). Separately, aberrant expression of CD28 on myeloma cells allowed their interaction with stromal DCs in the bone marrow microenvironment, resulting in DC expression of IDO and prolonged survival of the myeloma cells (Nair et al., 2011).
MAINTENANCE OF THE IDO-MEDIATED TOLEROGENIC ENVIRONMENT
IDO is expressed constitutively in physiological settings where maintenance of immune tolerance is critical to the survival of the organism, i.e., fetal tolerance and gut homeostasis (Matteoli et al., 2010; Munn et al., 1998; Onodera et al., 2009; Zhu, 2010). This observation has been extended to other non-physiological settings as well. For example, in a model of murine renal allograft acceptance, it was found that although the immune suppressive markers TGFβ and Foxp3 transiently increased after transplant acceptance, the expression of IDO in intragraft DCs was persistent and continued to increase beyond 150 days post-transplant (O’Sullivan et al., 2011). In the acute inflammatory setting, on the other hand, IDO is induced by transient signals such as IFNγ yet expression of IDO can remain high long after this initial signaling fades (Gu et al., 2010). Therefore, a clinically relevant question regarding IDO is how it is maintained in the long-term.
Several studies suggest that positive feedback pathways may be involved in maintaining a tolerogenic environment. A recent paper by Pallotta et al. described a mechanism for chronic expression of IDO in pDCs (Pallotta et al., 2011). This study revealed a novel function for IDO independent of its enzymatic activity. Specifically, the authors reported that in pDCs IDO can function as a signaling molecule via phosphylation of ITIMs found at sites distant from the catalytic domain. They demonstrated that binding of SHP to phosphorylated ITIMs on IDO initiated a signaling cascade that resulted in autocrine production of TGFβ by pDCs. In contrast to IFNγ, which only promotes a robust, but transient activation of the Ido1 promoter, autocrine TGFβ induced Ido1 promoter activity in pDCs in a prolonged fashion (Chen et al., 2011; Pallotta et al., 2011), thus providing a mechanism of IDO maintenance in pDCs.
Alternatively, the IDO metabolite, kynurenine, can bind to and activate the aryl-hydrocarbon receptor (Mezrich et al., 2010), and AhR activation in DCs can result in the induction of IDO (Benson et al., 2011; Mezrich et al., 2010). Therefore, it will be important to determine if those reagents that are potent inducers of IDO, might also induce increased AhR expression in DCs, thus maintaining IDO at high levels using this positive feed forward mechanism. Consistent with this notion, Nguyen et al. (2010) demonstrated that LPS or CpG can result in increased AhR expression in bone marrow derived DCs, along with increased IDO expression. Importantly, recent preliminary data from our laboratory revealed that IFNγ, a potent inducer of IDO, increased AhR expression in DCs and allowed these DCs to maintain high levels of IDO even after IFNγ signaling ceased (Harden JL and Egilmez NK, unpublished observations). Therefore, initial induction of IDO may be IFNγ mediated, but maintenance of IDO after IFNγ signaling has ceased may be through the kynurenine-AhR positive feedback loop.
In a murine model of GVHD, survival of mice was 100% when treated daily with tryptophan metabolites (Jasperson et al., 2009). However, when injections ceased, the mice rapidly succumbed to GVHD. In this study, the mechanism of action may have involved the direct inhibitory effects of the metabolites on T-cells and/or the maintenance of IDO via the kynurenine-AhR pathway as discussed above. Separately, another IDO-produced tryptophan metabolite, 3-hydroxyanthranilllic acid (3-HAA) has been found to induce TGFβ in DCs (Yan et al., 2010). Because TGFβ can also induce IDO-expression in DCs (Chen et al., 2011; Pallotta et al., 2011), it is possible that a TGFβ-IDO-tryptophan metabolite axis may also play a role in the maintenance of an immunosuppressive environment. In CD4+ T-cells, TGFβ has been found to increase AhR expression, allowing these T-cells to be more responsive to kynurenine via the AhR, and polarize to a T-regulatory cell phenotype (Mezrich et al., 2010). Whether TGFβ can similarly enhance AhR expression in DCs and thus potentially aid in IDO maintenance is not yet known.
TGFβ also promotes regulatory T cell differentiation, and therefore continual DC:T-regulatory cell interactions, possibly via binding of CTLA-4 to B7, might also help maintain the tolerogenicity of DCs. Indeed, it was found that IDO expressing DCs in accepted renal allografts were found in close proximity to T-regulatory cells, suggesting that mutual interactions between these cells resulting in a persistent immunosuppressive environment. Similarly, constitutive IDO expression by DCs in the mesenteric lymph nodes (MLN) was maintained by continual T-regulatory cell: DC interactions via CTLA-4 and B7 (Onodera et al., 2009). IDO expressing DCs in the MLN also produced CCL22, which recruited CCR4 expressing T-regulatory cells. MLN DCs expressed much lower amounts of IDO in CCR4−/− mice, and in mice with T-reg specific loss of CTLA-4 (Onodera et al., 2009). Therefore, in anatomical locations of natural IDO production, such as in the gastrointestinal tract, positive feedback interactions between T-regulatory cells and tryptophan metabolizing DCs may help maintain IDO expression.
Spreading of tolerance (also known as “infectious tolerance”) from one small subset of DCs to another or multiple subsets of DCs, may also play a role in DC acquisition of IDO-positivity and maintenance of IDO-mediated tolerogenicity over the long term. Expression of the AhR by different DC subsets may allow for infectious tolerance. Specifically, kynurenine produced by a unique DC subset could potentially bind to the AhR in non-IDO-producing DCs, and initiate tryptophan catabolism. Additionally, low tryptophan environments can influence DC differentiation, resulting in acquisition of an immunosuppressive phenotype characterized by expression of inhibitory receptors and reduced up-regulation of co-stimulatory molecules (Brenk et al., 2009). Tryptophan metabolites can also induce cell death of T-cells, B-cells, and NK cells, but not DCs (Terness et al., 2002), explaining how DCs can remain in the IDO-high environment and could perpetuate continued expression of IDO.
Infectious tolerance and transfer of IDO positivity from one DC to another may be very important in long-term maintenance of IDO in a particular microenvironment over a period of several months, considering the normal life span of an immature DC ranges from a few days to weeks, and even shorter once it is matured (Granucci and Zanoni, 2009; Kamath et al., 2002). To this end, exposure of newly infiltrated DCs to previously deposited tryptophan metabolites, or interaction with pre-existing “IDO-induced” T-regulatory cells could perpetuate and sustain an IDO-high environment.
UTILITY OF CONTROLLING IDO EXPRESSION BY DCs
Due to the capacity of an IDO-rich environment to promote tumor growth through several mechanisms (such as expansion of T-regs or inhibition of effector T-cells), the benefits of inhibiting IDO in order to promote an effective immune response are well-justified. Inhibition of IDO in both human and murine DCs can be achieved via use of small molecule inhibitors, such as 1-MT (1-methyl tryptophan) (Hwu et al., 2000), or shRNA/siRNA (Flatekval and Sioud, 2009; Huang et al., 2011; Yen et al., 2009). To this end, Phase II clinical trials utilizing D-1MT are currently underway in cancer patients (NCT00567931). At the same time, the ongoing controversy regarding the relative efficacy and specificity of isomers of 1MT (D-1MT versus that of L-1MT) in targeting of IDO1 versus IDO2 has led to the design of new and more specific small molecule inhibitors that are being developed for clinical testing (Liu et al., 2010).
Although several groups have demonstrated modulation of tumor growth via inhibition of IDO alone (Huang et al., 2011, Liu et al., 2010), the use of IDO inhibition in combination with other immune-modulations has produced the most impressive results (Gu et al., 2010; Sharma et al., 2009). For example, combining 1-MT with a dendritic cell/tumor cell fusion vaccine improved antitumor responses by blocking steady-state IDO activity (Ou et al., 2008). Similarly, we recently demonstrated that IDO-inhibition can effectively diminish treatment-induced homeostatic counter-regulation that follows IL-12 therapy (Gu et al., 2010). In these studies, administration of D-1MT during IL-12 therapy blocked the post-treatment T-regulatory cell rebound that was driven by the IL-12-IFNg-IDO axis, enhanced the cytotoxic CD8+ T-cell activity window and resulted in the complete cure of established tumors in 45% of the mice (Gu et al., 2010).
Separately, the efficacy of traditional anti-cancer chemotherapeutics, such as cyclophosphamide, paclitaxel, and gemcitabine, were greatly improved when combined with IDO inhibition, resulting in suppression of tumor growth in several models (Hou et al., 2007). IDO inhibition was also found to improve the efficacy of a non-immunotherapy (a nicotinamide phosphoribosyl transferase enzyme inhibitor which targets cancer cells directly), and the additive effect of 1-MT with this small molecule inhibitor required an immune-competent recipient (Yang et al., 2010).
CONCLUSIONS
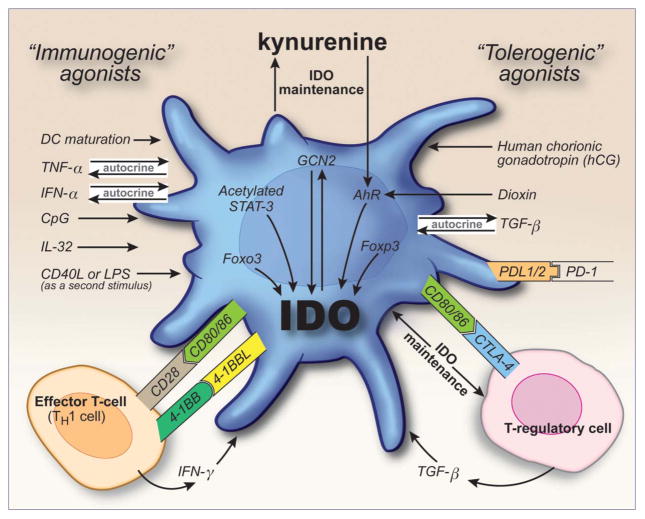
Dendritic cells are the master regulators of immune activity, and not only can induce an active immune response, but can also promote tolerogenicity. A major mechanism that DCs utilize to elicit immune tolerance involves the expression of the tryptophan metabolizing enzyme IDO. Dendritic cells can be induced to express IDO in multiple physiological settings, and both classical “tolerogenic” molecules and classical “immunogenic” molecules can elicit IDO (Figure 1 and Supplemental Table 1). The fact that such a multitude of cellular membrane ligands, soluble molecules and reagents can induce IDO expression in DCs highlights the importance of this enzyme in mediating immune tolerance. Additionally, induction of full tryptophan catabolism in DCs appears to often require multiple signals, similar to the co-stimulation requirement for T-cells. This mechanism may ensure that IDO expression is tightly controlled and is only elicited if it is truly required to dampen immune activity. Finally, dendritic cells are a heterogeneous population of cells, and the ability of DCs to express IDO appears to differ between distinct subsets.
Figure 1.
Induction and maintenance of IDO in dendritic cells. DCs can express the immunosuppressive enzyme, indoleamine 2,3-dioxygenase (IDO) in response to many immunogenic and tolerogenic molecules. Agonists for IDO induction in DCs include cytokines, Toll-like Receptor (TLR) ligands, co-stimulatory/co-inhibitory molecules and tryptophan catabolites. DCs may also secrete factors that work in an autocrine fashion to elicit IDO; conversely, other cells of the immune system, such as T-cells, can also influence the induction of tryptophan catabolism in DCs. Although IDO can be induced through short-lived signals, expression of IDO in DCs is often maintained long term. Plausible mechanisms involved in IDO maintenance include persistent T-regulatory cell:DC interactions, TGFβ signaling and/or the kynurenine-aryl-hydrocarbon receptor (AhR) pathway.
In many physiological settings, DC expression of IDO is beneficial to the organism, such as acceptance of the allogeneic fetus or in the maintenance of gut homeostasis. However, IDO also appears to be a critical feedback mediator in the termination of an immune response, which can be detrimental in some settings such as chronic infection and cancer. Moreover, once induced, IDO expression can be maintained long-term despite the typically short-lived signals provided by its agonists during the induction phase. The mechanisms underlying the sustained nature of IDO-mediated immune suppression are currently being elucidated, and several positive feedback loops have been identified.
Use of IDO inhibitors in the treatment of chronic infections or cancer may benefit patients by enhancing the intensity as well as the duration of cytotoxic immune effector activity. Conversely, the chronic nature of IDO expression may be an optimal target to purposely elicit long-term tolerogenicity, such as during transplant acceptance. In conclusion, further delineation of the mechanisms that regulate the induction and maintenance of IDO in DCs is likely to lead to the development of novel clinical interventions.
Supplementary Material
Footnotes
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Baban B, Hansen AM, Chandler PR, Manlapat A, Bingaman A, Kahler DJ, Munn DH, Mellor AL. A minor population of splenic dendritic cells expressing CD19 mediates IDO-dependent T cell suppression via type I IFN signaling following B7 ligation. Int Immunol. 2005;17:909–919. doi: 10.1093/intimm/dxh271. [DOI] [PubMed] [Google Scholar]
- Baban B, Chandler PR, Sharma MD, Pihkala J, Koni PA, Munn DH, Mellor AL. IDO activates regulatory T cells and block their conversion into Th17-like T cells. J Immunol. 2009;183(4):2475–2483. doi: 10.4049/jimmunol.0900986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baban B, Chandler PR, Johnson BA, III, Huang L, Li M, Sharpe ML, Francisco LM, Sharpe AH, Blazar BR, Munn DH, Mellor AL. Physiologic Control of IDO Competence in Splenic Dendritic Cells. J Immunol. 2011;187:2329–2335. doi: 10.4049/jimmunol.1100276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belladonna ML, Volpi C, Bianchi R, Vacca C, Orabona C, Pallotta MT, Boon L, Gizzi S, Fioretti MC, Grohmann U, Puccetti P. Cutting edge: Autocrine TGF-{beta} sustains default tolerogenesis by IDO-competent dendritic cells. J Immunol. 2008;181:5194–5198. doi: 10.4049/jimmunol.181.8.5194. [DOI] [PubMed] [Google Scholar]
- Belladonna ML, Orabona C, Grohmann U, Puccetti P. TGF-beta and kynurenine as the key to infectious tolerance. Trends Mol Med. 2009;15(2):41–49. doi: 10.1016/j.molmed.2008.11.006. [DOI] [PubMed] [Google Scholar]
- Benson JM, Shepherd DM. Dietary ligands of the aryl hydrocarbon receptor induce anti-inflammatory and immunoregulatoy effect on murine dendritic cells. Toxicol Sci. 2011;124(2):327–338. doi: 10.1093/toxsci/kfr249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boasso A, Royle CM, Doumazos S, Aquino VN, Biasin M, Piacentini L, Tavano B, Fuchs D, Mazzotta F, Lo Caputo S, Shearer GM, Clerici M, Graham DR. Overactivation of plasamacytoid dendritic cells inhibits antiviral T-cell responses: a model for HIV immunopathogenesis. Blood. 2011;118(19):5152–5162. doi: 10.1182/blood-2011-03-344218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenk M, Scheler M, Koch S, Neumann J, Takikawa O, Hacker G, Bieber T, von Bubnoff D. Tryptophan deprivation induces inhibitory receptors ILT3 and ILT4 on dendritic cells favoring the induction of human CD4+CD25+ Foxp3+ T regulatory cells. J Immunol. 2009;183(1):145–154. doi: 10.4049/jimmunol.0803277. [DOI] [PubMed] [Google Scholar]
- Buckwalter MR, Albert ML. Orchestration of the immune response by dendritic cells. Curr Biol. 2009;19(9):355–361. doi: 10.1016/j.cub.2009.03.012. [DOI] [PubMed] [Google Scholar]
- Chen WJ. IDO: more than an enzyme. Nat Immunol. 2011;12(9):809–811. doi: 10.1038/ni.2088. [DOI] [PubMed] [Google Scholar]
- Choi BK, Kim YH, Choi JH, Kim CH, Kim KS, Sung YC, Lee YM, Moffett JR, Kwon BS. Unified immune modulation by 4-1BB triggering leads to diverse effects on disease progression in vivo. Cytokine. 2011;55(3):420–428. doi: 10.1016/j.cyto.2011.05.015. [DOI] [PubMed] [Google Scholar]
- Chung DJ, Rossi M, Romano E, Ghith J, Yuan J, Munn DH, Young JW. Indoleamine 2,3-dioxygenase-expressing mature human monocyte-derived dendritic cells expand potent autologous regulatory T cells. Blood. 2009;114(3):555–563. doi: 10.1182/blood-2008-11-191197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, Hall J, Sun CM, Belkaid Y, Powrie F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007;204(8):1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daissormont IT, Christ A, Temmerman L, Sampedro Millares S, Seijkens T, Rousch M, Poggi M, Boon L, van der Loos C, Daemen M, Lutgens E, Halvorsen B, Aukrush P, Janssen E, Biessen EA. Plasmacytoid dendritic cells protect against atheroslerosis by tuning T-cell proliferation and activity. Circ Res. 2011;109(12):1387–1395. doi: 10.1161/CIRCRESAHA.111.256529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Haan JMM, Lehar SM, Bevan MJ. Cd8+ but not CD8− dendritic cells cross-prime cytotoxic T cells in vivo. J Exp Med. 2000;192(12):1685–1696. doi: 10.1084/jem.192.12.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Souze Sales J, Lara FA, Amadeu TP, de Oliveira Fulco T, da Costa Nery JA, Sampaio EP, Pinheiro RO, Sarno EN. The role of indoleamine 2, 3-dioxygenase in lepromatous leprosy immunosuppression. Clin Exp Immunol. 2011;165 (2):251–263. doi: 10.1111/j.1365-2249.2011.04412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong C. TH17 cells in development: an updated view of their molecular identity and genetic programming. Nat Rev Immunol. 2008;8:337–348. doi: 10.1038/nri2295. [DOI] [PubMed] [Google Scholar]
- Egilmez NK, Kilinc MO, Gu T, Conway TF. Controlled-release particulate cytokine adjuvants for cancer therapy. Endocr Metab Imm Disord Drug Targets. 2007;7 (4):266–270. doi: 10.2174/187153007782794335. [DOI] [PubMed] [Google Scholar]
- Fallarino F, Vacca C, Orabona C, Belladonna ML, Bianchi R, Marshall B, Keskin DB, Mellor AL, Fioretti MC, Grohmann U, Puccetti P. Functional expression of indoleamine 2,3-dioxygenase by murine CD8 alpha(+) dendritic cells. Int Immunol. 2002;14(1):65–68. doi: 10.1093/intimm/14.1.65. [DOI] [PubMed] [Google Scholar]
- Fallarino F, Grohmann U, Hwang KW, Orabona C, Vacca C, Bianchi R, Belladonna ML, Fioretti MC, Alegre ML, Puccetti P. Modulation of tryptophan catabolism by regulatory T cells. Nat Immunol. 2003;4(12):1206–1212. doi: 10.1038/ni1003. [DOI] [PubMed] [Google Scholar]
- Fallarino F, Grohmann U, You S, McGrath BC, Cavener DR, Vacca C, Orabona C, Bianchi R, Belladonna ML, Volpi C, Santamaria P, Fioretti M, Puccetti P. The combined effects of tryptophan starvation and tryptophan catabolites down-regulate T cell Receptor {zeta}-chain and induce a regulatory phenotype in naïve T cells. J Immunol. 2006;176:6752–6761. doi: 10.4049/jimmunol.176.11.6752. [DOI] [PubMed] [Google Scholar]
- Favre D, Mold J, Hunt PW, Kanwar B, Loke P, Seu L, Barbour JD, Lowe MM, Jayawardene A, Aweeka F, Huang Y, Douek DC, Brenchley JM, Martin JN, Hecht FM, Deeks SG, McCune JM. Tryptophan catabolism by indoleamine 2,3-dioxygenase 1 alters the balance of TH17 to regulatory T cells in HIV disease. Sci Transl Med. 2010;2(32):32–36. doi: 10.1126/scitranslmed.3000632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flatekval GF, Sioud M. Modulation of dendritic cell maturation and function with mono- and bifunctional small interfering RNAs targeting indoleamine 2,3-dioxygenase. Immunology. 2009;128:837–848. doi: 10.1111/j.1365-2567.2009.03093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forouzandeh F, Jalili RB, Germain M, Duronio V, Ghahary A. Skin cells, but not T cells, are resistant to indoleamine 2,3-dioxygenase (IDO) expressed by allogenic fibroblasts. Wound Repair Regen. 2008;16:379–387. doi: 10.1111/j.1524-475X.2008.00377.x. [DOI] [PubMed] [Google Scholar]
- Fricke I, Gabrilovich DI. Dendritic cells and tumor microenvironment: A dangerous liaison. Immunol Investig. 2006;35:459–483. doi: 10.1080/08820130600803429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frumento G, Rotondo R, Tonetti M, Damonte G, Benatti U, Ferrara GB. Tryptophan-derived catabolites are responsible for inhibition of T and natural killer cell proliferation induced by indoleamine 2,3-dioxygenase. J Exp Med. 2002;196 (4):459–468. doi: 10.1084/jem.20020121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granucci F, Zanoni I. The dendritic cell life cycle. Cell Cycle. 2009;8(23):3816–3821. doi: 10.4161/cc.8.23.9998. [DOI] [PubMed] [Google Scholar]
- Grohmann U, Orabona C, Fallarino F, Vacca C, Calcinaro F, Falorni A, Candeloro P, Belladonna ML, Bianchi R, Fioretti MC, Puccetti P. CTLA-4-Ig regulates tryptophan catabolism in vivo. Nat Immunol. 2002;3(11):1097–1-101. doi: 10.1038/ni846. [DOI] [PubMed] [Google Scholar]
- Grohmann U, Fallarino F, Bianchi R, Orabona C, Vacca C, Fioretti MC, Puccetti P. A defect in tryptophan catabolism impairs tolerance in nonobese diabetic mice. J Exp Med. 2003a;198(1):153–160. doi: 10.1084/jem.20030633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grohmann U, Bianchi R, Orabona C, Fallarino F, Vacca C, Micheletti A, Fioretti MC, Puccetti P. Functional plasticity of dendritic cell subsets as mediated by CD40 versus B7 activation. J Immunol. 2003b;171:2581–2587. doi: 10.4049/jimmunol.171.5.2581. [DOI] [PubMed] [Google Scholar]
- Gu T, Rowswell-Turner RB, Kilinc MO, Egilmez NK. Central role of IFNgamma-indoleamine 2,3-dioxygenase axis in regulation of interleukin-12-mediated antitumor immunity. Cancer Res. 2010;70(1):129–138. doi: 10.1158/0008-5472.CAN-09-3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habibi D, Jalili RB, Forouzandeh F, Ong CJ, Ghahary A. High expression of IMPACT protein promotes resistance to indoleamine 2,3-dioxygenase-induced cell death. J Cell Physiol. 2010;225:196–205. doi: 10.1002/jcp.22220. [DOI] [PubMed] [Google Scholar]
- Harden JL, Gu T, Kilinc MO, Rowswell-Turner RB, Virtuoso LP, Egilmez NK. Dichotomous effects of IFN-{gamma} on dendritic cell function determine the extent of IL-12-driven antitumor T cell immunity. J Immunol. 2011;187(1):126–132. doi: 10.4049/jimmunol.1100168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath WR, Carbone FR. Dendritic cell subsets in primary and secondary T cell responses at body surfaces. Nat Immunol. 2009;10(12):1237–1244. doi: 10.1038/ni.1822. [DOI] [PubMed] [Google Scholar]
- Heller MC, Drew CP, Jackson KA, Griffey S, Watson JL. A potential role for indoleamine 2,3-dioxygenase (IDO) in Rhodococcus equi infection. Vet Immunol Immunopathol. 2010;138(3):174–82. doi: 10.1016/j.vetimm.2010.07.013. [DOI] [PubMed] [Google Scholar]
- Hoshi M, Saito K, Hara A, Taguchi A, Ohtaki H, Tanaka R, Fujigaki H, Osawa Y, Takemura M, Matsunami H, Ito H, Seishima M. The absence of IDO up-regulates type I IFN production, resulting in suppression of viral replication in the retrovirus-infected mouse. J Immunol. 2010;185(6):3305–3312. doi: 10.4049/jimmunol.0901150. [DOI] [PubMed] [Google Scholar]
- Hou D, Muller AJ, Sharma MD, DuHadaway J, Banerjee T, Johnson M, Mellor AL, Prendergast GC, Munn DH. Inhibition of indoleamine 2,3-dioxygenase in dendritic cells by stereoisomers of 1-methyl-tryptophan correlates with antitumor responses. Cancer Res. 2007;67(2):792–801. doi: 10.1158/0008-5472.CAN-06-2925. [DOI] [PubMed] [Google Scholar]
- Huang TT, Yen MC, Lin CC, Weng TY, Chen YL, Lin CM, Lai MD. Skin delivery of short hairpin RNA of indoleamine 2,3-dioxygenase induces antitumor immunity against orthotopic and metastatic liver cancer. Cancer Sci. 2011;102(12):2214–2220. doi: 10.1111/j.1349-7006.2011.02094.x. [DOI] [PubMed] [Google Scholar]
- Hwu P, Du MX, Lapointe R, Do M, Taylor MW, Young HA. Indoleamine 2,3-dioxygenase production by human dendritic cells results in the inhibition of T cell proliferation. J Immunol. 2000;164(7):3596–3599. doi: 10.4049/jimmunol.164.7.3596. [DOI] [PubMed] [Google Scholar]
- Jasperson LK, Bucher C, Panoskaltsis-Mortari A, Mellor AL, Munn DH, Blazar BR. Inducing the tryptophan catabolic pathway, indoleamine 2,3-dioxygenase (IDO), for suppression of graft-versus-host disease (GVHD) lethality. Blood. 2009;114(24):5062–5070. doi: 10.1182/blood-2009-06-227587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BA, III, Baban B, Mellor AL. Targeting the immunoregulatory indoleamine 2,3-dioxygenase pathway in immunotherapy. Immunotherapy. 2009;1 (4):645–661. doi: 10.2217/IMT.09.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BA, III, Kahler DJ, Baban B, Chandler PR, Kang B, Shimoda M, Koni PA, Pihkala J, Vilagos B, Busslinger M, et al. B-lymphoid cells with attributes of dendritic cells regulate T cells via indolaemine 2,3-dioxygenase. Proc Natl Acad Sci USA. 2010;107:10644–10648. doi: 10.1073/pnas.0914347107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurgens B, Hainz U, Fuchs D, Felzmann T, Heitger A. Interferon-gamma-triggered indoleamine 2,3-dioxygenase competence in human monocyte-derived dendritic cells induces regulatory activity in allogenic T cells. Blood. 2009;114(15):3235–3243. doi: 10.1182/blood-2008-12-195073. [DOI] [PubMed] [Google Scholar]
- Kamath AT, Henri S, Battye F, Tough DF, Shortman K. Developmental kinetics and lifespan of dendritic cells in mouse lymphoid organs. Blood. 2002;100 (5):1734–1741. [PubMed] [Google Scholar]
- Kammerer U, Kruse A, Barrientos G, Arck PC, Blois SM. Role of dendritic cells in the regulation of maternal immune responses to the fetus during mammalian gestation. Immunol Invest. 2008;37:499–533. doi: 10.1080/08820130802191334. [DOI] [PubMed] [Google Scholar]
- Kilinc MO, Aulakh KS, Nair RE, Jones SA, Alard P, Kosiewicz MM, Egilmez NK. Reversing tumor immune suppression with intratumoral IL-12: activation of tumor-associated T effector/memory cells, induction of T suppressor apoptosis, and infiltration of CD8+ T effects. J Immunol. 2006;177(10):6962–6973. doi: 10.4049/jimmunol.177.10.6962. [DOI] [PubMed] [Google Scholar]
- Kilinc MO, Rowswell-Turner RB, Gu T, Virtuoso LP, Egilmez NK. Activated CD8+ T-effector/memory cells eliminate CD4+ CD25+ Foxp3+ T-suppressor cells from tumors via FasL mediated apoptosis. J Immunol. 2009;183(12):7656–7660. doi: 10.4049/jimmunol.0902625. [DOI] [PubMed] [Google Scholar]
- Kilinc MO, Gu T, Harden JL, Virtuoso LP, Egilmez NK. Central role of tumor-associated CD8+ T-ceffector/memory cells in restoring systemic antitumor immunity. J Immunol. 2009;182(7):4217–4225. doi: 10.4049/jimmunol.0802793. [DOI] [PubMed] [Google Scholar]
- Kuales MA, Wenzel J, Schmid-Wendtner MH, Bieber T, von Bubnoff D. Myeloid CD11c+ S100+ dendritic cells express indoleamine 2,3-dioxygenase at the inflammatory border to invasive lower lip squamous cell carcinoma. Histol Histopathol. 2011;8:997–1006. doi: 10.14670/HH-26.997. [DOI] [PubMed] [Google Scholar]
- Laskarin G, Kammerer U, Rukavina D, Thomson AW, Fernandez N, Blois SM. Antigen-presenting cells and materno-fetal tolerance: an emerging role for dendritic cells. Am J Reprod Immunol. 2007;68(3):255–267. doi: 10.1111/j.1600-0897.2007.00511.x. [DOI] [PubMed] [Google Scholar]
- Lee JH, Chen Y, Chan JL, Qian YW, Goydos JS. Molecular analysis of melanoma-induced sentinel lymph node immune dysfunction. Cancer Immunol Immunother. 2011;60(5):685–692. doi: 10.1007/s00262-011-0982-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Li B, Fan W, Geng L, Li X, Li L, Huang Z, Li S. CTLA4Ig gene transfer alleviates abortion in mice by expanding CD4+CD25+ regulatory T cells and inducing indoleamine 2,3-dioxygenase. J Reprod Immunol. 2009;80(1–2):1–11. doi: 10.1016/j.jri.2008.11.006. [DOI] [PubMed] [Google Scholar]
- Lipscomb MW, Taylor JL, Goldbach CJ, Watkins SC, Wesa AK, Storkus WJ. DC expressing transgene Foxp3 are regulatory APC. Eur J Immunol. 2010;40 (2):480–493. doi: 10.1002/eji.200939667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Shin N, Koblish HK, Yang G, Wang Q, Wang K, Leffet L, Hansbury MJ, Thomas B, Rupar M, Waeltz P, Bowman KJ, Polam P, Sparks RB, Yue EW, Li Y, Wynn R, Fridman JS, Burn TC, Combs AP, Newton RC, Scherle PA. Selective inhibition of IDO1 effectively regulates mediators of antitumor immunity. Blood. 2010;115(17):3520–3530. doi: 10.1182/blood-2009-09-246124. [DOI] [PubMed] [Google Scholar]
- Lu Y, Giver CR, Sharma A, Li JM, Darlak KA, Owen LM, Roback JD, Galipeau J, Wller EK. Interferon-gamma and indoleamine 2,3-dioxygenase signaling between donor dendritic cells and T cells regulates graft versus host and graft versus leukemia activity. Blood. 2012;119(4):1075–1085. doi: 10.1182/blood-2010-12-322891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald TT, Monteleone I, Fantini MC, Monteleone G. Regulation of homeostasis and inflammation in the intestine. Gastroenterology. 2011;140(6):1768–1775. doi: 10.1053/j.gastro.2011.02.047. [DOI] [PubMed] [Google Scholar]
- Maldonado-Lopez R, De Smedt T, Michel P, Godfroid J, Pajak B, Heirman C, Thielemans K, Leo O, Urbain J, Moser M. CD8{alpha}+ and CD8{alpha}-subclasses of dendritic cells direct the development of distinct T helper cells in vivo. J Exp Med. 1999;189(3):587–592. doi: 10.1084/jem.189.3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manlapat AK, Kahler DJ, Chandler PR, Munn DH, Mellor AL. Cell-autonomous contol of interferon type I expression by indoleamine 2,3-dioxygenase in regulatory CD19+ dendritic cells. Eur J Immunol. 2007;37(4):1064–1071. doi: 10.1002/eji.200636690. [DOI] [PubMed] [Google Scholar]
- Matteoli G, Mazzini E, Iliev ID, Mileti E, Fallarino F, Puccetti P, Chieppa M, Rescigno M. Gut CD103+ dendritic cells express indoleamine 2,3-dioxygenase which influences T regulatory/T effector cell balance and oral tolerance induction. Gut. 2010;56:595–604. doi: 10.1136/gut.2009.185108. [DOI] [PubMed] [Google Scholar]
- Mellor AL, Keskin DB, Johnson T, Chandler P, Munn DH. Cells expressing indoleamine 2,3-dioxygenase inhibit T cell responses. J Immunol. 2002;168:3771–3776. doi: 10.4049/jimmunol.168.8.3771. [DOI] [PubMed] [Google Scholar]
- Mellor AL, Chandler P, Baban B, Hansen AM, Marshall B, Pihkala J, Waldmann H, Cobbold S, Adams E, Munn DH. Specific subsets of murine dendritic cells acquire potent T-cell regulatory functions following CTLA4-mediated induction of indoleamine 2,3-dioxygenase. Int Immunol. 2004;16(10):1391–1401. doi: 10.1093/intimm/dxh140. [DOI] [PubMed] [Google Scholar]
- Mellor AL, Baban B, Chandler PR, Manlapat A, Kahler DJ, Munn DH. Cutting edge: CpG oligonucleotides induce splenic CD19+ dendritic cells to aquire potent indoleamine 2,3-dioxygenase-dependent T-cell regulatory functions via IFN type I signaling. J Immunol. 2005;175:5601–5605. doi: 10.4049/jimmunol.175.9.5601. [DOI] [PubMed] [Google Scholar]
- Mellor AL. Indoleamine 2,3-dioxygenase and regulation of T cell immunity. Biochem Biophys Res Commun. 2005;338(1):20–24. doi: 10.1016/j.bbrc.2005.08.232. [DOI] [PubMed] [Google Scholar]
- Mezrich JD, Fechner JH, Zhang X, Johnson BP, Burlingham WJ, Bradfield CA. An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J Immunol. 2010;185(6):3190–3198. doi: 10.4049/jimmunol.0903670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller AJ, Heseler K, Schmidt SK, Spekker K, Mackenzie CR, Daubener W. The missing link between indoleamine 2,3-dioxygenase mediated antibacterial and immunoregulatory effects. J Cell Mol Med. 2009;13(6):1125–1135. doi: 10.1111/j.1582-4934.2008.00542.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller AJ, Sharma MD, Chandler PR, DuHadaway JB, Everhart ME, Johnson BA, III, Kahler DJ, Pihkala J, Soler AP, Munn DH. Chronic inflammation that facilitates tumor progression creates local immune suppression by inducing indoleamine 2,3-dioxygenase. Proc Net Acad Sci USA. 2008;105 (44):17073–17078. doi: 10.1073/pnas.0806173105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munn DH, Zhou M, Attwood JT, Bondarev I, Conway SJ, Marshall B, Brown C, Mellor AL. Prevention of allogenic fetal rejection by tryptophan catabolism. Science. 1998;281(5380):1191–1193. doi: 10.1126/science.281.5380.1191. [DOI] [PubMed] [Google Scholar]
- Munn DH, Sharma MD, Hou D, Baban B, Lee JR, Antonia SJ, Messina JL, Chandler P, Koni PA, Mellor AL. Expression of indoleamine 2,3-dioxygenase by Plasmacytoid dendritic cells in tumor-draining lymph nodes. J Clin Invest. 2004;114(2):280–290. doi: 10.1172/JCI21583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munn DH, Sharma MD, Baban B, Harding HP, Zhang Y, Ron D, Mellor AL. GCN2 kinase in T cells mediates proliferative arrest and anergy induction in response to indoleamine 2,3-dioxygenase. Immunity. 2005;22(5):633–642. doi: 10.1016/j.immuni.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Munn DH. Lineage-specific transcription factors in unexpected places. Eur J Immunol. 2010;40(2):315–317. doi: 10.1002/eji.200940238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair JR, Carlson LM, Koorelle C, Rozanski CH, Byrne GE, Bergsagel PL, Shaughnessy JP, Jr, Boise LH, Chana-Khan A, Lee KP. CD28 expressed on malignant plasma cells induces a prosurvival and immunosuppressive microenvironment. J Immunol. 2011;187(3):1243–1253. doi: 10.4049/jimmunol.1100016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair RE, Kilinc MO, Jones SA, Egilmez NK. Chronic immune therapy induces a progressive increase in intratumoral T suppressor activity and a concurrent loss of tumor-specific CD8+ T-effects in her-2/neu transgenic mice bearing advanced spontaneous tumors. J Immunol. 2006;176(12):7325–7334. doi: 10.4049/jimmunol.176.12.7325. [DOI] [PubMed] [Google Scholar]
- Nguyen NT, Kimura A, Nakahama T, Chinen I, Masuda K, Nohara K, Fujii-Kuriyama Y, Kishimoto T. Aryl hydrocarbon receptor negatively regulates dendritic cell immunogenicity via a kynurenine-dependent mechanism. Proc Nat Acad Sci USA. 2010;107(46):19961–19966. doi: 10.1073/pnas.1014465107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Njau F, Geffers R, Thalmann J, Haller H, Wagner AD. Restriction of Chlamydia pneumoniae replication in human dendritic cell by activation of indoleamine 2,3-dioxygenase. Microbes Infect. 2009;11(13):1002–1010. doi: 10.1016/j.micinf.2009.07.006. [DOI] [PubMed] [Google Scholar]
- Norian LA, Rodriquez PC, O’Mara LA, Zabaleta J, Ochoa AC, Cella M, Allen PM. Tumor-infiltrating regulatoy dendritic cells inhibit CD8+ T cell function via L-arginine metabolism. Cancer Res. 2009;69(7):3086. doi: 10.1158/0008-5472.CAN-08-2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Sullivan BJ, Pai S, Street S, An X, MacDonald KP, Wong M, Strutton G, Gerondakis S, Steptoe RJ, de St Groth BF, Hill GR, Thomas R. Immunotherapy with costimulatory dendritic cells to control autoimmune inflammation. J Immunol. 2011;187(8):4018–4030. doi: 10.4049/jimmunol.1101727. [DOI] [PubMed] [Google Scholar]
- Ochiel DO, Ghosh M, Fahey JV, Guyre PM, Wira CR. Human uterine epithelial cell secretions regulate dendritic cells differentiation and responses to TLR ligands. J Leukoc Biol. 2010;88(3):435–444. doi: 10.1189/jlb.1009700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onodera T, Jang MH, Guo Z, Yamasaki M, Hirata T, Bai Z, Tsuji NM, Nagakubo D, Yoshie O, Sakaguchi S, Takikawa O, Miyasaka M. Constitutive expression of IDO by dendritic cells of mesenteric lymph nodes: functional involvement of the CTLA-4/B7 and CCL22/CCR4 interactions. J Immunol. 2009;183(9):5608–5614. doi: 10.4049/jimmunol.0804116. [DOI] [PubMed] [Google Scholar]
- Orabona C, Grohmann U, Belladonna ML, Fallarino F, Vacca C, Bianchi R, Bozza S, Volpi C, Salomon BL, Fioretti MC, Romani L, Puccetti P. CD28 induces immunostimulatory signals in dendritic cells via CD80 and CD86. Nat Immunol. 2004;5(11):1134–1142. doi: 10.1038/ni1124. [DOI] [PubMed] [Google Scholar]
- Ou X, Cai S, Liu P, Zeng J, He Y, Wu X, Du J. Enhancement of dendritic cell-tumor fusion vaccine potency by indoleamine-pyrrole 2,3-dioxygenase inhibitor, 1-MT. J Cancer Res Clin Oncol. 2008;134(5):525–533. doi: 10.1007/s00432-007-0315-9. [DOI] [PubMed] [Google Scholar]
- Pallotta MT, Orabona C, Volpi C, Vacca C, Belladonna ML, Bianchi R, Servillo G, Brunacci C, Calvitti M, Bicciato S, Mazza EM, Boon L, Grassi F, Fioretti MC, Fallarino F, Puccetti P, Grohmann U. Indoleamine 2,3-dioxygenase is a signaling protein in long-term tolerance by dendritic cells. Nat Immunol. 2011;12(9):870–878. doi: 10.1038/ni.2077. [DOI] [PubMed] [Google Scholar]
- Quintana FJ, Murugaiyan G, Farez MF, Mitsdoerffer M, Tukpah, Burns EJ, Weiner HL. An endogenous aryl hydrocarbon receptor ligand acts on dendritic cells and T cells to suppress experimental autoimmune encephalomyelitis. Proc Nat Acad Sci USA. 2010;107(48):20768–20773. doi: 10.1073/pnas.1009201107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reizis B, Colonna M, Trinchieri G, Barat F, Gilliet M. Plasmacytoid dendritic cells: one-trick ponies or workhorses of the immune system? Nat Rev Immunol. 2011;11:558–565. doi: 10.1038/nri3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma MD, Hou DY, Koni PA, Metz R, Chandler P, Mellor AL, He Y, Munn DH. Indoleamine 2,3-dioxygenase controls conversion of Foxp3+ Tregs to TH17-like cells in tumor-draining lymph nodes. Blood. 2009;113(24):6102–6111. doi: 10.1182/blood-2008-12-195354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simones T, Shepherd DM. Consequences of AhR activation in steady-state dendritic cells. Toxicol Sci. 2011;119(2):293–307. doi: 10.1093/toxsci/kfq354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AJ, Toledo CM, Wietgrefe SW, Duan L, Schacker TW, Reilly CS, Haase AT. The immunosuppressive role of IL-32 in lymphatic tissue during HIV-1 infection. J Immunol. 2011;186(11):6576–6584. doi: 10.4049/jimmunol.1100277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soliman H, Mediaville-Varela M, Antonia S. Indoleamine 2,3-Dioxygenase – Is It an Immun Suppressor? Cancer J. 2010;16:354–359. doi: 10.1097/PPO.0b013e3181eb3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman RM, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice. I Morphology, quantitation, tissue distribution. J Exp Med. 1973;137(5):1142–1162. doi: 10.1084/jem.137.5.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockinger B, Hirota K, Duarte J, Veldhoen M. External influences on the immune system via activation of the aryl hydrocarbon receptor. Semin Immunol. 2011;23 (2):99–105. doi: 10.1016/j.smim.2011.01.008. [DOI] [PubMed] [Google Scholar]
- Sucher R, Fischler K, Oberhuber R, Kronberger I, Margreiter C, Ollinger R, Schneeberger S, Fuchs D, Werner ER, Watschinger K, Zelger B, Tellides G, Pilat N, Pratschke J, Margreiter R, Wekerle T, Brandacher G. IDO and regulatory T cell support are critical for cytotoxic T lymphocyte-associated Ag-4 Ig-mediated long-term solid organ allograft survival. J Immunol. 2012;188(1):37–46. doi: 10.4049/jimmunol.1002777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun CM, Hall JA, Blank RB, Bouladoux N, Oukka M, Mora JR, Belkaid Y. Small interstine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med. 2007;204(8):1775–1785. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Chin YE, Weisiger E, Malter C, Tawara I, Toubai T, Gatza E, Mascagni P, Dinarello CA, Reddy P. Cutting edge: Negative regulation of dendritic cells through acetylation of the nonhistone protein STAT-3. J Immunol. 2009;182 (10):5899–5903. doi: 10.4049/jimmunol.0804388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terness P, Bauer TM, Rose L, Dufter C, Watzlik A, Simon H, Opelz G. Inhibition of Allogenic T Cell Proliferation by Indoleamine 2,3-Dioxygenase-expressing Dendritic Cells: Mediation of Suppression by Tryptophan Metabolites. J Exp Med. 2002;196(4):447–457. doi: 10.1084/jem.20020052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsampalas M, Gridelet V, Berndt S, Foidart JM, Geenen V, Perrier d’Hauterive S. Human chorionic gonadotropin: a hormone with immunological and angiogenic properties. J Reprod Immunol. 2010;85(1):93–8. doi: 10.1016/j.jri.2009.11.008. [DOI] [PubMed] [Google Scholar]
- Veldhoen M, Duarte JH. The aryl hydrocarbon receptor: fine-tuning the immune-response. Curr Opin Immunol. 2010;22(6):747–752. doi: 10.1016/j.coi.2010.09.001. [DOI] [PubMed] [Google Scholar]
- Wallet MA, Sen P, Tisch R. Immunoregulation of dendritic cells. Clin Med Res. 2005;3(3):166–175. doi: 10.3121/cmr.3.3.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan H, Versnel MA, Leijten LM, van Helden-Meeuwsen CG, Fekkes D, Leenen PJ, Khan NA, Benner R, Kiekens RC. Chorionic gonadotropin induces dendritic cells to express a tolerogenic phenotype. J Leukoc Biol. 2008;83(4):894–901. doi: 10.1189/jlb.0407258. [DOI] [PubMed] [Google Scholar]
- Watkins SK, Zhu Z, Riboldi E, Shafer-Weaver KA, Stagliano KE, Sklavos MM, Ambs S, Yagita H, Hurwitz AA. FOXO3 programs tumor-associated DCs to become tolerogenic in human and murine prostate cancer. J Clin Invest. 2011;121 (4):1361–1372. doi: 10.1172/JCI44325. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Wobser M, Voigt H, Houben R, Eggert AO, Freiwald M, Kaemmerer U, Kaempgen E, Schrama D, Becker JC. Dendritic cell based antitumor vaccination: impact of functional indoleamine 2,3-dioxygenase expression. Cancer Immunol Immunother. 2007;56(7):1017–24. doi: 10.1007/s00262-006-0256-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y, Zhang GX, Gran B, Fallarino F, Yu S, Li H, Cullimore ML, Rostami A, Xu H. IDO up-regulates regulatory T cells via tryptophan catabolite and suppresses encephalitogenic T cell responses in experimental autoimmune encepthalomyelitis. J Immunol. 2010;185(10):5953–5961. doi: 10.4049/jimmunol.1001628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang HJ, Yen MC, Lin CC, Lin CM, Chen YL, Weng TY, Huang TT, Wu CL, Lai MD. A combination of the metabolic enzyme inhibitor APO866 and the immune adjuvant L-1-methyl tryptophan induces additive antitumor activity. Exp Biol Med Maywood) 2010;235(7):869–876. doi: 10.1258/ebm.2010.010001. [DOI] [PubMed] [Google Scholar]
- Yen MC, Lin CC, Chen YL, Huang SS, Yang HJ, Chang CP, Lei HY, Lai MD. A novel cancer therapy by skin delivery of indoleamine 2,3-dioxygenase siRNA. Clin Cancer Res. 2009;15(2):641–649. doi: 10.1158/1078-0432.CCR-08-1988. [DOI] [PubMed] [Google Scholar]
- Zhang T, Fresney S, Welty E, Sangrampukar N, Rybak E, Zhou H, Cheng XF, Feng Q, Avon C, Laaris A, Whitters M, Nagelin AM, O’Hara RM, Jr, Azimzadeh AM. Selective CD28 blockade attenuates acute and chronic rejection of murine cardiac allografts in a CTLA-4-dependent manner. Am J Transplant. 2011;11(8):1599–1609. doi: 10.1111/j.1600-6143.2011.03624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu BT. Development of selective immune tolerance towards the allogenic fetus during pregnancy: Role of tryptophan catabolites. Int J Mol Med. 2010;25:831–835. doi: 10.3892/ijmm_00000411. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.