Abstract
Replication fork pausing drives genome instability, because any loss of paused replisome activity creates a requirement for reloading of the replication machinery, a potentially mutagenic process. Despite this importance, the relative contributions to fork pausing of different replicative barriers remain unknown. We show here that Deinococcus radiodurans RecD2 helicase inactivates Escherichia coli replisomes that are paused but still functional in vitro, preventing continued fork movement upon barrier removal or bypass, but does not inactivate elongating forks. Using RecD2 to probe replisome pausing in vivo, we demonstrate that most pausing events do not lead to replisome inactivation, that transcription complexes are the primary sources of this pausing, and that an accessory replicative helicase is critical for minimizing the frequency and/or duration of replisome pauses. These findings reveal the hidden potential for replisome inactivation, and hence genome instability, inside cells. They also demonstrate that efficient chromosome duplication requires mechanisms that aid resumption of replication by paused replisomes, especially those halted by protein–DNA barriers such as transcription complexes.
Keywords: DNA repair, genome stability, Rep, RNA polymerase, recombination
Faithful duplication of the genome is a key challenge to all organisms, and overcoming barriers to the timely progression of replication forks is a major part of this challenge. Template damage, proteins bound to the DNA, and non–B-form DNA structures all have the potential to halt movement of the replication machinery (1–3). If forks pause at such barriers and lose function, then replisome reloading, often via blocked fork processing by recombination enzymes, is required to resume genome duplication (4), an error-prone process associated with gross chromosomal rearrangements (5–7). However, pausing of replisomes does not necessarily lead to fork breakdown, because paused replisomes can continue duplication upon removal or bypass of the block (8–11). The balance between resumption of replication versus breakdown of paused forks is therefore a critical factor in the maintenance of genome stability.
Forks halted by nucleoprotein complexes can resume replication upon spontaneous dissociation of the nucleoprotein complex or active removal of the block by the replisome itself or accessory motors acting at the fork (9, 10, 12–14). Similarly, a replisome encountering a lesion that inhibits synthesis by one of the polymerases may continue replication downstream of the lesion by repriming DNA synthesis. Such repriming occurs regardless of whether the damage is on the leading or the lagging strand template, although recombination may be triggered at the gap left in the nascent strand (11, 15, 16).
Whether a replisome pauses at a barrier before resuming replication or loses activity is determined by the rates of barrier clearance/bypass and blocked replisome stability (17, 18). However, despite the critical importance of fork pausing for genome stability, the contributions of different types of barrier to replisome pausing remain unknown. In particular, although both transcription complexes and DNA damage present known challenges to fork movement in bacteria and eukaryotes (15, 19–21), the relative frequencies with which they affect fork progression in vivo is not known. This is in part because physical detection of fork pausing is only possible when such pausing occurs at specific locations in the genome and is sufficiently frequent and/or prolonged to facilitate detection. However, many pausing events might not meet these criteria. Furthermore, if a paused fork subsequently resumes translocation, either upon removal or bypass of the barrier, there would be no easily detectable phenotypic consequences.
Here we demonstrate that Deinococcus radiodurans RecD2 helicase inactivates paused but not elongating Escherichia coli replisomes in vitro. The basis of this inactivation is unknown, but this specificity provides a tool to probe the relative frequencies of replisome pausing in vivo. Wild-type Escherichia coli can survive expression of RecD2 but chromosomal DNA content is perturbed significantly, indicating that replisomes do pause frequently in vivo. Cells lacking a helicase, Rep, that clears protein–DNA barriers ahead of forks (10, 22) are hypersensitive to RecD2 expression. In contrast, defects in base or nucleotide excision repair do not render RecD2 toxic. These data indicate that protein–DNA complexes, not template damage, are the primary sources of replisome pausing in nonstressed cells and that most replicative barriers result in fork pausing but not inactivation. Thus, although there is a very considerable potential for triggering genomic instability during every cell cycle, this potential is only rarely realized because of the intrinsic stability of the replisome and the ability of a secondary motor to help drive forks along protein-bound DNA. Together, these two factors minimize the likelihood of fork pausing leading to replisome inactivation and the need to restart replication.
Results
RecD2 Inhibits Resumption of Replication by Paused Replisomes.
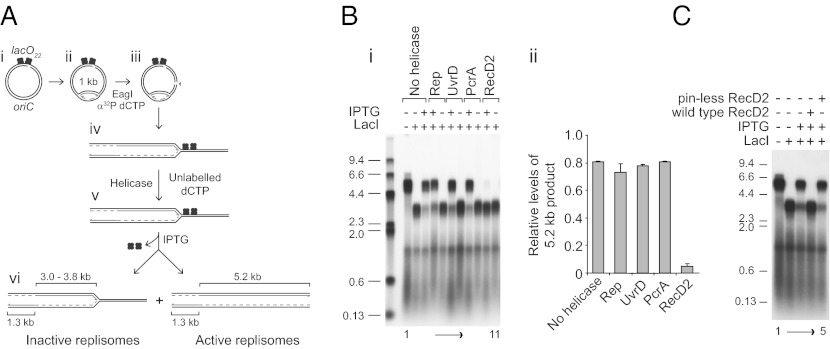
Superfamily 1 helicases that translocate 3′-5′ along ssDNA (E. coli Rep and UvrD and Bacillus stearothermophilus PcrA) promote movement of E. coli replisomes through nucleoprotein complexes, whereas D. radiodurans RecD2 and bacteriophage T4 Dda, 5′-3′ superfamily 1 helicases do not (10). Indeed, addition of RecD2 results in an apparent increase in the degree of replication blockage at protein–DNA complexes in vitro (10). We investigated the basis for this apparent increase in fork blockage by RecD2. We used a system in which replisomes could be blocked completely by a large array of lac repressor–operator complexes and then the block relieved by subsequent addition of isopropyl β-D-1-thiogalactopyranoside (IPTG), allowing monitoring of the ability of blocked replisomes to resume replication (18)(Fig. 1A and Fig. S1). Replisomes were reconstituted on plasmid templates bearing oriC and 22 tandem lac operators. In the absence of a topoisomerase, replisomes could proceed only a limited distance along the template owing to accumulation of positive supercoiling. Continued fork movement depended on cleavage of the template by a restriction enzyme to relieve the topological strain (17) (Fig. 1A, ii and iii and Fig. S1) with the site of cleavage allowing only one of the two forks to progress toward the lac operators (Fig. 1A, iii and iv).
Fig. 1.
RecD2 inactivates replication forks blocked by nucleoprotein complexes in vitro. (A) reaction scheme to monitor the ability of replisomes halted at LacI–lacO complexes to continue replication upon IPTG-induced dissociation of the barrier. (B, i) Denaturing agarose gel of replication products formed with pPM561 in the absence or the presence of 400 nM LacI with or without subsequent addition of 1 mM IPTG. Helicases added to 100 nM final concentration at step iv/v (A) are indicated. Sizes of markers in kilobases are indicated. (B, ii) Levels of the 5.2-kb leading strand products formed in the presence of repressor after subsequent addition of IPTG relative to those obtained in the absence of repressor (see lane 1 in B). (C) The effects of wild-type and pinless RecD2 on the ability of forks paused at LacI–lacO complexes to continue upon addition of IPTG.
In the absence of lac repressor, replication generated a population of lagging strands of ∼0.5 kb and leading strands of 1.3 and 5.2 kb (Fig. 1A, vi and B, lane 1). In the presence of repressor, 3.5-kb, rather than 5.2-kb, leading strands were generated (Fig. 1A, vi and B, lane 2 and Fig. S1), as expected if movement of replisomes was inhibited within the repressor–operator array. Subsequent addition of IPTG to these blocked forks relieved this inhibition (Fig. 1B, lane 3), reflecting the initial retention of activity of replisomes blocked at lac repressor–operator complexes (18).
The impact of helicases on the ability of blocked replisomes to resume replication upon addition of IPTG was analyzed. Rep, UvrD, and PcrA did not promote replication through the 22 repressor–operator complexes in the absence of IPTG (Fig. 1B, lanes 5, 7, and 9). Addition of IPTG allowed resumption of replication even in the presence of these helicases, indicating that Rep, UvrD, and PcrA did not inhibit resumption of replication upon removal of a block (Fig. 1 B, i, lanes 4, 6, and 8 and 1 B, ii). In contrast, RecD2 reduced severely the ability of replisomes to resume replication after IPTG addition (Fig. 1 B, i, compare lanes 10 and 11 with lanes 3 and 2, and 1 B, ii). However, a mutant RecD2 protein, pinless RecD2, that retains DNA binding but not helicase activity (23) failed to inhibit resumption of replication (Fig. 1C). The helicase activity of RecD2 thus inhibits continued movement of paused replisomes upon dissociation of a blocking nucleoprotein complex. T4 Dda, another superfamily 1 5′-3′ helicase, also inhibited resumption of fork movement (Fig. S2), demonstrating that fork inactivation is a general feature of this class of helicases rather than an activity associated specifically with RecD2.
RecD2 Inactivates Paused but Not Elongating Replisomes.
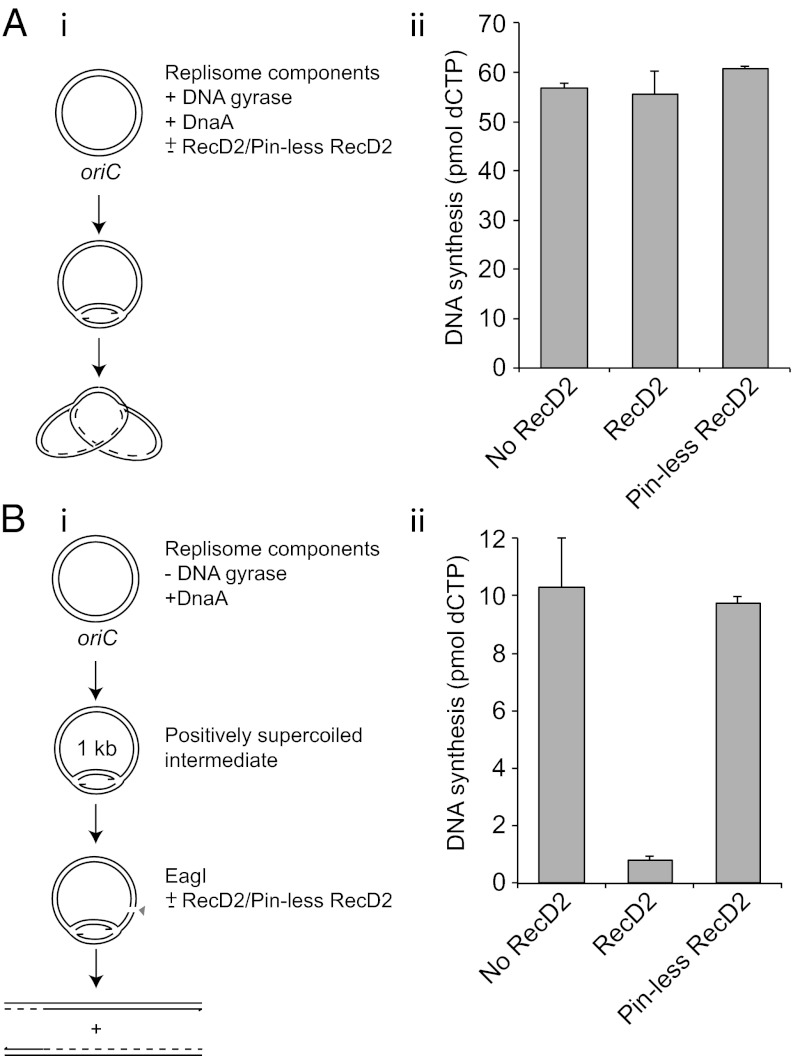
Inhibition of resumption of fork movement upon dissociation of the protein–DNA block could be explained by RecD2-catalyzed inactivation of paused replisomes, of elongating replisomes, or both. To distinguish between these possibilities we assessed the impact of RecD2 on the activity of elongating and of paused forks in two parallel experiments. First, the effects of wild-type and pinless RecD2 were analyzed on elongating replication forks whose movement around a supercoiled plasmid template lacking engineered barriers was sustained by DNA gyrase (Fig. 2 A, i). Addition of either wild-type or pinless RecD2 alongside the replication initiator DnaA had no significant impact on levels of DNA synthesis by elongating forks (Fig. 2 A, ii). Second, replication of the same supercoiled plasmid template was initiated by DnaA in the absence of a topoisomerase as in Fig. 1, resulting in fork stalling owing to topological strain (Fig. 2 B, i). Subsequent addition of a restriction enzyme relieved the strain and allowed paused forks that retained function to continue (Fig. S1). However, addition of wild-type, but not pinless, RecD2 alongside the restriction enzyme inhibited subsequent DNA synthesis (Fig. 2 B, ii). RecD2 helicase activity results, therefore, in inactivation of forks paused by positive supercoiling. Taken together, the data in Fig. 2 indicate that RecD2 inactivates paused but not elongating replisomes.
Fig. 2.
RecD2 inactivates blocked but not elongating replication forks. (A, i) Method to analyze the impact of RecD2 on elongating replication forks. (A, ii) DNA synthesis in the presence of DNA gyrase upon simultaneous addition of DnaA with or without either wild-type or pinless RecD2, each present at 100 nM final concentration. (B, i) Method to monitor the impact of RecD2 on the ability of forks stalled by positive supercoiling to continue DNA synthesis upon relief of topological strain. (B, ii) Levels of DNA synthesis upon addition of a restriction enzyme with or without either wild-type or pinless RecD2 (each at 100 nM).
RecD2 Inactivates Forks Paused by a Variety of Replicative Barriers.
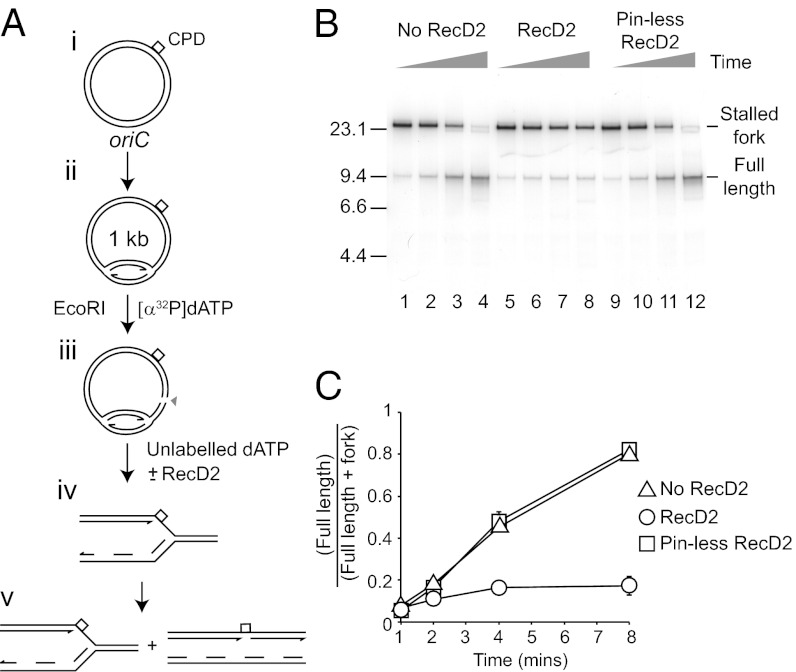
Different obstacles have different impacts on replisome movement. Nucleoprotein complexes and topological strain require removal of the original block for resumption of replication (Figs. 1 and 2). In contrast, DNA lesions pause forks but can eventually be bypassed. A cyclobutane pyrimidine dimer within the leading strand template pauses fork progression, but leading strand synthesis can be reinitiated downstream of the lesion over the course of several minutes (11). We tested, therefore, whether RecD2 inhibited the ability of replisomes paused at a pyrimidine dimer to bypass the lesion and continue replication.
Reactions were again initiated in the absence of a topoisomerase followed by restriction enzyme cleavage in the presence of labeled deoxynucleotide to allow forks to progress. The plasmid template contained a single pyrimidine dimer within the leading strand template (11). Following restriction enzyme cleavage for 50 s, excess unlabeled deoxynucleotide was added with or without RecD2 or pinless RecD2 and incubation continued. One minute after addition of the restriction enzyme all reactions contained predominantly stalled replication forks (Fig. 3B, lanes 1, 5, and 9) (11). In the absence of RecD2 the majority of stalled forks generated full-length products after 8 min (Fig. 3B, lanes 1–4). Wild-type, but not pinless, RecD2 inhibited this conversion of stalled forks into full-length products (Fig. 3B, compare lanes 5–8 with 9–12, and 3C). RecD2 helicase therefore prevents replisomes paused by a DNA lesion from bypassing the DNA damage.
Fig. 3.
RecD2 inactivates forks paused by template lesions. (A) reaction scheme to monitor replication fork pausing at, and bypass of, a cyclobutane pyrimidine dimer (CPD) within the leading strand template. (B) Native agarose gel of replication products formed on plasmid DNA harboring a pyrimidine dimer within the leading strand template. Time points were taken 1, 2, 4, and 8 min after addition of restriction enzyme and radiolabel. Positions of replication forks stalled at the pyrimidine dimer and full-length products generated by bypass of the lesion are indicated. Wild-type and pinless RecD2 were present at 100 nM, as indicated. (C) Accumulation of full-length products as a function of time.
The data in Figs. 1–3 indicate that RecD2 helicase activity results in loss of function of paused, but not elongating, replisomes regardless of the nature of the replicative barrier.
Absence of Rep Helicase Hypersensitizes Cells to recD2 Expression.
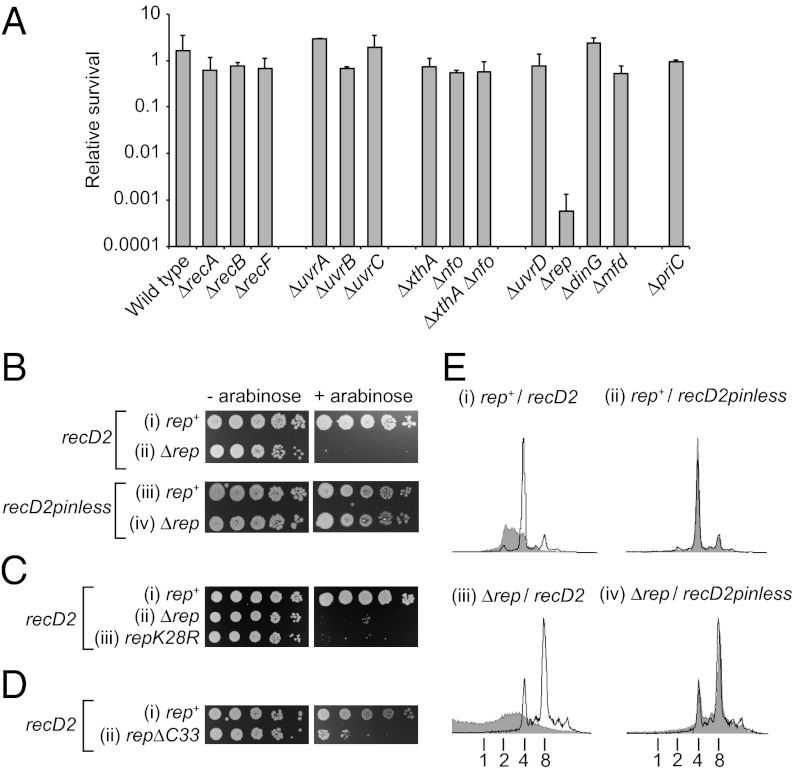
Inactivation of paused but not elongating replication forks by RecD2 in vitro provides a potential tool to probe fork pausing in vivo. Conversion of paused forks that retain the ability to continue replication into inactivated forks that cannot continue upon removal or bypass of blocks might present viability problems, the severity of which would depend upon the frequency and duration of fork pausing. However, induction of recD2 expression from a plasmid-based arabinose-inducible promoter had no significant impact on viability as monitored by colony-forming ability (10) (Fig. 4 A and B, i). Chromosomal DNA content of wild-type cells induced for recD2 expression was affected significantly, however, as monitored by flow cytometry under run-out conditions (Fig. 4E, i). Thus, RecD2 did have an impact upon chromosome duplication. This inhibition of chromosomal duplication required RecD2 helicase activity, not just DNA binding, because recD2pinless had no impact on DNA content (Fig. 4E, ii). Sensitivity to wild-type but not pinless RecD2 in vivo correlates with inactivation of paused forks by wild-type but not pinless RecD2 in vitro (Figs. 1C, 2B, and 3).
Fig. 4.
Expression of recD2 is toxic in strains lacking Rep. (A) Colony-forming ability of wild-type E. coli (BW25113) and otherwise isogenic strains bearing single gene deletions upon expression of recD2 using a plasmid-based arabinose-inducible system (pMG31). Survival is represented by the number of colonies formed upon induction of recD2 expression relative to the number of colonies formed in the absence of induction. Table S2 gives strain numbers. (B) Colony-forming ability of rep+ (TB28) and Δrep (N6577) strains harboring pBADrecD2 and pBADrecD2pinless. (C) Colony-forming ability of rep+ (MG1655), rep (N4982), and repK28R (SS1076) containing pBADrecD2. (D) Colony-forming ability of rep+ (MKG08) and repΔC33 (MKG10) upon recD2 overexpression (pMG31). (E) DNA content of (i and ii) wild-type (TB28) and (iii and iv) Δrep (N6577) cells without and with induction of expression (unshaded and shaded histograms, respectively) from pBADrecD2 and pBADrecD2pinless as monitored by flow cytometry. DNA content with respect to number of chromosome equivalents per cell is indicated below. Note that Δrep cells have a higher mean number of chromosomes per cell compared with rep+ (compare the uninduced samples in i and ii with iii and iv), as noted previously (39).
The ability to survive recD2 expression could be due to repair of RecD2-inactivated forks providing an efficient means of surviving such inactivation. Recombination enzymes provide multiple pathways to repair damaged replication forks (24, 25). However, no significant impact on viability was observed in the absence of any recombination gene tested, suggesting that fork processing is not critical for survival of cells expressing recD2 (Fig. 4A; ΔrecA, ΔrecB, and ΔrecF) (Table S1).
Alternatively, survival could reflect a low frequency and/or short duration of replication fork pauses in wild-type cells. Low levels of replisome pausing might be due to an inherently low frequency of pausing but might also be a consequence of multiple enzyme systems in wild-type cells that minimize fork pausing. We therefore analyzed the impact of recD2 expression in strains with a decreased ability to remove potential obstacles to replication. Lesions within the leading strand template cause replisome pausing before bypass of the lesion via repriming (11) (Fig. 3). However, absence of nucleotide excision repair did not render cells sensitive to RecD2 (Fig. 4A, ΔuvrA, ΔuvrB, and ΔuvrC). Absence of Mfd, a dsDNA translocase that promotes transcription-coupled repair of DNA lesions via UvrABC (26), also did not render cells sensitive to RecD2 (Fig. 4A). Thus, removal of bulky lesions from either nontranscribed or transcribed DNA was not required for tolerance of recD2 expression. Similarly, absence of one or both of the apurinic/apyrimidinic endonucleases required for base excision repair did not result in hypersensitivity to RecD2 (Fig. 4A, ΔxthA and Δnfo). Efficient removal of DNA lesions, and thus a reduction in their potential to pause forks, was not required therefore for tolerance of recD2 expression.
Rep helicase provides another means to reduce fork pausing by acting at the replisome to disrupt nucleoprotein complexes ahead of the replication fork (10, 22, 27). Wild-type but not pinless RecD2 had a severe impact on colony-forming ability in the absence of Rep (Fig. 4 A and B). Absence of Rep also caused hypersensitivity to T4 Dda (Fig. S3A). The toxicity of both RecD2 and Dda in Δrep cells correlated, therefore, with the ability of both helicases to inactivate replisomes in vitro. The requirement for Rep to ameliorate the impact of RecD2 on colony-forming ability was dependent on Rep helicase activity, not just DNA binding, because a rep allele bearing a mutation in the Walker A motif was sensitive to RecD2 (Fig. 4C, iii). Thus Rep does not merely block access of RecD2 to a DNA substrate. Chromosomal DNA content upon recD2 induction in Δrep cells was also reproducibly more severely affected compared with rep+ cells (Figs. 4E, i and iii and 5E, i and F, i).
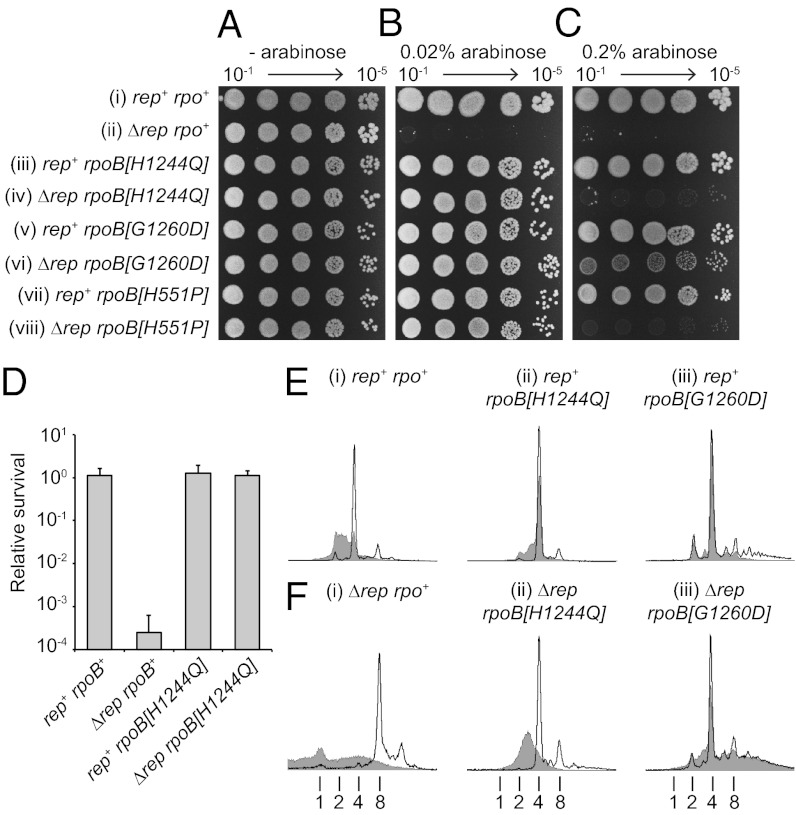
Fig. 5.
RecD2-directed lethality is associated with transcription complexes. (A–C) Colony-forming ability of rep+ and Δrep strains bearing either wild-type or mutant rpoB alleles upon no, low-, or high-level induction from pBADrecD2 (0%, 0.02% and 0.2% arabinose, respectively). Strains (i–viii) are TB28, N6577, PM486, N7604, AM2158, HB278, N7616, and HB280. (D) Colony-forming ability of rep+ and Δrep strains bearing rpoB+ or rpoB[H1244Q] upon induction of recD2 expression with 0.2% arabinose. Strain numbers are as in A–C. (E and F) DNA content of rep+ and Δrep strains bearing rpoB+, rpoB[H1244Q], or rpoB[G1260D] without and with induction of expression of recD2 with 0.2% arabinose (unshaded and shaded histograms, respectively). The number of chromosome equivalents per cell is indicated below. Note that both rpoB[H1244Q] and rpoB[G1260D] also reduced the median number of chromosome equivalents in Δrep cells in the absence of recD2 expression from eight to four, the same number as seen in rep+ cells (F, compare i with ii and iii). This may reflect suppression of the reduced rate of genome duplication in Δrep cells (30). Strain numbers are as in A–C.
Taken together, these data indicate that inactivation of paused but otherwise functional replisomes by RecD2 results in chromosomal defects that, although exhibited by wild-type cells, are exacerbated in the absence of Rep. The corollary of these observations is that many fork pausing events do not lead to replisome inactivation under normal circumstances in either rep+ or Δrep cells and that conversion of these paused forks into inactive forks by RecD2 is deleterious.
Clearance of Nucleoprotein Barriers Ahead of Forks Protects Against RecD2-Induced Cell Death.
Rep facilitates both clearance of nucleoprotein complexes ahead of forks (10, 22) and PriC-directed reloading of the replication apparatus (28, 29). However, the colony-forming ability of a strain lacking PriC was not reduced by RecD2 (Fig. 4A) or T4 Dda (Fig. S3B), indicating that failure of PriC-directed replisome reloading is not responsible for the inviability of Δrep cells expressing RecD2.
Efficient clearance of nucleoprotein complexes ahead of forks by Rep is dependent upon not only Rep helicase activity but also a physical interaction between the C terminus of Rep and the replicative helicase DnaB (10, 30). A rep allele lacking the DnaB interaction domain but retaining helicase activity (repΔC33) was sensitive to RecD2 expression (Fig. 4D), consonant with efficient clearance of protein–DNA complexes ahead of forks being needed to minimize RecD2-induced viability defects. Both UvrD and DinG helicases have also been implicated in promoting replication of protein-bound DNA (10, 22), but neither helicase is known to associate physically with the replisome (31). Overexpression of recD2 had no impact on colony-forming ability in the absence of either UvrD or DinG (Fig. 4A), supporting the conclusion that this toxicity is specific for the replisome-associated activity of Rep.
These data indicate that promotion of replisome movement by Rep along protein-coated DNA may be essential to avoid RecD2-directed cell death. A major source of nucleoprotein replicative barriers is transcription, with mutations within RNA polymerase subunits being able to suppress multiple defects in genome duplication (20, 32–34). One such mutation, rpoB[H1244Q], suppresses defects in genome duplication by reducing the stability of stalled transcription complexes (20) and inhibiting backtracking of RNA polymerase (35). We found that this mutation suppressed the RecD2-dependent killing of Δrep cells as evinced by colony-forming ability (Fig. 5 A–C, compare i and ii with iii and iv, and 5D). Flow cytometry was also used to analyze DNA content. Although these analyses used rifampicin, an inhibitor of transcription initiation, to prevent replication reinitiation and simplify DNA content analysis, comparison of rpoB+ with rpoB[H1244Q] revealed that rpoB[H1244Q] reduced the impact of RecD2 on chromosomal DNA content in both rep+ and Δrep cells (compare i and ii in Fig. 5 E and F). However, suppression by rpoB[H1244Q] in Δrep cells was only partial, with both colony sizes reduced and DNA content still perturbed significantly by high level expression of recD2 (Fig. 5C, iii and iv, and F, ii).
Other mutations in rpo genes with the ability to suppress genome duplication defects also restored colony-forming ability to Δrep cells, confirming that this suppression was not specific to rpoB[H1244Q] (Fig. 5B, v–viii). Indeed, rpoB[G1260D] (33) resulted in effective suppression of RecD2 toxicity even with high-level recD2 expression, as indicated by colony sizes and DNA content in Δrep cells (Fig. 5C, compare v and vi, and 5F, compare i and iii), suppression that was also apparent with dda expression in Δrep cells (Fig. S3C). This suppression of toxicity suggested that transcription elongation factors might also provide a key means of ameliorating the consequences of paused fork inactivation. However, the simultaneous absence of four factors known to reduce RNA polymerase pausing and backtracking (26) did not render cells sensitive to recD2 expression (Fig. S4).
These data indicate that transcription complexes are the primary cause of RecD2-directed DNA content defects in both rep+ and Δrep cells and of lethality in Δrep cells. Together with our demonstration that RecD2 inactivates paused forks in vitro, these findings indicate that the most significant cause of paused replisomes in otherwise unperturbed cells in vivo are transcription complexes. Moreover, the primary means of resuming replication from these paused replisomes is via an accessory replicative helicase.
Discussion
We have demonstrated that inactivation of paused but not elongating E. coli replisomes is effected in vitro by a heterologous helicase, RecD2. Using this as an in vivo probe we have shown that many pausing events do not lead to fork inactivation, that protein–DNA complexes, not DNA damage, are the main sources of these transient pauses in unperturbed cells, and that accessory replicative helicases are the primary means by which the frequency and/or the duration of such pauses are reduced.
Although frequent replisome pausing and subsequent resumption of replication by the same fork is often assumed for both prokaryotes and eukaryotes (14, 24), there is little direct evidence to support such views. Indeed, fork pausing is not an inherent property of the replisome itself (36). Use of RecD2 to deactivate paused but still active replisomes has allowed us to probe such pausing events in vivo. Expression of this helicase in wild-type cells, although not lethal, did have a dramatic impact on chromosomal DNA content (Fig. 4E). Thus, replisomes do pause and subsequently continue duplication at a frequency that has the potential to perturb chromosome metabolism significantly. Although in eukaryotes the multiple origins per chromosome together with dormant but activatable origins provide mechanisms not available in bacteria to rescue inactivated forks (25), it is becoming apparent that inactivation of paused forks contributes to gross chromosomal rearrangements in higher organisms (7). Our data provide a direct demonstration of the major hidden potential for this initiation of genome instability during the course of chromosome duplication.
The survival of wild-type cells upon exposure to RecD2 allowed screening for mechanisms that ameliorate the consequences of paused fork inactivation. Repair of RecD2-inactivated forks by recombination enzymes was not critical for survival (Fig. 4 and Table S1), suggesting that mechanisms that act before fork inactivation are important. Removal of DNA lesions was not required to survive recD2 expression, but Rep-promoted duplication of protein-bound DNA was critical for survival (Fig. 4 A, D, and E). Forks paused at both template damage and protein–DNA complexes were inactivated by RecD2 in vitro (Figs. 1 and 3), and so an inability to inactivate forks paused at DNA lesions could not explain this differential RecD2 toxicity. Protein–DNA complexes, rather than DNA lesions, therefore dominate the formation of paused but active replication forks in otherwise unperturbed cells. This conclusion is supported by the suppression of RecD2 toxicity in both wild-type and Δrep cells by mutations in RNA polymerase (Fig. 5), a known source of nucleoprotein barriers to replication in both prokaryotes and eukaryotes (19, 32). Moreover, cells lacking four factors known to underpin RNA polymerase movement did not display hypersensitivity to recD2 expression, in contrast to cells lacking Rep (Fig. S4). An accessory replicative helicase, rather than transcription elongation factors, provides therefore a key mechanism for minimizing replisome pausing.
The lack of requirement for transcription elongation factors to minimize formation of paused but still active forks is in apparent conflict with the requirement for such factors to minimize the need for repair of stalled forks by recombination enzymes (20). However, recombination enzymes such as RuvABC act on forks that no longer possess an active replisome (24) rather than on those that are paused but still retain function. The implication is that transcription factors are important within the context of inactive forks that require replisome reloading via recombination enzymes. Perhaps this requirement reflects the critical importance of such factors in minimizing the formation of backed-up arrays of stalled transcription complexes, formidable obstacles to replication that might not be surmountable by paused, active replisomes (20).
There is cross-talk between DNA damage, transcription complexes, and replication fork pausing. Lesions are potent blocks to RNA as well as DNA polymerases and could contribute to the generation of paused replisomes. However, the ability of cells with defects in excision repair, including transcription-coupled repair, to survive expression of recD2 indicates that transcription complexes stalled at DNA lesions are not major causes of paused but active replisomes (Fig. 4A). Other factors such as template-directed RNA polymerase pausing, a ubiquitous feature of gene expression, must therefore dominate the generation of transcriptional barriers to replication (35).
Our data indicate that protein–DNA complexes are the most frequently encountered sources of active replisome pausing in the absence of elevated levels of DNA-damaging agents. However, the relative importance of nucleoprotein complexes versus DNA lesions will be affected by environmental sources of DNA damage as well as intracellular factors. The duration as well as the frequency of replisome pausing at leading strand lesions must also be considered with respect to genome stability. Pausing at nucleoprotein complexes is dictated by the rate of dissociation of the protein from the duplex template, with accessory replicative helicases effectively reducing the half-life of the barrier (10). It is not known whether excision repair enzymes can access DNA lesions within the context of a paused replisome to similarly reduce the barrier half-life. Lesion-induced pausing of replisomes might therefore be less frequent but of greater duration than pausing at protein–DNA complexes in vivo.
Bacterial superfamily 1 helicases with a 3′-5′ polarity of translocation can promote fork movement along protein-bound DNA (10). Here we have shown that superfamily 1 5′-3′ helicases not only lack accessory helicase function in bacteria but also catalyze inactivation of paused replisomes. Although we have been unable to identify the mechanism by which RecD2 inactivates paused E. coli replisomes, our data provide a dramatic demonstration of the critical importance of regulating helicase activity. If RecD2 has the capacity to inactivate paused replisomes in D. radiodurans as well as E. coli, then RecD2 activity must be regulated stringently in this organism. Indeed, the extreme toxicity of RecD2 in E. coli suggests that multiple levels of regulation may be needed to ensure helicase activities are unleashed only where and when required. This challenge may be compounded in more complex organisms by the greater numbers and diversity of these potentially toxic motors needed to sustain nucleic acid metabolism.
Methods
Plasmids, Proteins, and Strains.
Construction of pPM561, pBADrecD2, pBADrecD2pinless, pBADdda, and pMG31 are described in SI Methods.
LacI, Rep, UvrD, β, HU, DnaA, DnaB, DnaC, DnaG, single-stranded binding protein (SSB), and DNA polymerase III α, ε, θ, τ, χ, ψ, δ, and δ′ subunits were purified as described (10, 13, 37, 38). B. stearothermophilus PcrA, D. radiodurans wild-type and pinless RecD2, E. coli DNA gyrase, and T4 Dda were kind gifts of Panos Soultanas (University of Nottingham, Nottingham, UK), Dale Wigley (Chester Beatty Laboratories, Institute of Cancer Research, London, UK), Tony Maxwell (John Innes Centre, Norwich, UK), and Kevin Raney (University of Arkansas for Medical Sciences, Little Rock, AR). EagI was supplied by New England Biolabs. All protein concentrations refer to the monomer.
Strains are listed in Table S2.
In Vitro Replication Assays.
Assays to monitor the functionality of forks blocked by nucleoprotein complexes or positive supercoiling are described in SI Methods. To analyze the impact of test helicases on the ability of replisomes to replicate plasmid DNA in the absence of a replicative block (Fig. 2A), reactions containing pPM561 were assembled containing DNA gyrase (140 nM) but without LacI and replication was initiated as outlined in SI Methods. For analysis of replisome stability at a cyclobutane pyrimidine dimer, proteins and DNA template were prepared as described (11) and reactions performed as described in SI Methods.
Colony Formation Assays and Flow Cytometry.
Strains were transformed with the indicated plasmids and colonies selected on LB agar containing either 100 μg⋅mL−1 ampicillin (pMG31) or 30 μg⋅mL−1 kanamycin (pBADrecD2, pBADrecD2pinless, and pBADdda) at 37 °C overnight. Single colonies were grown subsequently in LB broth plus either ampicillin or kanamycin at 37 °C to an A650 of 0.4 before serial 10-fold dilutions were made with 56/2 salts on ice. Five microliters of the dilutions were then spotted onto LB agar containing ampicillin or kanamycin plus the indicated concentrations of arabinose. Plates were incubated at 37 °C for 16 h. Flow cytometry was performed on midlog phase cultures after treatment with rifampicin and cephalexin as described in SI Methods.
Supplementary Material
Acknowledgments
We thank Panos Soultanas, Dale Wigley, Kevin Raney, and Tony Maxwell for supplying proteins; Steve Sandler for supplying SS1076; and Bénédicte Michel for supplying JJC735. This work was supported by Biotechnology and Biological Sciences Research Council Grants BB/G005915/1 and BB/I001859/1 (to P.M.), National Institutes of Health Grant GM34557 (to K.J.M.), and Medical Research Council Grant G0800970 (to R.G.L.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1303890110/-/DCSupplemental.
References
- 1.French S. Consequences of replication fork movement through transcription units in vivo. Science. 1992;258(5086):1362–1365. doi: 10.1126/science.1455232. [DOI] [PubMed] [Google Scholar]
- 2.Lindahl T, Wood RD. Quality control by DNA repair. Science. 1999;286(5446):1897–1905. doi: 10.1126/science.286.5446.1897. [DOI] [PubMed] [Google Scholar]
- 3.Paeschke K, Capra JA, Zakian VA. DNA replication through G-quadruplex motifs is promoted by the Saccharomyces cerevisiae Pif1 DNA helicase. Cell. 2011;145(5):678–691. doi: 10.1016/j.cell.2011.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heller RC, Marians KJ. Replisome assembly and the direct restart of stalled replication forks. Nat Rev Mol Cell Biol. 2006;7(12):932–943. doi: 10.1038/nrm2058. [DOI] [PubMed] [Google Scholar]
- 5.Horiuchi T, Fujimura Y. Recombinational rescue of the stalled DNA replication fork: A model based on analysis of an Escherichia coli strain with a chromosome region difficult to replicate. J Bacteriol. 1995;177(3):783–791. doi: 10.1128/jb.177.3.783-791.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lambert S, Watson A, Sheedy DM, Martin B, Carr AM. Gross chromosomal rearrangements and elevated recombination at an inducible site-specific replication fork barrier. Cell. 2005;121(5):689–702. doi: 10.1016/j.cell.2005.03.022. [DOI] [PubMed] [Google Scholar]
- 7.Mizuno K, Miyabe I, Schalbetter SA, Carr AM, Murray JM. Recombination-restarted replication makes inverted chromosome fusions at inverted repeats. Nature. 2013;493(7431):246–249. doi: 10.1038/nature11676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pomerantz RT, O’Donnell M. The replisome uses mRNA as a primer after colliding with RNA polymerase. Nature. 2008;456(7223):762–766. doi: 10.1038/nature07527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pomerantz RT, O’Donnell M. Direct restart of a replication fork stalled by a head-on RNA polymerase. Science. 2010;327(5965):590–592. doi: 10.1126/science.1179595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guy CP, et al. Rep provides a second motor at the replisome to promote duplication of protein-bound DNA. Mol Cell. 2009;36(4):654–666. doi: 10.1016/j.molcel.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yeeles JT, Marians KJ. The Escherichia coli replisome is inherently DNA damage tolerant. Science. 2011;334(6053):235–238. doi: 10.1126/science.1209111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ivessa AS, et al. The Saccharomyces cerevisiae helicase Rrm3p facilitates replication past nonhistone protein-DNA complexes. Mol Cell. 2003;12(6):1525–1536. doi: 10.1016/s1097-2765(03)00456-8. [DOI] [PubMed] [Google Scholar]
- 13.Payne BT, et al. Replication fork blockage by transcription factor-DNA complexes in Escherichia coli. Nucleic Acids Res. 2006;34(18):5194–5202. doi: 10.1093/nar/gkl682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alzu A, et al. Senataxin associates with replication forks to protect fork integrity across RNA-polymerase-II-transcribed genes. Cell. 2012;151(4):835–846. doi: 10.1016/j.cell.2012.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rupp WD, Howard-Flanders P. Discontinuities in the DNA synthesized in an excision-defective strain of Escherichia coli following ultraviolet irradiation. J Mol Biol. 1968;31(2):291–304. doi: 10.1016/0022-2836(68)90445-2. [DOI] [PubMed] [Google Scholar]
- 16.McInerney P, O’Donnell M. Functional uncoupling of twin polymerases: mechanism of polymerase dissociation from a lagging-strand block. J Biol Chem. 2004;279(20):21543–21551. doi: 10.1074/jbc.M401649200. [DOI] [PubMed] [Google Scholar]
- 17.Marians KJ, Hiasa H, Kim DR, McHenry CS. Role of the core DNA polymerase III subunits at the replication fork. α is the only subunit required for processive replication. J Biol Chem. 1998;273(4):2452–2457. doi: 10.1074/jbc.273.4.2452. [DOI] [PubMed] [Google Scholar]
- 18.McGlynn P, Guy CP. Replication forks blocked by protein-DNA complexes have limited stability in vitro. J Mol Biol. 2008;381(2):249–255. doi: 10.1016/j.jmb.2008.05.053. [DOI] [PubMed] [Google Scholar]
- 19.Deshpande AM, Newlon CS. DNA replication fork pause sites dependent on transcription. Science. 1996;272(5264):1030–1033. doi: 10.1126/science.272.5264.1030. [DOI] [PubMed] [Google Scholar]
- 20.Trautinger BW, Jaktaji RP, Rusakova E, Lloyd RG. RNA polymerase modulators and DNA repair activities resolve conflicts between DNA replication and transcription. Mol Cell. 2005;19(2):247–258. doi: 10.1016/j.molcel.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 21.Lehmann AR. Postreplication repair of DNA in ultraviolet-irradiated mammalian cells. J Mol Biol. 1972;66(3):319–337. doi: 10.1016/0022-2836(72)90418-4. [DOI] [PubMed] [Google Scholar]
- 22.Boubakri H, de Septenville AL, Viguera E, Michel B. The helicases DinG, Rep and UvrD cooperate to promote replication across transcription units in vivo. EMBO J. 2010;29(1):145–157. doi: 10.1038/emboj.2009.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saikrishnan K, Griffiths SP, Cook N, Court R, Wigley DB. DNA binding to RecD: Role of the 1B domain in SF1B helicase activity. EMBO J. 2008;27(16):2222–2229. doi: 10.1038/emboj.2008.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Atkinson J, McGlynn P. Replication fork reversal and the maintenance of genome stability. Nucleic Acids Res. 2009;37(11):3475–3492. doi: 10.1093/nar/gkp244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Petermann E, Helleday T. Pathways of mammalian replication fork restart. Nat Rev Mol Cell Biol. 2010;11(10):683–687. doi: 10.1038/nrm2974. [DOI] [PubMed] [Google Scholar]
- 26.McGlynn P, Savery NJ, Dillingham MS. The conflict between DNA replication and transcription. Mol Microbiol. 2012;85(1):12–20. doi: 10.1111/j.1365-2958.2012.08102.x. [DOI] [PubMed] [Google Scholar]
- 27.Atkinson J, Gupta MK, McGlynn P. Interaction of Rep and DnaB on DNA. Nucleic Acids Res. 2011;39(4):1351–1359. doi: 10.1093/nar/gkq975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sandler SJ. Multiple genetic pathways for restarting DNA replication forks in Escherichia coli K-12. Genetics. 2000;155(2):487–497. doi: 10.1093/genetics/155.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heller RC, Marians KJ. Unwinding of the nascent lagging strand by Rep and PriA enables the direct restart of stalled replication forks. J Biol Chem. 2005;280(40):34143–34151. doi: 10.1074/jbc.M507224200. [DOI] [PubMed] [Google Scholar]
- 30.Atkinson J, et al. Localization of an accessory helicase at the replisome is critical in sustaining efficient genome duplication. Nucleic Acids Res. 2011;39(3):949–957. doi: 10.1093/nar/gkq889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McGlynn P. Helicases at the replication fork. Adv Exp Med Biol. 2013;767:97–121. doi: 10.1007/978-1-4614-5037-5_5. [DOI] [PubMed] [Google Scholar]
- 32.McGlynn P, Lloyd RG. Modulation of RNA polymerase by (p)ppGpp reveals a RecG-dependent mechanism for replication fork progression. Cell. 2000;101(1):35–45. doi: 10.1016/S0092-8674(00)80621-2. [DOI] [PubMed] [Google Scholar]
- 33.Trautinger BW, Lloyd RG. Modulation of DNA repair by mutations flanking the DNA channel through RNA polymerase. EMBO J. 2002;21(24):6944–6953. doi: 10.1093/emboj/cdf654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baharoglu Z, Lestini R, Duigou S, Michel B. RNA polymerase mutations that facilitate replication progression in the rep uvrD recF mutant lacking two accessory replicative helicases. Mol Microbiol. 2010;77(2):324–336. doi: 10.1111/j.1365-2958.2010.07208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dutta D, Shatalin K, Epshtein V, Gottesman ME, Nudler E. Linking RNA polymerase backtracking to genome instability in E. coli. Cell. 2011;146(4):533–543. doi: 10.1016/j.cell.2011.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yao NY, Georgescu RE, Finkelstein J, O’Donnell ME. Single-molecule analysis reveals that the lagging strand increases replisome processivity but slows replication fork progression. Proc Natl Acad Sci USA. 2009;106(32):13236–13241. doi: 10.1073/pnas.0906157106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Atkinson J, et al. Stimulation of UvrD helicase by UvrAB. J Biol Chem. 2009;284(14):9612–9623. doi: 10.1074/jbc.M808030200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marians KJ. ϕ X174-type primosomal proteins: Purification and assay. Methods Enzymol. 1995;262:507–521. doi: 10.1016/0076-6879(95)62042-7. [DOI] [PubMed] [Google Scholar]
- 39.Lane HE, Denhardt DT. The rep mutation. III. Altered structure of the replicating Escherichia coli chromosome. J Bacteriol. 1974;120(2):805–814. doi: 10.1128/jb.120.2.805-814.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.